Abstract
Objective
Randomised trials of type I anti-CD20 antibodies rituximab and ocrelizumab failed to show benefit in proliferative lupus nephritis (LN). We compared obinutuzumab, a humanised type II anti-CD20 monoclonal antibody that induces potent B-cell depletion, with placebo for the treatment of LN in combination with standard therapies.
Methods
Patients with LN receiving mycophenolate and corticosteroids were randomised to obinutuzumab 1000 mg or placebo on day 1 and weeks 2, 24 and 26, and followed through week 104. The primary endpoint was complete renal response (CRR) at week 52. Exploratory analyses through week 104 were conducted. The prespecified alpha level was 0.2.
Results
A total of 125 patients were randomised and received blinded infusions. Achievement of CRR was greater with obinutuzumab at week 52 (primary endpoint, 22 (35%) vs 14 (23%) with placebo; percentage difference, 12% (95% CI −3.4% to 28%), p=0.115) and at week 104 (26 (41%) vs 14 (23%); percentage difference, 19% (95% CI 2.7% to 35%), p=0.026). Improvements in other renal response measures, serologies, estimated glomerular filtration rate and proteinuria were greater with obinutuzumab. Obinutuzumab was not associated with increases in serious adverse events, serious infections or deaths. Non-serious infusion-related reactions occurred more frequently with obinutuzumab.
Conclusions
Improved renal responses through week 104 were observed in patients with LN who received obinutuzumab plus standard therapies compared with standard therapies alone. Obinutuzumab was well tolerated and no new safety signals were identified.
Trial registration number
Keywords: lupus nephritis, lupus erythematosus, systemic, rituximab
Key messages.
What is already known about this subject?
Although two randomised, placebo-controlled clinical trials of the type I anti-CD20 antibodies rituximab and ocrelizumab in patients with lupus nephritis failed to show a difference vs placebo in the primary endpoint of complete renal response, subsequent analyses suggested that the rapidity, depth and duration of peripheral B-cell depletion was associated with renal response.
Obinutuzumab is a type II anti-CD20 antibody that results in greater B-cell depletion than rituximab; in a preclinical study, obinutuzumab was shown to be more effective than rituximab in a murine model of lupus nephritis.
What does this study add?
In this randomised, placebo-controlled, phase 2 trial (NOBILITY), obinutuzumab was superior to placebo for the achievement of complete and overall renal responses at week 52 when added to mycophenolate and corticosteroids; improved renal responses with obinutuzumab compared with placebo continued through week 104.
Obinutuzumab resulted in rapid and potent depletion of peripheral B cells without an increase in the incidence of serious adverse events, serious infections or death compared with placebo.
How might this impact on clinical practice or future developments?
Compared with standard-of-care therapy alone, NOBILITY showed that obinutuzumab on a background of standard-of-care therapies improved renal responses through 104 weeks without increasing the frequency of serious adverse events. Based on the results from this study, the use of obinutuzumab in proliferative lupus nephritis is being further evaluated in a global phase 3 study (NCT04221477).
Introduction
Proliferative lupus nephritis (LN) is the most common severe organ-threatening manifestation of systemic lupus erythematosus (SLE). The goal of treatment is to preserve kidney function and avoid the need for kidney replacement therapy while minimising the toxicities of therapy.1 2 The 15-year risk of patients with LN developing end-stage kidney disease (ESKD) is approximately 20%, with even greater risk occurring in class IV proliferative LN. This risk has not substantially lessened in the last 20 years despite the use of potent immunosuppressive therapies.3 4
B cells are recognised as key mediators of SLE pathogenesis.5 However, randomised, placebo-controlled trials of the type I anti-CD20 antibodies rituximab and ocrelizumab failed to demonstrate increases in rates of complete renal response (CRR) when added to standard-of-care immunosuppression.6–8 Substantial variability in the degree of B-cell depletion has been observed following rituximab administration to patients with SLE, and the presence of residual B cells in peripheral blood after rituximab treatment has been associated with inferior clinical responses in SLE and LN.9–12 Resistance to B-cell depletion by type I anti-CD20 antibodies in SLE may occur via Fcγ receptor IIB (FcγRIIB)–mediated internalisation of CD20, ineffective complement-dependent cytotoxicity, decreased engagement of effector cells due to natural killer cell defects or Fc receptor polymorphisms and acquired deficiencies in antibody-dependent cellular phagocytosis.12–15
Obinutuzumab is a humanised, type II anti-CD20 monoclonal antibody that has a distinct mode of binding to the CD20 antigen compared with type I anti-CD20 antibodies and is glycoengineered for greater affinity for the FcγRIII on effector cells. These properties promote greater antibody-dependent cellular cytotoxicity, superior direct B-cell killing, and, thus, less reliance on complement-dependent cytotoxicity than type I anti-CD20 antibodies.16 Because obinutuzumab does not elicit CD20 redistribution to membrane-bound lipid rafts or activate FcγRIIB, it is associated with reduced CD20 internalisation compared with type I anti-CD20 antibodies.14 17 18 Clinical superiority of obinutuzumab to rituximab for the treatment of chronic lymphocytic leukaemia and follicular lymphoma, when administered in combination with standard chemotherapy, has been demonstrated.19 20 Obinutuzumab exhibited greater B-cell cytotoxicity and activation of natural killer cells than rituximab in SLE patient samples and was more effective than rituximab in the treatment of murine LN.13 14 21
The NOBILITY trial was conducted to test the hypothesis that enhanced B-cell depletion with obinutuzumab would increase the rate of CRR when added to background standard of care compared with standard of care alone. We report the results of a phase 2, multicentre, randomised, double-blind trial comparing obinutuzumab with placebo in patients with proliferative LN treated with mycophenolate and corticosteroids.
