Abstract
Spinal cord injury (SCI) is the most common disabling spinal injury, a complex pathologic process that can eventually lead to severe neurological dysfunction. The Wnt/mTOR signaling pathway is a pervasive signaling cascade that regulates a wide range of physiological processes during embryonic development, from stem cell pluripotency to cell fate. Numerous studies have reported that Wnt/mTOR signaling pathway plays an important role in neural development, synaptogenesis, neuron growth, differentiation and survival after the central nervous system (CNS) is damaged. Wnt/mTOR also plays an important role in regulating various pathophysiological processes after spinal cord injury (SCI). After SCI, Wnt/mTOR signal regulates the physiological and pathological processes of neural stem cell proliferation and differentiation, neuronal axon regeneration, neuroinflammation and pain through multiple pathways. Due to the characteristics of the Wnt signal in SCI make it a potential therapeutic target of SCI. In this paper, the characteristics of Wnt/mTOR signal, the role of Wnt/mTOR pathway on SCI and related mechanisms are reviewed, and some unsolved problems are discussed. It is hoped to provide reference value for the research field of the role of Wnt/mTOR pathway in SCI, and provide a theoretical basis for biological therapy of SCI.
Keywords: Wnt signaling, mTOR, Spinal cord injury (SCI), Cascade reaction, Therapeutic target
1. Introduction
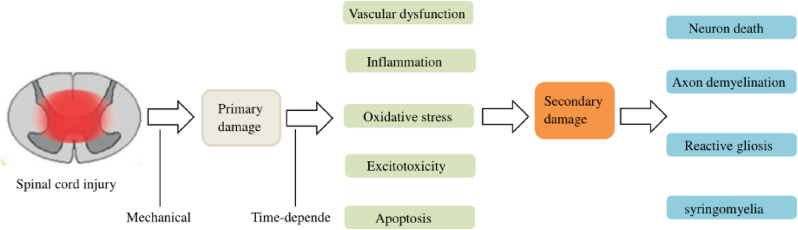
Spinal cord injury (SCI) is a highly disabling injury of the central nervous system (CNS) that can be caused by traffic accidents, falls, violence, or sports-related injuries.1 It is a huge challenge for clinical medicine and basic research and also places a heavy burden on patients and society.2, 3, 4 Due to the complexity of the pathogenesis of injury, both primary and secondary injuries are included.5 Primary injury refers to direct injury and necrosis of the spinal cord tissue and nerve cells caused by external mechanical forces immediately.6 Secondary injury happens because of primary injury delays and progressive tissue injury, occurring in a few hours or weeks. During secondary spinal cord bleeding, injury free oxygen and the excessive release of excitatory toxin and inflammation lead to a large number of neuron apoptosis and residual nerve fiber demyelination, and a series of biological events occurs7, 8, 9, 10, 11(Fig. 1). Secondary SCI is an irreversible process of dynamic regulation and therefore, it is difficult to recover after SCI.12, 13, 14 Consequently, secondary injury plays an important role in the occurrence of SCI and provides a potential clinical therapeutic target for SCI. However, the current treatment of SCI is mainly through conservative and surgical treatments for pain relief and symptom control, rather than through pathophysiological intervention. Therefore, a better understanding of its pathogenesis to delay or prevent nerve death and promote axon regeneration is essential for the effective treatment of SCI.
Fig. 1.
Pathophysiological process of SCI.
Wnt/mTOR seems to be a good candidate because its signaling pathway not only plays a major role in the development but also is involved in the adult nervous system.15,16 Wnt/mTOR is a class of glycoproteins that is widely involved in neural development, axon guidance, cell proliferation, and nerve cell survival.16, 17, 18 According to relevant research reports, the Wnt/mTOR signaling pathway is related to some functions of organisms, regulating key processes such as proliferation, autophagy, inflammation, neurogenesis and regeneration.19, 20, 21 Wnt/mTOR pathway is one of the most widely studied signaling pathways, which is involved in various diseases of trauma and central nervous system. They are affected in many neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, cerebral stroke, and Huntington's disease, and inhibition of mTOR activity can reduce neurodegenerative diseases associated with these diseases.22, 23, 24, 25, 26, 27, 28, 29 In addition, the Wnt/mTOR pathway plays an important role in neural development, synaptogenesis, neuron growth, differentiation and survival in the central nervous system (CNS).25,30, 31, 32, 33, 34, 35 Wnt/mTO has been identified as a key regulator of neuronal growth, differentiation, and survival.30 At the same time, there is growing evidence that Wnt signaling may be involved in disease progression in the spinal cord. Fernandez-Martos and Gonzalez-Fernandez et al.31,36 found that Wnt is expressed in the spinal cord of healthy adult rats and mice. In addition, other studies have shown that the Wnt signaling pathway is activated after SCI and that the activated Wnt signaling pathway is beneficial for functional recovery and axonal regeneration of the injured spinal cord.37, 38, 39mTOR regulates axonal regeneration in response to SCI and limits astrocyte scarring in injured spinal cord.40, 41, 42 Therefore, Wnt/mTOR signaling has been studied as a potential therapeutic target after SCI. In this paper, the current relationship between Wnt/mTOR pathway and spinal cord injury was studied to comprehensively understand the role of Wnt/mTOR pathway in SCI.
2. Wnt/mTOR pathway in SCI
The Wnt family of secreted cell-signaling proteins consists of classical (Wnt1, Wnt2, Wnt3, Wnt-3a, Wnt-8a, Wnt-8b, Wnt-10a, and Wnt-10b) and non-classical (Wnt4, Wnt-5a, Wnt-5b, Wnt-7a, Wnt-7b, and Wnt11) proteins; they function by activating both classical and non-classical pathways.43 The Wnt signaling pathway is a ubiquitous signaling cascade that regulates a wide range of physiological processes during embryonic development, from stem cell pluripotency to cell fate determination.44 The Wnt family of secreted cell-signaling proteins is mainly composed of at least 19 members in mammals, including Wnt1, Wnt2, Wnt3, and Wnt-3a, and plays an active role in multiple processes of cell growth and development.45,46 MTOR is a serine/threonine protein kinase, plays a key role in regulating cell metabolism, cell proliferation, cell death and survival, and is involved in transcription, mRNA conversion and translation, ribosome and other physiological processes. Biogenesis, vesicle transport, autophagy, and cytoskeletal tissue. Therefore, Wnt/mTOR signaling pathway has been identified as a key regulator of neuronal growth, differentiation and survival.30
2.1. Classical Wnt pathway
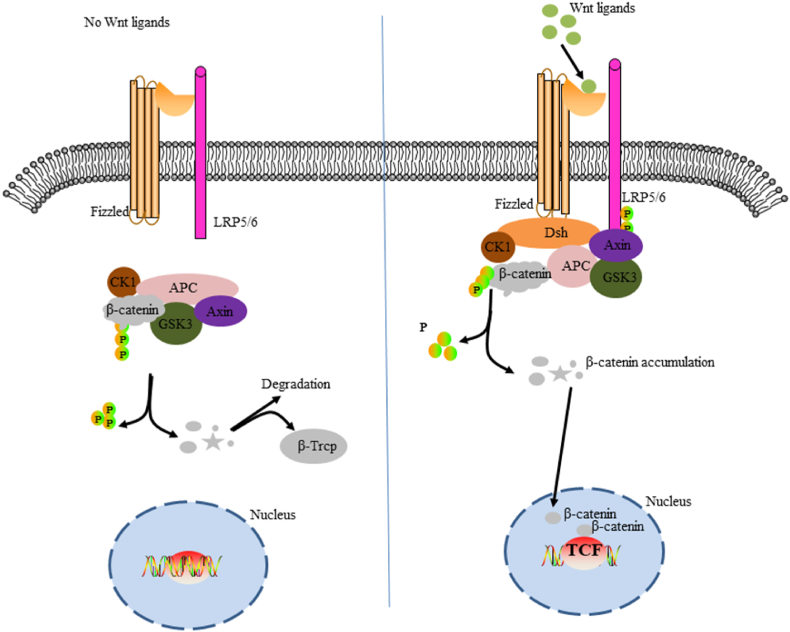
The classical Wnt pathway is the β-catenin signaling cascade. This pathway is marked by the accumulation and translocation of the adhesion-associated protein β-catenin in the nucleus. Wnt is a secretory glycoprotein that binds to the Fizzled (FZD) receptor and low-density lipoprotein receptor-associated protein 5/6 (LRP5/6) complex on the cell surface, leading to the accumulation of β-catenin in the nucleus, which leads to cell cycle activation and transcriptional regulation.47 The FZD receptor is the main binding site of Wnt,48 mainly containing seven transmembrane (7TM) and extracellular N-terminal cysteine-rich domain (CRD).49,50 However, in the absence of Wnt signaling, cytoplasmic β-catenin is degraded by β-catenin-destroying complexes such as Axin, adenomatous polyposis (APC), protein phosphatase 2A (PP2A), glycogen synthase kinase 3 (GSK3), and casein kinase 1α (CK1α). This leads to the failure of the classical Wnt pathway51,52 (Fig. 2).
