Abstract
In December 2019, a novel COVID-19 infection caused by SARS-CoV-2 has emerged as a global emergency. In a few months, the pathogen has infected millions of people in the world. Primarily SARS-CoV-2 infects the pulmonary system which ultimately leads to ARDS and lung failure. The majority of patients develop milder symptoms but the infection turns severe in a huge number of people, which ultimately results in enhanced mortality in COVID-19 patients. Co-morbid conditions, primarily cardiovascular complications and diabetes, have been reported to show a strong correlation with COVID-19 severity. Further, the onset of myocardial injury secondary to pulmonary damage has been observed in critically ill patients who have never reported heart-related ailments before. Due to drastic health risks associated with virus infection, the unprecedented disruption in normal business throughout the world has caused economic misery. Apparently, newer treatments are urgently needed to combat the virus particularly to reduce the severity burden. Therefore, understanding the crosstalk between lung and heart during COVID-19 might give us better clarity for early diagnosis followed by appropriate treatment in patients with the likelihood of developing severe symptoms. Accordingly, the present review highlights the potential mechanisms that may explain the crosstalk between lung and heart so that effective treatment/management strategies can be evolved swiftly in this direction.
Keywords: COVID-19, SARS-CoV-2, ARDS, cardiovascular disease, respiratory failure, lung-heart interplay
1. INTRODUCTION
The current outbreak of the novel coronavirus caused by Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS- CoV-2) originated in late December 2019 in Wuhan, China. Since then, within a span of three months, it was declared as a global health emergency and the destruction caused by the virus is known to the whole world [1]. According to the current statistics of WHO, a total of 40 million people have been infected and more than 1 million people have succumbed to the virus worldwide (Oct 19, 2020) [2].
Clinical representation of COVID-19 ranges from asymptomatic/mild influenza-like symptoms to deadly acute respiratory distress syndrome (ARDS). About 80% of the patients remain asymptomatic or develop milder symptoms and 20% of patients develop pulmonary infiltrates, out of which 2% progress to severe condition with an elevated risk of mortality [3]. Although the respiratory system appears to be the primary target of the infection, COVID-19 is now considered a systemic disease affecting multiple organs including the heart [4]. SARS-CoV-2 adversely affects the cardiovascular system thereby increasing morbidity in patients with underlying cardiovascular conditions and can even provoke the onset of acute myocardial injury and dysfunction [5]. Pulmonary injury and consequent cardiovascular issues might account for the severity of the disease. This review is aimed to discuss the crosstalk between lung and heart during the severe clinical discourse of COVID-19.
2. COVID-19 ASSOCIATED LUNG AND CARDIAC COMPLICATIONS
Respiratory failure is the most prominent problem reported in COVID-19 patients. Several studies report that majority of patients present no or mild pneumonia but some of them develop ARDS, the main cause of mortality [6-8]. The initial stage of the infection is characterized by the infiltration of SARS-CoV-2 in lung epithelium through binding to angiotensin converting enzyme receptor (ACE2) [9]. Common symptoms that are exhibited at the onset of illness are fever, chills, and shortness of breath [8, 10]. Along with this, a chest x-ray manifests patchy shadows in the lungs of infected patients [10]. At this stage, viral load is perhaps low but such individuals are able to transmit infection, and assessment of viral burden in this early phase might be helpful to predict consequent clinical course [3]. In the second stage, the virus invades gas exchange units and infects alveolar type II cells. The pathological result of SARS-CoV-2 infection is evident in the form of alveolar exudative inflammation and interstitial inflammation. Another important element of the injured lung is the inflammation and injury to the endothelial barrier and subsequent infiltration of monocytes, macrophages, lymphocytes, and neutrophils [11]. Histological analysis of lungs of COVID-19 patients revealed diffuse alveolar damage with fibrin rich hyaline membrane, which is one of the key characteristics of ARDS [3, 10]. The intensity of infiltrates and pulmonary damage increases with the progression of the disease. Clinical examination of severe ARDS is characterized by a decreased ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2: FiO2) accompanied by hypoxemia and low O2 levels in COVID-19 patients [12]. These observations were further found to be consistent with the computed tomography/lung ultrasound representing bilateral opacities and lung collapse like features in serious cases of COVID-19 [13]. As a whole, disruption in endothelial barrier, dysfunctional gas exchange and uncontrolled pulmonary inflammation are the highlighting features of ARDS associated with COVID-19.
Further, emerging data suggest that severe stage of COVID-19 can adversely impact cardiac health also. It has been observed that patients who suffer from cardiac or respiratory failure need ICU admissions and ventilator support for survival. Indeed, high mortality risk has been associated with such patients [14]. The concurrence of cardiovascular disease (CVD) during the current pandemic is not surprising as the association of CVD and respiratory illness is well known. Even during the MERS-CoV epidemic, a large proportion of patients represented the prevalence of CVD, hypertension and diabetes, suggesting CVD as an important predisposing factor for enhanced risk of respiratory viral infections such as MERS-CoV, SARS-CoV and the current pandemic SARS-CoV-2 [4, 15, 16]. In a study of 138 hospitalized COVID-19 patients, the major complications were found to be ARDS (27 patients), arrhythmia (23 patients) and acute cardiac injury (10 patients) [17]. Further, 64 out of these 138 subjects were having co-morbidity including hypertension [43 patients (ICU= 21)], diabetes [14 patients (ICU=8)] and CVD [20 patients (ICU=9)]. Infected patients who develop ARDS and having one or more underlying co- morbidities appeared as the crucial factors leading to severe/critical condition [17]. Another single-center case series conducted by Guo and the group revealed that elevated troponin T (TnT) level on hospital admission is one of the prominent features associated with co-morbid CVD and high mortality in COVID-19 patients [18].
