Abstract
Curcuma longa (Turmeric) is a tropical herbaceous perennial plant of the family Zingiberaceae and contains curcuminoids, sesquiterpenoids and monoterpenoids as its major components. Given the broad range of activities that Curcuma longa possesses and also its use as a traditional epilepsy remedy, this review attempts to systematically review the experimentally proven activities of Curcuma longa and its bioactive components, which are related to the management of epileptic seizures. Using the PRISMA model, five databases (Google Scholar, PubMed, ScienceDirect, SCOPUS and SpringerLink) were searched using the keywords [“Curcuma longa” AND “Epilepsy”] and [“Curcuma longa” AND “Seizures”], leaving 34 articles that met the inclusion criteria. The present systematic review elaborated on the experimentally proven potential of Curcuma longa components, such as an aqueous extract of Curcuma longa itself, Curcuma longa oil and active constituents like curcuminoids and bisabolene sesquiterpenoids found in Curcuma longa with anti-seizure potential. Using human equivalent dose calculations, human treatment parameters were suggested for each component by analysing the various studies in this review. This review also determined that the principal components possibly exert their anti-seizure effect via the reduction of corticosterone, modulation of neurotransmitters signalling, modulation of sodium ion channels, reduction of oxidative DNA damage, reduction of lipid peroxidation, upgregulation of brain-derived neurotrophic factor (BDNF) and γ-aminobutyric acid (GABA) mediated inhibition. It is anticipated that this review will help pave the way for future research into the development of Curcuma longa and its neuroactive constituents as potential drug candidates for the management of epilepsy.
Keywords: Turmeric, Curcuma longa, Epilepsy, Seizures, Curcumin, α-tumerone, β-turmerone, ar-turmerone, α-atlantone
1. INTRODUCTION
The human Central Nervous System (CNS) is critical to our body’s functions but is continuously assaulted by a host of different CNS disorders. An example of such a condition is epileptic seizures, typically termed as a short-lived occurrence of signs and/or symptoms due to aberrant, excessive or synchronous brain neuronal activity [1]. In comparison, epilepsy occurs when there is a high recurrence risk of another seizure after an unprovoked seizure; when two unprovoked seizures occur over 24 hours apart, the diagnosis of an epilepsy syndrome is made [2]. While epilepsy itself is currently incurable, anti-seizure medications (ASMs) can be used for symptomatic treatment by providing seizure control through methods such as blockading of sodium ion channels, activating of specific receptors or modulating the release of neurotransmitters [3]. Despite close to 30 ASMs have been discovered, the seizures in around 30% of epileptic patients cannot be satisfactorily controlled [4]. This drug resistance issue has spurred a search for alternative ASMs, and one possible search area could be plants.
Turmeric is the common name of Curcuma longa and is a tropical herbaceous perennial plant that belongs to the family Zingiberaceae and is native to South Asia [5]. The plant is widely cultivated in the region. The plant's rhizome functions as both a traditional food ingredient and a traditional remedy for ailments, such as biliary and hepatic disorders, abdominal pains [6] and even epilepsy [7]. The use of Curcuma Longa has also spread to the field of manufacturing, and it is used in perfumes, as a flavour additive for curries and mustards and a natural colouring agent due to the vibrant yellow powder obtained from crushing the rhizomes of the plant [8]. Given Curcuma Longa's popularity, it is hardly surprising that a substantial number of scientific studies have been conducted to determine its biological activities, which include anti-fungal [9], anti-cancer [10], anti-bacterial [11] and others, as well as therapeutic activities, such as wound healing and anti-lithogenic among many others [12].
The idea of plant-derived ASMs is not entirely novel as cannabidiol (CBD)-enriched cannabis oil from the infamous Cannabis sativa plant has been found to work well to treat refractory epilepsy [13]. The ASM losigamone derived from the kava-kava plant can also reduce seizure frequency [14]. Thus, given the broad range of activities that Curcuma Longa possesses and its use as a traditional epilepsy remedy, this review attempts to systematically review the experimentally proven activities of Curcuma longa and its bioactive components and the management of epileptic seizures to determine the possible mechanisms of action.
2. METHODS
2.1. Search Method
Five databases (Google Scholar, PubMed, ScienceDirect, SCOPUS and SpringerLink) were searched using the keywords [“Curcuma longa” AND “Epilepsy”] and [“Curcuma longa” AND “Seizures”]. The results were limited to articles published between January 2010 and April 2020 to allow for more recent articles while limiting the likelihood of inadvertently excluding older articles.
2.2. Study Selection and Inclusion Criteria
This systematic review has only considered original research articles' content as other publication types might not provide sufficient information for evaluation and comparison between the articles. Duplicated results were also removed as well as those irrelevant to Curcuma Longa or the field of neuroscience. The selection process was conducted according to the guidelines underlined by the PRISMA protocol [15]. The quality of the included articles was assessed using the Risk of Bias (RoB) tool developed by the SYstematic Review Center for Laboratory animal Experimentation (SYRCLE) [16]. Articles with both positive and negative outcomes were considered to reduce the possibility of publication bias.
3. RESULTS
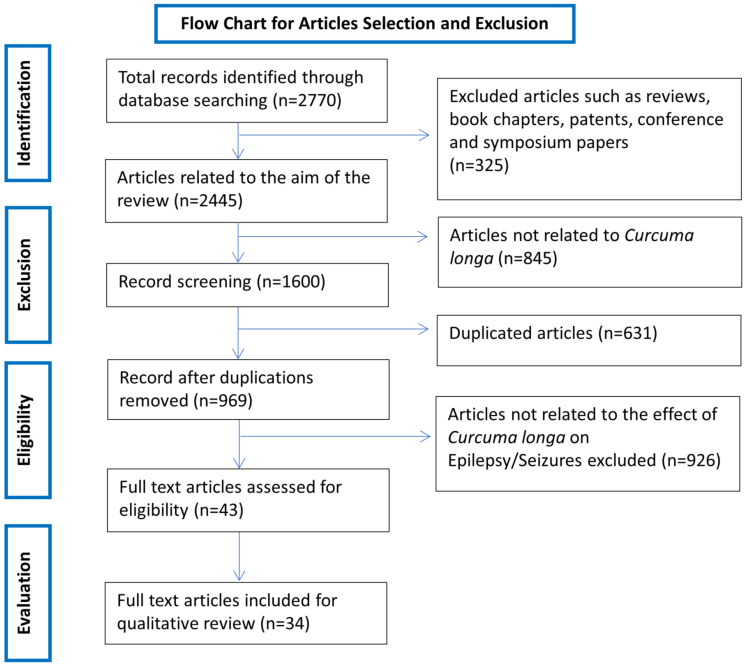
Using the chosen keywords to search the aforementioned databases resulted in a total of 2770 results. Of the 2770 results, 2000 were from Google Scholar (out of 3710 results as Google Scholar only allows the retrieval of the first 1000 results for each search), 8 from PubMed, 491 from ScienceDirect, 44 from SCOPUS and 227 from SpringerLink. After applying the exclusion criteria, 2727 results were removed, which included 631 duplicates and 2096 results not related to the scope of the review (Fig. 1). The remaining 43 results
underwent full-text evaluation, whereby the texts were searched for the principal component used in the study, the treatment regimen used, the seizure model used and major relevant study outcomes to compare and contrast them. Nine results were subsequently removed for ultimately being irrelevant to the aim of the review, leaving a total of 34 articles to be included in this review. The 34 articles are summarised in Table 1 and are discussed in greater detail throughout this systematic review.
The bioactive components of Curcuma Longa are variable due to the variety, location of cultivation and many other factors, though curcuminoids, sesquiterpenoids and monoterpenoids have been identified as the major components [17]. However, the major components of Curcuma Longa can vary between plant parts as well, leading to the suggestion that the active curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin) can be used as markers in the rhizome, powder and extracts of Curcuma Longa whereas the major ketonic sesquiterpenes (Ar-turmerone, α-turmerone and turmerone) can be used as markers for Curcuma longa oil and oleoresin products [18]. Nevertheless, the studies identified in our search overwhelmingly used the curcuminoid from curcumin. Only two studies used Curcuma longa extracts, one used Curcuma longa oil and two used the bisabolene sesquiterpenoids found in Curcuma longa.
3.1. Curcumin
This section is subdivided according to the seizure model used due to the large number of studies that fall under this category. Some studies utilised more than one model and are thus repeated under their respective subsections.
3.1.1. Pentylenetetrazol (PTZ) Induced Seizures
A total of 21 studies were found where turmeric principal constituents were evaluated using the PTZ model. These studies range from acute to chronic PTZ models, including kindling models. They also comprised of studies where turmeric-derived compounds were used either for single or repeated dose administration.
Chronic treatment of curcumin (20 mg/kg, i.p.) for two weeks reduced the duration of pentylenetetrazol (PTZ) induced generalised tonic-clonic seizures in Wistar rats that were potentiated by daily exposure to stress [19]. Curcumin pre-treatment also alleviated seizure activity and severity and delayed the onset of myoclonic jerks, whereas a single acute administration of curcumin had no significant effect on the seizures [19]. In another study, chronic oral treatment of curcumin at 100mg/kg in mice resulted in increased onset latency to myoclonic jerks and seizures [25]. The authors also noted that curcumin could have a synergistic effect with the standard ASM sodium valproate. A combination of 100 mg/kg of curcumin and a sub-therapeutic dose of sodium valproate (400 mg/kg) exhibited a similar anti-seizure activity compared to the therapeutic dose of sodium valproate alone. In addition, curcumin did not affect the learning and memory of the mice both before seizures were induced and after recovery from seizures. Agarwal et al. reported that an oral liposomal curcumin formulation significantly delayed the onset of myoclonic jerks and generalised seizures [27]. Their results also showed that liposomal curcumin given intravenously could dose-dependently increase the latency and decrease clonic seizures' duration during status epilepticus. The authors believed that this formulation of curcumin would have an enhanced effect over free curcumin as the phospholipid carriers could more easily cross the blood-brain barrier (BBB) with an improved half-life. However, the study does not mention when the liposomal curcumin was given in relation to the induction of seizures or how many doses were given.
When curcumin was given intraperitoneally to kindled male Wistar rats daily for 24 days, 200 mg/kg was found to be most effective in decreasing the mean frequency of PTZ-induced epileptiform activity. The dose of 100 mg/kg had a weaker effect and 50 mg/kg had no significant impact on the epileptiform activity [33]. Administering nitric oxide synthase inhibitors (N-nitro-L-arginine methyl ester, 7-Nitroindazole, or aminoguanidine) or a nitric oxide precursor (L-arginine) alone did not affect epileptiform activity. However, co-administration of N-nitro-L-arginine methyl ester and 7-Nitroindazole potentiated the effect of curcumin on epileptiform activity. In contrast, aminoguanidine had no impact on the effect of curcumin on epileptiform activity. L-arginine was found to reverse the effect of curcumin in the earlier stages and aggravated interictal activity but augmented curcumin and reduced interictal activity in the later stages of PTZ-induced epileptiform activity. It should be noted that this study [33] implies that neuronal nitric oxide synthase, and not inducible nitric oxide synthase, is at least partly responsible for the anti-epileptic effect of curcumin, but another study [31] in this review implies the opposite. The contradiction in the mechanism will be explored further in the discussion part of this review. In another study, curcumin was given orally to young male Wistar rats daily for up to 10 weeks, an hour before kindling with PTZ, and a dose-dependent protective effect was found against kindling in terms of reduced seizure scores [37]. Besides seizure score, a 300 mg/kg dose of curcumin was also found to significantly reduce the latency to myoclonic jerks, clonic and generalised tonic-clonic seizures, and decreased the number of myoclonic jerks. The study also found that curcumin dose-dependently reversed PTZ kindling induced hippocampal injury, hippocampal oxidative stress and hippocampal apoptosis.
When curcumin was given intraperitoneally to adult male Wistar rats at a dose of 100 mg/kg daily for 40 days, no significant effect on seizure score upon a single challenge dose of PTZ at day 40 after 30 days of concurrent PTZ kindling on alternate days, was discovered [39]. Moreover, the study also ran an array of mitochondrial complex activity tests on the rat hippocampus and cerebral cortex. It determined that curcumin restored NADH (Nicotinamide adenine dinucleotide): cytochrome c reductase and cytochrome c oxidase activity in both regions, despite the presence of PTZ. Similarly, the PTZ induced mitochondrial swelling and ultrastructural changes were also prevented by curcumin treatment. In addition, biochemical studies revealed that curcumin reduced oxidative stress levels in addition to increasing antioxidant defenses. Following that, neuronal cell death in both hippocampal and cerebral cortex regions was found to be reduced by curcumin treatment, in addition to PTZ induced memory impairment being ameliorated. In a separate follow-up experiment with an identical curcumin treatment and PTZ kindling regime by the same laboratory, curcumin was again found not to affect seizure score significantly, but a substantial amelioration of PTZ induced memory deficits was found in this study [40]. Curcumin treatment also produced a reduction in hippocampal and cerebral cortex inflammation as well as a significant reduction in glial and astrocyte activation was observed. In another study, curcumin was given orally to male Wistar rats daily for up to 43 days (till the development of PTZ kindling) and it was found that curcumin produced a dose-dependent anti-seizure effect [43]. Curcumin at a dose of 300 mg/kg significantly increased the latency to myoclonic jerks, clonic seizures, generalized tonic-clonic seizures as well as improved the seizure score and decreased the number of myoclonic jerks, with the lower doses also producing a lesser but still good anti-seizure effect. Curcumin was found to significantly reverse PTZ kindling induced cognitive impairment in a dose-dependent manner as it does not improve memory in healthy rats. Curcumin also dose-dependently reversed PTZ kindling induced oxidative stress.
