Abstract
Menopause is associated with changes consistent with cardiovascular aging. The effects of cardiac disease are multifaceted, affecting endothelial function, coronary artery physiology and metabolic dysfunction leading to structural changes in the coronary anatomy. A systematic review of literature from 1986 to 2019 was conducted using PubMed and Google Scholar. The search was directed to retrieve papers that addressed the changes in cardiovascular physiology in menopause and the current therapies available to treat cardiovascular manifestations of menopause. The metabolic and clinical factors secondary to menopause, such as dyslipidemia, insulin resistance, fat redistribution and systemic hypertension, contribute to the accelerated risk for cardiovascular aging and disease. Atherosclerosis appears to be the end result of the interaction between cardiovascular risk factors and their accentuation during the perimenopausal period. Additionally, complex interactions between oxidative stress and levels of L-arginine and ADMA may also influence endothelial dysfunction in menopause. The increased cardiovascular risk in menopause stems from the exaggerated effects of changing physiology on the cardiovascular system affecting peripheral, cardiac and cerebrovascular beds. The differential effects of menopause on cardiovascular disease at the subclinical, biochemical and molecular levels form the highlights of this review.
Keywords: Dyslipidemia, endothelial dysfunction, oxidative stress, metabolic syndrome, menopause, cardiovascular aging
1. INTRODUCTION
The life expectancy of women has increased over the years and is currently at 81years for American women [1]. Menopause (MP) is a normal physiologic event reflecting the loss of ovarian follicle function, decreasing ovarian hormone production, and permanent cessation of menstrual cycles. The average age of natural MP in industrialized countries is around 52 years. Hence, women spend about a third of their lives in MP. Quality of life (QoL) is very important in women as they age.
Coronary Vascular Disease (CVD) is the most common cause of death in post-menopausal women worldwide, even more than cases of breast or other gynecologic cancer deaths. Traditional CVD risk factors include age, smoking, sedentary lifestyle, poor diet, body mass index, hypertension, diabetes mellitus, dyslipidemia, and family history of premature CVD.
There are some sex differences in the incidence of cardiovascular disease (CVD), with women having a lower incidence of CVD before menopause (MP) compared to age- matched men. Pre-menopausal women have a low prevalence of CAD, likely due to the protective effects of estrogens in females [2, 3]. There is a marked increase in CAD in women after MP, typically seen about 10 years post MP [3, 4].
It is unlikely that MP per se leads to this change, but it is more likely that increasing adverse risk factors like dyslipidemia, insulin resistance, fat redistribution, high blood pressure after MP causing metabolic and vascular changes contributes to the accelerated risk for CAD and cardiovascular disease (CVD). These may be related to adverse peripheral artery endothelial function. The increased CVD risk is thought to begin in the menopausal transition period.
This review attempts to discuss the role of cardiovascular factors that contribute to the increased CVD risk, which begins with MP transition and into post-MP years. CVD risks increase at reproductive aging due to premature MP before age 40 years, early surgical MP by bilateral oophorectomy, or at natural MP.
2. VASCULAR AGING AND CORONARY ENDOTHELIAL FUNCTION
The progressive stiffening of the arteries with a decline in the ability of the vessels to dilate is called vascular aging. It progresses differently in men and women. At the onset of MP, there is accelerated vascular aging, which is different from the gradual loss of vascular function seen with chronologic aging. Vascular aging and endothelial dysfunction contribute to the development of CVD with MP. Vascular aging contributes to the development of hypertension and atherosclerosis [5]. Endothelial dysfunction is a contributor to vascular aging and a key initiator in the development of atherosclerosis [6].
Estrogen is crucial to maintain normal endothelial function. Estrogen increases the synthesis of nitric oxide (NO) by vascular endothelium, which then diffuses into vascular smooth muscle cells, causing its relaxation. This is called Endothelium-dependent vasodilation (EDV). Impaired EDV is a hallmark feature of endothelial dysfunction. Estrogen preserves endothelial function, and declining ovarian hormones with reproductive aging and MP rapidly affects EDV. MP has no effect on endothelial- independent smooth muscle function [7]. Estrogen decreases the synthesis of Endothelin-1 (a potent vasoconstrictor) by the endothelial cells. Hence in MP transition, there is a decrease in EDV and increased synthesis of endothelin 1, both promoting vasoconstriction. Studies have shown that estrogen has antioxidant and anti-inflammatory properties. Estrogen deficiency upregulates oxidative stress or systemic inflammation leading to decreased endothelial function [8]. Thus, estrogen has multiple effects like increasing NO synthesis, antioxidant and anti-inflammatory properties; its deficiency in MP causes endothelial dysfunction [9].
Endothelial dysfunction precedes the development of CAD (obstructive or non-obstructive). In women, endothelial dysfunction and its associated metabolic implications are of greater relevance (than obstructive CAD) in the development of CVD in women. The endothelium of blood vessels synthesizes and secretes substances involved in vasodilation and vasoconstriction. Hence, coronary endothelial function (CEF) serves as a marker for the vascular health of the coronary arteries. It is to be noted that the coronary and systemic vascular beds differ in their biology. Measures of endothelial function in these two vascular beds do not show any strong relations [10, 11]. It is known that normal CEF is affected by traditional and non-traditional CVD risk factors and may respond favorably to modification of these risk factors [12, 13]. Impaired function of coronary endothelium is an early mechanism that plays a role in the development of atherosclerosis and can predict CVD events [14-18]. So far, assessments of CEF was done by invasive methods, thus limiting its use to low risk populations.
