Abstract
Treatment options for patients infected with multi-drug resistant gram-negative bacteria harboring metallo-beta-lactamases (MBLs) requires precision therapy. We present the case of a 20 year-old male with a right distal femoral peri-prosthetic abscess with presumed infected hardware and osteomyelitis in whom four multi-drug resistant gram negative bacteria were isolated. The rapid identification of an MBL producing organism, novel combination of therapy, and prompt infection prevention enforcement and education led to appropriate treatment of our patient as well as prevention of spread of organisms during and after hospitalization. This case illustrated successful management of multiple challenges faced by patients infected and/or harboring extensively resistant bacteria.
Keywords: New Delhi metallo-beta-lactamase, Multi-drug resistance, Combination therapy, Infection control
Introduction
Carbapenem resistance due to carbapenemases is a global phenomenon and carbapenem-resistant Enterobacteriaceae (CRE) are included as an urgent threat by the Centers for Disease Control and Prevention [1], [2]. Multiple considerations play a role in assessing individual patient treatment options including resistance phenotype reported by clinical microbiology laboratories, knowledge of the patient’s geographic location, awareness of global antibiogram surveillance data, and, optimally, of the pathogen and characterization of resistance enzyme(s). We present the case of a 20 year-old male admitted to a New York City community hospital with progressive right knee and thigh pain found to have a right distal femoral peri-prosthetic abscess with presumed infected hardware and osteomyelitis. The bacterial culture demonstrated Klebsiella pneumoniae harboring New Delhi metallo (NDM) beta-lactamase. We address the management and unique combination therapy for treatment of this patient.
Case report
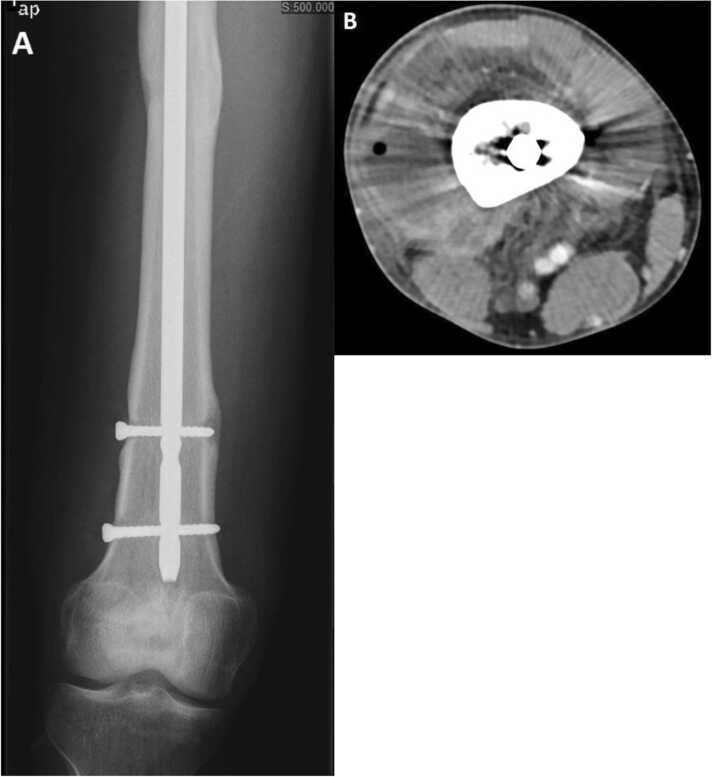
A 20 year old South Asian male suffered a right midshaft femur fracture due to a motorvehicle accident. He underwent open reduction and internal fixation with femoral rod placement at that time. He began having right leg pain associated with fever several weeks following surgery and was prescribed “oral antibiotic(s)” as per the patient and without symptom abatement. Six months later, he complained of swelling and pain of the right knee and thigh associated with subjective fevers. He was admitted to a hospital in South Asia and treated with unknown intravenous antibiotic(s) for a two week period. In spite of treatment, the right leg pain persisted after hospital discharge and worsened with ambulation. At that time, he travelled to the United States and consulted several orthopedic surgeons for a second opinion. He was informed that the fracture had radiographic evidence of delayed healing and was prescribed physical therapy and pain medications for relief. His pain worsened over the course of the year and progressed to inability to ambulate and increased swelling in his right thigh. He presented to the Emergency Department (ED) at our institution 18 months after initial repair with subjective fevers for several days and prominent swelling in the right distal thigh and right knee. Upon initial evaluation, he was afebrile (maximum temperature 37.7 °C) with other vital signs within normal range. Physical examination of the right distal thigh revealed diffuse swelling, (more prominent distally towards the knee), erythema around the medial distal femur, palpable effusion on right knee exam accompanied by tenderness to palpation and decreased range of motion. Pertinent laboratory results included leukocytosis (WBC 15.46 K/µL) with 70% neutrophils, 4% band forms, and a C-reactive protein of 17.2 mg/dL. Radiograph of the right femur demonstrated a healed shaft fracture with lucency surrounding the lower proximal and upper distal screws suggesting hardware loosening (Image 1a). The computerized tomography scan of the right lower extremity demonstrated a large rim-enhancing fluid collection surrounding the distal aspect of the femur measuring 11.0 cm by 7.4 cm by 7.0 cm most compatible with an abscess with a few small internal foci of air noted, but without definite evidence of osteomyelitis (Image 1b). The patient was admitted to the hospital and orthopedic and infectious diseases consultations were obtained.
