Summary
Background:
HIV incidence is increasing in Eastern Europe and Central Asia (EECA), primarily driven by injecting drug use. Coverage of antiretroviral therapy (ART) and opioid agonist therapy (OAT) are sub-optimal, with people who inject drugs (PWID) experiencing considerable incarceration. We evaluated whether using saved monies from decriminalising drug use and/or possession to scale-up ART and OAT could control HIV among PWID in EECA.
Methods:
A dynamic HIV transmission model among PWID incorporating incarceration, ART and OAT was calibrated to Belarus, Kazakhstan, Kyrgyzstan and St. Petersburg (Russia). Country-specific costs for OAT, ART and incarceration were collated/estimated. Compared to baseline, the model prospectively projected the life years gained (LYG), incremental costs (2018 Euros) and infections prevented over 2020-2040 for three scenarios: (1) Decriminalisation: Remove incarceration due to drug use and drug possession for personal use, reducing incarceration among PWID by 25-46%; (2) Public Health Approach: Use savings from decriminalisation to scale-up ART and OAT; and (3) Full Scale-up: scenario 2 plus invest additional resources to scale-up ART to 81% coverage and OAT to 40% coverage. The incremental cost-effectiveness ratio (ICER) per LYG for each scenario were calculated and compared to country-specific 1xGDP per-capita willingness-to-pay thresholds. Costs and LYG were discounted 3% annually.
Findings:
Current levels of incarceration, OAT and ART are estimated to cost €197-4,129 million over 2020-2040 across settings; 74.8-95.8% due to incarceration costs. Decriminalisation results in cost-savings (€38-773 million) but modest LYG (745-1,694). The Public Health Approach was cost-saving, allowing each country to reach 81% ART coverage and 29.7-41.8% OAT coverage, resulting in 17,768-148,464 LYG and 58.9-83.7% of infections prevented. Results were similar for the Full Scale-up scenario.
Interpretation:
Cost-savings from decriminalising drug use could dramatically reduce HIV transmission through increased OAT and ART coverage among PWID in EECA.
Funding:
Alliance for Public Health, US National Institute of Health, Economist Intelligence Unit
Keywords: HIV, people who inject drugs, Opioid substitution therapy, criminalisation, incarceration, anti-retroviral therapy, Eastern Europe and Central Asia
Introduction
HIV incidence and mortality have increased in Eastern Europe and Central Asia (EECA) since 20101. Injecting drug use (IDU) accounts for most (48%) new HIV infections1 with people who inject drugs (PWID) having high HIV prevalence (7.3-53.4%)2. This region is characterised by suboptimal HIV prevention and treatment3, with PWID experiencing substantial incarceration.2 HIV prevention strategies are lacking in prisons globally, including EECA3, with recent release associated with increased HIV transmission4.
ART dramatically reduces HIV morbidity and infectivity5. Opioid agonist treatment (OAT) reduces overdose mortality6, halves HIV acquisition risk6, improves the HIV continuum-of-care6 and reduces criminal activity/incarceration6,7, which could reduce HIV transmission3.
Existing analyses have demonstrated the considerable costs of global drug policy enforcement8 and criminalising drug users9, which our modelling has shown increases HIV transmission3. OAT and ART are cost-effective interventions10,11. Shifting the current policy from criminalising drug users to a public health approach involving scale-up of OAT and ART could be a cost-effective strategy for reducing HIV transmission rates in EECA.
Using dynamic HIV transmission modelling, we consider this question in four EECA settings (Belarus, St. Petersburg (Russia), Kyrgyzstan and Kazakhstan). These settings have a wide range of HIV prevalences (8-48%) and lifetime incarceration (34-76%) among PWID, while the coverage of OAT is low (<1-4%) or illegal (Russia) and ART is variable (27-42%)12-17. Table 1 compares other characteristics. In Russia, Kyrgyzstan and Kazakhstan, drug consumption and/or minor possession is punishable with fines or short detentions, while possession of larger quantities results in incarceration18. In Belarus, any drug offences result in incarceration18. In each setting over 2020-2040, we prospectively model the impact and cost-effectiveness of decriminalising drug use or possession and re-investing the costs saved into scaling up ART and OAT. Through this, we aim to evaluate whether reinvesting resources from criminalising drug use can achieve public health benefit.
Table 1:
Summary table of main model parameters, recent HIV prevalence and intervention coverage data (date in brackets), and model projections for specific unknown parameters in 2020 (denoted by an asterisk). Sources for data are given in the supplementary materials.