Arterial blood flow generates a frictional force per unit area on the endothelium of the vessel wall and is known as shear stress. Disturbed shear patterns act as weak stimuli to release vasoactive substances, cause endothelial inflammation and adhesion of leukocytes to the endothelium. This acts as a proatherogenic environment and can lead to increased production of superoxide radicals and contributes to endothelial dysfunction.
Recently, non-invasive methods to assess CEF with coronary MRI have been developed, and this enables the test to be more widely used [19]. In this technique, the changes in the lumen of coronary arteries (the cross-sectional coronary artery area CSA) and changes in blood flow (coronary blood flow CBF) are measured in response to isometric handgrip exercise (IHE), which is an endothelial-dependent stressor. These luminal and flow changes are dependent on coronary vasodilation caused by endothelial nitric oxide (NO), and thus is a marker for CEF. The changes in CSA with IHE reflect macrovascular endothelial reactivity, while changes in CBF are reflective of both macro and microvascular reactivity. Mathews et al., in the above study, concluded that there are sex differences in CEF, and pre-MP women have better CEF compared to post MP women. The peri MP may be associated with the onset of rapid adverse changes in CEF and MP accelerates the CV risks [20].
3. CHANGES IN LIPID PROFILE
Changes in the lipid profile of women are noted to start around the periMP years, with increases in total cholesterol (TC), LDL cholesterol, triglycerides (TD). The Study of Women’s Health Across the Nation (SWAN) study was a prospective study of MP transition in Caucasian and minority women (African American, Hispanic, Japanese, Chinese) not on hormone therapy. It provided evidence that MP transition is linked to adverse lipid profiles. It showed that TC, LDL and apolipoprotein- B all increase in the 1-year interval around the final menstrual period- independent of the age at which that occurs. All of these are linked to endothelial dysfunction and lead to atherosclerosis. An increase in LDL in the peri MP period is linked to carotid plaques post MP [21, 22]. These changes are distinct from the linear changes in chronologic aging.
The trend of HDL cholesterol or its presumed cardioprotective role is inconsistent over the MP transition. In young women, high HDL cholesterol plays an independent cardioprotective role. This may be because the HDL particles can promote cholesterol efflux, which is a means for HDL to remove cholesterol from peripheral cells [23, 24]. In peri and post MP women, high HDL cholesterol may be linked to higher CVD risk. Carotid intima-media thickness (cIMT) is a marker for vascular health and remodeling. In post MP women with higher HDL levels, the cIMT is greater. This may be due to changes in the quality of the HDL particles over the MP transition.
4. FAT DEPOTS OF HEART
Epicardial adipose tissue (EAT) directly covers the heart between the myocardium and visceral pericardium. Paracardial adipose tissue (PAT) located anterior to the EAT, outside the parietal pericardium, EAT and PAT are now recognized as novel coronary heart disease risk factors [25]. These fat depots may have a more adverse effect than visceral fat due to the proximity to the heart [26]. In the SWAN cardiovascular fat ancillary studies, late periMP/post-menopausal women had 9.9% more EAT and 20.7% more PAT than premenopausal women [28]. Thus, PAT may be a specific MP specific CHD risk marker [26].
5. SUBCLINICAL CVD
Markers like cIMT, coronary artery calcification (CAC), which is a marker of atherosclerotic plaques, aortic calcification, measures of vascular stiffness like aortic pulse wave velocity or flow-mediated dilation (suggestive of endothelial function) are suggestive of subclinical CVD. These can predict CV events. Late periMP, when dyslipidemia and metabolic syndrome worsen in women, is characterized by increased cIMT [27]. There also seems to be a link between the risk of endothelial dysfunction, and MP transition [28].
6. PREVALENCE OF METABOLIC SYNDROME
Metabolic syndrome is defined as a coexistence of several metabolic risk factors like hypertension, dyslipidemia, impaired glucose tolerance and central adiposity.
Estrogen plays an important part in fat storage and fat distribution. Before menopause, the fat is deposited in thighs, buttocks and hips. Women tend to gain weight (total body fat) during midlife and beyond as a function of chronologic aging. However, when women go through the MP transition, there is a change in the body composition (fat: lean body mass) as well as the distribution of fat. Many women in the MP transition and in post-MP complain of gaining weight in the midsection (android appearance) and trouble with losing weight despite maintaining a healthy lifestyle [29]. MP transition may thus contribute to an increase in visceral (abdominal) fat, insulin resistance, diabetes and inflammatory diseases, leading to the development or worsening of metabolic syndrome in women [29-33].
