Abstract
Understanding the similarities and differences between myocardial infarction with or without ST-segment elevation is an essential step for proper patients’ management in current practice. Both syndromes are caused by critical stenosis or total occlusion of coronary arteries (mostly due to thrombosis on atherosclerotic plaque), and manifest with a similar clinical presentation. Recent epidemiologic studies show that the relative incidence of ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) moves in an opposite fashion (decreasing and increasing respectively), with a prognosis that is worse at short-term follow-up for STEMI but comparable at long-term. Current management differs, as for STEMIs, immediate reperfusion is recommended, while for NSTEMIs, risk stratification is mandatory in order to stratify patients’ risk, and then decide the timing for coronary angiography. Periprocedural and technical aspects of the interventional management, as well as antithrombotic medications, are for the most similarly implemented in the two types of MI, with routine radial access, DES implant, and novel P2Y12 inhibitors representing the standard of care in both cases.
The following review article aims to compare the two types of MI, with and without persistent ST-segment elevation. The main purpose is to explore their similarities and differences and address areas of uncertainty with regards to clinical presentation, therapeutic management, and prognosis. The identification of high-risk NSTEMI patients is important as they may require an individualised approach that can substantially overlap with current STEMI recommendations, and their mortality remains high if their management is delayed.
Keywords: Acute myocardial infraction, de winter’s pattern, ECG, NSTEMI, STEMI, wellen’s syndrome
1. INTRODUCTION
Acute coronary syndrome (ACS) is the most common cardiovascular disease and one of the major causes of morbidity and mortality worldwide. The term acute myocardial infarction (AMI) is used when there is evidence of myocardial necrosis consequent to an ischaemic injury. The traditional AMI classification sharply distinguishes ST-segment elevation myocardial infarction (STEMI) from non-ST-segment elevation myocardial infarction (NSTEMI) based on the presence or absence of persistent ST-segment elevation on the electrocardiogram (ECG). Despite their different ECG presentation, both STEMI and NSTEMI share the same pathophysiologic grounds, and in most cases, are caused by acute thrombosis on a culprit coronary atherosclerotic plaque. In the case of STEMI, this often results in total occlusion of the culprit coronary artery [1]. On the other hand, in the case of NSTEMI, the angiographic pattern can be more heterogeneous and generally considered more susp icious for a critical stenosis than a total occlusion of the culprit vessel. However, a complete occlusion can also be an angiographic finding in approximately 25% of cases, identifying a high-risk subset (angiographically equivalent to STEMI) more vulnerable to ischemic myocardial injury [2, 3]. This net (ECG-based) separation of these two subsets of patients has been introduced for the purpose of selecting those deriving the main benefit from immediate thrombolytic reperfusion, and then maintained over time in the era of primary percutaneous coronary intervention (PCI).
Interestingly, the two types of MI share the same clinical presentation. Common characteristic is the onset of myocardial ischaemia that manifests with acute chest discomfort described as pain, pressure, tightness, and burning. Chest pain-equivalent symptoms may include dyspnoea, epigastric pain, and pain in the left arm. In STEMI cases, the pain has been reported to be more persistent and heavier, although a clear distinction is not possible. Some patients may present with less-typical symptoms such as abdominal pain, nausea/vomiting, fatigue, palpitations, or syncope, causing diagnostic challenges.
2. EPIDEMIOLOGY AND PROGNOSIS
The number of patients presenting with STEMI has been decreased considerably over the last decades, while the rate of NSTEMI slightly increased. Specifically, the incidence rates of STEMI decreased from 121 to 77 per 100.000, whereas for NSTEMI increased slightly from 126 to 132 per 100.000, between 1997 and 2005 [4, 5]. Important changes in public health policies prioritising primary prevention strategies for the prevention and control of coronary artery disease, as well as improved awareness of coronary risk factors, have had, as a result, the decline of the overall rate of STEMI [4, 6]. Nowadays, NSTEMI is the dominant phenotype in AMI, although its recent trend is difficult to interpret. In the last two decades, the massive evolution and use of high sensitivity troponin assays resulted in a more easy diagnosis of NSTEMI [7]. Due to this increase in diagnostic sensitivity, almost 30%-40% of patients who would have been diagnosed with unstable angina based on CPK results are now diagnosed with NSTEMI [8].
With regards to prognosis, STEMI patients appear to have higher short-term mortality compared with NSTEMI patients. This trend changes at 1- or 2-years follow-up when their mortality rates become comparable. This seems to be explained by differences in baseline patient characteristics, including older age and a greater prevalence of co-morbidities in the NSTEMI population [9, 10], but also by the fact that many NSTEMI patients remain undertreated because of non-proper risk stratification. Deaths in the early phase following NSTEMI are more attributable to ischaemia/thrombosis-related events, whereas, in the later phase, they are more likely to be associated with the progression of atherosclerosis and non-cardiovascular cause. The mortality in STEMI and NSTEMI patients is influenced by many clinical factors like advanced age, Killip class, time delay to treatment, treatment strategy, history of MI, diabetes mellitus, renal failure, number of diseased coronary arteries, and left ventricular ejection fraction (LVEF). Of note, in comparison with data from the previous decade, the 6-month mortality has decreased considerably for both STEMIs and NSTEMIs [11, 12]. Several registries have highlighted this decline in short- and long-term mortality, probably related to more effective and timelier reperfusion therapy, modern antithrombotic therapies, and new-generation drug-eluting stents [13, 14].
3. ELECTROCARDIOGRAPHY
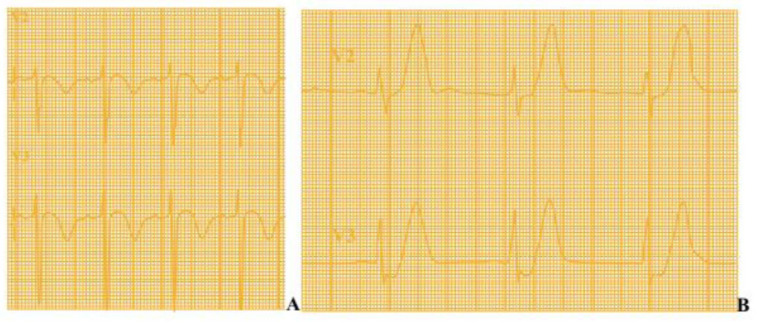
The 12-lead electrocardiogram (ECG) is a crucial tool in guiding the management of patients with ACS by sharply distinguishing between a STEMI or NSTE-ACS scenario, based on the presence (or lack) of persistent ST-segment elevation in two or more contiguous leads. If the standard leads are inconclusive and the patient has a clinical picture of ongoing myocardial ischaemia, additional leads should be recorded; left circumflex artery occlusion may be detected only in V7 - V9 or right ventricular MI only in V3R and V4R. The mechanism of regional ST-segment deviation during myocardial ischemia has been widely investigated and is related to regional loss of function of ion channels generating electrical gradients [15]. A new-onset ST-segment elevation is traditionally considered as highly suggestive for ongoing ischemia due to a total coronary occlusion that, if prolonged, causes an extensive and irreversible myocardial injury. In this case, an immediate recanalization is the only effective treatment to ensure myocardial salvage, preserve cardiac function, and improve clinical outcomes [16]. On the other hand, angiographic series showed that the absence of an ST-segment elevation during ACS more frequently reflects critical (and often complex) coronary stenoses, in the absence of total coronary occlusion [2]. A number of ECG patterns in NSTEMI patients have been suggested for high clinical risk (‘STEMI equivalents’) scenario. The presence of new T-wave inversion in anterior leads in patients admitted with ACS is highly suspicious for critical stenosis (or even occlusion) of the proximal LAD artery and associates with worse outcomes when patients were medically treated. This condition, now diffusely known as Wellen’s syndrome, is noted for having a significant portion of the left ventricle in jeopardy for progressing to anterior MI with no (or minimal) cardiac biomarkers elevation. The typical ECG pattern is characterized by deeply inverted symmetric T waves (75% of cases) or biphasic T waves (25% of cases) in leads V2-V3, normal precordial R-wave progression, in the absence of pathologic Q-waves and ST-elevation (Fig. 1A). More recently, de Winter et al. claimed that the presence of abnormally prominent (hyperacute) T-waves in leads V1-V4 preceded by an upsloping ST-D (de Winter’s pattern) can also associate with occluded or critically stenosed proximal LAD, with a significant risk for impending anterior wall MI (Fig. 1B) [17]. These conditions, characterized by anterior ECG abnormalities in the absence of a clear ST-segment elevation, are currently considered as a high-risk flag for adverse outcomes, and cardiologists are advised to refer these patients to urgent coronary angiography.
Fig. (1).