| Belarus | Kazakhstan | Kyrgyzstan | St. Petersburg, Russia | |
|---|---|---|---|---|
| Demographics/Fitting | ||||
| HIV Prevalence | 30.8%§ (2017) | 7.9% (95%CI 7.1-8.8%) (2018) | 14.3% (95%CI 12.5-16.3%) (2016) | 48.3% (95%CI 43.1-53.5%) (2017) |
| PWID population size | 75,000§ (2014) | 120,500 (100,000-150,000) (2016) | 25,000 (20,300-29,000) (2013) | 74,000 (60,700 - 87,300) (2009) |
| OAT coverage | 3.7%§ (2019) | 0.2%§ (2019) | 3.9%§ (2019) | 0 |
| ART coverage among current HIV+PWID | 40.5%§ (2018) | 28.5% (95%CI 24.1-33.2%) (2018) | 26.7% (95%CI 20.9-33.5%) (2016) | 42.1% (95%CI 34.8-49.7%) (2017) |
| Viral Suppression | 45.8%§ (2016) | 54.0% (95%CI 52.8-55.2%) (2018) | 88.6% (95%CI 86.1-90.7%) (2019) | 81.2% (95%CI 73.3-87.1%) (2017) |
| Lost to care rate from ART per year | 0.150-0.184 (2017) | 0.252§ (2017) | 0.027-0.051 (2017-2019) | 0.307 (95%CI 0.29-0.33) (2009) |
| Duration of Injecting in years | 6.9 (2017)-10.5 (2015) | 10 (5-30) (2018) | 10 (IQR 4-18) (2016) | 9.4 (2008)-13.8 (2012) |
| Percentage ever incarcerated | 76.2% (95%CI 68.0-82.9%) (2020) | 43.6% (95%CI 42.1-45.1%) (2018) | 45.8% (95%CI 43.2-48.6%) (2016) | 33.8% (95%CI 30.6-37.1%) (2012) |
| % Currently Incarcerated * | 35.2% (95%CrI 33.5 – 37.4%) | 30.4% (95%CrI 28.8 – 32.1%) | 16.7% (95%CrI 13.9 – 20.2%) | 16.3% (95%CrI 15.0 – 18.5%) |
| Average number of times incarcerated among those ever incarcerated* | 5.3 (95%CrI 4.9 – 5.6) | 4.6 (95%CrI 4.3 – 4.8) | 2.8 (95%CrI 2.1 – 3.4) | 2.7 (95%CrI 2.6 −3.0) |
| Average duration of a prison stay in years | 2.5 (IQR 2-3.5) (2020) | 3.4 § (2011) | 1.3-4.6 (2016-2020) | 2.4 (IQR 1.7-3.3) (2012-2013) |
| % of injecting career spent in prison among all PWID* | 36.2% (95%CrI 34.4 – 38.4%) | 32.2% (95%CrI 29.2 – 34.7%) | 15.7% (95%CrI 13.5 – 19.5%) | 17.4% (95%CrI 15.8 – 19.7%) |
| Incarceration rate per year * | 0.19 (95%CrI 0.17 – 0.20) | 0.09 (95%CrI 0.08 – 0.11) | 0.07 (95%CrI 0.04 – 0.09) | 0.046 (95%CrI 0.042 - 0.051) |
| Reincarceration rate per year * | 0.34 (95%CrI 0.32 – 0.37) | 0.47 (95%CrI 0.45 – 0.50) | 0.26 (95%CrI 0.17 – 0.37) | 0.16 (95%CrI 0.13 - 0.20) |
| Odds ratio for difference in HIV prevalence among ever versus never incarcerated PWID | 2.5 (95%CI 0.9-6.8) (2020) | 1.7 (95%CI: 1.1-2.7) (2009-2012) | 2.8 (95%CI: 2.0-3.9) (2016) | 1.7 (95%CI 1.3-2.3) (2012) |
| Relative risk for HIV transmission risk in prison compared to not in prison * | 0.35 (95%CrI 0.14 – 0.55) | 0.88 (95%CrI 0.70 – 1.07) | 2.58 (95%CrI 1.45 – 3.59) | 0.073 (95%CrI 0.00049 - 0.30) |
| Costs (all converted to 2018 euros) | ||||
| Cost of ART per person per year | €302 | €1230 | €363 | €1259 |
| Cost of OAT per person per year@ | €550 | €422 | €383 | €441 scaled from KAZ costs |
| Cost of prison per person per year@ | €5480 scaled from Azerbaijan | €5952 scaled from Russia costs | €1259 | €6641 |
| Arrest and conviction cost per person | €960 scaled from Russia costs | €1161 scaled from Russia costs | €2008 | €1371 |
| Average GDP per capita | €5419 | €8157 | €1123 | €9586 |
denotes median model projections which are from 2020, otherwise data estimates (date in brackets);
when country specific costs did not exist then available cost estimates from the country with the closest GDP was adapted as described in the methods;
denotes parameters where we did not have estimates of uncertainty and so uncertainty bounds were attached to them.
Methods
Model description
We developed a dynamic ordinary differential equation model of incarceration and HIV transmission among PWID to prospectively evaluate the costs and impact of criminalisation, ART and OAT on HIV transmission. The population was stratified by current and ex-injecting status, incarceration state, OAT status and HIV infection state (Figure 1).
Figure 1:

Model schematics for incarceration and injecting cessation components (a) and HIV infection and treatment components (b) of the full model.
Individuals enter the model through initiating IDU as uninfected PWID, with a proportion entering different incarceration strata. Individuals leave the model through non-HIV death and HIV-related death. PWID can also cease IDU and become ex-injectors. Model entry balances cessation of IDU and non-HIV deaths among PWID, but not HIV deaths. PWID are incarcerated and re-incarcerated at different rates, and released at a constant rate to enter the recently released compartment (6-months) before entering the ever-incarcerated category. We do not model incarceration of ex-injectors, assuming they experience negligible drug-related incarceration.
PWID transmit HIV in prison or the community (out of prison). Susceptible PWID are infected via IDU at a rate proportional to a setting’s (prison/community) HIV transmission rate and the proportion of PWID in each stage of HIV infection in that setting. We assume the injecting HIV transmission risk is the same for never incarcerated PWID and community PWID that have been incarcerated, but not recently, while it is increased in recently released PWID4. Incarcerated PWID are assumed to have a different transmission risk to community PWID. PWID and ex-injectors can also be infected sexually in the community, but not in prison.
Following HIV infection, individuals enter a short (~3 months) acute phase, with heightened infectivity, and then progress to chronic infection, which continues until they experience HIV-related mortality. Chronically infected individuals can initiate ART, which reduces their infectivity and HIV-related mortality5. Acutely infected individuals are not assumed to initiate ART because of its short duration. Individuals receiving ART can be lost-to-care and can then be re-enrolled as for ART-naïve individuals.
Although some PWID do not use opioids in our settings, evidence suggests most (83.1-99.3%) do13,14 and so we assume PWID can enrol onto OAT at rates dependent on their incarceration status and leave at fixed rates. A proportion remain on OAT when incarcerated, and similarly when released. Individuals on OAT have decreased mortality (supplementary Table A.7), except when starting/ceasing OAT when mortality is increased6. Being on OAT reduces their HIV transmission risk6; increases their rate of initiating ART and retention6; increases the proportion virally suppressed6; and decreases their rate of re-/incarceration7. We do not assume OAT increases injecting cessation as data is uncertain.