7. OXIDATIVE STRESS AND MP
Oxidative stress and aging go hand in hand. Overproduction of free radicals such as reactive oxygen species (ROS), and decreased antioxidant levels can lead to atherosclerosis [34]. This decline, combined with a gradual loss of estrogen in the female reproductive system, is highly associated with the various sequelae of MP such as heart disease and vasomotor disturbances in addition to non-cardiac effects such as osteoporosis. Estrogens exert an antioxidant effect at high concentrations by inhibiting the 8-hydroxylation of guanine DNA bases. Paradoxically at low concentrations, estrogen becomes pro-oxidative. Estrogens have been implicated in DNA adduct production as well as oxidation of bases via ROS formation [34]. Oxidative stress increases inflammatory cytokines and pro-oxidants such as glutathione, 4-hydroxynenal, and malonaldehyde [35], which in turn contribute to further pathology secondary to augmented inflammation.
Oxidative stress increases levels of oxidized LDL [35, 36]. Increased expression of AT1, the angiotensin receptor -1, leads to endothelial dysfunction and secondary increased vasoconstriction observed in atherosclerosis [37]. Decreased levels of nitric oxide play an important role in increased smooth muscle proliferation, inflammation, and atherogenic effects on the vasculature [38]. Nitric oxide exerts cardioprotective effects via inhibition of smooth muscle propagation [38]. Oxidative stress propagates vasomotor disturbances in MP such as hot flashes and night sweats particularly. Such vasomotor disturbances result in persistent increases in metabolism, leading to imbalances in pro-oxidants and antioxidants [38].
Disruption of the nitric oxide pathway in MP leads to endothelial dysfunction. The exact mechanisms still remain to be elucidated. Asymmetric dimethylarginine (ADMA) is an endogenous methylated arginine which competitively inhibits nitric oxide (NO) synthesis by competing with L –arginine, the substrate of NO. The competitive inhibition by ADMA leads to a decrease in NO production, translating into endothelial dysfunction contributing to atherosclerosis. ADMA has been implicated as an independent risk factor for cardiovascular disease. Hormone therapy (HT) lowers ADMA concentrations in healthy post-menopausal women. The effect of estrogens on ADMA levels, although small, is considered significant, as the physiological variation of ADMA is limited. Larger randomized trials are necessary to establish that estrogens significantly lower ADMA levels [39]. Current literature reports show that L- arginine appears to be decreased in MP transition. The ratio of L-arginine to L-arginine metabolism biomarker called citrulline, NG-mono-methyl-ւ-arginine [L-NMMA] was also decreased. The decreased ratio showed a significant positive correlation with flow-mediated vasodilation of the brachial artery. These findings could suggest a role for L-arginine deficiency in endothelial dysfunction noted in MP transition [40].
8. ESTROGEN DECLINE AND CEREBROVASCULAR DISEASE
Estrogens decrease vascular tone and therefore increase blood flow in the cerebrovascular system, while androgens increase tone. Increased angiogenesis is another function of estrogens and androgens. Inflammation and oxidative stress are reduced by estrogen and therefore exert a neuroprotective effect by preserving the blood-brain barrier and reduce oxidative stress. In the presence of cardiovascular comorbidities, MP changes in hormone levels contribute to cerebrovascular dysfunction and may influence adverse cognitive effects. Further research is needed in this area to elucidate pathophysiology, which in turn could lead to therapeutic developments [41, 42]. Estradiol or E2 declines rapidly over the menopausal transition, which influences cognition, mood and sleep [43]. Further studies are needed to elucidate the effect of E2 definitively in the human brain.
9. MANAGEMENT OF CARDIOVASCULAR SYMPTOMS IN MP
VMS (vasomotor symptom) is the major symptom in MP. Narrowing of the thermoneutral zone so that slight changes in core body temperature brings on compensatory flushing and sweating, leading to hot flashes and night sweats. VMS has been linked to CV risks [44, 45]. VMS also contributes to poor sleep quality, irritability, difficulty in concentration and overall reduced QoL [46]. Lifestyle changes, non-hormonal medications and systemic hormone therapy may be recommended for the management of VMS. The following discussion on the management of VMS and genitourinary syndrome of MP is based on the 2017 position statement from the North American MP Society [47].
Hormone therapy (HT) is not recommended for the primary prevention of any condition- like to preserve cardiovascular health, prevent osteoporosis, prevent memory loss, etc. However, the primary indication for HT is for the management of moderate to severe VMS. Hormone therapy is the gold standard for the relief of VMS. This may include the use of estrogen alone (Estrogen therapy ET) in women who have had a hysterectomy or estrogen and progesterone therapy (EPT) in women who still have their uterus. Women with a uterus need endometrial protection (against endometrial neoplasia) and are provided by either progestogens or the SERM bazedoxifene. Management of VMS requires the use of systemic hormones that may be given with different routes of administration, including oral (PO) and transdermal (T/D). In general, it is recommended to use the lowest dose of hormones needed for symptom relief for the shortest period of time needed. The type, dose, regimen and duration of use of HT should be individualized.
The decision to offer HT to a menopausal woman for management of VMS requires careful consideration of individual risks and benefits. Based on the Timing Hypothesis, the benefits of HT outweigh the risks in most healthy PM women under the age 60 years or less than 10 years from the final menstrual period. The Women’s Health Initiative (WHI) showed an increased risk of breast cancer with 3-5 years of EPT while 7 years of ET alone did not show the increased risk for breast cancer. It is to be noted that systemic HT is contraindicated in breast cancer survivors.
