Abstract
The transmembrane glycoprotein NSP4 functions as a viral enterotoxin capable of inducing diarrhea in young mice. It has been suggested that NSP4 may be a key determinant of rotavirus pathogenicity and a target for vaccine development. Twenty two G1P[6] rotaviruses from babies with and without diarrhea were comparatively analyzed along with reference strains and another 22 Taiwanese human rotaviruses of G and P combination types different from the G1P[6] type. The sequence variations in the NSP4 genes were studied by direct sequencing analysis of the amplicons of reverse transcription-PCR. Two genetic groups could be identified in this analysis. While the majority of these strains were closely related to the Wa strain, the G2 viruses were closely related to the S2 strain. Furthermore, phylogenetic analysis of the NSP4 gene among the G2 rotaviruses revealed three distinct lineages associated with DS-1, S2, and E210, respectively, as has been reported previously for the VP7 gene. However, we found no apparent correlation in the deduced amino acid sequences corresponding to the proposed enterotoxic peptide region between the rotaviruses recovered from individuals with and without diarrhea. The NSP4 gene product being a pathogenic determinant may not be a generalized phenomenon.
The rotavirus is one of the major etiologic agents of diarrheal disease, affecting mainly infants and young children (12). The rotavirus genome contains 11 segments of double-stranded RNA, and complete virus particles have a triple-layered protein capsid. VP4 and VP7 are two major structural proteins on the outer capsid. Among the group A rotaviruses, 14 G (VP7) serotypes and at least 20 P (VP4) genotypes have been defined on the basis of their reactivity with G serotype-specific neutralizing antibodies and the sequence variations of VP4 gene, respectively (8, 12).
The rotavirus genome also encodes five nonstructural proteins. The nonstructural protein NSP4, which is encoded by gene segment 10 for most strains of rotavirus, is a glycoprotein anchoring the membrane of the endoplasmic reticulum (8). It has been demonstrated in previous studies by these authors that NSP4 serves as an intracellular receptor for double-layered rotavirus particles and interacts with viral capsid proteins during viral morphogenesis (2, 23). NSP4 has been proposed as a possible viral enterotoxin capable of inducing diarrhea in young mice (3). Specifically, a peptide consisting of amino acids (aa) 114 to 135 on NSP4 has been shown to trigger a signal transduction pathway that increases intracellular calcium levels in cells by mobilizing calcium from the endoplasmic reticulum, thus stimulating chloride secretion (3, 7, 22). Furthermore, amino acid changes in this region have been associated with alterations in the toxigenic activity of NSP4 and virulence of rotavirus (3, 27). Finally, antibodies against NSP4 might diminish the frequency and severity of rotavirus diarrhea (3). Thus, further study of NSP4 as a key pathogenic determinant of human rotavirus infections might justify it as a suitable target for vaccine development (16).
In a previous study, we reported results of rotavirus surveillance in the neonatal nursery of Changhua Christian Hospital (CCH), located in the central part of Taiwan, Republic of China. Stool specimens from every baby born from October 1994 to May 1995 were tested (5). Rotaviruses with G1P[6] specificity were found to be associated with these hospital-acquired infections (C. N. Lee, C. C. Lin, C. L. Kao, and H. N. Chen, Abstr. Xth Int. Congr. Virol. Int. Union Microbiol. Soc., abstr. PW03-11, p. 102, 1996). While more than half of these rotavirus-positive neonates suffered from fever, diarrhea, or dehydration, a considerable proportion of the infected neonates remained well during this outbreak (5). In this study, we analyzed the genetic sequence of the NSP4 genes of the rotaviruses recovered from babies with and without diarrhea. Other locally isolated human rotavirus strains with different combinations of G and P types were also compared, along with reference strains.
MATERIALS AND METHODS
Rotavirus samples.
All rotavirus samples used in this study were collected from CCH in central Taiwan and from the National Taiwan University Hospital (NTUH) located in the northern city of Taipei (Table 1). These samples had been shown to be rotavirus positive with enzyme-linked immunosorbent assay kits: Test Pack Rotavirus (Abbott Laboratories, Chicago, Ill.) for CCH samples and Rotaclone (Meridian Diagnostic, Cincinnati, Ohio) for NTUH samples. Coinfection of these children with other enteric pathogens had been ruled out by negative bacterial cultures and negative testing for astrovirus (IDEIA Astrovirus; DAKO, Cambridgeshire, England) and for adenovirus type 40/41 (Meridian Diagnostic). All the original fecal samples contained sufficient rotavirus particles for the viral RNA to be made visual by silver staining after polyacrylamide gel electrophoresis.
TABLE 1.