Model parameterisation and calibration
The model was parameterised and calibrated to each setting using context-specific data as summarised in Table 1 and described fully in the Appendix. This included context-specific data from bio-behavioural surveys (BBS) undertaken among PWID and prisoners, program data on numbers of PWID on OAT and ART, and PWID population size estimates. Survey datasets included: five BBS (2002-2017) and three cohort study among PWID in St. Petersburg; four BBS (2009-2018) among PWID and one prison BBS (2011) in Kazakhstan; two BBS (2015-2017) and one behavioural survey (2020) in Belarus; and three BBS among PWID (2010-2016) and three prison BBS (2014-2020) in Kyrgyzstan. Other survey data was also used but just for HIV prevalence estimates. Full details of the data sources are in Supplementary table 1. Prior ranges were assigned to most parameters based on the data from each setting, although some were uninformative if only calibration data could inform that parameter. As described in the following paragraphs, each setting’s model was calibrated to context-specific data on the incarceration dynamics of PWID (three calibration targets usually at one time point), PWID population size (one target), trends in the coverage of OAT and ART (two targets over multiple time points), and several HIV prevalence measures among PWID (three targets; some over multiple time points). The calibration involved the full model and various incarceration sub-model(s) using an Approximate Bayesian Computation sequential Monte Carlo scheme. This produced 1000 parameter sets that fit the calibration data.
For each setting, incarceration sub-model(s) simulated the incarceration dynamics in each setting, stratified by duration of injecting. They were calibrated to data by injecting duration and overall on the proportion of community PWID that have ever been incarcerated, and the number of times prisoners (Kyrgyzstan) or community PWID (other settings) have been incarcerated (supplementary figure 1). This produced estimates for the (re-)incarceration rates and average duration in prison, which were used in the full model.
The full model was calibrated to HIV prevalence data from each setting (figure 2), the proportion of HIV transmission that is sexual and the odds ratio for the difference in HIV prevalence between PWID that have ever or never been incarcerated (Table 1). This produced estimates for the baseline HIV transmission risks due to sex among community PWID and due to injecting among community (not recently released) and incarcerated PWID, which were allowed to vary freely. Within this, the difference in HIV prevalence between PWID that have ever or never been incarcerated was important for estimating the difference in transmission risk between PWID in community and prison. Because levels of sexual HIV transmission among PWID is uncertain, we assumed that 5-25% (7-21% in Russia) of community HIV infections are sexually transmitted based on prior modelling from Russia19,20. These estimates are for all sexual HIV transmission and do not distinguish between homosexual or heterosexual contacts. HIV epidemics were seeded near the time of the first available HIV prevalence data, allowing a range in the seeded HIV prevalence based on data.
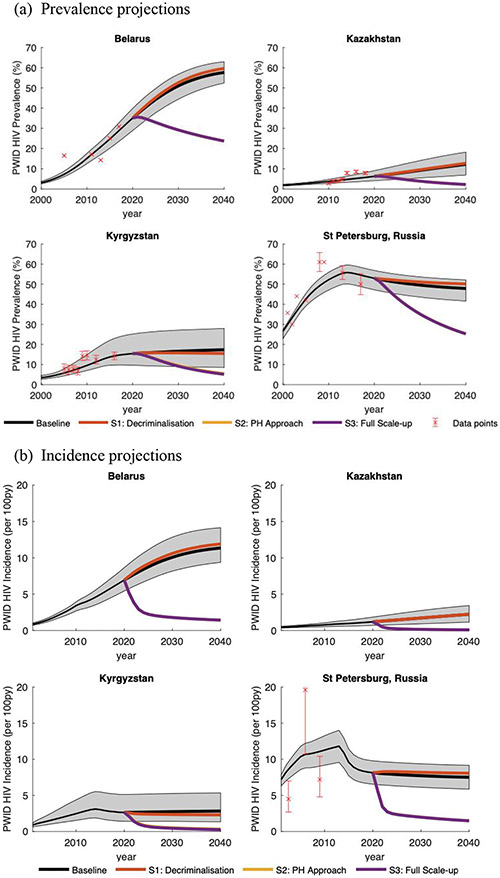
Figure 2:
A comparison of modelled HIV prevalence (a) and incidence (b) projections against data for each setting including the projected impact of different intervention scenarios from 2020 to 2040. The grey shaded area gives the 95% credibility intervals around the Baseline SQ model projections. The whiskers around different data points (red) denotes the 95% confidence intervals; if no whiskers are shown then no 95% confidence intervals could be estimated. Incidence data for St. Petersburg was not calibrated to.
The model was simultaneously calibrated to the estimated PWID population size and coverage data for ART (estimated coverage among all PWID infected with HIV) and OAT over time for each setting (supplementary figures 1). This estimated the ART and OAT recruitment rates (time variable if necessary), with the lost-to-care rate for OAT and ART being derived from local data or model fitting. Country-specific viral suppression data (among those on ART, Table 1) were used to estimate the efficacy of ART for reducing HIV infectivity (see appendix). Some settings also had data on the number of PWID on OAT (Kyrgyzstan) and ART (Kyrgyzstan and St. Petersburg) in prison, which was used to estimate linkage to OAT and ART upon incarceration. No OAT was available in prison in Belarus, Kazakhstan and St. Petersburg. We assumed the same lost-to-care rate for OAT and ART in prison as the community.
To validate our models, HIV projections were also compared against epidemiological data not used in the model calibration, including three HIV incidence estimates from St. Petersburg and four HIV prevalence estimates among prisoners for Kazakhstan and Kyrgyzstan (more details in supplementary materials).
Cost assumptions
We adopted a societal perspective to compare the costs of the current criminalisation approach with a scenario where criminal sanctions for drug use and/or personal possession are removed and OAT and ART are scaled-up. The costs for ART, OAT, and incarceration are given in Table 1 in 2018 Euros.
Estimates for the yearly cost of ART and facility-based OAT generally came from a Global Fund led resource optimisation analysis21, including the costs of drugs, tests and staff resources, but not overheads. The costs for introducing OAT in St. Petersburg were estimated by multiplying the OAT costs for Kazakhstan (similar GDP) by the ratio of their per-capita GDPs in 2018.
The costs of incarceration included the cost for arresting and convicting someone for drug-related crimes and the yearly costs of being in prison, both from national data. Estimates of the arrest and conviction costs for Russia and Kyrgyzstan came from a report by the Eurasian Harm Reduction Network22, with Russian costs being adapted for Belarus and Kazakhstan (as above). Yearly prison costs were available for Russia from a Council for Europe report from 201523 and for Kyrgyzstan from Government budget data for 2019. These estimates include costs for security, prison health care, overheads, food, and other support. Cost estimates for Belarus and Kazakhstan were adapted from Azerbaijan23 and Russia, respectively, because they have similar GDP.