Lower doses of HT are associated with a lower risk for venous thromboembolism (VTE), less unscheduled vaginal bleeding and less breast tenderness [48, 49]. Lower doses of HT may take 6-8 weeks to provide symptom relief. The formulations of estrogen may include: Oral conjugated equine estrogen (CEE) 0.3mg, oral 17 beta-estradiol 0.5 mg, estradiol patch 0.025mg. If progestogens are indicated for the patient, it may be in the form of oral medroxyprogesterone acetate (MPA), oral progesterone.
The use of progestogens (natural progesterone or synthetic progestogens) alone is a treatment option for VMS; however, they are not as effective as estrogen therapy and have limited long-term safety data. The concern with long- term use is the risk for breast pathology.
Formulations of progestogens include oral MPA 10 mg/day, oral megestrol acetate 20mg or micronized progesterone 300 mg nightly [50-53].
The combination of a SERM (Selective Estrogen Receptor Modulator) called Bazedoxifene with (CEE) is Tissue Selective Estrogen Complex (TSEC) that is FDA approved for management of VMS in women with a uterus. It has the additional benefit of the prevention of osteoporosis. Here, bazedoxifene offers endometrial protection. Hence additional progestogen is not indicated.
The government approved Bioidentical Hormone Therapy (BHT), i.e., hormones similar to endogenous hormones include formulations of estradiol, estrone and micronized progesterone are monitored for their purity, safety and efficacy. The “Compounded BHT” that are marketed as BHT is not approved by the FDA. There are unique concerns here, esp. of safety. These are usually prepared by a pharmacist in a compounding pharmacy based on the provider’s prescription. The compounded BHT may combine many hormones (estrone, estradiol, estriol, DHEA, testosterone and progesterone). Hence the purity, efficacy or safety of ingredients cannot be relied on. The concentration of hormones in these formulations is also uncertain as is its bioavailability. Hence there is a potential for overdosing or underdosing. Compounded BHT usually does not outline its risks. They may contain unapproved combinations of medications and may be used for untested routes of administration, including hormone pellets, troches or subdermal implants [54-56]. Compounded BHT has minimal government regulation and monitoring. They present safety concerns due to potential over or under dosing, presence of potential impurities, unknown sterility of ingredients, lack of safety /efficacy data and label outlining the risks of use.
Compounded BHT should only be considered if patients cannot tolerate the FDA-approved hormones due to issues like an allergy to components or if there is a lack of a dose or formulation of the FDA-approved hormones.
10. MANAGEMENT OF GENITOURINARY SYNDROME OF MP
Genitourinary syndrome of MP (GSM) is a collection of symptoms caused by estrogen deficiency that includes changes in the clitoris, labia, vestibule, vagina, urethra and bladder. Patients may report genital, vaginal dryness, burning, itching, irritation-urinary symptoms of urinary urgency, frequency, dysuria and frequent UTIs. Sexual symptoms include secondary dyspareunia from vaginal dryness. Unlike VMS that improves over the course of time, GSM does not improve with time but instead gets progressively worse over time. Vaginal lubricants and moisturizers may be tried initially for symptom relief. ET is the most effective treatment for GSM [57, 58].
Low-dose vaginal ET is generally safe and effective for the treatment of GSM [59, 60]. Topical ET may be delivered as tablets, suppositories, ring, or creams. The formulation may be estradiol or CEE. Topical ET usually has minimal systemic absorption. It is to be noted that with topical ET, it is not required to use progestogens for endometrial protection, even in women who have a uterus. There are no safety data for topical ET beyond 1 year of use. In women with a history of breast cancer, topical ET for GSM should be prescribed after consulting the patient’s oncologist. In women on aromatase inhibitors, even topical ET is of concern [61, 62].
HT does not have FDA approval for treating any urinary health issues. However, studies show that vaginal ET can improve urge urinary incontinence, overactive bladder and recurrent UTIs. This may possibly due to increased vascularity around the urethra and bladder neck and promoting relaxation of the detrusor muscle. However, systemic HT does not improve urinary incontinence and may actually worsen stress urinary incontinence [63, 64].
Ospemifene is a SERM that is FDA approved for the relief of moderate to severe dyspareunia associated with vulvovaginal atrophy [65]. Being a SERM, it has a class effect of increasing venous thromboembolism. 1-year follow-up studies have not shown an increase in endometrial hyperplasia or cancer [66]. Intravaginal DHEA is also FDA approved for the relief of GSM and dyspareunia in post-menopausal women. DHEA is a precursor hormone that gets converted into estrogen and testosterone in the vagina [67]. Since it is not estrogen when used, the label does not carry the warnings associated with estrogens.