When curcumin was given intraperitoneally to swiss albino mice daily up to 15 days in a study, it was found that PTZ induced seizure severity was reduced in a dose-dependent manner in terms of seizure score after administration of a sub-convulsive PTZ dose to PTZ kindled mice [34]. The study also found that the depressive behaviour and memory deficit induced by PTZ were both attenuated in a dose-dependent manner by curcumin. The effect of curcumin on seizures, depressive behaviour, and memory deficit was all found to increase with increasing treatment duration up to the study's 15 days limit. The whole-brain biochemical estimations showed that curcumin increased the levels of brain serotonin and norepinephrine as well as reduced acetylcholinesterase activity and nitrosative stress (nitrite level) after 15 days of treatment. In another study, oral curcumin was given for 35 days to inbred PTZ kindled male Swiss albino mice, and a dose-dependent decrease in the seizure incidence and severity was observed [28]. The 200 mg/kg dose produced a seizure protection effect comparable to the standard ASM diazepam (3 mg/kg). The study also found that after 35 days, all the curcumin doses significantly decreased malondialdehyde (lipid peroxidation marker) and restored the depressed glutathione levels in PTZ kindled mice's brain tissue.
When free curcumin was given intraperitoneally to male NMRI daily for 10 days in a study before kindling with PTZ every alternate day, there was no anti-seizure effect determined and no effect on memory was observed [36]. Free curcumin was also found to have a slight but non-significant reduction in the level of cell death and glial activation in the hippocampus. The same study also tested the effect of curcumin-loaded nanoparticles in an identical manner and found that a 25 mg/kg dose significantly reduced the seizure-induced behavioural signs and hence the seizure score, improved spatial learning and memory as well as significantly reduced hippocampal cell death and glial activation. In another study, curcumin-loaded nanoparticles were given intraperitoneally to male NMRI mice at a dose of 12.5 mg/kg for ten days before starting PTZ kindling and 20 days afterwards; it was found that the treatment significantly alleviated PTZ induced hippocampal neuronal cell death [42]. This observation was also supported by the downregulated hippocampal level of tumour necrosis factor-alpha (Tnf-α). In contrast, klotho (life-extension factor) levels and erythropoietin (neuroprotective) were upregulated, reversing the effects of PTZ. The study also tested free curcumin under the same conditions and found a similar effect on the parameters measured but to a significantly lesser degree.
When curcumin was given orally to male albino mice (Laka strain) before infusing PTZ intravenously, only the 80 mg/kg curcumin dose significantly decreased the PTZ threshold required for tonic onset extension but no effect on either myoclonic jerks or generalised clonus was observed [30]. The lower doses were ineffective on all three parameters and the same was also true for the higher 120 mg/kg dose, however the authors did not explore the reason for this. In addition, the study also determined that the PTZ threshold for the onset of tonic extension increased at every 15-minute timepoint up to a maximum at 45 minutes post 80 mg/kg curcumin administration and decreased thereafter. All subsequent testing was done with piperine (an inhibitor of hepatic and intestinal glucuronidation to increase curcumin bioavailability) which was given orally 15 minutes before curcumin administration; curcumin itself was given orally 45 minutes before intravenous PTZ. This subsequent testing revealed that both a nonselective adenosine A1/A2 receptor antagonist and a selective adenosine A1 receptor antagonist blocked curcumin's effect on the PTZ seizure threshold. They also found the reverse to be true, with a nonselective adenosine A1/A2 receptor agonist and a selective adenosine A1 receptor agonist both potentiating the effect of even a sub-effective dose of curcumin (40 mg/kg). In contrast, both a selective adenosine A2A antagonist and a selective adenosine A2A agonist did not affect the action of curcumin. The study also confirmed using a peripheral adenosine receptor antagonist that the effects of curcumin were mediated via central adenosine receptors.
When curcumin was given intraperitoneally to male NMRI albino mice in a study, it was found that curcumin administration 75 minutes before the infusion of PTZ did not significantly alter the seizure threshold [31]. In contrast, when nanoparticles of curcumin C3 complex (a standardised product with a specific ratio of curcumin, demethoxycurcumin and bisdemethoxycurcumin [53]) were given intraperitoneally to male NMRI albino mice, the study found a dose-dependent increase in seizure threshold, with 80 mg/kg being the most effective. It was also found that the 80 mg/kg dose of nanoparticles significantly increased the seizure threshold starting from 45 minutes following administration, with a maximal effect at 60 minutes. In the case of the nanoparticles, they found that L-arginine given 15 minutes before the nanoparticles dose-dependently reversed the effect of nanoparticle pre-treatment on the seizure threshold. In contrast, both the non-selective nitric oxide synthase inhibitor and the selective inducible nitric oxide synthase inhibitor potentiated a sub-effective nanoparticle dose of 10 mg/kg in a dose-dependent manner. The study also noted that a selective neuronal nitric oxide synthase inhibitor did not significantly affect the seizure threshold. As previously mentioned, the results of this study [31] contradict that of another study in this review [33].
When curcumin was given intraperitoneally to male mice 25 minutes beforehand, a study found that seizure and tonic-clonic latency was significantly increased, and the duration of both tonic and tonic-clonic seizures was reduced considerably, though the loss of balance and mortality were not significantly affected by curcumin [32]. The study also found that curcumin was inhibited and even reversed in some instances when brain serotonin levels were depleted over four days. In addition, the serotonin 5-HT1A, 5-HT2C and 5-HT4 receptor antagonists individually diminished the effect of curcumin, but the 5-HT7 antagonist potentiated it. However, antagonising all the four serotonin receptors at once prevented the effect of curcumin, and only the expression level of 5-HT7 was reduced in the hippocampus. In another study, a curcuminoids mixture from Curcuma longa containing curcumin, demethoxycurcumin and bisdemethoxycurcumin was used to pre-treat seven days post-fertilisation (dpf) zebrafish larvae of the Tg (fli 1a: EGFP)y1 strain by adding it directly into the water, for an hour in darkness before the addition of PTZ [46]. Every dose of the curcuminoids mixture showed significant anticonvulsant activity in a dose-dependent manner.
When wild type zebrafish larvae were exposed to curcumin and micronized curcumin at a dose of 1 µM in the water for 30 minutes, a study found that both did not induce behavioural alterations in terms of travelled distance and the number of times that the larvae crossed from the centre of the well to the periphery [50]. A different set of larvae was similarly exposed to curcumin but exposed to PTZ 30 minutes after the curcumin treatment. Micronized curcumin significantly reduced the occurrence of seizures in terms of seizure stages as well as increased the latency to the different stages. In contrast, curcumin did not affect the occurrence of seizures but did increase the latency to the different stages. The authors of the study also repeated their experiment in adult zebrafish using the intraperitoneal route at a dose of 0.5 µM and found that both curcumin and micronized curcumin reduced the travelled distance of the adult zebrafish but did not have any effect on the number of times that the zebrafish crossed the top, centre and bottom of the tank. In the PTZ seizure model, micronized curcumin significantly reduced the occurrence of the most severe stage III seizures and delayed the onset of each stage.
In contrast, curcumin did not affect seizure occurrence but significantly delayed the onset of each seizure stage. In another study, curcumin was given orally to male Wistar rats at a dose of 300 mg/kg, 60 minutes before PTZ seizure induction, and a significant increase in the myoclonic jerk latency that was comparable to a sub-therapeutic dose of the standard ASM valproate was found [49]. When the sub-therapeutic dose of valproate was co-administered with 300 mg/kg of curcumin, the study found that the myoclonic jerk latency increase was more remarkable than when they were given individually. However, there was no significant difference found in the serum level of valproate. Whole-brain estimation of oxidative stress parameters revealed a similar story whereby a moderate reduction was demonstrated when curcumin or valproate was given alone, and a more significant reduction was observed when given together. The study also measured the rats' learning and memory ability after seizure induction compared to before induction but found no significant improvement in the PTZ induced learning and memory deficiencies when the sub-therapeutic dose of valproate or curcumin was given alone. In contrast, a significant improvement was found when the two were co-administered.
3.1.2. Kainic Acid-Induced Seizures
Kainic acid, a neurotoxic analogue of glutamate, is commonly used to induced seizures in rodent models. Curcumin treatment at 100 mg/kg daily for seven days could modulate kainic acid-induced epileptogenesis by upregulating the anti-inflammatory cytokines Interleukin 10 Receptor Subunit Beta (Il10rb), Chemokine (C-X-C motif) ligand 16 (Cxcl16), and Cxcl17 as well as protect against hippocampal cell death by up-regulating nicastrin [22]. Curcumin treatment for 2 weeks was also able to reduce abnormal electroencephalography (EEG) spike frequency and spontaneous recurrent seizures as measured via seizure scoring [38]. Moreover, the learning and memory deficit induced by kainic acid was almost completely reversed by curcumin treatment. In addition, analysis of hippocampal tissue showed that curcumin significantly reduced the elevated Interleukin 1 beta (Il-1β) and Tnf-α levels caused by kainic acid as measured via ELISA three days after kainic acid administration. Gfap and Nissl staining seven days after kainic acid administration showed that curcumin reduced astrocyte activation (and hence pro-inflammatory cytokine production) as well as neuronal loss in the dentate hilus (DH) and CA3 regions of the hippocampus as a result of kainic acid. The study also found less mossy fibre sprouting because of kainic acid.
In another study, curcumin was given at a dose of 200 mg/kg either seven days before the administration of kainic acid (protected group) or three days after the administration (treated group) [51]. However, it was not clear from the statistical analysis if there was a significant difference in outcomes between the two treatment conditions, and they were often mentioned together in the paper during a discussion of the outcomes. Hence, this review has considered both conditions to not be significantly different from each other in terms of outcome. Both time points have also been deemed to be not substantially different from each other due to a lack of clarity in the statistical analysis and a lack of differentiation between the authors' two time-points. The study found that curcumin treatment reduced the level of oxidative stress and inflammation as measured by the serum level of sialic acid, TNF-α and Il-1β as well as the brain tissue levels of superoxide dismutase, catalase and glutathione peroxidase, L- malondialdehyde, glutathione, nitric oxide, 8-Oxo-2'-deoxyguanosine, activator protein 1 and myeloperoxidase. The caspase 3 activity level and DNA fragmentation were also reduced by curcumin treatment, which implies a reduction in kainic acid-induced neuronal death. Histopathological examination of the brain tissue showed that curcumin treatment reduced several pathological alterations such as haemorrhage, oedema, neural degeneration and encephalomalacia, to differing degrees.
3.1.3. Pilocarpine Induced Seizures
Pilocarpine induces status epilepticus condition in rodents characterized by tonic-clonic generalised seizures. Typically, pilocarpine injections induce epilepsy-like condition mimicking clinical epilepsy in patients. Pre-treatment of oral curcumin at doses of 50 - 200 mg/kg for 3 days decreased the occurrence and latency of both lithium – pilocarpine-induced seizures and status epilepticus in a dose-dependent manner [20]. The study also showed that curcumin could ameliorate status epilepticus induced cognitive dysfunction as measured via the Morris water maze and also oxidative stress in both the hippocampus and striatum of the brain. Curcumin 80 mg/kg daily for 21 days restored hippocampal Na+/K+-ATPase activity to normal, and this was associated with a decrease in cellular excitability and, hence, seizure appearance propagation [26]. The study also found that curcumin reversed the oxidative stress caused by pilocarpine in terms of the oxidative stress markers nitric oxide, glutathione and malondialdehyde. In addition, although the study found that acetylcholinesterase levels were slightly increased by curcumin; the decrease in catalase levels by pilocarpine was actually potentiated by curcumin, although the authors have suggested that catalase does not play a major role in the rat brain in response to oxidative stress. Curcumin treatment also protected from seizure-like behaviour, such as facial automatisms, forelimb clonus, rearing and falling [45]. The study determined an alteration in the equilibrium ratio between inhibitory amino acids (γ-aminobutyric acid [GABA], glycine, taurine and glutamine) and excitatory amino acids (glutamate and aspartate) levels by enhancing the former and inhibiting the later in the rat hippocampus and cortex. In addition, light microscopy examinations of the hippocampus and cortex showed that pilocarpine-induced neuronal histological changes and death were reduced by curcumin treatment.
Another study showed that curcumin treatment markedly increased the number of surviving hippocampal neurons as determined via Nissl staining and the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay, starting from the 24-hour time point in almost all four regions of the post-SE hippocampus (CA1, CA3, dentate gyrus, and hilus). Curcumin treatment resulted in an induction of autophagy proteins (beclin-1 and LC3BII/LC3BI) as determined via Western blotting and also inhibited the upregulation of necroptosis proteins (mixed lineage kinase domain-like pseudokinase [MLKL] and receptor-interacting serine/threonine-protein kinase 1 [RIP-1]) as determined via immunohistochemistry. Similarly, the number of autophagosomes as determined via transmission electron microscopy was also found to be decreased by curcumin treatment.
Curcumin nanoparticle treatment reduced excitability in the cortex and hippocampus by preventing a pilocarpine-induced reduction in acetylcholinesterase activity, although it was unable to do so for Na+/K+-ATPase. The study also found that curcumin exhibited anti-inflammatory activity and reversed the pilocarpine-induced increase in cortical malondialdehyde levels but not in the hippocampus, whereas the level of glutathione was only slightly increased in both regions. In contrast, Tnf-α levels in the hippocampus measured using ELISA were markedly reduced, whereas cortical levels were still elevated by pilocarpine. The decrease in the hippocampus's nitric oxide level due to pilocarpine was also prevented by curcumin treatment, but the decrease was not significant in the cortex. The study also determined that curcumin treatment produced an anti-apoptotic effect in the cortex and hippocampus, as estimated using ELISA determined caspase-3 levels.
