Abstract
Glioma, also known as glioblastoma multiforme (GBM), is the most prevalent and most lethal primary brain tumor in adults. Gliomas are highly invasive tumors with the highest death rate among all primary brain malignancies. Metastasis occurs as the tumor cells spread from the site of origin to another site in the brain. Metastasis is a multifactorial process, which depends on alterations in metabolism, genetic mutations, and the cancer microenvironment. During recent years, the scientific study of non-coding RNAs (ncRNAs) has led to new insight into the molecular mechanisms involved in glioma. Many studies have reported that ncRNAs play major roles in many biological procedures connected with the development and progression of glioma. Long ncRNAs (lncRNAs), microRNAs (miRNAs), and circular RNAs (circRNAs) are all types of ncRNAs, which are commonly dysregulated in GBM. Dysregulation of ncRNAs can facilitate the invasion and metastasis of glioma. The present review highlights some ncRNAs that have been associated with metastasis in GBM. miRNAs, circRNAs, and lncRNAs are discussed in detail with respect to their relevant signaling pathways involved in metastasis.
Keywords: glioblastoma, non-coding RNA, metastasis, microRNA, long non-coding RNA, circular RNA
Graphical abstract
These recent advances will shed more light on a thorough understanding of ncRNA function and the possible underlying molecular mechanisms involved in the regulation of metastasis. Their successful translation will depend on further interdisciplinary collaboration to improve tolerance, specificity, and delivery.
Introduction
Glioma is one of the most common primary tumors in the central nervous system (CNS) (∼30%) and is the most aggressive and lethal. Glioma is further histologically divided into five distinct subtypes: oligodendroglioma; astrocytoma; medulloblastoma; ependymoma; and glioblastoma multiforme (GBM).1,2 Despite recent development in treatment options for glioma, including various chemotherapy regimens, advanced radiotherapy, and surgical resection techniques, patients with glioma often have a poor prognosis.3 Accurate classification of the tumor and categorization of patients require a comprehensive understanding of the tumor properties and biology. During the last several decades, many studies have been carried out on gliomas to discover novel molecular biomarkers through the identification of abnormal gene expression, epigenetic alterations, and genetic mutations.4
GBM or grade IV glioma is the most common type of brain cancer. GBM is one of the deadliest malignant tumors, with a very short life expectancy. The 5-year survival of patients with GBM is only about 5%. Up to 60 Gy of radiotherapy delivered over 3 weeks accompanied by daily administration of temozolomide (TMZ), followed by an additional six cycles of adjuvant TMZ, is the standard therapy for patients with a sufficient performance status.5 Recent clinical trials have demonstrated improved survival with the administration of electric field therapy, called “tumor-treating fields.” The survival of glioblastoma patients could be prolonged by approximately 5 months by the addition of electric field therapy to the standard therapy.6
Abnormal regulation of proliferation and inhibition of apoptosis are vital processes in tumorigenesis that ultimately lead to the development of tumors. Metastasis is accepted to be one of the hallmark features of cancer.7 A major obstacle for the treatment of metastatic tumors is the biologic heterogeneity of the tumor cells. This biologic heterogeneity also contributes to the late diagnosis of many cancers. Oncogenes are tumor-promoter genes, which often control cellular proliferation and apoptosis. Scientists have recently focused on discovering specific biomarkers that can be related to genes that encode protein production in cancer.8 The human genome can be divided into two broad categories of sequences: (1) a minor group of sequences that are protein-coding genes, accounting for perhaps 20,000 genes or 2% of the entire genome and (2) a major group of sequences that encode non-coding RNA molecules, which are not translated into proteins. Non-coding RNAs (ncRNAs) regulate the translation of other RNAs and control the production of functional proteins from protein-coding transcripts.9 Many studies have shown the diagnostic or prognostic potential of ncRNAs.10, 11, 12, 13
Long ncRNAs (lncRNAs) are about 200 nt in length and are mostly polyadenylated transcripts. These transcripts are usually translated by RNA polymerase II and can be regulated by many different transcription factors. Among all the various ncRNAs, lncRNAs account for the largest fraction (>80%) and have greater variability compared with microRNAs (miRNAs).14 lncRNAs have been found to play a crucial role in the conversion of mRNAs into small interfering RNAs. miRNAs have been recently shown to control lncRNAs, and conversely, lncRNAs are able to regulate the expression of miRNAs, thus forming a two-way regulatory network with major roles in many cellular pathophysiological processes.15 This cross-talk between miRNAs and lncRNAs means that miRNA can induce destruction of lncRNAs, while lncRNAs can alter the levels of miRNAs and regulate their function, such as through sponging the miRNAs.16 Moreover, lncRNAs can compete with miRNAs for binding to mRNAs, while miRNAs can be produced from lncRNAs.17 This cross-regulatory interaction between miRNAs and lncRNAs and its abnormal expression have potential diagnostic and therapeutic implications in many cancers, including glioma.18, 19, 20 ncRNAs, such as lncRNAs and miRNAs, may be used as prognostic and predictive biomarkers in glioma patients, as their effects on gene expression can affect glioma progression and metastasis.
Circular RNAs (circRNAs) are a type of ncRNA that has only recently been discovered and are highly conserved across different species. circRNA expression patterns are tissue specific and tumor-stage dependent.21, 22, 23 circRNAs may have different functions, including sponging miRNAs, regulating gene transcription, and binding to proteins, and some circRNAs can be translated to proteins. These encoded peptides may serve as a new class of drug targets.24,25 circRNAs are frequently found in neural tissue and are expressed differentially in different parts of the brain. This finding may be due to the fact that there are a variety of different protein-coding genes, which produce different circRNAs, splicing factors, and RNA-binding proteins (RBPs), which regulate the formation of circRNAs.26
Piwi-interacting RNAs (piRNAs) are another type of ncRNA that are 26–30 nt in length and bind to Piwi proteins. These short RNAs were originally discovered in germline cells and are considered to be key regulators for germline maintenance.27 A growing body of evidence has now extended our knowledge of the biological significance of piRNAs, because they can also regulate gene expression in somatic cells through transposon silencing, epigenetic programming, DNA rearrangement, mRNA turnover, and translational control. Accumulating evidence has revealed that the dysregulation of piRNAs may cause epigenetic changes and contribute to many diseases.28
Herein, we highlight the role of some ncRNAs that have been associated with metastasis in GBM. miRNAs, circRNAs, and lncRNAs will be discussed in detail. Due to the high importance of RNAs in gliomas, many studies have been conducted on ncRNAs; however, in the present study, the effects of ncRNAs on metastasis-related genes and pathways are discussed, which distinguishes this review from others.
Metastasis and glioma
Glioma stem cells (GSCs) with a low proliferation rate are found in hypoxic areas of GBM tumors, such as periarteriolar pits.29,30 GSCs express angiomotin, thrombospondin, and ephrin (EphA5), which inhibit the formation of new blood vessels.31 GSC niches and normal hematopoietic stem cell (HSC) niches are similar in that both are located in periarteriolar regions, are hypoxic, and consist of similar functional proteins that attract biomolecules and receptors that bind to the stem cells.32 It has been shown that the mesenchymal stem cells (MSCs) present in GSC niches promote a proliferative and regenerative environment, which can stimulate GSCs in vitro, possibly through the interleukin-6 (IL-6)/STAT3 signaling pathway, with the resultant induction of glioma-associated human MSCs (GA-hMSCs) in vivo.33 CD105-positive MSCs are located in the periarteriolar space and express abundant amounts of SDF-1α and OPN. These two molecules are chemoattractant receptors that attract CD44+ and CXCR4+ GSCs to the periarteriolar niche; therefore, GSCs are protected from chemotherapy and remain in an inactive state.
GSCs in the quiescent state require the production of angiogenic factors to switch into a more proliferative and metastatic phenotype. This switch to a more aggressive and proliferative state has been demonstrated in many studies, particularly in patients with recurrent GBM,34 possibly through the proneural-mesenchymal transition (PMT). This shift was reported to be induced by microenvironmental cues, including those arising from stromal cells.34,35 Irradiation can also cause the PMT, which is followed by increased expression of CD44 and YKL-40 (chitinase-3-like protein 1), as well as nuclear factor κB (NF-κB), STAT3, and CEBPB, which are master transcription factors in PMT.34,36, 37, 38 This shift was reported to be associated with enhanced activity of the YAP/TAZ signaling axis and increased colony formation and resistance in GSCs.34 This assumption was in agreement with a study by Elena et al. indicating that the aggressive mesenchymal phenotype in GBM was associated with over-expression of the YKL-40 marker, which led to extracranial metastasis. In their study, extracranial metastasis appeared at a median 8.5 months after GBM was initially diagnosed, with an estimated survival time of 12 months.39 When Sullivan et al. compared primary GBM cells to GBM circulating tumor cells (CTCs) isolated from both patients and mouse-patient-derived xenograft (PDX) models, they found that MES markers were more abundant than N/PN markers in the metastatic cells (CTCs).40
The use of RNA in situ hybridization in primary GBM tumor cells revealed a subgroup of mesenchymal cells with high migratory properties. Metastatic lesions predominantly showed a mesenchymal phenotype, along with extra mutations in a small group of patients with systemic spread of GBM. It seems that the niche of the metastatic sites governs the progression of metastasis. After this initial spread, an angiogenic transition would be able to activate the quiescent cells,31,41 leading to their removal from the niche.30,42 This leads to more rapid proliferation and colonization of the GSCs in distant organs. In order to achieve an extracranial metastasis, the degradome (expression profile of proteases) of the metastatic GSCs has to adapt itself to the new microenvironment in distant organs.