Patient underwent immediate removal of the hardware and evacuation of an abscess around the right distal femur. Intraoperatively, copious amounts of purulent fluid was noted around the intramedullary nail extending to the femur under the anterior compartment. The nails and screws were removed in entirety, all loculations were broken up and curetted, and necrotic tissue was excised followed by wound closure. Intraoperative specimens were collected and sent to the clinical microbiology laboratory for bacterial, fungal, and acid-fast bacilli cultures. Vancomycin and meropenem were empirically administered post-operatively. Gram-negative rods were isolated within 24 hours and identified as Klebsiella pneumoniae as well as extended spectrum beta-lactamase producing Enterobacter cloacae by the Vitek 2 System (bioMérieux, Inc., Durham, NC) (Table 1). Further analysis demonstrated three distinct subpopulations of Klebsiella pneumoniae, one of which was carbapenem-resistant (CRE). Cepheid Xpert® CARBA-R assay performed on the GeneXpert® system (Cepheid, Sunnyvale, CA) identified the presence of New Delhi metallo-beta-lactamase (NDM). The fungal and acid-fast bacilli smears were negative and cultures remained without growth.
Table 1.
Organisms identified from the purulent material in the right distal femoral peri-prosthetic abscess before treatment, and minimum inhibitory concentration of tested antibiotics.
|
Klebsiella pneumoniae #1 NDM |
Klebsiella pneumoniae #2 |
Klebsiella pneumoniae #3 |
Enterobacter cloacae ESBL |
|
|---|---|---|---|---|
| Antibiotic | Minimum Inhibitory Concentration (μg/ml) | |||
| amikacin | ≥ 64 R | ≤ 2 S | ≥ 64 R | ≥ 64 R |
| aztreonam | ≥ 64 R | ≤ 1 S | R | ≥ 64 R |
| cefazolin | ≥ 64 R | 8 R | ≥ 64 R | ≥ 64 R |
| cefepime | ≥ 64 R | ≤ 1 S | 32 R | ≥ 64 R |
| cefoxitin | ≥ 64 R | 32 R | ≥ 64 R | ≥ 64 R |
| ceftazidime | ≥ 64 R | ≤ 1 S | ≥ 64 R | ≥ 64 R |
| ceftriaxone | ≥ 64 R | ≤ 1 S | ≥ 64 R | ≥ 64 R |
| ertapenem | ≥ 8 R | ≤ 0.5 S | ≥ 8 R | ≤ 0.5 S |
| gentamicin | ≥ 16 R | ≥ 16 R | ≥ 16 R | ≥ 16 R |
| levofloxacin | 4 I | 1 S | 1 S | 4 I |
| meropenem | ≥ 16 R | ≤ 0.25 S | ≥ 16 R | ≤ 0.25 S |
| tobramycin | ≥ 16 R | 4 S | ≥ 16 R | ≥ 16 R |
| trimethoprim/sulfamethoxazole | ≥ 320 R | ≥ 320 R | ≤ 20 S | ≥ 320 R |
| polymyxin B | 0.38 N/A | Not tested | 0.38 N/A | Not tested |
| ampicillin/sulbactam | ≥ 32 R | ≥ 32 R | ≥ 32 R | Not tested |
| tetracycline | ≥ 16 R | ≥ 16 R | ≤ 1 S | ≥ 16 R |
| piperacillin/tazobactam | ≥ 128 R | 16 S | ≥ 128 R | ≥ 128 R |
Antimicrobial therapy was adjusted to a combination of aztreonam (2 g intravenously every 8 hours), ceftazidime-avibactam (2–0.5 g intravenously every 8 hours), polymyxin-B (2.5 mg/kg intravenously once followed by 1.25 mg/kg intravenously every 12 hours) and rifampin (300 mg orally every 12 hours) to address the microbiologic findings and antimicrobial susceptibilities. The patient was placed under strict contact precautions in view of the detection of CRE. On post-op day 5 (and following three days of the quadruple combination antimicrobial treatment), the patient complained of oozing around the surgical wound site. He underwent a second incision and drainage of the right thigh abscess which was found to have re-accumulated. Gram-stain demonstrated few white blood cells without organisms and the subsequent bacterial culture was negative. He was eventually transferred to a short-term rehabilitation facility where he successfully completed six weeks of combination antibiotic therapy (three weeks of aztreonam, ceftazidime-avibactam, polymyxin B, and rifampin followed by three weeks of aztreonam and ceftazidime-avibactam). The wound healed uneventfully after the second procedure. He remained on strict contact precautions there until completion of antibiotics and is reported to have regained full function of his limb at the end of antimicrobial therapy.
