Abstract
The immune response following acute stroke has received significant attention. The spleen is an important immune organ, and more and more studies have shown that brain-spleen crosstalk after stroke plays an important role in its development and prognosis. There are many mechanisms of spleen activation after stroke, including activation of the sympathetic nervous system, the production of chemokines, and antigen presentation in the damaged brain. The changes in the spleen after stroke are mainly reflected in morphology, changes to immune cells, and cytokine production. Once activated, the spleen contracts, undergoes cellular changes, and releases inflammatory cytokines. Some studies have also shown that spleen cells specifically migrate to the site of primary brain injury. The size of the spleen is also negatively correlated with infarct volume — the more serious the spleen atrophy, the larger the infarct volume. Therefore, a comprehensive understanding of the dynamic response of the spleen to stroke will not only enable understanding of the evolution of ischemic brain injury but will also enable the identification of potential targets for stroke treatment. Here, we review recent basic and clinical drug studies on the spleen as a target for the treatment of stroke, focusing on therapeutic strategies for regulating the splenic response and inhibiting secondary brain injury.
Keywords: Stroke, spleen, immune response, splenic atrophy, splenectomy, stem cell therapy
1. INTRODUCTION
The central nervous system (CNS) and the immune system interact in complex ways after stroke [1]. Stroke-induced inflammatory processes, including innate and adaptive immune mechanisms, are responses to tissue damage caused by a lack of blood supply. They are also considered key factors in the pathophysiology of ischemic stroke at all stages [2]. In addition to the inflammatory response in the brain, there is growing evidence that systemic immune changes occur in peripheral organs and tissues, including the spleen, bone marrow, and lymph nodes after stroke. CNS and various peripheral immune cells become activated and play an important role in brain damage and repair. The spleen is a major reservoir of immune cells, and it releases immune cells into the blood circulation to protect against infection and injury, including damage to the central nervous system [3]. Preclinical studies and a large number of animal experiments have shown that in the acute phase of stroke, the spleen will contract sharply [4-6]. In one study, there was a negative correlation between the size of the spleen and infarct volume after tMCAO; the more serious the atrophy of spleen, the larger the infarct volume [4]. A growing number of studies have focused on the splenic response after stroke and have emphasized the role of the spleen in mediating secondary brain injury associated with the peripheral immune system [7]. Therefore, studying the evolution of the splenic response after stroke not only contributes to an in-depth understanding of the development of ischemic brain injury but also provides a potential target for the treatment of stroke. This review focuses on the crosstalk between the brain and spleen after stroke.
Additionally, we review animal and clinical studies using the spleen as a target for stroke treatment, including the use of splenectomy and stem cell therapy. Targeting the spleen may help to regulate post-stroke inflammation and inhibit secondary brain injury. However, these treatments are accompanied by several clinical challenges.
2. THE STRUCTURE AND FUNCTION OF SPLEEN
The spleen consists mainly of red pulp (RP) and white pulp (WP), which differ in function and structure. Between these two regions is the rodent marginal zone (MZ) and human perilobular zone [8, 9]. Unlike the lymph nodes, the spleen lacks afferent lymphatic vessels. Therefore, all cells and antigens enter the spleen via blood circulation. Splenic RP extracts aged, dead, or opsonized cells from the circulation, and pathogens and tissue damage are detected at the same time. The RP contains macrophages, which filter the blood as it passes through the spleen, and antibody-producing B cells, which quickly enter the circulation. The WP is the main immunologically active region of the spleen and serves as the communication hub of various immune cells, including T cells, B cells, macrophages/monocytes (MMs), and dendritic cells (DCs). The RP also has concentrated areas of lymphocytes. The proximity of these cells makes it easy for T cells and B cells to interact with each other, resulting in activation of the adaptive immune response and easy access to lymphocytes for macrophages and DCs. T cells are concentrated around arterioles, forming lymphoid sheaths. This results in T cells being positioned very close to the blood supply, allowing DCs to come into contact with immature T cells, which leave the spleen once activated. B cells are organized into B cell follicles, where B cell responses are honed by homotypic transformation and increased antigen specificity. These processes require T cells, and the highly specialized structure of the spleen allows these cells to be in close proximity. The MZ is located at the junction of the RP and WP. Lymphocytes in this zone are more sparse than they are in the WP and consist mainly of B cells, but there are also more macrophages [3, 10].
Sympathetic nerve fibers account for 98% of the nerve fibers innervating the spleen [11], and norepinephrine is their main neurotransmitter. The immune response is regulated through these splenic fibers [12]. Most of these fibers are found in the WP and regulate lymphocytes, eosinophils, mast cells, and macrophages [13]. The activation of the sympathetic nervous system leads to spleen contraction through α-1 receptors on smooth muscle cells. In addition, increased cortisol production [14] leads to activation of the hypothalamic-pituitary-adrenal axis [15], overactivation of the sympathetic nervous system (SNS) [14], and loss of parasympathetic tone. The splenic sympathetic nerves mainly impact immune function via two mechanisms: by regulating splenic blood perfusion and by regulating splenic immune cell function.
3. BRAIN-SPLEEN COMMUNICATION AFTER STROKE
3.1. Brain-spleen Interactions after Stroke
After a stroke, brain damage can lead to spleen atrophy. The spleens of rats decrease in size and weight after middle cerebral artery occlusion (MCAO) [4, 5]. The spleens began to shrink temporarily from 24 to 48 hours post permanent MCAO (pMCAO), reached their minimum size at 48 hours, and returned to their pre-stroke size by 96 hours [16]. The spleens of mice also atrophied and decreased in weight after stroke [17-19]. One study showed that the spleens of mice began to decrease in weight from 3 hours to 7 days after transient MCAO (tMCAO) [20]. Clinical studies have also evaluated changes in the splenic volume of stroke patients. One study showed that the loss of spleen volume in patients with ischemic stroke began less than 6 hours after the stroke. The splenic atrophy continued to about 3 days after the stroke, then the spleens increased gradually from days 4 to 8 and subsequently returned to their pre-stroke state [21]. The change in spleen volume with time was biphasic. Spleen volume initially decreased with time, reached its lowest point at 48 hours after the onset of stroke, and then increased [6]. Vahidy et al. found that 40% of stroke patients had a splenic contraction, which was associated with higher NIHSS at admission and during hospitalization. African-Americans, elderly patients, and patients with a previous history of stroke had a significantly higher risk of splenic contraction after stroke [22]. The spleen volume after stroke varied with gender but did not correlate with age, stroke subtype, or cerebral infarction volume [6]. Early splenic contraction (< 24 hours) was not associated with systemic inflammatory response syndrome [23].
There was a negative correlation between the size of the spleen and infarct volume after tMCAO-the more serious the atrophy of the spleen, the larger the infarct volume [4]. Splenectomy can reduce the volume of the cerebral infarction and improve neurological recovery [19, 24-30]. Sahota et al. have shown that the spleen volume continues to decrease in some patients, which is associated with an increased risk of adverse clinical outcomes [21].
3.2. The Effect of Brain Injury on the Spleen after Stroke
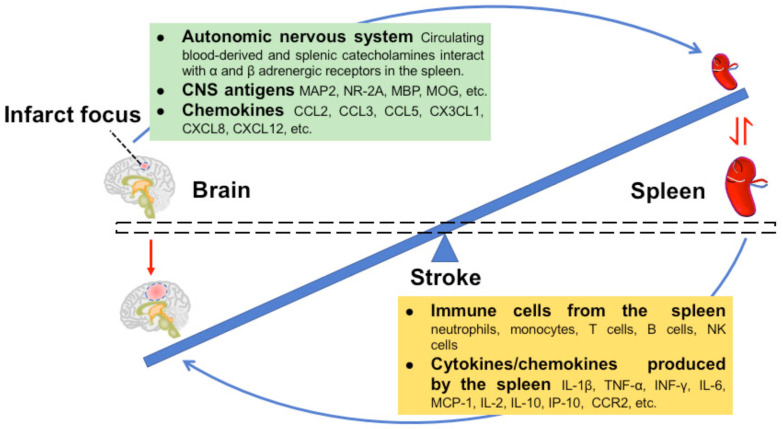
It has been shown that several events, including autonomic nervous system (ANS) activation, CNS antigen release, and chemokine/chemokine receptor interactions, are essential for effective brain-spleen crosstalk after stroke.
3.2.1. Autonomic Nervous System
Recent studies have shown a link between ANS activity and the peripheral immune response [31, 32]. The spleen is highly innervated by the sympathetic neural network [13]. Activation of the SNS after MCAO leads to an increase in the level of catecholamine in the spleen, which is directly innervated by the splenic nerve. However, splenic denervation before MCAO did not change the infarct volume or spleen size. In addition, the levels of catecholamines in the systemic circulation increased after release from the adrenal medulla after MCAO, and these catecholamines bind to α and β adrenergic receptors in the spleen. The activation of the α1-adrenergic receptor (α1-AR) in the splenic smooth muscle capsule resulted in the contraction of the splenic capsule, which led to the reduction in splenic volume [33]. An α1-AR antagonist blocked the reduction of splenic volume. Blocking α and β adrenergic receptors significantly inhibited splenic contraction and reduced the infarct volume [5]. Other studies have shown that the reduction in spleen size induced by MCAO is related to changes in metanephrine (MN), normetanephrine (NMN), and cytokines. The β adrenergic receptor antagonist Propranolol partially reversed the immunosuppression and splenic volume reduction [34]. These findings suggest that circulating blood-derived catecholamines regulate the splenic response to stroke by interacting with α and β adrenergic receptors.