Quite alarmingly, in addition to the negative impact of existing CVD on the severity of COVID-19, new cardiac problems have surfaced in people upon catching the SARS- CoV-2 infection. Huang et al. have reported that 12% of COVID-19 cases experience cardiac injury [8]. The major cardiac manifestations identified upon SARS-CoV2 infection are acute myocardial injury and arrhythmia [19]. Interestingly, the sudden onset of cardiac damage in SARS- CoV-2 patients remains asymptomatic and can be detected only by the analysis of laboratory markers. As described in a study of 138 hospitalized patients with COVID-19 in Wuhan China, the cardiac injury was witnessed by elevated troponin I (TnI) or electrocardiogram (ECG) abnormalities in 7.2% of patients and 22% out of which required ICU admission. In other cases, cardiac symptoms were represented in terms of chest pain, palpitations and arrhythmia. Moreover, progression to the severe condition has been linked with arrhythmias as its presence was found to be higher in ICU admitted patients (44.4%) than in those not requiring ICU (8.9%) [17]. In another observational study of 210 confirmed cases of SARS-CoV-2, 52 (7%) patients were found to be critically sick and 12 (23%) out of 52 suffered from an acute cardiac injury. The study further noted that 9 (75%) of 12 patients who experienced cardiac damage did not survive, suggesting higher mortality in COVID-19 patients with cardiovascular complications [20]. Endomyocardial autopsies data also corroborated these findings, indicating cardiac damage caused by myocardial inflammation and cell necrosis in COVID-19 patients [21].
Overall, SARS-CoV-2 appeared to have a serious impact on the health & functioning of the cardiovascular system. However, close analysis is required to verify that COVID-19 can trigger any kind of cardiac damage, as it is not clear whether the heart was hale and healthy before the contraction of the virus or simply the patients were not aware of any abnormality at the heart level until they were hospitalized. This aspect calls for attention to regular follow-up of the heart, especially of people with growing age; this could help in detecting people who tend to develop critical clinical course during respiratory viral infections.
3. LUNG AND HEART CROSSTALK: POTENTIAL MECHANISMS
The current pandemic is new to all of us. Till date, researchers are aware that the severity of COVID-19 is associated with respiratory and cardiac complications; but how viral infection severely affects the cardiovascular system remains a question. Various studies are ongoing to decode the mechanism related to COVID-19 pathophysiology and its impact on the heart. At this time, the knowledge gained from past epidemics and clinical presentation of COVID-19 patients has led to some speculation regarding heart-lung connection during infection. Accordingly, we would like to throw light on potential pathways that may explain the lung- heart crosstalk to a certain extent.
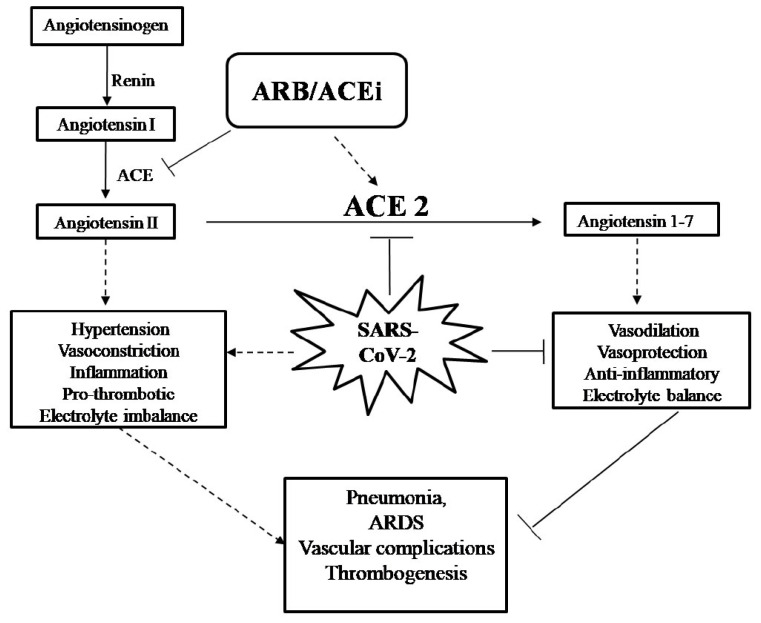
Role of ACE2 and ACEi/ARB: ACE2 receptor appears to play an important role in the invasion of SARS-CoV-2 as the receptors are present on the epithelial and endothelial lining of multiple vital organs such as lung and heart [22]. SARS-CoV-2 has a high-affinity binding site for ACE2 receptor and reduces ACE2 expression afterwards. ACE2 is a counter-regulatory enzyme of the RAAS (Renin-angiotensin-aldosterone system) pathway (Fig. 1), which is responsible for cleavage of AngII to Ang (1-7) [23]. Ang (1-7) is known to exert an anti-inflammatory effect through the restoration of electrolyte balance, vascular damage and regulation of blood pressure. All of these are elementary/primary processes of cardiovascular equilibrium [24]. On the other hand, ACE enzyme converts angiotensin to aldosterone and consequently results in vasoconstriction and inflammation. To facilitate anti-hypertensive effects, patients with CVD, diabetes, and hypertension are usually kept under chronic medication of angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs). The consumption of these drugs ultimately enhances the levels of the negative regulator of RAAS, i.e. ACE2. The presence of increased ACE2 expression particularly on the endothelial lining of vascular system make them prone to SARS-CoV-2 invasion and subsequent infection [22, 25]. To the best of our knowledge, there is one study that has examined the effects of ACEi/ARBs usage and mortality/severity in COVID-19 patients. The study has been conducted on 1178 COVID-19 patients, out of which 362 were hypertensive and 115 of 362 were on ACEi/ARBs medication. Interestingly, no difference in the disease succession or enhanced risk of in-hospital mortality was observed among the hypertensive patients whether or not they were on ACEi/ARBs medication prior to SARS-CoV-2 infection [26].