Fig. 2.
Schematic diagram of classical Wnt/β-catenin pathway cascade. The figure on the left shows the inactivation of the Wnt pathway. In the absence of Wnt signaling, β-catenin in the cytoplasmic is recognized, folded, and phosphorylated by a destructive complex composed of scaffold proteins Axin, APC, GSK3β, and CK1 and is targeted for degradation by β-Trcp-mediated proteosomal mechanisms. The figure on the right shows activation of the Wnt pathway. The signal induces dual phosphorylation of LRP6 by CK1 and GSK3-β via the FZD receptor and LRP5/6 co-receptor complex, which allows the protein complex containing Aneurin to transfer from the cytoplasm to the plasma membrane. DSH is also recruited to the cell membrane and binds to the FZD receptor, and Akin binds to phosphorylated LRP5/6. The complex is formed on the FZD/LRP5/6 membrane to induce the stabilization of β-catenin by the separation and/or degradation of axon. β-catenin, which accumulates in the cytoplasm, translocates into the nucleus and, together with the transcription factor TCF, drives downstream target gene expression.
2.2. Nonclassical Wnt pathways
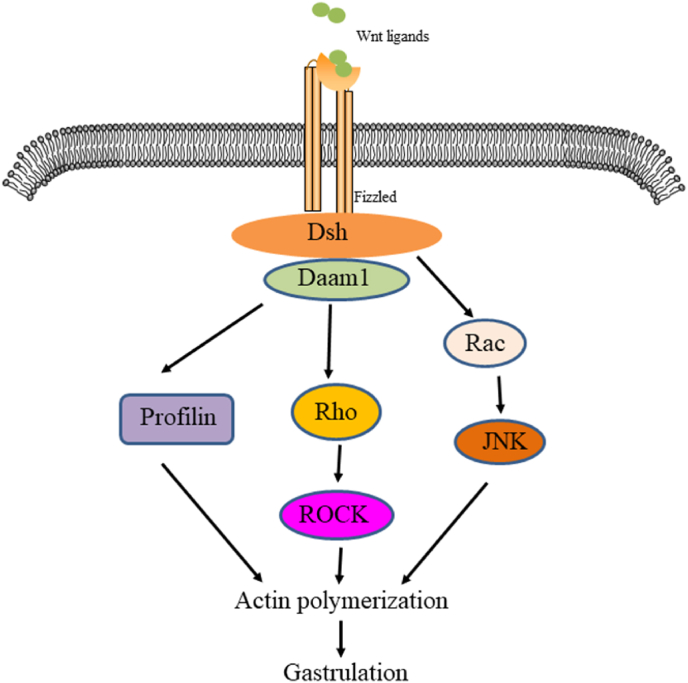
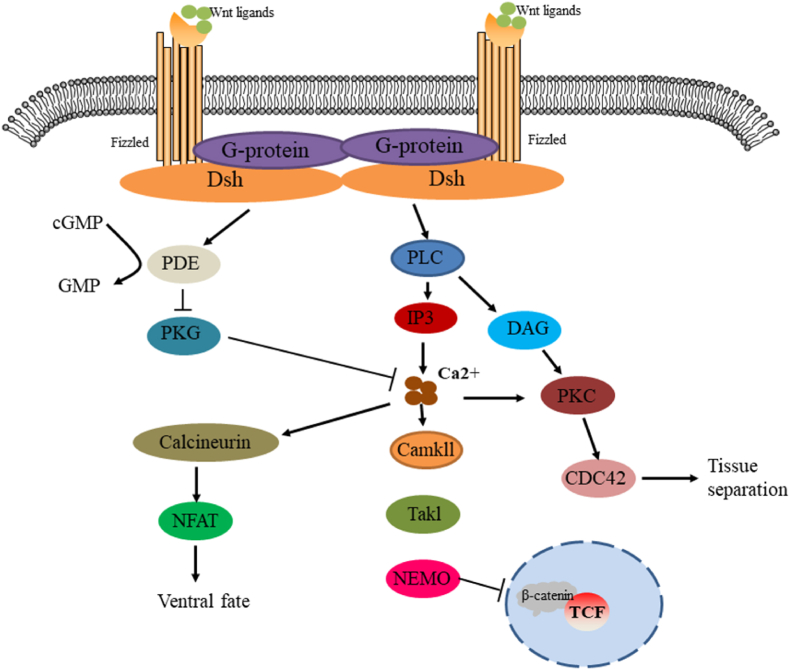
The nonclassical Wnt pathway is often referred to as the β-catenin-independent pathway, which can be further divided into the Wnt/plane-cell polarity (PCP) pathway and the Wnt/Ca2+ pathway. In the Wnt/PCP pathway, after the Wnt molecule binds to the FZD receptor, it recruits DVL and further activates small GTPases Rho and Rac, triggering the recruitment of downstream Rho-associated kinases (ROCK) and c-Jun N-terminal kinases (JNK), thereby allowing cytoskeletal recombination (Fig. 3). In the Wnt/Ca2+ pathway, when Wnt binds to the FZD receptor, DVL is activated, and the activated DVL recruits phospholipase C (PLC). PLC converts phosphatidylinositol 4, 5-diphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 stimulates the release of Ca2+ in the endoplasmic reticulum, and DAG and Ca2+ co-activate downstream protein kinase C (PKC), calcineurin phosphatase (CaN), and Ca2+/calmodulin-dependent protein kinase II (CaMKII), thereby regulating intracellular calcium flux and downstream calcium-dependent cytoskeleton and/or transcriptional responses (Fig. 4).
Fig. 3.
The PCP pathway transition cascade is shown here. Wnt signals are transduced through the FZD receptor pathway, independent of LRP5/6, leading to the activation of DSH. DSH mediates the activation of Rho through DAAM1, which activates ROCK. DAAM1 also mediates actin polymerization via the actin binding protein profilin. DSH also mediates activation of RAC, which in turn activates JNK. Signals from ROCK, JNK, and profilin are integrated into cytoskeletal changes in cell polarization and movement during proto-gut formation.
Fig. 4.
Schematic diagram of Wnt/Ca2+ signaling cascade. The FZD Wnt signal mediates the activation of DSH through the activation of G proteins. The disheveled protein activates phosphodiesterase (PDE), which inhibits PKG and in turn inhibits Ca2+ release. DSH activates IP3 via PLC, leading to the release of intracellular Ca2+, which activates CaMKII and CaN. CaN activates NF-AT to modulate the fate of ventral cells. CaMKII activated TAK and NLK and inhibited β-catenin/TCF function to negatively regulate dorsal axis formation. DAG activates Cdc42 via PKC to mediate tissue separation and cell movement during proto-gut formation.
2.3. MTOR pathway
mTOR is a serine/threonine protein kinase and the core of the signaling network, belonging to the phosphoinositol 3-kinase (PI3K) related protein kinase (PIKK) family. mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) protein complex bind to form the mTORC signaling pathway, which consists of its subunits Raptor and Rictor. MTORC1 phosphorylates downstream effectors, such as the P70 ribosomal S6 protein kinase (p70S6K), and further regulates mRNA translation. Therefore, mTORC1 plays an important role in promoting protein synthesis. On the other hand, mTORC2 has been shown to phosphorylate members of the AGC kinase family, including protein kinase B (Akt), which has been implicated in a variety of pathologic conditions. MTORC2 is considered to be a regulator of actin cytoskeleton.53 Phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) is one of the main pathways that activate mTOR. MTOR plays an important role in various physiological functions of the central nervous system, including regulating the growth and survival of neuronal cells and the development of axons and dendrites.54,55
3. Expression of Wnt/mTOR after SCI
Previous studies have shown that the Wnt/mTOR signaling pathway is closely related to the development of the nervous system and plays a key role in embryo development, tissue regeneration and bone growth.45,46 In CNS diseases, the Wnt family mainly affects the proliferation, composition, and survival of nerve cells. In the CNS, Wnt/mTOR signaling pathway expressed in the dorsal region of the spinal cord, which regulates the expansion and differentiation of spinal neurons and promotes the continuous differentiation of spinal precursor cells.20 Studies have reported that the Wnt/mTOR pathway promotes axon conduction, stimulates axon regeneration, reduces neuronal death, and ultimately promotes the recovery of neural function after SCI.56, 57, 58, 59 Wei et al.60 showed that Wnt/mTOR signal expression in the head and tail spinal segments increased significantly within 7 days after SCI, remained at a high level, and then decreased. In addition, Gonzalez et al.61 proposed that the expression model of Wnt/mTOR signaling was expressed in a time-dependent manner after SCI. Therefore, we can confirm that the expression of Wnt/mTOR pathway increases after SCI and that it is involved in a variety of pathological mechanisms after injury.
