Abstract
Aortic dissection is an emergent medical condition, generally affecting the elderly, characterized by a separation of the aortic wall layers and subsequent creation of a pseudolumen that may compress the true aortic lumen. Predisposing factors mediate their risk by either increasing tension on the wall or by causing structural degeneration. They include hypertension, atherosclerosis, and a number of connective tissue diseases. If it goes undetected, aortic dissection carries a significant mortality risk; therefore, a high degree of clinical suspicion and a prompt diagnosis are required to maximize survival chances. Imaging methods, most commonly a CT scan, are essential for diagnosis; however, several studies have also investigated the effect of several biomarkers to aid in the detection of the condition. The choice of intervention varies depending on the type of dissection, with open surgical repair remaining of choice in those with type. In dissections, however, the role of conventional open surgery has considerably diminished in complicated type B dissections, with endovascular repair, a much less invasive technique, proving to be more effective. In uncomplicated type B dissections, where medical choice reigned supreme as the optimal intervention, endovascular repair is being explored as a viable option which may reduce long- term mortality outcomes, although the ideal intervention in this situation is far from settled.
Keywords: Aortic dissection, acute aortic syndrome, endovascular surgery, aortic replacement, cardiovascular emergency, D-dimer
1. INTRODUCTION
Acute Aortic Dissection (AD) is a highly fatal cardiovascular emergency that is defined by the progressive separation of the layers of the aorta by a column of blood as a result of the degeneration of the aortic media [1, 2]. With rapid detection and treatment, the outlook of AD patients is considerably improved; therefore, a flux of new diagnostic modalities has been investigated to aid in the prompt detection of aortic dissection. These diagnostic methods not only include imaging tools to better visualize the location as well as the extent of the dissection, but also different chemical biomarkers that can be used to aid in the diagnostic process.
In addition, there have been several advances in the realm of treatment, with newer, less invasive approaches such as endovascular repair gaining favor over the more traditional choice of open surgical repair, as in the case of complicated type B dissections [3]. In other cases, as in uncomplicated type B dissections, endovascular repair is being trialed in an area where treatment was generally restricted to conservative medical management.
The relevance of this urgent medical condition is also likely to increase over the coming decades with changing population demographics, as it is predominantly a disease of the elderly. Furthermore, traditionally cited incidence and mortality rates may well be underestimates of the true burden of this disease [4].
The aim of this article is to review the epidemiology, etiology, diagnostics, and treatment options for aortic dissection, as well as highlight recent changes which may impact the burden posed by the disease.
2. EPIDEMIOLOGY
Aortic dissection is an uncommon condition with a relatively high mortality rate, making it difficult to ascertain its overall incidence as many patients die before they are diagnosed [2, 5]. Untreated, aortic dissection is a fatal condition, with an estimated mortality rate of 40% on initial presentation; this rate, however, increases by 1% every hour, and can reach an annual mortality rate of up to 90% [6]. The most accurate estimate of incidence comes from population-based studies. Between 1980 and 2015, population-based studies across Europe and North America reported an annual incidence ranging from 2.5 to 15 per 100,000 [4, 5, 7-11].
3. CLASSIFICATION
The anatomic classification of AD is important for accurate diagnosis and management. The two main classification systems categorize AD based on either the site of origin of the intimal tear - DeBakey [12] or the involvement of the ascending aorta - Stanford Classification [13]. The DeBakey classification has the advantage of being more precise in identifying the site of the lesion [2]. Type I dissections involve the ascending aorta and progress towards the aortic arch and descending aorta, type II dissections involve the ascending aorta only, and type III dissections originate in the descending aorta and progress distally [12]. The Stanford Classification categorizes AD into type A, which includes all dissections involving the ascending aorta (DeBakey type I and II), and type B, which includes dissections involving only the descending aorta (DeBakey type III) [13]. In a triage setting, the Stanford classification has the advantage of classifying ADs into cases which require surgical intervention - type A, and those which can be managed conservatively- type B (with the exception of complicated type B) [14].
However, rare cases have been identified to have dissection components in the aortic arch and descending aorta while sparing the ascending aorta [15-17]. This category fits into neither of the traditional classifications. A recent study by Sievers et al. modified the Stanford classification to include this type of dissection under the category of non-A non-B. The study then developed a classification system based on the type, site of entry, and malperfusion of the AD called the TEM system [17]. This novel classification system is comprehensive in that it transcends the traditional anatomical classification by integrating a clinical perspective (malperfusion), thus providing information on both the anatomical and clinical extent of the disease. Further studies, however, are needed to tailor this new system to the decision-making process for the management of ADs.
AD can also be classified according to the time of onset of symptoms into acute, sub-acute, and chronic; however, a standard time-frame for each category has not yet been established [18]. According to previous studies by Hirst et al. and DeBakey et al., the acute period is < 2 weeks between the onset of symptoms and diagnosis, and a period > 2 weeks was considered chronic [19, 20]. A recent analysis of the IRAD put forward a new classification system based on patient survival rates, with the Kaplan-Meier curve inflection points being used to define the temporal cut off points as follows: the hyper-acute period (< 24 hours), acute period (2 days to 1 week), sub-acute period (8 days to 1 month), and the chronic period (> 1 month) [21]. This temporal classification plays an important role in refining the assessment of patient survival chances, which in turn can help direct the management of each case [17, 20].
4. THE ROLE OF MECHANICAL FACTORS AND DEGENERATIVE CHANGES IN THE PATHOGENESIS OF AORTIC DISSECTION
The core physiopathological principle underlying AD is an increase in pressure leading to the separation of the layers of the media which creates a false lumen within the aortic wall. There are two main factors related to its development: structural weakness of the aortic wall, and increased wall tension. Many connective tissue components are implicated as culprits in the pathogenesis of AD, and several connective tissue diseases such as Marfan and Ehler-Danlos syndromes are important predisposing factors. Firstly, damaged interlaminar elastic fibers weaken the structural integrity of the aortic media [22]. Secondly, Fibrillin, a glycoprotein that plays a role in organizing elastic fibers by forming scaffolds around elastin, may also play a role [23]. The degeneration of the aortic media is also facilitated/accelerated by Medin, a fibril protein which forms oligomers that damage the aortic wall, via two mechanisms: Cytotoxicity of smooth muscle cells, and increased induction of matrix metalloproteinases (MMP) [24]. The strong association between hypertension and AD highlights the role of wall tension in the pathology of the disease. Hypertension, the most commonly associated condition with AD, illustrates the importance of wall tension; however, most hypertensive patients do not have dissections, thus illustrating the importance of degenerative changes - to which hypertension itself may be a contributor [25].
In addition to hypertension, the role of other biomechanical factors must not be overlooked. For instance, the motion of the aortic root during systole can significantly increase the longitudinal stress placed upon the aortic wall, with the point of maximal increase closely corresponding to the most common location of type A dissection [26]. The geometric properties of the wall itself also play an important role in dissection risk. For instance, the diameter of the aorta is positively correlated with dissection risk and has been used as a marker for surgical intervention [27]. Additionally, the thickness of the wall itself may predispose it to dissection, with studies showing a thinner tunica media amongst AD patients [28, 29].
It is important to note that it is the complex interaction between the different factors -rather than an individual factor in and of itself- that provides a satisfactory explanation for the onset of AD; for instance, the aortic diameter alone, long used as a marker of dissection risk, may be insufficiently predictive of said risk [30, 31].
Two other entities closely related to AD are aortic Intramural Hematomas (IMH) and penetrating atherosclerotic aortic ulcers (PAU). IMH may be due to rupture of the vasa vasorum leading to an accumulation of blood within the medial layer of the aorta with a usual lack of an intimal tear, although it may progress to full AD in a number of cases [32]. In a PAU, an aortic plaque ruptures into the underlying media, creating an ulcer within the aortic wall, and possibly an IMH [18]. In contrast to classical acute AD, which usually involves the first few centimeters of the ascending aorta, these subtypes most commonly affect the descending portion [32, 33].
5. DEMOGRAPHICS & RISK FACTORS
According to the IRAD, type A dissections make up almost two-thirds of the series population (67%), with the remaining third being type B (33%) [34]. Most population-based studies were in line with this distribution (type A being the most common) [4, 5, 9, 11]; however, McClure et al. and Fusako et al. reported a higher incidence of type B (61% and 54.5% respectively) [10, 35], suggesting that the population demographics of different countries could play a role in influencing the relative distributions of each type.
5.1. Sex & Age
Most studies agree that the incidence of ADs is higher in men, with a male to female ratio of almost 2:1 [36-38]. Dissections most commonly occur in the elderly with a mean age of 63 years [34]. According to the IRAD, women diagnosed with an AD are generally older than men (mean of 67 and 63 respectively) and tend to have a worse prognosis as an atypical presentation, and by extension, a delayed diagnosis is more common [34, 39].
5.2. Risk Factors
Hypertension poses the greatest risk for AD, with one study reporting a hazard ratio of 2.64 [9]. It is also the most commonly occurring risk factor, observed in more than 55% of patients in population-based studies [4, 10, 11, 38, 40], and 76% of patients in the IRAD series [34]. Whether hypertension is more strongly associated with a specific type of dissection is still unclear as studies on different populations report conflicting results [10, 41]. Smoking and dyslipidemia (low ApoA1), were also found to be significant risk factors amongst several studies, both of which pose double the risk for AD [9].
5.3. Predisposing Conditions
Several predisposing medical conditions are associated with AD, including genetic disorders, inflammatory vasculitides, as well as other conditions such as pregnancy, trauma, history of cardiac surgery, stimulant abuse, and infection.
5.3.1. Connective Tissue Disorders
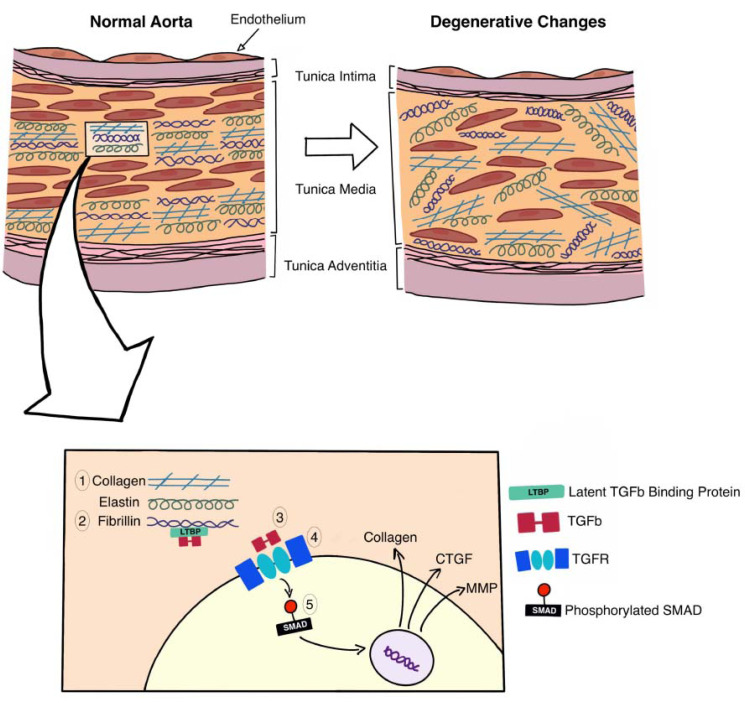
Connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome (LDS), and type IV Ehlers-Danlos Syndrome (EDS) are well known predisposing factors for both aortic aneurysms and dissections Fig. (1) [1, 2, 39].
Fig. (1).