A. Wellen’s syndrome: Deeply inverted T waves in leads V2 and V3 (may also be seen in leads V1, V4, V5, and V6) OR biphasic T waves (with initial positivity and terminal negativity) in V2 and V3. B. De Winter's T Waves: Prominent (hyperacute) T-waves in leads V1-V4 preceded by an upsloping ST-Depression >1mm at the J- point in the precordial leads. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. MANAGEMENT
The management of patients with STEMI and NSTEMI has evolved considerably over the last decades. The use of primary PCI for STEMI is nowadays considered as the first- line treatment in patients within 12 hours of symptom onset, provided it can be performed within 120 minutes from diagnosis and is associated with a reduction in mortality compared with other reperfusion strategies [18]. If a timely primary PCI cannot be performed (time to intervention more than 2 hours), fibrinolytic therapy is recommended as an alternative reperfusion therapy (within 12 h of symptom onset) in patients without contraindications. Primary PCI may also be considered in patients with more than 12 h of symptoms onset if there is ECG evidence of ongoing ischaemia, ongoing chest pain, and/or heart failure, shock, or malignant arrhythmias [18].
For NSTEMI, multiple algorithms for guiding invasive management have been investigated over the years. Based on current recommendations, the first step is to define the risk of each patient in the acute setting and then proceed with an immediate, early or delayed invasive strategy according to the risk class [19]. Risk stratification is defined based on pain characteristics, severity of pain, clinical findings, ECG changes, biochemical markers and risk scores like the Thrombolysis in Myocardial Infarction (TIMI) risk score, and the Global Registry of Acute Coronary Events (GRACE 2.0) risk calculation score.
Patients with very high-risk NSTEMI should undergo urgent coronary angiography within less than 2 hours after the initial hospital admission [20], and ideally within 24 hours [21, 22]. In a post hoc analysis of the ACUITY trial, a delay to PCI of more than 24 hours was identified as an independent predictor of 30-day and 1-year mortality in those patients presenting with high-risk features [23]. Patients with recurrent symptoms or at least one intermediate-risk criterion should receive coronary angiography within 72 hours of first presentation [24, 25]. Low-risk patients may be treated conservatively, and the indication for an invasive evaluation can be done based on the evidence of myocardial ischaemia during a non-invasive stress testing (Table 1).
Table 1.
Recommendations for invasive coronary angiography and revascularization in NSTEMI.
| Recommendations for Invasive Coronary Angiography and Revascularization in NSTEMI |
|---|
| Immediate Invasive Strategy (<2h) at least one of the following very-high-risk criteria: |
| Haemodynamic Instability or Cardiogenic Shock |
| Recurrent or Ongoing Chest Pain Refractory to Medical Treatment |
| Life Threatening Arrythmias or Cardiac Arrest |
| Mechanical Complications of MI |
| Recurrent Dynamic ST- or T-Wave Changes |
| Early Invasive Strategy (<24h) at least one of the following high-risk criteria: |
| GRACE score >140 |
| Rise or Fall in Cardiac Troponin Compatible with MI |
| Dynamic ST- or T-Wave Changes (symptomatic or silent) |
| Selective Invasive Strategy (<72h) at least one of the following intermediate-risk criteria: |
| Diabetes Mellitus |
| Renal Insufficiency (eGFR <60 mL/min/1.73 m2) |
| LVEF <40% or Congestive Heart Failure |
| Early Post-Infarction Angina |
| Recent PCI, Prior CABG |
| GRACE Risk Score >109 and <140 |
Note: Adapted from 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC).
With regards to peri-procedural technical aspects, the radial approach is considered the default access site nowadays in all the ACS patients undergoing PCI by experienced radial operators [26-28]. Radial access is associated with lower risks of bleeding, vascular complications, need for transfusion, and death in the whole setting of ACS. Another potential benefit of radial access is its association with a reduced risk of acute kidney injury (AKI), as reported in another large study [29].
New-generation DES is the first choice in all ACS patients. These devices have shown superior safety and improved efficacy compared with first-generation DES and bare-metal stents (BMS), with respect to lower risks of stent thrombosis, prevention of restenosis, and need for repeat revascularization. Most of the evidence comes from landmark trials performed in STEMI patients [30, 31], but several studies also investigated a mixed ACS population [32, 33]. Therefore, stenting with new-generation DES is recommended for any PCI irrespective of the type of MI, clinical presentation, lesion type, planned non-cardiac surgery, anticipated duration of DAPT, and concomitant anticoagulant therapy.
The concept of thrombus aspiration has been deeply investigated in the STEMI setting and its routine use nowadays is not recommended [34, 35], and should be considered only in the case of large residual thrombus burden. Despite lower evidence, this treatment modality cannot also be recommended in the NSTE-ACS [36]. In the recent TATORT-NSTEMI trial on thrombectomy in NSTEMI patients, routine thrombus aspiration before PCI did not improve clinical outcome at 12-month follow-up (Table 2) [37].
Table 2.
Main similarities and differences in pathophysiology, angiography, electrocardiography, and clinical features between STEMI and NSTEMI.
| - | STEMI | NSTEMI |
|---|---|---|
| Pathophysiology | - | |
| Cause | Atherosclerosis (plaque rupture, plaque erosion) | |
| Macroscopically | Total coronary occlusion | Subtotal occlusion 25% of the cases present with total occlusion |
| Angiographic Findings | Total coronary occlusion | Heterogenous angiographic pattern Critical (and often complex) coronary stenosis 25% of the cases present with total occlusion |
| ECG Findings | Persistent (>20 min) ST-segment elevation New or presumed new LBBB Isolated posterior MI |
Transient (<20 min) ST-segment elevation Persistent or transient ST-segment depression T-wave inversion, flat T waves, or pseudonormalization of T waves Normal ECG |
| Clinical Features | - | |
| Clinical Picture | Acute chest discomfort is described as pain, pressure, tightness, and burning. Chest pain-equivalent symptoms may include dyspnoea, epigastric pain, and pain in the left arm | |
| Corresponding Risk Factors | The same risk factors of coronary artery disease | |
| Secondary Prevention | The same principles of secondary prevention | |
The post-intervention management of both types of AMI is similar and cardiac rehabilitation is recommended for all patients following an acute myocardial infarction. Furthermore, the benefits of lifestyle modification and pharmacotherapy for secondary prevention continues to be important. Primary prevention against sudden death is strongly recommended with implantable cardioverter-defibrillators (ICDs) for patients with severe myocardial impairment and LVEF below 30-35% at least 6 weeks after myocardial infarction and irrespective of the type of AMI [38].
5. ANTITHROMBOTIC MEDICATIONS
Mainly similarities, but also important differences in antithrombotic medications can be found between the STEMI and NSTE-ACS settings. Generally, the choice of treatment, the combination of drugs, the timing for administration, and the in-hospital and long-term duration depend on patient’s characteristics, clinical setting, comorbidities, and modality of revascularisation [39]. Platelet activation and aggregation are crucial in the process of thrombus generation in any type of AMI. Therefore, dual antiplatelet therapy (DAPT), consisting of the combination of aspirin and P2Y12 inhibitors, is the cornerstone treatment for patients with AMI, especially among those undergoing PCI. The goal of this treatment is to reduce the risk of future cardiovascular events and, additionally, prevent stent thrombosis.
Aspirin is the basis of treatment for inhibition of thromboxane A2 generation. Aspirin treatment is started with a loading dose (150-300 mg p.o. or 75-250 mg i.v.), followed by maintenance treatment which is normally complete with a dose ≥ 75 mg/d. (Table 3). The preferred P2Y12 inhibitors in both STEMI and NSTEMI are nowadays prasugrel (60mg loading dose and 10 mg maintenance dose once daily p.o.) and ticagrelor (180 mg p.o. loading dose and 90 mg maintenance dose twice daily) (Table 3). Both drugs have a more rapid onset of action, better absorbable and a stronger antiplatelet effect than clopidogrel, and they have proved to be superior for improving clinical outcomes [40, 41]. Prasugrel has been tested against 300 mg loading and 75 mg/day maintenance dose of Clopidogrel in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38, included both STEMI and NSTEMI patients. Prasugrel treated patients had less cardiovascular events at a 15-month follow-up (11.2% vs 9.3%) driven mainly by a significant reduction in periprocedural MI (from 11.2% to 9.3%). However, these benefits came with an increased risk of major and minor bleeding. Prasugrel is contraindicated in patients aged more than 75 years old, in patient with bodyweight lower than 60 kg and in these with previous stroke or transient ischaemic attack [41], and only indicated in those with known coronary anatomy [42].
Table 3.