The yearly costs of OAT, ART and being in prison were applied to any individual in these modelled states, while the arrest and conviction cost was applied when anyone became incarcerated. Costs were tracked among PWID and ex-injectors with all costs and outcomes discounted 3% annually.
Model analyses
For each setting, the model estimated the impact and costs over 20-years (2020-2040) for the following scenarios:
SQ. Baseline:
Current levels of incarceration and OAT for PWID and ART for PWID and ex-injectors.
S1. Decriminalisation:
Removal of incarceration due to criminal sanctions for drug use and/or possession for personal use while maintaining current levels of OAT and ART
S2. Public Health Approach:
previous decriminalisation scenario with cost-savings from reducing incarceration diverted to first increasing coverage of ART in community and prison (UNAIDS 90/90/90 target of 81%) and then, if funds permit, to increasing coverage of OAT in community and prison (WHO target of 40%).
S3. Full Scale-up:
decriminalisation scenario with OAT and ART scaled up to WHO/UNAIDS target coverage levels in community and prison.
For the decriminalisation scenario, we used survey estimates for the proportion of last incarcerations due to drug possession for St. Petersburg (46.4%12) or substance related offences in Kyrgyzstan (24.8%24) to estimate how incarceration may reduce with decriminalisation in these settings. For Belarus and Kazakhstan, we conservatively assumed 24.8%. Cost projections for St. Petersburg were also scaled-up to estimate nationwide costs for Russia, assuming 1,881,000 PWID2. For each scenario, we considered the breakdown of costs to determine where costs are saved and estimated the percentage of infections prevented and life-years gained (LYG) compared to the baseline SQ scenario. Compared to the baseline scenario and previous scenario, we estimated the incremental cost-effectiveness ratio (ICER) in terms of the cost per LYG. These were compared to the willingness-to-pay threshold of GDP per-capita. All model analyses were done in MATLAB R2020a.
Uncertainty and Sensitivity analyses
ANCOVA analyses were carried out to determine which parameter uncertainties contributed most to the variability in the incremental costs and LYG, all for Public Health Approach compared to Baseline. One-way sensitivity analyses also determined the effect of specific parameter or scenario changes:
Limited Public Health Approach where only ART is scaled-up due to OAT remaining illegal (Russia) or drug use shifting to non-opioids.
Include HIV testing cost for recruiting someone on to ART (3.5-19.0 euro per test21 multiplied by estimated number needing testing to identify one undiagnosed PWID; see appendix)
Proportion of incarcerations due to drug offences changed to 46.4% in Belarus and Kazakhstan, instead of 24.8%;
Assume Kyrgyzstan yearly ART costs (€363) in Kazakhstan and St. Petersburg, instead of €1230 and €1259, respectively;
Assume cheapest yearly OAT costs (€383) in Belarus, Kazakhstan and St. Petersburg, instead of €550, €422 and €441, respectively;
5% discounting of costs and 0% for life years as recommended for Russia, instead of 3%;
0% discounting of costs and life years;
Include 20% overheads on OAT and ART costs, instead of none;
Change incarceration costs by +/−30%;
Time horizons of 5 and 10 years, instead of 20 years.
Role of the Funding Source
The primary funders EIU and APH contributed to the analysis plan and write up. University of Bristol had final responsibility for the analysis, write up and decision to publish.
Results
The model agreed with HIV prevalence and other calibration data from each setting (Figure 2 and supplementary figure 1), with projections suggesting median HIV prevalence ranged from 6% in Kazakhstan to 53% in St. Petersburg in 2020, with all epidemics being fairly stable, except Belarus which is increasing. The models for Kazakhstan, St. Petersburg and Kyrgyzstan were also consistent with available HIV prevalence and incidence data that was not used for model calibration (Figure 1b for St. Petersburg and supplementary figure 2 for Kazakhstan and Kyrgyzstan). Across the four settings, 16.3-35.2% (range across medians) of PWID were currently incarcerated, with HIV transmission risk generally being lower (RR=0.073-0.88; Table 1) in prison than the community, except in Kyrgyzstan (RR=2.58). If incarcerated and recently released PWID had the same transmission risk as PWID who have never been incarcerated then 13.0-48.3% fewer infections would occur in Kyrgyzstan and Kazakhstan over 2020-2040, while 12.7-14.2% more infections would occur in Belarus and St Petersburg (supplementary figure 3).
The current baseline situation was estimated to cost from €197 million in Kyrgyzstan to €4,129 million in Kazakhstan over 2020-2040 (Table 2), with the cost for St. Petersburg extrapolating to €42.8 billion for all Russia. This equated to a yearly cost per PWID of €535-2,255, with incarceration making up 74.8-95.8% of these costs (Figure 3). If we removed incarceration due to drug use and/or drug possession for personal use (Decriminalisation; S1), then these overall costs reduced by 16.9-26.1% or €38-773 million due to reduced prison costs (Figure 3 and Table 2), with the extrapolated savings for Russia being €11.1 billion. Decriminalisation achieved a small number of LYG (745-1,694) in each setting, with infections averted in Kyrgyzstan (Figure 4) but not elsewhere because of reduced levels of injecting risk in prison compared to the community. If these cost-savings were diverted to scaling-up ART and then OAT (towards UNAIDS/WHO targets; S2 Public Health Approach) in the community and prison, then ART could scale-up to 81% coverage in all settings and OAT could scale-up to 29.7-41.8% coverage depending on setting (Table 2). The scale-up in coverage was achieved by 2024 in all settings. Over 2020-2040, this saved 17,768-148,464 life-years compared to baseline (839-1576 LYG per 1000 PWID), prevented 58.9-83.7% of new HIV infections (Figure 4) and decreased HIV incidence by 74.2-87.3% by 2030 and 79.4-92.9% by 2040 (Figure 2b). Compared to baseline, this Public Health Approach was cost-saving in all settings over 20-years due to reductions in future ART use.