CONCLUSION
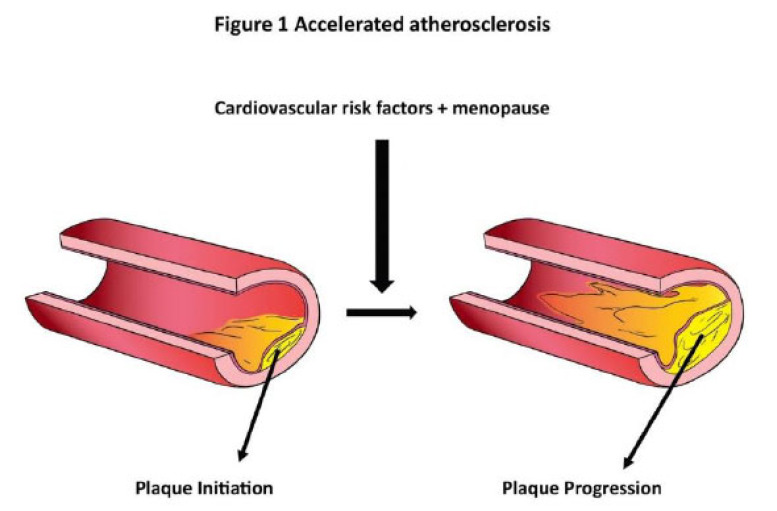
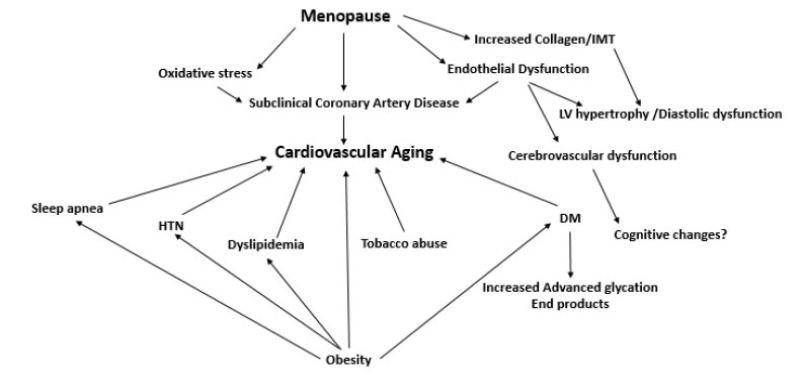
Accelerated atherosclerosis (Fig. 1) appears to be the end result of a complex interaction between cardiovascular risk factors and their accentuation during the perimenopausal period. Additionally, complex interactions between oxidative stress and levels of L-arginine and ADMA may also influence endothelial dysfunction in menopause. The increased cardiovascular risk in MP stems from the exaggerated effects of changing physiology on the cardiovascular system, which affects vasculature in the peripheral, cardiac and cerebrovascular systems [43, 68]. Changes in lipid profile, vascular stiffness, metabolic parameters, advanced glycation end products (AGE) and oxidative stress (Fig. 2) all contribute to worsening cardiovascular risk in women in the perimenopausal period [68-71]. Treatment strategies should include tight control of cardiovascular risk factors to prevent accelerated cardiovascular disease in menopausal women.
Fig. (1).
Shows the possible impact of cardiovascular risk factors and menopause on the progression of atherosclerosis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
Shows the accentuation of cardiovascular risk factors in the setting of menopause.
FUTURE DIRECTIONS
Dynamic changes in estradiol and follicle-stimulating hormone levels that lead to vasomotor symptoms should be monitored and treated effectively. Research into developing metabolic markers should be augmented so that possible prophylactic therapeutics can be developed to combat the metabolic syndrome that is accentuated during MP. The female gender-specific aspects and drug therapy in women need more research investigations. Tight regulation of inflammation influences a balanced immune response. The nuclear factor erythroid 2-like 2 (Nrf2) and its role in the inflammation from the standpoint of the development of therapeutics need further investigation. The biochemical basis of nrf-2 activation needs to be studied in the human system. Nrf-2 pathway modulation and its role in cardiovascular aging need further exploration [72] and specifically during MP. Another aspect of metabolic change in MP that needs more detailed investigation is the role of particle size of low, intermediate and high- density lipoproteins in influencing cardiovascular risk [73]. Investigating metabolic changes at the cellular and molecular levels using nuclear magnetic resonance or more sophisticated artificial intelligence (AI) techniques to identify diagnostic /therapeutic targets will pave the way for better surveillance and improving the cardiovascular risk profile in women undergoing MP [74]. Though AI has limitations in that it needs large datasets and accessibility to clinical workflow, it is definitely a technique to be utilized in the future.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
All authors have given consent to the publication of this manuscript.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Arias E, Xu J. National Vital Statistics Reports, CDC. United States life tables. 2017. 2019; 68(7): 1-65. [PubMed] [Google Scholar]
- 2.Mosca L., Hammond G., Mochari-Greenberger H., Towfighi A., Albert M.A. American Heart Association Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on High Bloo. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation. 2013;127(11):1254–1263, e1-e29. doi: 10.1161/CIR.0b013e318287cf2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerner D.J., Kannel W.B. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26-year follow-up of the Framingham population. Am. Heart J. 1986;111(2):383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 4.Stampfer M.J., Colditz G.A. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev. Med. 1991;20(1):47–63. doi: 10.1016/0091-7435(91)90006-P. [DOI] [PubMed] [Google Scholar]
- 5.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 6.Rossi R., Nuzzo A., Origliani G., Modena M.G. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J. Am. Coll. Cardiol. 2008;51(10):997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Taddei S., Virdis A., Ghiadoni L., Mattei P., Sudano I., Bernini G., Pinto S., Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28(4):576–582. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 8.Sumino H., Ichikawa S., Kasama S., Takahashi T., Kumakura H., Takayama Y., Kanda T., Kurabayashi M. Different effects of oral conjugated estrogen and transdermal estradiol on arterial stiffness and vascular inflammatory markers in postmenopausal women. Atherosclerosis. 2006;189(2):436–442. doi: 10.1016/j.atherosclerosis.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Stanhewicz A.E., Wenner M.M., Stachenfeld N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018;315(6):H1569–H1588. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iantorno M., Hays A.G., Schär M., Krishnaswamy R., Soleimanifard S., Steinberg A., Stuber M., Gerstenblith G., Weiss R.G. Simultaneous non-invasive assessment of systemic and coronary endothelial function. Circ Cardiovasc Imaging. 2016;9(3):e003954. doi: 10.1161/CIRCIMAGING.115.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson T.J., Uehata A., Gerhard M.D., Meredith I.T., Knab S., Delagrange D., Lieberman E.H., Ganz P., Creager M.A., Yeung A.C., et al. Close relation of endothelial function in the human coronary and peripheral circulations. J. Am. Coll. Cardiol. 1995;26(5):1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 12.Reriani M.K., Lerman L.O., Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomarkers Med. 2010;4(3):351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadi H.A., Carr C.S., Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005;1(3):183–198. [PMC free article] [PubMed] [Google Scholar]