Main similarities and differences in treatment and outcome between STEMI and NSTEMI.
| - | STEMI | NSTEMI |
|---|---|---|
| Management | Immediate reperfusion by primary percutaneous coronary intervention (PCI) or, if not available in a timely manner, by fibrinolytic therapy. | Depending on the risk stratification (see Table 1) Immediate invasive strategy (<2h) in very high-risk patients. Routine early invasive strategy in high-risk patients (<24h). Selective invasive strategy in intermediate / low-risk patients. |
| Procedural Aspects of the Percutaneous Coronary Intervention Strategy | - | |
| Access | Radial access is recommended over femoral access if performed by an experienced radial operator. | |
| Type of Stent | Stenting with new-generation DES is recommended over BMS for any PCI irrespective of clinical presentation, lesion type, planned non- cardiac surgery, anticipated duration of DAPT, concomitant anticoagulant therapy. | |
| Thrombus Aspiration | It is not recommended routinely. | |
| Adjunctive Antithrombotic Medication | Is crucial and mandatory to inhibit platelet activation and anticoagulation. | |
| Antiplatelet Treatment, type of P2Y12 | Aspirin is recommended for all patients without contraindications at an initial oral loading dose of 150-300 mg (or 75-250 mg i.v.), and at a maintenance dose of 75-100 mg daily long-term. Prasugrel or Ticagrelor is the first choice option. Prasugrel should be considered in preference to ticagrelor for NSTE-ACS patients who proceed to PCI. Ticagrelor irrespective of the planned treatment strategy (invasive or conservative) (180 mg LD, 90 mg b.i.d.). Prasugrel in P2Y12 receptor inhibitor-naive patients proceeding to PCI (60 mg LD, 10 mg/d as the standard dose, 5 mg/d for patients aged >_75 years or with a bodyweight. Clopidogrel (300 - 600 mg LD, 75 mg daily dose), only when prasugrel or ticagrelor are not available, cannot be tolerated, or are contraindicated. Prasugrel is not recommended in NSTEMI patients in whom coronary anatomy is not known. |
|
| Peri-interventional Anticoagulant Treatment |
Heparin is indicated. Enoxaparin (i.v.) should be considered as an alternative to heparin in STEMI patients and in NSTEMI patients pre-treated with subcutaneous enoxaparin. Fondaparinux is not recommended in STEMI patients. Bivalirudin is considered a second line anticoagulant agent. GP IIb/IIIa inhibitors should be considered for bail-out if there is evidence of no-reflow or a thrombotic complication. |
|
| Post Interventional Management | DAPT is recommended for 12 months unless there are contraindications such as the excessive risk of bleeding. | |
| - | Low-dose rivaroxaban may be considered for patients at high ischaemic risk and low bleeding risk, receiving aspirin and clopidogrel. | |
| Prognosis and Outcome | - | |
| Short-term Mortality | Higher 30-days mortality in STEMI patients. | |
| Long-term Mortality | Similar long-term mortality in STEMI and NSTEMI patients. | |
Ticagrelor has been investigated in the Study of Platelet Inhibition and Patient Outcomes (PLATO) where again mixed population of STEMI and NSTEMI patients were tested against Clopidogrel. At 12 months, the primary endpoint - a composite of death from vascular causes, myocardial infarction, or stroke -occurred in 9.8% of patients receiving ticagrelor as compared with 11.7% of those receiving clopidogrel. While minor bleeding was more common with ticagrelor, the major bleeding risk was comparable to that with clopidogrel. Ticagrelor is recommended for all AMI patients, regardless the type (STEMI or NSTEMI), regardless of the initial treatment strategy, including those pre-treated with clopidogrel [40].
Head to head comparison of these two novel P2Y12 inhibitors, showed that among patients who presented with acute coronary syndromes with or without ST-segment elevation, the incidence of death, myocardial infarction, or stroke was significantly lower among those who received prasugrel than among those who received ticagrelor. Interestingly, the incidence of major bleeding was not significantly different between the two groups [43]. Based on the results of this study, the more recent ESC guidelines recommend prasugrel as the preferred P2Y12 receptor inhibitor for NSTE-ACS patients who proceed to PCI [19].
Despite the dominant role of these two more potent novel P2Y12 inhibitors, clopidogrel still has important role in the antiplatelet management in AMI whenever prasugrel and ticagrelor are not available or contraindicated [44]. Importantly, clopidogrel is the P2Y12 inhibitor of choice whenever triple therapy is needed in patients requiring oral anticoagulation (OAC) after PCI and AMI. In these patients, triple therapy with aspirin, clopidogrel and an OAC for longer than 1 month and up to 6 months should be considered, always after careful examination of their bleeding risk [45].
Cangrelor, a potent intravenous reversible P2Y12 inhibitor with a rapid onset and offset of action, has been examined in three randomized controlled trials enrolling patients with PCI for stable angina or ACS against clopidogrel loading or placebo [46-48]. A meta-analysis of these studies, in which 69% of patients were undergoing PCI for AMI (both STEMI and NSTEMI), observed a 19% relative risk reduction in periprocedural death, MI, ischaemia-driven revascularization, and stent thrombosis (Cangrelor 3.8% vs clopidogrel 4.7%; p = 0.007), with a 39% relative risk reduction in stent thrombosis alone [49]. TIMI bleeds were increased, but there was no increase in the rate of transfusions. At present, Cangrelor is indicated at the time of PCI in patients who have not been exposed to pre-treatment with oral P2Y12 inhibitors.
Periprocedural anticoagulation is recommended for all patients in addition to antiplatelet therapy during primary PCI or PCI for NSTEMI (Table 3). Unfractionated heparin (UFH) is primary recommended in all cases of PCI with a standard dose of 50-70 IU/kg, allowing an additional dose of bail-out GP IIb/IIIa inhibitors and this recommendation is the same for primary PCI as for PCI in NSTEMI [50]. Regarding other anticoagulant options, enoxaparin should be considered as an alternative to UFH treatment in STEMI patients [51], while in NSTEMI cases, enoxaparin should be considered in patients pre-treated with subcutaneous enoxaparin [52]. Fondaparinux did not show any benefit in the setting of primary PCI and is, therefore, not recommended in STEMI patients [53] but is widely used in NSTEMI patients. In NSTEMI, whenever fondaparinux is used as a pre-interventional anticoagulation therapy, a single bolus UFH (85 IU/kg, or 60 IU in the case of concomitant use of GP IIb/IIIa receptor inhibitors) is indicated [54]. With regards to the bivalirudin, recent data showed benefit regarding the bleeding complications with a cost of a higher risk of acute stent thrombosis. In the recent MATRIX trial, including both NSTEMI (47%) and STEMI (53%) patients, bivalirudin did not reduce the incidence of the primary endpoint compared to UFH [55], thus bivalirudin is considered a second line anticoagulant agent. Finally, with regards to GP IIb/IIIa inhibitors in PCI-treated patients, currently recommended only as a bail-out in high thrombotic settings and are not indicated in patients where the coronary anatomy is not known [56]. Of note, most of the relevant GP IIb/IIIa inhibitor trials are old and come before the establishment and the routine use of the novel oral P2Y12 Inhibitors.
Irrespective of the final revascularization strategy, the standard of care and the default DAPT duration in patients with AMI is 12 months. The individualization of the decision with shorter (6 months) or longer (>12 months) should be considered based on the concomitant bleeding risk and thrombotic risk of each patient [45]. Patients with complex PCI have a higher risk of ischemic events and they benefit from long-term DAPT only if they are considered a low bleeding risk. Prospective data from the Dual Antiplatelet Therapy (DAPT) study [57] and Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) study [58] now have confirmed the benefits of prolonged DAPT to prevent MACE (the risks of stent thrombosis, major adverse cardiovascular and cerebrovascular events and the risk of cardiovascular death, myocardial infarction, or stroke respectively) in patients with AMI with an increased risk of bleeding. These data suggested that when concordant, bleeding, more than ischemic risk, should inform decision-making on the duration of DAPT [59].
6. COMPLICATIONS
STEMIs and NSTEMIs also share the same complications, although their rate can differ. Cardiogenic shock (CS) complicates 6-10% of all STEMI cases and 3-5% of NSTEMIs and remains a leading cause of death, with in-hospital mortality rates 50% [60, 61]. Ventricular failure is the major cause of cardiogenic shock in AMI. Mechanical complications of AMI represent less frequent causes of CS [ventricular septal rupture (4%), free wall rupture (2%), and acute severe mitral regurgitation (7%)]. They may occur in the first days following STEMI and are less often in NSTEMIs. In patients with cardiogenic shock, irrespectively of the type of the AMI, immediate coronary angiography is indicated, and PCI is the most frequently used revascularization modality [62]. In the cases of multivessel disease in settings of CS, the current recommendations suggest a significant clinical benefit of a culprit-lesion-only strategy [63].
No-reflow develops in almost 5% of the patients with AMI during PCI and is characterised by inadequate myocardial reperfusion at the microcirculatory level [64]. Depending on the definitions used, 10-40% of patients undergoing primary PCI for STEMI may show evidence of no-reflow [65]. Similar rates of distal embolization of thrombotic material and no-reflow phenomenon observe in NSTEMI, ranging from 15% to 40% [66].