3.2.2. CNS Antigens
After ischemia, the injured brain secretes a variety of antigens which activate the adaptive immune response and recruit immune cells from the spleen. In acute stroke, new antigens such as microtubule associated protein 2 (MAP2), N-methyl-D-aspartaic acid receptor subunit 2 (NR-2A), myelin basic protein (MBP), and myelin oligodendrocyte glycoprotein (MOG) are released into the periphery and captured by antigen presenting cells (APC), especially DCs and macrophages. This response is thought to eventually trigger the activation of the T cell-dependent adaptive immune response in the T cell region [35, 36]. Mice with smaller infarct sizes had greater self-responsiveness to MAP2 and MBP. This autoimmune reaction is maintained by CD4+ and CD8+ T cells and CD19+ B cells in the spleen in the first 10 days after stroke [37]. The autoimmune response of splenic CD4+ T cells to neuron and myelin antigens in the early stage of stroke correlates with better recovery [38]. In previous studies, MOG-reactive splenocytes secreting toxic Th1 cytokines, such as interferon-γ (INF-γ) and tumor necrosis factor-α (TNF-α), have been adoptively transferred into severe combined immunodeficient (SCID) mice. Compared to the control group, the cerebral ischemic area, neurological defects, and immune cell accumulation were increased in the adoptive transfer group [39]. In another study, MBP-specific splenocytes were obtained from donor animals one month post-stroke and passed on to immature recipient animals during cerebral ischemia (CI). The animals receiving MBP-specific TH1 or TH17 cells had a poor prognosis, and the degree of injury was related to the robustness of the MBP-specific TH1 and TH17 responses [40].
3.2.3. Chemokines/chemokine Receptors
After ischemia, the expression of chemokines in the brain and blood increases [41-44]. These chemokines, including chemokine (C-C motif) ligand 2 (CCL2), CCL3, CCL5, CX3CL1, CXCL8, and CXCL12, are secreted by damaged CNS cells to recruit inflammatory cells into the injured brain. The corresponding chemokine receptors in splenocytes are also increased after ischemia/reperfusion (I/R) [44]. CCL2 can effectively mediate the infiltration of MMs and neutrophils during CI [45]. Some MMs and neutrophils in the spleen express CCR2. Splenic monocytes enter the ischemic focus after ischemia [46], and these cells migrate from the spleen to the ischemic brain through the CCL2-CCR2 axis [18, 45, 47]. In addition, the CXCL12-CXCR4 axis is closely related to the pathology of ischemic stroke. CI results in a rapid and lasting increase in CXCL12 in the ischemic penumbra [48]. When a CXCR4 antagonist was used to block the interaction between CXCR4 and CXCL12, not only were cerebral inflammation and cerebral infarction reduced after tMCAO, but splenic atrophy was prevented and splenocyte death was delayed. Therefore, CXCL12 and CXCR4 may play a role in regulating the splenic response after stroke [49].
In short, ANS activation after stroke leads to increased levels of catecholamines in the spleen and circulation. Catecholamines interact with α and β adrenergic receptors of splenic smooth muscle, resulting in splenic contraction. Ischemia leads to the release of CNS antigens produced by the brain to the periphery, which can recruit immune cells responses in the spleen. Chemokines produced by ischemic brain tissue recruit more spleen immune cells into the ischemic brain and aggravate inflammatory response.
3.3. The Effect of the Spleen on the Brain after Stroke
Both cellular and humoral immune responses occur in the spleen in response to brain injury after stroke. When the spleen atrophies, many splenic cells are released into the circulation and migrate to the damaged cerebral hemisphere. The humoral response after stroke is characterized by the production of inflammatory cytokines and chemokines in the spleen.
3.3.1. Timing of the Splenic Response to Stroke
The splenic response after a stroke occurs in the early stage after MCAO, and studies have shown that splenectomy in the early stages of stroke can reduce the infiltration of peripheral immune cells into the ischemic brain. One study found that immediate splenectomy after ischemia reduced the infiltration of T cells, B cells, and monocytes into the brain tissue, reduced the early infarct volume, and partially improved neurological function. When performed 3 days after ischemia, splenectomy had no corresponding effect [50].
3.3.2. Immune Cells in the Spleen
The spleen is the largest reservoir of innate immune cells. Many splenic immune cells undergo changes after stroke, including lymphocytes, monocytes, neutrophils, and NK cells. During splenic contraction, spleen cells are released into the circulation and infiltrate into the damaged brain tissue (Table 1). Splenectomy results in a decrease in the number of T cells, neutrophils, and macrophages in ischemic brain tissue [26]. Clinically, the spleens of patients with ischemic stroke may initially contract and then expand again after onset, leading to ischemic brain injury [21].
Table 1.
Response of the immune cells from the spleen at different time points after stroke.
Studies have shown that splenic lymphocytes, monocytes, and neutrophils enter the bloodstream and migrate into the brain 48 hours after MCAO, but they appear to remain within the vasculature. NK cells and monocytes can be seen at 48 hours, and NK cells, T cells, and monocytes can be seen at 96 hours post MCAO [16]. Neutrophils are among the first immune cells to respond to ischemic damage, and they infiltrate the damaged brain within hours after a stroke. In patients with acute ischemic stroke, neutrophils gather in the ischemic area within 24 hours after the onset of symptoms [51]. In an animal model, neutrophil infiltration reached its peak at 1-3 days after cerebral infarction. When performed 2 weeks before pMCAO, splenectomy reduced the number of neutrophils in the injured brain tissue [24]. Neutrophil infiltration plays an important role in the pathological process of stroke [24, 51]. Peripheral blood monocytes/macrophages migrate and infiltrate into ischemic brain tissue through the CCL2-CCR2 axis and promote brain inflammation and tissue injury after stroke [18]. In one study, the Ly6Chigh monocyte subset in spleen decreased from day 1 to day 3, and the number of Ly6Clow monocytes decreased from 3 hours to 7 days after stroke. The number of peripheral monocytes in the brain began to increase on the 1st day and reached its peak on the 3rd day.
The Ly6Chigh and Ly6Clow monocytes in the post-stroke brain all originated from the spleen, and the deployment of spleen-derived MMs is consistent with their accumulation in the brain after ischemia. Splenectomy reduced the accumulation of MMs in the ischemic brain at 1 and 3 days post-ischemia [20]. It has also been shown that clodronate liposomes can reduce the number of macrophages in the spleens of mice by 80%, resulting in a reduction in macrophage infiltration into the ischemic brain tissue and thus promoting tissue repair and remodeling after cerebral ischemia [52]. Systemic injection of low-dose lipopolysaccharide (LPS) after MCAO in mice can induce the Ly6Chigh monocyte response, which has a protective effect on the brain. Infarct volume was significantly smaller in both LPS-preconditioned mice and in mice receiving adoptively transferred LPS-treated monocytes. Applied cell tracking studies have shown that these cells originate in the spleen. They migrate from the spleen to the brain and meninges, where they inhibit post-ischemic inflammation and the flow of neutrophils into the brain parenchyma. These protective effects disappeared when splenectomy was performed 14 days before ischemia, indicating the necessity of the spleen in mediating these effects [46]. Studies have shown that at 96 hours post-stroke, spleen monocytes and neutrophils in aged mice are decreased, while T and B cells did not change. The reduction was cell-specific rather than secondary to the overall contraction of the spleen. Splenectomy before stroke significantly reduced the number of CD45high cells in the brain tissue at 96 hours post stroke. These results indicate that the infiltrating spleen-derived immune cells in the brains of aged mice are mainly monocytes and neutrophils [30].
In clinical studies, spleen volume was negatively correlated with the percentage of blood lymphocytes in patients with early stroke, suggesting that greater spleen contraction resulted in the migration of more lymphocytes into the bloodstream [6]. One study used labeled splenic cells to show that spleen-derived T cells could be seen in the brain at 96 hours after MCAO [16]. Recombinant T cell receptor ligands (RTLs) are partial agonists that redirect autoreactive T cells to become nonpathogenic. In young mice, RTL1000 inhibited splenocyte outflow and reduced the frequencies of T cells and macrophages in the spleen. Elderly mice treated with RTL1000 showed a significant decrease in inflammatory cells in the brain and an inhibition of splenic atrophy. We found a significant reduction in the frequency of CD4+ T cells with RTL treatment in both young and old mice. RTL treatment also significantly reduced the percent of CD8+ T cells, CD3+ total T cells, and CD11b+ myeloid cells in young mice only. There were major differences in splenocyte activation between the younger and older mice in response to stroke [53]. The protective effect of splenectomy 2 weeks before ischemia on the brain is accompanied by a decrease in the number of T cells at the site of brain injury [26]. Immediate splenectomy after ischemia, but not delayed splenectomy, reduced the infiltration of T cells and B cells into the brain tissue [29]. Splenic irradiation also decreased the number of T cells infiltrating into the brain [25]. Many studies have confirmed that T lymphocytes play a harmful role in I/R injury [54-56]. However, studies have demonstrated harmful and beneficial effects of Th1 and Th2 cells in vivo and in vitro [54]. In addition, certain T cell subsets, such as regulatory T cells (Tregs), may play a protective role in the pathological process of stroke [57, 58]. The number of Tregs in the spleen increase after stroke, and they are relatively resistant to apoptosis and other mechanisms that lead to a decrease in the number of viable splenocytes. This may reflect an endogenous protection mechanism [17]. In addition, it is known that splenic T lymphocytes can be activated by APCs, especially DCs. An increase in the number of DCs was observed for both pMCAO and tMCAO in mice [59]. Therefore, DCs can present antigens to T lymphocytes in the spleen and activate acquired immunity after stroke. However, the exact effect of splenic DCs on the prognosis of stroke is not clear. Spleen atrophy is accompanied by the apoptosis of spleen cells and loss of B cells in the germinal center. In mice, B cells account for around 60% of spleen and blood mononuclear cells. By 96 hours post-stroke, the percentage of B cells decreased by about half to 30% [17]. Studies have shown that μMT-/- mice with B-cell deficiency have larger infarct volumes, higher mortality, and more severe functional defects [60]. After experimental stroke in mice, the B cell populations limited the infarct volume and functional neurological dysfunction and inhibited the activation and recruitment of inflammatory T cells, macrophages, and microglia into the central nervous system infarction. These regulatory activities are related to an increase in the proportion of CD19+ B cells secreting interleukin (IL)-10 [61]. In addition, after experimental stroke, a β-adrenergic receptor antagonist (Propranolol) prevented the loss of MZ B cells in the spleen and maintained the level of IgM [62]. B cells are the most common type of lymphocyte in the spleen, but few studies have focused on the role of B cells in stroke injury. The effects of spleen-derived B cells on ischemic brain tissue are worthy of further study.