Discussion
Recognition and therapy for the treatment of patients infected with carbapenemase harboring organisms requires strong clinical suspicion taking into account the patient’s geographic origins and/or travel history, antibiogram data (including international, national and local data), understanding of innate resistance mechanisms of the bacterium isolated, and ability to rapidly identify the specific enzyme(s) involved [1], [3]. A recent paper reported use of pertinent patient risk factors to be a predictor of ability to identify whether carbapenem-resistant Enterobacteriaceae (CRE) are caused by carbapenemase producing bacteria [4]. The authors reported an 18-fold greater odds that the mechanism of such resistance was due to a carbapenemase as opposed to a non-CP-CRE if patients underwent recent hospitalization in a foreign country [4]. Recent case reports document the isolation of a pan-resistant NDM producing Klebsiella pneumoniae from a patient in Washoe County, Nevada who received inpatient healthcare exposure in India and a traveler from Bangladesh to New York City with a complex orthopedic infection associated with extensive osteomyelitis due to bacteria harboring NDM and oxacillinase type-181 carbapenemases [5], [6].
The patient we describe presented with multiple challenges: (1) a broad microbiologic differential diagnosis based on his geographic origin, chronicity of symptoms and with multiple prior courses of unknown antibiotics; (2) chronic extensive orthopedic intra-operative findings requiring additional interventions; (3) availability of advanced molecular microbiologic rapid identification platforms for multi-drug resistant bacteria; (4) extensive multi-drug resistant bacterial populations requiring novel approach to combination therapy; (5) need for strict infection control practices to be implemented in an urgent fashion.
We quickly considered infection with carbapenemase producing bacteria as our infectious diseases division has a long history in translational research focused on multidrug resistant bacteria, mechanisms of resistance, and novel antimicrobial combinations. Rapid identification by the clinical microbiology of the presence of the NDM enzyme facilitated targeted antimicrobial therapy and immediate infection prevention precautions. Ceftazidime-avibactam was the first approved and at that time the only available beta-lactam-beta-lactamase inhibitor combination with activity against serine-based Klebsiella pneumoniae carbapenemases (KPC), OXA-48 like enzymes, and AMP C beta-lactamases. However, there are no clinically available beta-lactamase inhibitors that inactivate MBLs. Published studies report successful use of combination ceftazidime-avibactam and aztreonam to treat patients infected with MBLs [7], [8], [9], [10], [11], [12]. In vitro data suggests the combination is stable in the presence of the enzymes as aztreonam is not hydrolyzed by MBLs and avibactam will inhibit most aztreonam-hydrolyzing betalactamases. Resistance to ceftazidime-avibactam has been reported in the literature based a variety of mechanisms [13], [14], [15]. We chose to add polymyxin B and rifampin during the initial few weeks of treatment to better address the potentially higher early inoculum and possible presence of multi-drug resistant sub-populations of bacteria. We based our additions on prior published in vitro and in vivo synergy studies [16], [17], [18], [19], [20].