7. DISCUSSION
STEMI and NSTEMI have probably more in common than in difference (Tables 2 and 3). They share the same pathophysiology, risk factors, demographics, and clinical presentation. Additionally, the two entities have similar long-term prognosis, even though different management is recommended. Antithrombotic medications, the peri- and post-interventional management, as well as long-term treatment, are substantially the same. Another common point is that secondary prevention is crucial for both entities and they have exactly the same secondary prevention therapies to avoid recurrent ischaemic events.
The major difference between the two entities is related to the timing of the revascularisation. Based on different ECG presentation and ‘expected’ angiographic findings, current guidelines recommend a differential approach in these two scenarios: (a) an emergent coronary angiography with primary PCI is considered mandatory in STEMI, to restore epicardial coronary patency and reinstate myocardial perfusion promptly [18]; conversely, (b) a routine immediate reperfusion is not requested for NSTEMI, with a time-to-angiography depending on clinical and laboratory data [19]. As there is no debate about the benefits of primary PCI in STEMI, an immediate reperfusion is not accepted so far in the heterogeneous group of NSTEMI patients. However, the NSTEMI subgroup with very high-risk characteristics Table 1 has a similar time frame revascularisation recommendation with STEMI patients (<120 min) and this suggestion has been included both in ESC and ACC/AHA recent guidelines [19, 67].
Nowadays, there is a consistent evidence that early coronary angiography (first 24 hours after hospital admission) in high-risk NSTEMI patients is associated with reduced all- cause mortality and cardiovascular mortality at long-term follow-up [19]. Several studies have demonstrated that early angiography and revascularization reduce the risk of refractory ischemia, recurrent myocardial infarction, repeat hospitalization, and death [68-70]. The question that still exists is if the motto ‘time is muscle’ that we use in STEMI can be addressed in the NSTEMI group. Despite less recent data pointed out that there is no difference in the extent of myocardium necrosis or major adverse events between those treated within the first 2 h vs 24-48 h [71], some more recent evidence showed that there is a trend to a reduction in major ischemic events (mostly new myocardial infarctions) in those subjected to angiography and revascularization within the first 2 h. The recently published RIDDLE-NSTEMI trial, showed that an immediate invasive strategy (median time 1.4 hours) was superior in improving cardiovascular outcomes compared with a delayed invasive strategy (median time 61.0 hours), mainly driven by a reduction in the rates of reinfarction. Interestingly, only one-third of the patients in this trial were high risk (based on a GRACE score >140) [72].
Although this binary ‘in or out’ approach in AMI is still appealing for clinical and operational purposes, with the limitations we addressed above, the ability of ECG in discriminating between the presence or absence of acute coronary occlusion (based on the solely up- or downwardly ST-deviation) is also questionable. Indeed, in a considerable proportion of NSTE-ACS patients (approximating at 25%) the ECG fails this critical task and is then contradicted by the angiographic examination identifying a total culprit occlusion. .More than two-thirds of these cases present with either a right coronary or left circumflex artery involvement, with a predominant inferolateral and posterior infarct location [2, 3, 73-75]. Matching these data, total occlusion of the left circumflex is infrequently reported in studies investigating STEMI patients (only 15% of cases) [74] due to the silent nature of perfused territory on 12-lead ECG [2, 73].
These patients with ‘missed STEMI’ and total culprit occlusion systematically receive a delayed (but guidelines-compliant) invasive management, being referred to coronary angiography 24-48 hours after initial presentation [2, 3, 73-77]. This point raises considerable concerns due to the established detrimental impact of an acute culprit occlusion on outcomes and potential benefit from earlier reperfusion. Among the 13,608 patients enrolled in the TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis In Myocardial Infarction 38) [2], 1,198 (or 8.8%) presented with an anterior ST-depression, of whom 314 (or 26.2%) showed culprit occlusion at coronary angiography (performed at a median time from ECG of 29.4 h), with a left circumflex involvement in 50% of the cases. 30-day outcomes (namely, death and MI) were significantly worse in patients with culprit occlusion as compared with those without, confirming prognostic relevance of this factor and the current inadequate management of these patients [2].
Additional ECG criteria have been proposed to unravel this diagnostic knot and narrow the diagnostic gap between ECG and angiography, including the magnitude/location of ST-depression, ST-elevation in aVR, R/S ratio in leads V1-V2, T-wave width/morphology following ST-depression (i.e., de Winter’s sign), but evidence remains non-conclusive [73-76, 78-81]. The use of additional posterior (V7-V9) and right (especially V4R) precordial leads is of valid help in these cases, potentially unmasking STEMI cases concealed on the standard 12-leads recording, but also this approach is considered unsatisfactory [82, 83].
Therefore, patients presenting with NSTE-ACS and culprit occlusion represent a blind spot to the guidelines, whose identification remains challenging, and for whom a dedicated (pre-angiographic) diagnostic/therapeutic track is not currently defined [19, 39]. Although universally accepted and relatively easy-to-use (especially in emergency setting), the current STEMI/NSTE-ACS classification primarily based on an ECG (ST- centred) analysis, entails relevant limitations and does not fully meet contemporary clinical needs. Such a practical (but somehow artificial) classification is fated to remain physician-friendly but potentially counterproductive for the patients in the modern era of extensive availability and use of PCI.
Finally, one other additional group of patients, where STEMI and NSTEMI population overlap are the patients with transient (< 20 min) ST elevation (tSTEMI). The management of this subgroup is still a therapeutic challenge as it is unclear what is the optimal timing of revascularization and whether they should be treated with a STEMI-like or NSTEMI-like approach. This group compromises about 6-7% of AMI patients. The data so far are conflicting in this group of patients regarding the management. Some old data have shown that tSTEMI was associated with less myocardial damage, less extensive coronary artery disease, higher TIMI flow in the culprit artery, and better cardiac function [84]. The recent TRANSIENT trial showed that infarct size in transient STEMI is small and is not influenced by an immediate or delayed invasive strategy. In addition, short-term MACE was low and not different between the treatment groups [85]. Other studies have showed that tSTEMI patients have a relatively benign course with a better outcome than both STEMI and NSTEMI patients when treated by an early invasive approach [86].
CONCLUSION
Undoubtedly STEMI and NSTEMI have many similarities with regards the pathophysiology, epidemiology, clinical presentation, and management. NSTEMI is associated with a more favourable prognosis than STEMI, but the risk of in-hospital death for these high-risk NSTEMI patients is also high. It is very important to recognise early these high-risk STEMI equivalents because they experience a similar degree of myocardial damage and thus would benefit from urgent PCI. Early revascularization may limit infarct expansion and improve the overall prognosis in high-risk NSTEMI patients. Someone could argue that the high-risk group of NSTEMI patients and STEMI patients are overlapping clinical entities in the acute coronary syndrome spectrum of disease.
Recognition of STEMI-equivalents like NSTEMI with missed total occlusion represents a chance for earlier intervention with prompt coronary angiography. These patients need as soon as possible reperfusion and their mortality remains high if there is no treatment. To date, implementing an appropriate algorithm for the correct and timely identification of the full spectrum of ACS patients suffering an acute coronary occlusion (regardless of ST-segment deviation) is crucial to facilitate earlier revascularization (following a “STEMI-like pathway”) and improve prognosis in contemporary practice.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest, financial or otherwise.