Spleen-derived NK cells were found in the brain 48 hours and 96 hours after MCAO [16]. NK cells are cytotoxic cells, and studies have shown that chemokines produced by ischemic neurons cause NK cells to migrate and permeate into the brain, where they promote further brain damage [63-66].
3.3.3. Cytokines/Chemokines Produced by the Spleen
Activated immune cells in the spleen increase levels of cytokines in the blood after stroke, which in turn leads to higher levels of cytokines in the brain (Table 2). At 6 and 22 hours post MCAO, the levels of TNF-α, IFN-γ, IL-6, monocyte chemoattractant protein-1 (MCP-1), and IL-2 secreted by activated splenocytes in stroke-injured mice were significantly increased, while the level of anti-inflammatory cytokine IL-10 was also increased in varying degrees [44]. The levels of TNF-α, IFN-γ, and IL-6 in the brains of mice increased significantly in the early stage of stroke [27, 44]. However, the inflammatory cytokines in the brain decreased significantly at 96 hours post-stroke [17]. INF-γ increased in the spleen in the early stage after MCAO and then in the brain. IFN-γ was reduced in the infarct post-MCAO after splenectomy [19, 27]. In addition, many of these splenocyte-derived inflammatory cytokines and chemokines, such as INF-γ and interferon-inducible protein-10 (IP-10), have been shown to play a key role in stroke-induced neurodegeneration [27, 67]. After cerebral ischemia or hemorrhagic stroke in mice or rats, the mRNA levels of IL-1β, TNF-α, INF-γ, IL-4, and IL-6 in the spleen increased [55, 68, 69]. In clinical studies, the levels of some cytokines were increased in patients with splenic contraction compared to patients without splenic atrophy, including INF-γ, IL-6, IL-10, IL-12, and IL-13 [22]. Splenectomy can also affect cytokine levels in the brain after stroke. On the 4th day after MCAO, many pro-inflammatory cytokines (including TNF-α, IL-12, IL-1a, IL-13, and IL-6) were significantly lower in the peripheral blood of splenectomized elderly mice than normal mice, suggesting that splenectomy led to a reduction in the peripheral pro-inflammatory state in splenectomized elderly mice [30]. In addition, splenectomy resulted in lower levels of IL-1β, TNF-α, and INF-γ in the brain but higher levels of IL-10 [19, 26, 27]. Additionally, INF-γ activated the expression of the inflammatory chemokine interferon-inducible protein 10 (IP-10). Splenectomy results in decreased IP-10 in the infarcted area following MCAO [67]. IP-10 increases blood-brain barrier damage induced by natural killer (NK) cells through CXCR3 [63]. From these studies, we can infer that the spleen can affect systemic inflammation and brain inflammation after stroke by releasing immune cells and cytokines. These are potential targets of new treatment strategies for stroke.
Table 2.
Changes of spleen-derived cytokines/chemokines in brain and spleen at different time points after stroke.
To sum up, the size of the spleen is also negatively correlated with infarct volume. Some studies used cell tracking techniques and splenectomy to observe the changes of spleen-derived immune cells and cytokines/chemokines in spleen and brain tissue at different time points after stroke. These studies suggest that with 3 days after stroke, the spleen releases immune cells and cytokines/chemokines into circulation and finally recruits to ischemic brain tissue. These immune cells and cytokines play an important role in the inflammatory response of ischemic brain parenchyma in the early stage after stroke. Finally, it leads to the enlargement of the volume of cerebral infarction (Fig. 1).
Fig. (1).
Crosstalk between the spleen and brain in the acute phases of stroke. The ischemic brain induces a reduction in spleen volume by stimulating the autonomic nervous system and releasing chemokines and CNS antigens, which cause the spleen to release immune cells and cytokines into the blood. The immune cells and cytokines traffic to the ischemic brain tissue and aggravate the cerebral infarction. This results in a cycle of injury from brain-to-spleen-to-brain. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. THERAPEUTIC STRATEGIES TARGETING THE SPLENIC RESPONSES TO STROKE
In previous studies, it has been found that the evolving cerebral ischemic injury elicits a cycle of injury from brain-to-spleen-to-brain [70]. The evolving brain injury sends a “signal” to activate the spleen, which leads to apoptosis and a sharp loss of immune cells. The activated spleen also releases cells into the bloodstream, which are then transported to the brain through the capillaries. If any intermediate link of the brain-spleen-brain cycle is blocked, it may reduce brain injury after stroke and play a neuroprotective role (Table 3).
Table 3.
Treatments of stroke with spleen as target.
| Treatment | Target Cells or Moleculars | Effects on the Spleen | Effects on the Injured Brain | Refs. | ||||
|---|---|---|---|---|---|---|---|---|
| Splenectomy | All the splenocytes | - | • Reduced infarct volume and edema, improved neurobehavioral and infarct outcomes. A reduction in peripheral immune cell infiltration into the brain and decreased levels of peripheral inflammatory cytokines after stroke. • It could not promote long-term functional recovery after MCAO. |
[19, 24, 26, 27, 28, 29,30, 69, 71, 72] | ||||
| Stem cells therapy | Spleen cells | Prior to migrating to the spleen. Rescued the spleen weight, splenic CD8+T-cells. Attenuated splenic activations of TNF-α, IL-6, NF-κB |
• Decrease the infarct volume and have significant recovery in behavioral performance. • Reduced the brain oedema and the initial neurologic deficits, infalmmatory infiltrations and apoptosis. |
[4, 68, 69, 81, 82, 83, 85, 86, 88, 89] | ||||
| LPS-Preconditioning | Ly6Chigh monocytes from the spleen | Mobilized a Ly6Chigh monocytes from the spleen to brain and meninges after stroke. | Reduced infarct volume. Increased Ly6Chigh monocytes in brain and meninges, where they suppressed postischemic inflammation and neutrophil influx into the brain parenchyma after stroke. | [46] | ||||
| remote ischemic preconditioning (RIPC) | Lymphocytes in spleen | Increased splenic volume with an expansion of splenic lymphocytes 3 days after MCAO. promptly increased the percentages of CD3+CD8+cytotoxic T (Tc) cells in the spleen with a relatively delayed elevation in CD3+CD161+ natural killer T (NKT) and CD3-CD45RA+ B lymphocytes. |
Reduced infarct volume and neurological deficit and reduced brain infiltration of Tc and NKT cells. | [93] | ||||
| remote ischemic limb conditioning (RLC) | CCR2+ monocyte subset in spleen | Adoptive transfer of CCR2-deficient monocytes abolished RLC-mediated protection in splenectomized mice. Shifted circulating monocytes to a CCR2+ pro-inflammatory monocyte subset |
Reduced acute brain injury, swelling, and improved motor/gait function in chronic stroke. | [94] | ||||
| carvedilol | Pan adrenergic receptors in the spleen | Prevented the reduction in spleen size. |
Significantly reduced infarct volume | [5] [34] |
||||
| prazosin | α1 receptor in the spleen | Prevented the reduction in spleen size |
No effect | |||||
| propranolol | β receptor in the spleen | No effect or Partly reverse the immunodepression and the reduction in spleen volume | No effect | |||||
| Simvastatin | Mitochondria of splenocytes | Reduced stroke-induced spleen atrophy and splenic apoptosis via increased mitochrondrial antiapoptotic Bcl-2 expression and decreased proapoptotic Bax translocation from cytosol into mitochondria. | Inhibited brain interferon-γ (3 days) and reduced infarct volume and neurological deficits (5 days) after stroke. | [19] | ||||
| Treatment | Target Cells or Moleculars | Effects on the Spleen | Effects on the Injured Brain | Refs. | ||||
| αCD147(an antiCD147 antibody) | monocytes/macrophages in spleen | Reduced the early splenic inflammatory response. Reduced the early proinflammatory activation of splenic monocytes/macrophages after tMCAO |
• Reduced Ly6Chi monocyte/macrophages in the brain. • CD147 as a key mediator of the spleen’s inflammatory activation in response to cerebral ischemia. |
[95] | ||||
| Recombinant mouse IL-33 | splenic T cells | Reduced INF-γ+ Tcells and incteased Foxp3+ T cells in the spleen tissue. Deceased the production of INF-γ and increased the secretion of IL-4, IL-10, and TGF-β from at 24 h after MCAO. |
Attenuated neurological deficit scores and infarct volumes after MCAO. | [97] | ||||
| RTL551 RTL1000 |
Spleinc T cells | Mitigated splenic atrophy. Significantly Increased the splenic cells in the spleen after MCAO. Reduced expression of the chemokine receptors, CCR5 in the spleen. |
• Reduced cortical and total stroke lesion size by approximately 50%, inhibited the accumulation of inflammatory cells, particularly macrophages/activated microglial cells and dendritic cells. • Reduced the frequency of the activation marker, CD44, on T-cells in the blood and in the ischemic hemisphere. |
[53, 99, 100, 101] | ||||
| selective endovascular cooling | Splenic cells | Promotes anti-inflammatory IL-10 elevation in the spleen. Hypothermia-exposed splenocytes co-cultured with primary rat neurons upregulate BNDF and IL-10 and improve cell viability following OGD. | Increase BDNF expression in the motor cortex, striatum, and hippocampal CA3. | [102] | ||||
| Clodronate liposomes | Macrophages in spleen | Depleted 80% of the macrophages in the spleen | Reduced macrophage infiltration in the brain. Enhanced the microvessel density in the peri-infarct region, decreased brain atrophy, and promoted neurological recovery. | [52] | ||||
4.1. Splenectomy
Many studies have used splenectomy to study the role and pathological mechanisms of the spleen during a stroke. Several studies have shown that splenectomy can reduce the volume of cerebral infarction and have neuroprotective effects. However, some studies have found that splenectomy does not provide neuroprotection for cerebral infarction. These differing results are related to the time of splenectomy and the ischemic model used.