Methods
Study design
This multicentre, double-blind, phase 2, randomised, controlled trial was performed at 43 sites in North America, South America, Europe and Israel. This trial was executed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All patients provided informed consent. Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Patients
Eligible adults were aged 18–75 years, had SLE by American College of Rheumatology classification 1997 criteria,22 kidney biopsy evidence of International Society of Nephrology/Renal Pathology Society 200323 class III or IV active or active/chronic LN within 6 months of screening (concomitant class V was permitted), urine protein-to-creatinine ratio (UPCR) >1 from a 24-hour urine collection, and estimated glomerular filtration rate (eGFR) of ≥30 mL/min/1.73 m2. The full protocol is available in online supplemental file.
annrheumdis-2021-220920supp001.pdf (120.7KB, pdf)
Randomisation and masking
Patients were randomly assigned (1:1) to receive either obinutuzumab 1000 mg or placebo infusions. Randomisation was performed using an interactive web response system and stratified by race (Afro-Caribbean/African American vs others) and region (USA vs non-USA). Randomisation codes were kept within the interactive web response system for patients and investigators to remain masked to treatment allocation. The sponsor was masked to treatment allocation up to the week 52 database lock.
Procedures
Obinutuzumab was administered as a blinded intravenous infusion of 1000 mg on day 1 and weeks 2, 24 and 26, after premedication with blinded methylprednisolone 80 mg intravenous to reduce the risk of infusion-related reactions. Patients randomly assigned to placebo received an intravenous placebo infusion on day 1 and weeks 2, 24 and 26 after infusion of placebo methylprednisolone. All patients received mycophenolate mofetil (MMF) (target dose 2–2.5 g/day or equivalent dose of mycophenolic acid). Protocol-mandated corticosteroid treatment included methylprednisolone (a total of 1000–3000 mg intravenous) and an oral corticosteroid regimen (initial prednisone dose: 0.5 mg/kg/day, maximum 60 mg/day, with taper to 7.5 mg/day by week 12). It was recommended that patients receive antimalarial medications, an ACE inhibitor or angiotensin receptor blocker, calcium and vitamin D at stable doses throughout the study. All patients were followed in a blinded fashion through week 104, and patients with persistent B-cell depletion were followed for safety and B-cell measurements thereafter.
Urinary protein excretion (measured by UPCR from a 24-hour urine collection and/or spot UPCR, preferably from a first morning void), serum creatinine, levels of autoantibodies and serum complement components were assessed at weeks 4, 12, 24, 36, 52, 76 and 104. Peripheral blood B-cells were measured at baseline and at weeks 2, 4, 12, 24, 52 and 104. Laboratory assessments were performed at a centralised laboratory. B cells were measured using a validated 6-colour, lyse/no-wash flow cytometry assay. Complement components were measured by immunonephelometry and anti-dsDNA titres by ELISA.
Outcome measures
The primary endpoint at week 52 was the proportion of patients who achieved CRR, a composite measure requiring UPCR <0.5, normal renal function (serum creatinine ≤ULN) without worsening of baseline serum creatinine by more than 15%, and inactive urinary sediment (<10 red blood cells (RBCs)/high-power field (HPF) without RBC casts). Patients who received rescue therapies such as cyclophosphamide, rituximab, tacrolimus or pulse-dose corticosteroids (equivalent to methylprednisolone 500 mg or greater) after baseline or who withdrew from the study prematurely were imputed as non-responders for all subsequent response endpoints.
Major secondary endpoints at week 52 were proportion of patients achieving a partial renal response (PRR), a composite measure requiring ≥50% reduction in UPCR from baseline to a value <1 (to <3 if baseline UPCR was ≥3), serum creatinine not increased >15% from baseline and urinary RBCs <10/HPF or ≤50% increase over the baseline value; proportion of patients achieving an overall renal response (ORR), which was met if CRR or PRR was achieved; proportion of patients achieving variations of the definition of CRR, including modified CRR (mCRR), a composite measure requiring UPCR <0.5 g/g and serum creatinine ≤ULN; changes in C3, C4 and anti-dsDNA antibody levels from baseline; and time to CRR and ORR. Additional prespecified endpoints included change in eGFR from baseline (as calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation) and achievement of renal responses at other time points. A post hoc endpoint, the proportion of patients achieving UPCR <0.8 g/g, was added based on the predictive value of this cut-off for long-term outcome.24 Exploratory analyses were conducted to assess these measures at week 104.
Statistical analyses
Assuming a proportion of CRR responders at week 52 of 30% in the placebo group6 7 and 50% in the obinutuzumab group (difference, 20%), 60 patients in each group were projected to yield 83% power to detect a significant difference using the Cochran-Mantel-Haenszel (CMH) test at a two-sided alpha of 0.2 for this proof-of-concept study. To control for type I error rate for the primary and secondary endpoints, hypothesis testing was conducted using a fixed sequence method, proceeding sequentially in a prespecified order starting from the primary endpoint and testing each endpoint after achieving statistical significance on the previous endpoint at an alpha of 0.2. Type I error rate was not controlled for exploratory analyses.