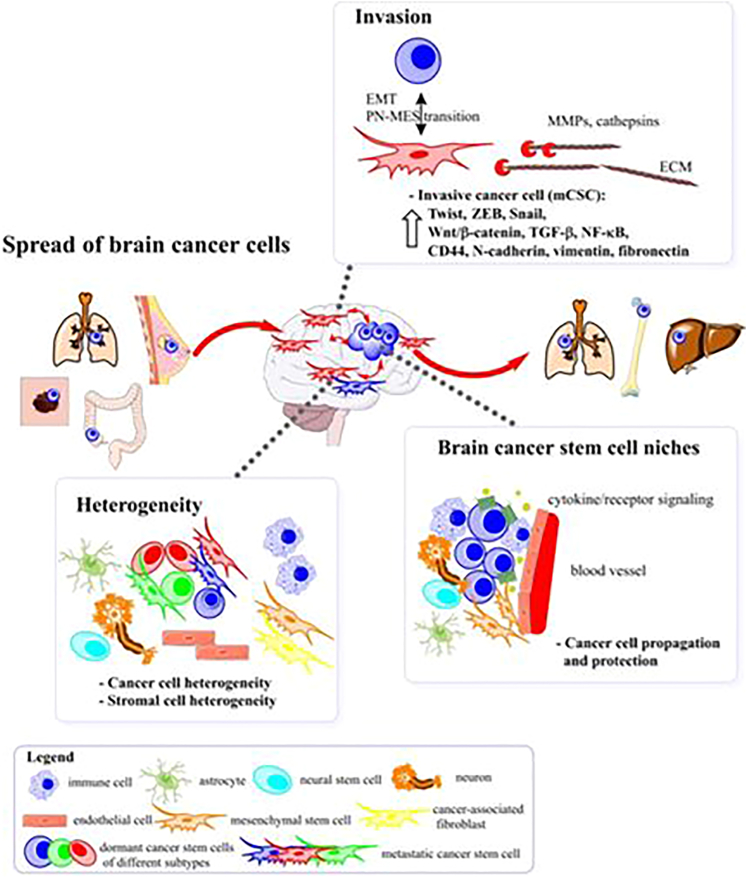
It can be concluded that, although still not completely identified, the metastatic niche may be considered as a novel therapeutic target in GBM patients with distant metastasis (Figure 1). Zhao et al. described some examples of niche-targeting therapeutic methods against GSCs that have been tested using complex in vivo models and organoid cultures and might be applicable in the clinical setting.43 This approach could be used as an adjuvant therapy in combination with radiotherapy or surgical resection and could inhibit the development of recurrence and metastasis.
Figure 1.
Possible routes of spread of malignant cells in GBM
The microenvironment of brain cancer involves various subtypes of tumor cells in addition to different subtypes of stromal cells, which are physiologically located in the brain (neurons, astrocytes, and microglia) or invade the brain during the progression of the tumor (fibroblasts, mesenchymal stem cells, lymphocytes, and macrophages). Dormant cancer stem cells (dCSCs) within a number of tumors (colorectal, lung, breast, and melanoma) could initially transform into metastatic cancer stem cells (mCSCs) to develop a niche by colonizing the brain tissue. On the other hand, dormant glioblastoma stem cells (dGSCs) are present in their conserved niche in the brain; however, exogenous stimuli, such as chemotherapy, radiotherapy, hypoxia, or alterations in the endogenous microenvironment, may enhance the proliferation and invasion of these malignant cells. The PMT with the resultant transformation of dGSCs into mGSCs is triggered by cytokine production and signaling.
Extracranial metastasis is rare, occurring in up to 0.5% of patients with GBM. Although GBM tumors are locally invasive, they rarely spread beyond the brain.44 However, the rapidly progressive and destructive nature of the tumor in the brain results in an unfavorable prognosis and earlier death compared with systemic metastasis of other cancers. Extracranial metastasis is usually not detected at autopsies, as the underlying cause of death is already known.44 Therefore, two important questions arise: the first is whether spontaneous metastasis in GBM patients is actually as rare as supposed, and the second is, if so, which of the phases of metastasis prevents the development of spread to other organs? Metastasis may occur in GBM patients undergoing craniotomy and surgical resection as GBM and/or GSC cells may be released into blood vessels during surgery. This notion was initially suggested by the presence of GBM metastases in skin scars and soft tissue in proximity to the original site of the craniotomy,44 as well as presence of extracranial metastases located within ventriculo-peritoneal shunts.45,46 However, the spreading of GBM cells beyond the brain has not been clearly established by laboratory evidence.
Possible barriers to metastasis in GBM patients are (1) presence of the blood-brain barrier (BBB), providing protection; (2) lack of lymphatic vessels located in the CNS preventing lymphatic metastasis; (3) inhibition of growth of GBM cells outside the brain caused by the immune system; and (4) possible inability of GBM cells to invade or destroy the extracellular matrix (ECM) in organs other than the brain.
Current experimental studies suggest that the BBB is unable to effectively prevent penetration of cells through it to reach the extracranial space.47 Moreover, intercellular interactions between differentiated MSCs (pericytes), cancer associated fibroblasts (CAFs), and even tumor cells themselves may disrupt the blood vessels in GBM tissue.47 In the CNS of mice and humans, functioning lymphatic vessels were found to be present, which are covered by typical endothelial cells in dural venous sinuses with subsequent involvement of deep cervical lymph nodes.48 The idea that lymph node metastasis may occur in GBM patients prior to any surgical resection has been confirmed by previous studies.49 Lymphatic metastases account for approximately 50% of GBM metastasis, followed by pleural/lung (50%), bone (31%), liver (12%), and skin, respectively.44 In addition to lymphatic vessels, GBM and GSC metastasis can occur by invasion into the cerebrospinal fluid (CSF). CSF acts physiologically as a “cushion” for the CNS as it maintains the ionic balance of the extracellular space and contains proteins with specific functions, including insulin-like growth factor 1 (IGF1) and 2 (IGF2) and Sonic hedgehog, which contribute to maintenance of neural stem cell properties in their niches located in the subventricular space.50 These factors are also up-regulated in GSCs in some GBM patients.51 They can also condition metastatic GSCs (mGSCs), causing neural stem cell properties to be preserved in their subventricular zone niche.50 Hematogenous metastasis occurs when GSCs reach the systemic blood circulation via the CSF and lymphatic vessels. In a study by Müller et al., CTCs were found in the systemic blood circulation in approximately 21% of patients with GBM, as shown by a specific neural biomarker glial fibrillary acidic protein (GFAP), in conjunction with mutations or over-expression of the EGFR gene. This suggests that EGFR gene mutation is important for GBM hematogenous metastasis.52 Levels of CTCs were generally not elevated after surgical resection. Metastases that do not lead to any clinical symptoms are often ignored, despite the unfavorable overall survival of GBM patients. However, evaluation of CTCs may be beneficial in GBM patients with a long life expectancy (see sections below).
GSCs are able to protect themselves from the immune system within the systemic blood circulation,53 because they can stimulate the production of myeloid-derived suppressor cells (MDSCs), contributing to the ability of GSCs to evade natural killer (NK) cells.54,55 Apparently, GSCs lack Toll-like receptors on their surface, which contributes to avoidance from attack by the immune system.56 In patients with a normal immune system, the majority of circulating tumor cells are identified and killed by NK cells.57 However, immuno-deficient patients, including those undergoing chemotherapy or radiotherapy, have a higher likelihood to suffer disseminated metastases. This suggests a correlation between the competence of the immune response and metastasis risk in GBM patients.
There are several studies that have examined survival and metastasis in GBM patients, with different numbers of patients and ages at the time of diagnosis. Lun et al. carried out a study on 88 patients and found that the median overall survival of GBM patients was only approximately 10 months after diagnosis and about 8.5 months from the diagnosis of metastasis.58 Among different metastatic sites examined, metastasis to the lung was associated with the worst prognosis. Intensive therapy, including the application of shunts for the CSF, increased the overall survival of patients with metastases. This finding questioned the assumption that treatment of GBM patients may enhance the chance of metastasis and result in a worse prognosis. A meta-analysis by Pietschmann et al. on 150 patients found a median survival of about 6 months from detection of metastasis.59 A cohort study compared 84 patients with GBM metastasis with patients without metastasis and found that younger patients with GBM metastasis had a longer overall survival, presumably due to the presence of an effective immune response. Notably, resection of the tumor increased the time interval to occurrence of metastasis outside the brain compared with biopsy alone. Surgical excision of the tumor combined with chemotherapy or radiotherapy may be associated with an even longer interval before occurrence of metastasis and better overall survival. This notion was corroborated by a meta-analysis on 115 younger GBM patients, which found that the time interval between detection of metastasis and death was longer when patients had surgical excision of the mass or received chemotherapy or radiotherapy.60 In another study, metastasis to the liver was associated with the worst outcome and shortest overall survival. However, treatment with bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, may paradoxically enhance progression of the disease through hypoxia induction, which may be associated with earlier metastasis.61
In conclusion, these clinical studies suggest that GBM cells may spread beyond the brain via CSF and lymphatic vessels, finally reaching the bloodstream or other lymph nodes. Notably, hematogenous metastases do not appear to be encouraged by surgical excision of the tumor and may even be associated with a better prognosis because they stimulate the immune response.
Non-coding RNAs and glioma
The uncontrolled proliferation, invasion, and migration of cancer cells are considered to be the hallmark features of tumors. The survival of cancer patients is affected by the migration of the malignant cells and their ability to invade into other organs.62 Specific interactions between tumor cells and other normal cells, as well as the ECM, in combination with other physiological processes results in the active movement of cells.63 The epithelial-mesenchymal transition (EMT) is initiated through alteration of transcription factors (Snail, ZEB, and Twist) and secretion of various growth factors, including fibroblast growth factor (FGF)-2, transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), and VEGF. Tumor cells also produce different types of proteases that facilitate the invasion of malignant cells into healthy brain tissue by degrading the ECM.64 Furthermore, glioblastomas are associated with disturbances in apoptosis, including increased levels of anti-apoptotic proteins, such as phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and the anti-apoptotic family protein B-cell lymphoma 2 (Bcl-2).65 The regulatory mechanisms that control the cell cycle via retinoblastoma (RB) and p53 proteins are not active, leading to uncontrolled proliferation and tumor progression.66 Loss of cellular tissue integrity, dysregulated cell death, and uncontrolled proliferation are all hallmarks of cancer development. ncRNAs play a crucial role in regulating these cellular characteristics in cancer patients.67
There is an expanding number of lncRNAs that have been found to be highly expressed in GBM patients, which highlights their important role in proliferation and development of cancer (Figure 2). miRNAs have been studied for a long time, and a substantial amount of data about their target genes and signaling pathways now exists. There still remain some missing gaps about how miRNAs interact with lncRNAs, even after lncRNAs were discovered. An association between miRNAs and lncRNAs has not been found in all cases. The current knowledge about the interaction of miRNAs and their target genes with lncRNAs and signaling pathways with relevance to glioma is summarized in Figure 3. lncRNAs, miRNAs, and their target genes particularly affect the signaling pathways, Wnt/β catenin, Notch, and PI3K/Akt/mTOR. A number of lncRNAs and miRNAs have the ability to control multiple different signaling pathways. Although these ncRNAs have a complex network, they may act as novel predictive or prognostic biomarkers in patients with cancer (Figure 3).