Rotavirus strains used for NSP4 gene analysis
| Straina | Recovery of strain
|
G typec | P typec | NSP4 typed | Accession no. | ||
|---|---|---|---|---|---|---|---|
| Ageb of child | Yr | Symptom seen in child | |||||
| CH2 | 4D | 1995 | − | 1 | [6] | B | AF173179 |
| CH7 | 4D | 1995 | − | 1 | [6] | B | AF173180 |
| CH26 | 4D | 1995 | − | 1 | [6] | B | AF173181 |
| CH31 | 6D | 1995 | − | 1 | [6] | B | AF173182 |
| CH52 | 3D | 1995 | − | 1 | [6] | B | AF173183 |
| CH55 | 5D | 1995 | − | 1 | [6] | B | AF173184 |
| CH61 | 4D | 1994 | − | 1 | [6] | B | AF173185 |
| CH81 | 4D | 1995 | − | 1 | [6] | B | AF173186 |
| CH83 | 5D | 1995 | − | 1 | [6] | B | AF173187 |
| CH92 | 4D | 1995 | − | 1 | [6] | B | AF173188 |
| CH96 | 5D | 1995 | + | 1 | [6] | B | AF173195 |
| CH98 | 5D | 1995 | + | 1 | [6] | B | AF173196 |
| CH107 | 14D | 1995 | − | 1 | [6] | B | AF173189 |
| CH113 | 4D | 1995 | − | 1 | [6] | B | AF173190 |
| CH119 | 5D | 1995 | − | 1 | [6] | B | AF173191 |
| CH142 | 4D | 1995 | + | 1 | [6] | B | AF173197 |
| CH145 | 4D | 1994 | + | 1 | [6] | B | AF173198 |
| CH612 | 4D | 1994 | − | 1 | [6] | B | AF173192 |
| CH613 | 6D | 1994 | − | 1 | [6] | B | AF173193 |
| CH619 | 6D | 1994 | − | 1 | [6] | B | AF173194 |
| CH624 | 9D | 1994 | + | 1 | [6] | B | AF173199 |
| TE56 | 14D | 1993 | + | 1 | [6] | B | AF173200 |
| CH631 | 2Y 6M | 1994 | + | 1 | [8] | B | AF173201 |
| CH638 | 3Y | 1994 | + | 1 | [8] | B | AF173202 |
| TA1 | 1Y 9M | 1978 | + | 1 | [8] | B | AF173203 |
| TA10 | 10Y | 1981 | + | 1 | [8] | B | AF173204 |
| TD11 | 4M | 1992 | + | 1 | [8] | B | AF173205 |
| TE2 | 9M | 1993 | + | 1 | [8] | B | AF173206 |
| TG59 | 11M | 1995 | + | 1 | [8] | B | AF173207 |
| TA3 | 9M | 1981 | + | 2 | [4] | A | AF174298 |
| TA26 | 1Y 11M | 1982 | + | 2 | [4] | A | AF174299 |
| TA34 | 6M | 1983 | + | 2 | [4] | A | AF174300 |
| TD7 | 2Y | 1992 | + | 2 | [4] | A | AF174301 |
| TE68 | 11M | 1993 | + | 2 | [4] | A | AF174302 |
| TE83 | 5M | 1993 | + | 2 | [4] | A | AF174303 |
| TF85 | 2Y | 1994 | + | 2 | [4] | A | AF174304 |
| TA371 | 16D | 1989 | + | 3 | [8] | B | AF173208 |
| TA372 | 1M | 1989 | + | 3 | [8] | B | AF173209 |
| TA373 | 9M | 1989 | + | 3 | [8] | B | AF173210 |
| TI23 | 1Y 9M | 1997 | + | 3 | [8] | B | AF173211 |
| TI25 | 2Y 6M | 1997 | + | 3 | [8] | B | AF173212 |
| TA74 | 1Y | 1985 | + | 4 | [8] | B | AF173213 |
| TA77 | 6Y | 1985 | + | 4 | [8] | B | AF173214 |
| TA79 | 1Y 4M | 1985 | + | 4 | [8] | B | AF173215 |
Strains with a name beginning with CH were collected from the CCH. The rest of the strains were collected from NTUH.
D, days; M, months; Y, years.
G and P types have been determined by RT-PCR.
NSP4 genotypes determined in this study; the types were designated as described by Kirkwood and Palombo (18).
Fecal samples were diluted to approximately 10% (wt/vol) in Dulbecco's phosphate-buffered saline (138 mM NaCl, 2.7 mM KCl, 1.2 mM KH2PO4, 8.1 mM Na2HPO4, 0.9 mM CaCl2, 0.5 mM MgCl2), pH 7.2, and clarified by low-speed centrifugation. The supernatant was collected and stored at −70°C.
RNA extraction and purification.
Rotavirus RNA was extracted and purified from stool samples according to a procedure described previously (15). Rotavirus particles were concentrated and precipitated by adding ammonium sulfate (to a final concentration of 35% [wt/vol]) in 1 ml of clarified stool suspension. After thorough mixing, the precipitated rotavirus particles were sedimented at 10,000 × g for 30 min. The supernatant was then removed, and the pellet was suspended in 100 μl of TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). The rotavirus particles were then lysed by adding 25 μl of 5× extraction buffer to achieve a final concentration of 0.02 M Tris-HCl (pH 7.4), 0.15 M NaCl, 0.01 M MgCl2, 1% sodium dodecyl sulfate, and 2% (wt/vol) Ficoll. The viral RNA was extracted with phenol-chloroform two or three times, depending on the clarity of the samples, and further purified with chromatographic cellulose fiber powder CF11 (Whatman, Springfield Mill, England) to remove the inhibitors present in stool samples (25). Fifty microliters of the RNA was mixed with 15 mg of CF11, 2.5 μl of 5 M NaCl, and 9.25 μl of 100% ethanol. The mixture was rotated for 90 min at 4°C, and the RNA bound to CF11 was pelleted and washed three times with 500 μl of ethanol-containing STE (0.1 M NaCl, 50 mM Tris-HCl [pH 7.0], 1 mM EDTA). Finally, the RNA was eluted from the CF11 by adding 300 μl of ethanol-free STE. After 5 min of centrifugation, the supernatant RNA was collected and precipitated with ethanol in the presence of 2.5 M ammonium acetate. The RNA pellet was washed and suspended in 20 μl of TE buffer.