3.1.4. Iron Induced Seizures
When curcumin supplemented food pellets were given for six months to one-month-old male Wistar rats at a concentration of 1500 parts per million (ppm) (or approximately 100 mg/kg based on the author estimated rat daily food intake), a study found a significant reduction (but not cessation) in the development and occurrence of iron chloride seizures (induced at the end of the fifth month of treatment) as determined via cortical electroencephalographic and multiple-unit activity measurements [41]. The study also determined that the basal electrical activity of the brain was not affected by curcumin treatment. Besides that, the mRNA (RT-PCR) and protein levels (immunohistochemistry) of Nav1.1 (Sodium Voltage-Gated Channel Alpha Subunit 1, Scn1a) were determined to be reduced in the cortex but not the hippocampus. In contrast, the mRNA expression levels of Nav1.6 (Scn8a) were found to be unchanged, yet protein levels were reduced in both the cortex and hippocampus; these results were described as inconclusive by the authors.
3.1.5. Penicillin Induced Seizures
When curcumin was given intraperitoneally to adult male Wistar rats at doses of 50, 100 or 200 mg/kg ten minutes after the intracortical administration of penicillin to induce seizures, a study found that 100 or 200 mg/kg of curcumin significantly reduced both the frequency and the amplitude of spike waves [29]. The study also found that a sub-therapeutic dose of curcumin (50 mg/kg) could still potentiate the effect of the ASM diazepam at therapeutic doses.
3.1.6. Electricity Induced Seizures
When curcumin was given orally to three to four months old Swiss albino mice at doses of 50 or 100 mg/kg for 14 consecutive days in a study, both doses did not significantly affect Maximal Electroshock Seizure (MES) induced tonic hind limb extension [25]. However, the study found that the 100 mg/kg dose significantly reduced the clonic phase duration. The study also found that neither curcumin dose affected the mice's learning and memory, as measured using the elevated plus-maze, before seizures were induced nor after the recovery from seizures.
In the presence of a liposomal curcumin formulation given orally to inbred male Swiss albino mice at doses of 25 or 50 mg/kg, a study found that the current threshold required to induce seizures in the Increasing Current Electroshock Seizures (ICES) test was significantly increased in a dose-dependent manner [27]. The study's authors believed that this formulation of curcumin had an enhanced effect over free curcumin as the phospholipid carriers could more easily cross the blood-brain barrier (BBB) and exhibited a prolonged half-life. However, the study does not mention when the liposomal curcumin was given in relation to the induction of seizures or how many doses were given.
When curcumin was given intraperitoneally to adult male Swiss mice at a dose of 300 mg/kg at four different pre-treatment times of 15,30, 60 or 120 minutes, a study found that curcumin had no anti-convulsive effect regardless of pre-treatment time in the MES model as defined by the occurrence of tonic hind limb extension [35].
When curcumin was given orally to male Wistar rats at a dose of 300 mg/kg at 60 minutes before seizure induction using the MES model, a study found a 33% protection against tonic hind limb extension that was comparable to sub-therapeutic doses of the standard ASMs phenytoin and phenobarbitone given 30 minutes before PTZ seizure induction, but inferior to the 50% afforded by the ASM carbamazepin [49]. When the sub-therapeutic dose of carbamazepine was co-administered with 300 mg/kg of curcumin, the study found that the protection was greater than when they were given individually, though there was no significant difference found in the serum level of the ASMs. The whole brain estimation of oxidative stress parameters revealed that the levels of malondialdehyde and glutathione demonstrated a moderate reduction in oxidative stress when curcumin was given alone, but not when the sub-therapeutic dose of ASMs was given alone. However, a significant decrease in oxidative stress was seen when the two were given together. The study also measured the rats' learning and memory ability after seizure induction compared to before induction using the elevated plus maze and passive avoidance tests but found no significant improvement in the MES induced learning and memory deficiencies when the sub-therapeutic dose of ASMs or curcumin was given alone. In contrast, a significant improvement was found when the two were co-administered.
3.1.7. Non-In Vivo Models
Using Glide to run molecular docking studies against the human 4-aminobutyrate-aminotransferase, a study found that curcumin binds to human 4-aminobutyrate-aminotransferase, which is an enzyme that catalyses the breakdown of the inhibitory neurotransmitter GABA. The study found that the contact points of this binding were Leu299, Leu301, Ala135, Gln267, Arg164 and Tyr161 [21].
Using patch-clamp electrophysiology on Human Embryonic Kidney cells 293 (HEK293) that express the calcium impermeable homomeric GluA1 and calcium-permeable heteromeric GluA1/A2 L-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) excitatory receptors, a study found that curcumin at a dose of 20 µM did not significantly change several gating biophysical properties that were peak current, deactivation and desensitisation [48]. As a comparison, the study also found that certain five-membered ring heterocyclic moiety derivatives of curcumin had inhibitory effects on the gating biophysical properties, but these derivatives are not naturally found in Curcuma longa and thus will not be considered further in this review.
3.2. Curcuma longa Crude Extract
An aqueous extract of Curcuma longa produced via soxhlet extraction in distilled water was given orally once daily to adult albino mice for 21 days in a study at the doses of 50, 100 or 200 mg/kg [23]. The study found that 100 and 200 mg/kg of the extract could delay the onset of pentylenetetrazol-induced seizures, though 50 mg/kg of the extract had no significant effect. In contrast, the same 100 mg/kg dose did not affect tonic hind limb extension in the MES test, but 200 mg/kg of the extract was sufficient to significantly reduce the duration. The authors noted that none of the doses used in their study could completely prevent the occurrence of seizures in either model.
A methanolic extract of Curcuma longa at concentrations of 3.1, 6.2 or 12.5 µg/ml was used to pre-treat seven dpf zebrafish larvae of the Tg (fli 1a: EGFP)y1 strain by adding it directly into the water, for an hour in darkness in a study [46]. Only a 12.5 µg/ml concentration showed significant anticonvulsant activity to significantly counteract the increase in locomotor activity induced by PTZ.
3.3. Curcuma longa Oil
Curcuma longa oil was used to pre-treat seven dpf larval zebrafish of the Tg (fli 1a: EGFP)y1 strain at concentrations of 2.5, 5.0 or 10.0 µg/ml by adding it directly into the water, for an hour in darkness in a study [46]. Only a 10 µg/ml concentration showed significant anticonvulsant activity in terms of significantly counteracting the increase in locomotor activity induced by pentylenetetrazol. However, Curcuma longa oil by itself was found to increase locomotor activity. The same study also determined the effect of Curcuma longa oil on EEG recordings under identical conditions as previously described. Curcuma longa oil was found to partly protect against pentylenetetrazol seizures in terms of significantly reducing the number and the duration of ictal-like discharges as well as shortening the cumulative duration of all forms of epileptiform discharges [46]. The study also confirmed that Curcuma longa oil is not pro-convulsive as it did not significantly affect the number and duration of interictal-like spikes compared to vehicle control. The same study also used Curcuma longa oil to pre-treat 10-week-old male C57Bl/6 mice intravenously for 10 minutes at doses of 50 and 100 mg/kg before pentylenetetrazol was infused intravenously into the mice. The study found that 50 mg/kg of Curcuma longa oil increased the PTZ dose required to trigger all behavioural endpoints (ear twitch, myoclonic twitch, tail twitch, forelimb clonus, falling, tonic hindlimb extension and death), whereas 100 mg/kg significantly delayed seizure generation for all seizure parameters including death.
3.4. Curcuma longa Bisabolene Sesquiterpenoids
The bisabolene sesquiterpenoids α,β-turmerone, ar-turmerone and α-atlantone were isolated from Curcuma longa oil using reversed-phase HPLC in a study [46]. The study pre-treated seven dpf larval zebrafish of the Tg (fli 1a: EGFP)y1 strain with the bisabolene sesquiterpenoids by adding it directly into the water, for an hour in darkness at the doses of 5, 11, 23 or 46 µM. The study found that α,β-turmerone significantly counteracted the increase in locomotor activity induced by PTZ at 46 µM, ar-turmerone at 23 µM and α-atlantone at 23 µM, though all three also slightly increased locomotor activity when given alone. The same study also used the bisabolene sesquiterpenoids to pre-treat 10-week-old male C57Bl/6 mice intravenously for 10 minutes before PTZ was infused intravenously into the mice. The study found that 50 mg/kg of ar-turmerone or 100 mg/kg of α,β-turmerone significantly increased the PTZ dose required to trigger all behavioural endpoints (ear twitch, myoclonic twitch, tail twitch, forelimb clonus, falling, tonic hindlimb extension and death), though they did not test α-atlantone due to the small amount available.
In another study, the bisabolene sesquiterpenoid ar-turmerone was also intraperitoneally given to 10-week-old male NMRI mice at doses of 0.01, 0.1, 1, 20 or 50 mg/kg 30 minutes before electrical stimulation using the 6-Hz psychomotor seizure assay [47]. Mice given ar-turmerone at a dose of at least 0.1 mg/kg displayed normal exploratory and locomotion behaviour and were all protected from electrically induced sudden behavioural arrest, whisker trembling, head nodding, facial and mouth jerking, forelimb clonus and dorsiflexion of the tail (Straub tail). When given intraperitoneally 24 hours before electrical stimulation, 50 mg/kg of ar-turmerone still protected 70% of the mice. The same dose of ar-tumerone was also found to persist in the mouse brains as early as 15 minutes after administration and for at least 24 hours using reversed-phase HPLC. The same study also treated 10-week-old C57Bl/6 mice with ar-tumerone via the intraperitoneal route and found that a 10-minute pre-treatment of 1 mg/kg of at-tumerone was sufficient to raise the PTZ dose necessary to cause tonic hind limb extension and death, whereas a 20 mg/kg dose had the same effect only on death. When given intraperitoneally 10 minutes beforehand, a 50 mg/kg dose of ar-tumerone was found not to affect the balance of 10-week-old C57Bl/6 mice in the beam walking test in contrast with the established ASM diazepam which impairs balance. The authors of the study also found that when 46 µM of ar-tumerone was added into water containing seven dpf AB strain zebrafish larvae for an hour before subsequent exposure to PTZ, ar-tumerone counteracted PTZ induced c-fos upregulation but did not affect the expression of the GABAA receptor and Il-10, as determined using RT PCR. Interestingly, they found that ar-tumerone alone upregulated brain-derived neurotrophic factor (bdnf) and this upregulation was even more prominent when also exposed to PTZ.
4. DISCUSSION
This systematic review has discovered a significant number of studies that experimentally demonstrate the potential of Curcuma longa and its bioactive components for the management of epileptic seizures. However, all the studies in this review were non-clinical studies that did not involve human testing, which is a major stumbling block towards the goal of managing epileptic seizures in human patients. In addition, relying on published works carries the inherent risk of publication bias as studies demonstrating a lack of positive results are less likely to be published. Therefore, the three studies with negative results in this review [31, 35, 36] will be weighed and examined equally for any contradictions to the other positive findings. Nevertheless, there could be some insights gained by analyzing all the results of the substantial number of studies in this review. Two of these major insights would be treatment parameters as well as possible mechanisms of action and will be discussed below.
4.1. Treatment Parameters for Curcuma longa and Its Neuroactive Components for the Management of Epileptic Seizures
To compare treatment parameters between the different studies, four main points were considered, namely which component of Curcuma longa has been used, treatment parameters for that component (dose, route and duration), seizure model (induction method, dose, route and duration) and finally the animal model used. Next, non-animal in vivo models were excluded due to lower clinical relevance (the seizure phenotype cannot be expressed) and the difficulties in converting the treatment parameters to in vivo models due to pharmacokinetic differences. This exclusion leaves three in vivo animal models, rats, mice and zebrafish, with rats and mice being the overwhelmingly dominant animal models. Unfortunately, given that there are currently no guidelines for converting zebrafish doses to mammalian equivalents [54], this analysis is left with the mice and rat models for which human equivalent dose calculations exist [55, 56]. The next exclusion criterion would be the use of different formulations (such as micronized, nanoparticles, liposomal) or synthetic analogs of Curcuma longa components as these parameters are more likely to be study-specific and are not components of Curcuma longa in a sense that they are not naturally occurring. After applying all the exclusion criteria, the selected studies along with the main parameters are tabulated in Table 2. The human treatment parameters are based on the lowest dose and shortest treatment duration for a given administration route if multiple studies are being considered for a particular model. These treatment parameters make the simplistic assumption that the base seizure severity of all the studies for a given model is similar and that the treatment outcomes are similar. Thus, these treatment parameter recommendations should be treated as a starting point for further exploration and refinement of the dose, treatment duration, and possibly the route of administration.
The first seizure model is the PTZ induced seizure model in which PTZ antagonizes the GABAA receptor to produce primary generalized seizures [57]. The studies in this review used PTZ in three different ways, single-dose, continuous intravenous infusion, or kindling (repeated, intermittent administration of sub-convulsive doses that ultimately produce a tendency to develop seizures [58]. The second seizure model is the kainic acid-induced seizure model, as kainic acid is a cyclic analog of L-glutamate and an agonist of ionotropic kainic acid receptors, which causes excitatory responses in cortical neurons, making it a model of temporal lobe epilepsy [59]. The third seizure model is the pilocarpine-induced seizure model of epilepsy as pilocarpine is a muscarinic receptor agonist and may be administered with lithium to increase rats' susceptibility to this model of temporal lobe epilepsy [60]. The fourth seizure model utilized by the selected studies is the iron-induced seizure model, which is believed to occur due to cortical neurons' reaction to iron-induced oxidative stress and is said to be a model of post-traumatic epilepsy [61]. The fifth seizure model is the penicillin-induced seizure model which appears to interact with
GABA to reduce its inhibitory activity via penicillin’s β-lactam ring [62], and is a model of focal motor seizures [63]. The sixth seizure model is the MES-induced seizure model, whereby electrical stimulation is used to predict effective drugs against tonic-clonic (grand mal) type generalized seizures [64]. The seventh model is the 6 Hz corneal stimulation model, which produces seizures that resemble the behaviors seen in human limbic epilepsy [65].
4.1.1. Treatment Parameters for Curcumin
This section is subdivided according to seizure type due to the large number of studies that fall under this category.