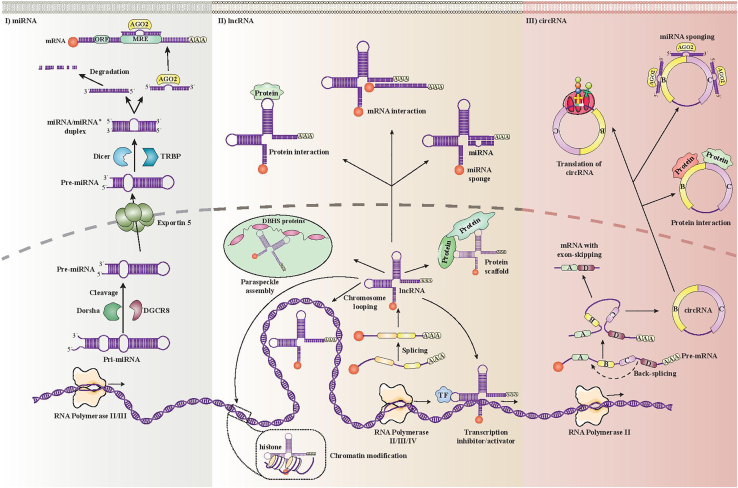
Figure 2.
Biogenesis and function of ncRNAs
miRNAs, lncRNAs, and circRNAs. (I) RNA polymerase II/III transcribes miRNAs initially as primary miRNAs (pri-miRNAs). pri-miRNAs are composed of a stem-loop structure. DiGeorge syndrome critical region 8 (DGCR8) and Drosha cut the stem of pri-miRNA in the nucleoplasm, which results in production of precursor miRNA (premiRNA). Exportin-5 contributes to the removal of pre-miRNAs from the nucleus. The pre-miRNA is further processed in the cytoplasm through the activity of transactivation response element RNA-binding protein (TRBP) and Dicer. A duplex composed of two miRNAs (miRNA/miRNA∗) is created after pre-miRNA loop is cleaved. The strand attaches to Argonaute 2 (AGO2), which is the major protein of the RNA-induced silencing complex (RISC). miRNAs are included in the RISC. They can regulate gene expression in a posttranscriptional manner through formation of miRNA response elements (MREs) by binding to specific sites located on the 3′ untranslated region (3′ UTR) of mRNA. (II) lncRNAs have a similar biogenesis to mRNAs, in that a number of lncRNA transcripts are polyadenylated at 3′ and 5′ ends, coated, and spliced. (III) circRNAs can be transcribed from protein-coding genes that contain introns or exons. “Back-splicing” can be triggered by various mechanisms, including dimerization of proteins, pairing of introns, or lariat intron formation.
Nevertheless, other types of ncRNAs that have been less well investigated may function as biomarkers in glioma patients. These ncRNAs include circRNAs, small nucleolar RNAs (snoRNAs), small Cajal body-specific RNAs (scaRNAs), and piRNAs. snoRNAs have many biological functions, such as structural alteration of other RNAs, miRNA precursors, assembly, and activation of telomerase enzymes.68 A genome-wide analysis study of small ncRNAs in pediatric glioma patients found that 118 separate members were over-expressed (72 CD-Box, 26 HACA-Box, 3 scaRNAs, and 17 snoRNAs) and 39 separate members were under-expressed (one snoRNA and 38 CD-Box).69 The snoRNA SNORD was found to control the EMT and to affect the response to therapy with temozolomide.70 SNORD76 (C/D box snoRNA U76) was inversely associated with HOTAIR expression and could affect the proliferation of cells in vivo and in vitro. The up-regulated SNORD76 level resulted in a decreased expression of p107, cyclin A1, and cyclin B1 genes and an increased expression of Rb gene.71 High expression of snoRNA SNHG18 may lead to radiotherapy resistance of glioma cells by inhibiting Semaphorin 5A.72 There is no evidence yet regarding the expression pattern of SNHG18 in glioma tissue. The snoRNA SNHG1 was found to be up-regulated in glioma cells and was associated with enhanced proliferation and decreased apoptosis of tumor cells. However, the precise cellular mechanisms and biological targets remain to be elucidated.73 scaRNAs play biological roles in glioma; however, their role remains poorly understood. In addition, scaRNAs may play an important role in the pathogenesis of other malignancies.74,75 circRNAs are a type of ncRNA that may function as specific sponges of miRNAs.76,77 A group of circRNAs has been recently shown to have a crucial role in the development of glioma. circ-TTBK2 is over-expressed in glioma patients and in vitro in U87 and U251 cell lines. The under-expression of circ-TTBK2 along with up-regulation of miR-217 resulted in inhibition of cancer growth in vivo.78 Moreover, circFBXW7 was under-expressed in glioma tissue in comparison to normal adjacent brain tissue. In addition, up-regulated levels of circFBXW7 correlated with a more favorable overall survival in glioma patients.79 circZNF292 is another ncRNA that promotes the formation of new blood vessels. Moreover, this circRNA can stimulate cell cycle progression and proliferation acting via the Wnt/β-catenin signaling pathway and was up-regulated in U251 and U87MG cell lines.80 Unfortunately, the expression level of circZNF292 in glioma patients has not been clearly identified.81 piRNAs are a group of small ncRNAs that is produced from RNA precursors different from the precursors of miRNAs.82 piRNAs contribute to regulation of gene expression through inducing alterations at the epigenetic level,83 leading to destruction of mRNAs.84 PIWI protein and piRNAs form a complex that controls differentiation, proliferation, and apoptosis of cells.85, 86, 87 Furthermore, piRNAs can regulate other biological processes through interaction with siRNAs and miRNAs.88,89 The PIWIL1/MEG3/miR-330/RUNX3 signaling axis controls the blood-tumor barrier, demonstrating the significance of ncRNAs in cellular processes.90
Biogenesis of non-coding RNAs
miRNAs are the group of ncRNAs that have been most well studied. The initial phase of miRNA formation involves a structure composed of two stem loops (known as pri-miRNA). This two-stem-loop pri-mRNA is cleaved into a single-stem-loop structure (pre-miRNA) by the activity of the complex between DGCR8 and Drosha. This pre-miRNA is further cleaved by Dicer to a double-stranded structure (miRNA). This miRNA is added to a member of the protein family called Argonaute, which leads to formation of the miRNA-induced silencing complex (miRISC) (Figure 2). In addition to the miRISC, miRNAs are able to modulate posttranscriptional expression of genes via a mechanism mediated by another Argonaute family protein, which results in fragmentation and suppression of mRNA sequences (Figure 2).91
RNA polymerase II enzyme contributes to the production of lncRNAs through transcription of genes located in exonic, intergenic, or distal protein-producing sequences of the human genome. This pre-mature lncRNA becomes polyadenylated at the 3′ end and attached to methyl-guanosine at the 5′ end.92 This structure is often subjected to alternative splicing to form different proteins.93 Alternative splicing can be categorized into three pathways. Initially, lncRNAs undergo interactions with different splicing factors, which leads to the production of RNA-RNA complexes along with molecules of pre-mRNA. They can finally regulate the remodeling of chromatin; thereby, mixing of the target genes is achieved.94
Traditionally, the functions of lncRNAs have been classified either as cis (when the target gene is located adjacent to the lncRNA sequence) or trans (when the target gene is located far away from the lncRNA sequence). cis lncRNAs typically stimulate or suppress transcription of adjacent genes by mechanisms such as triggering various epigenetic alterations in chromatin, modifying the DNA structure (looping of chromosomes), and interacting with different transcription factors. The trans function of lncRNAs mainly takes place in the nucleus, such as assembling the paraspeckles or interacting with different proteins in the nucleus. Various lncRNAs are able to leave the nucleus and sequester miRNAs within the cytoplasm, which leads to miRNA inhibition. Moreover, they can increase or decrease the half-life of proteins in the cytoplasm by interacting with them or cause stimulation or inhibition of mRNAs.
circRNAs are derived from pre-mRNA, and they are produced by the activity of group I or II ribozyme catalysts or splicing of spliceosomes.95 Spliceosomes may contribute to circRNA biogenesis, because both circRNAs and linear transcripts were decreased after suppression of canonical spliceosomes.96 circRNAs can arise from intronic, 3′ UTRs, 5′ UTRs, and also intergenic regions; however, they are predominantly transcribed from exons that encode proteins. Canonical and non-canonical cleavage processes contribute to the formation of circRNAs. Different from the orthodox splicing of cognate linear mRNAs, circRNAs can originate from a single gene locus through selection of alternative back-splicing sites, which are available in the CIRCpedia database.97
Up to now, circRNAs have been classified into three different types: intronic RNAs (ciRNAs); exonic circRNAs (ecircRNAs);98 and exon-intron circRNAs (ElciRNAs). One study suggested that ecircRNAs comprise the majority of circRNAs in plants and animals, and about 83% of them share sequences with genes encoding proteins.99 A number of ecircRNAs interact with RBPs and/or miRNAs. In addition, many ecircRNAs encircle another exon containing a canonical translation start codon.100
MicroRNAs and metastasis in glioma
miR-376a-3p has been identified as an important miRNA involved in several malignancies.101,102 A high concentration of miR-376a-3p in the nucleus or cytoplasm was associated with increased tumor aggressiveness and could be used as a prognostic marker for glioma patients.101,102 According to bioinformatics, KLF15 is the downstream gene that binds to miR-376a-3p. Krüppel-like transcription factors (KLFs) are a family of transcription factors with a structure containing a C2H2 zinc finger domain. They are widely distributed in eukaryotes, comprising 9 unique proteins forming 18 KLF members.103,104 The hematopoietic, respiratory, and immune systems are all controlled by KLF15. Furthermore, KLF15 can influence tumor development by modulating the expression of downstream genes.105
Chen et al. identified the role of miR-376a-3p in modulating the invasiveness and migration of glioma cells and suggested potential mechanisms.106 Levels of miR-376a-3p were measured in glioma samples from 39 patients. The clinical histories of the patients were collected, and the relationship between the clinical presentation and the levels of miR-376a-3p was investigated. Then, the effects of miR-376a-3p on regulating the metastatic and proliferative properties of T98-G and U251 cell lines were investigated. Bioinformatics analysis was used to detect genes that could interact with miR-376a-3p. KLF15 was reported to be involved in the growth of glioma, and this was controlled by miR-376a-3p. miR-376a-3p was found to be down-regulated in glioma cells, and low levels were associated with higher metastatic capacity and a poor survival in glioma patients. In addition, higher levels of miR-376a-3p inhibited metastasis and proliferation in glioma tissue. KLF15 is a gene that can bind to miR-376a-3p and was inversely correlated with miR-376a-3p. KLF15 was up-regulated in glioma patients. In vitro, exogenous KLF15 had the ability to block miR-376a-3p effects on regulating glioma cell properties. Moreover, miR-376a-3p was associated with hematogenous metastasis via lymph nodes and distant metastasis, which was inversely correlated with KLF15.106
miR-623 expression has been shown to be down-regulated in many malignancies, including lung and stomach cancer. This miRNA has been shown to act as a tumor-suppressor gene.107,108 Earlier studies showed that mimics of miR-623 could reduce proliferation, migration, invasion, and colony formation of glioma cells. The volume of the tumor in an intracranial xenograft model in mice was significantly reduced when a treatment was applied that could up-regulate miR-940, suggesting that miR-940 may be proposed as glioma therapy.109,110 In addition, there is an ever-expanding list of miRNAs that have been associated with metastasis in GBM patients as summarized in Table 1.