- 14.Reriani M., Sara J.D., Flammer A.J., Gulati R., Li J., Rihal C., Lennon R., Lerman L.O., Lerman A. Coronary endothelial function testing provides superior discrimination compared with standard clinical risk scoring in prediction of cardiovascular events. Coron. Artery Dis. 2016;27(3):213–220. doi: 10.1097/MCA.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(69170):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 16.Schächinger V., Britten M.B., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 17.Suwaidi J.A., Hamasaki S., Higano S.T., Nishimura R.A., Holmes D.R., Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- 18.von Mering G.O., Arant C.B., Wessel T.R., McGorray S.P., Bairey Merz C.N., Sharaf B.L., Smith K.M., Olson M.B., Johnson B.D., Sopko G., Handberg E., Pepine C.J., Kerensky R.A. National Heart, Lung, and Blood Institute. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 19.Mathews L., Iantorno M., Schär M., Bonanno G., Gerstenblith G., Weiss R.G., Hays A.G. Coronary endothelial function is better in healthy premenopausal women than in healthy older postmenopausal women and men. PLoS One. 2017;12(10):e0186448. doi: 10.1371/journal.pone.0186448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews K.A., Kuller L.H., Sutton-Tyrell K., et al. Changes in cardiovascular risk factors during the periMPand post MP and coronary artery atherosclerosis in healthy women. Stroke. 2001;32(5):1104–1111. doi: 10.1161/01.STR.32.5.1104. [DOI] [PubMed] [Google Scholar]
- 21.Matthews K.A., Crawford S.L., Chae C.U., Everson-Rose S.A., Sowers M.F., Sternfeld B., Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J. Am. Coll. Cardiol. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews K.A., El Khoudary S.R., Brooks M.M., Derby C.A., Harlow S.D., Barinas-Mitchell E.J., Thurston R.C. Lipid changes around the final menstrual period predict carotid subclinical disease in postmenopausal women. Stroke. 2017;48(1):70–76. doi: 10.1161/STROKEAHA.116.014743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Khoudary S.R. HDL and the menopause. Curr. Opin. Lipidol. 2017;28(4):328–336. doi: 10.1097/MOL.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 24.El Khoudary S.R., Hutchins P.M., Matthews K.A., Brooks M.M., Orchard T.J., Ronsein G.E., Heinecke J.W. Cholesterol efflux capacity and subclasses of HDL particles in healthy women transitioning through menopause. J. Clin. Endocrinol. Metab. 2016;101(9):3419–3428. doi: 10.1210/jc.2016-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iacobellis G., Gao Y.J., Sharma A.M. Do cardiac and perivascular adipose tissue play a role in atherosclerosis? Curr. Diab. Rep. 2008;8(1):20–24. doi: 10.1007/s11892-008-0005-2. [DOI] [PubMed] [Google Scholar]
- 26.El Khoudary S.R., Shields K.J., Janssen I., Hanley C., Budoff M.J., Barinas-Mitchell E., Everson-Rose S.A., Powell L.H., Matthews K.A. Cardiovascular fat, MP and sex hormones in women: the SWAN cardiovascular fat ancillary study. J. Clin. Endocrinol. Metab. 2015;100(9):3304–3312. doi: 10.1210/JC.2015-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Khoudary S.R., Wildman R.P., Matthews K., Thurston R.C., Bromberger J.T., Sutton-Tyrrell K. Progression rates of carotid intima- media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20(1):8–14. doi: 10.1097/gme.0b013e3182611787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samargandy S., Matthews K., Janssen I., et al. Central arterial stiffness increases within one- year interval of the final menstrual period in midlife women: Study of Women’s Health Across the Nation (SWAN) heart. Circulation. 2018;137:AP362. [Google Scholar]
- 29.Lovejoy J.C., Champagne C.M., de Jonge L., Xie H., Smith S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. J. Obes. 2008;32(6):949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews K.A., Abrams B., Crawford S., Miles T., Neer R., Powell L.H., Wesley D. Body mass index in mid-life women: relative influence of menopause, hormone use, and ethnicity. Int. J. Obes. Relat. Metab. Disord. 2001;25(6):863–873. doi: 10.1038/sj.ijo.0801618. [DOI] [PubMed] [Google Scholar]
- 31.Abdulnour J., Doucet E., Brochu M., Lavoie J.M., Strychar I., Rabasa-Lhoret R., Prud’homme D. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause. 2012;19(7):760–767. doi: 10.1097/gme.0b013e318240f6f3. [DOI] [PubMed] [Google Scholar]