Table 2:
Median percentage incarcerated and coverage of OAT and ART (community and prison) for each intervention scenario with corresponding mean total and incremental costs, life years gained (LYG), and incremental cost-effectiveness ratio (ICER) per life year gained over 20 years from 2020-2040. All costs and LYG are discounted 3% annually.
| Intervention coverage and % incarcerated in 2040 |
Comparisons compared to BASELINE | Comparisons compared to PREVIOUS SCENARIO |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | % Incarcerated |
Coverage ART*** |
Coverage OAT*** |
Total Costs (Euros) |
Total Life Years |
Incremental Costs (Euro) |
Life Years Gained |
ICER (Cost per LYG) |
Incremental Costs (Euro) |
Life Years Gained |
ICER (Cost per LYG) |
| Belarus | |||||||||||
| Baseline SQ | 34.6% | 39.6% | 0.4% | 2,534,578,868 | 1,905,493 | 0 | 0 | ||||
| S1: Decriminalisation | 27.3% | 43.7% | 0.6% | 2,103,723,830 | 1,906,415 | −430,855,039 | 923 | Cost saving | −430,855,039 | 923 | Cost saving |
| S2: PH approach | 25.5% | 83.8% | 41.2% | 2,345,128,162 | 2,005,618 | −189,450,706 | 100,126 | Cost saving | 241,404,332 | 99,203 | 2,433 |
| S3: Full scale-up | 25.5% | 83.8% | 41.2% | 2,345,128,162 | 2,005,618 | −189,450,706 | 100,126 | Cost saving | 0 | 0 | N/A |
| Kazakhstan | |||||||||||
| Baseline SQ | 30.0% | 21.2% | 0.2% | 4,128,681,587 | 3,736,146 | 0 | 0 | 0 | 0 | ||
| S1: Decriminalisation | 22.8% | 25.3% | 0.2% | 3,355,446,703 | 3,737,840 | −773,234,884 | 1,694 | Cost saving | −773,234,884 | 1,694 | Cost saving |
| S2: PH approach | 21.8% | 86.5% | 41.8% | 3,691,945,813 | 3,884,610 | −436,735,773 | 148,464 | Cost saving | 336,499,111 | 146,769 | 2,293** |
| S3: Full scale-up | 21.8% | 86.5% | 41.8% | 3,691,945,813 | 3,884,610 | −436,735,773 | 148,464 | Cost saving | 0 | 0 | N/A |
| Kyrgyzstan | |||||||||||
| Baseline SQ | 16.5% | 44.0% | 6.8% | 197,853,088 | 794,721 | 0 | 0 | 0 | 0 | ||
| S1: Decriminalisation | 12.3% | 46.6% | 6.3% | 159,842,974 | 795,466 | −38,010,115 | 745 | Cost saving | −38,010,115 | 745 | Cost saving |
| S2: PH approach | 11.7% | 88.0% | 29.7% | 185,326,522 | 812,488 | −12,526,567 | 17,768 | Cost saving | 25,483,548 | 17,023 | 1,497* |
| S3: Full scale-up | 11.5% | 90.0% | 41.1% | 198,376,963 | 818,332 | 523,875 | 23,611 | 22** | 13,050,442 | 5,844 | 2,233* |
| St. Petersburg | |||||||||||
| Baseline SQ | 16.1% | 35.1% | 0 | 1,638,734,392 | 1,592,757 | 0 | 0 | 0 | 0 | ||
| S1: Decriminalisation | 8.5% | 40.0% | 0 | 1,211,257,416 | 1,594,060 | −427,476,976 | 1,303 | Cost saving | −427,476,976 | 1,303 | Cost saving |
| S2: PH approach | 8.2% | 79.7% | 39.5% | 1,596,859,627 | 1,691,858 | −41,874,765 | 99,101 | Cost saving | 385,602,211 | 97,798 | 3,943 ** |
| S3: Full scale-up | 8.2% | 79.8% | 40.3% | 1,600,037,977 | 1,692,821 | −38,696,415 | 100,064 | Cost saving | 3,178,351 | 963 | 3,300 ** |
and ** signifies the intervention is cost effective compared to the threshold of three and one times per capita GDP for each country (see Table 1), respectively.
ART coverage increases greater than 81% due to the benefits of OAT on ART recruitment and retention. Coverage of OAT increases above 40% due to dynamic effects in the model over time.
Figure 3:
Projected costs (Euros) and their breakdown for each intervention scenario in each setting. All costs are discounted 3% annually.
S1: Decriminalisation is the scenario where incarceration due personal drug possession and use is removed; S2: Public Health (PH) Approach is the scenario where the costs saved from reducing incarceration are used to increase the coverage of ART and then OAT; S3: Full Scale-up is the scenario where extra resources are also used to increase OAT up to the full target coverage as advised by WHO (40%)
Figure 4:
Model projections of the percentage change in new HIV infections over 2020 to 2040 compared to Baseline due to each intervention scenario in each setting. A negative change denotes that HIV infections are averted. The whiskers denote the 95% credibility intervals around the model projections.
S1: Decriminalisation is the scenario where incarceration due personal drug possession and use is removed; S2: Public Health (PH) Approach is the scenario where the costs saved from reducing incarceration are used to increase the coverage of ART and then OAT; S3: Full Scale-up is the scenario where extra resources are also used to increase OAT up to the full target coverage as advised by WHO (40%)
To further scale-up OAT to 40% coverage in community and prison (S3 Full Scale-up), then overall costs are increased by €3.1 and 13.1 million in St. Petersburg and Kyrgyzstan, respectively, compared to the Public Health Approach (less than baseline cost in St Petersburg and €0.5 million more than baseline in Kyrgyzstan; Table 2), while no extra investment was needed in Kazakhstan or Belarus because OAT had already reached 40% coverage in scenario S2. This scenario averted 59.0-83.7% of new HIV infections compared to baseline and achieved 963-17,023 LYG (15-274 per 1000 PWID) compared to the Public Health Approach (Table 2 and Figure 4) for Kyrgyzstan and St Petersburg. Compared to baseline, this scenario was cost-saving for St. Petersburg and cost $22/LYG for Kyrgyzstan, while compared to S2 it cost less than the GDP for St Petersburg and two times GDP for Kyrgyzstan. Across countries, the Full Scale-up scenario will have reduced incidence by 74.2-87.3% after 10-years and 79.9-92.9% after 20-years (Figure 2b).