Table 1.
Metastasis-related microRNAs in GBM
| miRNA | Expression status (up/down) | Target | Model (in vitro, in vivo, human) | Cell line | Ref |
|---|---|---|---|---|---|
| miR-376a-3p | down | KLF15 | in vitro, human | U251, T98G | Chen et al.106 |
| miR-4530 | down | RTEL1 | in vitro, human, | U251, T98G | Wang et al.111 |
| miR-623 | up | TRIM44 | in vitro, in vivo | LN229, U251MG | Cui et al.112 |
| miR-450a-5p | down | EGFR | in vitro | A172, SHG-44 | Liu et al.113 |
| miR-32 | down | EZH2 | in vitro, human | U87, U251, A172, U118 | Peng et al.114 |
| miR-767-5p | down | SUZ12 | in vitro, human | T98, A172, U87, LN229, U251 U118 | Zhang et al.115 |
| miRNA-320c | down | cyclin D1, CDK6, MMP2, MMP9, N-cadherin, integrin β1 | human | Lv et al.116 | |
| miR-3653 | down | human | Chen et al.117 | ||
| miR-93-5p | down | MMP2 | in vitro, human | U87-MG | Wu et al.118 |
| miR-375 | down | RWDD3 | in vitro, human | U251, U87 | Ji et al.119 |
| miR-140 | down | ADAM9 | in vitro, human | U87, U251, U373, U118, A172, LN18 | Liu et al.120 |
| miR-187 | down | SMAD1 | in vitro, human | U87, U251 | Gulinaer et al.121 |
| miR-141 | down | TGF-β2 | in vitro, human | U251, U87, U118, LN18 | Peng et al.122 |
| miR-200b | down | ZEB2 | in vitro, human | U251, U87 | Li et al.123 |
| miR-138 | down | IGF2BP2 | in vitro, human | Res186, Res259 | Yang et al.124 |
| miR-22 | down | SNAIL1 | in vitro | U-87, U-118 MG, M059K, Hs 683 | Zhou et al.125 |
| miR-508-5p | down | in vitro, human | Liu et al.126 | ||
| miR-132 | down | TTK | in vitro, human | U-87 | Thunshelle et al.127 |
| miR-101-3p | down | TRIM44 | in vitro | U87MG, U251MG, U118MG, T98 | Li et al.128 |
| miR-378 | down | IRG1 | in vitro, human | SHG44, A172, LN229, LN18 | Shi et al.129 |
| miR-758-5p | down | ZBTB20 | in vitro, human | U118, LN-299, H4, A172, U87-MG, U251 | Liu et al.130 |
| miR-27b | up | Spry2 | in vitro, human | U87, U251, SHG44 | Liu et al.131 |
| miR-424 | down | KIF23 | in vitro, human | A172, SHG-44, T98, LN18, LN229 | Zhao et al.132 |
| miR-154-5p | down | PIWIL1 | in vitro, human | U251, U87, A172, LN229, SNB19, LN308 | Wang et al.133 |
| miRNA-132 | down | MMP16 | in vitro, human | A172, SHG44, U87 | Wang et al.134 |
| miR-200b | down | CREB1 | in vitro, human | U87, SF126, U251, SF767 | Peng et al.135 |
| miR-374b | down | EGFR | in vitro, human | U251, U87 | Pan et al.136 |
| miR-622 | down | ATF2 | in vitro, human | U87, U251, A172, U118, LN229 | Zhang et al.137 |
| miR-29a | up | PTEN | in vitro, human | U87, U251, LN229 | Zhao et al.138 |
| miR-422a | down | IGF1, IGF1R | in vitro, human | U87, U251 | Wang et al.139 |
| miR-139-3p | down | NOB1 | in vitro, human | U251, U87MG, TJ905, SHG44 | Shi et al.140 |
| miR-491 | down | Wnt/β-catenin | in vitro, human | s LN18, LN229 | Meng et al.141 |
| miR-1290 | up | LHX6 | in vitro, in vivo, human | LN-229, U87 | Yan et al.142 |
| miR-7 | down | EGFR | in vitro, in vivo, human | U-87MG, U-118MG | Wang et al.143 |
| miR-351 | down | NAIF1 | in vitro | U87, U251 | Wu et al.144 |
| miR-150-3p | down | SP1 | in vitro, human | U251, U87MG, A172, SWO-38, SHG44 | Tan et al.145 |
| miR-133b | down | Sirt1 | in vitro, human | U87 | Li et al.146 |
| miR-30b-3p | up | RECK | in vitro, human | SHG44, U251, U87 A172 | Jian et al.147 |
| miR-144 | down | FGF7, CAV2 | in vitro, human | U251, LN229, LN18 | Liu et al.148 |
| miR-30a | down | Wnt5a | in vitro, human | T98G, SHG44, U251, U87, U373 | Zhang et al.149 |
| miR-221/222 | up | TIMP2 | in vitro, human | U87, U251, SHG-44, BT325, A172 | Yang et al.150 |
| hsa-mir-127 | up | REPIN1 | in vitro | U87, LN-229 | Wang and Lin151 |
| miR-202 | down | MTDH | in vitro, human | A172, U87, U251, U373, LN229 | Yang et al.152 |
| miR-219 | down | SALL4 | in vitro, human | A172, U87, U251, U373 | Jiang et al.153 |
| miR-204-5p | down | RAB22A | in vitro, human | LN-229, U87 | Xia et al.154 |
| miR-188 | down | IGF2BP2 | in vitro, human | U87, U251, U118, LN229, LN18 | Ding et al.155 |
| miR-200c | down | MSN | in vitro, in vivo, human | H4, U251 | Qin et al.156 |
| miR-320 | down | E2F1 | in vitro, human | U251, SHG-44 | Sun et al.157 |
| miR-139-3p | down | MDA9/syntenin | in vitro, human | U87MG, U251MG, U118, A172 | Tian et al.158 |
| miR-637 | down | Akt1 | in vitro, human | U251, U87 | Que et al.159 |
| miR-376a | down | SP1 | in vitro, human | U138, U251, LN229, T98 | Li et al.160 |
| miR-590-3p | down | ZEB1, ZEB2 | in vitro, human | U87MG, A172 | Pang et al.161 |
| miR-16 | down | SALL4 | in vitro, human | U251, U87 | Han et al.162 |
| miR-98 | down | RKIP | in vitro, human | U251, U87, SHG44 | Chen et al.163 |
| miR-370 | down | β-catenin | In vitro, human | U251, U87 | Lu et al.164 |
| miR-139-5p | down | ZEB1, ZEB2 | in vitro, human | U87, A172 | Yue et al.165 |
| miR-217 | up | YWHAG | in vitro, in vivo, human | U87 MG, U118 MG, U251, U87 | Wang et al.166 |
| miR-548b | down | MTA2 | in vitro, in vivo, human | U87, T98G, U373, LN229, SNB19, U251 | Pan et al.167 |
| miR-663 | down | TGF-β1 | in vitro, human | A172, U87 | Zhang et al.168 |
| miR-489 | down | SPIN1 | in vitro, in vivo, human | U87, T98, U251 | Li et al.169 |
| miR-10b | up | TGF-β1 | in vitro, in vivo, human | U87, U251 | Ma et al.170 |
| miR-20a | up | TIMP-2 | in vitro, in vivo, human | U87 | Wang et al.171 |
| miR-106a | up | TIMP-2 | in vitro, in vivo, human | U87 | Wang et al.171 |
| miR-146b | up | MMP16 | in vitro | U87, U373, U138, U118, SW1783, SW1088 | Xia et al.172 |
| miR-203 | down | GAS41/miR10b | in vitro | U87, HNGC2 | Pal et al.173 |
| miR-204 | down | ezrin | in vitro, human | U87, U118, U138, U87, SW1088, SW1783, CCF-STTG1 | Mao et al.174 |
| miR-873 | down | IGF2BP1 | in vitro, human | A172, T98G, U87, U373, U251, U138 | Wang et al.175 |
| miR-144-3p | down | FZD7 | in vitro, human | Cheng et al.176 | |
| miR-124 | down | PPP1R3L | in vitro, human | U251, U373 | Zhao et al.177 |
| miR-351 | down | NAIF1 | in vitro | U87, U251 | Wu et al.178 |
| hsa-miR-9 | down | MAPKAP | in vitro, human | T98G, U251, SF295 | Ben-Hamo et al.179 |
miR-382 inhibits the development and metastasis of GBM and could be a novel therapeutic target to increase efficacy of GBM therapy.180 Tripartite pattern containing 44 (TRIM44) is a member of the TRIM family of proteins and is involved in various disorders, including viral infections, developmental abnormalities, and neurodegenerative diseases.181, 182, 183 The presence of an E3 site in their structure allows E3 ubiquitin ligase activity, which can be regulated after translation. Earlier studies found that TRIM44 over-expression was present in many cancers, which stimulated development, proliferation, and progression of the cell cycle. Furthermore, over-expression of TRIM44 could promote invasion and migration of cancer cells and increase the metastatic potential of the tumor.184, 185, 186 In conclusion, inhibiting the expression of TRIM44 could be useful in the management of tumor metastasis and also inhibit tumor growth.