Efficacy analyses were done in a modified intention-to-treat population consisting of all randomised patients who had received ≥1 dose of study drug. Safety analyses were grouped according to the treatment received. Infusion-related reactions were defined as any adverse event that occurred during or within 24 hours after infusion of obinutuzumab or placebo and was judged to be related to the infusion. Descriptive statistics were used to evaluate safety.
Renal response endpoints and other categorical variables were evaluated by CMH test accounting for the stratification factors. Change from baseline endpoints were analysed by analysis of covariance model with baseline measurement and the stratification factors as covariates. All statistical analyses were performed using SAS, V.9.4. An independent data monitoring committee regularly reviewed unblinded interim data.
Results
Patients
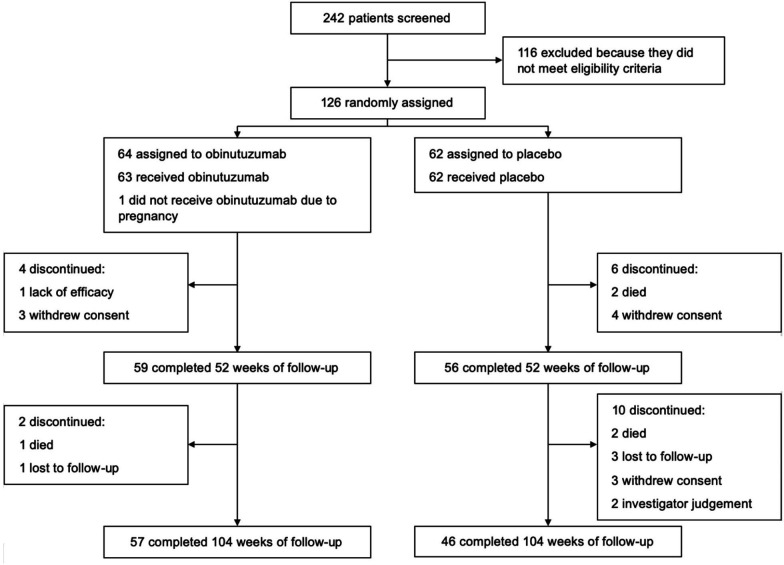
Patients were enrolled from November 2015 through December 2017. Final data collection was on 19 December 2019. Two hundred and forty-two patients were screened, of whom 125 were randomised and received placebo (n=62) or obinutuzumab (n=63) in addition to mycophenolate and corticosteroids in Latin America and the Caribbean (n=85), Europe and Israel (n=25) and the USA (n=15). The most common reason for screen failure was failure to meet the eligibility criteria. A total of 115 patients (92%) completed 52 weeks, and 103 patients (82%) completed 104 weeks of the protocol (figure 1).
Figure 1.
Patient flow diagram.
Women comprised 85% of the study cohort, and the mean age was 33 years. Seventy-three per cent self-identified as Hispanic or Latino, and 43% were white. A total of 74% had class IV LN; the remainder had class III LN. Concomitant class V LN was present in 30%. Mean baseline values (±SD) were UPCR: 3.12±2.56; serum creatinine: 0.84±0.33 mg/dL; and eGFR: 102.0±31.7 mL/min/1.73 m2. The patients’ disease characteristics at baseline were similar between treatment groups (table 1).
Table 1.
Baseline characteristics and demographics
| Obinutuzumab (n=63) |
Placebo (n=62) |
|
| Age—years | 33.1±9.8 | 31.9±10.1 |
| Female—no (%) | 55 (87) | 51 (82) |
| Region—no (%) | ||
| Latin America and the Caribbean | 38 (60) | 47 (76) |
| Europe and Israel | 18 (29) | 7 (11) |
| USA | 7 (11) | 8 (13) |
| Hispanic or Latino ethnicity—no (%) | 42 (67) | 49 (79) |
| Race—no (%) | ||
| White | 28 (44) | 26 (42) |
| American Indian or Alaska Native | 11 (18) | 17 (27) |
| Black or African American | 6 (10) | 5 (8) |
| Asian | 3 (5) | 2 (3) |
| Other or unknown | 15 (24) | 12 (20) |
| Prior history of lupus nephritis—no (%) | 32 (51) | 32 (52) |
| Class IV lupus nephritis—no (%) | 40 (64) | 35 (57) |
| Concomitant class V lupus nephritis—no (%) | 20 (32) | 17 (27) |
| Serum creatinine—mg/dL | 0.87±0.34 | 0.80±0.33 |
| eGFR—mL/min/1.73 m2 | 102.0±30.6 | 102.1±32.9 |
| UPCR—g/g | 3.3±2.7 | 2.9±2.5 |
| Anti-dsDNA Ab >30 IU/mL—no (%) | 42 (67) | 46 (74) |
| C3 <90 mg/dL—no (%) | 43 (68) | 37 (60) |
| C4 <16 mg/dL—no (%) | 37 (59) | 44 (71) |
eGFR was calculated using the CKD-EPI creatinine equation.
Ab, antibody; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; ULN, upper limit of normal; UPCR, urine protein-to-creatinine ratio.