RT-PCR amplification of NSP4 gene.
Three microliters of purified rotavirus RNA were used as the template in the reverse transcription PCR (RT-PCR) mixture. This mixture contained 7% dimethyl sulfoxide 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.01% (wt/vol) gelatin, 1.5 mM MgCl2, 0.1% Triton-X-100, a 200 μM concentration of each of the deoxynucleoside triphosphates and a 200 mM concentration of each of the forward, 10BEG.16 (5′-TGTTCCGAGAGAGCGCGTG-3′; nucleotide 16 to 34), and reverse, 10END.722c (5′-GACCATTCCTTCCATTAAC-3′; nucleotide 722 to 740), primers (24, 27). This mixture was heated for 10 min at 97°C, cooled on ice for 5 min, and briefly centrifuged at full-speed in a Microcentrifuge (Eppendorf 5415C). Super RT (5 U; HT Biotechnology, Cambridge, England), Taq polymerase (1 U; HT Biotechnology), and human placental ribonuclease inhibitor (20 U; HT Biotechnology) were then added. The mixture was incubated in a thermal cycler (Model 480; Perkin-Elmer, Foster City, Calif.) at 42°C for 1 h, 30 cycles each at 95°C for 45 s, 49°C for 30 s, 72°C for 1.5 min, and one cycle at 72°C for 10 min. Fragments 725 bp in length were amplified. For sequencing, the PCR products were purified through QIAquick silica gel membrane (QIAGEN, Chatsworth, Calif.).
Cycle sequencing.
The purified PCR product was sequenced by using the sequencing kit with fluorescent dye terminators (Perkin-Elmer) according to the manufacturer's instructions. The primers used for sequencing were 10BEG.16, 10END.722c, 10.374 (5′-ATGATTGATAAACTAACTAC-3′), and 10.394c (5′-GTAGTTAGTTTATCAATCAT-3′). The thermal cycling conditions were set as recommended by the manufacturer: 96°C for 30 s, 50°C for 15 s, and 60°C for 4 min for 25 cycles (model 480 thermocycler; Perkin-Elmer). The products were purified by ethanol precipitation and then suspended in the loading buffer (100% formamide and 25 mM EDTA [pH 8.0] mixed in a 5:1 ratio). Samples were heated at 95°C for 2 min and loaded onto a 4.75% polyacrylamide gel. The sequence data were collected by an automatic DNA sequencer (model ABI-373A; Perkin-Elmer). Each gene fragment was sequenced at least twice.
Analysis of sequences.
The sequence data were analyzed by GeneWorks software (IntelliGenetics, Mountain View, Calif.). Phylogenetic analysis was performed using the neighbor-joining method and the Kimura two-parameter distance matrix listed in the MEGA analytical package (14). The following reference NSP4 sequences from standard reference strains (with GenBank accession numbers in parentheses) were used in comparison: 1076 (U59105), A28 (D01145), AU-1 (D89873), AU32 (D88830), E201 (U59106), E210 (U59107), FRV-1 (D89874), FRV64 (D88833), KUN (D88829), M37 (U59109), NCDV (X06806), OSU (D88831), RV3 (U42628), RV4 (U59108), RV5 (U59103), S2 (U59104), SA11 (K01138), ST3 (U59110), UK (K03384), VA70 (U83798), Wa (K02032), YO (AB008236).
Nucleotide sequence accession number.
The NSP4 gene sequences determined in this study were deposited in the GenBank sequence database. Table 1 lists the assigned accession numbers, AF173179 to AF173214 and AF174298 to AF174304, for Taiwanese rotavirus samples. The accession number for DS-1 is AF174305.
RESULTS
Sequence analysis of NSP4 genes of neonatal rotaviruses.
The nucleotide sequences of NSP4 gene were amplified by RT-PCR and were then sequenced. Excluding the primer sequences, a fragment of 687 bp was analyzed and compared. Of the 21 G1P[6] rotavirus strains collected from neonates admitted to the CCH in 1994 and 1995 (CH2 to CH624, Table 1), 5 were recovered from infants with diarrhea and 16 were from diarrhea-free infants. The nucleotide sequences of the NSP4 genes were highly similar, with a homology of greater than 99%. Only one other G1P[6] strain, TE56, collected from a neonate with diarrhea in NTUH in 1993, exhibited such similarity in its NSP4 gene.
Comparison of NSP4 genes from rotaviruses with different combinations of G and P types.