4. Wnt signaling pathway in SCI
Many studies have shown that Wnt signaling plays an important role in SCI. After spinal cord loss, Wnt signaling is involved in the regulation of neural stem cell proliferation and differentiation, axon regeneration, neuroinflammation, and pain through various mechanisms.
4.1. Promotion of proliferation and differentiation of neural stem cells
Neural stem cells can differentiate into nerve cells, astrocytes, and oligodendrocytes and have the function of self-renewal, thus providing a large number of brain tissue cells.62 During SCI, neuronal injury and necrosis, axon rupture, demyelination, and oligodendrocyte destruction directly lead to spinal cord conduction dysfunction. In addition, the lack of neuronal regeneration and late glial scarring, which inhibits axon regeneration, prevents the spinal cord from self-repairing during SCI.63, 64, 65, 66 The Wnt signaling pathway transmits growth stimulation signals and plays an important role in the development of the embryonic nervous system.67,68 Lie et al.69 demonstrated that adult hippocampal stem cells express Wnt receptors and signaling components and confirmed, in vitro and in vivo, that Wnt signaling is involved in neuronal determination and the regulation of directed proliferation of neuronal precursors during neurogenesis in adult hippocampal stem cells. In addition, Jiao et al.70 found that miR-124 can activate the Wnt/β-catenin pathway by targeting DACT1, promote the proliferation of neural stem cells, and induce their differentiation into neurons. It has also been reported that activation of the Wnt pathway can promote the differentiation of bone mesenchymal stem cells (MSCs) into neuron-like cells. When the Wnt pathway is inhibited, the differentiation of MSCs into neuron-like cells is eliminated.71 In addition, a recent study showed that salvianolic acid B (Sal B) can protect neurons from injury after SCI by activating the Wnt pathway, promoting nerve cell regeneration, and inhibiting nerve cell apoptosis.72 Therefore, Wnt signaling is thought to be involved in the proliferation and differentiation of endogenous MSCs after SCI. The exact mechanism of action may provide a potential clinical value for SCI therapy.
4.2. Promotion of axon regeneration
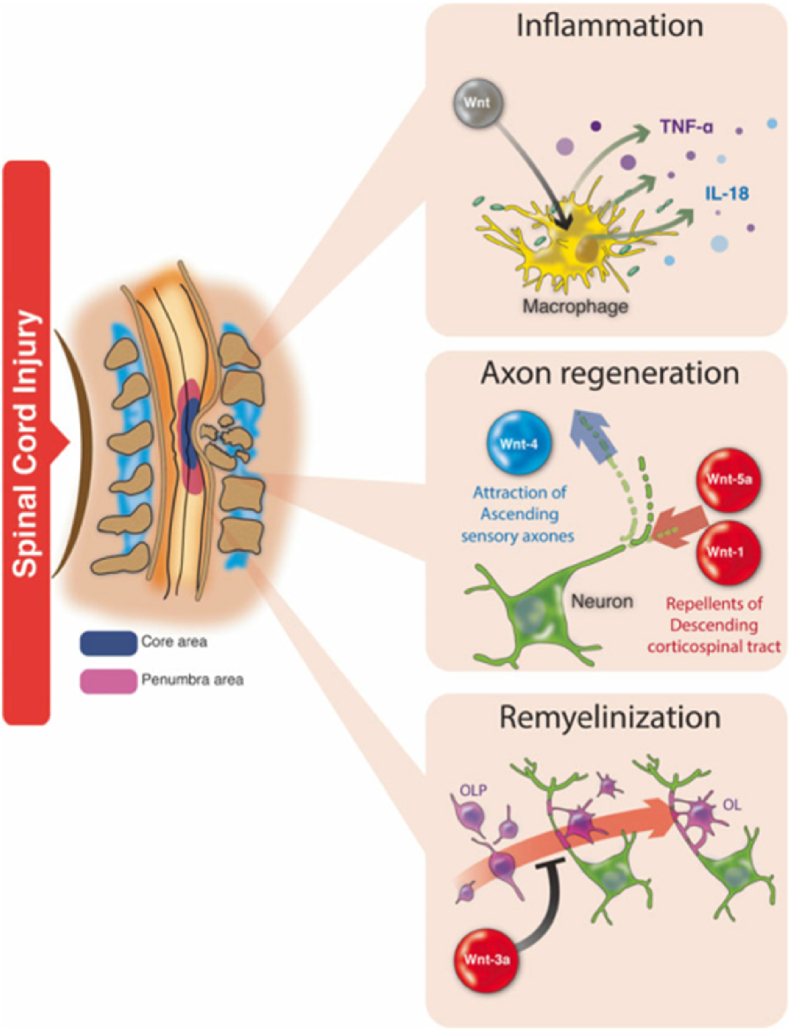
Another serious impairment of functional recovery after SCI is the development of colloid scars around the wound center, which strongly inhibits axonal regeneration.73,74 Therefore, promotion of axon regeneration is a prerequisite for effective recovery from SCI.75,76 During development, the Wnt pathway is a key factor controlling axonal growth after CNS injury.77, 78, 79 Wnt ligands inhibit GSK-3 activity through its classical signal transduction pathway and promote axonal regeneration, similar to neurotrophic factors.46 Endogenous and exogenous Wnt-2b and Wnt-3a directly promote β-catenin-dependent CNS regeneration in the retina of adult mammals.80,81 In a rat SCI model, the transplantation of Wnt-3a secretory fibroblasts promoted axon regeneration and improved motor function after recovery.58 A zebrafish SCI model has also been used to study the regeneration activity of Wnt signaling. Increased expression of β-catenin and Wnt-4b was observed after spinal transection of adult zebrafish and was associated with axon regeneration and functional recovery.82 Increased Wnt/β-catenin activity was observed in radial glial cells after SCI in zebrafish larvae, and Wnt signaling was found to be essential for neurogenesis and axon regeneration.83 In addition, SCI in zebrafish resulted in increased Wnt/β-catenin signaling in fibroblast-like cells adjacent to regenerated axons. Wnt signaling in these cells induces the expression and deposition of collagen XII at the injured site, and changes in the extracellular matrix promotes the growth of axons at the injured site.84 In addition, it has been reported that Wnt-induced pathways responsible for regulating axon growth during embryogenesis can be reused to promote axon growth after injury.85 At the same time, long-term lentivirus-mediated overexpression of Wnt improves motor recovery and increases myelin preservation and neuronal survival by promoting axon regeneration and inhibiting glial scar formation in astrocytes.86 The above experimental studies suggest that Wnt signaling, especially Wnt-3a signaling, plays an important role in axonal regeneration in the CNS; however, many scholars have put forward the opposite view. Li et al.87 also showed that overactivation of Wnt signaling inhibits axon growth, thereby affecting connective axon projection and nerve fiber formation. In addition, Liu et al.30 showed that the Wnt signaling pathway inhibits regeneration after SCI through rejection, and activation of the Wnt signaling pathway after injury leads to axonal contraction of the corticospinal tract and axonal burst bud suppression (Fig. 5). Although the mechanism of action of the Wnt pathway in axonal regeneration is still unclear and further studies are needed to elucidate the intracellular mechanisms by which Wnt signaling promotes axonal regeneration and functional improvement, this approach may be a promising therapeutic strategy for SCI.
Fig. 5.
Wnt signaling pathways in SCI. This signal modulates several processes associated with the progression of SCI, including axonal regeneration, myelin regeneration, and inflammation.
4.3. Regulation of neuroinflammatory response
Neuroinflammation is an important pathological process in SCI and other injury-induced diseases of the CNS.88 Neuroinflammation is defined as the inflammatory response occurring in the brain or spinal cord, which is mediated by cytokines (IL-1β, IL-6, and tumor necrosis factor (TNF-α)), chemokines, and reactive oxygen species. These mediators in neuroinflammation arise from the intrinsic glial cells (microglia and astrocytes) of the CNS.89 Microglia play an important role in neuroinflammation and secondary injury after nerve trauma.90 Studies have reported that the Wnt signaling pathway is closely related to the neuroinflammatory response regulated by activated microglial cells.91,92 Vallee et al.93 demonstrated disease-related β-catenin signaling in microglia in vivo by showing age-dependent accumulation of β-catenin in mice with Alzheimer's disease-like pathology (APDE9). In cultured mouse microglia expressing the Wnt FZD receptors 4, 5, 7, and 8, and LRP5/6, Wnt-3a was found to stabilize β-catenin and dose-dependently induced the phosphorylation of LPR6. It has disordered downstream activation, β-catenin stabilization, and nuclear input. Gene expression profiles showed that Wnt-stimulated microglia specifically increased the expression of pro-inflammatory immune response genes in microglia and enhanced de novo release of IL-6, IL-12, and TNF-α. In addition, Zhang et al.94 also concluded that typical activation of Wnt signaling stimulates the production of pro-inflammatory cytokines IL-18 and TNF-α. Furthermore, NR2B glutamate receptor and Ca2+ dependent signaling were modulated by Wnt-3a/FZ8/β-catenin signaling in the spinal cord. In addition, astrocytes play an important role in degenerative and traumatic diseases of the CNS. After SCI, astrocytes can release a large number of inflammatory factors that damage neurons,95 and activation of the Wnt pathway can inhibit the activation of astrocytes, thereby reducing the release of inflammatory factors.96 The above studies confirmed that the Wnt signaling pathway is involved in the regulation of the neurogenic inflammatory response. Although the specific mechanism of action remains unclear, these findings provide a potential clinical therapeutic value.