4.1.1.1. Treatment Parameters for Generalised Seizures (PTZ Induced)
Using the United States Food and Drug Administration human equivalent dose recommendations [56], a 12.20 mg/kg dose of curcumin given as an intraperitoneal injection 25 minutes beforehand [32] or a 3.226 mg/kg dose of curcumin given as an intraperitoneal injection daily for 14 days to a 60 kg human could treat single generalized seizure events [19, 24] (single dose PTZ acute seizure model). Single seizure events could also be treated by a 48.39 mg/kg dose of curcumin given orally 60 minutes beforehand [49] or an 8.130 mg/kg dose of curcumin given orally daily for 14 days [25]. Using the same recommendations, a 4.065 mg/kg dose of curcumin given as an intraperitoneal injection daily for 15 days [34] or a 1.016 mg/kg dose of curcumin given as an intraperitoneal injection daily for 30 days to a 60 kg human could be used to treat recurrent generalized seizure events [42] (PTZ kindling model). Recurrent generalized seizure events could also be treated by a 6.504 mg/kg dose given orally 45 minutes beforehand [30] (PTZ infusion model) or a 4.065 mg/kg dose given orally daily for 7 days [28] (PTZ kindling model) to a 60 kg human.
4.1.1.2. Treatment Parameters for Temporal Lobe Epilepsy (Kainic Acid-Induced)
Using the United States Food and Drug Administration human equivalent dose recommendations [56], a 16.13 mg/kg dose of curcumin given as an intraperitoneal injection daily to a 60 kg human for seven days [22] or a 16.26 mg/kg dose of curcumin given orally daily for three days [51] could treat temporal lobe epilepsy.
4.1.1.3. Treatment Parameters for Temporal Lobe Epilepsy (Pilocarpine Induced)
Using the United States Food and Drug Administration human equivalent dose recommendations [56], an 8.065 mg/kg dose of curcumin given orally daily to a 60 kg human for three days [20] could treat temporal lobe epilepsy.
4.1.1.4. Treatment Parameters for Post-Traumatic Epilepsy (Iron Induced)
Using the United States Food and Drug Administration human equivalent dose recommendations [56], a 16.13 mg/kg dose of curcumin intake daily by a 60 kg human for six months could treat post-traumatic epilepsy [41].
4.1.1.5. Treatment Parameters for Focal Motor Seizures (Penicillin Induced)
Using the United States Food and Drug Administration human equivalent dose recommendations [56], a 16.13 mg/kg dose of curcumin given as an intraperitoneal injection to a 60 kg human 10 minutes beforehand [29] could treat focal motor seizures.
4.1.1.6. Treatment Parameters for Tonic-Clonic (Grand Mal) Type Generalised Seizures (MES Induced)
Using the United States Food and Drug Administration human equivalent dose recommendations [56], a 48.39 mg/kg dose of curcumin given orally to a 60 kg human 60 minutes beforehand [49] or an 8.130 mg/kg dose of curcumin given orally daily to a 60 kg human for 14 days [25] could treat tonic-clonic (grand mal) type generalized seizures.
4.1.2. Treatment Parameters for Aqueous Curcuma longa Extract
Using the United States Food and Drug Administration human equivalent dose recommendations [56], an 8.130 mg/kg dose of aqueous Curcuma longa extract delivered as a daily intraperitoneal injection into a 60 kg human for 21 days [23] could treat tonic-clonic (grand mal) type generalized seizures and a 16.26 mg/kg dose [23] primary generalized seizures.
4.1.3. Treatment Parameters for Curcuma longa Oil
Using the United States Food and Drug Administration human equivalent dose recommendations [56], a 4.065 mg/kg intravenous infusion of Curcuma longa oil [46] into a 60 kg human could treat primary generalized seizures.
4.1.4. Treatment Parameters for α,β-turmerone
Using the United States Food and Drug Administration human equivalent dose recommendations [56], an 8.130 mg/kg continuous intravenous infusion of α,β-turmerone [46] into a 60 kg human could treat primary generalized seizures.
4.1.5. Treatment Parameters for Ar-turmerone
Using the United States Food and Drug Administration human equivalent dose recommendations [56], a 4.065 mg/kg continuous intravenous infusion of Ar-turmerone or an intraperitoneal injection with the same dose 30 minutes beforehand [46] into a 60 kg human could treat primary generalized seizures. A 4.065 mg/kg dose of Ar-turmerone delivered as an intraperitoneal injection 10 minutes beforehand [47] into a 60 kg human could treat human limbic epilepsy.
4.1.6. Overall Discussion of Treatment Parameters
Only three studies reported negative results in this review, which were only related to the usage of curcumin in the PTZ and MES models. Using these few cases in which treatment with curcumin was unsuccessful, a general observation could be made. When given as a single dose a short period (tens of minutes to several hours) before seizure induction, a high curcumin concentration is required to be effective. However, increasing the number of doses over a prolonged period (days to weeks) reduces the concentration of curcumin needed in each dose to be effective. This could be due to the well-known bioavailability problems of curcumin. However, it is safe in human clinical trials even at a high dose of 12 g per day [66], which could allow for the bioavailability problems to be offset by giving a higher dose. Curcumin has bioavailability problems as it is relatively poorly absorbed by the small intestine and undergoes extensive conjugative and reductive metabolism in the liver when given orally [67]. This correlates with the earlier observation that a high dose or repeated doses are required as this low bioavailability must be overcome. The curcumin treatment parameters recommended by this review also suggest the intraperitoneal route, which is rarely used in humans [68], but can result in a higher and faster peak plasma concentration as quickly as after 15 minutes in mice but it may decline rapidly thereafter in comparison to the oral route, in which the plasma concentration peaks after one hour but declines more gradually over several hours [69]. In addition, the half-life of curcumin when taken orally differs from species to species such as around 32 minutes for rats [70] and around six to seven hours in humans [71. However, the rapid degradation of curcumin may not be negative as it has been suggested that the efficacy of curcumin despite its degradation could be due to its degradation products being the active compounds rather than curcumin itself [72]. Alternative approaches to bioavailability have also been explored by some of the studies in this review, which are the creation of synthetic curcumin analogues [48] or different formulations [27, 36, 42, 44, 50]. When the studies in this review compared the different formulations of curcumin to the base free curcumin, they found that the curcumin formulation was more effective at a given dose [36, 42, 50]. The compound piperine, which is found in black pepper, can also greatly increase curcumin bioavailability due to its inhibition of hepatic and intestinal glucuronidation [73].
4.2. Possible Anti-Epileptic Mechanism of Action for Curcuma longa and Its Neuroactive Components for the Management of Epileptic Seizures
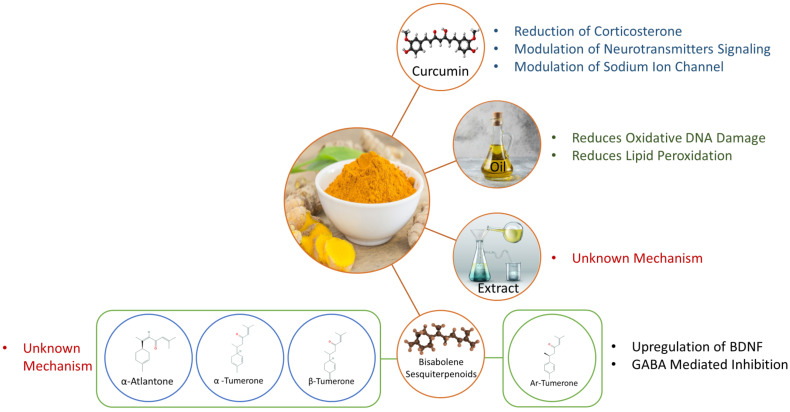
Similar to the determination of the suggested treatment parameters, this review will attempt to infer a mechanism of action for the various bioactive components by analyzing the proposed mechanism of action hypothesized by each study in this review and synthesizing them together to a better understanding of the overall mechanism. This synthesis will take into account all the studies in this review that have positive results as well as both directly (such as the measurement of oxidative stress markers for antioxidant activity) and indirectly (such as a decrease in oxidative stress being associated with a particular outcome) to determine mechanisms of action. Most of these studies analyzed either the whole brain or specific regions of the brain (commonly the hippocampus and the cortex) from one species (typically rats or mice), and so it is unclear if these mechanisms would apply outside the brain or in other species. The possible mechanisms are graphically summarized in Fig. 2, and are discussed in greater detail further below.
4.2.1. Possible Anti-Epileptic Mechanism of Action for Curcumin
Antioxidant Effect
-
Increases brain endogenous antioxidant defenses
i Increase in glutathione levels.
ii Activation of oxidative stress defensive genes.
-
Reduces brain oxidative stress
i Reduces lipid peroxidation.
ii Scavenges reactive oxygen species or reduces its production.
iii Reduces protein carbonylation.
-
Restores brain mitochondrial functions
i Restores activities of the mitochondrial complexes in the hippocampus and cerebral cortex due to a reduction in oxidative stress.
ii Prevents mitochondrial swelling due to membrane permeability changes.
iii Possibly due to phenolic and β-diketone curcumin functional groups which scavenge free radicals and ameliorate oxidative stress.
Anti-Inflammatory Effect
Upregulates anti-inflammatory cytokines
Downregulates pro-inflammatory cytokines
-
Inhibits the inflammatory signaling cascade
i Inhibits the mammalian target of rapamycin (mTOR) pathways and inflammatory mechanisms.
ii Inhibits the activation of astrocytes and microglia.
Neuroprotective Effect
-
Increases neurogenesis
i Reverses stress-induced decrease in progenitor cell proliferation in the subgranular zone.
ii Proliferation and recruitment of neural stem cells increased by overexpression of erythropoietin or klotho due to downregulation of Tnf-α via NFκB and the reduction in the level of the erythropoietin receptor.
-
iii Erythropoietin has neuroprotective properties.
Reduces apoptosis and necroptosis
Induces autophagy
Decreases cell death due to a reduction in oxidative stress
Upregulates nicastrin, CX3CL1 and CXCL16
Regulates microglial activation and microglial genes
Improvement of Cognition
Modulates BDNF, synapsin I and cyclic adenosine monophosphate response element-binding protein (CREB) levels
Reduces neuronal loss, gliosis, abnormal mossy fiber sprouting
Modulation of Neurotransmitter Signalling
-
Promotes neuronal inhibition
i Increases GABA synthesis (possibly by an increase in its precursor glutamine) at the expense of the major excitatory amino acids.
ii Direct or indirect activation of adenosine A1 but not A2A receptors.
iii Increases serotonin levels and/or serotonin Receptor 1A (HTR1A), HTR2C and HTR4 activation. Reduces HTR7 protein expression.
iv Inhibits monoamine oxidase A.
v Reduces acetylcholinesterase activity.
-
vi Inhibits the catecholaminergic mechanism.
Reduces neuroexcitation
i Reduces the activity of glutamate receptors (N-methyl-D-aspartate [NMDA], kainate) but does not appear to affect AMPA.
ii Reduces hyperexcitability by reducing excitatory glutamate activity and protects against glutamate-induced cytotoxicity by influencing intracellular calcium homeostasis via the modulation of taurine.
-
Reduction of nitric oxide production
i Mediated through the L-arginine – Nitric Oxide pathway.
ii Inhibition of nitrosative stress.
iii Conflicting reports on whether neuronal nitric oxide synthase or inducible nitric oxide synthase is responsible.
Downregulation of Sodium Ion Channels
It may be associated with the downregulation of Nav1.1 in the cortex
Downregulation of Corticosterone
Reduces corticosterone levels under stressful conditions to reduce stress potentiated seizure activity
Attenuates neuronal death in the hippocampal CA1 and CA3 areas by normalizing corticosterone blood serum levels
4.2.1.1. Overall Discussion of the Anti-Epileptic Mechanism of Curcumin
As represented in Fig. 2, both antioxidant effects [74] and anti-inflammatory effects [75] are associated with neuroprotection due to a reduction in neuronal loss, which is itself associated with an improvement in cognition [76, 77]. Similarly, the promotion of neuroinhibition and the reduction of neuroexcitation via the modulation of neurotransmitter signalling or sodium ion channels have the potential to help in the management of epileptic seizures as they are the basis by which many current ASMs exert their action [78]. The ability of curcumin to possibly reduce stress potentiated seizure activity is essential as conventional therapies focus on strategies to minimize stress rather than treating it pharmacologically, though the relationship between stress and seizures is not entirely clear [79]. While the associations are depicted in this way, significant relationships have been observed between the different mechanisms of action as neuronal cell death can also potentiate seizures, and also, seizures can also cause neuronal cell death [80]; hence, ameliorating one will also benefit the other. Inflammation [81] and oxidative stress [82] are also related to the occurrence of seizures, and BDNF plays a role in cognition and seizures, oxidative stress, and inflammation as well [83]. One of the studies in this review has also shown that normalizing corticosterone blood serum levels also attenuates hippocampal neuronal death [24] and further emphasizes how intertwined the various mechanisms are.
Also intertwined are epilepsy and its various comorbidities, which are exceedingly common in epilepsy patients and are categorised into medical (such as obesity and diabetes), psychiatric (such as depression and anxiety) and cognitive (such as learning disabilities and dementia) [84]. This association is perhaps what led some studies in this review to examine the effect of curcumin on depression [34] as well as the learning and memory aspect of cognitive dysfunction [20, 24, 25, 34, 36, 38, 39, 43] using rodent models. Those studies have identified ameliorative properties of curcumin on these comorbidities and thus raised the intriguing possibility of curcumin not only for seizure management but also the management of psychiatric and neurological comorbidities, such as depression and memory loss in particular.