The 22 local strains (7 G1P[8], 7 G2P[4], 5 G3P[8], and 3 G4P[8]) that contained variable combinations of G and P types and were recovered from children with acute gastroenteritis were compared for similarity of the NSP4 genes (Table 1). The degrees of similarity in the nucleotide sequence of the 22 Taiwanese G1P[6] strains with these G1, G3, and G4 strains ranged from 94.6 to 98.4%. The degrees of similarity of these Taiwanese G1P[6] strains with the reference G1P[8] strain Wa were higher than 97.5%. With three P[6] reference strains (M37, RV3, and ST3), the degrees of similarity ranged from 94.2 to 97.2%. The Taiwanese G1P[6] strains differed more in their NSP4 gene from the G2P[4] strains (TA3, TA26, TA34, TD7, TE68, TE83, and TF85), with degrees of similarity lower than 84%. Comparisons of the samples representing the most divergent sequences from different genotypes are summarized in Table 2.
TABLE 2.
Comparison of NSP4 genes from Taiwanese strains and reference strains
| Straina | G1
|
G2
|
G3
|
G4
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M37 | Wa | CH31 | CH81 | CH107 | CH142 | CH145 | TE56 | TA1 | TA10 | TE2 | E210 | RV5 | S2 | TA3 | TA26 | TE83 | RV3 | TA371 | ST3 | TA74 | |
| M37 | 96.6 | 96.6 | 96.6 | 96.6 | 96.6 | 96.6 | 96.6 | 94.9 | 96.6 | 93.1 | 84.6 | 82.3 | 83.4 | 82.3 | 83.4 | 84.6 | 94.9 | 93.7 | 94.3 | 95.4 | |
| Wa | 96.2b | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 98.3 | 98.9 | 96.6 | 85.7 | 83.4 | 84.6 | 83.4 | 84.6 | 85.7 | 97.1 | 97.1 | 95.4 | 98.9 | |
| CH31 | 94.2 | 97.5 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 98.3 | 98.9 | 96.6 | 85.7 | 83.4 | 84.6 | 83.4 | 84.6 | 85.7 | 97.1 | 97.1 | 95.4 | 98.9 | |
| CH81 | 95.3 | 98.4 | 99.6 | 100.0 | 100.0 | 100.0 | 100.0 | 98.3 | 98.9 | 96.6 | 85.7 | 83.4 | 84.6 | 83.4 | 84.6 | 85.7 | 97.1 | 97.1 | 95.4 | 98.9 | |
| CH107 | 95.3 | 98.7 | 99.0 | 99.3 | 100.0 | 100.0 | 100.0 | 98.3 | 98.9 | 96.6 | 85.7 | 83.4 | 84.6 | 83.4 | 84.6 | 85.7 | 97.1 | 97.1 | 95.4 | 98.9 | |
| CH142 | 95.3 | 98.4 | 99.6 | 99.9 | 99.3 | 100.0 | 100.0 | 98.3 | 98.9 | 96.6 | 85.7 | 83.4 | 84.6 | 83.4 | 84.6 | 85.7 | 97.1 | 97.1 | 95.4 | 98.9 | |
| CH145 | 95.2 | 98.3 | 99.4 | 99.7 | 99.1 | 99.7 | 100.0 | 98.3 | 98.9 | 96.6 | 85.7 | 83.4 | 84.6 | 83.4 | 84.6 | 85.7 | 97.1 | 97.1 | 95.4 | 98.9 | |
| TE56 | 95.5 | 98.8 | 99.1 | 99.4 | 99.7 | 99.4 | 99.3 | 98.3 | 98.9 | 96.6 | 85.7 | 83.4 | 84.6 | 83.4 | 84.6 | 85.7 | 97.1 | 97.1 | 95.4 | 98.9 | |
| TA1 | 92.9 | 95.6 | 94.6 | 94.9 | 95.2 | 95.0 | 94.8 | 95.3 | 97.1 | 94.9 | 85.7 | 84.0 | 84.6 | 84.0 | 84.6 | 85.7 | 95.4 | 95.4 | 93.7 | 97.1 | |
| TA10 | 97.7 | 96.8 | 95.3 | 95.6 | 95.9 | 95.6 | 95.5 | 96.1 | 93.7 | 95.4 | 85.1 | 82.9 | 84.0 | 82.9 | 84.0 | 85.1 | 96.0 | 96.0 | 94.9 | 97.7 | |
| TE2 | 94.3 | 97.1 | 95.6 | 95.9 | 96.2 | 96.0 | 95.8 | 96.4 | 93.6 | 94.9 | 82.9 | 81.7 | 82.9 | 81.7 | 82.9 | 83.4 | 94.9 | 99.4 | 93.1 | 95.4 | |
| E210 | 82.2 | 82.4 | 82.1 | 82.4 | 82.4 | 82.4 | 82.2 | 82.5 | 83.7 | 81.7 | 81.5 | 94.9 | 98.9 | 95.4 | 98.9 | 100.0 | 84.0 | 84.0 | 82.3 | 84.6 | |