Fig. (1).
Role of ACE2/ACEi/ ARB in COVID-19: In the RAAS system, angiotension 1 is hydrolysed by ACE to form Ang II, which further leads to the release of aldosterone and consequently causes vasoconstriction and electrolyte imbalance. On the other hand, ACE2 converts Ang II to Ang (1-7) peptide, thus supporting hypertensive effects. SARS-CoV-2 binds to ACE2 to get entry into the cell and downregulates/degrades ACE2 afterward, which ultimately enhances the activity of ACE and its inflammatory effects. ARB/ACEi are ACE inhibitors and promote vasodilation and anti-hypertensive effects.
Further, ACE2 is reported to be down-regulated upon SARS-CoV-2 infection resulting in loss of its protective action and thus promotes vasoconstriction as well as inflammation. In addition, loss of ACE2 activity is associated with exaggerated cardiac inflammation, pulmonary edema and reduction in lung function [27, 28]. This theory has also been supported by autopsy reports of the heart samples of SARS- CoV patients, where the presence of SARS-CoV was associated with marked down-regulation of ACE2 expression along with the release of inflammatory cells in cardiac tissues [29]. This is to note that failure of ACE2 concomitantly increases ACE activity, which acts opposite to ACE2 and enhances AngII production consequently leading to aldosterone release, high blood pressure due to vasoconstriction and re-absorption of water as well as sodium. This highlights the importance of electrolyte disturbances in the pathogenesis of COVID-19 patients. Moreover, a study has confirmed that alteration in electrolyte balance (decreased sodium, potassium and calcium) is associated with severe cases of COVID-19 [30]. Another report has revealed that the up-regulation of aldosterone leads to hypokalemia and BP variability, resulting in disruption of pulmonary vasoregulation and can promote thrombogenesis during COVID-19 [31, 32]. These studies indicate the importance of ACE2 in the maintenance of body fluid and appropriate functioning of vital organs, i.e. lung and heart. Additionally, previous studies have documented the protective potential of recombinant ACE2 in mitigation of COVID-19 independent lung injury and thus represent a potential treatment option for the current pandemic [33, 34]
As of now, due to insufficient evidence on the potential benefit or harm, there are no recommendations for additions or discontinuation of ACEi/ARBs in the COVID-19 setting. From the current understanding, ACE2 appears to be a double-edged sword as it not only helps in the invasion of the virus but also plays a protective role. These studies clearly suggest the intricate role of ACE2 in respiratory dysfunction and vascular complications during COVID-19 severity.
Immune dysregulation: The poor clinical outcome in infected patients has been observed to be a dysregulated immune response to a new viral antigen in the form of SARS- CoV-2. ICU admitted COVID-19 patients exhibit significant elevation in D-dimer, ferritin, IL-6, TnT, IL-2, IL-6, IL-7, G-CSF, MCP-1, IFN- γ, MIP-1α and TNF-α [35]. Particularly, IL-6 levels have been reported to increase sharply in severe patients of COVID-19 as compared to mild cases [36]. Moreover, pro-inflammatory cytokines and chemokines can further attract monocytes/macrophages to the site of infection, which initiates a pro-inflammatory feedback loop. In addition to innate immune response, the adaptive immune response seems to be another component involved in the clinical progression of COVID-19. The reduction in the lymphocyte population has been noted in severe COVID-19 patients. A study conducted by Qin and the group documented an evident reduction in the total number of B cell, T cells and NK cells in severe cases as compared to non-severe patients. Further, data on different subsets of T cells suggests a decrease in both helper T cells (CD3+ CD4+) and cytotoxic T cells (CD3+ CD8+) below normal levels. Despite the marked reduction in the total number of helper-T cells, the percentage of naïve T helper cells [Naïve T-helper cells (CD3+CD4+CD45RA+)/T-helper] was found to be increased with a concomitant decline in memory T cells [memory T-helper cells (CD3+ CD4+ CD45RO+)/T-helper] in severely affected patients [36]. Another study also supports that the reduction of CD4+ and CD8+ T cells is strongly correlated with the severity of COVID-19 [37]. It is worth mentioning that CD4+ and CD8+ cells provide a protective response by helping in the production of neutralizing antibodies and eradication of infected cells, respectively. Generally, naïve T cells help to fight against previously unknown infection by the release of cytokines in a tightly coordinated manner. On the contrary, memory T cells provide antigen-specific immune response [38]. Notably, the balance between naïve T cells and memory cells is crucial for the maintenance of efficient defensive response, disruption of which might be contributing to the SARS-CoV-2 associated severity and worse outcomes of patients.