Cross-section of the wall of the aorta, showing the role of various genes (associated with connective tissue disorders) in maintaining the integrity of the extracellular matrix. The wall of the aorta consists of three layers (intima, media, adventitia), genetic mutations associated with connective tissue disorders target the distorted remodeling of the aortic media, weakening the wall, thus leaving the patient susceptible to developing an aneurysm and/or dissection. The aortic media is composed of concentric layers of smooth muscle cells, within a dense Extracellular Matrix (ECM). The ECM consists of organized layers of elastin, fibrillin, and collagen, any derangement in the structure of these fibres leads to disorganization of the ECM and consequent weakening of the aorta. COL3A1 (1) encodes the alpha-1 procollagen chains, which are responsible for the formation of type III collagen, it is the gene defect found in Ehlers-Danlos type IV. The mutation responsible for this disease leads to an increase in the friability of the collagen molecules [47]. Fibrillin (2) is a structural protein which plays a major role in the sequestration and consequent regulation of the TGF-β (3) signaling pathway, the mutated form of fibrillin is found in Marfan Syndrome due to a mutation in the FBN1 gene [48]. The TGF-β signaling pathway plays a major role in connective tissue growth and maintenance of the ECM. TGF-β is released into the ECM as an inactive dimer bound to latent TGF-β binding protein (LTBP). In the ECM, the TGF-β/LTBP complex is sequestered by fibrillin; further interactions with other ECM components, e.g., integrins lead to the activation of the TGF-β. Once activated TGF-β binds to the heteromeric TGF-receptor complex (4), which then leads to the phosphorylation of SMAD proteins, a group of intracellular signal transducers [49]. The phosphorylated SMAD (5) are transported to the nucleus where they act as a transcription factor involved in the expression of proteins such as collagen, Connective Tissue Growth Factor (CTGF) [50], and matrix metalloproteinase (MMP) [51, 52]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Marfan syndrome is an autosomal dominant disorder caused by a mutation in the FBN1 gene which encodes fibrillin-1, a protein responsible for the structural integrity of the extracellular matrix, and the regulation of TGFβ. The underlying mechanism which leads to the development of an AD in Marfan syndrome is the upregulation of TGFβ and the subsequent release of matrix metalloproteinases and collagen growth factors [42]. According to the IRAD, around 5% of those with an AD have Marfan syndrome [34]. These patients present at a younger age (< 40 years old) and are more likely to present with larger diameter type A dissections [34, 43-45].
LDS has 6 subtypes classified according to the affected gene. Similar to Marfan syndrome, mutations in said genes are associated with the augmentation of the TGFβ signaling pathway [42]. LDS types 1 and 2 are caused by mutations in TGFBR1 and TGFBR2 genes, respectively, which encode for the TGF-β receptor subtypes [42, 45]. Moreover, the genetic mutations in types 4 and 5 - TGFB2 and TGFB3 encode the cytokines involved in the pathway, whereas the genes affected in types 3 and 6 (SMAD3 and SMAD2) encode the primary set of intracellular effectors in the signaling pathway [42, 45]. Around 98% of patients with LDS have aortic root aneurysms, making them highly susceptible to the development of an AD [1]; additionally, Loeys et al. reported that AD was responsible for 89% of deaths in a cohort of 90 LDS patients [46].
Type IV Ehlers-Danlos Syndrome, the vascular variant of EDS (vEDS), is characterized by vascular fragility leading to rupture and hemorrhage [42]. It is caused by a mutation in the COL3A1 gene which encodes type III collagen, a structural protein in the extracellular matrix. AD and dissecting aneurysms comprise around 48% of vascular complications associated with vEDS [47-52].
5.3.2. Bicuspid Aortic Valve
Another cause of AD at a young age is a Bicuspid Aortic Valve (BAV). As the most common congenital heart anomaly, BAV is responsible for the majority of morbidity and mortality attributed to congenital heart defects [53]. Patients with a BAV are reported to be eight times as likely to suffer an AD as the general population [54] and, similar to Marfan syndrome, those with a BAV tend to have a larger dissection [34].
5.3.3. Non-syndromic Familial Thoracic Aortic Aneurysm and Dissection
Non-syndromic Familial Thoracic Aortic Aneurysm and Dissection (FTAAD) is a group of inherited gene mutations that result in an increased risk of aortic aneurysms and dissections. Around 30% of patients with FTAAD harbor one of the 37 genes associated with the development of an aneurysm or dissection [55]. Similar to some connective tissue disorders, the mutated genes in FTAAD are associated with the TGFβ signaling pathway or the smooth muscle contraction mechanism [39].
6. COMPLICATIONS OF AORTIC DISSECTIONS
Possible complications of AD can be classified based on the affected organ system. Most complications result from malperfusion due to the redirection of blood, which if not reversed, and can lead to end-organ failure.
6.1. Cardiovascular Complications
Cardiac complications are the most frequently observed complications in AD [39]. Due to the close anatomical relationship between the ascending aorta and the heart, cardiac complications are more common in type A dissections [56].
Acute aortic regurgitation is the most frequently observed complication in type A dissections. It affects 40%-75% of patients and is the second most common cause of death due to AD. The severity of the regurgitation depends on the size of the AD and ranges from a subtle diastolic murmur to congestive heart failure and cardiogenic shock [1, 39, 56].
Compression of the coronary ostium by the expanded false lumen leads to various degrees of myocardial ischemia and infarction, it occurs in 10%-15% of patients with type A dissections [57]. Ischemia or infarction caused by dissection is almost identical to their primary counterparts both clinically and on electrocardiography (ECG); as a result, an AD is often misdiagnosed and/or incorrectly treated, which in turn, increases the fatality of the condition. Perhaps the most imminently threatening complication is cardiac tamponade. Around 33% of patients present with hemodynamically stable effusion due to the transudation of fluid from the false lumen into the pericardial cavity [1]; however, 8% to 10% are diagnosed with cardiac tamponade, which doubles their risk of mortality [58].
Congestive heart failure (CHF) is an uncommon but debilitating complication. It mainly occurs in type A AD and is usually a result of aortic regurgitation. In cases where regurgitation is not the primary lesion, other aetiologies have been suggested such as myocardial ischemia, uncontrolled hypertension, and pre-existing cardiomyopathy [39]. According to the IRAD, patients with acute heart failure are often diagnosed late as they do not present with the characteristic chest pain associated with dissection, and thus only seek medical attention when in the stage of cardiovascular shock [39, 59].
One vascular complication that has been sporadically reported in the literature is the Superior Vena Cava (SVC) syndrome, which may present as upper limb venous distension and facial swelling [60]. Importantly, some of these cases have been associated with an absence of chest pain [61, 62], a characteristic sign of aortic dissection. In addition, the most common cause of SVC syndrome is lung cancer, which shares smoking as a risk factor with aortic dissection; therefore, cases of aortic dissection presenting with SVC syndrome can be particularly challenging to diagnose [63].
6.2. Neurological Complications
Neurological complications occur equally among patients with type A and B dissections. They are often a result of malperfusion secondary to hypotension, nerve compression by the false lumen, or thromboembolic events. Minor neurologic events are common and occur in 40% of type A dissections [39]; while more serious complications such as stroke or spinal cord ischemia occur less frequently in around 10% and 1% of patients respectively [57].
6.3. Gastrointestinal Complications
Mesenteric ischemia is a serious complication of AD. It occurs in less than 5% of patients and is often misdiagnosed due to the ambiguous nature of abdominal pain. Di Eusanio et al. showed that patients with mesenteric ischemia are almost three times as likely to die while hospitalized compared to those without [64].
7. MANAGEMENT
7.1. Diagnosis & Screening of Aortic Dissections
7.1.1. Clinical Symptomatology
Aortic dissection presents with a wide array of symptoms, all of which depend on the underlying physiopathological derangement. The most common presentation of an AD is the characteristic of acute severe pain in the chest, back, or abdomen. This type of pain is uniquely characterized by being abrupt and of a tearing or stabbing nature [39, 56]. Chest pain is commonly reported in 80% of patients; however, it is a less common finding in type B dissections, which can often present with pain localized to the back or abdomen [34, 39]. Pain due to type B AD is often described as being of a migrating quality, suggesting that it follows the extent of the dissection [6]. Sensitivity and specificity analysis of acute chest pain revealed a negative predictive value of 99%, with a sensitivity of 82.9% and specificity of 70.7% [65], suggesting that it is very likely for a patient with an AD to present with acute chest pain. Those who do not present with this classic symptom are often the cases complicated by stroke, congestive heart failure, or syncope [1].
Although chest pain is the typical presentation of most AD, it can also be associated with numerous conditions such as Myocardial Infarction (MI), acute pericarditis, and pulmonary emboli. Different investigations such as electrocardiographs (ECG), Chest X-Rays (CXR), and Computed Tomography (CT) scans can be used to differentiate these etiologies.
In addition to pain, around 15% of patients with type A dissections will also present with syncope. The underlying causes of syncope are usually fatal such as cardiac tamponade and aortic rupture; consequently, this symptom is associated with increased in-hospital mortality and signals the need for immediate intervention [39, 58, 66].
Other clinical symptoms include pulse deficits and symptoms of end-organ ischemia. Though pulse deficits are an uncommon finding, they are highly suggestive of an AD, with one study reporting a positive likelihood ratio of 5.7 [6]. Acute symptoms related to end-organ ischemia are uncommon, they may include acute paraplegia, lower limb pain, decreased urine output, and reflex hypertension [39].
7.2. ECG
Though ECG findings often present within the first 12 hours of admission, they are of limited use in the definitive diagnosis of an AD [67]. An ECG is routinely used to exclude a myocardial infarction; however, data from the IRAD suggests that almost 19% of patients with an AD presented with ECG changes suggestive of myocardial ischemia, and 7% of patients had a coexisting MI [1, 34]. Other findings such as ventricular hypertrophy, Q wave abnormalities, and S-T changes in AD are nonspecific, and merely suggest the presence of a cardiac abnormality [67]. The ECG does, however, play an important role in assessing the prognosis of patients with AD. In a multivariate analysis conducted by Kimura et al. ST-abnormalities (elevation, depression, or negative T-waves) were independently associated with increased in-hospital mortality [68].
7.3. Diagnostic Imaging Studies
Imaging studies are the mainstay for the diagnosis of aortic diseases, as they provide valuable information about the site, size, shape, and extent of the vessel’s pathology. Regarding AD, imaging techniques can assess the characteristics and extent of the dissection as well as identify any complications such as regurgitation, affected aortic branches, and the presence of thrombi. There are a number of viable imaging techniques that can be used when dealing with a suspected case of AD; however, the different degrees of specificity and sensitivity Table (1), combined with the emergent nature of the condition, make the treating physician’s selection of the proper imaging modality a crucial decision [69-82].
Table 1.
The diagnostic accuracy of different AD testing modalities.
| Study | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Imaging | ||
| X-ray | ||
| Lovy 2013 [65] | 78.8 | 82.5 |
| Funakoshi 2018 [69] | 81 | 89 |
| Helical CT | ||
| Shiga 2006 [70] | 100 | 98 |
| MDCT | ||
| Mishra 2005 [71] | 100 | 100 |
| MRI | ||
| Shiga 2006 [70] | 98 | 98 |
| TEE | ||
| Shiga 2006 [70] | 98 | 95 |
| Pepi 2000 [72] | 100 | 100 |
| Biochemical Measurements | ||
| sELAF | ||
| Shinohara 2003 [73] | - | 99.8 |
| smMHC | ||
| Suzuki 2000 [74] | 90.9 | 98 |
| D-Dimer | ||
| Watanabe 2016 [75] | 95.2 | 60.4 |
| Li 2017 [76] | 94 | 56.8 |
| Nazerian 2017 [77] | 96.7 | 64 |
| Itagaki 2018 [78] | 98.9 | - |
| Matrix Metalloproteinase 8 (MMP8) | ||
| Giachino 2013 [79] | 100 | 9.5 |
| Matrix Metalloproteinase 9 (MMP9) | ||
| Giachino 2013 [79] | 96.2 | 16.2 |
| Li 2018 [79] | 68.2 | 84.1 |
| MiRNA 15a | ||
| Dong 2017 [80] | 75.7 | 82.5 |
| ST2 | ||
| Wang 2018 [81] | 99.1 | 84.9 |
| Morello 2020 [82] | 95.5 | 85.1 |
7.3.1. Chest X-ray
Chest X-Ray (CXR) is routinely performed in patients with chest pain of unknown etiology, making it perhaps the first imaging study performed on any patient with an AD. Mediastinal widening is the most important finding on CXR, as it is found in almost 60% of patients; however, a CXR alone is not enough to exclude the presence of an AD [83, 84].