| RV5 | 82.8 | 82.4 | 82.0 | 82.2 | 82.0 | 82.2 | 82.1 | 82.1 | 83.6 | 81.1 | 81.4 | 95.2 | 94.9 | 97.7 | 94.9 | 94.9 | 82.3 | 82.3 | 80.6 | 82.3 | |
| S2 | 82.5 | 82.4 | 82.1 | 82.4 | 82.1 | 82.4 | 82.2 | 82.2 | 83.3 | 82.1 | 81.8 | 97.7 | 94.9 | 95.4 | 98.9 | 98.9 | 83.4 | 83.4 | 81.7 | 83.4 | |
| TA3 | 81.5 | 82.0 | 82.0 | 82.4 | 82.0 | 82.2 | 82.1 | 82.1 | 83.6 | 80.8 | 81.4 | 95.3 | 99.0 | 94.8 | 95.4 | 95.4 | 82.3 | 82.3 | 80.6 | 82.3 | |
| TA26 | 82.4 | 82.0 | 81.7 | 82.0 | 81.7 | 82.0 | 81.8 | 81.8 | 83.1 | 81.7 | 81.4 | 98.3 | 95.2 | 99.1 | 95.3 | 98.9 | 83.4 | 83.4 | 81.7 | 83.4 | |
| TE83 | 82.4 | 82.0 | 81.4 | 81.7 | 81.7 | 81.7 | 81.5 | 81.8 | 83.6 | 81.7 | 81.1 | 99.0 | 94.8 | 97.5 | 94.9 | 98.1 | 84.0 | 84.0 | 82.3 | 84.6 | |
| RV3 | 96.1 | 98.3 | 96.7 | 97.1 | 97.1 | 97.1 | 96.9 | 97.2 | 94.6 | 95.9 | 96.4 | 82.2 | 82.1 | 82.8 | 82.1 | 82.4 | 82.1 | 95.4 | 93.7 | 96.0 | |
| TA371 | 94.8 | 97.5 | 96.1 | 96.4 | 96.7 | 96.4 | 96.2 | 96.8 | 94.6 | 95.3 | 98.8 | 82.5 | 81.5 | 82.8 | 81.5 | 82.4 | 82.1 | 96.8 | 93.7 | 96.0 | |
| ST3 | 95.2 | 97.1 | 95.9 | 96.2 | 96.7 | 96.2 | 96.2 | 96.1 | 93.4 | 94.5 | 95.2 | 81.4 | 81.5 | 81.1 | 81.5 | 80.6 | 81.2 | 96.7 | 95.3 | 94.3 | |
| TA74 | 95.6 | 99.3 | 97.5 | 97.8 | 98.1 | 97.8 | 97.8 | 98.3 | 95.1 | 96.2 | 96.5 | 82.1 | 81.8 | 82.1 | 81.6 | 81.7 | 81.7 | 97.7 | 96.9 | 96.5 | |
Taiwanese rotavirus strains are marked in bold. CH31, CH81, and CH107 were recovered from diarrhea-free infections. The rest of the Taiwanese strains were recovered from infections with diarrhea.
Values represent percentage identities between nucleotide and amino acid sequences. The nucleotide sequence identities are shown on the lower left portion, the amino acid sequence identities on the upper right portion.
Phylogenetic analysis of NSP4 gene.
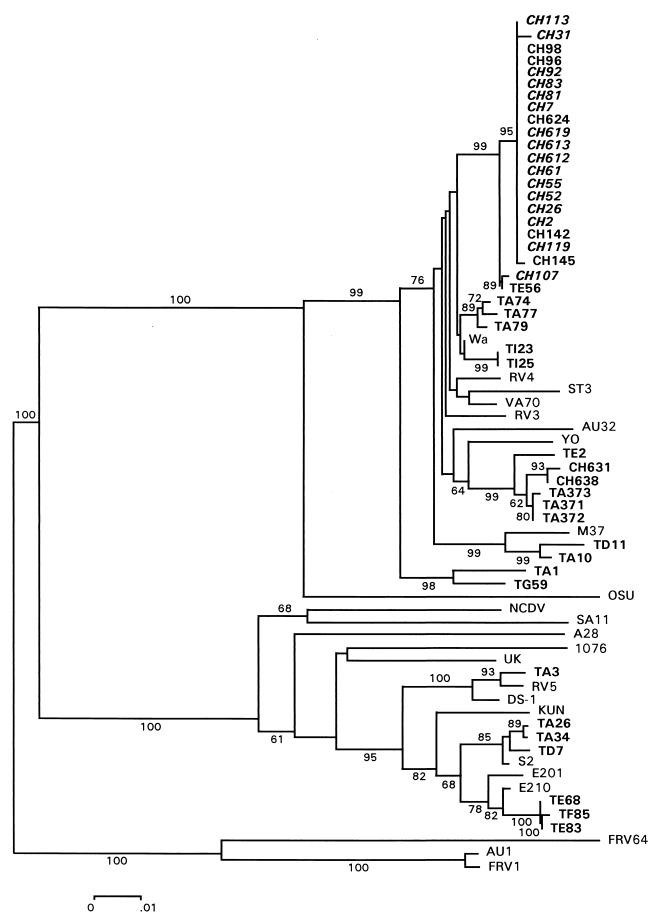
Phylogenetic analysis of the NSP4 genes for all the Taiwanese strains along with the reference human and animal strains revealed that all the neonatal G1P[6] strains clustered together, forming a distinct lineage within a broader cluster that includes all Taiwanese strains of G1P[8], G3P[8], and G4P[8] types, as well as the human reference strains with G1 (Wa, RV4, M37), G3 (RV3, YO), G4 (ST3, VA70), and G9 (AU32) specificities (Fig. 1). Two G1P[8] strains, TA10 and TD11, collected in 1981 and 1992, respectively, formed a distinct lineage along with M37. Two G1P[8] strains, TA1 (1978) and TG59 (1995), which were recovered from different epidemics separated by 17 years, were closely related and formed one distinct lineage. The G3P[8] strains of 1989 (TA371, TA372, and TA373) formed a separate lineage from those of 1997 (TI23 and TI25). In the same phylogenetic tree, all the G2 strains formed a totally separate cluster, in which the TA3 strain was close to reference G2 strains RV5 and DS-1; TA26, TA34, and TD7 clustered with S2; TE68, TE83, and TF85 clustered with E210 and E201 (Fig. 1). None of the Taiwanese strains associated closely with any of the human strains, A28, 1076, and AU1, or the animal strains, FRV1, FRV64, NCDV, OSU, SA11, and UK, analyzed in this phylogenetic tree.