4.1.1. The Timing of Splenectomy
In most studies, one of three different timings of splenectomy have been performed: pre-stroke splenectomy, splenectomy immediately before or after stroke, or post-stroke splenectomy. In most studies, splenectomy was performed 2 weeks before MCAO, which reduced the volume of the cerebral infarction, improved short-term neurological function [19, 24, 26, 27, 30, 71], and relieved brain edema after intracerebral hemorrhage [69]. It has also been reported that splenectomy just before or after stroke can reduce the infarct volume [28, 29], but with more severe neurological scores in the acute phase [72]. However, more studies have found that splenectomy at this stage did not alter the infarct volume [20, 29, 72] and had no effects on short-term and long-term neurological function [28, 29, 72]. Splenectomy 3 days after the stroke has no effect on cerebral infarction volume, motor-sensory function, or cognitive function [29]. Splenectomy is a major surgery in which the body compensates for spleen loss by temporarily increasing circulating immune cells within a week. The body also responds to the surgical insult after splenectomy. Therefore, splenectomy should be performed 2 weeks before MCAO to allow for recovery from the splenectomy.
4.1.2. Effect of Sex and Age on Splenectomy
Most studies have used male adult rats or mice to examine the neuroprotective effect of splenectomy on stroke. However, studies have found that while male mice can be protected from a stroke after splenectomy, but female mice are not [73]. There are more regulatory lymphocytes in the spleens of female mice than in those of male mice. The loss of these regulatory cells in female mice leads to the loss of protection against stroke [73, 74]. In addition, more T cells were activated in the spleens of male mice than in female mice after tMCAO [75]. Compared with young adult rats or mice, the splenic function of aged rats or mice may be different. Some studies have found that splenectomy reduces the infarct volume of aged male mice, improves behavioral results, and reduces the infiltration of immune cells into the brain [30]. Splenectomy on postnatal day-7 rats 3 days prior to hypoxia-ischemia ameliorated the infarct volume and reduced neurobehavioral deficits at 3 weeks [71]. Therefore, splenectomy also has a neuroprotective effect on neonatal rats and older mice.
4.1.3. Alternatives to Splenectomy
The risk of surgical splenectomy in stroke patients is a major obstacle to the elimination of splenic inflammation. One study used splenic irradiation instead of splenectomy as a non-invasive method to passivate the splenic response after stroke [25]. Rats received targeted splenic irradiation 3 or 4 hours after tMCAO, which significantly reduced the infarct size. Irradiation of the spleen 4 hours after tMCAO can provide tissue protection related to extensive apoptotic cell death in the spleen and reduce the infiltration of immune cells to the brain tissue. This considerable neuroprotection is not accompanied by side effects of the immune system or acute radiation syndrome.
In summary, splenectomy may have a short-term neuroprotective effect on stroke, but it does not provide long-term protection. In addition, immune regulation via the spleen
may play different roles in the different pathological periods of stroke, and the regulation of splenocyte function in different periods is more beneficial than simple splenectomy. In addition, clinical studies have shown that splenic injury and splenectomy are significantly associated with an increased risk of hemorrhagic and ischemic stroke [76]. Vascular events in patients with splenic injury and splenectomy may be caused by a variety of factors, including platelet activation, a hypercoagulable state, endothelial activation, and changes in lipid metabolism [77]. Splenectomy may lead to thrombocytopenia, leukocytosis, concentrated hemoglobin, hyperlipidemia, and elevated C-reactive protein levels [78-80]. All these changes are independently associated with an increased risk of vascular complications in patients undergoing splenectomy (Table 4).
Table 4.
Splenectomy and stroke outcome in rodents.
| Factors Associated with Splenectomy |
Time of
Splenectomy |
Species | Stroke Model | Survival | Outcomes | Beneficial/ Detrimental | Refs. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-stroke splenectomy |
14 days before MCAO | male SD rats | pMCAO | 48 hours, 96 hours | Decreased infarction volume in the brain (48 hours and 96 hours). Reduced neurodegeneration after ischemic insult, decreased numbers of activated microglia, macrophages and neutrophils present in the brain tissue (96 hours). | Beneficial | [5, 24] | ||||||||
| 14 days before MCAO | male SD rats | pMACO | 24 hours | Reduce cerebral infarct volumes, Lower numbers of T cells, neutrophils, and macrophages in brain tissue and lower levels of pro-cytokines, but higher levels of IL-10. | Beneficial | [26] | |||||||||
| 14 days before MCAO | male SD rats | pMCAO | 4 days | Decreased infarct size and the amount of IFNγ in the infarct post-MCAO. | Beneficial | [27] | |||||||||
| 14 days before MCAO | Male C57BL/6J mice | tMCAO | 1 days, 3 days, 5 days |
reduced infarct size (5 days), neurological deficit(1, 3, 5 days) and brain interferon-γ (3 days) after stroke | Beneficial | [19] | |||||||||
| 14 days before MCAO | male SD rats | pMCAO | 96 hours | Reduced the amount of IP-10 in the infarct area of rats post-MCAO | Beneficial | [67] | |||||||||
| 3 days before stroke | male SD rats | intracerebral haemorrhage (ICH) | 3 days | significantly decreased edema and brain water content. |
Beneficial | [69] | |||||||||
| upon stroke splenectomy |
Splenectomy just before MCAO | Male C57BL/6J mice | tMCAO 30min | 3 days, 7 days |
Not reduced infarct volume and swelling. | No effect | [20] | ||||||||
| Splenectomy immediately before MCAO | male SD rats | tMCAO 90min | 24, 72, 144 hours | reduced infarct volume(MRI). | Beneficial | [28] | |||||||||
| 1 day-7 days | No significant difference between both group behavioral scores at different time points. | No effect | |||||||||||||
| Splenectomy immediately before MCAO | Male Lewis rats | tMACO 120min | 3 and 6 hours | Worse neurological scores | Detrimental | [72] | |||||||||
| 72 hours | Similar infarct size in both groups | No effect | |||||||||||||
| 4 weeks | No effect on behavioral outcomes and immune response to myelin basic protein | ||||||||||||||
| Splenectomy right upon reperfusion | Male SD rats | tMCAO 90min | 3 days | significantly reduced the infarct size and immune cell infiltration 3d after MCAO, | Beneficial | [29] | |||||||||
| 28 days | but fails to reduce brain tissue loss at 28 days after MCAO | No effect | |||||||||||||
| 3-28 days | Result in a transient improvement in functional performance; however, it could not promote long-term functional recovery after MCAO | ||||||||||||||
| Post-stroke splenectomy |
3 days after MCAO | Male SD rats | tMCAO 90min | 5 days, 28 days |
Not reduced infarct volume (5 days) and brain tissue loss (28 days). | No effect | [29] | ||||||||
| 3-28 days | no effect on sensorimotor function or cognitive function. | ||||||||||||||
|
Factors Associated with
Splenectomy |
Time of
Splenectomy |
Species | Stroke Model | Survival | Outcomes | Beneficial/ Detrimental | Refs. | ||||||||
| splenectomy about gender | 14 days before MCAO | Male and female C57BL/6J mice | tMCAO 60min | 4 days | Decreased infarct size in males,not females. | Beneficial | [73] | ||||||||
| splenectomy about age | 14 days before MCAO | Older C57BL/6J male mice (18-22 months) |
tMCAO 60min | 4 days | Improved neurobehavioral and infarct outcomes. Reduced the number of peripheral immune cells infiltrating into the brain and decreased levels of peripheral inflammatory cytokines after stroke. | Beneficial | [30] | ||||||||
| 3 days before hypoxia-ischemia (HI) | Postnatal day-7 rats | Neonatal HI | 72 hours | Decreased infarct volume. | Beneficial | [71] | |||||||||
| 3 weeks | Diminished brain atrophy and Improved behavioral outcome. Improved short- and long-term body weight gain after HI. |
||||||||||||||
| Alternative therapy for splenectomy |
Splenic irradiation at 3 and 4hrs after the start of ischemia | Male SD rats | tMCAO 120min | 48 hours | Reduced cerebral infarct volumes in the rats irradiated at 3 hours | Beneficial | [25] | ||||||||
| 7 days | Reduced cerebral infarct volumes and counts of microglia, infiltrating T cells and apoptotic neurons in the rats irradiated at 4 hours | ||||||||||||||
| Splenectomy in other treatment model |
LPS-preconditioning (LPS-PC) | 14 days before MCAO | Male mice | tMCAO 35min | 72 hours | Eliminated the neuroprotection of LPS-PC after stroke. Similar large infarcts in both LPS- and saline-treated splenectomized mice. | Detrimental | [46] | |||||||
| Remote ischemic limb conditioning | Splenectomy and adoptive transfer of CCR2 KO splenocytes into C57BL/6 mice, just before MCAO, | Male and female C57BL/6J mice, CCR2 KO mice |
tMCAO 30min | 2 months | CCR2-deficient splenocytes transfer abolishes Remote Ischemic Limb Conditioning-mediated protection in splenectomized mice. | Detrimental | [93] | ||||||||
| Remote ischemic precondition (RIPC) of a limb |
1 day or 2 weeks before RIPC and MCAO | Male SD rats | tMCAO 90 min | 3 days | Reduced the protective effect of RIPC on ischemic brain injury and reversed the effects of RIPC on circulating immune cell composition. | Detrimental | [93] | ||||||||
| Human multipotent adult progenitor cells treatment |
14 days before | Male Long Evans rats |
tMCAO 90 min | 21days, 28 days |
Eliminated the improvement of stroke recovery treated with MAPC. | Detrimental | [85] | ||||||||
| Neural stem cell transplantation | 3 days before ICH | Male SD rats | ICH | 1 day, 3 days |
Eliminated the effect of NSCs on brain water content (3 days), perihematomal inflammatory cells (1 day), and initial neurologic deficits (3 days). |
Detrimental | [69] | ||||||||
4.2. Stem Cell Therapy
In animal models of stroke, treatments based on different types of stem cells have been studied for several years. The stem cell types studied include human umbilical cord blood cells (hUCBs) [4, 81], hematopoietic stem cells (HSCs) [68], human bone marrow stromal cells (hBMSCs) [82-84], neural stem cells (NSC) [69], multipotent adult progenitor cells (MAPC) [85], and human amnio epithelial cells (hAECs) [86]. Positive results have been obtained using stem cell therapy for the treatment of stroke, and this therapy can enhance the recovery of neurological function after stroke. In an animal model of acute brain injury, more and more evidence shows that intravenous injection of various types of stem cells can regulate not only the local microenvironment of the brain but also the peripheral systemic immune response. The spleen plays an important role in this process. The regulatory effect of stem cells on peripheral immunity may be one of the reasons why this intravenous therapy is more effective. Interestingly, when stem cells are systemically given, these stem cells have priority in entering the peripheral immune organs, especially the spleen. After entering the spleen, these stem cells interact with spleen cells to regulate the peripheral immune response after stroke, reduce damage to the ischemic center by the peripheral immune response, and thus play a neuroprotective role.