New strict infection control precautions were rapidly implemented to prevent transmission of these multidrug resistant organisms. The patient and family members received education about infections caused by bacteria harboring these enzymes and our infection preventionists educated all staff caring for the patient. Strict contact precautions included dedicated patient equipment and/or single use equipment. Education of health care staff extended to the short-term rehabilitation facility following his discharge from the hospital. This last process was a crucial part of our infection prevention practices as it may be overlooked during the transfer process and potentially lead to secondary transmission in the accepting facility.
The rapid identification of an MBL producing organism in our patient, novel combination focused therapy, and prompt infection prevention enforcement and education led to appropriate treatment of and an excellent outcome for our patient as well as prevention of spread of organisms during and after hospitalization. This case illustrated successful management of multiple challenges faced by patients infected and/or harboring extensively resistant bacteria. Fig. 1.
Fig. 1.
(A) X-ray of the distal right femur showing lucency around the screws; (B) Computed tomography of the distal right femur showing circumferential distal femoral abscess with air.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Consent to publish was not obtained since the case report does not contain any personal identifiers.
CRediT authorship contribution statement
Monica Cespedes Santana Participated in the diagnostic process, data collection, and initial writing of the manuscript. Ting Ting Wong Participated in the diagnostic process, data collection, and initial writing of the manuscript. Carl Urban Participated in the writing, review and editing of the manuscript, Noriel Mariano Participated in data analysis and editing of the manuscript. Janice Burns Participated in data analysis and editing of the manuscript, George Rodriguez Participated in the review and editing of the manuscript. Elan Goldwyn Participated in the diagnostic process and data collection, Nishant Prasad Participated in the review and editing of the manuscript. Sorana Segal-Maurer Participated in the review and editing of the manuscript.
Declaration of competing interest
The authors report no declarations of interest.
References
- 1.Bonomo R.A., Burd E.M., Conly J., Limbago B.M., Poirel L., Segre J.A., Westblade L.F. Carbapenemase- producing organisms: a global scourge. Clin Infect Dis. 2018;66:1290–1297. doi: 10.1093/cid/cix893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) CDC; Atlanta: 2019. Antibiotic resistance threats in the United States. [Google Scholar]
- 3.Nordman P., Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram- negative bacteria. Clin Infect Dis. 2019;69(S7):S521–S528. doi: 10.1093/cid/ciz824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simner P.J., Goodman K.E., Carroll K.C., Harris A.D., Han J.H., Tamma P.D. Using patient risk factors to identify whether carbapenem-resistant Enterobacteriaceae infections are caused by carbapenemase-producing organisms. Open Forum Infect Dis. 2018;5:1–4. doi: 10.1093/ofid/ofy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L., Todd R., Kiehlbauch J., Walters M., Kallen A. Pan-resistant New Dehli metallo-β-lactamase-producing Klebsiella pneumoniae-Washoe County, Nevada. MMWR. 2016;66:33. doi: 10.15585/mmwr.mm6601a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittal J., Szymczak W., Guo Y., Levi M., Chen L., Kreiswirth B., et al. Two for the price of one: Emerging carbapenemases in a returning traveller to New York City. BMJ Case Rep. 2018:1–4. doi: 10.1136/bcr-2018-225440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mojica M.F., Ouellette C.P., Leber A., Becknell M.B., Ardura M.I., Perez F., Shimamura M., Bonomo R.A., Aitken S.L., Shelburne S.A. Successful treatment of bloodstream infection due to metallo-β-lactamase-producing Stenotrophomonas maltophilia in a renal transplant patient. Antimicrob Agents Chemother. 