References
- 1.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013;368(21):2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 2.Pride Y.B., Tung P., Mohanavelu S., et al. Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST- segment depression: a TRITON-TIMI 38 sub-study. J Am Coll Cardiol Cardiovasc Interv. 2010;3(8):806–811. doi: 10.1016/j.jcin.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Wang T.Y., Zhang M., Fu Y., Armstrong P.W., Newby L.K., Gibson C.M., Moliterno D.J., Van de Werf F., White H.D., Harrington R.A., Roe M.T. Incidence, distribution, and prognostic impact of occluded culprit arteries among patients with non-ST-elevation acute coronary syndromes undergoing diagnostic angiography. Am. Heart J. 2009;157(4):716–723. doi: 10.1016/j.ahj.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 4.McManus D.D., Gore J., Yarzebski J., Spencer F., Lessard D., Goldberg R.J. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am. J. Med. 2011;124(1):40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widimsky P., Wijns W., Fajadet J., de Belder M., Knot J., Aaberge L., Andrikopoulos G., Baz J.A., Betriu A., Claeys M., Danchin N., Djambazov S., Erne P., Hartikainen J., Huber K., Kala P., Klinceva M., Kristensen S.D., Ludman P., Ferre J.M., Merkely B., Milicic D., Morais J., Noc M., Opolski G., Ostojic M., Radovanovic D., De Servi S., Stenestrand U., Studencan M., Tubaro M., Vasiljevic Z., Weidinger F., Witkowski A., Zeymer U., European Association for Percutaneous Cardiovascular Interventions Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur. Heart J. 2010;31(8):943–957. doi: 10.1093/eurheartj/ehp492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myerson M., Coady S., Taylor H., Rosamond W.D., Goff D.C., Jr, ARIC Investigators Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2009;119(4):503–514. doi: 10.1161/CIRCULATIONAHA.107.693879. [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K., Mair J., Giannitsis E., Mueller C., Lindahl B., Blankenberg S., Huber K., Plebani M., Biasucci L.M., Tubaro M., Collinson P., Venge P., Hasin Y., Galvani M., Koenig W., Hamm C., Alpert J.S., Katus H., Jaffe A.S., Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care How to use high-sensitivity cardiac troponins in acute cardiac care. Eur. Heart J. 2012;33(18):2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza M., Sarkisian L., Saaby L., Poulsen T.S., Gerke O., Larsen T.B., Diederichsen A.C., Jangaard N., Diederichsen S.Z., Hosbond S., Hove J., Thygesen K., Mickley H. Diagnosis of unstable angina pectoris has declined markedly with the advent of more sensitive troponin assays. Am. J. Med. 2015;128(8):852–860. doi: 10.1016/j.amjmed.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Mandelzweig L., Battler A., Boyko V., Bueno H., Danchin N., Filippatos G., Gitt A., Hasdai D., Hasin Y., Marrugat J., Van de Werf F., Wallentin L., Behar S., Euro Heart Survey Investigators The second Euro Heart Survey on acute coronary syndromes: Characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur. Heart J. 2006;27(19):2285–2293. doi: 10.1093/eurheartj/ehl196. [DOI] [PubMed] [Google Scholar]
- 10.Terkelsen C.J., Lassen J.F., Nørgaard B.L., Gerdes J.C., Jensen T., Gøtzsche L.B., Nielsen T.T., Andersen H.R. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur. Heart J. 2005;26(1):18–26. doi: 10.1093/eurheartj/ehi002. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen F., Butrymovich V., Kelbæk H., Wachtell K., Helqvist S., Kastrup J., Holmvang L., Clemmensen P., Engstrøm T., Grande P., Saunamäki K., Jørgensen E. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J. Am. Coll. Cardiol. 2014;64(20):2101–2108. doi: 10.1016/j.jacc.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Fokkema M.L., James S.K., Albertsson P., Akerblom A., Calais F., Eriksson P., Jensen J., Nilsson T., de Smet B.J., Sjögren I., Thorvinger B., Lagerqvist B. Population trends in percutaneous coronary intervention: 20-year results from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). J. Am. Coll. Cardiol. 2013;61(12):1222–1230. doi: 10.1016/j.jacc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Puymirat E., Simon T., Steg P.G., Schiele F., Guéret P., Blanchard D., Khalife K., Goldstein P., Cattan S., Vaur L., Cambou J.P., Ferrières J., Danchin N., USIK USIC 2000 Investigators. FAST MI Investigators Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA. 2012;308(10):998–1006. doi: 10.1001/2012.jama.11348. [DOI] [PubMed] [Google Scholar]
- 14.Gale C.P., Allan V., Cattle B.A., Hall A.S., West R.M., Timmis A., Gray H.H., Deanfield J., Fox K.A., Feltbower R. Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003-2010: a multilevel and relative survival analysis for the National Institute for Cardiovascular Outcomes Research (NICOR). Heart. 2014;100(7):582–589. doi: 10.1136/heartjnl-2013-304517. [DOI] [PubMed] [Google Scholar]
- 15.Di Diego J.M., Antzelevitch C. Acute myocardial ischemia: cellular mechanisms underlying ST segment elevation. J. Electrocardiol. 2014;47(4):486–490. doi: 10.1016/j.jelectrocard.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braunwald E. The open-artery theory is alive and well-again. N. Engl. J. Med. 1993;329(22):1650–1652. doi: 10.1056/NEJM199311253292211. [DOI] [PubMed] [Google Scholar]
- 17.de Winter R.J., Verouden N.J., Wellens H.J., Wilde A.A., Interventional Cardiology Group of the Academic Medical Center A new ECG sign of proximal LAD occlusion. N. Engl. J. Med. 2008;359(19):2071–2073. doi: 10.1056/NEJMc0804737. [DOI] [PubMed] [Google Scholar]
- 18.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., Hindricks G., Kastrati A., Lenzen M.J., Prescott E., Roffi M., Valgimigli M., Varenhorst C., Vranckx P., Widimský P., ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 19.Collet JP., Thiele H., Barbato E, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC), Euro Heart J, ehaa575. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 20.Kolte D., Khera S., Dabhadkar K.C., Agarwal S., Aronow W.S., Timmermans R., Jain D., Cooper H.A., Frishman W.H., Menon V., Bhatt D.L., Abbott J.D., Fonarow G.C., Panza J.A. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non-ST-elevation myocardial infarction. Am. J. Cardiol. 2016;117(1):1–9. doi: 10.1016/j.amjcard.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Mehta S.R., Granger C.B., Boden W.E., Steg P.G., Bassand J.P., Faxon D.P., Afzal R., Chrolavicius S., Jolly S.S., Widimsky P., Avezum A., Rupprecht H.J., Zhu J., Col J., Natarajan M.K., Horsman C., Fox K.A., Yusuf S., TIMACS Investigators Early versus delayed invasive intervention in acute coronary syndromes. N. Engl. J. Med. 2009;360(21):2165–2175. doi: 10.1056/NEJMoa0807986. [DOI] [PubMed] [Google Scholar]
- 22.Jobs A., Mehta S.R., Montalescot G., Vicaut E., Van’t Hof A.W.J., Badings E.A., Neumann F.J., Kastrati A., Sciahbasi A., Reuter P.G., Lapostolle F., Milosevic A., Stankovic G., Milasinovic D., Vonthein R., Desch S., Thiele H. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: a meta-analysis of randomised trials. Lancet. 2017;390(10096):737–746. doi: 10.1016/S0140-6736(17)31490-3. [DOI] [PubMed] [Google Scholar]
- 23.Sorajja P., Gersh B.J., Cox D.A., McLaughlin M.G., Zimetbaum P., Costantini C., Stuckey T., Tcheng J.E., Mehran R., Lansky A.J., Grines C.L., Stone G.W. Impact of delay to angioplasty in patients with acute coronary syndromes undergoing invasive management: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J. Am. Coll. Cardiol. 2010;55(14):1416–1424. doi: 10.1016/j.jacc.2009.11.063. [DOI] [PubMed] [Google Scholar]