4.2.1. Migration of Stem Cells after Intravenous Injection
There are many routes of stem cell administration [87], among which intracerebral focus injection and intravenous injection are the most commonly used. Studies have shown that after intravenous injection of stem cells, most of the stem cells first migrate to the spleen [68, 69, 81-83, 85].
Then, a small number of stem cells temporarily invade the injured brain tissue. The vast majority (> 95%) of the stem cells entered the spleen shortly after intravenous injection [82]. Another study showed that HSCs intravenously injected 24 hours after reperfusion were first detected 24 hours later in the spleen and then in the ischemic brain parenchyma [68]. In addition, compared to the sham-operated control group, the immune environment after cerebral infarction increased the migration of HSCs to the spleen 72 hours after reperfusion. In the absence of induced injury, cells will not preferentially gather in the spleen [68]. Unlike intravenous injection, stem cells introduced by intracerebral focus injection migrate from the brain to the spleen through the lymphatic vessels and via inflammatory signals [84].
4.2.2. Effects of Stem Cell Therapy on the Spleen after Stroke
After entering the spleen, stem cells interact with spleen cells. It has been found that NSCs injected intravenously come into direct contact with CD11b+ cells in the spleen [69], which partly indicates that the neuroprotective effect of NSCs in stroke involves their interaction with spleen cells. HUCB mononuclear cells change the cell populations in the peripheral circulation and spleen after pMCAO through interaction with various subsets [88]. One study suggested that when stem cells are intraperitoneally injected into mice with cerebral infarction, the neurogenic niche proliferation and glial brain response to human cord blood-derived CD34(+)-enriched mononuclear cells after neonatal stroke may involve interaction with the spleen and are sex-dependent [89]. Stem cells can rescue the loss in spleen weight caused by stroke [4, 69, 85, 86, 89] and regulate splenic inflammation.
4.2.3. Stem Cell Therapy can Regulate the Number and Response of Splenic Immune Cells after Stroke
Multiple studies have shown that stem cells can alter the numbers and functions of T cell subsets in the spleen. In one study, HUCBC treatment rescued splenic CD8+ T-cell counts [4]. Similarly, intravenous injection of MAPC increased the number of Treg cells in the spleen after stroke [85]. Another study showed that intravenous injection of HUCB significantly changed the response of splenic T cells to concanavalin A and decreased their proliferative activity [88]. It has been reported that intravenous injection of lineage-negative bone marrow-derived hematopoietic stem cells can weaken the peripheral immune response after ischemia and reduce the infiltration of immune cells into the ischemic hemisphere, especially T cells and macrophages. This mediates the neuroprotective effect in the subacute phase after ischemic stroke [68]. Stem cell therapy can also reduce inflammatory factors in the spleen and peripheral blood. Intravenous injection of MAPC increased IL-10 in the spleen and decreased the release of IL-1β from splenocytes [85]. Intravenous injection of hBMSCs reduced the level of TNF-α in the spleen by 40% after cerebral infarction [83]. Systemic administration of hUCB 24 hours after MCAO reduced the production of inflammatory factor IFN-γ and increased the production of anti-inflammatory cytokine IL-10. HSC treatment decreased the levels of TNF-α and IL-1β in the spleen after CI [68]. MAPC treatment decreased IL-6 and IL-1β and increased IL-10 in the serum [85]. In a model of intracerebral hemorrhage, NSC treatment reduced TNF-α and IL-6, as well as nuclear factor-kappa B (NF-κB) activation in the spleen [69].
4.2.4. Effect of Stem Cell Therapy on the Injured Brain after Stroke
Intravenous injection of stem cells reduces the volume of cerebral infarction and the amount of apoptotic cells and promotes the recovery of neural function [4, 68, 81-83, 85]. Human NSC treatment attenuated cerebral activations of TNF-α, IL-6, and NF-κB and reduced brain edema and inflammatory cell infiltration after intracerebral hemorrhage [69]. The effect of stem cell therapy on relieving brain edema, decreasing inflammatory infiltration, and promoting functional recovery disappears after splenectomy [83, 85]. Correlation analysis showed that the migration of hBMSCs to the spleen was negatively correlated with the infarct size, the area around the infarct focus, the amount of activated major histocompatibility complex (MHC) II-positive cells in the striatum, and the level of TNF-α in the spleen [83]. HSC treatment reduced the volume of the cerebral infarction, decreased the apoptosis of neuronal cells around the infarct focus, and decreased the infiltration of immune cells (T cells and macrophages) into the ischemic hemisphere [68]. HUCBC therapy counteracts T cell proliferation associated with MCAO by increasing IL-10 production and reducing INF-γ [4]. MAPC treatment restored the expression of multiple genes and pathways involved in the immune and inflammatory responses after stroke. The gene categories down-regulated by MAPC include leukocyte activation, antigen presentation, and immune response processing, which are related to signal pathways regulated by TNF-α, IL-1β, IL-6, and IFN-γ in the brain [85]. These studies suggest that stem cell therapy works to some extent by regulating the peripheral immune response after stroke and that it may work to promote brain repair, especially via the spleen. Therefore, the spleen is an important regulatory target for stem cells in the treatment of stroke.
4.3. Preconditioning and Postconditioning
Inducing ischemic tolerance by exposure to subinjurious stressors, also known as preconditioning (PC), is an effective mechanism to evoke endogenous neuroprotective programs [90]. In the brain, PC can be achieved through a variety of mediators and stressors, including ischemia, inflammatory mediators, metabolic blockers, anesthetics, cortical spread inhibition, and seizures [91]. It has been found that systemic injection of low-dose LPS induces Ly6Chigh monocytes and protects brain function after MCAO in mice. In addition, adoptive transplantation of monocytes isolated from preconditioned mice into naive mice 7 hours after transient MCAO reduced brain damage. Cell tracking studies have shown that protective monocytes are mobilized from the spleen to the brain and meninges, where they inhibit post-ischemic inflammation and neutrophil flow into the brain parenchyma. Splenectomy eliminated the neuroprotective effect of LPS-PC after stroke [46]. Therefore, this neuroprotective effect is dependent upon the intact spleen, and it is possible that monocytes may need to return to the spleen to obtain their neuroprotective phenotype. Secondly, adoptively transferred monocytes may instruct other immune cells in the spleen to change their inflammatory phenotypes. Remote ischemic preconditioning (RIPC) of a limb has been reported to protect against ischemic stroke [92]. RIPC increased the volume of the spleen and increased the number of splenic lymphocytes. In addition, splenectomy 1 day or 2 weeks before RIPC and MCAO reduced the protective effect of RIPC on ischemic brain injury and reversed the effect of RIPC on the circulating immune cell composition. RIPC significantly decreased the infiltration of cytotoxic T cells and NKT cells into the brain. However, prior splenectomy eliminated the infiltration of immune cells into the injured brain after RIPC and stroke [93]. A procedure similar to RIPC, called remote ischemic limb conditioning (RLC), is performed after ischemia and also triggers an endogenous tolerance mechanism. Mice subjected to post-stroke RLC displayed a shift in their circulating monocytes towards a CCR2+ pro-inflammatory subset, less acute brain injury and swelling in chronic stroke, and improved motor/gait function. Transfer of CCR2-deficient splenocytes eliminated the protective effect of RLC in splenectomized mice [94]. These studies suggest that the spleen plays a pivotal role in PC and RLC-mediated alterations of the peripheral immune system and subsequent neuroprotection. The neuroprotective effects of these preconditioning or postconditioning methods are achieved by regulating certain immune cells in the spleen.
4.4. Adrenergic Receptor Blockers
Activation of the SNS after stroke results in an increase in the level of catecholamine in the spleen and in the systemic circulation after release from the adrenal medulla. Catecholamine binds to the α and β adrenoceptors of the spleen to regulate the splenic response to stroke. Denervation before MCAO did not alter the cerebral infarct volume or splenic size. Injection of carvedilol, a pan-adrenergic receptor blocker, significantly decreased the shrinking of the spleen and the volume of the cerebral infarction. Treatment with prazosin (an α-1 receptor blocker) only prevented the spleen from shrinking. Treatment with propranolol (a β-receptor blocker) had no effect on these outcomes [5]. However, some studies have reported that propranolol partially reverses immunosuppression and spleen volume reduction after stroke [34]. The experimental stroke resulted in a significant loss of B cells in the splenic MZ, insufficient ability to capture blood-derived antigens, and inhibition of circulating IgM. These defects are accompanied by a spontaneous bacterial lung infection. Propranolol prevented the loss of MZ B cells in the spleen, maintained the IgM level, and reduced the bacterial burden after experimental stroke. These findings suggest that adrenergic MZ B cell loss contributes to infection susceptibility after stroke [62].