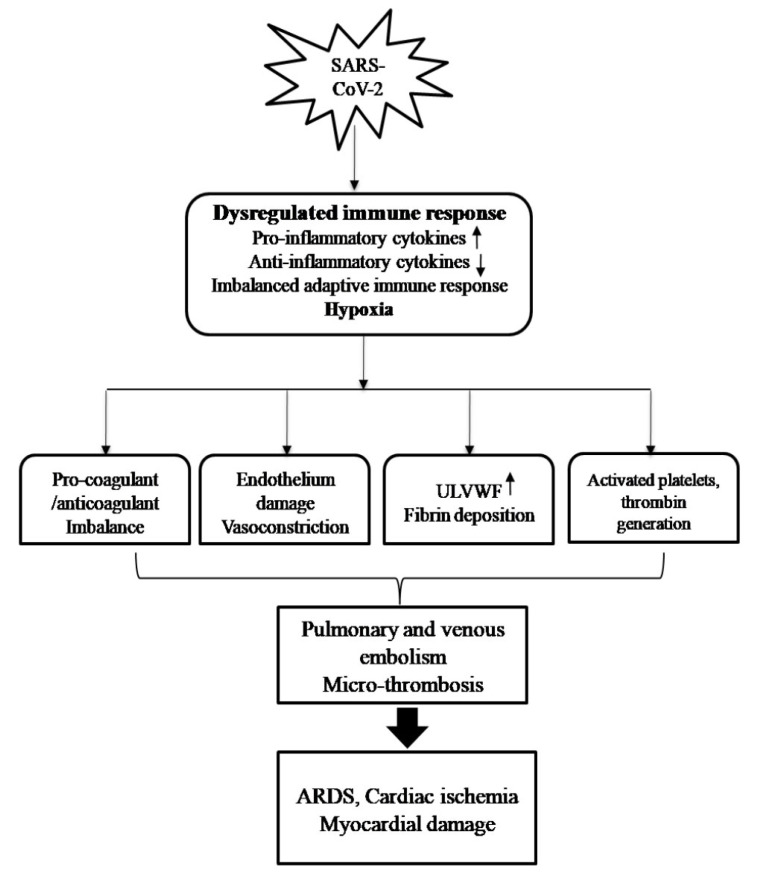
This dysregulated immune response in COVID-19 patients damages pulmonary infrastructure, eventually resulting in the development and progression of ARDS and extra-pulmonary damage [8]. Acute and chronic inflammation during respiratory infection has been previously associated with acute ischemic events [39]. During the infectious course of SARS-CoV-2, inflammation has emerged as a major player in the cascade of lung and heart damage. The aberrant inflammatory response in combination with hypoxia has been linked to endothelial dysfunction, imbalance in pro/anticoagulants and thrombin generation, which eventually leads to thrombo-embolic events in COVID-19 patients [40, 41]. Pulmonary and venous thromboembolism in COVID-19 patients further results in obstruction of oxygen and blood flow through vessels and can cause severe problems like cardiac ischemia and myocardial damage (Fig. 2) [42, 43]. A meta-analysis conducted by Chi and the group highlighted the significance of the thromboembolism in the pathogenesis of COVID-19 patients. Overall, the analysis was carried out on 1981 subjects based on data collected from 11 studies. In five of the studies (576 patients), data was procured entirely from ICU patients, whereas in six studies (1405 patients), patients were enrolled from ward settings out of which 10-38% required ICU admission subsequently. It was observed that despite prophylactic anticoagulant treatment, 24% of infected patients developed venous thromboembolism (VTE) and 12% represented pulmonary embolism (PE). The occurrence of VTE & PE was higher in ICU settings [30.4% (VTE) and 15.7% (PE)] than in the ward settings [13% (VTE) & 5.6% (PE)] [44]. Considering the high probability of VTE/PE in ICU admitted COVID-19 patients, the extraordinary stratification of high-risk population by the continuous diagnosis of coagulopathy may be needed. An improvement in prophylactic strategies in terms of dose and time period of thromboprophylaxis/anti-coagulants may also be explored. Further, the role of an aberrant immune response cannot be overlooked in coagulopathy and hence appropriate immune-based therapy may also be devised.
Fig. (2).
Impact of Immune dysregulation and hypoxia on lung- heart interplay during COVID-19: Abrupt inflammatory response in combination with hypoxia results in an imbalance of pro-coagulants/coagulant factors, endothelial damage and vasoconstriction. In addition, it causes activation of immune cells, deposition of fibrin, thrombin and release of ULVWF. These multiple factors cause physiological destruction in the form of thromboembolism/micro thrombosis and ensuing ARDS and cardiac damage.