4.4. Regulation of neuropathic pain
Wnt signaling was initially thought to be involved in regulating cellular processes such as proliferation, differentiation, and migration during neuronal development. However, recent studies have demonstrated an important role of Wnt signaling in the pathogenesis of pain induced by neurological and bone cancers.94,97 The Wnt signaling pathway is involved in the occurrence of neuropathic pain after nerve injury by stimulating the production of pro-inflammatory cytokines IL-18 and TNF-α in the spinal cord and dorsal root ganglion (DRG) neurons and plays an important role in the induction and persistence of neuropathic pain94,98. Neuronal injury leads to rapid and continuous activation of Wnt signaling, and blocking of the Wnt signaling pathway can inhibit the development and progression of neuropathic pain94,99,100. Neural damage and bone cancer lead to rapid and persistent upregulation of Wnts (such as Wnt-3a), as well as activation of the Wnt-Frizzled-β-catenin signaling pathway of the primary sensory neurons and the nociceptive neurons of the spinal dorsal horn, which contributes to the development and maintenance of hyperalgesia and mechanical abnormal pain. These responses may be significantly attenuated by spinal injection of a Wnt signaling inhibitor.97,101 A study by Itokazu et al.99 also showed that partial sciatic nerve ligation induced the upregulation of Wnt-3a expression in the spinal cord and inhibited Wnt/β-catenin signaling, thus attenuating the induction of neuropathic pain. Fan and Wang et al.102,103 found that Mir-216B–5P can alleviate neuropathic pain caused by chronic constrictive injury in female rats by targeting MAL2 and inactivating the Wnt/β-catenin signaling pathway. In addition, Liu et al.98 found that Wnt signaling may cause neuropathic pain by regulating the excitability of sensory neurons and synaptic plasticity of the spinal cord through atypical Wnt/Ryk signaling in rats. The Wnt pathway may also induce peripheral and central sensitization by increasing neuronal excitability in the chronic compression persistent DRG and synaptic strength in the spinal dorsal horn. Peripheral and central sensitizations lead to sustained and abnormal increases in neuronal excitability and increased levels of TNF-α and IL-18, leading to increased pain signal production; thus, it enhances neuropathic pain. Inhibition of Wnt pathway activity can reduce the severity and duration of neuropathic pain, and the pathway plays a key role in the generation and persistence of neuropathic pain after chronic compression of DRG.104 The above studies suggest that the Wnt pathway plays a key role in the pathogenesis of neuropathic pain, and targeting the pathway may be a potential target for the treatment of neuropathic pain.
5. Role of mTOR pathway in spinal cord injury
An in-depth understanding of various signaling pathways in the recent years has improved the treatment of spinal cord injury by interfering with nuclear factor-κB (NF-κB), mTOR, mitogen-activated protein kinase (MAPK) and other signaling pathways.105, 106, 107 Particularly, the mTOR signaling pathway plays an important role in the regulation of inflammation, nerve regeneration, and glial scar formation after SCI.
5.1. Regulation of mTOR signaling pathway on inflammatory response
The traumatic spinal cord induces local inflammatory responses through activated microglia-infiltrating neutrophils and macrophages. Furthermore, traumatic spinal cord has been reported to induce the expression of relevant pro-inflammatory cytokines.108 MTOR regulates the maturation of antigen-presenting cells including T and B cells.109 In one study, the mTOR pathway was found to increase the survival rate of EOC2 microglia during oxygen-glucose deprivation and enhanced the expression of nitric oxide synthase 2 (NOS2) in BV2 microglia during hypoxia. This suggests that mTOR is involved in microglial pro-inflammatory activation.110 MTOR inhibition may improve the anti-inflammatory effects by regulating T cells.111 Similarly, the inhibition of mTOR has been shown to control macrophage/microglia activation and reduce neuro-inflammation.112 MTOR inhibition may also reduce the pro-inflammatory cytokine production by macrophages.113 Some other studies have also reported the regulation of neuroinflammation by mTOR inhibitors, in SCI.114 Therefore, the inhibition of mTOR can reduce the inflammatory process after SCI.
5.2. Regulation of mTOR pathway on nerve regeneration
The major reasons effectuating the failure of central nerve regeneration are related to the inhibitory factors in the myelin sheath, formation of glial scars, and the weak growth ability of mature neurons.115 In the chronic stage, the mTOR signaling pathway regulates the regeneration of damaged nerve tissues. Immunohistochemical analysis showed that the mTOR signaling pathway exists at the level of the dorsal root ganglion and inner layer II spinal cord neurons in injury-specific C fiber neurons.116 In addition, mTOR inhibition contributes to axon regeneration. Following SCI, rapamycin induced inhibition of mTOR promotes axonal regeneration by inhibiting new protein synthesis and cell proliferation. MTOR is also associated with improved myelination and oligodendrocyte differentiation in the central nervous system.59 S6K1 (ribosomal protein S6 kinase 1) is an important downstream effector of mTOR.117 Studies have shown that S6K1 inhibition promotes the regeneration of the corticospinal tract and axon counts at 8 weeks after SCI.118 In another study, knocking out nodule sclerosis complex 1 (TSC1) was shown to reactivate mTOR, thereby promoting axonal regeneration.119
5.3. Regulation of mTOR pathway on glial scar
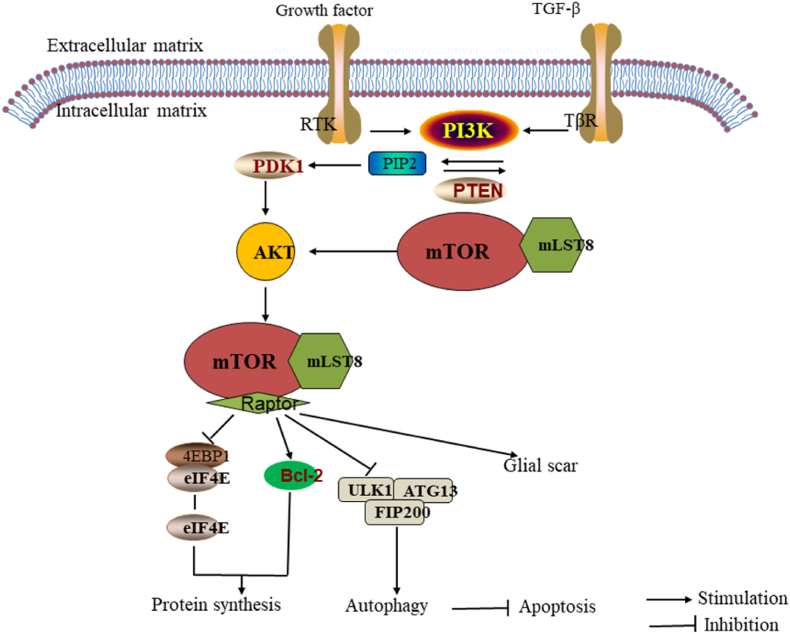
Glial scarring is another major obstacle to neuronal regeneration and recovery after spinal cord injury. Therefore, overcoming glial scarring is important for axon regeneration.120 Glial scars are composed of reactive astrocytes and connective tissue. The main component of the extracellular matrix is the chondroitin sulfate proteoglycan. More specifically, the astrocytes become hypertrophic and proliferate to form astrocyte-rich boundaries, resulting in a glial scar following SCI.121 MTOR has been reported to be involved in astrocyte proliferation by inducing the downstream cascade proteins and activating astrocytes.122 The mTOR-STAT3 pathway is activated while the Nogo receptor is blocked by Nogo-66. The mTOR-STAT3 pathway promotes the differentiation of neural progenitor cells into astrocyte lineages.123 Studies have shown that the astrocytes are upregulated by the epidermal growth factor (EGF). EGF phosphorylates Akt, leading to mTOR activation, which is an important pathway in astrocyte physiology.42 Several upstream regulators of mTOR are critical for astrocytes. Luan et al. found that the downregulation of PI3K/Akt/mTOR expression may inhibit glial scar formation.124 In addition, PTEN negatively regulates the PI3K/Akt/mTOR pathway, and therefore, it displays great potential to reduce glial scar formation.125 In addition, rapamycin, an mTORC1-sensitive allosteric inhibitor, was found to bind to the intracellular 12 kDa FK506 binding protein (FKBP12) to inhibit the proliferation of astrocytes and reduce GFAP-positive cells in the damaged region, followed by the progression of axonal remodeling.126 Autotrophic hypertrophy and re-entry into the cell cycle lead to reactive astrocyte hyperplasia. Cell cycle regulation is crucial to reduce scar formation. Rapamycin inhibits astrocyte proliferation by regulating the cell cycle In addition, a study found that rapamycin inhibits astrocyte proliferation by reducing the number of cells in the S phase.127 Together, these findings suggest that mTOR plays an important regulatory role in glial scar formation after spinal cord injury (Fig. 6), therefore, the inhibition of mTOR signaling may be a potential regimen for SCI treatment.