7.3.2. Computed Tomography
Computed tomography (CT) is the gold-standard method for radiodiagnosis of AD [18, 39, 84]. It has the advantage of being readily accessible, cheaper, and faster than an MRI [84]. The key finding on a CT is the presence of an intimal flap separating the true and false lumina [56]. Currently, helical CT (HCT) scans and multidetector CT (MDCT) are the conventionally used protocols for the diagnosis of AD. MDCT has the advantage of being almost eight times faster than traditional HCT [18], and has been reported to have a specificity and sensitivity of up to 100% [85]; whereas HCT is less specific (98%) (Shiga 2006). In addition to the standard MDCT protocol, an ECG-gated approach is highly recommended [18]. This approach reduces the presence of artifacts by taking slices in predetermined portions of the cardiac cycle, thus eliminating any misreading caused by the heart’s movement [84]. Another approach is the triple rule-out CT, which simultaneously examines the coronary arteries, aorta, and pulmonary arteries; it provides better coverage for the diagnosis of nonspecific chest pain. This approach, however, is of limited use as it subjects the patient to excess radiation and a larger dose of contrast dye [18, 84]. Additionally, given that it scans three vessels simultaneously, it uses a standardized protocol rather than the optimal protocol for each, making it less than ideal for the routine screening of AD [17].
7.3.3. MRA
Magnetic Resonance Angiography (MRA) is a reliable method for diagnosis with a sensitivity and specificity of 100% [70]. However, an MRA is significantly slower than a CT scan and does not allow for the appropriate monitoring of critical patients; as a result, its use is limited to patients who are stable or unsuitable for a CT scan, such as those with renal insufficiency or iodine dye allergy [86].
7.3.4. Echocardiography
Transthoracic echocardiography (TTE) is a second line method of diagnosis often done whilst waiting for a CT/MRA scan. Though its visualization of the dissection itself is limited, it provides useful data on the condition of the heart, as well as the presence and severity of aortic regurgitation [18]. In comparison to other diagnostic modalities, TTE has a relatively low sensitivity and specificity [39]. Aside from its limited visualization, TTE is also limited in patients with abnormal chest wall configurations.
On the other hand, transoesophageal echocardiography (TOE) is as reliable as a CT/MRA scan [18, 39, 70] and is ideal for patients who are unstable and cannot be transported. Shiga et al. reported the validity of a TOE to be similar to that of a CT/MRA; however, unlike the latter, the accuracy of a TOE is highly dependent on the skills of the operator [70]. Another disadvantage of the TOE is the presence of a ‘blind spot’, the area covered by the trachea and left bronchus, which makes it difficult to visualize the upper ascending aorta and proximal arch [18, 87]. This limitation, however, is overcome by the use of biplane and multiplane probes [72].
7.4. Laboratory Investigations
7.4.1. D-Dimer
D-dimer is a fibrin degradation product found in the blood associated with dissections, pulmonary emboli, thrombosis, and myocardial infarction amongst others [88]. It is the only laboratory investigation recommended by the European and American guidelines for the screening of AD [1, 39]. D-dimer levels increase rapidly in an acute AD as compared to other diseases; peripheral blood concentration is most relevant during the first hour of dissection [76, 89]. Li et al. showed that within the first 24 hours of a dissection, elevated D-dimer levels had a sensitivity of 94% and a negative predictive value of 96.6% at a 0.5 μg/ml cutoff level [76]. However, D-dimer levels did not differ significantly between patients with AD and pulmonary emboli, suggesting that although D-dimer testing can be useful in differentiating AD from coagulopathies other than pulmonary emboli, it should not be used as the sole diagnostic test [90]. Furthermore, several studies have shown that D-dimer levels can also be of prognostic value. Wen et al. found that increased D-dimer levels were independently associated with in-hospital mortality [91] and were able to predict mortality with a sensitivity and specificity of 90.3% and 75.9% respectively. This finding was recently corroborated by Itagaki et al. whose analysis also concluded that patients with a reduced D-dimer concentration (≤8.3 μg/mL) had more favorable postoperative outcomes and lower in-hospital mortality [78].
7.4.2. C-Reactive Protein
C-Reactive Protein (CRP) is an acute-phase reactant produced by the liver in response to cytokines. CRP has been extensively studied as a biomarker of general inflammation; additionally, it is an independent risk factor for vascular inflammation and a prognostic factor for cardiovascular events [92-94]. There is a growing interest in the role of CRP in the diagnosis and prognosis of AD. Admission CRP levels were found to be an independent risk factor for in-hospital death [91, 95, 96] and other complications such as impaired oxygenation [97, 98]. CRP levels can also predict long-term AD outcomes such as all-cause mortality, recurrence or rupture [95, 99]. Moreover, two recent meta-analyses showed increased in-hospital mortality [100, 101] and mid-term mortality in patients with elevated CRP on admission [100]; thus further supporting the role of CRP levels in risk stratification.
7.4.3. MMPs
Matrix metalloproteinases (MMP) are a family of enzymes responsible for the remodeling of the extracellular matrix; thus, they contribute to the degenerative pathogenesis of AD. Several studies have reported a significant increase in plasma MMP levels in patients with AD compared to a control population, the pooled results of which are reported in a meta-analysis by Takagi et al. [102]. A study by Giachino et al. showed that circulating MMP-8 and MMP-9 levels could be useful in ruling out AD with a sensitivity of 100% and 96.2% respectively; however, the high degree of sensitivity at the chosen cut off points came at the cost of very low specificity (9.5% and 16.2% respectively) [79]. Additionally, the study showed that ruling out AD using MMP levels below the respective cut-off points (3.6 ng/L for MMP-8 and 20 ng/L for MMP-9) would reduce the number of exploratory CT scans by 5.6% and 9.5% [79]. A more recent study reported MMP-9 to have a sensitivity of 68.2% and a specificity of 84.1% at a cut-off point of 379.47 ng/ml [103]. This discrepancy in results could be due to several variables, the most important of which are variations in patient populations. Though specific MMP levels might be useful in aiding the diagnosis of AD, large-scale prospective studies are needed in order to reach a consensus as the available data is inconsistent and limited by small patient populations and the retrospective nature of the studies.
7.4.4. Other Potential Biomarkers
Potential biomarkers that have been suggested include smooth muscle Myosin Heavy Chain (smMHC), calponin, and soluble elastin fragments (sELAF) [84].
smMHC is a byproduct of the degeneration and necrosis of smooth muscle cells of the aortic media [104]. Suzuki et al. reported the sensitivity and specificity of smMHC to be time-dependent, showing a gradual increase over the first 3 hours [74]. During the first 12 hours, smMHC had a sensitivity of 90% and specificity of 97%. smMHC also has the advantage of being easy and quick to measure making it an ideal candidate for clinical use [104].
Calponin is a regulatory protein, it is the counterpart of troponin in smooth muscles. There are three isoforms of calponin: acidic, basic, and neutral, with the first two having the most diagnostic value. Suzuki et al. reported that in type A AD, the concentration of acidic calponin doubled while that of the basic variant tripled within the first 6 hours and remained elevated for 12 hours [105].
The diagnostic potential of soluble elastin fragments (sELAFs) is a matter of debate. sELAF is a product of the degradation of the wall of the aorta, there is a physiological increase in the concentration of sELAF with aging, and due to the degenerative nature of the pathogenesis underlying an AD, there is a growing interest in its role as a diagnostic biomarker [104]. A study by Shinohara et al. demonstrated the beneficial role of sELAF in the screening and diagnosis of acute dissection, with 64% of AD patients showing elevated levels [73]. On the other hand, a more recent study by Akutsu et al. concluded that there was no significant difference between the sELAF levels of patients diagnosed with AD versus control patients [106], calling into question its clinical utility.
MicroRNAs (miRNA) are a class of small non-coding RNAs that regulate gene expression by mediating the degradation or suppression of their complementary mRNA molecules. miRNAs are a novel class of biomarkers that are currently of interest in the pathogenesis of many diseases. A study by Dong et al. found that miRNA-15a expression was upregulated in patients with AD compared to control patients; they also concluded that miRNA-15a could potentially aid in the diagnosis of AD, reporting a sensitivity and specificity of 75.7% and 82.5% respectively [80]. Further analysis of the same AD cohort found that when compared to patients with chest pain of non-AD etiology, elevated miRNA-15a levels were 100% specific in identifying AD patients [80]. Additionally, the authors were able to identify 3 alternative miRNA subtypes which were found to be elevated only in AD patients (miRNA-23a, let-7b, and hcmv-miR-US-33-5p) [80]. Unfortunately, studies on the role of miRNA in AD are scarce. Though it shows promise as a potential diagnostic biomarker, miRNA assays are time-consuming, expensive, and not always commercially available; hence, whether miRNA testing could be integrated into clinical practice is questionable, and requires extensive clinical studies in order to provide a better understanding of the role of this novel procedure.
Another novel biomarker of interest is ST2 (suppression of tumorgenicity 2) which is currently being investigated for its role in cardiovascular disease. Elevated ST2 levels denote myocardial stress and fibrosis. Wang et al. first evaluated the role of ST2 in the diagnosis of AD, reporting a sensitivity of 99.1% and specificity of 84.9% at a cut-off of 34.6 ng/mL [81]. More recently, Morello et al. evaluated the diagnostic accuracy of soluble ST2 at three different cut-off levels (≥ 12 ng/ml, ≥23.7 ng/ml, and ≥ 66.5 ng/ml). The results of their study showed a maximum sensitivity of 95.5% (at ≥ 12 ng/ml) and a maximum specificity of 85.1% (at ≥ 66.5 ng/ml); however, their cohort population was considerably different regarding ethnicity, gender, and age [82] all of which could explain the differences in the reported outcomes.
8. TREATMENT
The treatment of AD is stratified according to the location of the lesion: Type A necessitates surgical intervention, whereas type B, notwithstanding complicated cases, is amenable to medical treatment [39].
8.1. Type A Dissection
The surgical approach to type A dissection, first established by Debakey et al. is as follows: following surgical incision and establishment of cardiopulmonary bypass, the surgeon clamps the aorta just proximal to the left innominate, transects the region of dissection, repairs the tear, obliterates the false lumen and finally performs end-to-end anastomosis of the aorta [20].
In terms of the extent of the repair, a synthetic supracoronary graft may be used with or without replacement of the aortic root. The latter decision is influenced by whether the root is affected by a tear or an aneurysm, and the functionality of the aortic valve. In patients with Marfan syndrome, aortic root replacement is recommended to avoid future reoperations, which are much more common with the conservative supracoronary replacement approach [107].