Another noteworthy study conducted by Guagliumi revealed that the presence of microthrombi can be an important mechanism in causing myocardial infarction [45]. This study draws our attention towards an alternative facet of endothelial dysfunction characterized by the release of ultralarge von willebrand factor multimers (ULVWF) upon the influx of pro-inflammatory cytokine [46]. ULVWF acts as a connection between damaged/injured endothelial cells and activated immune cells such as neutrophils, macrophages and platelets. Severely-ill COVID-19 patients exhibit microthrombi like features with alteration of alveoli and pulmonary vasculature as a result of the attachment of platelets/ULVWF strings to the injured endothelium along with deposition of fibrin in alveoli [47]. Considering the above-mentioned events during SARS-CoV2, systemic inflammatory mediators appear to be the major culprit in causing pulmonary and cardiac damage, but so far, no study has evidenced the migration of inflammatory cells in the myocardium [48], and therefore, further studies are required to bridge the gaps.
Hypoxia linked cardiac damage: This physiological mechanism is another aspect of lung-heart interaction during COVID-19. Hypoxemia caused by acute respiratory illness results in decreased delivery of oxygen to cardiac tissues. On the other hand, systemic infection results in increased cardio-metabolic demand which ultimately leads to impaired myocardial oxygen consumption and energy expenditure [49]. Such imbalance may result in a predisposition to myocardial ischemia and can progress to myocardial injury, especially in patients having pre-existing CVD [50]. Additionally, hypoxemia caused by COVID-19 can trigger arterial fibrillation, a common form of arrhythmia that occurs among elders [51]. Such reports may partially explain how hypoxia caused by respiratory illness could lead to acute myocardial injury during ARDS.
4. IMPACT OF TREATMENTS/SIDE EFFECTS OF TREATMENTS
Various treatment strategies are being employed in patients with COVID-19 to control the devastation caused by the pandemic. Antimalarial drugs, chloroquine and hydroxychloroquine (HCQ) have been tested in COVID-19 patients and have shown promising results by decreasing viral load. The efficacy of drugs has been noticed in a small sample size and needs to be verified by involving a larger cohort size [52, 53]. At the same time, the cardiotoxicity of HCQ should not be ignored. It has been previously documented that long-term use of chloroquine may prolong the QT interval which can further trigger ventricular arrhythmia and atrioventricular conduction disturbances [19, 54]. In addition, cardiac myopathy caused by these drugs has been found to be associated with respiratory failure [55].
Another potential category of drug candidates that caught attention during the COVID-19 was found to possess antiviral/antibacterial activity. One of them is azithromycin which is known to be effective against Ebola and bacterial pneumonia. Furthermore, it has been used to treat non- COVID-19 ARDS and was found to be associated with a significant reduction in mortality and diminished time required for intubation [56]. During the current pandemic, a study conducted by Gautret et al. confirmed the protective potential of a combination of azithromycin and HCQ in a small cohort of 36 confirmed SARS-CoV-2 infected French patients [57]. On the contrary, a U.S. study performed by Magagnoli and the group reported that a combination of these two drugs failed to provide any benefit to 368 hospitalized patients of COVID-19 but surprisingly was associated with increased risk of mortality [58]. The previously known ability of azithromycin to increase the risk of QT interval extension, sudden cardiac death, and torsade de pointes may be the reason behind such untoward effects. In addition to azithromycin, remdesivir has also emerged as possible drug therapy. No major adverse effects of remdesivir have been observed when tested on animals except for a transient increase in respiratory rate. However, a study carried out on 53 COVID-19 patients who received remdesivir as a treatment represented ARDS (4%) and pneumothorax (4%). Along with respiratory toxicity, cardiovascular side-effects such as hypotension (8%), hypernatremia (6%) and atrial fibrillation were also observed in COVID-19 patients infused with remdesivir [59]. Another anti-viral combination which came into the picture is the combination of lopinavir–ritonavir. A study conducted by Cao et al. revealed that there were no benefits observed in the patients who received this combinatorial treatment (99 patients). Rather, the patients suffered from ARDS (12/99) along with prolonged QT interval (1/99) and disseminated interval coagulation (1/99) [60]. Despite the proven efficiency of these antiviral drugs in combating various types of viral infection(s), their efficacy against SARS-CoV-2 remains questionable. Further, given their reported adverse effects, caution must be taken while using such drugs in patients with severe COVID-19.
Heart and lung owing to their presence in the same thoracic cavity are obviously anatomically connected but their complex physiological interplay is critical to understand in order to reduce the severity of the COVID-19. Further, severe cases of ARDS require ventilation support for survival which can lead to variation in intrathoracic pressure and ventricular dysfunction, which subsequently impacts cardiovascular performance [61]. Moreover, it has been documented that hyperinflation of lungs in ventilator patients often impedes cardiac output [62]. Thus, continuous monitoring of cardio-respiratory parameters is required to ensure the proper functioning of both lungs and heart.
5. IMMUNE-BASED THERAPY: POTENTIAL STRATEGY TO HALT THE TRANSITION FROM MILD/MODERATE TO SEVERITY
Taking into account the impact of immune dysregulation that allows the COVID-19 disease to advance from an asymptomatic/moderate to severe stage, immune-based treatments can be taken into account. The effect of immune-therapies, such as monoclonal antibodies against IL-6, has been found effective against COVID-19 in single-center studies [63]. Additionally, the administration of IL-1R antagonist and a monoclonal antibody against IFN-γ was found to dampen the hyper-inflammatory response and reduce both requirement of mechanical ventilation in the ICU and mortality rate among patients with the severe form of COVID-19 in a cohort study [64]. Targeting IFN-γ can be another promising therapy as it has been observed to diminish inflammatory signals during severe inflammatory disease hemophagocytic lymphohistiocytosis (HLH) [65]. However, randomized clinical trials are under investigation to confirm the efficacy and safety of immunotherapy in the pandemic (Clinical trial Id: NCT04330638, NCT04324021).