2016;60:5130–5134. doi: 10.1128/AAC.00264-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasassri M.E., Boyce T.G., Norgan A.P., Cunningham S.A., Jeraldo P.R., Weissman S.J., Patel R., Banerjee R., Pogue J.M., Kaye K.S. An immunocompromised child with bloodstream infection caused by two Escherichia coli strains, one harboring NDM-5 and the other harboring OXA-48-like carbapenemase. Antimicrob Agents Chemother. 2016;60:3270–3275. doi: 10.1128/AAC.03118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall S., Hujer A.M., Rojas L.J., Papp-Wallace K.M., Humphries R.M., Spellberg B., Hujer K.M., Marshall E.K., Rudin S.D., Perez F., Wilson B.M., Wasserman R.B., Chikowski L., Paterson D.L., Vila A.J., van Duin D., Kreiswirth B.N., Chambers H.F., Fowler VG Jr, Jacobs M.R., Pulse M.E., Weiss W.J., Bonomo R.A. Can ceftazidime-avibactam and aztreonam overcome β- lactam resistance conferred by metallo-β- lactamases in Enterobacteriaceae? Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.02243-16. e02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzler E., Deraedt M.F., Harrington A.T., Danizger L.H. Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-β-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis. 2017;88:352–354. doi: 10.1016/j.diagmicrobio.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Shaw E., Rombauts A., Tubau F., Padullés A., Càmara J., Lozano T., Cobo-Sacristán S., Sabe N., Grau I., Rigo-Bonnin R., Dominguez M.A., Carratalà J. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTXM-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother. 2018;73:1104–1106. doi: 10.1093/jac/dkx496. [DOI] [PubMed] [Google Scholar]
- 12.Davido B., Fellous L., Lawrence C., Maxime V., Rottman M., Dinh A. Ceftazidime-avibactam and aztreonam, an interesting strategy to overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61:e01008–e01017. doi: 10.1128/AAC.01008-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries R.M., Hemarajata P. Resistance to ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00537-17. e00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields R.K., Potoski B.A., Haidar G., Hao B., Doi Y., Chen L., Press E.G., Kreiswirth B.N., Clancy C.J., Nguyen M.H. Clinical outcomes, drug toxicity and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis. 2016;63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papp- Wallace K.M., Winkler M.L., Taracila M.A., Bonomo R.A. Variants of β-lactamase KPC-2 that are resistant to inhibition by avibactam. Antimicrob Agents Chemother. 2015;59:3710–3717. doi: 10.1128/AAC.04406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulbin A., Bono C., Philp T., Mariano N., Urban C. Successful treatment of Klebsiella pneumoniae harboring a Klebsiella pneumoniae carbapenemase isolated from lumbar wound infection and blood in a patient with hardware retention. Case Rep Infect Dis. 2017;2017 doi: 10.1155/2017/9028543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban C., Mariano N., Rahal J.J. In vitro double and triple bactericidal activities of doripenem, polymyxin B, and rifampin against multidrug-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrob Agents Chemother. 2010;54:2732–2734. doi: 10.1128/AAC.01768-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tängdén T T., Hickman R.A., Forsberg P., Lagerbäck P., Giske C.G., Cars O. Evaluation of double-triple-antibiotic combinations for VIM- and NDM-producing Klebsiella pneumoniae by in vitro time-kill experiments. Antimicrob Agents Chemother. 2015;58:1757–1762. doi: 10.1128/AAC.00741-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura I., Sakamoto N., Ida Y., Imai R., Aoki K., Ando R., Yamaguchi T., Matsumura H., Matsumoto T. Combination therapy against polymicrobial infection, including by NDM-1 producing Enterobacteriaceae resistant to colistin. Antimicrob Agents Chemother. 2015;59:5092–5093. doi: 10.1128/AAC.00746-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulman Z.P., Satlin M.J., Chen L., Kreiswirth B.N., Shin B.S., Walsh T.J. New polymyxin strategies to fortify old allies in the war against KPC-2-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.02023-16. e02023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]