In one study, RT-PCR was used to measure miR-623 expression levels in GBM tissue. miR-623 up-regulation and its effects on proliferation, invasion, and migration of malignant cells was evaluated using transwell, colony formation, and MTS assays.112 Moreover, a subcutaneous mouse xenograft model was used to evaluate the effects in vivo. A dual-luciferase reporter assay was used to confirm miR-623-TRIM44 binding and western blotting to assess the effect of miR-623 on markers of the EMT. miR-623 was down-regulated in cell lines and samples from GBM patients. Over-expression of miR-623 or suppression of TRIM44 inhibited GBM cell proliferation, invasion, and migration. On the other hand, inhibition of miR-623 increased TRIM44 expression, the EMT, and the consequent progression of GBM. Expression of TRIM44 was suppressed by direct binding of miR-623 to the 3′ UTR region. Furthermore, in nude mice with a GBM xenograft, systemic administration of a miR-623 mimic inhibited tumor development and suppressed expression of TRIM44 protein. They also verified that high expression of miR-623 or low expression of TRIM44 inhibited the proliferation and migration of the glioma cell lines U251MG and LN229. They concluded that miR-623 could decrease EMT triggered by TRIM44 by direct targeting of the TRIM44 3′ UTR and could be a new therapeutic target for GBM treatment.112
Studies have found that miR-140 is often dysregulated in several cancers. Down-regulation of miR-140 has been identified in adenocarcinoma of the pancreatic duct,187 lung cancer,188,189 colorectal cancer,190 ovarian cancer,191 esophageal cancer,192 and cancer of the tongue.193 On the other hand, miR-140 has also been found to be highly expressed in some other tumors, such as spinal chordoma194 and breast cancer.195 Down-regulation of miR-140 enhanced invasion and EMT in esophageal cancer cells.192 Besides, up-regulation of miR-140 was associated with growth inhibition in tumor cells and less metastasis in hepatocellular carcinoma.196 miR-140 was found to act as a tumor suppressor in previous studies, indicating that restoration of the expression of miR-140 may help in cancer therapy. A variety of miR-140 target genes have been identified, such as iASPP,187 ATP6AP2,188 ATP8A1,189 VEGFA,190 PDGFRA,191 Slug,192 and IGF-1R.197
Nevertheless, miR-140 targets have not often been reported in glioma patients. One study reported that miR-140 acted in glioma cells by adversely modulating a newly discovered target, called ADAM9. A disintegrin and metalloproteinases (ADAMs) belong to the metzincin superfamily of matrix metalloproteinases.198 ADAM9 belongs to the ADAM family and contains an N-terminal pro-domain, which is accompanied by a metalloprotease domain, a disintegrin domain, a cysteine-rich region, a transmembrane domain, an epidermal growth factor similar region, and a tail with a possible SH3 ligand domain located in the cytoplasm.199,200 Accumulating evidence has shown the over-expression of ADAM9 in several human cancers, including renal cell carcinoma,201 prostate cancer,202 breast cancer,203 hepatocellular carcinoma,204 and pancreatic cancer.205 ADAM9 was found to be highly expressed in glioma samples and promoted migration and invasion in glioma cells.206,207 As a result, ADAM9 could be a potential therapeutic target for treatment of human tumors.
In a study by Liu et al., the miR-140 expression level was evaluated in glioma patients and the effects of miR-140 on proliferation, migration, and invasion of tumor cells in glioma tissue.120 They found significant down-regulation of miR-140 in glioma patient samples, which was associated with poor Karnofsky performance score (KPS) and World Health Organization (WHO) grades. Restoration of miR-140 expression significantly reduced the ability of glioma cells to proliferate, invade, and migrate. The ADAM9 gene was identified to be a new direct target gene of miR-140 in glioma patients. Moreover, silencing of ADAM9 mimicked the activity of miR-140 as a tumor-suppressor gene in glioma. Meanwhile, over-expression of ADAM9 abrogated the inhibitory effect of miR-140 in glioma cells. They concluded that miR-140 deregulation played a major role in the development of glioma and served as a tumor suppressor in glioma pathogenesis. miR-140 could inhibit expression of ADAM9 and thereby suppress proliferation, invasion, and migration of glioma cells. As a result, miR-140 could be a new potential target to develop promising treatment approaches for glioma patients.120
Deregulation of miR-133b has been found to play important roles in many human cancers.208, 209, 210 This process was mediated by direct targeting of the receptor tyrosine kinase MET.211 Previous studies have reported that miR-133b may play a key role in glioma development.212 In a study by Wang et al., miR-133b was shown to be significantly down-regulated in vitro in GBM cell lines and could directly target the human-Ether-à-Go-Go-related gene (hERG) channel, which increased apoptosis in U251 glioma cells after treatment with arsenic.212
In a study by Li et al., the ability of miR-133b to regulate glioma cell proliferation and invasion was investigated.146 miR-133b was considerably under-expressed in glioma samples in comparison with adjacent healthy tissue. Sirt1 was verified as a new direct target of miR-133b in U87 glioma cells. Up-regulation of miR-133b was associated with lower expression of Sirt1 and suppressed the invasion and proliferation of U87 glioma cells. This phenomenon could be partially rescued by induced over-expression of Sirt1. Furthermore, Sirt1 mRNA was found to be considerably up-regulated in glioma samples, compared with adjacent healthy tissue, and had an inverse correlation with the level of miR-133b in tumor cells. In conclusion, the role of miR-133b in modulating the development and metastasis of gliomas could be mediated by Sirt1 expression and could be a therapeutic target for glioma patients.146
Silent information regulator 1 (Sirt1) is a member of the family of the mammalian sirtuin proteins. Sirt1 serves as a histone deacetylase enzyme that is dependent on nicotinamide adenine dinucleotide (NAD+) and plays a key role in regulating the cell cycle and gene transcription.213 Sirt1 has been implicated in many cell biology processes, such as cell cycle progression, metabolism, proliferation, cell death pathways, differentiation, and senescence.214, 215, 216, 217, 218, 219, 220 Sirt1 has been found to have dual effects in human cancers. In one study by Kim et al., Sirt1 was found to serve as a tumor suppressor in breast cancer patients, suggesting that down-regulation of Sirt1 would reflect an unfavorable prognosis and lead to more metastasis.221 On the other hand, Sirt1 was over-expressed in colon cancer and was associated with mutations of P53 and the tumor, node, and metastasis (TNM) stage.222 According to Lu et al., Sirt1 reduced the development of gastric cancer by inhibiting the activation of NF-κB and STAT3,223 and they concluded that Sirt1 plays a complex role in cancer development. Whether Sirt1 could function as an oncogene in the pathogenesis of glioma was a major question in recent studies. Sirt1 increased proliferation and suppressed apoptosis in glioma tissue.224 In addition, Sirt1 silencing was found to promote the sensitivity of the CD133+ cells in glioma tissue to radiotherapy, both in vivo and in vitro.225 SIRT1 also facilitated p53 activity in the tumorigenesis of CNS stem cells.226
miR-27b is a ncRNA with a regulatory role in the development of many tumors. Wan et al. reported that miR-27b expression was significantly down-regulated in non-small cell lung cancer (NSCLC) cell lines, and up-regulation of miR-27b expression was associated with significant inhibition of the invasion and proliferation of tumor cells.227 This suggests that miR-27b acts as a tumor suppressor in the pathogenesis of NSCLC. Several studies have reported that miR-27b inhibited the progression and development of different cancers, such as neuroblastoma, prostate, and colorectal cancer.228, 229, 230 However, some other studies have suggested that miR-27b could enhance tumorigenesis. In one study by Jin et al., miR-27b was significantly over-expressed in other malignancies, such as breast cancer. Silencing of miR-27b expression significantly suppressed the development of breast cancer.231
miR-27b was significantly up-regulated in glioma samples in comparison with adjacent healthy brain tissue.131 Moreover, miR-27b has been reported to be highly expressed in samples from glioma patients and in glioma cell lines (U251, SHG44, and U87) compared with healthy astrocytes and adjacent brain tissue. Spry2 was verified as a new miR-27b target in glioma cell lines (U251), and the level of expression of Spry2 protein was inversely correlated with miR27b in glioma cells. In addition, miR-27b suppression and Spry2 up-regulation both inhibited glioma cell invasion. On the other hand, low expression of Spry2 abolished the miR-27b inhibitory effect on glioma cell invasion. The result of this study showed that miR-27b could directly inhibit the expression of Spry2 and increase glioma invasion. The results also suggest that miR-27b could be a molecular target to inhibit glioma metastasis and invasion.131
Sprouty homolog 2 (Spry2) belongs to the Sprouty family (named for Drosophila development) and contains a carboxy-terminal region that is cysteine-rich, which plays a crucial role in the suppression of receptor tyrosine kinase signaling.232 Spry2 contributes to the modulation of tumor cell invasion by regulating the mitogen-activated protein kinase (MAPK) signaling axis.233,234 The Spry2 protein level was recently shown to be notably down-regulated in glioma patients with a more invasive tumor type, confirming the regulatory function of Spry2 in glioma invasion.235
Table 1 lists various metastasis-related miRNAs reported to be involved in GBM.