- 24.Bavry A.A., Kumbhani D.J., Rassi A.N., Bhatt D.L., Askari A.T. Benefit of early invasive therapy in acute coronary syndromes: a meta- analysis of contemporary randomized clinical trials. J. Am. Coll. Cardiol. 2006;48(7):1319–1325. doi: 10.1016/j.jacc.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 25.Fox K.A., Clayton T.C., Damman P., Pocock S.J., de Winter R.J., Tijssen J.G., Lagerqvist B., Wallentin L., FIR Collaboration Long-term outcome of a routine versus selective invasive strategy in patients with non-ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J. Am. Coll. Cardiol. 2010;55(22):2435–2445. doi: 10.1016/j.jacc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Valgimigli M., Gagnor A., Calabró P., Frigoli E., Leonardi S., Zaro T., Rubartelli P., Briguori C., Andò G., Repetto A., Limbruno U., Cortese B., Sganzerla P., Lupi A., Galli M., Colangelo S., Ierna S., Ausiello A., Presbitero P., Sardella G., Varbella F., Esposito G., Santarelli A., Tresoldi S., Nazzaro M., Zingarelli A., de Cesare N., Rigattieri S., Tosi P., Palmieri C., Brugaletta S., Rao S.V., Heg D., Rothenbühler M., Vranckx P., Jüni P., MATRIX Investigators Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385(9986):2465–2476. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- 27.Valgimigli M., Frigoli E., Leonardi S., Vranckx P., Rothenbühler M., Tebaldi M., Varbella F., Calabrò P., Garducci S., Rubartelli P., Briguori C., Andó G., Ferrario M., Limbruno U., Garbo R., Sganzerla P., Russo F., Nazzaro M., Lupi A., Cortese B., Ausiello A., Ierna S., Esposito G., Ferrante G., Santarelli A., Sardella G., de Cesare N., Tosi P., van ’t Hof A., Omerovic E., Brugaletta S., Windecker S., Heg D., Jüni P., MATRIX Investigators Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet. 2018;392(10150):835–848. doi: 10.1016/S0140-6736(18)31714-8. [DOI] [PubMed] [Google Scholar]
- 28.Jolly S.S., Yusuf S., Cairns J., Niemelä K., Xavier D., Widimsky P., Budaj A., Niemelä M., Valentin V., Lewis B.S., Avezum A., Steg P.G., Rao S.V., Gao P., Afzal R., Joyner C.D., Chrolavicius S., Mehta S.R., RIVAL trial group Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 29.Vuurmans T., Byrne J., Fretz E., Janssen C., Hilton J.D., Klinke W.P., Djurdjev O., Levin A. Chronic kidney injury in patients after cardiac catheterisation or percutaneous coronary intervention: a comparison of radial and femoral approaches (from the British Columbia Cardiac and Renal Registries). Heart. 2010;96(19):1538–1542. doi: 10.1136/hrt.2009.192294. [DOI] [PubMed] [Google Scholar]
- 30.Räber L., Kelbæk H., Ostojic M., Baumbach A., Heg D., Tüller D., von Birgelen C., Roffi M., Moschovitis A., Khattab A.A., Wenaweser P., Bonvini R., Pedrazzini G., Kornowski R., Weber K., Trelle S., Lüscher T.F., Taniwaki M., Matter C.M., Meier B., Jüni P., Windecker S., COMFORTABLE AMI Trial Investigators Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308(8):777–787. doi: 10.1001/jama.2012.10065. [DOI] [PubMed] [Google Scholar]
- 31.Sabate M., Cequier A., Iñiguez A., Serra A., Hernandez-Antolin R., Mainar V., Valgimigli M., Tespili M., den Heijer P., Bethencourt A., Vazquez N., Gómez-Hospital J.A., Baz J.A., Martin-Yuste V., van Geuns R.J., Alfonso F., Bordes P., Tebaldi M., Masotti M., Silvestro A., Backx B., Brugaletta S., van Es G.A., Serruys P.W. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380(9852):1482–1490. doi: 10.1016/S0140-6736(12)61223-9. [DOI] [PubMed] [Google Scholar]
- 32.Bønaa K.H., Mannsverk J., Wiseth R., Aaberge L., Myreng Y., Nygård O., Nilsen D.W., Kløw N.E., Uchto M., Trovik T., Bendz B., Stavnes S., Bjørnerheim R., Larsen A.I., Slette M., Steigen T., Jakobsen O.J., Bleie Ø., Fossum E., Hanssen T.A., Dahl-Eriksen Ø., Njølstad I., Rasmussen K., Wilsgaard T., Nordrehaug J.E., NORSTENT Investigators NORSTENT Investigators. Drug-eluting or bare-metal stents for coronary artery disease. N. Engl. J. Med. 2016;375(13):1242–1252. doi: 10.1056/NEJMoa1607991. [DOI] [PubMed] [Google Scholar]
- 33.Valgimigli M., Tebaldi M., Borghesi M., Vranckx P., Campo G., Tumscitz C., Cangiano E., Minarelli M., Scalone A., Cavazza C., Marchesini J., Parrinello G., PRODIGY Investigators Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study (PROlonging Dual Antiplatelet Treatment After Grading stent-induced Intimal hyperplasia studY). JACC Cardiovasc. Interv. 2014;7(1):20–28. doi: 10.1016/j.jcin.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Fröbert O., Lagerqvist B., Olivecrona G.K., Omerovic E., Gudnason T., Maeng M., Aasa M., Angerås O., Calais F., Danielewicz M., Erlinge D., Hellsten L., Jensen U., Johansson A.C., Kåregren A., Nilsson J., Robertson L., Sandhall L., Sjögren I., Ostlund O., Harnek J., James S.K., TASTE Trial Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. 2013;369(17):1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 35.Jolly S.S., Cairns J.A., Yusuf S., Meeks B., Pogue J., Rokoss M.J., Kedev S., Thabane L., Stankovic G., Moreno R., Gershlick A., Chowdhary S., Lavi S., Niemelä K., Steg P.G., Bernat I., Xu Y., Cantor W.J., Overgaard C.B., Naber C.K., Cheema A.N., Welsh R.C., Bertrand O.F., Avezum A., Bhindi R., Pancholy S., Rao S.V., Natarajan M.K., ten Berg J.M., Shestakovska O., Gao P., Widimsky P., Džavík V., TOTAL Investigators Randomized trial of primary PCI with or without routine manual thrombectomy. N. Engl. J. Med. 2015;372(15):1389–1398. doi: 10.1056/NEJMoa1415098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagerqvist B., Fröbert O., Olivecrona G.K., Gudnason T., Maeng M., Alström P., Andersson J., Calais F., Carlsson J., Collste O., Götberg M., Hårdhammar P., Ioanes D., Kallryd A., Linder R., Lundin A., Odenstedt J., Omerovic E., Puskar V., Tödt T., Zelleroth E., Östlund O., James S.K. Outcomes 1 year after thrombus aspiration for myocardial infarction. N. Engl. J. Med. 2014;371(12):1111–1120. doi: 10.1056/NEJMoa1405707. [DOI] [PubMed] [Google Scholar]
- 37.Meyer-Saraei R., de Waha S., Eitel I., Desch S., Scheller B., Böhm M., Lauer B., Gawaz M., Geisler T., Gunkel O., Bruch L., Klein N., Pfeiffer D., Schuler G., Zeymer U., Thiele H. Thrombus aspiration in non-ST-elevation myocardial infarction - 12-month clinical outcome of the randomised TATORT-NSTEMI trial. Eur. Heart J. Acute Cardiovasc. Care. 2017;6(1):10–17. doi: 10.1177/2048872615617044. [DOI] [PubMed] [Google Scholar]
- 38.Priori S.G., Blomström-Lundqvist C., Mazzanti A., Blom N., Borggrefe M., Camm J., Elliott P.M., Fitzsimons D., Hatala R., Hindricks G., Kirchhof P., Kjeldsen K., Kuck K.H., Hernandez-Madrid A., Nikolaou N., Norekvål T.M., Spaulding C., Van Veldhuisen D.J., Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 2015;17(11):1601–1687. doi: 10.1093/europace/euv319. [DOI] [PubMed] [Google Scholar]
- 39.Neumann F-J., Sousa-Uva M., Ahlsson A., et al. ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018;39(42):3759. doi: 10.1093/eurheartj/ehy658. [DOI] [PubMed] [Google Scholar]
- 40.Wallentin L., Becker R.C., Budaj A., Cannon C.P., Emanuelsson H., Held C., Horrow J., Husted S., James S., Katus H., Mahaffey K.W., Scirica B.M., Skene A., Steg P.G., Storey R.F., Harrington R.A., Freij A., Thorsén M., PLATO Investigators Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 41.Wiviott S.D., Braunwald E., McCabe C.H., Montalescot G., Ruzyllo W., Gottlieb S., Neumann F.J., Ardissino D., De Servi S., Murphy S.A., Riesmeyer J., Weerakkody G., Gibson C.M., Antman E.M., TRITON-TIMI 38 Investigators Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 42.Montalescot G., Bolognese L., Dudek D., et al. ACCOAST Investigators. Pre-treatment with prasugrel in non-ST segment elevation acute coronary syndromes. N. Engl. J. Med. 2013;369:999–1010. doi: 10.1056/NEJMoa1308075. [DOI] [PubMed] [Google Scholar]