The ANCOVA analysis suggested that uncertainty in the ART initiation rate, incarceration rates and injecting transmission rates generally contributed most to variability in the incremental costs for the Public Health Approach (Supplementary figure 4). Uncertainty in the ART initiation rate, transmission rates, acute cofactors, and death rates contributed most to variability in the LYG.
All of the one-way sensitivity analyses still resulted in the Public Health Approach S2 being cost-saving compared to baseline, except when assuming shorter time horizons for St Petersburg, which resulted in ICERs of €3,044 and €316 per LYG compared to baseline for 5- and 10-year time horizon, respectively (supplementary Table S.1-S.4). However, some changes did affect the coverage of ART and OAT achieved in some settings, and so impact, with Kyrgyzstan achieving the UNAIDS/WHO coverage targets for both ART and OAT if incarceration is reduced by the same amount as in St Petersburg (46%), so increasing the number of infections averted from 69.4% to 74.5% (compared to baseline). Otherwise, including HIV testing costs or extra overhead costs for OAT and ART did not affect our cost projections (<5%), 5.1-15.1 percentage point fewer infections were averted if OAT could not be scaled-up, and the percentage of infections averted over 5-years (41.9-56.7%) was about one-third lower than over 20-years.
Discussion
Our analyses suggest that decriminalising drug use and investing the money saved into scaling-up ART and OAT could reduce HIV incidence by at least three-quarters among PWID in our modelled EECA settings, and ultimately be cost-saving. This strategy achieves the UNAIDS target of 90/90/90 coverage of ART in all settings by 2024 and achieves the WHO target of 40% OAT coverage in all settings with none or little additional investment.
Our analysis is novel in demonstrating the potential benefits of decriminalising drug use (and/or personal possession) and switching to a public health approach for PWID. Strengths of our analysis include using detailed country-specific data to model four contrasting settings in EECA, aiding generalizability of our results to other settings in EECA. We also include the effects of incarceration, which is rarely modelled despite it effecting most PWID2 and increasing HIV-risk3,4.
Limitations include data on some parameters. For instance, there were no data on the injecting HIV transmission risk in prison, which was estimated through calibrating the model to the difference in HIV prevalence between PWID who have been incarcerated and those that have not. Projections suggested transmission risk was greater in prison than the community in Kyrgyzstan but not in the other settings, consistent with data suggesting more PWID injected in prison in Kyrgyzstan (81%24) than St. Petersburg (25%12). We used systematic review data to estimate the increased risk of HIV transmission among recently released prisoners4, which included data from EECA but not our modelled countries. It is possible that recently released PWID in our countries may have differing levels of risk or it may last longer. This should not have affected our projections because the overall risk related to incarceration was constrained as described above. We also had little data on prison-based interventions; uncertainty was attached to these parameters. Little data existed on levels of sexual HIV transmission among PWID, and so we assumed similar levels to estimated for Russia (17-25%19,20). Our uncertainty analysis suggests these issues should not have affected our projections. Lastly, some cost estimates were converted from other settings or did not include overheads, while ART costs did not include HIV testing costs; sensitivity analyses suggested these limitations were not important.
There are also a number of potential benefits of decriminalisation and OAT/ART scale-up that we did not consider. Firstly, we did not consider other infectious diseases. In EECA, PWID have high prevalences of HCV and prisoners experience elevated TB transmission3, and so just focusing on HIV will have underestimated the benefits from our public health approach. Secondly, we did not consider potential benefits beyond PWID, which could include reduced HIV transmission to their sexual partners and so reduced ART costs. We also assumed no effect on levels of drug use following decriminalization because studies generally find no association25, notably in Portugal. Thirdly, whilst we modelled reduced incarceration of PWID due to decriminalisation of drug use, we did not incorporate the cost-savings from reducing incarceration among other people who use drugs. We also did not consider possible reductions in policing related to drug use, which could reduce policing costs and may reduce HIV transmission through lessening police harassment and its detrimental effects on behaviours and engagement in HIV services26. We also did not include productivity gains, which may give an added benefit from reducing incarceration27. Fourthly, although we included cost-savings from OAT reducing incarceration, we did not include cost-savings from reduced crime6. These omissions mean our estimations of the cost savings of decriminalisation and health benefits of scaling up OAT and ART will be conservative.
Lastly, we did not model the impact of SSP because there is uncertainty about how this may improve syringe availability in EECA due to most PWID (>75%) buying syringes from pharmacies28,29. However, they are still likely to have benefits, with data from Ukraine suggesting contact with harm reduction interventions increases HIV testing and ART coverage, reduces syringe sharing, and improves referral to OAT28. Although limited data from Russia shows similar associations27, no similar data exists for Kazakhstan, Belarus and Kyrgyzstan. Importantly, preliminary projections for Russia suggest that scaling-up SSP may cost one-tenth the cost of scaling-up OAT or ART but may avert ~40% of the impact27.
Unfortunately, it may also not be possible to increase OAT coverage in some settings. This would occur if OAT remained illegal in Russia, or if drug use transitioned towards non-opioids, as has occurred in some Russian cities13, but not St. Petersburg. Although this will diminish the impact achieved, large reductions in HIV incidence are still possible (64.6-82.6% over 20-years) through just scaling up ART.
Modelling analyses have already shown the beneficial impact and cost-effectiveness of interventions for preventing HIV transmission among PWID in EECA10,11,19,20. Other modelling has also shown the detrimental effect that incarceration can have on HIV transmission3 and the potential impact and cost-effectiveness of prison/jail interventions3,30. One analysis has also shown the large potential HIV-impact of diverting resources from drug control to prevention interventions for PWID31, although this analysis did not make country-level projections or consider the health impact of transitioning from criminalising drug use to a public health approach. To our knowledge, our analysis is the first to consider this question in four specific countries, incorporating the costs and numerous effects of incarceration, OAT and ART.