Long non-coding RNAs and metastasis in glioma
The lncRNA called FOXD2-AS1 (NR_026878) is located on chromosome 1p33 and contains 2,527 nt. It was discovered to be highly expressed in gastric cancer.236 In other studies, it was shown that FOXD2-AS1 could be a molecular marker for some cancers. FOXD2-AS1 expression was correlated with invasion, migration, and low apoptosis of cancer cells and a poor outcome of patients with these tumors.237, 238, 239, 240 FOXD2-AS1 could lead to glioma progression by modulating the PI3K/AKT signaling pathway and also the miR-185-5p/high-mobility group 2 (HMGA2) axis.241
One study was carried out to measure the expression of CDK2, P21, cyclinE1, matrix metalloproteinase 7 (MMP7), MMP9, neural and epithelial cadherins, vimentin, miR-506-5p, and FOXD2-AS1.242 mir-506-5p was found to be a direct target of FOXD2-AS1 by a luciferase reporter assay. They found that FOXD2-AS1 expression was significantly higher in glioma cells, particularly in the U251 cell line. Moreover, low expression of FOXD2-AS1 resulted in significant reduction of tumor invasion, cell migration and proliferation, and suppression of the EMT. In addition, FOXD2-AS1 could regulate the expression level of P21, cyclinE1, MMP7 and MMP9, and CDK2. The possible underlying mechanism was suggested to be that FOXD2-AS1 down-regulated the expression of miR-506-5p, an anti-oncogene in several human cancer types. Over-expression of mir-506-5p and transfection of FOXD2-AS1 had the opposite effects, in that high expression of miR-506-5p inhibited proliferation, invasion, and migration as well as EMT. In conclusion, FOXD2-AS1 could facilitate the EMT and the subsequent metastasis of glioma cells through inhibiting miR-506-5p. As a result, FOXD2-AS1 could be a new target in glioma treatment.242
The lncRNA cancer susceptibility candidate 2 (CASC2) located on chromosome 10q26 has been found to act as an anti-oncogene.243 Over-expression of CASC2 could target mir-193a and mir-21 and inhibit malignant behavior in glioma.244,245 The down-regulation of CASC2 was correlated with a shorter survival time in glioma patients.246 Although researchers are investigating the function and clinical implications of CASC2, its molecular mechanism remains understudied and needs more research. miR-18a-5p has been identified as an oncogene in several tumor types247 and is likely to play the same role in glioma.248,249
In a study by Wang et al., they showed that CASC2 could act as a tumor suppressor.250 CASC2 over-expression led to more apoptosis and increased expression of E-cadherin (but not N-cadherin) and vimentin in A172 and T98 glioma cell lines. Furthermore, over-expression of CASC2 suppressed cell migration, viability, and colony-forming ability in T98 and A172 cells. It was concluded that miR-18a was a downstream target of CASC2, and CASC2 expression was inversely correlated with mir-18a levels.250
Another highly conserved lncRNA in mammals is metastasis-associated lung adenocarcinoma transcript-1 (MALAT1), which is composed of about 8,000 nt.251 Studies reported that MALAT1 was highly expressed in different types of tumors, such as gastric adenocarcinoma, squamous cell carcinoma, and hepatocellular carcinoma, and played a crucial role in the development of these cancers.252, 253, 254 Furthermore, MALAT1 was associated with hyperproliferation and metastasis in lung cancer via regulating factors, such as p53 and c-MYC, and also played a role in the EMT.255 Furthermore, MALAT1 was associated with progression of glioma tumors,256 but the exact mechanism of MALAT1 in glioma is still uncertain. Ras-related protein1 (Rap1) is a globally expressed small guanosine triphosphatase (GTPase) that transduces signals from different receptors. Cellular functions, such as adhesion, polarity, and migration,257 can be modulated by the two RAP1 isoforms, Rap1A and Rap1B. These molecules have been reported to affect invasion, metastasis, and proliferation in different cancer types, including ovarian and colorectal cancer.258, 259, 260
Li et al. evaluated the role of MALAT1 and its mechanisms in glioma cells.261 In this study, they measured the expression levels of miR-101, Rap1B mRNA, and MALAT1 in U87 and U251 glioma cells. They found that both Rap1B and MALAT1 were up-regulated, while miR-101 expression was down-regulated in U87 and U251 cells. Knockdown of either Rap1B or MALAT1 reduced cell proliferation and promoted apoptosis. There was a correlation between the expression of Rap1B and MALAT1, and it was suggested that MALAT1 increased the expression of Rap1B through sponging miR-101 in U87 and U251 cells. Silencing of MALAT1 in glioma cell lines reduced proliferation and enhanced apoptosis, while suppression of miR-101 or over-expression of Rap1B had the opposite effects on proliferation and apoptosis. This finding showed a novel regulatory axis consisting of MALAT1, Rap1B, and miR-101, which could serve as a target in glioma treatment.261
mir-124 has also been found to be a potential target of MALAT1. miR-124 is widely distributed in brain tissue and also plays a major role in a number of human cancers.262,263 There is increasing evidence that miR-124 could inhibit invasion and suppress tumor growth in different cancer types, such as colorectal cancer, breast cancer, renal cancer, and cervical cancer.264, 265, 266, 267 Feng et al. suggested that the expression of miR-124 in breast cancer was lowered by the binding of MALAT to miR-124 and thus MALAT1 could be a potential endogenous regulator.268 miR-124 was identified as a direct target of MALAT1. It was revealed that the higher expression of MALAT1 in glioma tumors correlated with lower miR-124 expression. Previous research showed that ZEB2 was associated with pathologic and clinical features of human tumors, such as tumor grade, patient overall survival, patient prognosis, and neoplastic progression.269, 270, 271 In a study by Qi et al., it was found that the migration, invasion, and proliferation of glioma cells may be suppressed by down-regulation of ZEB2. In addition, down-regulation of ZEB2 resulted in G1/S cell-cycle arrest and more apoptosis in glioma cells.272 In different human tumors, ZEB2 was found to regulate various miRNAs, such as miR-132, miR-101, miR-144, and miR-141.273, 274, 275, 276 In a recent study, MALAT1 was found to affect ZEB2 expression via sponging miR-200 in clear-cell renal carcinoma.277
In a study by Cheng et al., the expression of MALAT1 was found to be increased in human glioma cells and tissues, and it was proposed to act as a functional oncogene. Moreover, MALAT1 was associated with poor outcomes in glioma patients, and silencing of MALAT1 resulted in reduced cell proliferation and caused cycle arrest and apoptosis. In addition, MALAT1 expression was correlated with glioma tumor volume. MALAT1 silencing reduced the tumor volume, while miR-24 had the opposite effect on tumor volume. miR-124 could reverse the silencing of MALAT1 and the subsequent tumor-suppressor effect in different human cancer xenografts. It has been shown that ZEB2 serves as a direct target of miR-124 and ZEB2 expression was down-regulated by miR-124 and also by MALAT1 over-expression-induced ZEB2 expression. In conclusion, this study revealed a new MALAT1/mir-124/ZEB2 axis that was correlated with glioma progression and could be a new therapeutic target in glioma.278
Long intergenic non-protein coding RNA689 (LINC00689) has been found to be involved in various human cancers, A clinical study carried out in Northern Han Chinese individuals showed that LINC00689 was a gene related to obesity.279 The stress and tumor necrosis factor alpha (TNF-α)-activated open reading frame (ORF) micropeptide (STORM) peptide is encoded by LINC00689 and can act in a similar manner to signal recognition particle 19 (SRP19) in controlling gene expression in eukaryotic cells.280 miR-338-3p was found to be a tumor suppressor in several cancer types.281, 282, 283 miR-338-3p inhibited migration and induced apoptosis in gastric cancer cells via affecting its target, protein-tyrosine phosphatase 1B (PTP1B).281 Furthermore, miR-338-3p suppressed the progression of hepatocellular carcinoma (HCC) cells and inhibited the Warburg effect via restoration of the activity of pyruvate kinase L/R (PKLR).282 mir-338-3p acts as an anti-oncogene by suppressing GBM invasion and proliferation and reducing ATP synthesis by targeting the pyruvate kinase M2 (PKM2)-β-catenin axis.283
The lncRNA SBF2-AS1 was found to sponge miR-338-3p in GBM cells.284 Afterward, PKM2 was reported to be a downstream target of the LINC00689-miR-338-3p axis in glioma patients. PKM2 was found to enhance proliferation, glycolysis, and metastasis of HCC tumor cells. In addition, PKM2 expression was correlated with glucose metabolism and malignant properties in glioma cells.285,286
In a study by Liu et al., the expression of LINC00689 was found to be higher in glioma tissue in comparison with normal tissue, on the basis of the GSE dataset (GEO: GSE4290). Then, they demonstrated experimentally that LINC00689 was highly expressed in glioma tissue and cell lines and LINC00689 expression was correlated with a larger tumor size (particularly >3 cm), poor prognosis, and a low KPS score.287 LINC00689 knockdown led to suppression of glioma cell proliferation, migration, and glycolysis. Moreover, LINC00689 knockdown was associated with significant inhibition of glioma tumor growth in vivo. LINC00689 enhanced the expression of PKM2 through a direct interaction with miR-338-3p, suggesting that LINC00689 plays a competing endogenous RNA (ceRNA) role in glioma. The effects of LINC00689 knockdown on proliferation, invasion, migration, and glycolysis of glioma cells could be abrogated by PKM2 restoration. In conclusion, the LINC00689/miR-338-3p/PKM2 axis plays a role in glioma progression.287 Table 2 lists some metastasis-related lncRNAs reported to be involved in GBM.
Table 2.