The contradiction on whether inhibition of neuronal nitric oxide synthase [33] or inducible nitric oxide synthase [31] is responsible for reducing nitric oxide levels could be explained by two significant differences in their treatment parameters. The first significant difference is the treatment compound whereby one study used pure curcumin, and another used a mixture of curcumin, demethoxycurcumin and bisdemethoxycurcumin. The treatment times also significantly different, with the combination being given 75 minutes beforehand and the pure curcumin being given daily for 24 days. As the effect of each component of the mixture and the effect of treatment duration have not been compared equivalently, it is difficult to identify the cause of this contradiction as the different components of the mixture differ in the strength of their effects [85] and could also differ in their mechanisms of action.
4.2.2. Possible Anti-Epileptic Mechanism of Action for Aqueous Curcuma longa Extract
The possible anti-epileptic mechanism of action for an aqueous extract of Curcuma longa could not be determined by the lone study in this review that used it.
4.2.3. Possible Anti-Epileptic Mechanism of Action for Curcuma longa Oil
The possible anti-epileptic mechanism of action for Curcuma longa oil is a neuroprotective action due to the suppression of oxidative DNA damage and lipid peroxidation.
4.2.4. Possible Anti-Epileptic Mechanism of Action for Bisabolene Sesquiterpenoids
The possible anti-epileptic mechanism of action for the bisabolene sesquiterpenoids is speculated to be due to their antioxidant effects. However, the exact mechanism of action is not known for α,β-turmerone and α-atlantone. For ar-tumerone, its anticonvulsant effects could be due to GABA-mediated inhibition and the upregulation of BDNF, which could have a neuroprotective effect. It is also possible that metabolites of ar-tumerone could be responsible for its apparent activity as ar-tumerone concentrations in the brain after treatment would be low.
CONCLUSION
The present systematic review indicates that an aqueous extract of Curcuma longa itself and Curcuma longa oil, as well as the curcumin and bisabolene sesquiterpenoids found in Curcuma longa, have been experimentally proven to have the potential for epileptic seizure management as well as the depression and memory loss comorbidities. Suggested human treatment parameters and the possible mechanism of action for their epileptic seizure management effects have also been discussed to pave the way for future research and ASM development.
Fig. (1).
Flow chart of the article selection and exclusion criteria based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
Summary of the possible mechanisms of action for the bioactive components of Curcuma longa as hypothesized by the studies analyzed in this systematic review. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
A summarized table of studies focused on active constituents of turmeric in experimental epilepsy models.
| Principal Component | Treatment Regimen | Seizure Model | Major Relevant Outcome/s | Refs. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Curcumin (20 mg/kg) | Intraperitoneal 14 days daily pre-treatment 30 minutes pre-treatment |
Wistar Rats PTZ (60 mg/kg, ip) |
• Curcumin pre-treatment for 14 days alleviated PTZ-induced seizure activity, increased myoclonic jerk latency as well as decreased the duration and severity of generalized tonic-clonic seizures under stressful conditions. • Curcumin pre-treatment for 30 minutes before PTZ administration did not affect the seizures under stressful conditions. |
[19] | |||||||||||||||
| Curcumin (50, 100, 200 mg/kg) | Oral, three days daily pre-treatment | Sprague-Dawley Rats Lithium (3 mEq/ml/kg, ip) + Pilocarpine (20 mg/ml/kg, sc) |
• Curcumin dose-dependently increased seizure and status epilepticus latency, decreased the percentage of seizures and status epilepticus in a dose-dependent manner as well as reduced the intensity and frequency of seizures and their resulting behavioral changes. • Curcumin significantly ameliorated status epilepticus induced cognitive dysfunction and oxidative damage in the hippocampus and striatum areas of the brain. |
[20] | |||||||||||||||
| Curcumin | Molecular docking against human 4-aminobutyrate-aminotransferase | - | • Curcumin binds to 4-aminobutyrate-aminotransferase with Leu299, Leu301, Ala135, Gln267, Arg164 and Tyr161 being the contact points. | [21] | |||||||||||||||
| Curcumin (100 mg/kg) | Intraperitoneal, seven days daily pre-treatment | Wistar Rats Kainic Acid (10 mg/kg, ip) |
• Curcumin upregulated genes related to anti-inflammatory cytokines (Il10rb, Cxcl16, and Cxcl17) to modulate the epileptogenic process and protected against hippocampal cell loss by upregulating nicastrin. • It likely also exerted neuroprotective effects by upregulating Cx3cl1 and Cxcl16. |
[22] | |||||||||||||||
| Curcuma longa aqueous extract (50, 100, 200 mg/kg) | Oral, 21 days daily pre-treatment | Albino Mice PTZ (80 mg/kg, ip) MES (150 mA for 0.2 seconds) |
• A 100 and 200 mg/kg dose of the extract delayed the onset of PTZ induced convulsions. • Only 200 mg/kg decreased the duration of MES induced convulsions. • No extract dose completely prevented the occurrence of convulsions. |
[23] | |||||||||||||||
| Curcumin (20 mg/kg) | Intraperitoneal 14 days daily pre-treatment 30 minutes pre-treatment |
Wistar Rats PTZ (60 mg/kg, ip) |
• The latency to the onset of myoclonic jerks and the latency to stage 3 seizures were increased by the 14-day pre-treatment. • The rate of myoclonic seizure occurrence and the seizure severity score were both reduced by the 14-day curcumin pre-treatment in both stress and non-stress conditions. • The 14-day curcumin pre-treatment also improved learning ability. • The 30 minutes pre-treatment dose had no significant effect. |
[24] | |||||||||||||||
| Curcumin (50, 100 mg/kg) | Oral, 14 days daily pre-treatment | Swiss Albino Mice PTZ (95 mg/kg, ip) MES (36 mA for 0.2 seconds) |
• Neither dose had a significant effect on MES induced tonic extension, but the 100 mg/kg dose significantly reduced the clonic phase. • The 100 mg/kg dose inhibited PTZ induced seizures. • Curcumin pre-treatment had no effect on memory retention. |
[25] | |||||||||||||||
| Curcumin (80 mg/kg) | Oral, 21 days daily pre-treatment | Wistar Albino Rats Pilocarpine (380 mg/kg, ip) |
• Curcumin pre-treatment ameliorated the pilocarpine-induced increase in nitric oxide levels, decrease in glutathione levels, decrease in catalase activity, and decrease in Na+, K+-ATPase activity. • Curcumin had no effect on acetylcholinesterase levels. • Curcumin worsened the pilocarpine-induced decline in catalase. |
[26] | |||||||||||||||
| Principal Component | Treatment Regimen | Seizure Model | Major Relevant Outcome/s | Refs. | |||||||||||||||
| Liposomal Curcumin (25, 50 mg/kg) | Oral for PTZ induced seizures and ICES, treatment duration and the number of doses were not given Intravenous for PTZ induced status epilepticus, and treatment duration were not given |
Swiss Albino Inbred Mice ICES (starting current of 2 mA for 0.2 seconds with a linear increase of 2mA/2 seconds PTZ (60 mg/kg, ip) PTZ Induced Status Epilepticus (80 mg/kg, sc) |
• Liposomal curcumin dose-dependently increased the seizure threshold current in the ICES model. • Liposomal curcumin at both doses increased the latency to PTZ induced myoclonic jerks and generalised seizures. • Liposomal curcumin dose-dependently increased the latency and decreased the duration of clonic seizures in PTZ induced status epilepticus. |
[27] | |||||||||||||||
| Curcumin (50, 100, 200 mg/kg) |
Oral, one-hour pre-treatment, daily for seven days up to 35 days | Swiss Albino Inbred Mice PTZ kindling (25 mg/kg, ip), every alternate day, one hour after daily curcumin pre-treatment |
• Curcumin administration dose-dependently decreased the seizure score from PTZ kindling from seven days onwards. • Curcumin dose-dependently decreased whole brain malondialdehyde levels. • All doses of curcumin increased whole brain glutathione levels. |
[28] | |||||||||||||||
| Curcumin (50, 100, 200 mg/kg) Curcumin (50 mg/kg) + Diazepam (5 µg) |
Intraperitoneal, 10 minutes after penicillin administration |
Wistar Rats Penicillin (200 IU, 1 µl, ic) |
• Curcumin at doses of 100 and 200 mg/kg significantly reduced the frequency and amplitude of spike waves. • Co-administration of sub-therapeutic dose of curcumin with diazepam enhanced the anti-epileptic effect of diazepam. |
[29] | |||||||||||||||
| Curcumin (20, 40, 80, 120 mg/kg) |
Oral, multiple 15-minute treatment timepoints up to 60 minutes |
Albino Mice (Laka Strain) PTZ (0.5%, iv infusion) |
• Curcumin had a maximal anti-convulsive effect 45 minutes after administration. • The optimal curcumin dose was 80 mg/kg, with neither higher nor lower doses having a significant effect on the convulsive phases. • The PTZ dose threshold required for the onset of the tonic extension was increased by PTZ. • Using a combination of A1 and A2 adenosine receptor agonists and antagonists, curcumin was found to exert its effect via a direct or indirect activation of the adenosine A1 receptor but not A2. • Piperine coadministration (to inhibit curcumin metabolism) did not change the curcumin bell-shaped dose-response curve seen against the PTZ induced tonic extensor phase. |
[30] | |||||||||||||||
| Curcumin C3 Complex (mixture of curcumin, demethoxycurcumin and bisdemethoxycurcumin) Nanoparticles (1, 5, 10, 20, 40, 80 mg/kg) Curcumin (40, 80 mg/kg) |
Nanoparticles - Intraperitoneal, multiple timepoints up to 120 minutes Curcumin - intraperitoneal, 75 minutes pre-treatment |
NMRI Albino Mice PTZ (0.5%, iv infusion) |
• Curcumin C3 Complex Nanoparticles had significant anti-convulsant properties in a dose-dependent manner at 20, 40 and 80 mg/kg. • The nanoparticles had a significant anticonvulsive effect at 45, 60 and 120 minutes after administration. • The nitric oxide donor dose--dependently reversed the anti-convulsant effect of the nanoparticles. • Both the non-selective nitric oxide synthase inhibitor and the selective inducible nitric oxide synthase inhibitor increased the seizure thresholds and potentiated a sub-effective dose of the nanoparticles in a synergistic manner. • Neither dose of curcumin had any effect on seizure thresholds. |
[31] | |||||||||||||||
| Principal Component | Treatment Regimen | Seizure Model | Major Relevant Outcome/s | Refs. | |||||||||||||||
| Curcumin (150 mg/kg) | Intraperitoneal, 25 minutes pre-treatment | Mice PTZ (80 mg/kg, ip) |
• Curcumin increased the seizure and tonic-clonic stage latency as well as reduced the duration of tonic-clonic seizures. • When brain serotonin levels were depleted, the anti-convulsant effect of curcumin was partially reversed. • Antagonising the serotonin 5-HT1A, 5-HT2C or 5-HT4 receptors individually diminished the anti-convulsant effect of curcumin but antagonising 5-HT7 potentiated it. • Antagonising 5-HT1A, 5-HT2C,5-HT4 and 5-HT7 together prevented the anticonvulsant effect of curcumin. • In hippocampal tissue, only 5-HT7 gene expression was reduced after administration of curcumin. |
[32] | |||||||||||||||
| Curcumin (50, 100, 200 mg/kg) | Intraperitoneal, 20 minutes pre-treatment, daily for 24 days | Wistar Rats PTZ (35 mg/kg, ip), every alternate day, 20 minutes after daily curcumin treatment |
• A 200 mg/kg curcumin dose most effectively decreased the mean frequency of PTZ-induced epileptiform activity, followed by 100 mg/kg, whereas 50 mg/kg was found ineffective. • Co-administering a non-selective nitric oxide synthase inhibitor with curcumin augmented the anti-epileptic activity of curcumin. • A selective neuronal nitric oxide synthase potentiated the anti-epileptic activity of curcumin, whereas a selective inducible nitric oxide synthase inhibitor did not have an effect. • Giving a nitric oxide precursor reversed the anti-epileptic activity of curcumin in the early stage and aggravated interictal activity but augmented the activity of curcumin in the late stage and also relieved interictal activity. |
[33] | |||||||||||||||
| Curcumin (50, 100, 200 mg/kg) | Intraperitoneal, 5, 10 or 15 days daily pre-treatment in kindled animals | Swiss Albino Mice PTZ (35 mg/kg, ip), considered kindled after two administrations 48 hours apart |
• Curcumin attenuated seizure severity as well as depression- like behaviour and memory impairment in a dose-dependent manner. • Curcumin increased whole brain serotonin and norepinephrine levels as well as reduced nitrite levels and acetylcholinesterase activity. |
[34] | |||||||||||||||
| Curcumin (300 mg/kg) | Intraperitoneal, 15, 30, 60 or 120 minutes pre-treatment | Swiss Mice MES (25 mA for 0.2 seconds, 50 Hz, 500 V) |
• No anti-convulsant effect of curcumin was found at any pre-treatment time. | [35] | |||||||||||||||
| Curcumin Loaded Nanoparticles (12.5, 25.0 mg/kg) Free Curcumin (12.5, 25 mg/kg) |
Intraperitoneal, one-hour pre-treatment, daily for 10 days | NMRI Mice PTZ (36.5 mg/kg, ip), every alternate day, one hour after daily curcumin administration |
• Free curcumin was found to not have an anti-seizure effect or memory improving ability. • The curcumin loaded nanoparticles at a dose of 25 mg/kg reduced the seizure-induced behavioural signs as well as improved spatial learning and memory. • Free curcumin slightly reduced the level of PTZ induced hippocampal cell death and glial activation, whereas the curcumin loaded nanoparticles greatly reduced both. |
[36] | |||||||||||||||
| Curcumin (100, 200, 300 mg/kg) | Oral, one-hour pre-treatment, daily for up to 10 weeks | Wistar Rats PTZ (35 mg/kg, ip), every alternate day, one hour after daily curcumin administration |