More recently, corticosteroids have gained much attention due to their ability to combat hyperinflammatory response. Earlier, corticosteroid use has been found to be associated with a lower incidence of myocardial infarction among hospital admitted patients with pneumonia [66]. In the current pandemic, the potential beneficial role of corticosteroids, namely dexamethasone (Dex), has been investigated in a clinical trial ‘RECOVERY’. After randomization, the trial enrolled 2104 in-hospital patients for Dex treatment and 4321 patients were given the standard (std.) care therapy. Diabetics (25% Dex vs. 24% std. therapy) and patients with heart diseases (28% Dex vs. 27% std. care) were also included in the study. Dex was given to severe patients at a dose of 6mg/day orally/intravenously for a total of 10 days. Outcomes of the study suggest that patients who received Dex displayed a lowered rate of mortality (29.3%) than the usual care group (41.4%) when compared with those who required invasive mechanical ventilation. The patients who received O2 support without mechanical ventilation represented a similar pattern with a mortality rate of 23.3% in the Dex group and 26.2% in the standard care group. In contrast, the patients who did not require any respiratory support did not show any significant difference in mortality rate (17.8% vs. 14.0%). This data somehow explains the importance of immunosuppression to cease the advancement of disease towards severity [67].
Though corticosteroids and immune-based therapy have shown promising results in COVID-19 patients, there are still some gaps that need to be examined for better clarity. The impact of Dex has not been elaborated on patients with pre-existing CVD or those who develop cardiac complications during the clinical course of COVID-19. Effectiveness of corticosteroid is reported in young patients and it remains to be seen whether the therapy will be equally effective against Covid-19 patients belonging to the older age group. However, reports on viral load have not been documented after receiving the drug. Therefore, documentation of viral burden can be an important asset to guide the appropriate treatment decision(s). Moreover, there can be a possibility of steroid linked complications which can only be known after long-term follow up of the patients.
Considering the variability of immune response among severe cases of COVID-19 patients, therapy should be devised accordingly. Along with this, continuous monitoring of viral burden and hyper inflammation is required to conclude the impact of any drug.
6. MANAGEMENT STRATEGIES
To date, there is no proven therapy available for COVID-19 patients and treatment has been mostly confined to supportive measures. Pre-existing poor health, advanced-age and compromised immune system have been shown to increase the risk of lung and heart complications and poor prognosis [5]. Hence, patients with underlying diabetes, hypertension and CVD should be prioritized for suitable treatments. Cardiac intensive care in the form of ACE inhibitors, ARB blockers, anti-arrhythmic, statins, ionotropes and anti-coagulation therapies is generally recommended to handle cardiac problems [68]. Hypoxemia following ARDS is frequent in COVID-19 patients and can be worsened by heart complications. Thus, oxygen therapy and mechanical ventilation can be used in patients with heart problems than those without any cardiac complications. Mechanical assisting devices such as extracorporeal membrane oxygenation (ECMO) and intra-aortic balloon pump are helpful tools to get through critical periods and can be considered in severe conditions [19]. Respiratory and cardiovascular complications are the indicators of progression to severe conditions and poor outcomes. Thus, it is critical to find out the exact mechanism and connection of lung and heart injury during infection to choose the appropriate therapy.
CONCLUSION AND FUTURE DIRECTIONS
At this time, it can be concluded that COVID-19 clinical symptoms represent a spectrum that ranges from asymptomatic to critical condition with poor outcomes. Though ARDS is a common problem in COVID-19 patients but the role of cardiac complication in the development of severe illness is critical. The infected patients exhibit worse outcomes that progress to severe illness with lung-cardiac problems and require ICU admissions. This highlights the need for an initial diagnosis of cardiac injury along with the respiratory dysfunction in patients after hospital admission to identify the patients having a tendency to progress towards the severe clinical condition. The execution of combined assessment of imaging, histopathology, ECG and levels of TnT, coagulation and inflammatory markers might act as a helpful tool in the categorization of patients with severe medical conditions. Additionally, the role of any treatment/drug in triggering events leading to complications in patients during SARS-CoV-2 infection must be examined. Further, the pathophysiological mechanism of COVID-19, as well as short and long-term prognosis in severely ill patients, needs to be explored to fully understand the role of the heart-lung axis during viral infection.
ACKNOWLEDGEMENTS
We also acknowledge the ICMR junior research fellowship to Gayatri Puri from ICMR, New Delhi, India.
LIST OF ABBREVIATIONS
- ACE2
Angiotensin Converting Enzyme 2
- ACEi
Angiotensin Converting Enzyme inhibitor
- Ang
Angiotensin
- ARB
Angiotensin Receptor Blocker
- ARDS
Acute Respiratory Distress Syndrome
- COVID-19
Coronavirus Disease 2019
- CVD
Cardiovascular Disease
- DAD
Diffuse Alveolar Damage
- Dex
Dexamethasone
- ECG
Electrocardiogram
- FiO2
Fraction of inspired Oxygen
- ICU
Intensive Care Unit
- MERS-CoV
Middle East Respiratory Syndrome Coronavirus
- PaO2
Partial pressure of Oxygen
- PE
Pulmonary Embolism
- RAAS
Renin-Angiotensin-Aldosterone System
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SARS-CoV
Severe Acute Respiratory Syndrome Coronavirus
- Std.