4.5. Other Methods Using the Spleen as a Therapeutic Target
Statins are widely used in the primary and secondary prevention of ischemic stroke. However, some studies have found that simvastatin increases the expression of anti-apoptotic Bcl-2 in mitochondria, reduces the translocation of pro-apoptotic Bax from the cytoplasm to the mitochondria, and reduces splenic atrophy and splenocyte apoptosis induced by stroke. Simvastatin inhibited brain INF-γ, reduced the infarct volume, and decreased neurological deficits after stroke. Splenectomy abolished the neuroprotective effect of simvastatin treatment [19]. This result gives us a new understanding of how simvastatin works in the treatment of stroke. Cluster of differentiation 147 (CD147) is a cell surface glycoprotein that has recently been shown to be an important mediator of inflammation and the immune response. Studies have found that CD147 plays an important role in promoting brain inflammation after ischemic stroke [95]. CD147 is the key mediator of splenic inflammation activation after cerebral ischemia. Treatment with αCD147 (an anti-CD147 antibody) reduced the early inflammatory response in the spleen after tMCAO. Inhibition of CD147 eliminated the inflammatory activation of splenic monocyte/macrophages (MMs) induced by cerebral ischemia and reduced the infiltration of Ly-6Chigh MMS into the brain [96]. A cytokine in the IL-1 family, IL-33, has been found to have protective effects on ischemic brain injury [97]. The long-term protective mechanism of IL-33 against ischemic stroke may partly involve the regulation of the splenic T cell response via inhibition of the Th1 response and promotion of the Treg response, suggesting that IL-33 may be a candidate drug for the treatment of stroke [98]. RTLs consist of part of the MHC class II molecule, which is connected to a peptide by covalent bonds. Treatment with RTL551, an I-Ab molecule linked to MOG-35-55 peptides, can reduce the extent of cortical and total stroke injury by about 50 percent. RTL551 treatment partially prevented the atrophy of the spleen and the decrease in cell count and reduced the expression of the chemokine receptor CCR5 in the spleen. RTL551 treatment also reduced the infiltration of peripheral immune cells into the brain after MCAO [99-101]. RTL1000 reduced the infarct volume and prevented splenic atrophy in young and old mice. In young mice, RTL1000 inhibited splenocyte outflow and reduced the frequencies of T cells and macrophages in the spleen. Older mice treated with RTL1000 showed a significant decrease in inflammatory cells in the brain. RTLs significantly impact multiple subsets of splenocytes in young mice while having much less impact on the splenocytes of older mice after MCAO. RTL1000 therapy prevents splenic atrophy by preventing cell death and likely by inhibiting splenocyte migration in young mice while only preventing splenocyte migration in older mice [53]. Selective intravascular cooling (SEC) involves the injection of cold saline into an artery to maintain body temperature while cooling locally. SEC had a neuroprotective effect after stroke. Animals subjected to SEC before stroke had increased expression of brain-derived neurotrophic factor (BDNF) in the ipsilateral and contralateral cerebral cortex, striatum, and hippocampus. SEC also upregulated splenic IL-10 to regulate the peripheral inflammatory response. In vitro experiments also confirmed that “cold” splenic cells from rats had a protective effect on primary neurons by up-regulating BDNF and IL-10 [102]. Clodronate liposome injection depleted 80% of the macrophages in the mouse spleen and reduced macrophage infiltration into the mouse brain. Macrophage depletion enhanced the microvessel density in the peri-infarct region, decreased brain atrophy, and promoted neurological recovery following MCAO [52]. The spleen is an important site of the peripheral immune response after stroke, these methods play a neuroprotective role in stroke by regulating the splenic inflammatory response.
Spleen has become a new target for the treatment of stroke. Spleen-related treatment can be divided into two aspects. One is to block the activation of the spleen by the CNS, such as the use of adrenergic receptor blockers. Another method is to regulate immune cells and cytokines/chemokines in the spleen, such as stem cell therapy, splenectomy, PC, RTLs, etc. Among them, splenectomy is difficult to implement in the clinic. Intravenous injection of stem cell therapy may be a very practical and effective method to interfere with the immune response of the spleen.
CONCLUSION
In the acute stage of stroke, under stimulation from various signals from the infarct center, the spleen shrinks, splenic immune cells are activated, cytokines are secreted, cells are released and migrate from the spleen to the circulation, and then the cells infiltrate into the ischemic brain. Cellular infiltration is thought to aggravate brain damage. However, there are many details of this process that are not entirely clear. For example, it is unclear how the immune cells interact in the spleen after stroke, how these cells differentially affect the injured brain in the acute and recovery stages, and the specific effects of spleen-derived B cells on the infarct center. In addition, it is not clear whether and how the spleen promotes nerve repair during the recovery from stroke. Therefore, regulating the immune response by inhibiting the early splenic response and peripheral inflammation and accelerating the initiation of anti-inflammatory/repair mechanisms to regulate the central immune response and nerve repair will have greater clinical effects. Presently, some experimental strategies targeting the splenic response have shown therapeutic effects during the acute phase of stroke, but translating these studies into clinical application is a long process. It is necessary to have a more in-depth and comprehensive understanding of the splenic reaction of stroke patients. If the responses and interactions of various immune cells in the spleen after stroke can be understood in detail, different cell subsets in the spleen can be targeted to inhibit harmful immune cells and stimulate beneficial immune cells. In the future, we believe that therapies that target the splenic immune response will become more efficacious and that more stroke patients will eventually benefit from these therapies.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was financially supported by the National Natural Science Foundation of China (NO.81771271).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kamel H., Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch. Neurol. 2012;69(5):576–581. doi: 10.1001/archneurol.2011.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mebius R.E., Kraal G. Structure and function of the spleen. Nat. Rev. Immunol. 2005;5(8):606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 4.Vendrame M., Gemma C., Pennypacker K.R., Bickford P.C., Davis Sanberg C., Sanberg P.R., Willing A.E. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp. Neurol. 2006;199(1):191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Ajmo C.T., Jr, Collier L.A., Leonardo C.C., Hall A.A., Green S.M., Womble T.A., Cuevas J., Willing A.E., Pennypacker K.R. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp. Neurol. 2009;218(1):47–55. doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu N.L., Kaiser B., Nguyen Y.V., Welbourne S., Lall C., Cramer S.C. The volume of the spleen and its correlates after acute stroke. J. Stroke Cerebrovasc. Dis. 2016;25(12):2958–2961. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennypacker K.R., Offner H. The role of the spleen in ischemic stroke. J. Cereb. Blood Flow Metab. 2015;35(2):186–187. doi: 10.1038/jcbfm.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronte V., Pittet M.J. The spleen in local and systemic regulation of immunity. Immunity. 2013;39(5):806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Krieken J.H., te Velde J. Normal histology of the human spleen. Am. J. Surg. Pathol. 1988;12(10):777–785. doi: 10.1097/00000478-198810000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lewis S.M., Williams A., Eisenbarth S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019;4(33):eaau6085. doi: 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R.L., Wilson S.P., Dzielak D.J., Yang W.H., Viveros O.H. Opioid peptides and noradrenaline co-exist in large dense-cored vesicles from sympathetic nerve. Neuroscience. 1982;7(9):2255–2261. doi: 10.1016/0306-4522(82)90135-X. [DOI] [PubMed] [Google Scholar]
- 12.Cano G., Sved A.F., Rinaman L., Rabin B.S., Card J.P. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J. Comp. Neurol. 2001;439(1):1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 13.Felten D.L., Felten S.Y., Carlson S.L., Olschowka J.A., Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J. Immunol. 1985;135(2) Suppl.:755s–765s. [PubMed] [Google Scholar]
- 14.Feibel J.H., Hardy P.M., Campbell R.G., Goldstein M.N., Joynt R.J. Prognostic value of the stress response following stroke. JAMA. 1977;238(13):1374–1376. doi: 10.1001/jama.1977.03280140052016. [DOI] [PubMed] [Google Scholar]
- 15.Fassbender K., Schmidt R., Mössner R., Daffertshofer M., Hennerici M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke. 1994;25(6):1105–1108. doi: 10.1161/01.str.25.6.1105. [DOI] [PubMed] [Google Scholar]
- 16.Seifert H.A., Hall A.A., Chapman C.B., Collier L.A., Willing A.E., Pennypacker K.R. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J. Neuroimmune Pharmacol. 2012;7(4):1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offner H., Subramanian S., Parker S.M., Wang C., Afentoulis M.E., Lewis A., Vandenbark A.A., Hurn P.D. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J. Immunol. 2006;176(11):6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 18.Bao Y., Kim E., Bhosle S., Mehta H., Cho S. A role for spleen monocytes in post-ischemic brain inflammation and injury. J. Neuroinflammation. 2010;7:92. doi: 10.1186/1742-2094-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin R., Zhu X., Liu L., Nanda A., Granger D., Li G. Simvastatin attenuates stroke-induced splenic atrophy and lung susceptibility to spontaneous bacterial infection in mice. Stroke. 2013;44(4):1135–1143. doi: 10.1161/STROKEAHA.111.000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim E., Yang J., Beltran C.D., Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow Metab. 2014;34(8):1411–1419. doi: 10.1038/jcbfm.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahota P., Vahidy F., Nguyen C., Bui T.T., Yang B., Parsha K., Garrett J., Bambhroliya A., Barreto A., Grotta J.C., Aronowski J., Rahbar M.H., Savitz S. Changes in spleen size in patients with acute ischemic stroke: A pilot observational study. Int. J. Stroke. 2013;8(2):60–67. doi: 10.1111/ijs.12022. [DOI] [PubMed] [Google Scholar]