- 43.Schupke S., Neumann F.J., Menichelli M., et al. ISAR-REACT 5 Trial Investigators. Ticagrelor or prasugrel in patients with acute coronary syndromes. N. Engl. J. Med. 2019;381:15241534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 44.Mehta S.R., Tanguay J.F., Eikelboom J.W., Jolly S.S., Joyner C.D., Granger C.B., Faxon D.P., Rupprecht H.J., Budaj A., Avezum A., Widimsky P., Steg P.G., Bassand J.P., Montalescot G., Macaya C., Di Pasquale G., Niemela K., Ajani A.E., White H.D., Chrolavicius S., Gao P., Fox K.A., Yusuf S., CURRENT-OASIS 7 trial investigators Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 45.Valgimigli M., Bueno H., Byrne R.A., Collet J.P., Costa F., Jeppsson A., Jüni P., Kastrati A., Kolh P., Mauri L., Montalescot G., Neumann F.J., Petricevic M., Roffi M., Steg P.G., Windecker S., Zamorano J.L., Levine G.N., ESC Scientific Document Group. ESC Committee for Practice Guidelines (CPG) ESC National Cardiac Societies 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018;39(3):213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 46.Harrington R.A., Stone G.W., McNulty S., White H.D., Lincoff A.M., Gibson C.M., Pollack C.V., Jr, Montalescot G., Mahaffey K.W., Kleiman N.S., Goodman S.G., Amine M., Angiolillo D.J., Becker R.C., Chew D.P., French W.J., Leisch F., Parikh K.H., Skerjanec S., Bhatt D.L. Platelet inhibition with cangrelor in patients undergoing PCI. N. Engl. J. Med. 2009;361(24):2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 47.Bhatt D.L., Lincoff A.M., Gibson C.M., Stone G.W., McNulty S., Montalescot G., Kleiman N.S., Goodman S.G., White H.D., Mahaffey K.W., Pollack C.V., Jr, Manoukian S.V., Widimsky P., Chew D.P., Cura F., Manukov I., Tousek F., Jafar M.Z., Arneja J., Skerjanec S., Harrington R.A., CHAMPION PLATFORM Investigators Intravenous platelet blockade with cangrelor during PCI. N. Engl. J. Med. 2009;361(24):2330–2341. doi: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
- 48.Bhatt D.L., Stone G.W., Mahaffey K.W., Gibson C.M., Steg P.G., Hamm C.W., Price M.J., Leonardi S., Gallup D., Bramucci E., Radke P.W., Widimský P., Tousek F., Tauth J., Spriggs D., McLaurin B.T., Angiolillo D.J., Généreux P., Liu T., Prats J., Todd M., Skerjanec S., White H.D., Harrington R.A., CHAMPION PHOENIX Investigators Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 2013;368(14):1303–1313. doi: 10.1056/NEJMoa1300815. [DOI] [PubMed] [Google Scholar]
- 49.Steg P.G., Bhatt D.L., Hamm C.W., et al. CHAMPION Investigators. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet 2013; 382: 1981-92. doi: 10.1016/S0140-6736(13)61615-3. [DOI] [PubMed] [Google Scholar]
- 50.Erlinge D., Omerovic E., Fröbert O., Linder R., Danielewicz M., Hamid M., Swahn E., Henareh L., Wagner H., Hårdhammar P., Sjögren I., Stewart J., Grimfjärd P., Jensen J., Aasa M., Robertsson L., Lindroos P., Haupt J., Wikström H., Ulvenstam A., Bhiladvala P., Lindvall B., Lundin A., Tödt T., Ioanes D., Råmunddal T., Kellerth T., Zagozdzon L., Götberg M., Andersson J., Angerås O., Östlund O., Lagerqvist B., Held C., Wallentin L., Scherstén F., Eriksson P., Koul S., James S. Bivalirudin versus heparin monotherapy in myocardial infarction. N. Engl. J. Med. 2017;377(12):1132–1142. doi: 10.1056/NEJMoa1706443. [DOI] [PubMed] [Google Scholar]
- 51.Montalescot G., Zeymer U., Silvain J., Boulanger B., Cohen M., Goldstein P., Ecollan P., Combes X., Huber K., Pollack C., Jr, Bénezet J.F., Stibbe O., Filippi E., Teiger E., Cayla G., Elhadad S., Adnet F., Chouihed T., Gallula S., Greffet A., Aout M., Collet J.P., Vicaut E., ATOLL Investigators Intravenous enoxaparin or unfractionated heparin in primary percutaneous coronary intervention for ST-elevation myocardial infarction: the international randomised open-label ATOLL trial. Lancet. 2011;378(9792):693–703. doi: 10.1016/S0140-6736(11)60876-3. [DOI] [PubMed] [Google Scholar]
- 52.Silvain J., Beygui F., Barthélémy O., Pollack C., Jr, Cohen M., Zeymer U., Huber K., Goldstein P., Cayla G., Collet J.P., Vicaut E., Montalescot G. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012;344:e553. doi: 10.1136/bmj.e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yusuf S., Mehta S.R., Chrolavicius S., Afzal R., Pogue J., Granger C.B., Budaj A., Peters R.J., Bassand J.P., Wallentin L., Joyner C., Fox K.A., OASIS-6 Trial Group Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006;295(13):1519–1530. doi: 10.1001/jama.295.13.joc60038. [DOI] [PubMed] [Google Scholar]
- 54.Steg P.G., Jolly S.S., Mehta S.R., Afzal R., Xavier D., Rupprecht H.J., López-Sendón J.L., Budaj A., Diaz R., Avezum A., Widimsky P., Rao S.V., Chrolavicius S., Meeks B., Joyner C., Pogue J., Yusuf S., FUTURA/OASIS-8 Trial Group Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010;304(12):1339–1349. doi: 10.1001/jama.2010.1320. [DOI] [PubMed] [Google Scholar]
- 55.Valgimigli M., Frigoli E., Leonardi S., Rothenbühler M., Gagnor A., Calabrò P., Garducci S., Rubartelli P., Briguori C., Andò G., Repetto A., Limbruno U., Garbo R., Sganzerla P., Russo F., Lupi A., Cortese B., Ausiello A., Ierna S., Esposito G., Presbitero P., Santarelli A., Sardella G., Varbella F., Tresoldi S., de Cesare N., Rigattieri S., Zingarelli A., Tosi P., van ’t Hof A., Boccuzzi G., Omerovic E., Sabaté M., Heg D., Jüni P., Vranckx P., MATRIX Investigators MATRIX Investigators. Bivalirudin or unfractionated heparin in acute coronary syndromes. N. Engl. J. Med. 2015;373(11):997–1009. doi: 10.1056/NEJMoa1507854. [DOI] [PubMed] [Google Scholar]
- 56.Stone G.W., Bertrand M.E., Moses J.W., Ohman E.M., Lincoff A.M., Ware J.H., Pocock S.J., McLaurin B.T., Cox D.A., Jafar M.Z., Chandna H., Hartmann F., Leisch F., Strasser R.H., Desaga M., Stuckey T.D., Zelman R.B., Lieber I.H., Cohen D.J., Mehran R., White H.D., ACUITY Investigators Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA. 2007;297(6):591–602. doi: 10.1001/jama.297.6.591. [DOI] [PubMed] [Google Scholar]
- 57.Mauri L., Kereiakes D.J., Yeh R.W., Driscoll-Shempp P., Cutlip D.E., Steg P.G., Normand S.L., Braunwald E., Wiviott S.D., Cohen D.J., Holmes D.R., Jr, Krucoff M.W., Hermiller J., Dauerman H.L., Simon D.I., Kandzari D.E., Garratt K.N., Lee D.P., Pow T.K., Ver Lee P., Rinaldi M.J., Massaro J.M., DAPT Study Investigators Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014;371(23):2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonaca M.P., Bhatt D.L., Cohen M., Steg P.G., Storey R.F., Jensen E.C., Magnani G., Bansilal S., Fish M.P., Im K., Bengtsson O., Oude Ophuis T., Budaj A., Theroux P., Ruda M., Hamm C., Goto S., Spinar J., Nicolau J.C., Kiss R.G., Murphy S.A., Wiviott S.D., Held P., Braunwald E., Sabatine M.S., PEGASUS-TIMI 54 Steering Committee and Investigators Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015;372(19):1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 59.Costa F., Van Klaveren D., Feres F., James S., Räber L., Pilgrim T., Hong M.K., Kim H.S., Colombo A., Steg P.G., Bhatt D.L., Stone G.W., Windecker S., Steyerberg E.W., Valgimigli M., PRECISE-DAPT Study Investigators Dual antiplatelet therapy duration based on ischemic and bleeding risks after coronary stenting. J. Am. Coll. Cardiol. 2019;73(7):741–754. doi: 10.1016/j.jacc.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 60.Holmes D.R., Jr, Berger P.B., Hochman J.S., Granger C.B., Thompson T.D., Califf R.M., Vahanian A., Bates E.R., Topol E.J. Cardiogenic shock in patients with acute ischemic syndromes with and without ST-segment elevation. Circulation. 1999;100(20):2067–2073. doi: 10.1161/01.CIR.100.20.2067. [DOI] [PubMed] [Google Scholar]
- 61.Goldberg R.J., Spencer F.A., Gore J.M., Lessard D., Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119(9):1211–1219. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hochman J.S., Buller C.E., Sleeper L.A., Boland J., Dzavik V., Sanborn T.A., Godfrey E., White H.D., Lim J., LeJemtel T. Cardiogenic shock complicating acute myocardial infarction-etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J. Am. Coll. Cardiol. 2000;36(3) Suppl. A:1063–1070. doi: 10.1016/S0735-1097(00)00879-2. [DOI] [PubMed] [Google Scholar]