• All curcumin doses decreased seizure score in a dose-dependent manner and protected against PTZ kindling. • Curcumin at a 300 mg/kg dose increased the latency to myoclonic jerks, clonic seizures, generalised tonic-clonic seizures as well as improved the seizure score and decreased the number of myoclonic jerks. • Curcumin reversed the PTZ kindling induced hippocampal injury, oxidative stress and apoptosis in a dose-dependent manner. |
[37] | |||||||||||||||
| Principal Component | Treatment Regimen | Seizure Model | Major Relevant Outcome/s | Refs. | |||||||||||||||
| Curcumin (100 mg/kg) | Intraperitoneal, 14 days daily pre-treatment after cessation of kainic acid-induced status epilepticus | Wistar Rats Kainic Acid (0.8 µg, ihc) |
• Kainic acid-induced elevation of Il-1β and Tnf-α was reduced by curcumin. • Kainic acid-induced astrocyte activation was reduced by curcumin. • Kainic acid-induced neuronal loss in the dentate hilus and CA3 regions was reduced by curcumin. • Kainic acid-induced mossy fibre sprouting was reduced by curcumin. • Kainic acid-induced abnormal EEG spike frequency and the rate of spontaneous recurrent seizure occurrence were reduced by curcumin. • Kainic acid-induced learning and memory deficit were reduced by curcumin. |
[38] | |||||||||||||||
| Curcumin (100 mg/kg) | Oral, 30 minutes pre-treatment, daily for 40 days | Wistar Rats PTZ (40 mg/kg, ip), every alternate day for 30 days, 30 minutes after daily curcumin administration |
• Curcumin did not affect the seizure score. • Curcumin restored the activities of the mitochondrial complexes in both the hippocampus and the cerebral cortex. • Curcumin decreased reactive oxygen species, protein carbonyl and lipid peroxidation levels and restored the level of glutathione. • Curcumin prevented PTZ induced mitochondrial swelling and restored other mitochondrial functions. • Curcumin attenuated PTZ induced neuronal cell death in both the hippocampus and the cerebral cortex. • Curcumin ameliorated PTZ induced impaired memory functions. |
[39] | |||||||||||||||
| Curcumin (100 mg/kg) | Oral, 30 minutes pre-treatment, daily for 40 days | Wistar Rats PTZ (40 mg/kg, ip), every alternate day for 30 days, 30 minutes after daily curcumin administration |
• Curcumin at 100 mg/kg had no anti-convulsant potential against a PTZ induced model of chronic epilepsy but prevented cognitive deficits. • Curcumin decreased pro-inflammatory cytokines and chemokine activation in the hippocampus and cortex. • Gfap and Iba-1 upregulation by PTZ was also attenuated by curcumin. • Curcumin also reduced the number of activated glial cells. |
[40] | |||||||||||||||
| Curcumin (approximately equivalent to 100 mg/kg) |
Curcumin supplemented food pellets, six months daily feeding | Wistar Rats FeCl3 (100 mM, ic), administered five months after the start of curcumin feeding |
• Curcumin reduced the development and occurrence of seizures but did not completely suppress it. • Curcumin reduced an epilepsy-related increase in multiple-unit activity (electrocorticography) but did not alter the basal electrical activity of the brain. • Curcumin reduced the mRNA and protein expression of Nav 1.1 in the cortex but not in the hippocampus. • Curcumin did not affect the mRNA expression of Nav 1.6 but reduced its protein levels in both the cortex and the hippocampus. |
[41] | |||||||||||||||
| Curcumin Loaded Nanoparticles (12.5 mg/kg) Free Curcumin (12.5 mg/kg) |
Intraperitoneal, one-hour pre-treatment, daily for 30 days | NMRI Mice PTZ (36.5 mg/kg, ip), every alternate day for 20 days after 10 days of curcumin administration, one hour after daily curcumin administration |
• Curcumin loaded nanoparticles alleviated PTZ induced neuronal cell death. • Curcumin loaded nanoparticles upregulated the levels of erythropoietin and klotho in mice receiving PTZ. • Curcumin loaded nanoparticles reduced the mRNA level of Tnf-α. • Free curcumin generally had the same effect as the nanoparticles, but typically to a lesser degree. |
[42] | |||||||||||||||
| Principal Component | Treatment Regimen | Seizure Model | Major Relevant Outcome/s | Refs. | |||||||||||||||
| Curcumin (100, 200, 300 mg/kg) | Oral, 30 minutes pre-treatment, daily for up to 43 days | Wistar Rats PTZ (30 mg/kg, ip), every alternate day until the development of kindling or up to day 43, 30 minutes after daily curcumin administration |
• Curcumin significantly increased the latency to myoclonic jerks, clonic seizures as well as generalised tonic-clonic seizures, improved seizure score and decreased the number of myoclonic jerks in a dose-dependent manner. • Curcumin reversed PTZ induced oxidative stress and cognitive impairment in a dose-dependent manner. • Curcumin at a 300 mg/kg dose did not change cognitive function in normal rats but improved cognition in kindled rats. • Curcumin prevented PTZ induced rise in whole-brain malondialdehyde and glutathione levels. |
[43] | |||||||||||||||
| Curcumin Nanoparticles (50 mg/kg) | Intraperitoneal, daily pre-treatment for four days | Wistar Rats Pilocarpine (380 mg/kg, ip), administered after the end of curcumin treatment |
• The nanoparticles prevented a pilocarpine-induced decrease in cortical and hippocampal acetylcholinesterase, but not the decrease in Na+/K+-ATPase activity. • The nanoparticles produced control like lipid peroxidation values in the cortex and nonsignificant change in the hippocampus as compared to the control. • The nanoparticles improved the decreased levels of cortical and hippocampal glutathione. • The nanoparticles prevented the decrease in hippocampal nitric oxide levels, but not cortical levels. • The nanoparticles reduced hippocampal Tnf-α levels to near control levels, but not cortical levels. • The nanoparticles attenuated the increase in cortical and hippocampal caspase-3 levels. |
[44] | |||||||||||||||
| Curcumin (80 mg/kg) | Intraperitoneal, daily for 21 days in rats with spontaneous recurrent seizures | Wistar Albino Rats Pilocarpine (380 mg/kg, ip), single-dose administration and left for 22 days to establish spontaneous recurrent seizures |
• Curcumin prevented the manifestation of seizures. • Cortical aspartic acid and glycine in rats given pilocarpine were restored to near control levels, whereas GABA levels were increased with curcumin treatment though the decrease in taurine levels was not prevented. • Hippocampal glutamate and glutamine levels in rats given pilocarpine were decreased by curcumin treatment, whereas GABA levels were increased together with aspartic acid and taurine concentrations to near normal levels. • Overall, curcumin treatment of rats given pilocarpine produced a state of inhibition in the hippocampus and cortex by altering the balance between inhibitory and excitatory amino acids. • Curcumin treatment reduced pilocarpine-induced pathological abnormalities in both the hippocampus and cortex. |
[45] | |||||||||||||||
|
Curcuma longa methanolic extract (3.1, 6.2, 12.5 µg/ml) Curcuminoids mixture (Curcumin, demethoxycurcumin and bisdemethoxycurcumin, 2.5, 5.0, 10.0 µg/ml) Curcuma longa oil Zebrafish: 2.5, 5.0, 10 µg/ml Mice: 50, 100 mg/kg) Ar-turmerone Zebrafish: 11.0, 23.0, 46.0 µM Mice: 50 mg/kg α,β-turmerone (1:1 mixture) Zebrafish: 5.0, 11.0, 23.0 µM Mice: 100 mg/kg α-atlantone (11.0, 23.0, 46.0 µM) |
Added into the water for zebrafish larvae, one-hour pre-treatment Intravenous infusion for mice, 10 minutes pre-treatment |
Zebrafish Larvae: Tg (fli 1a: EGFP)y1 strain PTZ (20 mM), added into the water C57Bl/6 Mice PTZ (7.5 mg/ml at 150 µl/minute, iv) |
• Curcuma longa methanolic extract had anti-convulsant activity in larval zebrafish at a 12.5 µg/ml dose. • The curcuminoids mixture produced an anti-convulsant effect at all doses in a dose-dependent manner. • Curcuma longa oil had anti-convulsant activity at 10 µg/ml. • α,β-turmerone, ar-turmerone and α-atlantone were determined to have anti-convulsant activity. • All constituents slightly increased zebrafish larvae locomotor activity despite the absence of PTZ, but EEG showed that the oil was not pro-convulsant. • Mice treated with the 50 mg/kg of Curcuma longa oil required a higher PTZ dose to trigger all behavioural endpoints, whereas a 100 mg/kg dose delayed seizure generation for all parameters and also death, with the required PTZ dose for all assessed events being increased. • Mice treated with ar-tumerone required an increased PTZ dose to trigger assessed events, whereas α,β-turmerone positively affected all seizure parameters. • α-atlantone was not tested in mice due to an insufficient amount being collected. |
[46] | |||||||||||||||
| Principal Component | Treatment Regimen | Seizure Model | Major Relevant Outcome/s | Refs. | |||||||||||||||
| Ar-tumerone Zebrafish: 46 µM C57BI/6 Mice: 50 mg/kg NMRI Mice: 1, 20, 50 mg/kg |
Added into the water for zebrafish larvae, one-hour pre-treatment Intraperitoneal for C57BI/6 mice, 30 minutes or 24 hours pre-treatment Intraperitoneal for NMRI mice, 10 minutes pre-treatment |
Zebrafish Larvae: AB strain PTZ (20 mM), added into the water C57Bl/6 Mice PTZ (7.5 mg/ml at 150 µl/minute, iv) NMRI Mice Low frequency, long duration corneal stimulation (6 Hz, 0.2 milliseconds rectangular pulse width, 3 seconds duration, 44 mA) |
• All C57BI/6 mice were protected from corneal stimulation by an ar-tumerone dose between 0.1 and 50 mg/kg with a 30 minutes pre-treatment time and 70% were protected after 24 hours pre-treatment with a 50 mg/kg dose. • A 1 mg/kg ar-tumerone dose raised the PTZ threshold required to trigger tonic hind limb extension and death, whereas 20 mg/kg increased the dose needed to cause death in the NMRI mice. • A 50 mg/kg dose of ar-tumerone did not produce any loss of balance in the C57Bl/6 mice. • Ar-tumerone at a 50 mg/kg dose was detectable in the brains of C57BI/6 mice as early as 15 minutes and as late as 24 hours after administration. • In zebrafish larvae, ar-tumerone downregulated C-fos expression and upregulated Bdnf in both the presence and absence of PTZ, whereas the expression patterns of the GABAA receptor and Il-10 were not affected. |
[47] | |||||||||||||||
| Curcumin (20 µM) | - | Human Embryonic Kidney cells 293 (HEK293) Patch-Clamp Electrophysiology, no seizures induced |
• Curcumin treatment did not significantly change peak current nor deactivation and desensitisation of the AMPA receptor. • Some derivatives of curcumin at the same dose did demonstrate an inhibitory effect. |
[48] | |||||||||||||||
| Curcumin (300 mg/kg) | Oral, one-hour pre-treatment | Wistar Rats PTZ (60 mg/kg, ip) MES (70 mA, 0.2 seconds) |
• Curcumin alone significantly increased myoclonic latency but only had a 50% protection rate against generalised tonic-clonic seizures and did not affect learning and memory. • Curcumin alone had a 33.33% protection rate against MES induced seizures. • Curcumin alone reversed the PZT/MES induced rise in malondialdehyde and decrease in glutathione levels. • Co-administration of curcumin with sub-therapeutic doses of ASMs potentiated the effect of ASMs and prevented the impairment of learning and memory. |
[49] | |||||||||||||||
| Curcumin Larvae: 1 µM Adult: 0.5 µM Micronized Curcumin Larvae: 1 µM Adult: 0.5 µM |
Larvae: Added into the water, 30 minutes pre-treatment Adult: Intraperitoneal, 30 minutes pre-treatment |
Wild-Type Zebrafish PTZ (3 mM), added into the water |
• Both curcumin and micronized curcumin treatment in zebrafish larvae did not alter the distance traveled and the number of line crossings. • In adult zebrafish, both curcumin and micronized curcumin treatment reduced locomotion but did not alter the number of line crossings. • Both micronized curcumin and curcumin administration to zebrafish larvae reduced seizure occurrence and slowed seizure stage progression. • Both micronized curcumin and curcumin administration to zebrafish adults slowed seizure stage progression. |
[50] | |||||||||||||||
| Curcumin (200 mg/kg) |
Oral, daily pre-treatment for seven or three days | Swiss Albino Mice Kainic acid (10 mg/kg, ip), administered after the completion of curcumin pre-treatment |
• Blood samples and brain tissue biochemical parameters showed that curcumin administration had antioxidant, anti-inflammatory and antiapoptotic effects. • Curcumin reduced the severity of pathological changes (mainly in haemorrhage, oedema, neural degeneration and encephalomalacia) in the brain to varying degrees. |
[51] | |||||||||||||||
| Curcumin (200, 300 mg/kg) |
Oral, daily pre-treatment for two weeks | Sprague-Dawley Rats Pilocarpine (20 mg/kg, ip), administered after the completion of curcumin treatment |
• Curcumin pre-treatment markedly decreased the number of apoptotic neurons and increased the number of surviving neurons in the hippocampus (CA1, CA3, dentate gyrus, hilus) after 24 and 72 hours post pilocarpine administration. • Upregulation of Mlkl and Rip-1 due to pilocarpine was inhibited by curcumin in a dose-dependent manner. • Curcumin pre-treatment upregulated the expression levels of Beclin-1 and LC3BII/LC3BI and increased the number of autophagosomes. |
[52] | |||||||||||||||
ASM: Anti-Seizure Medication, AMPA: L-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, Bdnf: brain-derived neurotrophic factor, Cxcl16: Chemokine (C-X-C motif) ligand 16, EEG: Electroencephalography, GABA: γ-aminobutyric acid, Gfap: Glial fibrillary acidic protein, Iba1: Ionized calcium binding adaptor molecule 1, Il-1β: Interleukin 1 beta, Il10rb: Interleukin 10 Receptor Subunit Beta, MES: Maximal ElectroShock, ICES: Increasing Current Electroshock Seizures, Mlkl: Mixed lineage kinase domain like pseudokinase, PTZ: Pentylenetetrazol, Rip-1: Receptor-interacting serine/threonine-protein kinase 1, Tnf-α: tumour necrosis factor alpha, ic: Intracortical, ihc: Intrahippocampal, ip: Intraperitoneal, iv: Intravenous, po: Oral, sc: Subcutaneous.