- 22.Vahidy F.S., Parsha K.N., Rahbar M.H., Lee M., Bui T.T., Nguyen C., Barreto A.D., Bambhroliya A.B., Sahota P., Yang B., Aronowski J., Savitz S.I. Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2016;36(6):1012–1021. doi: 10.1177/0271678X15607880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zha A., Vahidy F., Randhawa J., Parsha K., Bui T., Aronowski J., Savitz S.I. Association Between Splenic Contraction and the Systemic Inflammatory Response After Acute Ischemic Stroke Varies with Age and Race. Transl. Stroke Res. 2018;9(5):484–492. doi: 10.1007/s12975-017-0596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajmo C.T., Jr, Vernon D.O., Collier L., Hall A.A., Garbuzova-Davis S., Willing A., Pennypacker K.R. The spleen contributes to stroke-induced neurodegeneration. J. Neurosci. Res. 2008;86(10):2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrowski R.P., Schulte R.W., Nie Y., Ling T., Lee T., Manaenko A., Gridley D.S., Zhang J.H. Acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Transl. Stroke Res. 2012;3(4):473–481. doi: 10.1007/s12975-012-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B.J., Men X.J., Lu Z.Q., Li H.Y., Qiu W., Hu X.Q. Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin. Med. J. (Engl.) 2013;126(12):2354–2360. [PubMed] [Google Scholar]
- 27.Seifert H.A., Leonardo C.C., Hall A.A., Rowe D.D., Collier L.A., Benkovic S.A., Willing A.E., Pennypacker K.R. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab. Brain Dis. 2012;27(2):131–141. doi: 10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belinga V.F., Wu G.J., Yan F.L., Limbenga E.A. Splenectomy following MCAO inhibits the TLR4-NF-κB signaling pathway and protects the brain from neurodegeneration in rats. J. Neuroimmunol. 2016;293:105–113. doi: 10.1016/j.jneuroim.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Ran Y., Liu Z., Huang S., Shen J., Li F., Zhang W., Chen C., Geng X., Ji Z., Du H., Hu X. Splenectomy fails to provide long-term protection against ischemic stroke. Aging Dis. 2018;9(3):467–479. doi: 10.14336/AD.2018.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauhan A., Al Mamun A., Spiegel G., Harris N., Zhu L., McCullough L.D. Splenectomy protects aged mice from injury after experimental stroke. Neurobiol. Aging. 2018;61:102–111. doi: 10.1016/j.neurobiolaging.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ay I., Sorensen A.G., Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–115. doi: 10.1016/j.brainres.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenney M.J., Ganta C.K. Autonomic nervous system and immune system interactions. Compr. Physiol. 2014;4(3):1177–1200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aboud R., Shafii M., Docherty J.R. Investigation of the subtypes of alpha 1-adrenoceptor mediating contractions of rat aorta, vas deferens and spleen. Br. J. Pharmacol. 1993;109(1):80–87. doi: 10.1111/j.1476-5381.1993.tb13534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan F.L., Zhang J.H. Role of the sympathetic nervous system and spleen in experimental stroke-induced immunodepression. Med. Sci. Monit. 2014;20:2489–2496. doi: 10.12659/MSM.890844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuchida T., Parker K.C., Turner R.V., McFarland H.F., Coligan J.E., Biddison W.E. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc. Natl. Acad. Sci. USA. 1994;91(23):10859–10863. doi: 10.1073/pnas.91.23.10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planas A.M., Gómez-Choco M., Urra X., Gorina R., Caballero M., Chamorro Á. Brain-derived antigens in lymphoid tissue of patients with acute stroke. J. Immunol. 2012;188(5):2156–2163. doi: 10.4049/jimmunol.1102289. [DOI] [PubMed] [Google Scholar]
- 37.Ortega S.B., Noorbhai I., Poinsatte K., Kong X., Anderson A., Monson N.L., Stowe A.M. Stroke induces a rapid adaptive autoimmune response to novel neuronal antigens. Discov. Med. 2015;19(106):381–392. [PMC free article] [PubMed] [Google Scholar]
- 38.Mracsko E., Liesz A., Karcher S., Zorn M., Bari F., Veltkamp R. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav. Immun. 2014;41:200–209. doi: 10.1016/j.bbi.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Ren X., Akiyoshi K., Grafe M.R., Vandenbark A.A., Hurn P.D., Herson P.S., Offner H. Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metab. Brain Dis. 2012;27(1):7–15. doi: 10.1007/s11011-011-9267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zierath D., Schulze J., Kunze A., Drogomiretskiy O., Nhan D., Jaspers B., Dressel A., Becker K. The immunologic profile of adoptively transferred lymphocytes influences stroke outcome of recipients. J. Neuroimmunol. 2013;263(1-2):28–34. doi: 10.1016/j.jneuroim.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamagami S., Tamura M., Hayashi M., Endo N., Tanabe H., Katsuura Y., Komoriya K. Differential production of MCP-1 and cytokine-induced neutrophil chemoattractant in the ischemic brain after transient focal ischemia in rats. J. Leukoc. Biol. 1999;65(6):744–749. doi: 10.1002/jlb.65.6.744. [DOI] [PubMed] [Google Scholar]
- 42.Zaremba J., Ilkowski J., Losy J. Serial measurements of levels of the chemokines CCL2, CCL3 and CCL5 in serum of patients with acute ischaemic stroke. Folia Neuropathol. 2006;44(4):282–289. [PubMed] [Google Scholar]
- 43.Cowell R., Xu H., Galasso J., Silverstein F. Hypoxic-ischemic injury induces macrophage inflammatory protein-1alpha expression in immature rat brain. Stroke. 2002;33(3):795–801. doi: 10.1161/hs0302.103740. [DOI] [PubMed] [Google Scholar]
- 44.Offner H., Subramanian S., Parker S.M., Afentoulis M.E., Vandenbark A.A., Hurn P.D. Experimental stroke induces massive, rapid activation of the peripheral immune system. J. Cereb. Blood Flow Metab. 2006;26(5):654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 45.Dimitrijevic O., Stamatovic S., Keep R., Andjelkovic A. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38(4):1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Bonilla L., Brea D., Benakis C., Lane D.A., Murphy M., Moore J., Racchumi G., Jiang X., Iadecola C., Anrather J. Endogenous protection from ischemic brain injury by preconditioned monocytes. J. Neurosci. 2018;38(30):6722–6736. doi: 10.1523/JNEUROSCI.0324-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schilling M., Strecker J.K., Ringelstein E.B., Schäbitz W.R., Kiefer R. The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res. 2009;1289:79–84. doi: 10.1016/j.brainres.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 48.Hill W.D., Hess D.C., Martin-Studdard A., Carothers J.J., Zheng J., Hale D., Maeda M., Fagan S.C., Carroll J.E., Conway S.J. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 2004;63(1):84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 49.Ruscher K., Kuric E., Liu Y., Walter H.L., Issazadeh-Navikas S., Englund E., Wieloch T. Inhibition of CXCL12 signaling attenuates the postischemic immune response and improves functional recovery after stroke. J. Cereb. Blood Flow Metab. 2013;33(8):1225–1234. doi: 10.1038/jcbfm.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seifert H.A., Offner H. The splenic response to stroke: from rodents to stroke subjects. J. Neuroinflammation. 2018;15(1):195. doi: 10.1186/s12974-018-1239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price C., Menon D., Peters A., Ballinger J., Barber R., Balan K., Lynch A., Xuereb J., Fryer T., Guadagno J., Warburton E. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. 2004;35(7):1659–1664. doi: 10.1161/01.STR.0000130592.71028.92. [DOI] [PubMed] [Google Scholar]
- 52.Ma Y., Li Y., Jiang L., Wang L., Jiang Z., Wang Y., Zhang Z., Yang G.Y. Macrophage depletion reduced brain injury following middle cerebral artery occlusion in mice. J. Neuroinflammation. 2016;13:38. doi: 10.1186/s12974-016-0504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dotson A.L., Zhu W., Libal N., Alkayed N.J., Offner H. Different immunological mechanisms govern protection from experimental stroke in young and older mice with recombinant TCR ligand therapy. Front. Cell. Neurosci. 2014;8:284. doi: 10.3389/fncel.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu L., Xiong X., Zhang H., Xu B., Steinberg G., Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke. 2012;43(7):1941–1946. doi: 10.1161/STROKEAHA.112.656611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurn P.D., Subramanian S., Parker S.M., Afentoulis M.E., Kaler L.J., Vandenbark A.A., Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J. Cereb. Blood Flow Metab. 2007;27(11):1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yilmaz G., Arumugam T.V., Stokes K.Y., Granger D.N. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113(17):2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 57.Liesz A., Suri-Payer E., Veltkamp C., Doerr H., Sommer C., Rivest S., Giese T., Veltkamp R.J.N. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 2009;15(2):192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 58.Li P., Gan Y., Sun B.L., Zhang F., Lu B., Gao Y., Liang W., Thomson A.W., Chen J., Hu X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann. Neurol. 2013;74(3):458–471. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelderblom M., Leypoldt F., Steinbach K., Behrens D., Choe C., Siler D., Arumugam T., Orthey E., Gerloff C., Tolosa E., Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 60.Ren X., Akiyoshi K., Dziennis S., Vandenbark A.A., Herson P.S., Hurn P.D., Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J. Neurosci. 2011;31(23):8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bodhankar S., Chen Y., Vandenbark A.A., Murphy S.J., Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metab. Brain Dis. 2013;28(3):375–386. doi: 10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCulloch L., Smith C.J., McColl B.W. Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nat. Commun. 2017;8:15051. doi: 10.1038/ncomms15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Gao Z., Wang D., Zhang T., Sun B., Mu L., Wang J., Liu Y., Kong Q., Liu X., Zhang Y., Zhang H., He J., Li H., Wang G. Accumulation of natural killer cells in ischemic brain tissues and the chemotactic effect of IP-10. J. Neuroinflammation. 2014;11:79. doi: 10.1186/1742-2094-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gan Y., Liu Q., Wu W., Yin J.X., Bai X.F., Shen R., Wang Y., Chen J., La Cava A., Poursine-Laurent J., Yokoyama W., Shi F.D. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc. Natl. Acad. Sci. USA. 2014;111(7):2704–2709. doi: 10.1073/pnas.1315943111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z.K., Xue L., Wang T., Wang X.J., Su Z.Q. Infiltration of invariant natural killer T cells occur and accelerate brain infarction in permanent ischemic stroke in mice. Neurosci. Lett. 2016;633:62–68. doi: 10.1016/j.neulet.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Dou B., Zhou W., Li S., Wang L., Wu X., Li Y., Guan H., Wang C., Zhu S., Ke Z., Huang C., Wang Z. Buyang huanwu decoction attenuates infiltration of natural killer cells and protects against ischemic brain injury. Cell. Physiol. Biochem. 2018;50(4):1286–1300. doi: 10.1159/000494587. [DOI] [PubMed] [Google Scholar]