FIG. 1.
Phylogenetic analysis of the nucleotide sequences of the NSP4 gene of rotavirus. A phylogenetic tree was constructed based on the neighbor-joining method within the MEGA package. Percentage bootstrap values above 60% are shown at branch nodes. The scale bar represents a 1% nucleotide difference. The Taiwanese strains recovered from diarrhea-free infections are indicated by boldface italic type. The other Taiwanese rotavirus strains are indicated by boldface type.
Amino acid sequence alignment.
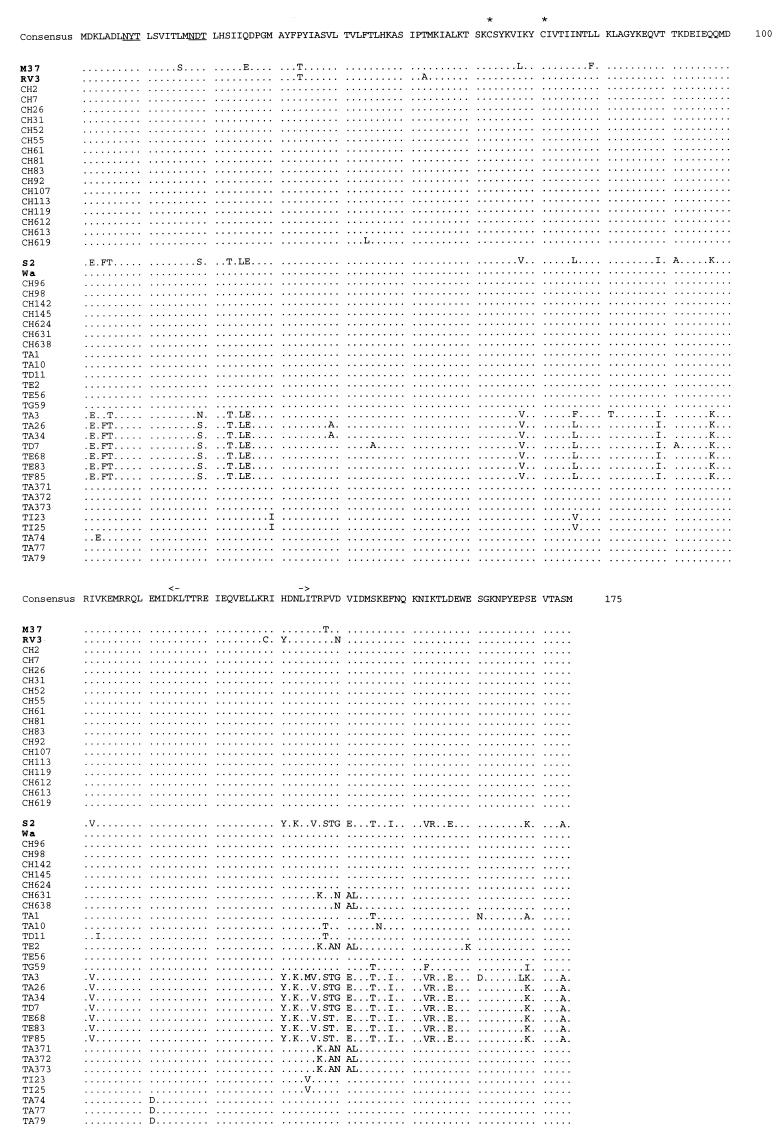
The deduced amino acid sequences of the NSP4 genes of 44 Taiwanese strains were aligned with the avirulent reference strains, M37 and RV3, and virulent reference strains, S2 and Wa. In all the strains analyzed, the 2 N-linked glycosylation sites (aa 8 and 18) and two cysteine residues (aa 63 and 71) were all conserved (Fig. 2). The three hydrophobic domains (aa 7 to 21, 28 to 47, and 67 to 85) (8) were also mostly conserved. No discernible differences in the amino acid sequences of NSP4 were noted between the GIP[6] strains recovered from unaffected neonates and those from the diarrheal neonates. The G2 strains differed from all other strains by more than 25 residues. Variations could be found in the regions of the putative VP4 binding site (aa 112 to 146), double-layered particle binding site (161 to 175), and the predicted amphipathic alpha-helix (aa 93 to 133) (8). In the region proposed as a toxic peptide (aa 114 to 135), however, all of the Taiwanese G1P[6] strains shared the exact amino acid sequence as that of the M37 and Wa strains, with a His residue at position 131 in lieu of a Tyr, as in all G2 strains.
FIG. 2.