- 32.Witteman J.C., Grobbee D.E., Kok F.J., Hofman A., Valkenburg H.A. Increased risk of atherosclerosis in women after the menopause. BMJ. 1989;298(6674):642–644. doi: 10.1136/bmj.298.6674.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bittner V. Menopause, age, and cardiovascular risk: a complex relationship. J. Am. Coll. Cardiol. 2009;54(25):2374–2375. doi: 10.1016/j.jacc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z., Chandrasena E.R., Yuan Y., Peng K.W., van Breemen R.B., Thatcher G.R., Bolton J.L. Redox cycling of catechol estrogens generating apurinic/apyrimidinic sites and 8-oxo-deoxyguanosine via reactive oxygen species differentiates equine and human estrogens. Chem. Res. Toxicol. 2010;23(8):1365–1373. doi: 10.1021/tx1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Signorelli S.S., Neri S., Sciacchitano S., Pino L.D., Costa M.P., Marchese G., Celotta G., Cassibba N., Pennisi G., Caschetto S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53(1):77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Tchernof A., Calles-Escandon J., Sites C.K., Poehlman E.T. Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coron. Artery Dis. 1998;9(8):503–511. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 37.Arnal J.F., Scarabin P.Y., Trémollières F., Laurell H., Gourdy P. Estrogens in vascular biology and disease: where do we stand today? Curr. Opin. Lipidol. 2007;18(5):554–560. doi: 10.1097/MOL.0b013e3282ef3bca. [DOI] [PubMed] [Google Scholar]
- 38.Kronenberg F. Hot flashes: epidemiology and physiology. Ann. N. Y. Acad. Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 39.Karkanaki A., Vavilis D., Traianos A., Kalogiannidis I., Panidis D. Hormone therapy and asymmetrical dimethylarginine in postmenopausal women. Hormones (Athens) 2010;9(2):127–135. doi: 10.14310/horm.2002.1262. [DOI] [PubMed] [Google Scholar]
- 40.Klawitter J., Hildreth K.L., Christians U., Kohrt W.M., Moreau K.L. A relative L-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol. Rep. 2017;5(17):e13409. doi: 10.14814/phy2.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robison L.S., Gannon O.J., Salinero A.E., Zuloaga K.L. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019;1710:43–60. doi: 10.1016/j.brainres.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Gannon O.J., Robison L.S., Custozzo A.J., Zuloaga K.L. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem. Int. 2019;127:38–55. doi: 10.1016/j.neuint.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Russell J.K., Jones C.K., Newhouse P.A. The role of estrogen in brain and cognitive aging. Neurotherapeutics. 2019;16(3):649–665. doi: 10.1007/s13311-019-00766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurston R.C., Sutton-Tyrrell K., Everson-Rose S.A., Hess R., Matthews K.A. Hot flashes and subclinical cardiovascular disease: findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118(12):1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thurston R.C., Sutton-Tyrrell K., Everson-Rose S.A., Hess R., Powell L.H., Matthews K.A. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18(4):352–358. doi: 10.1097/gme.0b013e3181fa27fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson H.D. Menopause. Lancet. 2008;371(9614):760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 47.The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753. doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 48.Canonico M., Plu-Bureau G., Lowe G.D., Scarabin P.Y. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227–1231. doi: 10.1136/bmj.39555.441944.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ettinger B. Rationale for use of lower estrogen doses for postmenopausal hormone therapy. Maturitas. 2007;57(1):81–84. doi: 10.1016/j.maturitas.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 50.Schiff I., Tulchinsky D., Cramer D., Ryan K.J. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244(13):1443–1445. doi: 10.1001/jama.1980.03310130021021. [DOI] [PubMed] [Google Scholar]
- 51.Hitchcock C.L., Prior J.C. Oral micronized progesterone for vasomotor symptoms-a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19(8):886–893. doi: 10.1097/gme.0b013e318247f07a. [DOI] [PubMed] [Google Scholar]
- 52.Prior J.C., Nielsen J.D., Hitchcock C.L., Williams L.A., Vigna Y.M., Dean C.B. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: a 1-year randomized double-blind trial following premenopausal ovariectomy. Clin. Sci. (Lond.) 2007;112(10):517–525. doi: 10.1042/CS20060228. [DOI] [PubMed] [Google Scholar]
- 53.Goodwin J.W., Green S.J., Moinpour C.M., Bearden J.D., III, Giguere J.K., Jiang C.S., Lippman S.M., Martino S., Albain K.S. Phase III randomized placebo-controlled trial of two doses of megestrol acetate as treatment for menopausal symptoms in women with breast cancer: Southwest Oncology Group Study 9626. J. Clin. Oncol. 2008;26(10):1650–1656. doi: 10.1200/JCO.2006.10.6179. [DOI] [PubMed] [Google Scholar]
- 54.Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J. Womens Health (Larchmt.) 2007;16(5):600–631. doi: 10.1089/jwh.2006.0311. [DOI] [PubMed] [Google Scholar]