Fig. 6.
Mechanism of mTOR signaling pathway in autophagy, apoptosis and glial scar formation.
5.4. Regulation of mTOR pathway on angiogenesis of injured spinal cord
The mTOR signaling pathway plays an important role in regulating angiogenesis in normal as well as cancerous tissues.128 The mTOR pathway regulates the expression of angiogenic factors such as vascular endothelial growth factor (VEGF), nitric oxide, and angiogenin128,129. Previous studies have shown that the inhibition of the mTOR pathway reduces angiogenesis and secretion of angiogenic factors.130 SCI leads to the destruction of vascular structures during primary injury.131 It has been widely reported that the stimulation of post-traumatic angiogenesis by angiogenic factors (such as VEGF) promotes nerve regeneration and improves functional recovery after spinal cord injury. Optimizing angiogenesis-targeting therapy after spinal cord injury is key therapeutic strategy.132,133 Therefore, the modulation of the mTOR signaling pathway to stimulate angiogenesis may be a promising method to promote nerve regeneration after SCI.
5.5. Role of mTOR pathway in autophagy and apoptosis after SCI
Autophagy is an intracellular mechanism involved in the degradation of organelle-dependent pathways and removal of dysfunctional cellular components to maintain metabolism and homeostasis. Apoptosis or programmed cell death, includes a series of characteristic cell changes, and plays a crucial role in cell death and autophagy.134 The kinase complex needs to pre-stimulate autophagy; ULK1/Atg13/FIP200 (unc-51-like kinase 1/mammalian autophagy-related gene 13/focal adhesion kinase family interacting protein of about 200 kDa) is directly phosphorylated and inhibited by mTORC1.135 Studies have proposed that the mTOR signaling pathway is involved in the regulation of autophagy and apoptosis after spinal cord injury. The activation of the mTOR pathway may increase the phosphorylation of P70 S6 kinase (p70S6K) protein, leading to the decreased expression of LC-3 II and Beclin-1 in spinal cord injury. At the same time, TUNEL-positive cells were increased, apoptosis was promoted, and nerve tissue damage associated with dyskinesia was increased after SCI. However, the harmful effects of mTOR pathway activation, in SCI, were inhibited by rapamycin and simvastatin. They can also combat apoptosis and reduce nerve tissue damage associated with dyskinesia after SCI.35,107,126 In contrast, some studies have demonstrated that the activation of the Akt/mTOR signaling pathway inhibits excessive autophagy and enhances functional recovery after SCI.136,137 Therefore, the mTOR signaling pathway is involved in the regulation of autophagy and apoptosis after SCI. Interestingly, there is a close relationship between apoptosis and autophagy in spinal cord injury, and inducing autophagy can produce neuroprotective effects by inhibiting apoptosis in rats with acute spinal cord injury138, 139, 140 (Fig. 6).
6. The role of related herbal medicines in Wnt/mTOR signaling pathway
The current treatment of SCI mainly includes early surgical treatment and conservative treatment. However, there has been little intervention in the pathophysiological processes of SCI. One of the main goals of the SCI therapy is to improve the microenvironment and promote regeneration of the injured site. In recent years, traditional Chinese medicine (TCM) has attracted significant attention in SCI therapy.141 Studies have found that intervention with some Chinese herbs (such as curcumin and resveratrol), in the middle and late stages of SCI, plays a certain role in improving the microenvironment and promotes the regeneration of injured sites displaying their potential as a future therapeutic agents for SCI.
6.1. Effect of resveratrol and Wnt/mTOR signaling pathway on SCI
Resveratrol is a natural polyphenol antioxidant in Traditional Chinese medicine. Its active ingredients are synthesized from a variety of plants such as knotweed and blueberry.142 Resveratrol has a wide range of biological activities and pharmacological functions, including anti-inflammatory, antioxidant, and improvement of microcirculation.143, 144, 145, 146 Resveratrol has been found to have a protective effect in animal models of neuronal degeneration.147 Relevant studies have identified resveratrol as a potential therapeutic agent for SCI therapy, and its therapeutic effect has been confirmed by behavioral score.148 It has also been shown to regulate the Wnt/mTOR pathway through a variety of mechanisms, such as PI3K and Akt. PI3K and Akt are upstream activators of mTOR. Additionally, some other studies have found that resveratrol is involved in various pathological processes, such as apoptosis, autophagy, inflammation, and improvement of scar tissue after SCI.149 Following SCI, resveratrol may inhibit the phosphorylation of PI3K, Akt, and mTOR, thereby improving the ratio of Bcl2/Bax and reducing the expression of Caspase-3, exerting an anti-apoptotic effect.150,151 Several studies have reported that autophagy plays an important role in SCI. Zhou et al. reported an increase in autophagy after resveratrol treatment.152 The mechanism of autophagy induction by resveratrol mainly involves the inhibition of the mTOR-ULK1 pathway, which reduces the phosphorylation barrier of UNC-51-like autophagy-activated kinase 1 (ULK1), thereby inducing autophagy.153 Similarly, resveratrol inhibits the proliferation of pathological scar fibroblasts by decreasing the expression of mTOR and its downstream molecule p70S6K.154 These studies confirmed that resveratrol is important adjunctive therapeutic for spinal cord injury and may help in the treatment and repair for spinal cord injury.
6.2. Effects of curcumin and Wnt/mTOR signaling pathway on SCI
Curcumin is a highly pleiotropic molecule from Curcuma Longa with strong anti-inflammatory properties,155 and its pleiotropic activity seems to affect a variety of signaling pathways activated in SCI.156,157 Studies have reported that curcumin can inhibit the Wnt/mTOR signaling pathway, promote motor function recovery after injury, reduce neuronal apoptosis, and play a neuroprotective role.158 Although there are relatively few reports discussing the role of curcumin in the treatment of SCI through the Wnt/mTOR signaling pathway, the role of curcumin in SCI has been confirmed.159 A large number of studies have reported that curcumin improves the prognosis of SCI and reduces secondary spinal cord injury mainly through its antioxidant, anti-inflammatory, and anti-apoptosis effects.160, 161, 162 Based on the above studies, it is clear that curcumin plays an important regulatory role in SCI. Although there are few studies on the interaction between curcumin and the Wnt/mTOR signaling pathway in SCI, but it may be speculated that the Wnt/mTOR signaling pathway is a potential therapeutic target of curcumin in the treatment of SCI by improving the spinal cord microenvironment.
7. Discussion
The Wnt/mTOR signaling pathway is a universal signaling cascade that is involved in the physiological and pathological processes of a variety of diseases, and in recent years, an increasing number of studies have shown that Wnt/mTOR signaling plays a crucial role in the treatment and progression of SCI. Wnt/mTOR signaling has been established to play an important role in neural development, synaptogenesis, neuronal growth, differentiation, and survival. However, there are relatively few studies on Wnt/mTOR in spinal cord, and the specific mechanism of SCI has not been fully elucidated. In addition, the exact role of Wnt/mTOR signaling in spinal cord injury is controversial. Most studies have shown that the activation of Wnt/mTOR signaling can alleviate SCI and contribute to axon regeneration and neurological function recovery, but relatively few studies have shown that the activation of Wnt/mTOR has a negative effect on SCI. Therefore, future studies need to focus on the detailed mechanisms of Wnt/mTOR to develop targeted therapies for SCI. However, the following issues need to be included into the focus of further research before further research. First, most studies on the effects of Wnt/mTOR on SCI are at the cellular stage, and further studies using preclinical animal studies are needed to improve the transition to the clinic. Secondly, the implementation of Wnt/mTOR therapy requires an appropriate delivery system, so how to select an appropriate vector to ensure successful delivery of Wnt/mTOR to the desired target is the direction of future research. Thirdly, whether Wnt/mTOR signal is a potential therapeutic target in spinal cord injury still needs to be verified by an independent cohort. So as to intervene SCI from the pathophysiological level. However, at present, the role of Wnt/mTOR pathway and spinal cord injury remains mostly in vitro and animal models, and there are few clinical studies on the activation or inhibition of Wnt/mTOR pathway on SCI. Therefore, future clinical studies are needed to evaluate the feasibility, effectiveness and safety of targeting Wnt/mTOR signaling in the treatment of SCI. In conclusion, Wnt/mTOR signaling plays an important role in spinal cord injury and may be a potential therapeutic target for SCI to intervene in the treatment and neurological recovery after SCI from the pathophysiological level.