Alignment of the deduced amino acid sequences of the NSP4 gene of rotaviruses. Twenty-two G1P[6], 7 G1P[8], 7 G2P[4], 5 G3P[8], and 3 G4P[8] rotavirus strains from Taiwan were compared with reference strain M37, RV3, S2, and Wa. Consensus sequence was derived from all of these strains. Only amino acids that differed from the consensus sequence are shown. The avirulent strains are shown in the upper portion of each panel; the virulent strains are shown in the lower portion of each panel. The N-linked glycosylation sites are underlined; the cysteines are marked by asterisks. The region of proposed enterotoxic peptide is marked between the arrows.
DISCUSSION
In this study, two genetic groups of the NSP4 gene could be recognized in these human rotaviruses from Taiwan. The majority of the rotavirus strains analyzed here were the non-G2 strains that were closely related to the non-G2 reference strains, such as Wa, YO, or ST3. The G2 strains were closely related to the S2 strain.
Neonates at CCH, when infected by G1P[6] viruses, showed a variable spectrum of clinical outcomes. Coinfection by other pathogens that might explain such variable clinical outcomes was unlikely because those diagnostic procedures used for other common causes of diarrhea, viral or bacterial agents, revealed no other likely sources of infection. Similarly, no differences in feeding methods or metabolic status between the babies with diarrhea and those without were found. Although not examined in this study, maternal antibodies to rotavirus might have a variable effect in protecting neonates from different strains of rotavirus (17, 19–21). Differences in the genetic background of these neonates might also affect the clinical outcome. This possibility needs to be further investigated.
The genetic comparisons of NSP4 revealed that genes from unaffected neonates were nearly identical to those from neonates and children with diarrhea. These results are consistent with those of other investigators (10) and suggest that the NSP4 gene may not be the only pathogenic determinant of rotavirus. This is also supported by studies in a mouse model (1), where it was suggested that the enterotoxigenic properties of NSP4 do not play a critical role in the pathogenesis of murine rotavirus diarrhea. In view of these findings, the possible selection of NSP4 as a target for vaccine development requires further scrutiny. As an alternative to NSP4, other rotavirus genes (VP3, VP4, VP7, NSP1, and NSP2) have also been associated with pathogenicity in various animal models (4, 9, 11). Further studies of these genes and NSP4 to delineate the molecular basis of rotavirus pathogenesis will be needed to develop effective control strategies.
The genetic divergence of the NSP4 genes between the two genetic groups exceeded 16%. The amino acid variations between the strains of these two genetic groups could be found in the proposed biologically important domains, the VP4 binding site and the double-layered particle binding site (8). It remains to be examined whether these variations would affect the biological role of NSP4. Previously, the NSP4 genes of human rotaviruses have been categorized into two main genetic groups by some investigators (6, 10, 13). Most human rotaviruses were categorized as group B, whereas G2 viruses, together with many animal rotaviruses, were categorized as group A (13). Kirkwood and Palombo suggested that the human G2 rotaviruses may have derived their NSP4 genes from different animal rotaviruses (13). In our phylogenetic tree of the NSP4 gene, the genetic relationships of human G2 rotaviruses were comparable to those in the phylogenetic tree of VP7 gene (26). Zao et al. in 1999 indicated that a Taiwanese G2 strain, TA3, recovered in 1981, was closely related to RV5 in its VP7 gene; thereafter, the G2 strains were closely related to S2 (26). Within the S2 lineage, the G2 strains (TE68, TE83, and TF85) responsible for the epidemic in 1993 were more closely related to E210. TE68, TE83, TF85, and E210 have all been reported as reassortants of rotavirus (18, 26). The similarity between VP7 and NSP4 genes in the phylogenetic trees may imply that the two genes coevolve in these G2 rotavirus reassortants.
In this study, the nucleotide sequence of the NSP4 genes was determined for 44 human rotavirus strains belonging to genotypes of several G and P combinations recovered from Taiwanese children with or without diarrhea. Results in this study demonstrate that the genetic relationships among strains from different epidemics can be resolved by phylogenetic analysis of this gene. NSP4 gene analysis provides further insight into the genetic relation among rotaviruses worldwide but so far has failed to reveal pathogenic correlates among rotaviruses of Taiwan origin.
ACKNOWLEDGMENTS
We are grateful to Mei-Shang Ho for precious comments and advice on language usage.
We thank the National Science Council of the Republic of China for financially supporting this research under contract NSC88-2314-B002-257.