Metastasis-related lncRNAs in GBM
| lncRNAs | Expression status | Targets | Model (in vitro, in vivo, human) | Type of cell line | Ref |
|---|---|---|---|---|---|
| FOXD2-AS1 | up | miR-506-5p | in vitro | U251, SHG44, LN229, T98G | Zhao et al.242 |
| NBAT1 | down | miR-21/SOX7 | in vitro, human | AM38, Gli-6, GSC11, A172 | Guan et al.288 |
| HCG11 | down | miR-4425/MTA3 | in vitro, in vivo, human | A172, U251, U87MG, U118 | Zhang et al.289 |
| MEG3 | down | miR-96-5p/MTSS1 | in vitro, human | GSC11, M059J, D54 | Zhang and Guo290 |
| DANCR | up | miR-33a-5p | in vitro, in vivo, human | U87, U251, T98G, LN22 9 | Yang et al.291 |
| lncRNA001089 | down | in vitro, in vivo, human | U251 | Perez-Laguna et al.292 | |
| HOTTIP | up | miR-101/ZEB1 | in vitro, human | U87, U251 | Zhang et al.293 |
| CASC19 | miR-454-3p/RAB5A | in vitro, human | Wu et al.294 | ||
| MALAT1 | up | miR-124/ZEB2 | in vitro, in vivo, human | U251 | Cheng et al.295 |
| GHET1 | up | miR-216a | in vitro | U251 | Cao et al.296 |
| CASC2 | down | miR-18a | in vitro, in vivo, human | T98, A172 | Wang et al.250 |
| MALAT1 | up | Rap1B, miR-101 | in vitro, human | U87, U251 | Xiang et al.297 |
| H19 | up | miR-29a | in vitro, in vivo, human | U87MG | Jia et al.298 |
| MALAT1 | up | miR-101 | in vitro | U251, U87 | Li et al.261 |
| XIST | up | miR-429 | in vitro, in vivo, human | A172, U251 | Cheng et al.299 |
| TSLNC8 | down | in vitro, human | BE-2C, BT325, SHG-44, CHG-5 U25-MG, SWO38 | Chen and Yu300 | |
| lncRNA-LYPLAL1-2 | down | miR-127/YWHAG | in vitro, in vivo, human | U87, U251 | Zheng et al.301 |
| LINC00961 | down | in vitro, human | U251, A172, U-118, U87 | Abedi-Gaballu et al.302 | |
| UBE2R2-AS1 | down | miR-877-3p/TLR4 | in vitro, human | U251, A-172, U373, U87-MG | Xu et al.303 |
| FOXD2-AS1 | up | miR-185 | in vitro, human | U251,LN18, T98G, A172, LN22 | Dong et al.304 |
| SNHG18 | ENO1 | in vitro | M059J, M059K, U87 | Zheng et al.305 | |
| TP73-AS1 | up | miR-124 | in vitro, human | U87, U118, U251, U373, SHG-44 | Xiao et al.306 |
| SPRY4-IT1 | up | in vitro, human | U251, SF295 | Liu et al.307 | |
| GAS5 | down | miR-18a-5p | in vitro, in vivo, human | U251, U87 | Liu et al.308 |
| ZEB1-AS1 | up | miR-200c/141-ZEB1 | in vitro, in vivo, human | U87, U251, LN18, U118, T98G | Meng et al.309 |
| LINC01426 | up | PI3K/Akt | in vitro, human | PG1, A172, LN229, U251, LN118, H4 | Wang et al.310 |
| LINC00689 | up | miR-338-3p/PKM2 | in vitro, in vivo, human | U87, U251 | Liu et al.287 |
| NEAT1 | up | miR-139-5p/CDK6 | in vitro, in vivo, human | U251, SHG-44, TJ905 | Wu et al.311 |
| MALAT1 | up | miR-199a/ZHX1 | in vitro, in vivo, human | U87-MG, U251, T98G, A172 | Liao et al.312 |
| LIFR-AS1 | down | miR-4262/NF-κB | in vitro, human | A172, U87, U251, LN229 | Ding et al.313 |
| MALAT1 | up | in vitro, human | primary | Ma et al.256 | |
| MALAT1 | NF-κB, p53 | in vitro, in vivo | U87, A172, U251 | Voce et al.314 | |
| XIST | up | miR-133a/SOX4 | in vitro | U251 | Luo et al.315 |
| HOXA11-AS | up | miR-130a-5p-HMGB2 | in vitro, in vivo, human | U251, U87MG | Xu et al.316 |
| MALAT1 | down | ERK/MAPK | in vitro, in vivo, human | U87, U251 | |
| MALAT1 | down | miR-155 | in vitro, human | U87, SHG139 | Cao et al.317 |
| EGOT | down | in vitro, human | A172, U251, U87, SHG44, | Wu et al.318 | |
| HOXD-AS1 | up | miR-130a | in vitro, human | U87, U251 | Chen et al.319 |
| GAS5-AS1 | down | miR-106b-5p/TUSC2 | in vitro, in vivo, human | U251 | Huang et al.320 |
| PVT1 | up | UpF1 | in vitro, human | U87, LN229 | Lv et al.321 |
| NEF | down | TGF-β1 | in vitro, human | Hs 683, CCD-25Lu | Wang et al.322 |
| SAMD12-AS1 | up | P53 | in vitro, human | U251, U87, T98-G, A172 | Jia et al.323 |
| FOXD2-AS1 | up | miR-185-5p/HMGA2 | in vitro, in vivo, human | U87, A172, U251, T98G | Ni et al.241 |
| MEG3 | down | miR-21-3p | in vitro | U87, U251 | Qin et al.324 |
| ANCR | up | EZH2, PTEN | in vitro, human | U87, U251, SHG44, U118 | Cheng et al.325 |
| Linc01116 | up | miR-31 | in vitro, in vivo, human | SHG-44, U87, U25, U118 MG | Zhang et al.326 |
| LSINCT5 | up | miR-451 | in vitro, human | GL15 | Liu et al.327 |
| lincRNA-p21 | miR-34c | in vitro, in vivo | U87, U251 | Yang et al.328 | |
| AWPPH | up | HIF1α | in vitro, human | Hs 683, CCD-25Lu | Zhang et al.329 |
| MACC1-AS1 | up | MACC1 | in vitro, human | U251, T98G, A172, SHG44 | Zheng et al.330 |
| H19 | up | miR-342/Wnt5a/β-catenin | in vitro, in vivo, human | A172, LN229, U251 | Zhou et al.331 |
| AC016405.3 | down | miR-19a-5p, TET2 | in vitro, human | U87MG | Ren and Xu332 |
| MIR4697HG | down | miR-766-5p/PRR12 | in vitro | U343, Hs683, LN25, A172, LN18, U87, U251 | Mao et al.333 |
| LINC00466 | up | miR-508/CHEK1 | in vitro, human | A172, T98G, LN299, U251, LN18 | Li et al.334 |
| SNHG16 | up | miR-490/PCBP2 | in vitro, human | T98G, U251 | Kong et al.335 |
| MALAT1 | up | in vitro, human | primary | Argadal et al.336 | |
| LINC01614 | up | miR-383/ADAM12 | in vitro, human | LN18, U251, T98G, LN229, A172 | Wang et al.250 |
| MALAT1 | up | miR-384/GOLM1 | in vitro, in vivo, human | SHG-44, LN229 | Alcon-Giner et al.337 |
| GAPLINC | up | miR-331-3p | in vitro, human | T98G, U251, LN18, LN229, A172 | Chen et al.338 |
| H19 | up | miR-140, iASPP | in vitro, human | U373, A172, U251, T98G, U87MG | Zhao et al.339 |
| FTH1P3 | up | miR-224-5p/TPD52 | in vitro, human | U251 | Zhang et al.340 |
| HOXA11-AS | up | miR-214-3p/EZH2 | in vitro, in vivo, human | U251, U87, LN229, SHG-44, A172 | Xu et al.341 |
| SNHG7 | up | miR-5095, Wnt/β-catenin | in vitro, in vivo, human | A172, U87, T98G, SHG44 | Ren et al.342 |
| UCA1 | up | miR-182, iASPP | in vitro, human | U373MG, T98MG, SWO38, U251, SHG44 | He et al.343 |
| LINC01260 | down | CARD11, NF-κB | in vitro, in vivo, human | U251 | Wu et al.344 |
| RP5-833A20.1 | down | microRNA-382-5p, NFIA | in vitro, human | U251 | Kang et al.345 |
| UCA1 | miR-204-5p/ZEB1 | in vitro, in vivo | SHG44, U87MG | Liang et al.346 | |
| SNHG5 | up | miR-205-5p/ZEB2 | in vitro, human | Meng et al.347 | |
| MALAT1 | up | in vitro | SHG139, SHG139S | Han et al.348 | |
| LINC-PINT | down | Wnt/β-catenin | in vitro, in vivo | U87, LN229, U373, A172, U251, T98, U118 | Zhu et al.349 |
circRNAs and metastasis in glioma
Previous studies have confirmed the regulatory role of circRNAs in tumor malignancy and metastasis to adjacent tissues.350 An increasing number of studies suggest that circRNAs can cause tumor drug resistance and increase metastasis and recurrence.351 Certain specific circRNAs, such as circ_0067934,352 circ_0014359,353 and circZNF264,354 have been found to be involved in glioma progression. One theory is that circRNAs may act as ceRNAs to sponge miRNAs and thereby reduce the aggressive malignant properties of cancer cells by regulation of mRNA expression.355 For example, circFOXO3 encouraged invasion and cell proliferation by sponging both miR-182 and miR-433 in glioma.356 Xing et al. reported that circFOXO3 could promote esophageal squamous cell carcinoma (SCC) progression via the miR-23a-3p/PTEN axis.357 Li et al. found that increased expression of circU2AF1 promoted glioma progression and was correlated with a poor clinical prognosis.358 A study by Lei et al. reported that circ_0076248 could encourage glioma progression by increasing SIRT1 due to the sponging of miR-181a.359 Another study suggested that circFBXW7 could be a therapeutic target to inhibit glioma progression.360 Yang and colleagues used RNA sequencing to determine circRNA profiles in glioma samples compared with normal adjacent tissue and found that circFBXW7 was expressed higher in normal samples compared with glioma. Higher levels of circFBXW7 were correlated with longer patient survival and a better prognosis.79
Gao et al. evaluated the function of circFBXW7 in glioma and its underlying mechanism.361 The expression of circFBXW7, miR-23a-3p, and PTEN was measured by qRT-PCR in glioma tissue and cell lines. The proliferation of glioma cells was measured by Cell Counting Kit 8 (CCK8) assay. The migration and invasion of glioma cells were measured by transwell assays. Possible interactions between circFBXW7, PTEN, and miR-23a-3p were evaluated using a dual-luciferase reporter assay. Western blotting measured the expression of these proteins. A mouse xenograft model of glioma was used to investigate circFBXW7 function in vivo. They found that circFBXW7 expression was significantly lower in glioma cell lines and tumor tissue compared with normal tissue. High expression of circFBXW7 had an inhibitory effect on glioma cell migration, proliferation, and invasion. Furthermore, miR-23a-3p was shown to be a direct target of circFBXW7 using bioinformatics analysis and dual-luciferase reporter assay. The binding of miR-23a-3p to the PTEN 3′ UTR might be inhibited by the activity of circFBXW7. They concluded that circFBXW7 reduced glioma metastasis and proliferation by directly sponging miR-23a-3p, thus increasing PTEN. circFBXW7 up-regulation inhibited glioma growth and metastasis in vivo. Over-expression of circFBXW7 resulted in decreased expression of Ki67, miR-23a-3p, and N-cadherin but increased levels of PTEN and E-cadherin, which was in agreement with the in vitro findings. They suggested that circFBXW7 could be a new therapeutic and diagnostic target in glioma patients.361
New studies have reported that the inhalational anesthetic sevoflurane (Sev) could suppress the progression of tumors through modulating miRNAs. Sun et al. found that Sev inhibited the invasion and migration of colorectal cancer cells by modulating the miRNA-34a/ADAM10 axis.362 A study by Gao et al. reported that Sev could inhibit metastasis and proliferation of glioma cells by targeting the miRNA-124-3p/ROCK1 axis.363 Recently, Xie et al. reported that miR-628-5p could suppress cell proliferation in glioma.364 However, the exact mechanism of how Sev affects miR-628-5p and the progression of glioma are not yet clear.