Standard
- TnT
Troponin T
- TnI
Troponin I
- UVWF
Ultralarge Von Willebrand Factor (ULVWF)
- VTE
Venous Thromboembolism
- WHO
World Health Organization
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The present work was supported by funds from the Department of Biotechnology, Government of India (BT/PR17968/MED/122/33/2016), and UGC-SAP to Dr. Amarjit Singh Naura.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Worldometer COVID-19 coronavirus pandemic. Available from: https://www.worldometers.info/coronavirus/. [Accessed Oct 19, 2020]
- 3.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L., Schwartz A., Uriel N. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 5.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zangrillo A., Beretta L., Scandroglio AM., Monti G., Fominskiy E., Colombo S., Morselli F., Belletti A., Silvani P., Crivellari M., Monaco F., Azzolini M. L., Reineke R., Nardelli P., Sartorelli M., Votta C. D., Ruggeri A., Ciceri F., De Cobelli F., Tresoldi M., Dagna L., Rovere-Querini P., Serpa Neto A., Bellomo R., Landoni G. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22(3):200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zumla A., Niederman M.S. Editorial: The explosive epidemic outbreak of novel coronavirus disease 2019 (COVID-19) and the persistent threat of respiratory tract infectious diseases to global health security. Curr. Opin. Pulm. Med. 2020;26(3):193–196. doi: 10.1097/MCP.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain A. COVID-19 and lung pathology. Indian J. Pathol. Microbiol. 2020;63(2):171–172. doi: 10.4103/IJPM.IJPM_280_20. [DOI] [PubMed] [Google Scholar]
- 12.Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit. Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopal K., Keller S.P., Akkanti B., Bime C., Loyalka P., Cheema F.H., Zwischenberger J.B., El Banayosy A., Pappalardo F., Slaughter M.S., Slepian M.J. Advanced pulmonary and cardiac support of covid-19 patients: emerging recommendations from ASAIO-A “Living Working Document”. ASAIO J. 2020;66(6):588–598. doi: 10.1097/MAT.0000000000001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A systematic review and meta-analysis. Int. J. Infect. Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C.M., Wong R.S., Wu E.B., Kong S.L., Wong J., Yip G.W., Soo Y.O., Chiu M.L., Chan Y.S., Hui D., Lee N., Wu A., Leung C.B., Sung J.J. Cardiovascular complications of severe acute respiratory syndrome. Postgrad. Med. J. 2006;82(964):140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of Patients With coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng Y.J., Wei Z.Y., Qian H.Y., Huang J., Lodato R., Castriotta R.J. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc. Pathol. 2020;47:107228. doi: 10.1016/j.carpath.2020.107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., Sepe P.A., Resasco T., Camporotondo R., Bruno R., Baldanti F., Paolucci S., Pelenghi S., Iotti G.A., Mojoli F., Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-Angiotensin-Aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qaradakhi T., Gadanec L.K., McSweeney K.R., Tacey A., Apostolopoulos V., Levinger I., Rimarova K., Egom E.E., Rodrigo L., Kruzliak P., Kubatka P., Zulli A. The potential actions of angiotensin-converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin. Exp. Pharmacol. Physiol. 2020;47(5):751–758. doi: 10.1111/1440-1681.13251. [DOI] [PubMed] [Google Scholar]
- 25.Khashkhusha T.R., Chan J.S.K., Harky A. ACEi and ARB with COVID-19. J. Card. Surg. 2020;35(6):1388. doi: 10.1111/jocs.14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for Coronavirus Disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X., Li S., Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020;96(1137):403–407. doi: 10.1136/postgradmedj-2020-137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 29.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39(7):618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann. Clin. Biochem. 2020;57(3):262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vicenzi M., Di Cosola R., Ruscica M., Ratti A., Rota I., Rota F., Bollati V., Aliberti S., Blasi F. The liaison between respiratory failure and high blood pressure: Evidence from COVID-19 patients. Eur. Respir. J. 2020;56(1):2001157. doi: 10.1183/13993003.01157-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 33.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R., Wang Y., Li J., Han H., Xia Z., Liu F., Wu K., Yang L., Liu X., Zhu C. Decreased T cell populations contribute to the increased severity of COVID-19. Clin. Chim. Acta. 2020;508:110–114. doi: 10.1016/j.cca.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surh C.D., Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Van Eeden S., Leipsic J., Paul Man S.F., Sin D.D. The relationship between lung inflammation and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2012;186(1):11–16. doi: 10.1164/rccm.201203-0455PP. [DOI] [PubMed] [Google Scholar]