Efficacy
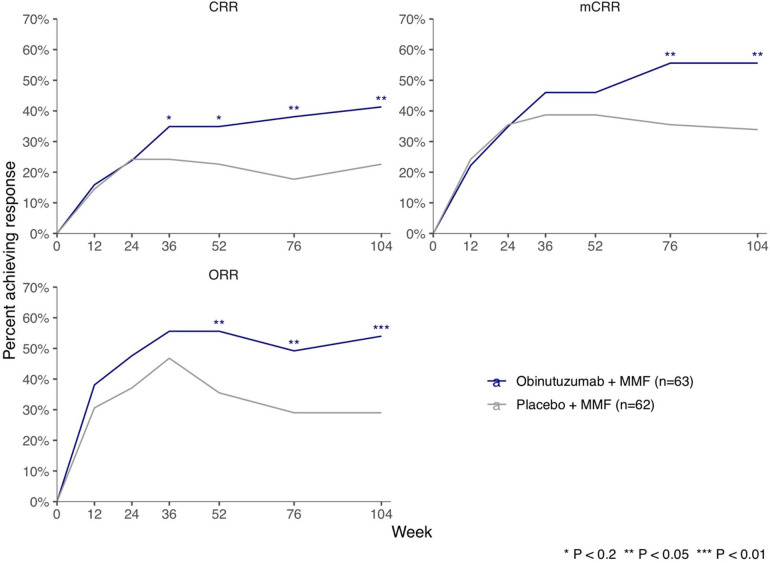
A significantly greater proportion of patients in the obinutuzumab group achieved CRR than in the placebo group at week 52 (primary endpoint, 22 of 63 patients (35%) in the obinutuzumab group vs 14 of 62 patients (23%) in the placebo group; percentage difference, 12% (95% CI −3.4% to 28%), p=0.115) and week 104 (26 of 63 patients (41%) in the obinutuzumab group vs 14 of 62 patients (23%) in the placebo group; percentage difference, 19% (95% CI 2.7% to 35%), p=0.026) (table 2, figure 2). A significantly greater proportion of patients in the obinutuzumab group achieved CRR and ORR (CRR or PRR) at weeks 52, 76 and 104 and mCRR at weeks 76 and 104 (figure 2).
Table 2.
Primary and secondary endpoints at weeks 52 and 104
| Week 52 | Week 104* | |||||||
| Obinutuzumab (n=63) | Placebo (n=62) |
Difference (95% CI) | P value | Obinutuzumab (n=63) | Placebo (n=62) |
Difference (95% CI) | P value | |
| Primary endpoint | ||||||||
| CRR, n (%) | 22 (35) | 14 (23) | 12 (−3.4 to 28) | 0.115 | 26 (41) | 14 (23) | 19 (2.7 to 35) | 0.026 |
| Secondary endpoints | ||||||||
| mCRR, n (%) | 29 (46) | 24 (39) | 7 (−10 to 25) | 0.373 | 35 (56) | 21 (34) | 22 (5 to 39) | 0.015 |
| ORR (CRR or PRR), n (%) | 35 (56) | 22 (36) | 20 (3.0 to 37) | 0.025 | 34 (54) | 18 (29) | 25 (8.2 to 42) | 0.005 |
| Change in C3 from baseline, mean† (SE) | 30 (3.4) | 12 (3.5) | 18 (8.0 to 27) | <0.001 | 29 (3.4) | 11 (3.4) | 19 (8.9 to 28) | <0.001 |
| Change in C4 from baseline, mean† (SE) | 9.7 (1.3) | 0.8 (1.3) | 8.8 (5.2 to 12) | <0.001 | 9.6 (1.3) | 0.4 (1.3) | 9.3 (5.7 to 13) | <0.001 |
| Change in log anti-dsDNA titre from baseline, mean† (SE) | −0.91 (0.12) | −0.10 (0.12) | −0.81 (−1.1 to 0.48) | <0.001 | −1.1 (0.13) | −0.05 (0.13) | −1.0 (−1.4 to 0.67) | <0.001 |
| Renal response components | ||||||||
| UPCR <0.5, n (%) | 33 (52) | 24 (39) | 14 (−3.6 to 31) | 0.102 | 39 (62) | 23 (37) | 25 (7.8 to 42) | 0.005 |
| SCr ≤15% increase from baseline and ≤ULN | 48 (76) | 38 (61) | 15 (−1.2 to 31) | 0.080 | 45 (71) | 32 (52) | 20 (3.1 to 37) | 0.019 |
| Urinary RBCs <10/HPF without RBC casts | 52 (83) | 51 (82) | 0.3 (−13 to 13) | 0.987 | 49 (78) | 41 (66) | 12 (−4.0 to 27) | 0.154 |
| No rescue immunosuppression or early discontinuation | 57 (91) | 53 (86) | 5 (−6.4 to 16) | 0.414 | 51 (81) | 38 (61) | 20 (4.1 to 35) | 0.012 |
| CRR in prespecified subgroups | ||||||||
| Baseline proteinuria, n (%) | ||||||||
| UPCR <3 (n=73) | 13 (38) | 12 (31) | 7.5 (−14 to 29) | 0.468 | 16 (47) | 12 (31) | 16 (−5.9 to 39) | 0.147 |
| UPCR ≥3 (n=47) | 8 (31) | 2 (10) | 21 (−0.5 to 43) | 0.163 | 8 (31) | 2 (10) | 21 (−0.5 to 43) | 0.098 |
| Baseline biopsy class, n (%) | ||||||||
| Class III (n=31) | 5 (36) | 6 (35) | 0.4 (−33 to 34) | 0.952 | 3 (21) | 7 (41) | −19 (−52 to 12) | 0.338 |
| Class IV (n=94) | 17 (35) | 8 (18) | 17 (−0.5 to 34) | 0.068 | 23 (47) | 7 (16) | 31 (14 to 49) | 0.001 |
| Baseline biopsy class, n (%) | ||||||||
| No class V (n=88) | 17 (40) | 9 (20) | 20 (0.8 to 38) | 0.054 | 17 (40) | 10 (22) | 17 (−1.7 to 36) | 0.117 |
| Class V (n=37) | 5 (25) | 5 (29) | −4.4 (−33 to 24) | 0.825 | 9 (45) | 4 (24) | 22 (−8.2 to 51) | 0.187 |
| Post hoc endpoints | ||||||||
| UPCR <0.8, n (%) | 41 (65) | 31 (50) | 15 (-2.1 to 32) | 0.085 | 45 (71) | 28 (45) | 26 (9.6 to 43) | 0.003 |
For all response analyses, non-response imputation was used after rescue immunosuppression or early discontinuation.