Funding
This work has been co-financed by the National Natural Science Foundation of China (No.31960175).
Author's contribution and acknowledgment
All authors read and approved the final manuscript.
Research involving human and animal participants
This article does not contain any studies with animals performed by any of the authors.
Declaration of competing interest
The manuscript has not been published before and is not being considered for publication elsewhere. All authors have contributed to the creation of this manuscript for important intellectual content and read and approved the final manuscript. We declare there is no conflict of interest.
Contributor Information
Peng Cheng, Email: chengpeng0508@126.com.
Hai-Yang Liao, Email: 17379742435@163.com.
Hai-Hong Zhang, Email: 840620262@qq.com.
References
- 1.Zhang J., Li S., Wu Y. Recovery of spinal cord injury following electroacupuncture in rats through enhancement of Wnt/β-catenin signaling. Mol Med Rep. 2017;16(2):2185–2190. doi: 10.3892/mmr.2017.6801. [DOI] [PubMed] [Google Scholar]
- 2.Dumont R.J., et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24(5):254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Zhou K., et al. Spatiotemporal expression of Ski after rat spinal cord injury. Neuroreport. 2017;28(3):149–157. doi: 10.1097/WNR.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R., et al. Advances in the management of spinal cord injury. J Am Acad Orthop Surg. 2010;18(4):210–222. doi: 10.5435/00124635-201004000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Tator C.H. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5(4):407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 6.Lukovic D., et al. Concise review: reactive astrocytes and stem cells in spinal cord injury: good guys or bad guys? Stem Cell. 2015;33(4):1036–1041. doi: 10.1002/stem.1959. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y.H., Ha K.Y., Kim S.I. Spinal cord injury and related clinical trials. Clin Orthop Surg. 2017;9(1):1–9. doi: 10.4055/cios.2017.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon B.K., et al. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4(4):451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Zechner D., et al. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258(2):406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 10.Crowe M.J., et al. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3(1):73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 11.Osterholm J.L., Mathews G.J. Altered norepinephrine metabolism following experimental spinal cord injury. 1. Relationship to hemorrhagic necrosis and post-wounding neurological deficits. J Neurosurg. 1972;36(4):386–394. doi: 10.3171/jns.1972.36.4.0386. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja C.S., et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80(3s):S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 13.Rouanet C., et al. Traumatic spinal cord injury: current concepts and treatment update. Arq Neuropsiquiatr. 2017;75(6):387–393. doi: 10.1590/0004-282X20170048. [DOI] [PubMed] [Google Scholar]
- 14.Ahuja C.S., et al. Traumatic spinal cord injury. Nat Rev Dis Prim. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 15.Inestrosa N.C., Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11(2):77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 16.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Yamagami T., et al. Transient activation of Wnt/β-catenin signaling reporter in fibrotic scar formation after compression spinal cord injury in adult mice. Biochem Biophys Res Commun. 2018;496(4):1302–1307. doi: 10.1016/j.bbrc.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman P.E., et al. Highly conserved molecular pathways, including Wnt signaling, promote functional recovery from spinal cord injury in lampreys. Sci Rep. 2018;8(1):742. doi: 10.1038/s41598-017-18757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchetti B., Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol Med. 2013;19(3):144–156. doi: 10.1016/j.molmed.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David M.D., Cantí C., Herreros J. Wnt-3a and Wnt-3 differently stimulate proliferation and neurogenesis of spinal neural precursors and promote neurite outgrowth by canonical signaling. J Neurosci Res. 2010;88(14):3011–3023. doi: 10.1002/jnr.22464. [DOI] [PubMed] [Google Scholar]
- 21.Varela-Nallar L., et al. Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci U S A. 2010;107(49):21164–21169. doi: 10.1073/pnas.1010011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nusse R., Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. Embo j. 2012;31(12):2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cisternas P., et al. Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol. 2014;49(1):574–589. doi: 10.1007/s12035-013-8540-5. [DOI] [PubMed] [Google Scholar]
- 24.Varela-Nallar L., Inestrosa N.C. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci. 2013;7:100. doi: 10.3389/fncel.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ríos J.A., et al. Is Alzheimer's disease related to metabolic syndrome? A Wnt signaling conundrum. Prog Neurobiol. 2014;121:125–146. doi: 10.1016/j.pneurobio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Ravikumar B., et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 27.Santini E., et al. Inhibition of mTOR signaling in Parkinson's disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2(80):ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- 28.Ma T., et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spilman P., et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., et al. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28(33):8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González-Fernández C., et al. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 2014;31(6):565–581. doi: 10.1089/neu.2013.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S., et al. Fezf2 promotes neuronal differentiation through localised activation of Wnt/β-catenin signalling during forebrain development. Development. 2014;141(24):4794–4805. doi: 10.1242/dev.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosso S.B., Inestrosa N.C. WNT signaling in neuronal maturation and synaptogenesis. Front Cell Neurosci. 2013;7:103. doi: 10.3389/fncel.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva-Alvarez C., et al. Canonical Wnt signaling protects hippocampal neurons from Aβ oligomers: role of non-canonical Wnt-5a/Ca(2+) in mitochondrial dynamics. Front Cell Neurosci. 2013;7:97. doi: 10.3389/fncel.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekiguchi A., et al. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma. 2012;29(5):946–956. doi: 10.1089/neu.2011.1919. [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Martos C.M., et al. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One. 2011;6(11):e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libro R., et al. Is the Wnt/β-catenin pathway involved in the anti-inflammatory activity of glucocorticoids in spinal cord injury? Neuroreport. 2016;27(14):1086–1094. doi: 10.1097/WNR.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 38.Lu G.B., et al. Methylprednisolone promotes recovery of neurological function after spinal cord injury: association with Wnt/β-catenin signaling pathway activation. Neural Regen Res. 2016;11(11):1816–1823. doi: 10.4103/1673-5374.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyashita T., et al. Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma. 2009;26(7):955–964. doi: 10.1089/neu.2008.0776. [DOI] [PubMed] [Google Scholar]
- 40.Park K.K., et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu K., et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13(9):1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Codeluppi S., et al. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci. 2009;29(4):1093–1104. doi: 10.1523/JNEUROSCI.4103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Q., et al. The many postures of noncanonical Wnt signaling in development and diseases. Biomed Pharmacother. 2017;93:359–369. doi: 10.1016/j.biopha.2017.06.061. [DOI] [PubMed] [Google Scholar]
- 44.Abou Ziki M.D., Mani A. The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome. Nutr Res. 2019;70:18–25. doi: 10.1016/j.nutres.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willert K., Nusse R. Wnt proteins. Cold Spring Harbor Perspect Biol. 2012;4(9):a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X., et al. Characterization of tiki, a new family of Wnt-specific metalloproteases. J Biol Chem. 2016;291(5):2435–2443. doi: 10.1074/jbc.M115.677807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark H.F., et al. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. 1995;9(12):1530–1542. doi: 10.1101/gad.9.12.1530. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh J.C., et al. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci U S A. 1999;96(7):3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhanot P., et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382(6588):225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 51.He X., et al. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131(8):1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 52.Gordon M.D., Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281(32):22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 53.Yang Q., Guan K.L. Expanding mTOR signaling. Cell Res. 2007;17(8):666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 54.Gong X., et al. Activating the translational repressor 4E-BP or reducing S6K-GSK3β activity prevents accelerated axon growth induced by hyperactive mTOR in vivo. Hum Mol Genet. 2015;24(20):5746–5758. doi: 10.1093/hmg/ddv295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaur A., Sharma S. Mammalian target of rapamycin (mTOR) as a potential therapeutic target in various diseases. Inflammopharmacology. 2017;25(3):293–312. doi: 10.1007/s10787-017-0336-1. [DOI] [PubMed] [Google Scholar]
- 56.Yin Z.S., et al. Repair effect of Wnt3a protein on the contused adult rat spinal cord. Neurol Res. 2008;30(5):480–486. doi: 10.1179/174313208X284133. [DOI] [PubMed] [Google Scholar]
- 57.Park J.H., et al. Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fibroblasts. Acta Neurochir (Wien) 2013;155(5):809–816. doi: 10.1007/s00701-013-1663-7. [DOI] [PubMed] [Google Scholar]