An ascending dissection generally requires the use of a Dacron graft to replace a portion of the aortic arch. The extent of aortic arch replacement, however, is still a matter of controversy. The most conservative approach is an end- to-end anastomosis. More commonly, a surgeon would replace the proximal part of the aortic arch only, however, this risks leaving a residual dissection more distally. To solve this issue, a more radical approach would be to perform a total arch replacement. The rationale behind this approach is based on the hazards of leaving behind a residual false lumen following surgery. First, patients with a patent residual false lumen suffer greater aortic dilation than those without [108]; however, Takahara et al. showed that even when the total arch replacement approach was adopted, more than a quarter of patients still had residual false lumina [109]. In addition, Shiono et al. demonstrated no statistically significant differences in the percentages of patent false lumina when total arch replacement was compared to ascending/hemiarch replacement, though that may have been due to a relatively small sample size of 134 patients, of whom only 29 underwent total arch replacement [110, 111]. Second, the presence of a partial thrombus could potentially lead to a cul de sac, which would lead to a build-up of pressure within the false lumen and potentially rupture the aorta, thus leading to higher mortality [112]. Furthermore, the same investigators had also demonstrated that partial thrombosis was less common with a total arch replacement. Despite these potential advantages, there is little evidence to support the wide adoption of total arch replacement. Kazui et al. showed no outcome differences between different degrees of arch replacement [113]; additionally, more recent studies remain critical of the more complex and radical surgical technique [114, 115]. In the most recent study to date, Hsu et al. showed that although total arch replacement was significantly associated with improved remodeling on multivariate logistic regression, it was nevertheless associated with an increase in mortality and stroke rates [114]. The findings of Hsu et al. corroborate previous findings by Lio et al., [114] who also showed that total arch replacement is associated with a statistically significant increase in mortality when compared to hemiarch replacement using multivariate models. Thus, for the time being, total arch replacement is not a routinely recommended approach and should be reserved for more select cases. However, it is important to note that the level of evidence regarding the extent of replacement is far from ideal; although the aforementioned studies did adjust for confounding factors, they are greatly limited by their observational nature, which inevitability poses a risk of residual confounding variables having altered the results. Nevertheless, in the absence of sufficiently high-quality evidence from randomized trials, and in light of the available data, total arch replacements should remain a niche option reserved for select patients.
One issue that remains unsettled is the best access route for cannulation, with studies reporting conflicting results. Two recent meta-analyses showed decreased mortality and stroke rates with axillary as opposed to femoral cannulation [116, 117]; however, a more recent analysis of the German registry for acute AD type A showed no significant differences according to cannulation methods [118]. One advantage of the traditional femoral approach is that it is less time consuming; however, the femoral artery is also more commonly atherosclerosed than the axillary artery, thus making it a less accessible option.
In cases complicated by obstruction of the coronary ostia, the prognosis is particularly grim as the coronary malperfusion portends higher mortality rates; therefore, coronary artery bypass grafting may be warranted to restore revascularization [119]. Another possible complication of AD is cardiac tamponade [109], which can lead to shock and thus require immediate surgical intervention. One modality for dealing with cardiac tamponade is pericardiocentesis, where suction of the blood from the pericardium can help relieve a hemodynamically unstable patient [120]. However, further loss of blood into the pericardium with consequent cardiovascular collapse is a possible consequence of this procedure, as a result, it should be reserved for acutely unstable cases.
During the period of circulatory arrest, brain tissue is at a particularly high risk of ischemia. As such, cerebral protection strategies, aiming at the preservation of cerebral perfusion during this critical period, have been developed. There is great variation in these approaches, with anterograde, retrograde, and cardiopulmonary bypass only approaches all being practiced. Furthermore, anterograde perfusion may be accomplished via either unilateral or bilateral approaches, further compounding the variability of possible approaches. Studies show that anterograde cerebral perfusion becomes an especially vital component in long-lasting procedures, with negligible differences between bilateral and unilateral approaches rendering the choice largely a matter of the surgeon’s preference [121].
Recent trends in type A management demonstrate increased uptake of surgical intervention with decreasing mortality rates, as opposed to the increasingly abandoned conservative approach, in which the high mortality rates remain nearly unchanged. These changes are not only attributed to improvements in surgical care and technique, but also improvements in diagnostic imaging, which facilitate more rapid surgical correction [41].
8.2. Type B Dissection
8.2.1. Uncomplicated Type B Dissection
Uncomplicated type B dissection is generally managed more conservatively, with medical therapy having two aims: A reduction in blood pressure and heart rate to 100 to 120 mmHg (though the exact range varies across different guidelines) and 50-60 bpm, respectively, and analgesic control [39, 122]. The former is generally achieved via the use of beta-blockers, with calcium channel blockers being a reasonable alternative in cases where beta-blocker use is relatively contraindicated, as in patients with severe asthma. Findings from the IRAD registry show that beta-blockers and calcium blockers, but not ACEI, are associated with decreased mortality in this category of patients [123]. If more intensive blood pressure-lowering therapy is required, intravenous nitroprusside may be used; however, care must be taken that beta-blockers are administered before nitroprussides, to avoid a reflex release of catecholamines due to vasodilation, and therefore precipitate increased left ventricular ejection forces and propagation of the dissection [124]. The rationale behind blood pressure-lowering therapy is to minimize the risk of rupture and dissection propagation; however, there may be a risk of underperfusion and ischemia if the blood pressure drops too low. Supporting this view, analysis of the IMPROVE trial had shown that a decrease in blood pressure to 70 mmHg was associated with greater mortality [125]. It must also be noted that although there is some evidence to support the aforementioned heart rate goals [126], the evidence base for blood pressure goals is largely poor, with the ongoing RAID trial attempting to remedy this gap in the literature [127]. With respect to analgesic control, opiates are recommended to alleviate the patient’s pain and consequently reduce sympathetic stimulation, thus serving to augment heart rate and blood pressure control [122].
Another approach that has gained popularity is thoracic endovascular aortic repair (TEVAR) [3], with recent studies challenging the well-established tradition of opting for conservative medical therapy in uncomplicated type B dissection, particularly in chronic patients. The initial two-year analysis of the INSTEAD trial, which compared TEVAR with medical therapy, showed improved remodeling in favor of TEVAR but failed to demonstrate improved survival outcomes [128]; however, five years follow up of the trial demonstrated both improved mortality and remodeling outcomes with TEVAR [129, 130]. In contrast, endovascular therapy is less promising in the acute uncomplicated setting, where the ADSORB trial failed to show improved survival outcomes. However, similar to the initial two years follow up of the INSTEAD trial, the ADSORB trial showed improved remodeling [131], which has been demonstrated to be associated with better outcomes [131]. The statistical analysis of the trial revealed that only 43% of TEVAR patients lacked false lumen thrombi, as opposed to 97% for those placed solely on medical therapy. Differences in the other two components of the composite endpoint were not statistically significant. It is worth noting that the authors of the ADSORB trial, which only included 61 patients, had powered the study for a primary endpoint of remodeling or rupture, rather than for mortality, thus highlighting the need for sufficiently powered trials to compare medical treatment against an endovascular repair in the acute uncomplicated setting.
8.2.2. Complicated Type B Dissection
Complicated type B dissection, marked by malperfusion or rupture, necessitates surgical intervention. Traditionally, an open surgical approach was used to resect the tear and replace the aorta. In recent years, however, the endovascular intervention has grown in popularity and supplanted open surgical repair [3, 41]. The main aim of endovascular stent grafting is the obliteration of the dissection tear and depressurization of the false lumen, allowing the re-establishment of flow in the true aortic lumen, and therefore leading to the reversal of end-organ ischemia. A modification to this approach involves not only stent coverage of the primary dissection, but a further provisional extension of the stent beyond said dissection. To our knowledge, there are no two-armed randomized trials comparing the traditional and extended stenting approaches, though results from single-armed studies show satisfactory results [132-134]. A retrospective study comparing the two approaches showed that not only the distal stenting approach provides better hemodynamic outcomes (higher true volume lumen and false lumen thrombosis), but it also boasts lower rates of reintervention and distal dissections. However, there were no significant differences with regards to mortality, renal failure, paraplegia or aortic ruptures [135].
Studies have shown that TEVAR boasts significantly better survival outcomes as compared to open surgical repair [136-138]. Furthermore, beyond the endpoint of mortality, patients undergoing TEVAR are significantly less likely to experience respiratory, cardiac, and neurological complications (including a lower risk of paraplegia, which can be severely debilitating to patients). These advantages, coupled with shorter procedure times and reduced length of hospital stay, make TEVAR a significantly more attractive option than open surgical repair [139]. It is worth noting that much of the available data stems from observational studies rather than randomized clinical trials, which limits the reliability and strength of evidence thereof; however, the consistency of the results across studies boosts the robustness of the conclusions.
9. FUTURE RECOMMENDATIONS
Great strides have been made in the management of AD over the past decade, including both diagnostic and interventional procedures. Nevertheless, several outstanding issues remain to be resolved. Firstly, there is still insufficient progress on the utilization of biomarkers for the detection of AD, with D-dimers being the only measure approved by European and American guidelines for screening. Despite boasting a high specificity, D-dimers are not specific for the diagnosis of AD, as they may be elevated in other emergent conditions such as pulmonary emboli; therefore, they cannot be relied upon as the sole measure by which to guide management in the acute setting. It is therefore important that other biomarkers should be further investigated and, if sufficiently valuable, be integrated as screening protocols for suspected cases. This includes the use of isolated markers as well as the investigation of maximally useful combinations of markers. This is particularly the case when taking into consideration the acuteness of AD, as in such emergent conditions a reliable and rapid diagnosis is invaluable.
Another area in need of further investigation is the management of acute uncomplicated type B dissection, where endovascular intervention has recently challenged medical management as being the optimal choice. Studies such as the ADSORB trial have indeed reported improved remodeling outcomes with TEVAR; however, in light of being underpowered for mortality outcomes, it could not demonstrate improved outcomes in that particular regard. This highlights the need for clinical trials sufficiently powered for mortality to establish a more definitive stance regarding the ideal intervention. Furthermore, as regarding TEVAR, there is a need for further evidence that can guide patient selection and time of the endovascular intervention to maximize patient benefit. Regarding medical management, there is a need for further evidence on which current blood pressure control recommendations can be based, though the ongoing RAID trial may hopefully provide answers in that regard.
CONCLUSION
In conclusion, acute AD is a highly fatal medical emergency requiring a high degree of clinical suspicion on the part of the physician, as prompt medical intervention is of paramount importance. To that end, advances in imaging and diagnostic modalities have played an important part in shaping the management of suspected cases. In addition, the promise of diagnostic biomarkers could play a role in improving outcomes. In terms of treatment, recent trends show increasing adoption of endovascular approaches, which promise to improve patient outcomes.
ACKNOWLEDGEMENTS
We would like to acknowledge Omar Shazly and Yousra Abed for their help with the graphical figure (Figure 1 in the manuscript).