*Week 104 analyses were exploratory and not adjusted for multiplicity.
†Adjusted mean from analysis of covariance model adjusting baseline measurement and stratification factors race and region.
CRR, complete renal response (which required UPCR <0; CRR, complete renal response; HPF, high-power field; mCRR, modified CRR; ORR, overall renal response; PRR, partial renal response; RBC, red blood cell; SCr, serum creatinine; UPCR, urine protein-to-creatinine ratio.
Figure 2.
Renal responses over time. CRR, complete renal response; mCRR, modified CRR; MMF, mycophenolate mofetil; ORR, overall renal response.
In prespecified subgroup analyses, the benefit of obinutuzumab over placebo at 104 weeks was greatest among patients with baseline UPCR ≥3 and those with class IV (as compared with class III) LN on renal biopsy (table 2). While obinutuzumab was not associated with increased CRR among patients with concomitant class V LN at week 52, the treatment effects of obinutuzumab over placebo among patients with and without concomitant class V disease were similar at week 104. A post hoc analysis showed that, compared with placebo, obinutuzumab was associated with greater achievement of UPCR <0.8 at week 104 (45 of 63 patients (71%) in the obinutuzumab group vs 28 of 62 patients (45%) in the placebo group; percentage difference, 26% (95% CI 9.6% to 43%), p=0.003).
Compared with placebo, obinutuzumab resulted in greater improvements from baseline in C3, C4 and anti-dsDNA antibodies at weeks 4 through 104 and UPCR at weeks 52 through 104 (table 2, figure 3). Obinutuzumab also resulted in greater improvement in eGFR at week 4 and weeks 24 through 104 (adjusted mean difference, 9.7 mL/min/1.73 m2 (95% CI 1.7 to 18), p=0.017). In the placebo group only, mean eGFR was decreased compared with baseline from week 24 through week 104 (figure 3).
Figure 3.
Change from baseline in laboratory parameters. Mean change from baseline was calculated with the last observation carried forward for missing data. If treatment failure occurred, the last measurement prior to treatment failure was used. eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; UPCR, urine protein-to-creatinine ratio.
By week 104, nine patients (14%) in the obinutuzumab group and 15 patients (24%) in the placebo group received one or more rescue therapies. Of these, six patients in the obinutuzumab group and 11 patients in the placebo group received rescue with cyclophosphamide or anti-CD20 therapy. The median initial (day 1) prednisone dose was 30 mg/day, and the median (IQR) cumulative corticosteroid exposure was 6561 (5938–7473) and 6672 (5785–7380) mg of prednisone equivalent in the obinutuzumab and placebo groups, respectively, inclusive of both oral and intravenous corticosteroid doses through week 104. Through week 104, the median MMF dose was 2.0 g/day in both groups. Thirty-eight patients (30%) required one or more MMF dose reductions due to adverse events, and nine patients (7%) received mycophenolic acid at some point during the trial.
Obinutuzumab resulted in rapid and sustained depletion of peripheral CD19+ B cells to ≤5 cells/µL (figure 4). In the obinutuzumab group, 98% were depleted at week 2, after one infusion, and 94% were depleted at week 52. At the next measurement, week 104, similar rates of B-cell depletion were seen in the obinutuzumab and placebo groups (16% and 12%, respectively). Depletion of memory B cells, naïve B cells, and plasmablasts, and increases in serum BAFF, were also observed with obinutuzumab (online supplemental figure 1). Obinutuzumab was associated with a rapid and sustained decrease in IgM levels compared with placebo; at week 104, the proportions of patients with IgM below the lower limit of normal were 33% and 8% for obinutuzumab and placebo groups, respectively (online supplemental table 1). In contrast, the prevalence of low IgG decreased over time in both treatment groups (9% and 4% in the obinutuzumab and placebo groups, respectively, had IgG below the lower limit of normal at week 104). Titres of preformed antibodies against tetanus, rubella and mumps did not differ between treatment groups over time (data not shown).