In the face of evidence that international drug policy has negatively impacted public health and leads to violation of human rights32, there have been calls from WHO and United Nations for the decriminalisation of drug use and personal possession. Data from numerous countries show that this legal reform should not increase drug use25. Indeed, evidence from Portugal and the Czech Republic suggests that such reforms could reduce problematic drug use, drug-related harms and prison overcrowding32. Reductions in the incidence of HIV32 have also been achieved through concurrent increases in harm reduction services. Our findings suggest similar benefits could be achieved in EECA if they decriminalise drug use and/or possession and utilise the freed-up resources to rapidly scale-up ART and OAT. This has been achieved in settings like Ukraine where OAT scale-up has occurred through changes in legislation, introduction of a national health system and changes to OAT prescribing during the COVID-19 pandemic33. This transition could have massive health benefits, enabling considerable progress towards eliminating HIV among PWID. In the current economic climate, this gives important pointers for how the health of PWID can be improved at no extra cost, and provides incentives for better advocacy work to bring about law enforcement reform, to develop strategies to reduce stigma in the law enforcement sector, and to improve investment in harm reduction services.
Supplementary Material
Research in context.
We searched PubMed for studies (on 27 November 2020) that modelled the impact and/or costs of reducing the incarceration of drug use for reducing transmission of infectious diseases among people who inject drugs (PWID), using the terms (“prison*” OR “jail” OR “incarceration” OR “imprison*”OR “detained” OR “crim*” OR “decrim*” OR “polic*” OR “arrest*”) AND (model* or cost*) AND (“PWID” OR "IDU" OR "IVDU" OR "injection drug" OR "injecting drug" OR "intravenous drug" OR "people who inject drugs").
We identified 18 studies that included incarceration in models of disease transmission for PWID, with these studies either modelling HIV (9 studies), HCV (10 studies) or HBV (1 study), with two studies modelling both HIV and HCV. Of these, nine studies considered the impact of reducing or stopping incarceration of PWID, or increasing the coverage of interventions that reduce incarceration, either opioid agonist therapy (OAT) or prison diversion programs. These studies were from varied settings, including the US (4 studies), Ukraine (3), Mexico (2), Iran (1), Thailand (1), Australia (1) and Scotland (2). These analyses generally showed that reducing or halting incarceration of PWID could prevent a large proportion of new HIV or HCV infections among this group, and interventions that reduce incarceration can have sizeable impact. Only one analysis considered the costs related to interventions to reduce incarceration, with this analysis estimating the cost-effectiveness of a police diversion intervention in King County, Washington state, USA. The intervention was modelled as increasing the uptake (2-times) to and reducing attrition (by 50%) from community interventions and reducing the chance of re-incarceration (by 42%) for those enrolled (25% of those with misdemeanour crimes). The study showed that the intervention could be cost-effective for reducing HIV and HCV transmission (and overdose) when only health care costs were included, but became much more cost-effective (but not cost-saving) when including the costs saved from reducing imprisonment.
Added value of this study.
To our knowledge, this is the first study to evaluate the potential cost-savings from decriminalising drug use or drug possession for personal use, and the benefits of diverting those savings to scaling-up prevention and treatment interventions to reduce disease transmission. Our analysis undertakes detailed modelling for four countries in the hardest hit global region for injecting drug use driven HIV epidemics, Eastern Europe and Central Asia, aiding generalisability to that region. Our analysis shows that Belarus, Kyrgyzstan and Kazakhstan could save up to three-quarters of a billion euros over 20 years from decriminalising injecting drug use (and personal possession), increasing to 11 billion euros in Russia. These savings are sufficient to scale-up anti-retroviral therapy up to the UNAIDS target of 90/90/90 (90% viral suppression and 81% coverage) and to scale-up OAT to higher levels (29.7-41.8%), with model projections suggesting this would decrease HIV incidence by 79.4-92.9% over 20 years.
Implications of all the available evidence.
The current global drug policy to criminalise drug use is estimated to cost $100 billion per year. This is much greater than the global investment in harm reduction interventions for PWID, with global coverage levels being low and many countries experiencing high prevalence or explosive HIV epidemics among PWID, especially in EECA. Our analyses highlight the cost savings and considerable health benefits that could result from decriminalising drug use and drug possession for personal use, as advocated by the World Health Organisation and United Nations. Legal reform has occurred in numerous countries without detrimental effects, while countries such as Portugal have achieved considerable benefits through decriminalising drug use. Our analysis gives further important evidence for decriminalising drug use and how this could fund important interventions to control HIV and other diseases in PWID at no extra cost, something that is needed in this climate of restricted aid funding.
Acknowledgements:
The study was commissioned by the Economist Intelligence Unit (EIU) and sponsored by Alliance for Public Health (Funded by the Global Fund to fight AIDS, Tuberculosis and Malaria), which is a leading non-governmental organisation aiming to make a significant impact on the epidemics of HIV/AIDS and other serious infectious diseases in the EECA region and globally. JS, ZW and PV acknowledge support from the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at the University of Bristol. PV also acknowledges support from the NIHR funded EPIToPe project and the NIHR HTA project (NIHR128513). PV and JS acknowledge support from U.S. National Institute for Drug Abuse (NIDA grant number R01 AI147490, R01 DA037773, R21 DA046809 and R01 DA047952,). JS, TL, FLA and PV acknowledge support from NIDA (R01 DA033679). FLA is supported on research related to this grant from the National Institute on Drug Abuse (K24 DA017072, R01 DA025943, R01 DA029910, R01 DA030768, R01 DA030762, R21 DA041953, R21 DA047902). JC acknowledges support from K01DA043421. This work was carried out using the computational facilities of the Advanced Computing Research Centre, University of Bristol – http://www.bristol.ac.uk/acrc/.
Footnotes
Declaration of interests: FLA has grants from Gilead and Merck and is on advisory boards to Gilead, Merck and Abbvie. PV has received research support from Gilead Sciences and honoraria from AbbVie and Gilead, in relation to hepatitis C virus treatment. RH has received research support from Gilead Sciences unrelated to this work. All other authors declare no conflicts of interest.
Data sharing statement: Model code will be made available immediately following publication. The code will be shared with researchers who provide a methodologically sound proposal approved by ZW and PV. Proposals should be directed to zoe.ward@bristol.ac.uk and peter.vickerman@bristol.ac.uk; requesters will need to sign a data access agreement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. UNAIDS DATA 2020. https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf.