- 40.Joly B.S., Siguret V., Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med. 2020;46(8):1603–1606. doi: 10.1007/s00134-020-06088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomasoni D., Italia L., Adamo M., Inciardi R.M., Lombardi C.M., Solomon S.D., Metra M. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur. J. Heart Fail. 2020;22(6):957–966. doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chi G., Lee J.J., Jamil A., Gunnam V., Najafi H., Memar Montazerin S., Shojaei F., Marszalek J. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: A systematic review and meta-analysis. J. Clin. Med. 2020;9(8):E2489. doi: 10.3390/jcm9082489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guagliumi G., Sonzogni A., Pescetelli I., Pellegrini D., Finn A.V. Microthrombi and ST-segment-elevation myocardial infarction in COVID-19. Circulation. 2020;142(8):804–809. doi: 10.1161/CIRCULATIONAHA.120.049294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.M., Meziani F., CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., Peccatori J., D’Angelo A., De Cobelli F., Rovere-Querini P., Tresoldi M., Dagna L., Zangrillo A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu H., Rhee J.W., Cheng P., Waliany S., Chang A., Witteles R.M., Maecker H., Davis M.M., Nguyen P.K., Wu S.M. Cardiovascular complications in patients with COVID-19: Consequences of viral toxicities and host immune response. Curr. Cardiol. Rep. 2020;22(5):32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: Acute and long-term implications. Eur. Heart J. 2020;41(19):1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehra M.R., Ruschitzka F. COVID-19 illness and heart failure: A missing link? JACC Heart Fail. 2020;8(6):512–514. doi: 10.1016/j.jchf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang C., Jin Z. An Acute respiratory infection runs into the most common noncommunicable epidemic-COVID-19 and cardiovascular disease. JAMA Cardiol. 2020;5(7):743–744. doi: 10.1001/jamacardio.2020.0934. [DOI] [PubMed] [Google Scholar]
- 52.Meo S.A., Klonoff D.C., Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020;24(8):4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 53.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 54.Boukhris M., Hillani A., Moroni F., Annabi M.S., Addad F., Ribeiro M.H., Mansour S., Zhao X., Ybarra L.F., Abbate A., Vilca L.M., Azzalini L. Cardiovascular implications of the COVID-19 pandemic: A global perspective. Can. J. Cardiol. 2020;36(7):1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siddiqui A.K., Huberfeld S.I., Weidenheim K.M., Einberg K.R., Efferen L.S. Hydroxychloroquine-induced toxic myopathy causing respiratory failure. Chest. 2007;131(2):588–590. doi: 10.1378/chest.06-1146. [DOI] [PubMed] [Google Scholar]
- 56.Kawamura K., Ichikado K., Takaki M., Sakata Y., Yasuda Y., Shingu N., Tanaka A., Hisanaga J., Eguchi Y., Anan K., Nitawaki T., Suga M. Efficacy of azithromycin in sepsis-associated acute respiratory distress syndrome: a retrospective study and propensity score analysis. Springerplus. 2016;5(1):1193. doi: 10.1186/s40064-016-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J. W., Sutton S. S., Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020;1(1):114–127. doi: 10.1016/j.medj.2020.06.001. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheifetz I.M. Cardiorespiratory interactions: The relationship between mechanical ventilation and hemodynamics. Respir. Care. 2014;59(12):1937–1945. doi: 10.4187/respcare.03486. [DOI] [PubMed] [Google Scholar]
- 62.Mahmood S.S., Pinsky M.R. Heart-lung interactions during mechanical ventilation: The basics. Ann. Transl. Med. 2018;6(18):349. doi: 10.21037/atm.2018.04.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Airò P., Bazzani C., Beindorf E.A., Berlendis M., Bezzi M., Bossini N., Castellano M., Cattaneo S., Cavazzana I., Contessi G.B., Crippa M., Delbarba A., De Peri E., Faletti A., Filippini M., Filippini M., Frassi M., Gaggiotti M., Gorla R., Lanspa M., Lorenzotti S., Marino R., Maroldi R., Metra M., Matteelli A., Modina D., Moioli G., Montani G., Muiesan M.L., Odolini S., Peli E., Pesenti S., Pezzoli M.C., Pirola I., Pozzi A., Proto A., Rasulo F.A., Renisi G., Ricci C., Rizzoni D., Romanelli G., Rossi M., Salvetti M., Scolari F., Signorini L., Taglietti M., Tomasoni G., Tomasoni L.R., Turla F., Valsecchi A., Zani D., Zuccalà F., Zunica F., Focà E., Andreoli L., Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19(7):102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., Sacco E., Naccache J.M., Bézie Y., Laplanche S., Le Berre A., Le Pavec J., Salmeron S., Emmerich J., Mourad J.J., Chatellier G., Hayem G. Anakinra for severe forms of COVID-19: A cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Salama Z.T. Emapalumab: First global approval. Drugs. 2019;79(1):99–103. doi: 10.1007/s40265-018-1046-8. [DOI] [PubMed] [Google Scholar]
- 66.Cangemi R., Falcone M., Taliani G., Calvieri C., Tiseo G., Romiti G.F., Bertazzoni G., Farcomeni A., Violi F., SIXTUS Study Group Corticosteroid use and incident myocardial infarction in adults hospitalized for community-acquired pneumonia. Ann. Am. Thorac. Soc. 2019;16(1):91–98. doi: 10.1513/AnnalsATS.201806-419OC. [DOI] [PubMed] [Google Scholar]
- 67.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J., RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 - Preliminary Report. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su Y-B., Kuo M-J., Lin T-Y., Chien C-S., Yang Y-P., Chou S-J., Leu H-B. Cardiovascular manifestation and treatment in COVID-19. J. Chin. Med. Assoc. 2020;83(8):704–709. doi: 10.1097/JCMA.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]