Figure 4.
Proportions of patients with B-cell depletion over time. B-cell depletion is defined as an absolute CD19 count ≤5 cells/µL. MMF, mycophenolate mofetil.
Safety
One patient randomised to placebo inadvertently received obinutuzumab infusions during the first cycle and was included in the obinutuzumab group for safety analyses. Through week 104, 58 of 64 patients (91%) in the obinutuzumab group and 54 of 61 patients (89%) in the placebo group had at least one adverse event (table 3). Sixteen of 64 patients (25%) in the obinutuzumab group and 18 of 61 patients (30%) in the placebo group had at least one serious adverse event (table 3); 5 of 64 patients (8%) in the obinutuzumab group and 11 of 61 patients (18%) in the placebo group had at least one serious infection. The most frequent adverse events with obinutuzumab were urinary tract infections and bronchitis.
Table 3.
Safety summary through week 104
| Obinutuzumab n=64 | Placebo n=61 | |
| Any adverse event | 58 (91) | 54 (89) |
| Deaths | 1 (2) | 4 (7) |
| Serious adverse events | 16 (25) | 18 (30) |
| Serious infection adverse events | 5 (8) | 11 (18) |
| Infection adverse event | 48 (75) | 38 (62) |
| Most common adverse events* | ||
| Urinary tract infection | 15 (23) | 13 (21) |
| Bronchitis | 12 (19) | 5 (8) |
| Herpes zoster | 9 (15) | 6 (10) |
| Abdominal pain | 7 (11) | 3 (5) |
| Infusion-related reaction | 7 (11) | 6 (10) |
| Nausea | 6 (9) | 3 (5) |
| Upper respiratory tract infection | 6 (9) | 5 (8) |
| Hypertension | 6 (9) | 3 (5) |
| Anaemia | 5 (8) | 4 (7) |
| Nasopharyngitis | 5 (8) | 6 (10) |
| Pharyngitis | 5 (8) | 4 (7) |
| Arthralgia | 5 (8) | 4 (7) |
| Headache | 5 (8) | 4 (7) |
| Conjunctivitis | 4 (6) | 2 (3) |
| Influenza | 4 (6) | 2 (3) |
| Neutropaenia | 3 (5) | 3 (5) |
| Diarrhoea | 3 (5) | 5 (8) |
| Peripheral oedema | 3 (5) | 3 (5) |
| Gastroenteritis | 3 (5) | 6 (10) |
| Sinusitis | 3 (5) | 0 |
| Insomnia | 3 (5) | 4 (7) |
| Frequent urination | 3 (5) | 0 |
| Cough | 3 (5) | 1 (2) |
| Infusion-related reaction† | 10 (16) | 6 (10) |
| Serious infusion related reaction | 0 | 0 |
| Progressive multifocal leukoencephalopathy | 0 | 1 (2) |
Data are n (%) of patients. One patient randomised to placebo inadvertently received obinutuzumab during the first cycle. This patient is included in the obinutuzumab group for safety analyses.
*Events that occurred in at least 5% of patients in the obinutuzumab group.
†Includes all treatment-related adverse events that occurred in the 24 hours from the start of blinded obinutuzumab or placebo infusions.
Infusion-related reactions, defined as any treatment-related adverse event that occurred within 24 hours of a blinded infusion, occurred in 10 of 64 patients (16%) in the obinutuzumab group and 6 of 61 patients (10%) in the placebo group. These events included headache, tachycardia, nausea and hypertension, and were most common with the first infusion. None were serious and all resolved with supportive care.
Five deaths occurred through week 104, one in the obinutuzumab group (gastrointestinal perforation) and four in the placebo group (gastrointestinal haemorrhage, refractory SLE, progressive multifocal leukoencephalopathy (PML), respiratory infection). The fatal case of PML occurred in a patient assigned to placebo who received cyclophosphamide rescue approximately 6 months prior to the diagnosis of PML.
Discussion
Obinutuzumab was superior to placebo for the achievement of CRR and ORR in patients with proliferative LN when added to mycophenolate and corticosteroids. Greater improvements in anti-dsDNA antibodies, C3, C4, eGFR and proteinuria were also observed with obinutuzumab. Obinutuzumab resulted in rapid and potent depletion of peripheral CD19+ B cells without an increase in the incidence of serious adverse events, serious infections or death compared with placebo. The treatment effect of obinutuzumab appeared to be greatest among patients with high levels of proteinuria at baseline and those with class IV LN. A similar treatment benefit was seen at week 104 among patients with and without concomitant class V disease.
We hypothesised that deeper and more durable depletion of B cells with obinutuzumab would result in superior clinical responses. NOBILITY used a similar design and patient population as the LUNAR trial, and comparison of CD19+ B cell data suggests that obinutuzumab results in more rapid, deep, and durable peripheral B-cell depletion than rituximab (online supplemental table 2).6 The results from NOBILITY support prior reports correlating the degree and duration of B-cell depletion to clinical responses in LN.9–11 Though all four doses of obinutuzumab were completed by 6 months, there was increasing clinical benefit through 24 months, implying that prolonged time may be required for healing of the kidney and achievement of CRR. Observational data from other studies indicate that short-term responses are predictive of improved long-term kidney outcomes, and, consistent with this, obinutuzumab was associated with greater preservation of eGFR over 2 years.24 25 Taken together, these observations suggest the addition of obinutuzumab to standard therapy may more effectively prevent damage accrual and thus be more likely to preserve kidney function.