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hiratzka L.F., Bakris G.L., Beckman J.A., et al. ACCF / AHA Guideline 2010 ACCF / AHA / AATS / ACR / ASA / SCA / SCAI / SIR / STS / SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease A Report of the American College of Cardiology Foundation / American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2011;121(13):e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 2.LeMaire S.A., Russell L. Epidemiology of thoracic aortic dissection. Nat. Rev. Cardiol. 2011;8(2):103–113. doi: 10.1038/nrcardio.2010.187. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu H., Endo S., Natsugoe S., et al. Thoracic and cardiovascular surgery in Japan in 2016: Annual report by The Japanese Association for Thoracic Surgery. Gen. Thorac. Cardiovasc. Surg. 2019;67(4):377–411. doi: 10.1007/s11748-019-01068-9. [DOI] [PubMed] [Google Scholar]
- 4.Howard D.P.J., Banerjee A., Fairhead J.F., Perkins J., Silver L.E., Rothwell P.M. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013;127(20):2031–2037. doi: 10.1161/CIRCULATIONAHA.112.000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacini D., Di Marco L., Fortuna D., Belotti L.M., Gabbieri D., Zussa C., Pigini F., Contini A., Barattoni M.C., De Palma R., Di Bartolomeo R. Acute aortic dissection: epidemiology and outcomes. Int. J. Cardiol. 2013;167(6):2806–2812. doi: 10.1016/j.ijcard.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Klompas M. Does this patient have an acute thoracic aortic dissection? JAMA. 2002;287(17):2262–2272. doi: 10.1001/jama.287.17.2262. [DOI] [PubMed] [Google Scholar]
- 7.Clouse W.D., Hallett J.W., Jr, Schaff H.V., Spittell P.C., Rowland C.M., Ilstrup D.M., Melton L.J., III Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin. Proc. 2004;79(2):176–180. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- 8.Olsson C., Thelin S., Ståhle E., Ekbom A., Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 9.Landenhed M., Engström G., Gottsäter A., Caulfield M.P., Hedblad B., Newton-Cheh C., Melander O., Smith J.G. Risk profiles for aortic dissection and ruptured or surgically treated aneurysms: a prospective cohort study. J. Am. Heart Assoc. 2015;4(1):e001513. doi: 10.1161/JAHA.114.001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClure R.S., Brogly S.B., Lajkosz K., Payne D., Hall S.F., Johnson A.P. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: A population-based study. J. Thorac. Cardiovasc. Surg. 2018;155(6):2254–2264.e4. doi: 10.1016/j.jtcvs.2017.11.105. [DOI] [PubMed] [Google Scholar]
- 11.Melvinsdottir I.H., Lund S.H., Agnarsson B.A., Sigvaldason K., Gudbjartsson T., Geirsson A. The incidence and mortality of acute thoracic aortic dissection: results from a whole nation study. Eur. J. Cardiothorac. Surg. 2016;50(6):1111–1117. doi: 10.1093/ejcts/ezw235. [DOI] [PubMed] [Google Scholar]
- 12.Debakey M.E., Henly W.S., Cooley D.A., Morris G.C., Jr, Crawford E.S., Beall A.C., Jr Surgical management of dissecting aneurysms of the aorta. J. Thorac. Cardiovasc. Surg. 1965;49:130–149. doi: 10.1016/S0022-5223(19)33323-9. [DOI] [PubMed] [Google Scholar]
- 13.Daily P.O., Trueblood H.W., Stinson E.B., Wuerflein R.D., Shumway N.E. Management of acute aortic dissections. Ann. Thorac. Surg. 1970;10(3):237–247. doi: 10.1016/S0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 14.Philip J.L., De Oliveira N.C., Akhter S.A., Rademacher B.L., Goodavish C.B., DiMusto P.D., Tang P.C. Cluster analysis of acute ascending aortic dissection provides novel insight into mechanisms of distal progression. J. Thorac. Dis. 2017;9(9):2966–2973. doi: 10.21037/jtd.2017.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Segesser L.K., Killer I., Ziswiler M., Linka A., Ritter M., Jenni R., Baumann P.C., Turina M.I. Dissection of the descending thoracic aorta extending into the ascending aorta. A therapeutic challenge. J. Thorac. Cardiovasc. Surg. 1994;108(4):755–761. doi: 10.1016/S0022-5223(94)70304-3. [DOI] [PubMed] [Google Scholar]
- 16.Rylski B., Pérez M., Beyersdorf F., Reser D., Kari F.A., Siepe M., Czerny M. Acute non-A non-B aortic dissection: incidence, treatment and outcome. Eur. J. Cardiothorac. Surg. 2017;52(6):1111–1117. doi: 10.1093/ejcts/ezx142. [DOI] [PubMed] [Google Scholar]
- 17.Sievers H.H., Rylski B., Czerny M., Baier A.L.M., Kreibich M., Siepe M., Beyersdorf F. Aortic dissection reconsidered: type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact. Cardiovasc. Thorac. Surg. 2020;30(3):451–457. doi: 10.1093/icvts/ivz281. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein S.A., Evangelista A., Abbara S., Arai A., Asch F.M., Badano L.P., Bolen M.A., Connolly H.M., Cuéllar-Calàbria H., Czerny M., Devereux R.B., Erbel R.A., Fattori R., Isselbacher E.M., Lindsay J.M., McCulloch M., Michelena H.I., Nienaber C.A., Oh J.K., Pepi M., Taylor A.J., Weinsaft J.W., Zamorano J.L., Dietz H., Eagle K., Elefteriades J., Jondeau G., Rousseau H., Schepens M. Multimodality imaging of diseases of the thoracic aorta in adults: From the American society of echocardiography and the European association of cardiovascular imaging: Endorsed by the society of cardiovascular computed tomography and society for cardiova. J. Am. Soc. Echocardiogr. 2015;28(2):119–182. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Hirst A.E.J., Jr, Johns V.J.J., Jr, Kime S.W.J., Jr Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore) 1958;37(3):217–279. doi: 10.1097/00005792-195809000-00003. [DOI] [PubMed] [Google Scholar]
- 20.DeBakey M.E., Beall A.C., Jr, Cooley D.A., et al. Dissecting aneurysms of the aorta. Surg. Clin. North Am. 1966;46(4):1045–1055. doi: 10.1016/S0039-6109(16)37946-4. [DOI] [PubMed] [Google Scholar]
- 21.Booher A.M., Isselbacher E.M., Nienaber C.A., Trimarchi S., Evangelista A., Montgomery D.G., Froehlich J.B., Ehrlich M.P., Oh J.K., Januzzi J.L., O’Gara P., Sundt T.M., Harris K.M., Bossone E., Pyeritz R.E., Eagle K.A. The IRAD classification system for characterizing survival after aortic dissection. Am. J. Med. 2013;126(8):730.e19–730.e24. doi: 10.1016/j.amjmed.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Nakashima Y., Sueishi K. Alteration of elastic architecture in the lathyritic rat aorta implies the pathogenesis of aortic dissecting aneurysm. Am. J. Pathol. 1992;140(4):959–969. [PMC free article] [PubMed] [Google Scholar]
- 23.Matt P., Huso D.L., Habashi J., Holm T., Doyle J., Schoenhoff F., Liu G., Black J., Van Eyk J.E., Dietz H.C. Murine model of surgically induced acute aortic dissection type A. J. Thorac. Cardiovasc. Surg. 2010;139(4):1041–1047. doi: 10.1016/j.jtcvs.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng S., Larsson A., Wassberg E., Gerwins P., Thelin S., Fu X., Westermark P. Role of aggregated medin in the pathogenesis of thoracic aortic aneurysm and dissection. Lab. Invest. 2007;87(12):1195–1205. doi: 10.1038/labinvest.3700679. [DOI] [PubMed] [Google Scholar]
- 25.Angouras D., Sokolis D.P., Dosios T., Kostomitsopoulos N., Boudoulas H., Skalkeas G., Karayannacos P.E. Effect of impaired vasa vasorum flow on the structure and mechanics of the thoracic aorta: implications for the pathogenesis of aortic dissection. Eur. J. Cardiothorac. Surg. 2000;17(4):468–473. doi: 10.1016/S1010-7940(00)00382-1. [DOI] [PubMed] [Google Scholar]
- 26.Beller C.J., Labrosse M.R., Thubrikar M.J., Robicsek F. Role of aortic root motion in the pathogenesis of aortic dissection. Circulation. 2004;109(6):763–769. doi: 10.1161/01.CIR.0000112569.27151.F7. [DOI] [PubMed] [Google Scholar]
- 27.Davies R.R., Goldstein L.J., Coady M.A., Tittle S.L., Rizzo J.A., Kopf G.S., Elefteriades J.A. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann. Thorac. Surg. 2002;73(1):17–27. doi: 10.1016/S0003-4975(01)03236-2. [DOI] [PubMed] [Google Scholar]
- 28.Shiran H., Odegaard J., Berry G., Miller D.C., Fischbein M., Liang D. Aortic wall thickness: an independent risk factor for aortic dissection? J. Heart Valve Dis. 2014;23(1):17–24. doi: 10.1016/S0735-1097(12)60832-8. [DOI] [PubMed] [Google Scholar]
- 29.Van Puyvelde J., Verbeken E., Verbrugghe P., Herijgers P., Meuris B. Aortic wall thickness in patients with ascending aortic aneurysm versus acute aortic dissection. Eur. J. Cardiothorac. Surg. 2016;49(3):756–762. doi: 10.1093/ejcts/ezv197. [DOI] [PubMed] [Google Scholar]
- 30.Pape L.A., Tsai T.T., Isselbacher E.M., Oh J.K., O’gara P.T., Evangelista A., Fattori R., Meinhardt G., Trimarchi S., Bossone E., Suzuki T., Cooper J.V., Froehlich J.B., Nienaber C.A., Eagle K.A. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116(10):1120–1127. doi: 10.1161/CIRCULATIONAHA.107.702720. [DOI] [PubMed] [Google Scholar]
- 31.Neri E., Barabesi L., Buklas D., Vricella L.A., Benvenuti A., Tucci E., Sassi C., Massetti M. Limited role of aortic size in the genesis of acute type A aortic dissection. Eur. J. Cardiothorac. Surg. 2005;28(6):857–863. doi: 10.1016/j.ejcts.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Evangelista A., Mukherjee D., Mehta R.H., O’Gara P.T., Fattori R., Cooper J.V., Smith D.E., Oh J.K., Hutchison S., Sechtem U., Isselbacher E.M., Nienaber C.A., Pape L.A., Eagle K.A. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111(8):1063–1070. doi: 10.1161/01.CIR.0000156444.26393.80. [DOI] [PubMed] [Google Scholar]
- 33.D’Annoville T., Ozdemir B.A., Alric P., Marty-Ané C.H., Canaud L. Thoracic endovascular aortic repair for penetrating aortic ulcer: literature review. Ann. Thorac. Surg. 2016;101(6):2272–2278. doi: 10.1016/j.athoracsur.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 34.Evangelista A., Isselbacher E.M., Bossone E., Gleason T.G., Eusanio M.D., Sechtem U., Ehrlich M.P., Trimarchi S., Braverman A.C., Myrmel T., Harris K.M., Hutchinson S., O’Gara P., Suzuki T., Nienaber C.A., Eagle K.A. Insights from the international registry of acute aortic dissection: A 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846–1860. doi: 10.1161/CIRCULATIONAHA.117.031264. [DOI] [PubMed] [Google Scholar]
- 35.Sato F., Kitamura T., Kongo M., Okinaka T., Onishi K., Ito M., Isaka N., Nakano T. Newly diagnosed acute aortic dissection: characteristics, treatment modifications, and outcomes. Int. Heart J. 2005;46(6):1083–1098. doi: 10.1536/ihj.46.1083. [DOI] [PubMed] [Google Scholar]
- 36.Howard D.P.J., Sideso E., Handa A., Rothwell P.M. Incidence, risk factors, outcome and projected future burden of acute aortic dissection. Ann. Cardiothorac. Surg. 2014;3(3):278–284. doi: 10.3978/j.issn.2225-319X.2014.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nienaber C.A., Fattori R., Mehta R.H., Richartz B.M., Evangelista A., Petzsch M., Cooper J.V., Januzzi J.L., Ince H., Sechtem U., Bossone E., Fang J., Smith D.E., Isselbacher E.M., Pape L.A., Eagle K.A. Gender-related differences in acute aortic dissection. Circulation. 2004;109(24):3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- 38.Mészáros I., Mórocz J., Szlávi J., Schmidt J., Tornóci L., Nagy L., Szép L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117(5):1271–1278. doi: 10.1378/chest.117.5.1271. [DOI] [PubMed] [Google Scholar]