Table 2.
Details of the treatment parameters and the seizure model for selected relevant rodent studies in this systematic review. A green background represents working treatments and a red background represents non-working treatments. For working treatments, the lowest working dose/duration and the highest dose/duration used for non-working treatments is given.
| Component | Dose (mg/kg) | Route | Treatment Duration | Seizure Induction Method | Convulsant Dose | Seizure Route | Duration of Seizure Induction | Animal | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Curcumin [19] | 20 | ip | 14 days | PTZ | 60 mg/kg | ip | Single dose | Wistar Rats | |||||
| Curcumin [20] | 50 | po | 3 days | Lithium + Pilocarpine |
3 mEq/ml/kg + 20 mg/ml/kg |
ip + sc | Single dose | SD Rats | |||||
| Curcumin [22] | 100 | ip | 7 days | Kainic Acid | 10 mg/kg | ip | Single dose | Wistar Rats | |||||
| CL Extract [23] | 200 | po | 21 days | MES | - | - | - | Albino Mice | |||||
| CL Extract [23] | 100 | po | 21 days | PTZ | 80 mg/kg | ip | Single dose | Albino Mice | |||||
| Curcumin [24] | 20 | ip | 14 days | PTZ | 60 mg/kg | ip | Single dose | Wistar Rats | |||||
| Curcumin [25] | 100 | po | 14 days | PTZ | 95 mg/kg | ip | Single Dose | Swiss Albino Mice | |||||
| Curcumin [25] | 100 | po | 14 days | MES | - | - | - | Swiss Albino Mice | |||||
| Curcumin [26] | 80 | po | 21 days | Pilocarpine | 380 mg/kg | ip | Single dose | Wistar Albino Rats | |||||
| Curcumin [28] | 50 | po | 7 days | PTZ | 25 mg/kg | ip | 35 days alternately | Swiss Albino Mice | |||||
| Curcumin [29] | 100 | ip | 10 minutes (single dose) | Penicillin | 200 IU, 1 µl | ic | Single dose | Wistar Rats | |||||
| Curcumin [30] | 80 | po | 45 minutes (single dose) | PTZ | 0.5% (w/v) | Infusion | Continuous (up to three minutes or onset of extension phase) | Albino Mice | |||||
| Curcumin [31] | 80 | ip | 75 minutes (single dose) | PTZ | 0.5% (w/v) | Infusion | Continuous (up to onset of forelimb clonus) | NMRI Mice | |||||
| Curcumin [32] | 150 | ip | 25 minutes (single dose) | PTZ | 80 mg/kg | ip | Single dose | Mice | |||||
| Curcumin [33] | 100 | ip | 24 days | PTZ | 35 mg/kg | ip | 24 days alternately | Wistar Rats | |||||
| Curcumin [34] | 50 | ip | 15 days | PTZ | 35 mg/kg | ip | 15 days alternately | Swiss Albino Mice | |||||
| Curcumin [35] | 300 | ip | 120 minutes (single dose) | MES | - | - | - | Swiss Mice | |||||
| Curcumin [36] | 25 | ip | 10 days | PTZ | 36.5 mg/kg | ip | 10 days alternately | NMRI Mice | |||||
| Curcumin [37] | 100 | po | 10 weeks | PTZ | 35 mg/kg | ip | 10 weeks alternately | Wistar Rats | |||||
| Curcumin [38] | 100 | ip | 14 days | Kainic Acid | 0.8 µg | ihc | Single dose | Wistar Rats | |||||
| Curcumin [39] | 100 | po | 40 days | PTZ | 40 mg/kg | ip | 30 days alternately | Wistar Rats | |||||
| Curcumin [40] | 100 | po | 40 days | PTZ | 40 mg/kg | ip | 30 days alternately | Wistar Rats | |||||
| Curcumin [41] | 100 | Food | 6 months | FeCl3 | 100 mM | ic | Single dose | Wistar Rats | |||||
| Curcumin [42] | 12.5 | ip | 30 days | PTZ | 36.5 mg/kg | ip | 20 days alternately | NMRI Mice | |||||
| Curcumin [43] | 100 | po | 43 days | PTZ | 30 mg/kg | ip | 43 days alternately | Wistar Rats | |||||
| Component | Dose (mg/kg) | Route | Treatment Duration | Seizure Induction Method | Convulsant Dose | Seizure Route | Duration of Seizure Induction | Animal | |||||
| Curcumin [45] | 80 | po | 21 days | Pilocarpine | 380 mg/kg | ip | Single dose | Wistar Albino Rats |
|||||
| CL Oil [46] | 50 | iv | - | PTZ | 7.5 mg/ml, 150 μl/min |
iv | Continuous (infused until death occurs between three to five minutes after starting) | C57BI/6 Mice | |||||
| α,β-turmerone [46] | 100 | iv | - | PTZ | 7.5 mg/ml, 150 μl/min |
iv | Continuous (infused until death occurs between three to five minutes after starting) | C57BI/6 Mice | |||||
| Ar-Tumerone [46] | 50 | iv | - | PTZ | 7.5 mg/ml, 150 μl/min |
iv | Continuous (infused until death occurs between three to five minutes after starting) | C57BI/6 Mice | |||||
| Ar-Tumerone [47] | 50 | ip | 10 minutes (single dose) | 6 Hz Corneal Stimulation | - | - | - | NMRI Mice | |||||
| Ar-Tumerone [47] | 50 | ip | 30 minutes (single dose) | PTZ | 7.5 mg/ml, 150 μl/min |
iv | Continuous | C57BI/6 Mice | |||||
| Curcumin [49] | 300 | po | 60 minutes (single dose) | PTZ | 60 mg/kg | ip | Single dose | Wistar Rats | |||||
| Curcumin [49] | 300 | po | 60 minutes (single dose) | MES | - | - | - | Wistar Rats | |||||
| Curcumin [51] | 200 | po | 3 days | Kainic Acid | 10 mg/kg | ip | Single dose | Swiss Albino Mice |
|||||
| Curcumin [52] | 200 | po | 2 weeks | Lithium + Pilocarpine | 125 mg/kg + 20 mg/kg |
ip | Single dose | SD Rats | |||||
Abbreviations: CL: Curcuma longa, MES: Maximal ElectroShock, PTZ: Pentylenetetrazol, SD: Sprague-Dawley, ic: Intracortical, ihc: Intrahippocampal, ip: Intraperitoneal, po: Oral, sc: Subcutaneous.
ACKNOWLEDGEMENTS
Fig. (2) uses images designed by Freepik (http://www.freepik.com).
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was funded by the Tropical Medicine and Biology Platform of Monash University, Malaysia (TMB-2020-SG3185140921-BCKM/MFS).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Fisher R.S., van Emde Boas W., Blume W., Elger C., Genton P., Lee P., Engel J., Jr Epileptic seizures and epilepsy: definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., Engel J., Jr, Forsgren L., French J.A., Glynn M., Hesdorffer D.C., Lee B.I., Mathern G.W., Moshé S.L., Perucca E., Scheffer I.E., Tomson T., Watanabe M., Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 3.Rogawski M.A., Löscher W., Rho J.M. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb. Perspect. Med. 2016;6(5):a022780. doi: 10.1101/cshperspect.a022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao F., Koepp M.J., Zhou D. Pharmaco-fMRI: A tool to predict the response to antiepileptic drugs in epilepsy. Front. Neurol. 2019;10:1203–1203. doi: 10.3389/fneur.2019.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad S., Aggarwal B.B. Turmeric, the golden spice.Herbal medicine: biomolecular and clinical aspects, 2nd. CRC Press/Taylor & Francis; 2011. [Google Scholar]
- 6.Zhang W-Y., Wang H-F., Chen G., Zhang O., Bai S., Pei Y-H. Two new bisabolane sesquiterpenoids from Curcuma longa. J. Asian Nat. Prod. Res. 2014;16(3):271–274. doi: 10.1080/10286020.2013.875010. [DOI] [PubMed] [Google Scholar]
- 7.Debjit Bhowmik C., Kumar K.S., Chandira M., Jayakar B. Turmeric: A herbal and traditional medicine. Arch. Appl. Sci. Res. 2009;1(2):86–108. [Google Scholar]
- 8.Hatcher H., Planalp R., Cho J., Torti F.M., Torti S.V. Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C., Long L., Zhang F., Chen Q., Chen C., Yu X., Liu Q., Bao J., Long Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS One. 2018;13(3):e0194284–e0194284. doi: 10.1371/journal.pone.0194284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lateh L., Yuenyongsawad S., Chen H., Panichayupakaranant P. A green method for preparation of curcuminoid-rich Curcuma longa extract and evaluation of its anticancer activity. Pharmacogn. Mag. 2019;15(65):730. doi: 10.4103/pm.pm_162_19. [DOI] [Google Scholar]
- 11.Joshi B., Pandya D., Mankad A. Comparative study of phytochemical screening and antibacterial activity of curcuma longa (L.) and curcuma aromatica (Salib.). Faslnamah-i Giyahan-i Daruyi. 2018;6(6):145–148. [Google Scholar]
- 12.Cikrikci S., Mozioglu E., Yilmaz H. Biological activity of curcuminoids isolated from Curcuma longa. Rec. Nat. Prod. 2008;2(1):19. [Google Scholar]
- 13.Tzadok M., Uliel-Siboni S., Linder I., Kramer U., Epstein O., Menascu S., Nissenkorn A., Yosef O.B., Hyman E., Granot D., Dor M., Lerman-Sagie T., Ben-Zeev B. CBD-enriched medical cannabis for intractable pediatric epilepsy: The current Israeli experience. Seizure. 2016;35:41–44. doi: 10.1016/j.seizure.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen H., He H., Xiao Y., Luo M., Luo H., Wang J. Losigamone add‐on therapy for focal epilepsy. Cochrane Database Syst. Rev. 2019;(12) doi: 10.1002/14651858.CD009324.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooijmans C.R., Rovers M.M., de Vries R.B.M., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14(1):43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosoky N.S., Setzer W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients. 2018;10(9):1196. doi: 10.3390/nu10091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S., Yuan W., Deng G., Wang P., Yang P., Aggarwal B. 2011.