- 67.Seifert H.A., Collier L.A., Chapman C.B., Benkovic S.A., Willing A.E., Pennypacker K.R. Pro-inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J. Neuroimmune Pharmacol. 2014;9(5):679–689. doi: 10.1007/s11481-014-9560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwarting S., Litwak S., Hao W., Bähr M., Weise J., Neumann H. Hematopoietic stem cells reduce postischemic inflammation and ameliorate ischemic brain injury. Stroke. 2008;39(10):2867–2875. doi: 10.1161/STROKEAHA.108.513978. [DOI] [PubMed] [Google Scholar]
- 69.Lee S.T., Chu K., Jung K.H., Kim S.J., Kim D.H., Kang K.M., Hong N.H., Kim J.H., Ban J.J., Park H.K., Kim S.U., Park C.G., Lee S.K., Kim M., Roh J.K. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131(Pt 3):616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 70.Offner H., Vandenbark A.A., Hurn P.D. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158(3):1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fathali N., Ostrowski R.P., Hasegawa Y., Lekic T., Tang J., Zhang J.H. Splenic immune cells in experimental neonatal hypoxia-ischemia. Transl. Stroke Res. 2013;4(2):208–219. doi: 10.1007/s12975-012-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zierath D., Shen A., Stults A., Olmstead T., Becker K. Splenectomy does not improve long-term outcome after stroke. Stroke. 2017;48(2):497–500. doi: 10.1161/STROKEAHA.116.016037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dotson A.L., Wang J., Saugstad J., Murphy S.J., Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J. Neuroimmunol. 2015;278:289–298. doi: 10.1016/j.jneuroim.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seifert H.A., Benedek G., Liang J., Nguyen H., Kent G., Vandenbark A.A., Saugstad J.A., Offner H. Sex differences in regulatory cells in experimental stroke. Cell. Immunol. 2017;318:49–54. doi: 10.1016/j.cellimm.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banerjee A., Wang J., Bodhankar S., Vandenbark A.A., Murphy S.J., Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl. Stroke Res. 2013;4(5):554–563. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin J.N., Lin C.L., Lin M.C., Lai C.H., Lin H.H., Yang C.H., Kao C.H. Increased risk of hemorrhagic and ischemic strokes in patients with splenic injury and splenectomy: a nationwide cohort study. Medicine (Baltimore) 2015;94(35):e1458. doi: 10.1097/MD.0000000000001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crary S.E., Buchanan G.R. Vascular complications after splenectomy for hematologic disorders. Blood. 2009;114(14):2861–2868. doi: 10.1182/blood-2009-04-210112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Troendle S.B., Adix L., Crary S.E., Buchanan G.R. Laboratory markers of thrombosis risk in children with hereditary spherocytosis. Pediatr. Blood Cancer. 2007;49(6):781–785. doi: 10.1002/pbc.21319. [DOI] [PubMed] [Google Scholar]
- 79.Visudhiphan S., Ketsa-Ard K., Piankijagum A., Tumliang S. Blood coagulation and platelet profiles in persistent post-splenectomy thrombocytosis. The relationship to thromboembolism. Biomed. Pharmacother. 1985;39(6):264–271. [PubMed] [Google Scholar]
- 80.Aviram M., Brook J.G., Tatarsky I., Levy Y., Carter A. Increased low-density lipoprotein levels after splenectomy: a role for the spleen in cholesterol metabolism in myeloproliferative disorders. Am. J. Med. Sci. 1986;291(1):25–28. doi: 10.1097/00000441-198601000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Vendrame M., Cassady J., Newcomb J., Butler T., Pennypacker K., Zigova T., Sanberg C., Sanberg P., Willing A. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35(10):2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 82.Keimpema E., Fokkens M.R., Nagy Z., Agoston V., Luiten P.G., Nyakas C., Boddeke H.W., Copray J.C. Early transient presence of implanted bone marrow stem cells reduces lesion size after cerebral ischaemia in adult rats. Neuropathol. Appl. Neurobiol. 2009;35(1):89–102. doi: 10.1111/j.1365-2990.2008.00961.x. [DOI] [PubMed] [Google Scholar]
- 83.Acosta S., Tajiri N., Hoover J., Kaneko Y., Borlongan C. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. 2015;46(9):2616–2627. doi: 10.1161/STROKEAHA.115.009854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu K., Lee J.Y., Kaneko Y., Tuazon J.P., Vale F., van Loveren H., Borlongan C.V. Human stem cells transplanted into the rat stroke brain migrate to the spleen via lymphatic and inflammation pathways. Haematologica. 2019;104(5):1062–1073. doi: 10.3324/haematol.2018.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang B., Hamilton J.A., Valenzuela K.S., Bogaerts A., Xi X., Aronowski J., Mays R.W., Savitz S.I. Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune Response from the Spleen. Stem Cells. 2017;35(5):1290–1302. doi: 10.1002/stem.2600. [DOI] [PubMed] [Google Scholar]
- 86.Evans M.A., Broughton B.R.S., Drummond G.R., Ma H., Phan T.G., Wallace E.M., Lim R., Sobey C.G. Amnion epithelial cells - a novel therapy for ischemic stroke? Neural Regen. Res. 2018;13(8):1346–1349. doi: 10.4103/1673-5374.235223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z., He D., Zeng Y.Y., Zhu L., Yang C., Lu Y.J., Huang J.Q., Cheng X.Y., Huang X.H., Tan X.J. The spleen may be an important target of stem cell therapy for stroke. J. Neuroinflammation. 2019;16(1):20. doi: 10.1186/s12974-019-1400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Golden J.E., Shahaduzzaman M., Wabnitz A., Green S., Womble T.A., Sanberg P.R., Pennypacker K.R., Willing A.E. Human umbilical cord blood cells alter blood and spleen cell populations after stroke. Transl. Stroke Res. 2012;3(4):491–499. doi: 10.1007/s12975-012-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kadam S., Chen H., Markowitz G., Raja S., George S., Verina T., Shotwell E., Loechelt B., Johnston M., Kamani N., Fatemi A., Comi A. Systemic injection of CD34(+)-enriched human cord blood cells modulates poststroke neural and glial response in a sex-dependent manner in CD1 mice. Stem Cells Dev. 2015;24(1):51–66. doi: 10.1089/scd.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iadecola C., Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat. Neurosci. 2011;14(11):1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirino T. Ischemic tolerance. J. Cereb. Blood Flow Metab. 2002;22(11):1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- 92.Hess D.C., Blauenfeldt R.A., Andersen G., Hougaard K.D., Hoda M.N., Ding Y., Ji X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015;11(12):698–710. doi: 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- 93.Chen C., Jiang W., Liu Z., Li F., Yang J., Zhao Y., Ran Y., Meng Y., Ji X., Geng X., Du H., Hu X. Splenic responses play an important role in remote ischemic preconditioning-mediated neuroprotection against stroke. J. Neuroinflammation. 2018;15(1):167. doi: 10.1186/s12974-018-1190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang J., Balkaya M., Beltran C., Heo J.H., Cho S. Remote postischemic conditioning promotes stroke recovery by shifting circulating monocytes to CCR2+ proinflammatory subset. J. Neurosci. 2019;39(39):7778–7789. doi: 10.1523/JNEUROSCI.2699-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin R., Xiao A., Chen R., Granger D., Li G. Inhibition of CD147 (Cluster of Differentiation 147) ameliorates acute ischemic stroke in mice by reducing thromboinflammation. Stroke. 2017;48(12):3356–3365. doi: 10.1523/JNEUROSCI.2699-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin R., Zhong W., Liu S., Li G. CD147 as a key mediator of the spleen inflammatory response in mice after focal cerebral ischemia. J. Neuroinflammation. 2019;16(1):198. doi: 10.1186/s12974-019-1609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo Y., Zhou Y., Xiao W., Liang Z., Dai J., Weng X., Wu X. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 2015;1597:86–94. doi: 10.1016/j.brainres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Xiao W., Guo S., Chen L., Luo Y. The role of Interleukin-33 in the modulation of splenic T-cell immune responses after experimental ischemic stroke. J. Neuroimmunol. 2019;333:576970. doi: 10.1016/j.jneuroim.2019.576970. [DOI] [PubMed] [Google Scholar]
- 99.Subramanian S., Zhang B., Kosaka Y., Burrows G., Grafe M., Vandenbark A., Hurn P., Offner H. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40(7):2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dziennis S., Mader S., Akiyoshi K., Ren X., Ayala P., Burrows G., Vandenbark A., Herson P., Hurn P., Offner H.J.M.d. Therapy with recombinant T-cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice. Metab. Brain Dis. 2011;26(2):123–133. doi: 10.1007/s11011-011-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akiyoshi K., Dziennis S., Palmateer J., Ren X., Vandenbark A.A., Offner H., Herson P.S., Hurn P.D. Recombinant T cell receptor ligands improve outcome after experimental cerebral ischemia. Transl. Stroke Res. 2011;2(3):404–410. doi: 10.1007/s12975-011-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corey S., Abraham D.I., Kaneko Y., Lee J.Y., Borlongan C.V. Selective endovascular cooling for stroke entails brain-derived neurotrophic factor and splenic IL-10 modulation. Brain Res. 2019;1722:146380. doi: 10.1016/j.brainres.2019.146380. [DOI] [PubMed] [Google Scholar]