- 39.Erbel R., Aboyans V., Boileau C., Bossone E., Bartolomeo R.D., Eggebrecht H., Evangelista A., Falk V., Frank H., Gaemperli O., Grabenwöger M., Haverich A., Iung B., Manolis A.J., Meijboom F., Nienaber C.A., Roffi M., Rousseau H., Sechtem U., Sirnes P.A., Allmen R.S., Vrints C.J. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur. Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 40.Reutersberg B., Salvermoser M., Trenner M., Geisbüsch S., Zimmermann A., Eckstein H.H., Kuehnl A. Hospital incidence and in-hospital mortality of surgically and interventionally treated aortic dissections: secondary data analysis of the nationwide German diagnosis-related group statistics From 2006 to 2014. J. Am. Heart Assoc. 2019;8(8):e011402. doi: 10.1161/JAHA.118.011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pape L.A., Awais M., Woznicki E.M., Suzuki T., Trimarchi S., Evangelista A., Myrmel T., Larsen M., Harris K.M., Greason K., Di Eusanio M., Bossone E., Montgomery D.G., Eagle K.A., Nienaber C.A., Isselbacher E.M., O’Gara P. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J. Am. Coll. Cardiol. 2015;66(4):350–358. doi: 10.1016/j.jacc.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 42.Meester J.A.N., Verstraeten A., Schepers D., Alaerts M., Van Laer L., Loeys B.L. Differences in manifestations of Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Ann. Cardiothorac. Surg. 2017;6(6):582–594. doi: 10.21037/acs.2017.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Beaufort H.W.L., Trimarchi S., Korach A., Di Eusanio M., Gilon D., Montgomery D.G., Evangelista A., Braverman A.C., Chen E.P., Isselbacher E.M., Gleason T.G., De Vincentiis C., Sundt T.M., Patel H.J., Eagle K.A. Aortic dissection in patients with Marfan syndrome based on the IRAD data. Ann. Cardiothorac. Surg. 2017;6(6):633–641. doi: 10.21037/acs.2017.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley T.J., Bowdin S.C., Morel C.F.J., Pyeritz R.E. The expanding clinical spectrum of extracardiovascular and cardiovascular manifestations of heritable thoracic aortic aneurysm and dissection. Can. J. Cardiol. 2016;32(1):86–99. doi: 10.1016/j.cjca.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Verstraeten A., Alaerts M., Van Laer L., Loeys B. Marfan syndrome and related disorders: 25 years of gene discovery. Hum. Mutat. 2016;37(6):524–531. doi: 10.1002/humu.22977. [DOI] [PubMed] [Google Scholar]
- 46.Loeys B.L., Schwarze U., Holm T., Callewaert B.L., Thomas G.H., Pannu H., De Backer J.F., Oswald G.L., Symoens S., Manouvrier S., Roberts A.E., Faravelli F., Greco M.A., Pyeritz R.E., Milewicz D.M., Coucke P.J., Cameron D.E., Braverman A.C., Byers P.H., De Paepe A.M., Dietz H.C. Aneurysm syndromes caused by mutations in the TGF-β receptor. N. Engl. J. Med. 2006;355(8):788–798. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 47.Eagleton M.J. Arterial complications of vascular Ehlers-Danlos syndrome. J. Vasc. Surg. 2016;64(6):1869–1880. doi: 10.1016/j.jvs.2016.06.120. [DOI] [PubMed] [Google Scholar]
- 48.Dietz H.C., Cutting G.R., Pyeritz R.E., Maslen C.L., Sakai L.Y., Corson G.M., Puffenberger E.G., Hamosh A., Nanthakumar E.J., Curristin S.M., et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352(6333):337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 49.MacFarlane E.G., Haupt J., Dietz H.C., Shore E.M. TGF-β family signaling in connective tissue and skeletal diseases. Cold Spring Harb. Perspect. Biol. 2017;9(11):1–42. doi: 10.1101/cshperspect.a022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loeys B.L., Chen J., Neptune E.R., Judge D.P., Podowski M., Holm T., Meyers J., Leitch C.C., Katsanis N., Sharifi N., Xu F.L., Myers L.A., Spevak P.J., Cameron D.E., De Backer J., Hellemans J., Chen Y., Davis E.C., Webb C.L., Kress W., Coucke P., Rifkin D.B., De Paepe A.M., Dietz H.C. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005;37(3):275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 51.Leivonen S.K., Chantry A., Häkkinen L., Han J., Kähäri V.M. Smad3 mediates transforming growth factor-β-induced collagenase-3 (matrix metalloproteinase-13) expression in human gingival fibroblasts. Evidence for cross-talk between Smad3 and p38 signaling pathways. J. Biol. Chem. 2002;277(48):46338–46346. doi: 10.1074/jbc.M206535200. [DOI] [PubMed] [Google Scholar]
- 52.Hall M.C., Young D.A., Waters J.G., Rowan A.D., Chantry A., Edwards D.R., Clark I.M. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-β 1. J. Biol. Chem. 2003;278(12):10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- 53.Martín M., Lorca R., Rozado J., Alvarez-Cabo R., Calvo J., Pascual I., Cigarrán H., Rodríguez I., Morís C. Bicuspid aortic valve syndrome: a multidisciplinary approach for a complex entity. J. Thorac. Dis. 2017;9(Suppl. 6):S454–S464. doi: 10.21037/jtd.2017.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michelena H.I., Khanna A.D., Mahoney D., Margaryan E., Topilsky Y., Suri R.M., Eidem B., Edwards W.D., Sundt T.M., III, Enriquez-Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306(10):1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 55.Brownstein A.J., Ziganshin B.A., Kuivaniemi H., Body S.C., Bale A.E., Elefteriades J.A. Genes associated with thoracic aortic aneurysm and dissection: an update and clinical implications. Aorta (Stamford) 2017;5(1):11–20. doi: 10.12945/j.aorta.2017.17.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukui T. Management of acute aortic dissection and thoracic aortic rupture. J. Intensive Care. 2018;6(1):15. doi: 10.1186/s40560-018-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jánosi R.A., Buck T., Erbel R. Mechanism of coronary malperfusion due to type-a aortic dissection. Herz. 2009;34(6):478. doi: 10.1007/s00059-009-3272-z. [DOI] [PubMed] [Google Scholar]
- 58.Gilon D., Mehta R.H., Oh J.K., Januzzi J.L., Jr, Bossone E., Cooper J.V., Smith D.E., Fang J., Nienaber C.A., Eagle K.A., Isselbacher E.M. Characteristics and in-hospital outcomes of patients with cardiac tamponade complicating type A acute aortic dissection. Am. J. Cardiol. 2009;103(7):1029–1031. doi: 10.1016/j.amjcard.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Januzzi J.L., Eagle K.A., Cooper J.V., Fang J., Sechtem U., Myrmel T., Evangelista A., Oh J.K., Llovet A., O’Gara P.T., Nienaber C.A., Isselbacher E.M. Acute aortic dissection presenting with congestive heart failure: results from the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2005;46(4):733–735. doi: 10.1016/j.jacc.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 60.Gupta S., Varadarajulu R., Mehta S.R., Mehdi S., Kumar K. Dissecting aortic aneurysm presenting as superior vena cava syndrome. Med. J. Armed Forces India. 2002;58(3):273–274. doi: 10.1016/S0377-1237(02)80150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernández Alonso L., Flórez Pelaez S., Cerezal Garrido J., Echevarría Uribarri J.R., Fulquet Carreras E., Herreros González J. Superior vena cava syndrome as initial manifestation of acute aortic dissection: a case report and review of the literature. Med. Interna. 1997;14(12):633–635. [PubMed] [Google Scholar]
- 62.Raja F.S., Islam A., Khan M., Abbasi I. Type A aortic dissection presenting as superior vena cava syndrome. CJEM. 2013;15(1):59–62. doi: 10.2310/8000.2012.110609. [DOI] [PubMed] [Google Scholar]
- 63.Parish J.M., Marschke R.F., Jr, Dines D.E., Lee R.E. Etiologic considerations in superior vena cava syndrome. Mayo Clin. Proc. 1981;56(7):407–413. [PubMed] [Google Scholar]
- 64.Di Eusanio M., Trimarchi S., Patel H.J., Hutchison S., Suzuki T., Peterson M.D., Di Bartolomeo R., Folesani G., Pyeritz R.E., Braverman A.C., Montgomery D.G., Isselbacher E.M., Nienaber C.A., Eagle K.A., Fattori R. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2013;145(2):385–390.e1. doi: 10.1016/j.jtcvs.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 65.Lovy A.J., Bellin E., Levsky J.M., Esses D., Haramati L.B. Preliminary development of a clinical decision rule for acute aortic syndromes. Am. J. Emerg. Med. 2013;31(11):1546–1550. doi: 10.1016/j.ajem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nallamothu B.K., Mehta R.H., Saint S., Llovet A., Bossone E., Cooper J.V., Sechtem U., Isselbacher E.M., Nienaber C.A., Eagle K.A., Evangelista A. Syncope in acute aortic dissection: diagnostic, prognostic, and clinical implications. Am. J. Med. 2002;113(6):468–471. doi: 10.1016/S0002-9343(02)01254-8. [DOI] [PubMed] [Google Scholar]
- 67.Hirata K., Wake M., Kyushima M., Takahashi T., Nakazato J., Mototake H., Tengan T., Yasumoto H., Henzan E., Maeshiro M., Asato H. Electrocardiographic changes in patients with type A acute aortic dissection. Incidence, patterns and underlying mechanisms in 159 cases. J. Cardiol. 2010;56(2):147–153. doi: 10.1016/j.jjcc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Kosuge M., Uchida K., Imoto K., Hashiyama N., Ebina T., Hibi K., Tsukahara K., Maejima N., Masuda M., Umemura S., Kimura K. Frequency and implication of ST-T abnormalities on hospital admission electrocardiograms in patients with type A acute aortic dissection. Am. J. Cardiol. 2013;112(3):424–429. doi: 10.1016/j.amjcard.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 69.Funakoshi H., Mizobe M., Homma Y., Nakashima Y., Takahashi J., Shiga T. The diagnostic accuracy of the mediastinal width on supine anteroposterior chest radiographs with nontraumatic Stanford type A acute aortic dissection. J Gen Fam Med. 2018;19(2):45–49. doi: 10.1002/jgf2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shiga T., Wajima Z., Apfel C.C., Inoue T., Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch. Intern. Med. 2006;166(13):1350–1356. doi: 10.1001/archinte.166.13.1350. [DOI] [PubMed] [Google Scholar]
- 71.Mishra M., Khurana P., Meharwal Z.S., Trehan N. A comparative study of imaging techniques in aortic dissection. Innovations (Phila.) 2005;1(1):40–47. doi: 10.1177/155698450500100106. [DOI] [PubMed] [Google Scholar]
- 72.Pepi M., Campodonico J., Galli C., Tamborini G., Barbier P., Doria E., Maltagliati A., Alimento M., Spirito R. Rapid diagnosis and management of thoracic aortic dissection and intramural haematoma: a prospective study of advantages of multiplanevs.biplane transoesophageal echocardiography. Eur. J. Echocardiogr. 2000;1(1):72–79. doi: 10.1053/euje.2000.0002. [DOI] [PubMed] [Google Scholar]