- 19.Haghighizad H., Touhidi A., Pourmotabbed A., Moradpour F., Nedaei S.E., Pourmotabbed T. Curcumin improves chronic stress induced potentiated seizure activity in experimental model of epilepsy. J. Neurol. Sci. 2017;34(1):76–85. [Google Scholar]
- 20.Ahmad M. Protective effects of curcumin against lithium-pilocarpine induced status epilepticus, cognitive dysfunction and oxidative stress in young rats. Saudi J. Biol. Sci. 2013;20(2):155–162. doi: 10.1016/j.sjbs.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijayakumar S., Kasthuri G., Prabhu S., Manogar P., Parameswari N. Screening and identification of novel inhibitors against human 4-aminobutyrate-aminotransferase: A computational approach. Egyptian Journal of Basic and Applied Sciences. 2018;5(3):210–219. doi: 10.1016/j.ejbas.2018.05.008. [DOI] [Google Scholar]
- 22.Hui-Yin Y., Ahmad N., Azmi N., Makmor-Bakry M. Curcumin: The molecular mechanisms of action in inflammation and cell death during kainate-induced epileptogenesis. Indian Journal of Pharmaceutical Education and Research. 2018;52(1):32–41. doi: 10.5530/ijper.52.1.4. [DOI] [Google Scholar]
- 23.Sharma A., Rauniar G.P. Anticonvulsive effects of purified curcuma longa in mice. Int. J. Health Sci. Res. 2016;6(4):236–241. [Google Scholar]
- 24.Touhidi A., Haghighizad H., Pourmotabbed A. Effect of curcumin on passive avoidance learning disorders induced by seizure activity under chronic restraint stress in rats. Neurological Sciences and Neurophysiology. 2018;35(2):77–83. doi: 10.5152/NSN.2018.10203. [DOI] [Google Scholar]
- 25.Anovadiya A.P., Sanmukhani J.J., Vadgama V.K., Tripathi C.B. Evaluation of antiepileptic and memory retention activity of curcumin per se and incombination with antiepileptic drugs. Asian J. Pharm. Clin. Res. 2013;•••:6. [Google Scholar]
- 26.Ezz H.S., Khadrawy Y.A., Noor N.A. The neuroprotective effect of curcumin and Nigella sativa oil against oxidative stress in the pilocarpine model of epilepsy: a comparison with valproate. Neurochem. Res. 2011;36(11):2195–2204. doi: 10.1007/s11064-011-0544-9. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal N.B., Jain S., Nagpal D., Agarwal N.K., Mediratta P.K., Sharma K.K. Liposomal formulation of curcumin attenuates seizures in different experimental models of epilepsy in mice. Fundam. Clin. Pharmacol. 2013;27(2):169–172. doi: 10.1111/j.1472-8206.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal N.B., Jain S., Agarwal N.K., Mediratta P.K., Sharma K.K. Modulation of pentylenetetrazole-induced kindling and oxidative stress by curcumin in mice. Phytomedicine. 2011;18(8-9):756–759. doi: 10.1016/j.phymed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Tamaddonfard E., Erfanparast A., Hamzeh-Gooshchi N., Yousofizadeh S. Effect of curcumin, the active constituent of turmeric, on penicillin-induced epileptiform activity in rats. Avicenna J. Phytomed. 2012;2(4):196–205. [PMC free article] [PubMed] [Google Scholar]
- 30.Akula K.K., Kulkarni S.K. Effect of curcumin against pentylenetetrazol-induced seizure threshold in mice: possible involvement of adenosine A1 receptors. Phytother. Res. 2014;28(5):714–721. doi: 10.1002/ptr.5048. [DOI] [PubMed] [Google Scholar]
- 31.Aminirad A., Mousavi S.E., Fakhraei N., Mousavi S.M., Rezayat S.M. The role of nitric oxide in anticonvulsant effect of nanocurcumine on pentylenetetrazole-induced seizure in mice. Neurosci. Lett. 2017;651:226–231. doi: 10.1016/j.neulet.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Arbabi Jahan A., Rad A., Ghanbarabadi M., Amin B., Mohammad-Zadeh M. The role of serotonin and its receptors on the anticonvulsant effect of curcumin in pentylenetetrazol-induced seizures. Life Sci. 2018;211:252–260. doi: 10.1016/j.lfs.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Zhu W., Su J., Liu J., Jiang C. The involvement of neuronal nitric oxide synthase in the anti-epileptic action of curcumin on pentylenetetrazol-kindled rats. Biomed. Mater. Eng. 2015;26(Suppl. 1):S841–S850. doi: 10.3233/BME-151376. [DOI] [PubMed] [Google Scholar]
- 34.Choudhary K.M., Mishra A., Poroikov V.V., Goel R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013;704(1-3):33–40. doi: 10.1016/j.ejphar.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Luszczki J.J., Wlodarczyk M., Glensk M., Marzęda E., Durmowicz D., Florek-Łuszczki M. Effects of alizarin, betulin, curcumin, diosmin, linalool, menthofuran, α-terpineol, theobromine, β-thujaplicin and vanillin against maximal electroshock-induced seizure in mice. Journal of Pre-Clinical and Clinical Research. 2013;7(1):40–42. doi: 10.26444/jpccr/71433. [DOI] [Google Scholar]
- 36.Hashemian M., Anissian D., Ghasemi-Kasman M., Akbari A., Khalili-Fomeshi M., Ghasemi S., Ahmadi F., Moghadamnia A. A., Ebrahimpour A. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. 2017. [DOI] [PubMed]
- 37.Saha L., Chakrabarti A., Kumari S., Bhatia A., Banerjee D. Antiapoptotic and neuroprotective role of Curcumin in Pentylenetetrazole (PTZ) induced kindling model in rat. Indian J. Exp. Biol. 2016;54(2):133–141. [PubMed] [Google Scholar]
- 38.Jiang Z., Guo M., Shi C., Wang H., Yao L., Liu L., Xie C., Pu S., LaChaud G., Shen J., Zhu M., Mu L., Ge H., Long Y., Wang X., Song Y., Sun J., Hou X., Zarringhalam A., Park S.H., Shi C., Shen H., Lin Z. Protection against cognitive impairment and modification of epileptogenesis with curcumin in a post-status epilepticus model of temporal lobe epilepsy. Neuroscience. 2015;310:362–371. doi: 10.1016/j.neuroscience.2015.09.058. [DOI] [PubMed] [Google Scholar]
- 39.Kaur H., Bal A., Sandhir R. Curcumin supplementation improves mitochondrial and behavioral deficits in experimental model of chronic epilepsy. Pharmacol. Biochem. Behav. 2014;125:55–64. doi: 10.1016/j.pbb.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Kaur H., Patro I., Tikoo K., Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 2015;89:40–50. doi: 10.1016/j.neuint.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Kumar V., Prakash C., Singh R., Sharma D. Curcumin’s antiepileptic effect, and alterations in Nav1.1 and Nav1.6 expression in iron-induced epilepsy. Epilepsy Res. 2019;150:7–16. doi: 10.1016/j.eplepsyres.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Mansoor S.R., Hashemian M., Khalili-Fomeshi M., Ashrafpour M., Moghadamnia A.A., Ghasemi-Kasman M. Upregulation of klotho and erythropoietin contributes to the neuroprotection induced by curcumin-loaded nanoparticles in experimental model of chronic epilepsy. Brain Res. Bull. 2018;142:281–288. doi: 10.1016/j.brainresbull.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Mehla J., Reeta K.H., Gupta P., Gupta Y.K. Protective effect of curcumin against seizures and cognitive impairment in a pentylenetetrazole-kindled epileptic rat model. Life Sci. 2010;87(19-22):596–603. doi: 10.1016/j.lfs.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Khadrawy Y.A., Sawie H.G., Hosny E.N. Neuroprotective effect of curcumin nanoparticles against rat model of status epilepticus induced by pilocarpine. J. Complement. Integr. Med. 2018;15(3) doi: 10.1515/jcim-2017-0117. [DOI] [PubMed] [Google Scholar]
- 45.Noor N.A., Aboul Ezz H.S., Faraag A.R., Khadrawy Y.A. Evaluation of the antiepileptic effect of curcumin and Nigella sativa oil in the pilocarpine model of epilepsy in comparison with valproate. Epilepsy Behav. 2012;24(2):199–206. doi: 10.1016/j.yebeh.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Orellana-Paucar A.M., Serruys A.S.K., Afrikanova T., Maes J., De Borggraeve W., Alen J., León-Tamariz F., Wilches-Arizábala I.M., Crawford A.D., de Witte P.A.M., Esguerra C.V. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish and mouse seizure models. Epilepsy Behav. 2012;24(1):14–22. doi: 10.1016/j.yebeh.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Orellana-Paucar A.M., Afrikanova T., Thomas J., Aibuldinov Y.K., Dehaen W., de Witte P.A., Esguerra C.V. Insights from zebrafish and mouse models on the activity and safety of ar-turmerone as a potential drug candidate for the treatment of epilepsy. PLoS One. 2013;8(12):e81634. doi: 10.1371/journal.pone.0081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qneibi M., Hamed O., Fares O., Jaradat N., Natsheh A.R., AbuHasan Q., Emwas N., Al-Kerm R., Al-Kerm R. The inhibitory role of curcumin derivatives on AMPA receptor subunits and their effect on the gating biophysical properties. Eur. J. Pharm. Sci. 2019;136:104951. doi: 10.1016/j.ejps.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Reeta K.H., Mehla J., Pahuja M., Gupta Y.K. Pharmacokinetic and pharmacodynamic interactions of valproate, phenytoin, phenobarbitone and carbamazepine with curcumin in experimental models of epilepsy in rats. Pharmacol. Biochem. Behav. 2011;99(3):399–407. doi: 10.1016/j.pbb.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Bertoncello K.T., Aguiar G.P.S., Oliveira J.V., Siebel A.M. Micronization potentiates curcumin’s anti-seizure effect and brings an important advance in epilepsy treatment. Sci. Rep. 2018;8(1):2645. doi: 10.1038/s41598-018-20897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hussein S.A., Abdel-mageid A., Abd-Elhamed O.M., Amin A., Al-Harthy H.S. Neuroprotective effect of curcumin on kainic acid model of epilepsy in male swiss albino mice. International Journal of Chemical and Natural Science. 2016;4:447–460. [Google Scholar]
- 52.Wang J., Liu Y., Li X.H., Zeng X.C., Li J., Zhou J., Xiao B., Hu K. Curcumin protects neuronal cells against status-epilepticus-induced hippocampal damage through induction of autophagy and inhibition of necroptosis. Can. J. Physiol. Pharmacol. 2017;95(5):501–509. doi: 10.1139/cjpp-2016-0154. [DOI] [PubMed] [Google Scholar]
- 53.https://www.sabinsa.com/newsroom/sabinsa-products-faq/curcumin-c3-complex-faq [Accessed 8th June]
- 54.Vaz R.L., Outeiro T.F., Ferreira J.J. Zebrafish as an animal model for drug discovery in parkinson’s disease and other movement disorders: a systematic review. Front. Neurol. 2018;9(347):347. doi: 10.3389/fneur.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.United States Food and Drug Administration Guidance for industry: estimating the maximum safe starting dose in adult healthy volunteer. 2005.
- 57.Lüttjohann A., Fabene P.F., van Luijtelaar G. A revised Racine’s scale for PTZ-induced seizures in rats. Physiol. Behav. 2009;98(5):579–586. doi: 10.1016/j.physbeh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Dhir A. 2012.
- 59.Lévesque M., Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci. Biobehav. Rev. 2013;37(10 Pt 2):2887–2899. doi: 10.1016/j.neubiorev.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Müller C.J., Bankstahl M., Gröticke I., Löscher W. Pilocarpine vs. lithium-pilocarpine for induction of status epilepticus in mice: development of spontaneous seizures, behavioral alterations and neuronal damage. Eur. J. Pharmacol. 2009;619(1-3):15–24. doi: 10.1016/j.ejphar.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Sharma V., Babu P.P., Singh A., Singh S., Singh R. Iron-induced experimental cortical seizures: electroencephalographic mapping of seizure spread in the subcortical brain areas. Seizure. 2007;16(8):680–690. doi: 10.1016/j.seizure.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Sutter R., Rüegg S., Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: A systematic review. Neurology. 2015;85(15):1332–1341. doi: 10.1212/WNL.0000000000002023. [DOI] [PubMed] [Google Scholar]
- 63.Beyazcicek E., Ankarali S., Beyazcicek O., Ankarali H., Demir S., Ozmerdivenli R. Effects of thymoquinone, the major constituent of Nigella sativa seeds, on penicillin-induced epileptiform activity in rats. Neurosciences (Riyadh) 2016;21(2):131–137. doi: 10.17712/nsj.2016.2.20150781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castel-Branco M., Alves G., Figueiredo I., Falcão A., Caramona M. 2009.
- 65.Kaminski R.M., Livingood M.R., Rogawski M.A. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45(7):864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- 66.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 67.Dei Cas M., Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients. 2019;11(9):2147. doi: 10.3390/nu11092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner P.V., Brabb T., Pekow C., Vasbinder M.A. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011;50(5):600–613. [PMC free article] [PubMed] [Google Scholar]
- 69.Pan M-H., Huang T-M., Lin J-K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999;27(4):486–494. [PubMed] [Google Scholar]
- 70.Gutierres V.O., Campos M.L., Arcaro C.A., Assis R.P., Baldan-Cimatti H.M., Peccinini R.G., Paula-Gomes S., Kettelhut I.C., Baviera A.M., Brunetti I.L. Curcumin pharmacokinetic and pharmacodynamic evidences in streptozotocin-diabetic rats support the antidiabetic activity to be via metabolite(s). Evid. Based Complement. Alternat. Med. 2015;2015:678218. doi: 10.1155/2015/678218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jäger R., Lowery R.P., Calvanese A.V., Joy J.M., Purpura M., Wilson J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014;13:11–11. doi: 10.1186/1475-2891-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jankun J., Wyganowska-Świątkowska M., Dettlaff K., Jelińska A., Surdacka A., Wątróbska-Świetlikowska D., Skrzypczak-Jankun E. Determining whether curcumin degradation/condensation is actually bioactivation. Int. J. Mol. Med. 2016;37(5):1151–1158. doi: 10.3892/ijmm.2016.2524. [Review]. [DOI] [PubMed] [Google Scholar]
- 73.Prasad S., Tyagi A.K., Aggarwal B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teleanu R.I., Chircov C., Grumezescu A.M., Volceanov A., Teleanu D.M. Antioxidant Therapies for neuroprotection-a review. J. Clin. Med. 2019;8(10):1659. doi: 10.3390/jcm8101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khansari P.S., Halliwell R.F. Mechanisms underlying neuroprotection by the NSAID mefenamic acid in an experimental model of stroke. Front. Neurosci. 2019;13(64):64. doi: 10.3389/fnins.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loris Z.B., Pieper A.A., Dietrich W.D. The neuroprotective compound P7C3-A20 promotes neurogenesis and improves cognitive function after ischemic stroke. Exp. Neurol. 2017;290:63–73. doi: 10.1016/j.expneurol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Meng X.J., Wang F., Li C.K. Resveratrol is neuroprotective and improves cognition in pentylenetetrazole-kindling model of epilepsy in rats. Indian J. Pharm. Sci. 2014;76(2):125–131. [PMC free article] [PubMed] [Google Scholar]
- 78.Cook A.M., Bensalem-Owen M.K. Mechanisms of action of antiepileptic drugs. Clin. Pract. 2011;8(3):307. [Google Scholar]
- 79.Novakova B., Harris P.R., Ponnusamy A., Reuber M. The role of stress as a trigger for epileptic seizures: a narrative review of evidence from human and animal studies. Epilepsia. 2013;54(11):1866–1876. doi: 10.1111/epi.12377. [DOI] [PubMed] [Google Scholar]
- 80.Walker M. Neuroprotection in epilepsy. Epilepsia. 2007;48(s8) Suppl. 8:66–68. doi: 10.1111/j.1528-1167.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 81.Rana A., Musto A.E. The role of inflammation in the development of epilepsy. J. Neuroinflammation. 2018;15(1):144–144. doi: 10.1186/s12974-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin E-J., Jeong J.H., Chung Y.H., Kim W-K., Ko K-H., Bach J-H., Hong J-S., Yoneda Y., Kim H-C. Role of oxidative stress in epileptic seizures. Neurochem. Int. 2011;59(2):122–137. doi: 10.1016/j.neuint.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lima Giacobbo B., Doorduin J., Klein H.C., Dierckx R.A.J.O., Bromberg E., de Vries E.F.J. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol. Neurobiol. 2019;56(5):3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seidenberg M., Pulsipher D.T., Hermann B. Association of epilepsy and comorbid conditions. Future Neurol. 2009;4(5):663–668. doi: 10.2217/fnl.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amalraj A., Pius A., Gopi S., Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - a review. J. Tradit. Complement. Med. 2016;7(2):205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]