B-cell depletion with obinutuzumab was not associated with increases in serious adverse events at 2 years. Obinutuzumab was associated with an increased prevalence of low IgM, but not low IgG, compared with baseline, and was not associated with reductions in concentrations of pre-existing protective antibodies, a pattern consistent with the preservation of CD20-negative long-lived plasma cells. Similar to a previous study of obinutuzumab in patients with ESKD prior to kidney transplantation,26 there were no severe infusion-related reactions or cases of severe thrombocytopaenia or neutropaenia, the most common severe toxicities seen with obinutuzumab in CLL and NHL (Gazyva US Prescribing Information; Gazyvaro EMA Summary of Product Characteristics). In CLL and NHL, patients with high levels of circulating malignant B cells appear to be at greatest risk for infusion-related reactions, which occur as a result of rapid lysis of B cells with release of proinflammatory cytokines.27 Pretreatment quantitative and/or qualitative differences in circulating B cells therefore provide a potential mechanistic basis for the apparent lower incidence and severity of infusion-related reactions and cytopeanias with obinutuzumab in non-malignant conditions. In addition, high-dose background corticosteroids may have reduced the frequency and severity of infusion-related reactions as suggested by a non-randomised study in patients with CLL comparing prolonged corticosteroid premedication or standard premedication prior to the first obinutuzumab infusion.28
Approximately two-thirds of patients in this study were enrolled from Latin American countries, and similar to other recent LN studies, only a small proportion of our study population was of African ancestry.29 30 This proof-of-concept study does not permit conclusions to be drawn regarding differences in treatment effect by region or ancestry. The use of blinded preinfusion methylprednisolone (active in the obinutuzumab group, placebo in the placebo group) prior to infusions at baseline and weeks 2, 24 and 26 could have biased towards a clinical benefit of obinutuzumab, although the durability of the observed treatment effect (through week 104) and the similarity of cumulative corticosteroid exposure between treatment groups argue against a substantial effect from this difference. Finally, this study had a limited sample size, a prespecified alpha level of 0.2, and no typeI error control for analyses after week 52; hence, these results require confirmation in a larger study.
Results from the present study indicate that B cells play a key role in LN pathogenesis and demonstrate that obinutuzumab contributes to improved clinical responses without increasing the frequency of serious safety events. Despite widespread use of immunosuppressive therapies for LN, the risk of ESKD has not been substantially reduced in recent decades.4 This underscores the critical need for more efficacious and safer therapies for patients with proliferative LN. The use of obinutuzumab in proliferative LN is being further evaluated in a global phase 3 study (NCT04221477).
Footnotes
Handling editor: Josef S Smolen
Presented at: This work was presented at the American Society of Nephrology (ASN) Kidney Week 2019, American College of Rheumatology (ACR) Annual Meeting 2019 (Furie R, Aroca G, Alvarez A, Fragoso-Loyo H, Zuta Santillan E, Rovin B, Schindler T, Hassan I, Cascino M, Garg J, Malvar A. A Phase II Randomised, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Obinutuzumab or Placebo in Combination with Mycophenolate Mofetil in Patients with Active Class III or IV Lupus Nephritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10)), ASN Kidney Week 2020, and the ACR Convergence 2020 (Furie R, Aroca G, Alvarez A, Fragoso-Loyo H, Zuta Santillan E, Rovin B, Brunetta P, Schindler T, Hassan I, Cascino M, Garg J, Malvar A. Two-Year Results from a Randomised, Controlled Study of Obinutuzumab for Proliferative Lupus Nephritis [(abstract]). Arthritis Rheumatol. 2020; 72 (suppl 10).
Contributors: RAF: conceptualisation, formal analysis, investigation methodology, writing–original draft, review, and editing; GA: resources, writing–review and editing; MC: medical monitoring, data review, data interpretation, writing–review and editing; JG: conceptualisation, protocol development, data review, data interpretation, writing–review and editing; BR: data review, data interpretation, manuscript preparation; AA: data review, data interpretation, manuscript preparation; HF-L: data review, data interpretation, manuscript preparation; EZ-S: data review, data interpretation, manuscript preparation; TS: study implementation, data review, analysis and interpretation, manuscript review; PB: original hypothesis, funding approval, protocol development, study design and implementation; CML: data review, data interpretation, manuscript preparation; IH: formal analysis, writing–review and editing; verified the underlying data; AM: writing review–and editing.
Funding: This study was sponsored by F. Hoffmann-La Roche Ltd. Support for editorial writing assistance, furnished by Health Interactions, Inc., was provided by F. Hoffmann-La Roche Ltd.
Competing interests: RAF reports personal fees from Genentech/Roche during the conduct of the study and outside the submitted work and grants from Genentech/Roche. MDC and JPG are employees and shareholders of Genentech/Roche. BHR reports personal fees from Genentech, during the conduct of the study; personal fees from Aurinia, personal fees from Bristol Myers Squibb, personal fees from Biogen, personal fees from Pfizer, personal fees from Lilly, personal fees from GlaxoSmithKline, personal fees from Mallinckrodt, personal fees from EMD Serono, personal fees from Omeros, personal fees from Calliditas, personal fees from Retrophin, personal fees from BioMarin, outside the submitted work. PB was an employee and shareholder of Genentech during the design and enrolment period. TS and IH are employees and shareholders of Roche. GA, AA, HF-L, EZ-S and AM have nothing to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The protocol was approved by the Institutional Review Board or Ethics Committee at each site.