- 58.Suh H.I., et al. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir (Wien) 2011;153(5):1003–1010. doi: 10.1007/s00701-011-0945-1. [DOI] [PubMed] [Google Scholar]
- 59.Kanno H., et al. The role of mTOR signaling pathway in spinal cord injury. Cell Cycle. 2012;11(17):3175–3179. doi: 10.4161/cc.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei H., et al. Comparative profiling of microRNA expression between neural stem cells and motor neurons in embryonic spinal cord in rat. Int J Dev Neurosci. 2010;28(6):545–551. doi: 10.1016/j.ijdevneu.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez P., et al. Spatio-temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats. PLoS One. 2012;7(12):e50793. doi: 10.1371/journal.pone.0050793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee I.C., Wu H.J., Liu H.L. Dual-frequency ultrasound induces neural stem/progenitor cell differentiation and growth factor utilization by enhancing stable cavitation. ACS Chem Neurosci. 2019;10(3):1452–1461. doi: 10.1021/acschemneuro.8b00483. [DOI] [PubMed] [Google Scholar]
- 63.Zheng G., et al. Carbon monoxide releasing molecule-3 alleviates neuron death after spinal cord injury via inflammasome regulation. EBioMedicine. 2019;40:643–654. doi: 10.1016/j.ebiom.2018.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X.J., et al. Polysialic-acid-based micelles promote neural regeneration in spinal cord injury therapy. Nano Lett. 2019;19(2):829–838. doi: 10.1021/acs.nanolett.8b04020. [DOI] [PubMed] [Google Scholar]
- 65.Yamashita T. Neogenin is a determining factor for regenerating neurons following spinal cord injury. Neuroscience. 2019;408:448–449. doi: 10.1016/j.neuroscience.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 66.Wang X., et al. A novel artificial nerve graft for repairing long-distance sciatic nerve defects: a self-assembling peptide nanofiber scaffold-containing poly(lactic-co-glycolic acid) conduit. Neural Regen Res. 2014;9(24):2132–2141. doi: 10.4103/1673-5374.147944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiNuoscio G., Atit R.P. Wnt/β-catenin signaling in the mouse embryonic cranial mesenchyme is required to sustain the emerging differentiated meningeal layers. Genesis. 2019;57(1):e23279. doi: 10.1002/dvg.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brafman D., Willert K. Wnt/β-catenin signaling during early vertebrate neural development. Dev Neurobiol. 2017;77(11):1239–1259. doi: 10.1002/dneu.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lie D.C., et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 70.Jiao S., et al. miR-124 promotes proliferation and neural differentiation of neural stem cells through targeting DACT1 and activating Wnt/β-catenin pathways. Mol Cell Biochem. 2018;449(1-2):305–314. doi: 10.1007/s11010-018-3367-z. [DOI] [PubMed] [Google Scholar]
- 71.Hu Y., et al. Fasudil may induce the differentiation of bone marrow mesenchymal stem cells into neuron-like cells via the Wnt/β-catenin pathway. Mol Med Rep. 2019;19(4):3095–3104. doi: 10.3892/mmr.2019.9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou H., et al. Salvianolic acid B activates Wnt/β-catenin signaling following spinal cord injury. Exp Ther Med. 2020;19(2):825–832. doi: 10.3892/etm.2019.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Profyris C., et al. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15(3):415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 74.Silver J., Miller J.H. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 75.Li P., Teng Z.Q., Liu C.M. Extrinsic and intrinsic regulation of axon regeneration by MicroRNAs after spinal cord injury. Neural Plast. 2016:1279051. doi: 10.1155/2016/1279051. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song Y.H., et al. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv Drug Deliv Rev. 2019;148:38–59. doi: 10.1016/j.addr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ciani L., Salinas P.C. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6(5):351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 78.Charron F., Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132(10):2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- 79.Fenstermaker A.G., et al. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30(47):16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kubo F., Nakagawa S. Wnt signaling in retinal stem cells and regeneration. Dev Growth Differ. 2008;50(4):245–251. doi: 10.1111/j.1440-169X.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 81.Osakada F., et al. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27(15):4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strand N.S., et al. Wnt/β-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochem Biophys Res Commun. 2016;477(4):952–956. doi: 10.1016/j.bbrc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Briona L.K., et al. Wnt/ß-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev Biol. 2015;403(1):15–21. doi: 10.1016/j.ydbio.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wehner D., et al. Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat Commun. 2017;8(1):126. doi: 10.1038/s41467-017-00143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia A.L., et al. A growing field: the regulation of axonal regeneration by Wnt signaling. Neural Regen Res. 2018;13(1):43–52. doi: 10.4103/1673-5374.224359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.González P., et al. Frizzled 1 and Wnt1 as new potential therapeutic targets in the traumatically injured spinal cord. Cell Mol Life Sci. 2020;77(22):4631–4662. doi: 10.1007/s00018-019-03427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Q., et al. Wnt3a ectopic expression interferes axonal projection and motor neuron positioning during the chicken spinal cord development. J Mol Neurosci. 2018;64(4):619–630. doi: 10.1007/s12031-018-1060-z. [DOI] [PubMed] [Google Scholar]
- 88.Duarte F.C.K., et al. Association between naturally occurring spine osteoarthritis in geriatric rats and neurogenic inflammation within neurosegmentally linked skeletal muscle. Exp Gerontol. 2019;118:31–38. doi: 10.1016/j.exger.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Norden D.M., et al. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64(2):300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rahimi V.B., et al. Protective effects of hydro-ethanolic extract of Terminalia chebula on primary microglia cells and their polarization (M(1)/M(2) balance) Mult Scler Relat Disord. 2018;25:5–13. doi: 10.1016/j.msard.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 91.L'Episcopo F., et al. Microglia polarization, gene-environment interactions and Wnt/β-catenin signaling: emerging roles of Glia-neuron and Glia-stem/neuroprogenitor crosstalk for dopaminergic neurorestoration in aged parkinsonian brain. Front Aging Neurosci. 2018;10:12. doi: 10.3389/fnagi.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matias D., et al. Microglia-glioblastoma interactions: new role for Wnt signaling. Biochim Biophys Acta Rev Cancer. 2017;1868(1):333–340. doi: 10.1016/j.bbcan.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Vallée A., et al. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer's disease. Acta Biochim Biophys Sin (Shanghai) 2017;49(10):853–866. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y.K., et al. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013;123(5):2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pekny M., Wilhelmsson U., Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 96.Sonn I., et al. Polarization of reactive astrocytes in response to spinal cord injury is enhanced by M2 macrophage-mediated activation of Wnt/β-catenin pathway. Mol Neurobiol. 2020;57(4):1847–1862. doi: 10.1007/s12035-019-01851-y. [DOI] [PubMed] [Google Scholar]
- 97.Ji R.R., Xu Z.Z., Gao Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu S., et al. Wnt/Ryk signaling contributes to neuropathic pain by regulating sensory neuron excitability and spinal synaptic plasticity in rats. Pain. 2015;156(12):2572–2584. doi: 10.1097/j.pain.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 99.Itokazu T., et al. Involvement of Wnt/β-catenin signaling in the development of neuropathic pain. Neurosci Res. 2014;79:34–40. doi: 10.1016/j.neures.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Shi Y., et al. Regulation of Wnt signaling by nociceptive input in animal models. Mol Pain. 2012;8:47. doi: 10.1186/1744-8069-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang S.J. Synaptic activity-regulated Wnt signaling in synaptic plasticity, glial function and chronic pain. CNS Neurol Disord - Drug Targets. 2014;13(5):737–744. doi: 10.2174/1871527312666131223114457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan X., et al. WITHDRAWN: MiR-216b-5p attenuates chronic constriction injury-induced neuropathic pain in female rats by targeting MAL2 and inactivating Wnt/β-catenin signaling pathway. Neurochem Int. 2020:104930. doi: 10.1016/j.neuint.2020.104930. [DOI] [PubMed] [Google Scholar]
- 103.Wang W., Li R. MiR-216a-5p alleviates chronic constriction injury-induced neuropathic pain in rats by targeting KDM3A and inactivating Wnt/β-catenin signaling pathway. Neurosci Res. 2021;170:255–264. doi: 10.1016/j.neures.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y., et al. The Wnt/β-catenin pathway regulated cytokines for pathological neuropathic pain in chronic compression of dorsal root ganglion model. Neural Plast. 2021:6680192. doi: 10.1155/2021/6680192. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan J., et al. Protective effects of hyperbaric oxygen treatment against spinal cord injury in rats via toll-like receptor 2/nuclear factor-κB signaling. Int J Clin Exp Pathol. 2014;7(5):1911–1919. [PMC free article] [PubMed] [Google Scholar]
- 106.Yu C.G., et al. Involvement of ERK2 in traumatic spinal cord injury. J Neurochem. 2010;113(1):131–142. doi: 10.1111/j.1471-4159.2010.06579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao K., et al. Neuroprotective effect of simvastatin via inducing the autophagy on spinal cord injury in the rat model. BioMed Res Int. 2015;2015:260161. doi: 10.1155/2015/260161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sahin B., et al. The effects of medroxy progesterone acetate on the pro-inflammatory cytokines, TNF-alpha and IL-1beta in the early phase of the spinal cord injury. Neurol Res. 2011;33(1):63–67. doi: 10.1179/016164110X12807570510095. [DOI] [PubMed] [Google Scholar]