- 2.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388(10050):1228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone J, Fraser H, Lim AG, et al. Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18(12):1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larney S, Toson B, Burns L, Dolan K. Effect of prison-based opioid substitution treatment and post-release retention in treatment on risk of re-incarceration. Addiction. 2012;107(2):372–80. [DOI] [PubMed] [Google Scholar]
- 8.The Alternative World Drug Report – 2nd Edition. Counting the costs. Steve Rolles, George Murkin, Martin Powell, Danny Kushlick, Nicky Saunter and Jane Slater, Transform Drug Policy Foundation; 2016. http://www.countthecosts.org/sites/default/files/AWDR-2nd-edition.pdf. [Google Scholar]
- 9.Merkinaite S. A war against people who use drugs: the costs. Eurasian Harm Reduction Network (EHRN), Vilnius: 2012. Available at: www.harm-reduction.org. [Google Scholar]
- 10.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8(3):e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mabileau G, Scutelniciuc O, Tsereteli M, et al. Intervention Packages to Reduce the Impact of HIV and HCV Infections Among People Who Inject Drugs in Eastern Europe and Central Asia: A Modeling and Cost-effectiveness Study. Open forum infectious diseases. 2018;5(3):ofy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cepeda JA, Niccolai LM, Lyubimova A, Kershaw T, Levina O, Heimer R. High-risk behaviors after release from incarceration among people who inject drugs in St. Petersburg, Russia. Drug Alcohol Depend. 2015;147:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plavinsky SL, Ladnaya NN, Barinova AN and Zaitseva EE (2017). Prevalence HIV infection and risky behaviours among vulnerable groups in 7 regions Russian Federartion, bio-behavioural results research, 2017 (http://www.ohi.ru/media/k2/attachments/enzs2.pdf), Open Institute of Health (OIH). [Google Scholar]
- 14.Kyrgyzstan Republican AIDS Center (2016). Bio-behavioural survey among people who inject drugs 2016. [Google Scholar]
- 15.Kazakh Scientific Center of Dermatology and Infectious diseases. Results epidemiological monitoring of the prevalence of HIV infection among PWID, 2018. Ministry of Health. [Google Scholar]
- 16.Ketchina EA. Report on Result of IBBS among key population at risk of HIV infection in Belarus, 2017. RSPCMT; 2019. [Google Scholar]
- 17.Minsk Harm reduction interventions. Minsk behavioural survey among people who inject drugs. 2020.
- 18.Penalties for drug law offences in East Europe and Central Asia at a glance. Eurasian Harm Reduction Association (EHRA), Vilnius, Lithuania. https://harmreductioneurasia.org/drug-laws/#Possession. [Google Scholar]
- 19.Mills HL, White E, Colijn C, Vickerman P, Heimer R. HIV transmission from drug injectors to partners who do not inject, and beyond: modelling the potential for a generalized heterosexual epidemic in St. Petersburg, Russia. Drug Alcohol Depend. 2013;133(1):242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cepeda JA, Eritsyan K, Vickerman P, et al. Potential impact of implementing and scaling up harm reduction and antiretroviral therapy on HIV prevalence and mortality and overdose deaths among people who inject drugs in two Russian cities: a modelling study. Lancet HIV. 2018;5(10):e578–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resource optimization to maximize the HIV response in Eastern Europe and Central Asia: Findings from Optima HIV modeling analyses across 11 countries in Eastern Europe and Central Asia 2020. Available at: http://optimamodel.com/pubs/EECA_English_2020.pdf.
- 22.Merkinaite S. A war against people who use drugs: the costs. Eurasian Harm Reduction Network (EHRN), Vilnius: 2012. Available at: www.harm-reduction.org. [Google Scholar]
- 23.Aebi MF, Tiago MM & Burkhardt C (2016). SPACE I – Council of Europe Annual Penal Statistics: Prison populations. Survey 2015. Strasbourg: Council of Europe. [Google Scholar]
- 24.Azbel L, Polonsky M, Wegman M, et al. Intersecting epidemics of HIV, HCV, and syphilis among soon-to-be released prisoners in Kyrgyzstan: Implications for prevention and treatment. Int J Drug Policy. 2016;37:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheim Al, Maghsoudi N, Marshall Z, Churchill S, Ziegler C, Werb D. Impact evaluations of drug decriminalisation and legal regulation on drug use, health and social harms: a systematic review. BMJ Open. 2020;10(9):e035148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker P, Beletsky L, Avalos L, et al. Policing Practices and Risk of HIV Infection Among People Who Inject Drugs. Epidemiol Rev. 2020;42(1):27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Economist Intelligence Unit (2021). Drug control policies in EECA: The economic, health and social impact. London: The EIU. [Google Scholar]
- 28.Trickey A, Semchuk N, Saliuk T, et al. Has resourcing of non-governmental harm-reduction organizations in Ukraine improved HIV prevention and treatment outcomes for people who inject drugs? Findings from multiple bio-behavioural surveys. J Int AIDS Soc. 2020;23(8):e25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busza J, Douthwaite M, Bani R, Scutelniciuc O, Preda M, Simic D. Injecting behaviour and service use among young injectors in Albania, Moldova, Romania and Serbia. Int J Drug Policy. 2013;24(5):423–31. [DOI] [PubMed] [Google Scholar]
- 30.Bernard CL, Rao IJ, Robison KK, Brandeau ML. Health outcomes and cost-effectiveness of diversion programs for low-level drug offenders: A model-based analysis. PLoS Med. 2020;17(10):e1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook C, Lines R, Wilson DP. A no-brainer for ending AIDS: the case for a harm reduction decade. J Int AIDS Soc. 2016;19(1):21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csete J, Kamarulzaman A, Kazatchkine M, et al. Public health and international drug policy. Lancet. 2016;387(10026):1427–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meteliuk A, Galvez de Leon SJ, Madden LM, et al. Rapid transitional response to the COVID-19 pandemic by opioid agonist treatment programs in Ukraine. J Subst Abuse Treat. 2021;121:108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.