References
- 1. Hahn BH, McMahon MA, Wilkinson A, et al. American College of rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res 2012;64:797–808. 10.1002/acr.21664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 3. Hanly JG, O'Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology 2016;55:252–62. 10.1093/rheumatology/kev311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol 2016;68:1432–41. 10.1002/art.39594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dörner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther 2011;13:243. 10.1186/ar3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum 2012;64:1215–26. 10.1002/art.34359 [DOI] [PubMed] [Google Scholar]
- 7. Mysler EF, Spindler AJ, Guzman R, et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum 2013;65:2368–79. 10.1002/art.38037 [DOI] [PubMed] [Google Scholar]
- 8. Gregersen JW, Jayne DRW. B-Cell depletion in the treatment of lupus nephritis. Nat Rev Nephrol 2012;8:505–14. 10.1038/nrneph.2012.141 [DOI] [PubMed] [Google Scholar]
- 9. Vital EM, Dass S, Buch MH, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 2011;63:3038–47. 10.1002/art.30466 [DOI] [PubMed] [Google Scholar]
- 10. Gomez Mendez LM, Cascino MD, Garg J, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol 2018;13:1502–9. 10.2215/CJN.01070118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Md Yusof MY, Shaw D, El-Sherbiny YM, et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann Rheum Dis 2017;76:1829–36. 10.1136/annrheumdis-2017-211191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anolik JH, Campbell D, Felgar RE, et al. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum 2003;48:455–9. 10.1002/art.10764 [DOI] [PubMed] [Google Scholar]
- 13. Reddy V, Cambridge G, Isenberg DA, et al. Internalization of rituximab and the efficiency of B cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol 2015;67:2046–55. 10.1002/art.39167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reddy V, Klein C, Isenberg DA, et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology 2017;56:1227–37. 10.1093/rheumatology/kex067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahuja A, Teichmann LL, Wang H, et al. An acquired defect in IgG-dependent phagocytosis explains the impairment in antibody-mediated cellular depletion in lupus. J Immunol 2011;187:3888–94. 10.4049/jimmunol.1101629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010;115:4393–402. 10.1182/blood-2009-06-225979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tipton TRW, Roghanian A, Oldham RJ, et al. Antigenic modulation limits the effector cell mechanisms employed by type I anti-CD20 monoclonal antibodies. Blood 2015;125:1901–9. 10.1182/blood-2014-07-588376 [DOI] [PubMed] [Google Scholar]
- 18. Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 2013;12:2031–42. 10.1158/1535-7163.MCT-12-1182 [DOI] [PubMed] [Google Scholar]
- 19. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014;370:1101–10. 10.1056/NEJMoa1313984 [DOI] [PubMed] [Google Scholar]
- 20. Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 2017;377:1331–44. 10.1056/NEJMoa1614598 [DOI] [PubMed] [Google Scholar]
- 21. Marinov AD, Wang H, Bastacky SI, et al. The type II anti-CD20 antibody Obinutuzumab (GA101) is more effective than rituximab at depleting B cells and treating disease in a murine lupus model. Arthritis Rheumatol 2021;73:826–36. 10.1002/art.41608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 23. Weening JJ, D'Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004;65:521–30. 10.1111/j.1523-1755.2004.00443.x [DOI] [PubMed] [Google Scholar]
- 24. Dall'Era M, Cisternas MG, Smilek DE, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus nephritis cohort. Arthritis Rheumatol 2015;67:1305–13. 10.1002/art.39026 [DOI] [PubMed] [Google Scholar]
- 25. Tamirou F, Lauwerys BR, Dall'Era M, et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN Nephritis Trial. Lupus Sci Med 2015;2:e000123. 10.1136/lupus-2015-000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Redfield RR, Jordan SC, Busque S, et al. Safety, pharmacokinetics, and pharmacodynamic activity of obinutuzumab, a type 2 anti-CD20 monoclonal antibody for the desensitization of candidates for renal transplant. Am J Transplant 2019;19:3035–45. 10.1111/ajt.15514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freeman CL, Morschhauser F, Sehn L, et al. Cytokine release in patients with CLL treated with obinutuzumab and possible relationship with infusion-related reactions. Blood 2015;126:2646–9. 10.1182/blood-2015-09-670802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pejsa V, Lucijanic M, Vrkljan Vuk A, et al. Prolonged methylprednisolone premedication prior to obinutuzumab in patients with chronic lymphocytic leukemia. Leuk Lymphoma 2020;61:934–9. 10.1080/10428194.2019.1702182 [DOI] [PubMed] [Google Scholar]
- 29. Furie R, Rovin BH, Houssiau F, et al. Two-Year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 2020;383:1117–28. 10.1056/NEJMoa2001180 [DOI] [PubMed] [Google Scholar]
- 30. Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (Aurora 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021;397:2070–80. 10.1016/S0140-6736(21)00578-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2021-220920supp001.pdf (120.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).