- 55.Files J.A., Ko M.G., Pruthi S. Bioidentical hormone therapy. Mayo Clin. Proc. 2011;86(7):673–680. doi: 10.4065/mcp.2010.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhavnani B.R., Stanczyk F.Z. Misconception and concerns about bioidentical hormones used for custom-compounded hormone therapy. J. Clin. Endocrinol. Metab. 2012;97(3):756–759. doi: 10.1210/jc.2011-2492. [DOI] [PubMed] [Google Scholar]
- 57.Portman D.J., Glass M.L. Vulvovaginal atrophy terminology consensus conference panel. Genitourinary symptom of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American MP Society. Menopause. 2014;21:1063–1068. doi: 10.1097/GME.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 58.Lethaby A., Ayeleke R.O., Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst. Rev. 2016;8(8):CD001500. doi: 10.1002/14651858.CD001500.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahn D.D., Carberry C., Sanses T.V., Mamik M.M., Ward R.M., Meriwether K.V., Olivera C.K., Abed H., Balk E.M., Murphy M. Society of gynecologic surgeons systematic review group. vaginal estrogen for genitourinary syndrome of menopause: A systematic review. Obstet. Gynecol. 2014;124(6):1147–1156. doi: 10.1097/AOG.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santen R.J. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric. 2015;18(2):121–134. doi: 10.3109/13697137.2014.947254. [DOI] [PubMed] [Google Scholar]
- 61.Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902. doi: 10.1097/GME.0b013e3182a122c2. [DOI] [PubMed] [Google Scholar]
- 62.Farrell R. American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. Farrell R. ACOG Committee Opinion No. 659 summary: the use of vaginal estrogen in a woman with a history of estrogen-dependent breast cancer. Obstet. Gynecol. 2016;127(3):618–619. doi: 10.1097/AOG.0000000000001349. [DOI] [PubMed] [Google Scholar]
- 63.Dueñas-Garcia O.F., Sullivan G., Hall C.D., Flynn M.K., OʼDell K. Pharmacological agents to decrease new episodes of recurrent lower urinary tract infections in postmenopausal women. A systematic review. Female Pelvic Med. Reconstr. Surg. 2016;22(2):63–69. doi: 10.1097/SPV.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 64.Cody J.D., Jacobs M.L., Richardson K., Moehrer B., Hextall A. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst. Rev. 2012;10:CD001405. doi: 10.1002/14651858.CD001405.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Constantine G., Graham S., Portman D.J., Rosen R.C., Kingsberg S.A. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18(2):226–232. doi: 10.3109/13697137.2014.954996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon J., Portman D., Mabey R.G., Jr Ospemifene Study Group. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281. doi: 10.1016/j.maturitas.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Labrie F., Archer D.F., Koltun W., Vachon A., Young D., Frenette L., Portman D., Montesino M., Côté I., Parent J., Lavoie L., Beauregard A., Martel C., Vaillancourt M., Balser J., Moyneur É. VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243–256. doi: 10.1097/GME.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 68.Mozos I., Malainer C., Horbańczuk J., Gug C., Stoian D., Luca C.T., Atanasov A.G. Inflammatory markers for arterial stiffness in cardiovascular diseases. inflammatory markers for arterial stiffness in cardiovascular diseases, front immunol. 2017;8:1058. doi: 10.3389/fimmu.2017.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pertynska-Marczewska M., Diamanti-Kandarakis E. Aging ovary and the role for advanced glycation end products. Menopause. 2017;24(3):345–351. doi: 10.1097/GME.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 70.Pertynska-Marczewska M., Merhi Z. Relationship of advanced glycation end products with cardiovascular disease in menopausal women. Reprod. Sci. 2015;22(7):774–782. doi: 10.1177/1933719114549845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Q., Ferreira D.L.S., Nelson S.M., Sattar N., Ala-Korpela M., Lawlor D.A. Metabolic characterization of menopause: cross-sectional and longitudinal evidence. BMC Med. 2018;16(1):17. doi: 10.1186/s12916-018-1008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nair N., Gongora E. Oxidative stress and cardiovascular aging: interaction between NRF-2 and ADMA. Curr. Cardiol. Rev. 2017;13(3):183–188. doi: 10.2174/1573403X13666170216150955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El Khoudary S.R., Ceponiene I., Samargandy S., et al. High-density lipoprotein metrics and atherosclerotic risk in women: do menopause characteristics matter? MESA Arterioscler Thromb Vasc Biol. 2018;38:2236–2244. doi: 10.1161/ATVBAHA.118.311017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan Y., Zhang J-W., Zang G-Y., Pu J. The primary use of artificial intelligence in cardiovascular diseases: what kind of potential role does artificial intelligence play in future medicine? J. Geriatr. Cardiol. 2019;16(8):585–591. doi: 10.11909/j.issn.1671-5411.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]