- 73.Shinohara T., Suzuki K., Okada M., Shiigai M., Shimizu M., Maehara T., Ohsuzu F. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler. Thromb. Vasc. Biol. 2003;23(10):1839–1844. doi: 10.1161/01.ATV.0000085016.02363.80. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki T., Katoh H., Tsuchio Y., Hasegawa A., Kurabayashi M., Ohira A., Hiramori K., Sakomura Y., Kasanuki H., Hori S., Aikawa N., Abe S., Tei C., Nakagawa Y., Nobuyoshi M., Misu K., Sumiyoshi T., Nagai R. Diagnostic implications of elevated levels of smooth-muscle myosin heavy-chain protein in acute aortic dissection. The smooth muscle myosin heavy chain study. Ann. Intern. Med. 2000;133(7):537–541. doi: 10.7326/0003-4819-133-7-200010030-00013. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe H., Horita N., Shibata Y., Minegishi S., Ota E., Kaneko T. Diagnostic test accuracy of D-dimer for acute aortic syndrome: systematic review and meta-analysis of 22 studies with 5000 subjects. Sci. Rep. 2016;6:26893. doi: 10.1038/srep26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li W., Huang B., Tian L., Yang Y., Zhang W., Wang X., Chen J., Sun K., Hui R., Fan X. Admission D-dimer testing for differentiating acute aortic dissection from other causes of acute chest pain. Arch. Med. Sci. 2017;13(3):591–596. doi: 10.5114/aoms.2017.67280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nazerian P., Mueller C., Soeiro A.M., Leidel B.A., Salvadeo S.A.T., Giachino F., Vanni S., Grimm K., Oliveira M.T., Jr, Pivetta E., Lupia E., Grifoni S., Morello F. ADvISED Investigators. Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes the ADvISED prospective multicenter study. Circulation. 2018;137(3):250–258. doi: 10.1161/CIRCULATIONAHA.117.029457. [DOI] [PubMed] [Google Scholar]
- 78.Itagaki R., Kimura N., Mieno M., Hori D., Itoh S., Akiyoshi K., Yuri K., Tanno K., Kawahito K., Yamaguchi A. Characteristics and treatment outcomes of acute type A aortic dissection with elevated D-dimer concentration. J. Am. Heart Assoc. 2018;7(14):e009144. doi: 10.1161/JAHA.118.009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giachino F., Loiacono M., Lucchiari M., Manzo M., Battista S., Saglio E., Lupia E., Moiraghi C., Hirsch E., Mengozzi G., Morello F. Rule out of acute aortic dissection with plasma matrix metalloproteinase 8 in the emergency department. Crit. Care. 2013;17(1):R33. doi: 10.1186/cc12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dong J., Bao J., Feng R., Zhao Z., Lu Q., Wang G., Li H., Su D., Zhou J., Jing Q., Jing Z. Circulating microRNAs: a novel potential biomarker for diagnosing acute aortic dissection. Sci. Rep. 2017;7(1):12784. doi: 10.1038/s41598-017-13104-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y., Tan X., Gao H., Yuan H., Hu R., Jia L., Zhu J., Sun L., Zhang H., Huang L., Zhao D., Gao P., Du J. Magnitude of soluble ST2 as a novel biomarker for acute aortic dissection. Circulation. 2018;137(3):259–269. doi: 10.1161/CIRCULATIONAHA.117.030469. [DOI] [PubMed] [Google Scholar]
- 82.Morello F., Bartalucci A., Bironzo M., Santoro M., Pivetta E., Ianniello A., Rumbolo F., Mengozzi G., Lupia E. Prospective diagnostic accuracy study of plasma soluble ST2 for diagnosis of acute aortic syndromes. Sci. Rep. 2020;10(1):3103. doi: 10.1038/s41598-020-59884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Earl-Royal E., Nguyen P.D., Alvarez A., Gharahbaghian L. Detection of type B aortic dissection in the emergency department with point-of-care ultrasound. Clin Pract Cases Emerg Med. 2019;3(3):202–207. doi: 10.5811/cpcem.2019.5.42928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coyle S., Moriarty T., Melody L., Ryan D. Diagnostic testing in acute aortic dissection. Curr. Emerg. Hosp. Med. Rep. 2014;2(2):97–103. doi: 10.1007/s40138-014-0044-8. [DOI] [Google Scholar]
- 85.Mishra M., Khurana P., Meharwal Z.S., Trehan N. A comparative study of imaging techniques in aortic dissection, DeBakey type I: intraoperative live three-dimensional epicardial echocardiography, multiplane transesophageal echocardiography, and multislice computed tomography. Innov Technol Tech Cardiothorac Vasc Surg. 2005 doi: 10.1097/01243895-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Wang G.X., Hedgire S.S., Le T.Q., Sonis J.D., Yun B.J., Lev M.H., Raja A.S., Prabhakar A.M. MR angiography can guide ED management of suspected acute aortic dissection. Am. J. Emerg. Med. 2017;35(4):527–530. doi: 10.1016/j.ajem.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 87.Evangelista A., Flachskampf F.A., Erbel R., Antonini-Canterin F., Vlachopoulos C., Rocchi G., Sicari R., Nihoyannopoulos P., Zamorano J., Pepi M., Breithardt O.A., Plonska-Gosciniak E. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur. J. Echocardiogr. 2010;11(8):645–658. doi: 10.1093/ejechocard/jeq056. [DOI] [PubMed] [Google Scholar]
- 88.Dong J., Duan X., Feng R., Zhao Z., Feng X., Lu Q., Jing Q., Zhou J., Bao J., Jing Z. Diagnostic implication of fibrin degradation products and D-dimer in aortic dissection. Sci. Rep. 2017;7:43957. doi: 10.1038/srep43957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rogers A.M., Hermann L.K., Booher A.M., Nienaber C.A., Williams D.M., Kazerooni E.A., Froehlich J.B., O’Gara P.T., Montgomery D.G., Cooper J.V., Harris K.M., Hutchison S., Evangelista A., Isselbacher E.M., Eagle K.A. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123(20):2213–2218. doi: 10.1161/CIRCULATIONAHA.110.988568. [DOI] [PubMed] [Google Scholar]
- 90.Diercks D.B., Promes S.B., Schuur J.D., Shah K., Valente J.H., Cantrill S.V. Clinical policy: critical issues in the evaluation and management of adult patients with suspected acute nontraumatic thoracic aortic dissection. Ann. Emerg. Med. 2015;65(1):32–42.e12. doi: 10.1016/j.annemergmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Wen D., Du X., Dong J.Z., Zhou X.L., Ma C.S. Value of D-dimer and C reactive protein in predicting inhospital death in acute aortic dissection. Heart. 2013;99(16):1192–1197. doi: 10.1136/heartjnl-2013-304158. [DOI] [PubMed] [Google Scholar]
- 92.Kaptoge S., Di Angelantonio E., Pennells L., Wood A.M., White I.R., Gao P., Walker M., Thompson A., Sarwar N., Caslake M., Butterworth A.S., Amouyel P., Assmann G., Bakker S.J., Barr E.L., Barrett-Connor E., Benjamin E.J., Björkelund C., Brenner H., Brunner E., Clarke R., Cooper J.A., Cremer P., Cushman M., Dagenais G.R., D’Agostino R.B., Sr, Dankner R., Davey-Smith G., Deeg D., Dekker J.M., Engström G., Folsom A.R., Fowkes F.G., Gallacher J., Gaziano J.M., Giampaoli S., Gillum R.F., Hofman A., Howard B.V., Ingelsson E., Iso H., Jørgensen T., Kiechl S., Kitamura A., Kiyohara Y., Koenig W., Kromhout D., Kuller L.H., Lawlor D.A., Meade T.W., Nissinen A., Nordestgaard B.G., Onat A., Panagiotakos D.B., Psaty B.M., Rodriguez B., Rosengren A., Salomaa V., Kauhanen J., Salonen J.T., Shaffer J.A., Shea S., Ford I., Stehouwer C.D., Strandberg T.E., Tipping R.W., Tosetto A., Wassertheil-Smoller S., Wennberg P., Westendorp R.G., Whincup P.H., Wilhelmsen L., Woodward M., Lowe G.D., Wareham N.J., Khaw K.T., Sattar N., Packard C.J., Gudnason V., Ridker P.M., Pepys M.B., Thompson S.G., Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012;367(14):1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Willerson J.T., Ridker P.M. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21) Suppl. 1:II2–II10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 94.Buckley D.I., Fu R., Freeman M., Rogers K., Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009;151(7):483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 95.Mori K., Tamune H., Tanaka H., Nakamura M. Admission values of d-dimer and c-reactive protein (Crp) predict the long-term outcomes in acute aortic dissection. Intern. Med. 2016;55(14):1837–1843. doi: 10.2169/internalmedicine.55.6404. [DOI] [PubMed] [Google Scholar]
- 96.Schillinger M., Domanovits H., Bayegan K., Hölzenbein T., Grabenwöger M., Thoenissen J., Röggla M., Müllner M. C-reactive protein and mortality in patients with acute aortic disease. Intensive Care Med. 2002;28(6):740–745. doi: 10.1007/s00134-002-1299-1. [DOI] [PubMed] [Google Scholar]
- 97.Komukai K., Shibata T., Mochizuki S. C-reactive protein is related to impaired oxygenation in patients with acute aortic dissection. Int. Heart J. 2005;46(5):795–799. doi: 10.1536/ihj.46.795. [DOI] [PubMed] [Google Scholar]
- 98.Sugano Y., Anzai T., Yoshikawa T., Satoh T., Iwanaga S., Hayashi T., Maekawa Y., Shimizu H., Yozu R., Ogawa S. Serum C-reactive protein elevation predicts poor clinical outcome in patients with distal type acute aortic dissection: association with the occurrence of oxygenation impairment. Int. J. Cardiol. 2005;102(1):39–45. doi: 10.1016/j.ijcard.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 99.Sakakura K., Kubo N., Ako J., Wada H., Fujiwara N., Funayama H., Ikeda N., Nakamura T., Sugawara Y., Yasu T., Kawakami M., Momomura S. Peak C-reactive protein level predicts long-term outcomes in type B acute aortic dissection. Hypertension. 2010;55(2):422–429. doi: 10.1161/HYPERTENSIONAHA.109.143131. [DOI] [PubMed] [Google Scholar]
- 100.Vrsalović M., Vrsalović Presečki A. Admission C-reactive protein and outcomes in acute aortic dissection: a systematic review. Croat. Med. J. 2019;60(4):309–315. doi: 10.3325/cmj.2019.60.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsieh W.C., Henry B.M., Hsieh C.C., Maruna P., Omara M., Lindner J. Prognostic role of admission C-reactive protein level as a predictor of in-hospital mortality in type-A acute aortic dissection: A meta-analysis. Vasc. Endovascular Surg. 2019;53(7):547–557. doi: 10.1177/1538574419858161. [DOI] [PubMed] [Google Scholar]
- 102.Takagi H., Hari Y., Nakashima K., Kuno T., Ando T. Matrix metalloproteinases and acute aortic dissection: Et Tu, Brute? Interact. Cardiovasc. Thorac. Surg. 2020;30(3):465–476. doi: 10.1093/icvts/ivz286. [DOI] [PubMed] [Google Scholar]
- 103.Li T., Jing J.J., Yang J., Sun L.P., Gong Y.H., Xin S.J., Yuan Y. Serum levels of matrix metalloproteinase 9 and toll-like receptor 4 in acute aortic dissection: a case-control study. BMC Cardiovasc. Disord. 2018;18(1):219. doi: 10.1186/s12872-018-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wen D., Zhou X.L., Li J.J., Hui R.T. Biomarkers in aortic dissection. Clin. Chim. Acta. 2011;412(9-10):688–695. doi: 10.1016/j.cca.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki T., Distante A., Zizza A., Trimarchi S., Villani M., Salerno Uriarte J.A., de Luca Tupputi Schinosa L., Renzulli A., Sabino F., Nowak R., Birkhahn R., Hollander J.E., Counselman F., Bossone E., Eagle K. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur. Heart J. 2008;29(11):1439–1445. doi: 10.1093/eurheartj/ehn162. [DOI] [PubMed] [Google Scholar]
- 106.Akutsu K., Yamanaka H., Katayama M., Yamamoto T., Takayama M., Osaka M., Sato N., Shimizu W. Usefulness of measuring the serum elastin fragment level in the diagnosis of an acute aortic dissection. Am. J. Cardiol. 2016;118(9):1405–1409. doi: 10.1016/j.amjcard.2016.07.052. [DOI] [PubMed] [Google Scholar]