- 63.Thiele H., Akin I., Sandri M., Fuernau G., de Waha S., Meyer-Saraei R., Nordbeck P., Geisler T., Landmesser U., Skurk C., Fach A., Lapp H., Piek J.J., Noc M., Goslar T., Felix S.B., Maier L.S., Stepinska J., Oldroyd K., Serpytis P., Montalescot G., Barthelemy O., Huber K., Windecker S., Savonitto S., Torremante P., Vrints C., Schneider S., Desch S., Zeymer U., CULPRIT-SHOCK Investigators CULPRIT-SHOCK Investigators. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N. Engl. J. Med. 2017;377(25):2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 64.Niccoli G., Burzotta F., Galiuto L., Crea F. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 2009;54(4):281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 65.Bouleti C., Mewton N., Germain S. The no-reflow phenomenon: State of the art. Arch. Cardiovasc. Dis. 2015;108(12):661–674. doi: 10.1016/j.acvd.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Vlaar P.J., Diercks G.F., Svilaas T., Vogelzang M., de Smet B.J., van den Heuvel A.F., Anthonio R.L., Jessurun G.A., Tan E.S., Suurmeijer A.J., Zijlstra F. The feasibility and safety of routine thrombus aspiration in patients with non-ST-elevation myocardial infarction. Catheter. Cardiovasc. Interv. 2008;72(7):937–942. doi: 10.1002/ccd.21717. [DOI] [PubMed] [Google Scholar]
- 67.Amsterdam E.A., Wenger N.K., Brindis R.G., Casey D.E., Jr, Ganiats T.G., Holmes D.R., Jr, Jaffe A.S., Jneid H., Kelly R.F., Kontos M.C., Levine G.N., Liebson P.R., Mukherjee D., Peterson E.D., Sabatine M.S., Smalling R.W., Zieman S.J. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J., Qiao SB., Zhu J. Chinese Cooperative Group of the Timing of Intervention in Acute Coronary Syndrome. Outcome of patients with non-ST segment elevation acute coronary syndrome undergoing early or delayed intervention. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38(10):865–9. [PubMed] [Google Scholar]
- 69.Katritsis D.G., Siontis G.C., Kastrati A., van’t Hof A.W., Neumann F.J., Siontis K.C., Ioannidis J.P. Optimal timing of coronary angiography and potential intervention in non-ST-elevation acute coronary syndromes. Eur. Heart J. 2011;32(1):32–40. doi: 10.1093/eurheartj/ehq276. [DOI] [PubMed] [Google Scholar]
- 70.Navarese E.P., Gurbel P.A., Andreotti F., Tantry U., Jeong Y.H., Kozinski M., Engstrøm T., Di Pasquale G., Kochman W., Ardissino D., Kedhi E., Stone G.W., Kubica J. Optimal timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes: a systematic review and meta-analysis. Ann. Intern. Med. 2013;158(4):261–270. doi: 10.7326/0003-4819-158-4-201302190-00006. [DOI] [PubMed] [Google Scholar]
- 71.Montalescot G., Cayla G., Collet J.P., Elhadad S., Beygui F., Le Breton H., Choussat R., Leclercq F., Silvain J., Duclos F., Aout M., Dubois-Randé J.L., Barthélémy O., Ducrocq G., Bellemain-Appaix A., Payot L., Steg P.G., Henry P., Spaulding C., Vicaut E., ABOARD Investigators Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA. 2009;302(9):947–954. doi: 10.1001/jama.2009.1267. [DOI] [PubMed] [Google Scholar]
- 72.Milosevic A., Vasiljevic-Pokrajcic Z., Milasinovic D., Marinkovic J., Vukcevic V., Stefanovic B., Asanin M., Dikic M., Stankovic S., Stankovic G. Immediate versus delayed invasive intervention for non-STEMI patients: the RIDDLE-NSTEMI study. JACC Cardiovasc. Interv. 2016;9(6):541–549. doi: 10.1016/j.jcin.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 73.Krishnaswamy A., Lincoff A.M., Menon V. Magnitude and consequences of missing the acute infarct-related circumflex artery. Am. Heart J. 2009;158(5):706–712. doi: 10.1016/j.ahj.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 74.Kim M.C., Ahn Y., Rhew S.H., Jeong M.H., Kim J.H., Hong Y.J., Chae S.C., Kim Y.J., Hur S.H., Seong I.W., Chae J.K., KAMIR Investigators Impact of total occlusion of an infarct-related artery on long-term mortality in acute non-ST-elevation myocardial infarction patients who underwent early percutaneous coronary intervention. Int. Heart J. 2012;53(3):160–164. doi: 10.1536/ihj.53.160. [DOI] [PubMed] [Google Scholar]
- 75.Khan A.R., Golwala H., Tripathi A., Bin Abdulhak A.A., Bavishi C., Riaz H., Mallipedi V., Pandey A., Bhatt D.L. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur. Heart J. 2017;38(41):3082–3089. doi: 10.1093/eurheartj/ehx418. [DOI] [PubMed] [Google Scholar]
- 76.Warren J., Mehran R., Yu J., Xu K., Bertrand M.E., Cox D.A., Lincoff A.M., Manoukian S.V., Ohman E.M., Pocock S.J., White H.D., Stone G.W. Incidence and impact of totally occluded culprit coronary arteries in patients presenting with non-ST-segment elevation myocardial infarction. Am. J. Cardiol. 2015;115(4):428–433. doi: 10.1016/j.amjcard.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 77.Nikus K.C., Eskola M.J., Niemelä K.O., Sclarovsky S. How to use ECG for decision support in the catheterization laboratory. Cases with ST-segment depression acute coronary syndrome. J. Electrocardiol. 2004;37(4):247–255. doi: 10.1016/j.jelectrocard.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 78.Boden W.E., Spodick D.H. Diagnostic significance of precordial ST-segment depression. Am. J. Cardiol. 1989;63(5):358–361. doi: 10.1016/0002-9149(89)90346-9. [DOI] [PubMed] [Google Scholar]
- 79.Shah A., Wagner G.S., Green C.L., Crater S.W., Sawchak S.T., Wildermann N.M., Mark D.B., Waugh R.A., Krucoff M.W. Electrocardiographic differentiation of the ST-segment depression of acute myocardial injury due to the left circumflex artery occlusion from that of myocardial ischemia of nonocclusive etiologies. Am. J. Cardiol. 1997;80(4):512–513. doi: 10.1016/S0002-9149(97)00406-2. [DOI] [PubMed] [Google Scholar]
- 80.Casas R.E., Marriott H.J., Glancy D.L. Value of leads V7-V9 in diagnosing posterior wall acute myocardial infarction and other causes of tall R waves in V1-V2. Am. J. Cardiol. 1997;80(4):508–509. doi: 10.1016/S0002-9149(97)00404-9. [DOI] [PubMed] [Google Scholar]
- 81.Kanemoto N., Wang Y., Fukushi H., Ibukiyama C., Takeuchi T., Sato T., Takahashi T. Electrocardiographic characteristics of patients with left circumflex-related myocardial infarction in the acute phase without tented T waves or definite ST elevation. J. Cardiol. 1995;26(3):149–158. [PubMed] [Google Scholar]
- 82.Braat S.H., Brugada P., de Zwaan C., Coenegracht J.M., Wellens H.J. Value of electrocardiogram in diagnosing right ventricular involvement in patients with an acute inferior wall myocardial infarction. Br. Heart J. 1983;49(4):368–372. doi: 10.1136/hrt.49.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grothoff M., Elpert C., Hoffmann J., Zachrau J., Lehmkuhl L., de Waha S., Desch S., Eitel I., Mende M., Thiele H., Gutberlet M. Right ventricular injury in ST-elevation myocardial infarction: risk stratification by visualization of wall motion, edema, and delayed-enhancement cardiac magnetic resonance. Circ Cardiovasc Imaging. 2012;5(1):60–68. doi: 10.1161/CIRCIMAGING.111.967810. [DOI] [PubMed] [Google Scholar]
- 84.Meisel S.R., Dagan Y., Blondheim D.S., Dacca S., Shochat M., Kazatsker M., Asif A., Frimerman A., Shotan A. Transient ST-elevation myocardial infarction: clinical course with intense medical therapy and early invasive approach, and comparison with persistent ST-elevation myocardial infarction. Am. Heart J. 2008;155(5):848–854. doi: 10.1016/j.ahj.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 85.Lemkes J.S., Janssens G.N., van der Hoeven N.W., van de Ven P.M., Marques K.M.J., Nap A., van Leeuwen M.A.H., Appelman Y.E.A., Knaapen P., Verouden N.J.W., Allaart C.P., Brinckman S.L., Saraber C.E., Plomp K.J., Timmer J.R., Kedhi E., Hermanides R.S., Meuwissen M., Schaap J., van der Weerdt A.P., van Rossum A.C., Nijveldt R., van Royen N. Timing of revascularization in patients with transient ST-segment elevation myocardial infarction: a randomized clinical trial. Eur. Heart J. 2019;40(3):283–291. doi: 10.1093/eurheartj/ehy651. [DOI] [PubMed] [Google Scholar]
- 86.Blondheim D.S., Kleiner-Shochat M., Asif A., Kazatsker M., Frimerman A., Abu-Fanne R., Neiman E., Barel M., Levy Y., Amsalem N., Shotan A., Meisel S.R. Characteristics, management, and outcome of transient ST-elevation versus persistent ST-elevation and Non-ST-elevation myocardial infarction. Am. J. Cardiol. 2018;121(12):1449–1455. doi: 10.1016/j.amjcard.2018.02.029. [DOI] [PubMed] [Google Scholar]