REFERENCES
- 1.Angel J, Tang B, Feng N, Greenberg H B, Bass D. Studies of the role for NSP4 in the pathogenesis of homologous murine rotavirus diarrhea. J Infect Dis. 1998;177:455–458. doi: 10.1086/517374. [DOI] [PubMed] [Google Scholar]
- 2.Au K S, Chan W K, Burns J W, Estes M K. Receptor activity of rotavirus nonstructural glycoprotein NS28. J Virol. 1989;63:4553–4562. doi: 10.1128/jvi.63.11.4553-4562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball J M, Tian P, Zeng C Q Y, Morris A P, Estes M K. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 4.Burke B, Desselberger U. Rotavirus pathogenicity. Virology. 1996;218:299–305. doi: 10.1006/viro.1996.0198. [DOI] [PubMed] [Google Scholar]
- 5.Chen H N, Dennehy P H, Oh W, Lee C N, Huang M L, Tsao L Y. Outbreak and control of a rotavirus infection in a nursery. J Formosan Med Assoc. 1997;96:884–889. [PubMed] [Google Scholar]
- 6.Cunliffe N A, Woods P A, Leite J P G, Das B K, Ramachandran M, Bhan M K, Hart C A, Glass R I, Gentsch J R. Sequence analysis of NSP4 gene of human rotavirus allows classification into two main genetic groups. J Med Virol. 1997;53:41–50. [PubMed] [Google Scholar]
- 7.Dong Y J, Zeng C Q Y, Ball J M, Estes M K, Morris A P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C mediated inositol 1,4,5-triphosphate production. Proc Natl Acad Sci USA. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estes M K. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1625–1655. [Google Scholar]
- 9.Greenberg H B, Clark H F, Offit P A. Rotavirus pathology and pathophysiology. Curr Top Microbiol Immunol. 1994;185:255–283. doi: 10.1007/978-3-642-78256-5_9. [DOI] [PubMed] [Google Scholar]
- 10.Horie Y, Masamune O, Nakagomi O. Three major alleles of rotavirus NSP4 proteins identified by sequence analysis. J Gen Virol. 1997;78:2341–2346. doi: 10.1099/0022-1317-78-9-2341. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino Y, Saif L J, Kang S Y, Sereno M M, Chen W K, Kapikian A Z. Identification of group A rotavirus genes associated with virulence of a porcine rotavirus and host range restriction of a human rotavirus in the gnotobiotic piglet model. Virology. 1995;209:274–280. doi: 10.1006/viro.1995.1255. [DOI] [PubMed] [Google Scholar]
- 12.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1657–1708. [Google Scholar]
- 13.Kirkwood C D, Palombo E A. Genetic characterization of the rotavirus nonstructural protein, NSP4. Virology. 1997;236:258–265. doi: 10.1006/viro.1997.8727. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetic Analysis, version 1.01. University Park, Pa: The Pennsylvania State University; 1993. [Google Scholar]
- 15.Lee C N, Kao C L, Ning H C, Fuh H L, Lee C Y. Identification of VP7 serotypes of human rotaviruses by enzyme-linked immunosorbent assay and reverse transcription-polymerase chain reaction. Acta Paediatr Taiwanica. 1997;38:454–462. [PubMed] [Google Scholar]
- 16.Nakagomi O, Nakagomi T. Rotavirus vaccines: a perspective. Microbiol Immunol. 1996;40:701–709. doi: 10.1111/j.1348-0421.1996.tb01130.x. [DOI] [PubMed] [Google Scholar]
- 17.Offit P A, Clark H F. Maternal antibody-mediated protection against gastroenteritis due to rotavirus in newborn mice is dependent on both serotype and titer of antibody. J Infect Dis. 1985;152:1152–1158. doi: 10.1093/infdis/152.6.1152. [DOI] [PubMed] [Google Scholar]
- 18.Palombo E A, Bugg H C, Masendycz P J, Coulson B S, Barnes G L, Bishop R F. Multiple-gene rotavirus reassortants responsible for an outbreak of gastroenteritis in central and northern Australia. J Gen Virol. 1996;77:1223–1227. doi: 10.1099/0022-1317-77-6-1223. [DOI] [PubMed] [Google Scholar]
- 19.Sheridan J F, Smith C C, Manak M M, Aurelian L. Prevention of rotavirus-induced diarrhea in neonatal mice born to dams immunized with empty capsids of simian rotavirus SA-11. J Infect Dis. 1984;149:434–438. doi: 10.1093/infdis/149.3.434. [DOI] [PubMed] [Google Scholar]
- 20.Snodgrass D R, Madeley C R, Wells P W, Angus K W. Human rotavirus in lambs: infection and passive protection. Infect Immun. 1977;16:268–270. doi: 10.1128/iai.16.1.268-270.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snodgrass D R, Wells P W. Passive immunity in rotaviral infections. J Am Vet Med Assoc. 1978;173:565–568. [PubMed] [Google Scholar]
- 22.Tian P, Hu Y, Schilling W P, Lindsay D A, Eiden J, Estes M K. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J Virol. 1994;68:251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian P, Estes M K, Hu Y, Ball J M, Zeng C Q Y, Schilling W P. The rotaviral nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward R L, Mason B B, Bernstenin D I, Sander D S, Smith V E, Zandle G A, Rappaport R S. Attenuation of a human rotavirus vaccine candidate did not correlate with mutations in the NSP4 protein gene. J Virol. 1997;71:6267–6270. doi: 10.1128/jvi.71.8.6267-6270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilde J, Eiden J, Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990;28:1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zao C L, Yu W N, Kao C L, Lee C Y, Lee C N. Sequence analysis of VP1 and VP7 genes suggests occurrence of a reassortant of G2 rotavirus responsible for an epidemic of gastroenteritis. J Gen Virol. 1999;80:1407–1415. doi: 10.1099/0022-1317-80-6-1407. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Zeng C Q Y, Dong Y, Ball J M, Saif L J, Morris A P, Estes M K. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virus virulence. J Virol. 1998;72:3666–3672. doi: 10.1128/jvi.72.5.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]