- 107.Rylski B., Bavaria J.E., Beyersdorf F., Branchetti E., Desai N.D., Milewski R.K., Szeto W.Y., Vallabhajosyula P., Siepe M., Kari F.A. Type A aortic dissection in Marfan syndrome: extent of initial surgery determines long-term outcome. Circulation. 2014;129(13):1381–1386. doi: 10.1161/CIRCULATIONAHA.113.005865. [DOI] [PubMed] [Google Scholar]
- 108.Fattori R., Bacchi-Reggiani L., Bertaccini P., Napoli G., Fusco F., Longo M., Pierangeli A., Gavelli G. Evolution of aortic dissection after surgical repair. Am. J. Cardiol. 2000;86(8):868–872. doi: 10.1016/S0002-9149(00)01108-5. [DOI] [PubMed] [Google Scholar]
- 109.Takahara Y., Sudo Y., Mogi K., Nakayama M., Sakurai M. Total aortic arch grafting for acute type A dissection: analysis of residual false lumen. Ann. Thorac. Surg. 2002;73(2):450–454. doi: 10.1016/S0003-4975(01)03422-1. [DOI] [PubMed] [Google Scholar]
- 110.Shiono M., Hata M., Sezai A., Niino T., Yagi S., Negishi N. Validity of a limited ascending and hemiarch replacement for acute type A aortic dissection. Ann. Thorac. Surg. 2006;82(5):1665–1669. doi: 10.1016/j.athoracsur.2006.05.112. [DOI] [PubMed] [Google Scholar]
- 111.Song S.W., Chang B.C., Cho B.K., Yi G., Youn Y.N., Lee S., Yoo K.J. Effects of partial thrombosis on distal aorta after repair of acute DeBakey type I aortic dissection. J. Thorac. Cardiovasc. Surg. 2010;139(4):841–7.e1. doi: 10.1016/j.jtcvs.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 112.Kazui T., Washiyama N., Bashar A.H.M., Terada H., Suzuki T., Ohkura K., Yamashita K. Surgical outcome of acute type A aortic dissection: analysis of risk factors. Ann. Thorac. Surg. 2002;74(1):75–81. doi: 10.1016/S0003-4975(02)03603-2. [DOI] [PubMed] [Google Scholar]
- 113.Hsu C.P., Huang C.Y., Wu F.Y. Relationship between the extent of aortic replacement and stent graft for acute DeBakey type I aortic dissection and outcomes: Results from a medical center in Taiwan. PLoS One. 2019;14(1):e0210022. doi: 10.1371/journal.pone.0210022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lio A., Nicolò F., Bovio E., Serrao A., Zeitani J., Scafuri A., Chiariello L., Ruvolo G. Total archversushemiarch replacement for type A acute aortic dissection: A single-center experience clinical investigation. Tex. Heart Inst. J. 2016;43(6):488–495. doi: 10.14503/THIJ-15-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tiwari K.K., Murzi M., Bevilacqua S., Glauber M. Which cannulation (ascending aortic cannulation or peripheral arterial cannulation) is better for acute type A aortic dissection surgery? Interact. Cardiovasc. Thorac. Surg. 2010;10(5):797–802. doi: 10.1510/icvts.2009.230409. [DOI] [PubMed] [Google Scholar]
- 116.Ren Z., Wang Z., Hu R., Wu H., Deng H., Zhou Z., Hu X., Jiang W. Which cannulation (axillary cannulation or femoral cannulation) is better for acute type A aortic dissection repair? A meta-analysis of nine clinical studies. Eur. J. Cardiothorac. Surg. 2015;47(3):408–415. doi: 10.1093/ejcts/ezu268. [DOI] [PubMed] [Google Scholar]
- 117.Conzelmann L.O., Weigang E., Mehlhorn U., Abugameh A., Hoffmann I., Blettner M., Etz C.D., Czerny M., Vahl C.F. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur. J. Cardiothorac. Surg. 2016;49(2):e44–e52. doi: 10.1093/ejcts/ezv356. [DOI] [PubMed] [Google Scholar]
- 118.Kawahito K., Adachi H., Murata S., Yamaguchi A., Ino T. Coronary malperfusion due to type A aortic dissection: mechanism and surgical management. Ann. Thorac. Surg. 2003;76(5):1471–1476. doi: 10.1016/S0003-4975(03)00899-3. [DOI] [PubMed] [Google Scholar]
- 119.Chiappini B., Schepens M., Tan E., Dell’ Amore A., Morshuis W., Dossche K., Bergonzini M., Camurri N., Reggiani L.B., Marinelli G., Di Bartolomeo R. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur. Heart J. 2005;26(2):180–186. doi: 10.1093/eurheartj/ehi024. [DOI] [PubMed] [Google Scholar]
- 120.Cruz I, Stuart B, Caldeira D, et al. Controlled pericardiocentesis in patients with cardiac tamponade complicating aortic dissection: experience of a centre without cardiothoracic surgery. Eur Hear journal Acute Cardiovasc Care. 2015;4(2):124–128. doi: 10.1177/2048872614549737. [DOI] [PubMed] [Google Scholar]
- 121.Krüger T., Weigang E., Hoffmann I., Blettner M., Aebert H. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation. 2011;124(4):434–443. doi: 10.1161/CIRCULATIONAHA.110.009282. [DOI] [PubMed] [Google Scholar]
- 122.Hiratzka L.F., Bakris G.L., Beckman J.A., et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: Executive summary: A report of the American College Of Cardiology Foundation/American Heart Association Task Force on practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 123.Suzuki T., Isselbacher E.M., Nienaber C.A., Pyeritz R.E., Eagle K.A., Tsai T.T., Cooper J.V., Januzzi J.L., Jr, Braverman A.C., Montgomery D.G., Fattori R., Pape L., Harris K.M., Booher A., Oh J.K., Peterson M., Ramanath V.S., Froehlich J.B. Type-selective benefits of medications in treatment of acute aortic dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am. J. Cardiol. 2012;109(1):122–127. doi: 10.1016/j.amjcard.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 124.Hebballi R., Swanevelder J. Diagnosis and management of aortic dissection. Contin. Educ. Anaesth. Crit. Care Pain. 2009;9(1):14–18. doi: 10.1093/bjaceaccp/mkn044. [DOI] [Google Scholar]
- 125.Powell J.T., Hinchliffe R.J., Thompson M.M., Sweeting M.J., Ashleigh R., Bell R., Gomes M., Greenhalgh R.M., Grieve R.J., Heatley F., Thompson S.G., Ulug P. Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br. J. Surg. 2014;101(3):216–224. doi: 10.1002/bjs.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kodama K., Nishigami K., Sakamoto T., Sawamura T., Hirayama T., Misumi H., Nakao K. Tight heart rate control reduces secondary adverse events in patients with type B acute aortic dissection. Circulation. 2008;118(14) Suppl.:S167–S170. doi: 10.1161/CIRCULATIONAHA.107.755801. [DOI] [PubMed] [Google Scholar]
- 127.Zhou J.C., Zhang N., Zhang Z.H., Wang T.T., Zhu Y.F., Kang H., Zhang W.M., Li D.L., Li W.D., Liu Z.J., Qian X.M., Zhang M.Y., Wang J., Zhou M., Yang Z.T., Yu Y.X., Li H.Y., Zhang J., Wang Y.G., Gao J.P., Ling L., Pan K.H. Intensive blood pressure control in patients with acute type B aortic dissection (RAID): study protocol for randomized controlled trial. J. Thorac. Dis. 2017;9(5):1369–1374. doi: 10.21037/jtd.2017.03.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nienaber C.A., Rousseau H., Eggebrecht H., Kische S., Fattori R., Rehders T.C., Kundt G., Scheinert D., Czerny M., Kleinfeldt T., Zipfel B., Labrousse L., Ince H. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120(25):2519–2528. doi: 10.1161/CIRCULATIONAHA.109.886408. [DOI] [PubMed] [Google Scholar]
- 129.Nienaber C.A., Kische S., Rousseau H., Eggebrecht H., Rehders T.C., Kundt G., Glass A., Scheinert D., Czerny M., Kleinfeldt T., Zipfel B., Labrousse L., Fattori R., Ince H. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ. Cardiovasc. Interv. 2013;6(4):407–416. doi: 10.1161/CIRCINTERVENTIONS.113.000463. [DOI] [PubMed] [Google Scholar]
- 130.Brunkwall J., Kasprzak P., Verhoeven E., Heijmen R., Taylor P., Alric P., Canaud L., Janotta M., Raithel D., Malina W., Resch T., Eckstein H.H., Ockert S., Larzon T., Carlsson F., Schumacher H., Classen S., Schaub P., Lammer J., Lönn L., Clough R.E., Rampoldi V., Trimarchi S., Fabiani J.N., Böckler D., Kotelis D., Böckler D., Kotelis D., von Tenng-Kobligk H., Mangialardi N., Ronchey S., Dialetto G., Matoussevitch V. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur. J. Vasc. Endovasc. Surg. 2014;48(3):285–291. doi: 10.1016/j.ejvs.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 131.Tsai T.T., Evangelista A., Nienaber C.A., Myrmel T., Meinhardt G., Cooper J.V., Smith D.E., Suzuki T., Fattori R., Llovet A., Froehlich J., Hutchison S., Distante A., Sundt T., Beckman J., Januzzi J.L., Jr, Isselbacher E.M., Eagle K.A. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N. Engl. J. Med. 2007;357(4):349–359. doi: 10.1056/NEJMoa063232. [DOI] [PubMed] [Google Scholar]
- 132.Lombardi J.V., Cambria R.P., Nienaber C.A., Chiesa R., Teebken O., Lee A., Mossop P., Bharadwaj P. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J. Vasc. Surg. 2012;55(3):629–640.e2. doi: 10.1016/j.jvs.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 133.Alsac J.M., Girault A., El Batti S., Abou Rjeili M., Alomran F., Achouh P., Julia P., Fabiani J.N. Experience of the Zenith Dissection Endovascular System in the emergency setting of malperfusion in acute type B dissections. J. Vasc. Surg. 2014;59(3):645–650. doi: 10.1016/j.jvs.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 134.Lombardi J.V., Cambria R.P., Nienaber C.A., Chiesa R., Mossop P., Haulon S., Zhou Q., Jia F. Aortic remodeling after endovascular treatment of complicated type B aortic dissection with the use of a composite device design. J. Vasc. Surg. 2014;59(6):1544–1554. doi: 10.1016/j.jvs.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 135.He H., Yao K., Nie W.P., Wang Z., Liang Q., Shu C., Dardik A. Modified petticoat technique with pre-placement of a distal bare stent improves early aortic remodeling after complicated acute Stanford type B aortic dissection. Eur. J. Vasc. Endovasc. Surg. 2015;50(4):450–459. doi: 10.1016/j.ejvs.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 136.Yuan X., Mitsis A., Ghonem M., Iakovakis I., Nienaber C.A. Conservative management versus endovascular or open surgery in the spectrum of type B aortic dissection. J. Vis. Surg. 2018;4:59–9. doi: 10.21037/jovs.2018.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fattori R., Tsai T.T., Myrmel T., Evangelista A., Cooper J.V., Trimarchi S., Li J., Lovato L., Kische S., Eagle K.A., Isselbacher E.M., Nienaber C.A. Complicated acute type B dissection: is surgery still the best option?: a report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc. Interv. 2008;1(4):395–402. doi: 10.1016/j.jcin.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 138.Brunt M.E., Egorova N.N., Moskowitz A.J. Propensity score-matched analysis of open surgical and endovascular repair for type B aortic dissection. Int. J. Vasc. Med. 2011;2011:364046. doi: 10.1155/2011/364046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng D., Martin J., Shennib H., Dunning J., Muneretto C., Schueler S., Von Segesser L., Sergeant P., Turina M. Endovascular aortic repair versus open surgical repair for descending thoracic aortic disease a systematic review and meta-analysis of comparative studies. J. Am. Coll. Cardiol. 2010;55(10):986–1001. doi: 10.1016/j.jacc.2009.11.047. [DOI] [PubMed] [Google Scholar]