- 109.Powell J.D., et al. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dello Russo C., et al. Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochem Pharmacol. 2009;78(9):1242–1251. doi: 10.1016/j.bcp.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 111.Choi Y.H., et al. Inhibition of protein kinase C delta attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF pathway. PLoS One. 2013;8(11):e81773. doi: 10.1371/journal.pone.0081773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie L., et al. mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. J Immunol. 2014;192(12):6009–6019. doi: 10.4049/jimmunol.1303492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie S., et al. Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PLoS One. 2014;9(4):e94496. doi: 10.1371/journal.pone.0094496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cordaro M., et al. KU0063794, a dual mTORC1 and mTORC2 inhibitor, reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. Mol Neurobiol. 2017;54(4):2415–2427. doi: 10.1007/s12035-016-9827-0. [DOI] [PubMed] [Google Scholar]
- 115.Leibinger M., et al. Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell Death Dis. 2013;4(9):e805. doi: 10.1038/cddis.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Asante C.O., Wallace V.C., Dickenson A.H. Mammalian target of rapamycin signaling in the spinal cord is required for neuronal plasticity and behavioral hypersensitivity associated with neuropathy in the rat. J Pain. 2010;11(12):1356–1367. doi: 10.1016/j.jpain.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang H., et al. Inhibition of Gli1-mediated prostate cancer cell proliferation by inhibiting the mTOR/S6K1 signaling pathway. Oncol Lett. 2017;14(6):7970–7976. doi: 10.3892/ol.2017.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Ali H., et al. The mTOR substrate S6 kinase 1 (S6K1) is a negative regulator of axon regeneration and a potential drug target for central nervous system injury. J Neurosci. 2017;37(30):7079–7095. doi: 10.1523/JNEUROSCI.0931-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choi Y.J., et al. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22(18):2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ren J., et al. TCTP expression after rat spinal cord injury: implications for astrocyte proliferation and migration. J Mol Neurosci. 2015;57(3):366–375. doi: 10.1007/s12031-015-0628-0. [DOI] [PubMed] [Google Scholar]
- 121.Milbreta U., et al. Astrocytic and vascular remodeling in the injured adult rat spinal cord after chondroitinase ABC treatment. J Neurotrauma. 2014;31(9):803–818. doi: 10.1089/neu.2013.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo D., Zou J., Wong M. Rapamycin attenuates acute seizure-induced astrocyte injury in mice in vivo. Sci Rep. 2017;7(1):2867. doi: 10.1038/s41598-017-03032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang B., et al. Nogo-66 promotes the differentiation of neural progenitors into astroglial lineage cells through mTOR-STAT3 pathway. PLoS One. 2008;3(3):e1856. doi: 10.1371/journal.pone.0001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luan Y., Chen M., Zhou L. MiR-17 targets PTEN and facilitates glial scar formation after spinal cord injuries via the PI3K/Akt/mTOR pathway. Brain Res Bull. 2017;128:68–75. doi: 10.1016/j.brainresbull.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 125.Chen C.H., et al. The role of the PI3K/Akt/mTOR pathway in glial scar formation following spinal cord injury. Exp Neurol. 2016;278:27–41. doi: 10.1016/j.expneurol.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 126.Goldshmit Y., et al. Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci. 2015;68:82–91. doi: 10.1016/j.mcn.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 127.Li C.Y., et al. Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen-glucose deprivation and reoxygenation. Neurochem Int. 2015;83–84:9–18. doi: 10.1016/j.neuint.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 128.Karar J., Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhong H., et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60(6):1541–1545. [PubMed] [Google Scholar]
- 130.Guba M., et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 131.Mautes A.E., et al. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80(7):673–687. [PubMed] [Google Scholar]
- 132.Facchiano F., et al. Promotion of regeneration of corticospinal tract axons in rats with recombinant vascular endothelial growth factor alone and combined with adenovirus coding for this factor. J Neurosurg. 2002;97(1):161–168. doi: 10.3171/jns.2002.97.1.0161. [DOI] [PubMed] [Google Scholar]
- 133.Widenfalk J., et al. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120(4):951–960. doi: 10.1016/s0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 134.Avin-Wittenberg T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019;42(3):1045–1053. doi: 10.1111/pce.13404. [DOI] [PubMed] [Google Scholar]
- 135.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang H.Y., et al. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol. 2013;48(3):452–464. doi: 10.1007/s12035-013-8432-8. [DOI] [PubMed] [Google Scholar]
- 137.Walker C.L., et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7(1):e30012. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Z.Y., et al. Beclin-1-mediated autophagy protects spinal cord neurons against mechanical injury-induced apoptosis. Apoptosis. 2014;19(6):933–945. doi: 10.1007/s10495-014-0976-1. [DOI] [PubMed] [Google Scholar]
- 139.Tang P., et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49(1):276–287. doi: 10.1007/s12035-013-8518-3. [DOI] [PubMed] [Google Scholar]
- 140.Guo Y., et al. G-CSF promotes autophagy and reduces neural tissue damage after spinal cord injury in mice. Lab Invest. 2015;95(12):1439–1449. doi: 10.1038/labinvest.2015.120. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Q., et al. Therapeutic effects of traditional Chinese medicine on spinal cord injury: a promising supplementary treatment in future. Evid Based Complement Alternat Med. 2016:8958721. doi: 10.1155/2016/8958721. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Engelhard K., et al. Influence of propofol on neuronal damage and apoptotic factors after incomplete cerebral ischemia and reperfusion in rats: a long-term observation. Anesthesiology. 2004;101(4):912–917. doi: 10.1097/00000542-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 143.Posey K.L., et al. Antioxidant and anti-inflammatory agents mitigate pathology in a mouse model of pseudoachondroplasia. Hum Mol Genet. 2015;24(14):3918–3928. doi: 10.1093/hmg/ddv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Holthoff J.H., et al. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012;81(4):370–378. doi: 10.1038/ki.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sobotková A., et al. Antioxidants change platelet responses to various stimulating events. Free Radic Biol Med. 2009;47(12):1707–1714. doi: 10.1016/j.freeradbiomed.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hong Y.B., et al. Inhibition of cell proliferation by a resveratrol analog in human pancreatic and breast cancer cells. Exp Mol Med. 2009;41(3):151–160. doi: 10.3858/emm.2009.41.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Han Y.S., et al. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol. 2004;141(6):997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ates O., et al. Effects of resveratrol and methylprednisolone on biochemical, neurobehavioral and histopathological recovery after experimental spinal cord injury. Acta Pharmacol Sin. 2006;27(10):1317–1325. doi: 10.1111/j.1745-7254.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 149.Zhou J., et al. Beneficial effects of resveratrol-mediated inhibition of the mTOR pathway in spinal cord injury. Neural Plast. 2018;2018:7513748. doi: 10.1155/2018/7513748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu C., et al. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 151.Chen Q., et al. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS One. 2010;5(12):e15288. doi: 10.1371/journal.pone.0015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao H., et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience. 2017;348:241–251. doi: 10.1016/j.neuroscience.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 153.Mariño G., Madeo F., Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Curr Opin Cell Biol. 2011;23(2):198–206. doi: 10.1016/j.ceb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 154.Tang Z.M., Zhai X.X., Ding J.C. Expression of mTOR/70S6K signaling pathway in pathological scar fibroblasts and the effects of resveratrol intervention. Mol Med Rep. 2017;15(5):2546–2550. doi: 10.3892/mmr.2017.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schraufstatter E., Bernt H. Antibacterial action of curcumin and related compounds. Nature. 1949;164(4167):456. doi: 10.1038/164456a0. [DOI] [PubMed] [Google Scholar]
- 156.Aggarwal B.B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 157.Begum A.N., et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Therapeut. 2008;326(1):196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li W., et al. Curcumin promotes functional recovery and inhibits neuronal apoptosis after spinal cord injury through the modulation of autophagy. J Spinal Cord Med. 2021;44(1):37–45. doi: 10.1080/10790268.2019.1616147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sanivarapu R., Vallabhaneni V., Verma V. The potential of curcumin in treatment of spinal cord injury. Neurol Res Int. 2016;2016:9468193. doi: 10.1155/2016/9468193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Limcharoen T., et al. Improved antiallodynic, antihyperalgesic and anti-inflammatory response achieved through potential prodrug of curcumin, curcumin diethyl diglutarate in a mouse model of neuropathic pain. Eur J Pharmacol. 2021;899:174008. doi: 10.1016/j.ejphar.2021.174008. [DOI] [PubMed] [Google Scholar]
- 161.Yardım A., et al. Protective effects of curcumin against paclitaxel-induced spinal cord and sciatic nerve injuries in rats. Neurochem Res. 2021;46(2):379–395. doi: 10.1007/s11064-020-03174-0. [DOI] [PubMed] [Google Scholar]
- 162.Ormond D.R., et al. Recovery from spinal cord injury using naturally occurring antiinflammatory compound curcumin: laboratory investigation. J Neurosurg Spine. 2012;16(5):497–503. doi: 10.3171/2012.1.SPINE11769. [DOI] [PubMed] [Google Scholar]