Magnesium transporter 1 (MAGT1) has been shown to be associated with several human cancers. Zheng et al. reported that high expression of MAGT1 was a poor prognostic indicator in colorectal cancer.365 Wang et al. showed that miRNA-199a-5p suppressed progression of glioma by inhibiting MAGT1.366
Li et al. evaluated the effect of Sev on glioma progression using bioinformatics analysis and experimental studies.367 They used flow cytometry for apoptosis; western blotting for protein levels of hexokinase 2 (HK2), MAGT1, Bcl-2, and BCL2-associated X (Bax) in glioma samples; and CCK8 assay for cell viability. The transwell assay measured cell migration and invasion. Colorimetric assay kits were utilized to measure lactate synthesis and glucose metabolism. The levels of circRNA1656 (also known as circ-0002755) and mir628-5p were measured by qRT-PCR, and the interactions between miR-628-5p, circ-0002755, and MAGT1 were confirmed by dual-luciferase reporter assays. AA mouse xenograft tumor model was employed to investigate the function of circ-0002755 in vivo. They found that Sev suppressed viability, invasion, and migration and decreased lactate production and glucose consumption, while increasing apoptosis. Administration of Sev significantly reduced the expression of circ-0002755, while circ-0002755 was notably over-expressed in glioma tissues. They reported a three-way interaction between circ-0002755, mir628-5p, and MAGT1 and suggested that Sev could regulate the progression of glioma via the circ_0002755/miR-628-5p/MAGT1 axis. In addition, Sev suppressed the progression of glioma tumors in vivo.367
Recent studies have shown that circRNA Scm-like with 4 Mbt domain 2 (circ_SFMBT2) can act as a sponge of miR-182-5p, with a regulatory function in gastric adenocarcinoma growth.368 There is not much data about the putative downstream molecules of circ_SFMBT2 and the underlying mechanisms in glioma development, but the role of miR-182-5p in the pathogenesis of bladder and gastric cancer has been shown.368,369 miR-182-5p can control the growth of various kinds of tumors via metastasis suppressor 1 (MTSS1);370, 371, 372 MTSS1 has been correlated with tumor metastasis and progression in different cancers by complex interactions with the actin cytoskeleton.371,372 circ_SFMBT2 was shown to affect the growth of gastric cancer cells through targeting miR-182-5p.368
Zhang and colleagues investigated circ_SFMBT2 expression in glioma tissues.373 In their study, lower expression of circ_SFMBT2 was detected compared with normal astrocyte cells, and circ_SFMBT2 over-expression inhibited glioma cell growth in vitro and reduced metastasis. mir182-5p could be a downstream molecule of circ_SFMBT2, and they suggested that the circ_SFMBT2/mir-182-5p/MTSS1 axis could play a role in glioma treatment.373
circRNA homeodomain interacting protein kinase 3 (circHIPK3) and miR-124-3p were found to promote glioma development.374 In addition, miR-524-5p was found to regulate glioma cell proliferation.375 Kinesin family member 2A (KIF2A) plays an important role in cancer progression. Zhang et al. found that KIF2A could affect the prognosis of patients with nasopharyngeal carcinoma.376 KIF2A down-regulation inhibited gastric cancer invasion377 and could suppress oral SCC through inhibiting the PI3K/protein kinase B pathway.378 It was shown that KIF2A silencing could suppress migration and metastasis of glioma cells, while KIF2A knockdown could stimulate apoptosis in vitro.379
In a study by Yin et al., the effects of miR-524-5p, KIF2A, and circHIPK3 regulatory network on the development of TMZ-resistant glioma were investigated. This might shed light on the mechanisms involved in TMZ resistance and could improve TMZ treatment efficacy in glioma patients.380 qRT-PCR was used to measure serum levels of mRNAs, circRNAs, and miRNAs. The MTT assay was used to measure cell proliferation, and the TMZ half inhibitory concentration (IC50), western blotting, dual-luciferase reporter assay, flow cytometry, and transwell assays were employed to measure the level of protein expression, apoptosis, and metastasis, respectively. The results showed that circHIPK3 knockdown increased TMZ sensitivity in glioma and affected proliferation, apoptosis, and metastasis via the miR-524-5p/KIF2A-mediated PI3K/AKT pathway. This could be a new approach for diagnosis and therapy of TMZ-resistant glioma.380 Table 3 lists some metastasis-related circRNAs reported to be involved in GBM.
Table 3.
Metastasis-related circular RNAs involved in GBM
| circRNA | Expression status (up/down) | Target | Model (in vitro, in vivo, human) | Cell line | Ref |
|---|---|---|---|---|---|
| circCPA4 | up | let-7 | in vitro, in vivo, human | U25, U87 | Peng et al.114 |
| circ_SFMBT2 | down | miR-182-5p | in vitro, human | D54, A172, U251 | Zhang et al.381 |
| circMMP9 | up | miR-124 | in vitro, in vivo, human | U87, U251, SHG44, A172, SNB19 | Wang et al.382 |
| circFBXW7 | down | miR-23a-3p/PTEN | in vitro, in vivo, human | U251, U87, SHG44 | Gao et al.383 |
| circHIPK3 | up | miR-124-3p | in vitro, human | T98G | Xia et al.384 |
| circ_0002755 | up | miR-628-5p/MAGT1 | in vitro, in vivo, human | A-172, SHG-44 | Li et al.367 |
| circMMP1 | up | miR-433/HMGB3 | in vitro, in vivo, human | U251, LN229 | Yin and Liu385 |
| circTTBK2 | up | miR-145-5p/CPEB4 | in vitro, in vivo, human | T98G, LN229 | Liu et al.386 |
| hsa_circ_0030018 | up | miR-1297/RAB21 | in vitro, in vivo, human | Song et al.387 | |
| circHIPK3 | up | miR-524-5p/KIF2A | in vitro, human | A172, U251 | Yin and Cui380 |
| circ_101064 | up | miR-154-5p/PIWIL1 | in vitro, human | U251, U87 | Zhou et al.125 |
| circ-U2AF1 | up | hsa-miR-7-5p | in vitro, in vivo, human | U251, U87 | Hamblin388 |
| hsa_circ_0088732 | up | miR-661/RAB3D | in vitro, in vivo, human | LN229, U251, A172 U87-MG | Jin et al.389 |
| circPVT1 | up | miR-199a-5p | in vitro, human | U539, U251 | Chi et al.390 |
| circSCAF11 | up | miR-145-5p/miR-145-5p | in vitro, in vivo, human | T98G, LN229 | Yin et al.391 |
| hsa_circ_0067934 | up | PI3K-AKT | in vitro, human | LN18, U251, LN229, T98G, A172 | Xin et al.392 |
| circ_0029426 | up | miR-197 | in vitro, human | Zhang et al.393 |
Conclusion
It is now known that ncRNAs are involved in all phases of metastasis and can regulate invasion, migration, colony formation, and the development of the metastatic mass. ncRNAs may form complex interactions with other RNAs, DNA, and proteins to regulate the metastatic process. The exact signaling axes that are mediated by specific ncRNAs involved in the regulation of metastasis in individual cancer types should be elucidated by functional genomics assays. The latest advances in genome-editing techniques, such as CRISPR-Cas9, have made it possible to rapidly modify the human genome and could be used to study ncRNAs. Combining practical genetic screening with single-cell-based assays and confirming the results in animal models is the most attractive approach. These recent advances will shed more light on a thorough understanding of ncRNA function and the possible underlying molecular mechanisms involved in the regulation of metastasis. Clinical studies should be undertaken to verify the critical functions of ncRNAs in glioma development and metastasis. ncRNAs display a specific expression profile in many malignancies, which suggests they can serve as promising diagnostic and prognostic biomarkers in cancer as well as potential promising therapeutic targets for future cancer treatment.
ncRNAs could be a promising tool for targeted therapy combined with other new promising therapies. The development of a complete network of all ncRNAs involved in glioma formation and progression could supplement other therapeutic approaches (such as immunotherapy and gene therapy) and could also be used as a stratification tool for individualized treatment, resulting in an improved antitumor effect. The successful application of ncRNA-based therapeutics requires an unprecedented interdisciplinary approach, including technical advancements in molecular biology, immunology, pharmacology, chemistry, and nanotechnology. An optimal ncRNA therapeutic agent must be extensively tested for immunogenicity, chemically modified to improve its pharmacokinetics and pharmacodynamics, and delivered with consideration of its biodistribution and intracellular uptake mechanisms. It would need to specifically and potently interact with its intended target and be dosed at an appropriate level to trigger the desired effect. Studies in each of these areas should be carried out for each separate ncRNA therapeutic agent, but their successful translation will depend on further interdisciplinary collaboration to improve tolerance, specificity, and delivery.
Acknowledgments
Author contributions
M.R.H., H.M., S.M.M., F.D., S.M.A.M., S.H., P.G., and N.R. contributed in data collection and manuscript drafting. M.D., F.B., and M.M.-T. critically revised the manuscript. All authors approved the final version for submission.
Declaration of interests
M.R.H. declares the following potential conflicts of interest: scientific advisory boards: Transdermal Cap Inc., Cleveland, OH; BeWell Global Inc., Wan Chai, Hong Kong; Hologenix Inc., Santa Monica, CA; LumiThera Inc., Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc., Bee Cave, TX; Medical Coherence, Boston, MA; NeuroThera, Newark, DE; JOOVV Inc., Minneapolis-St. Paul, MN; AIRx Medical, Pleasanton, CA; FIR Industries, Inc., Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc., Lansing, MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp., Incline Village, NV; Niraxx Light Therapeutics, Inc., Boston, MA; consulting: Lexington Int, Boca Raton, FL; USHIO Corp., Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V., Eindhoven, the Netherlands; Johnson & Johnson Inc., Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany; and stockholdings: Global Photon Inc., Bee Cave, TX and Mitonix, Newark, DE. The other authors declare no competing interests.
Contributor Information
Neda Rahimian, Email: rahimian.n@iums.ac.ir.
Michael R. Hamblin, Email: hamblin.lab@gmail.com.
Hamed Mirzaei, Email: mirzaei-h@kaums.ac.ir, h.mirzaei2002@gmail.com.
References
- 1.Bagherian A., Mardani R., Roudi B., Taghizadeh M., Banfshe H.R., Ghaderi A., Davoodvandi A., Shamollaghamsari S., Hamblin M.R., Mirzaei H. Combination therapy with nanomicellar-curcumin and temozolomide for in vitro therapy of glioblastoma multiforme via Wnt signaling pathways. J. Mol. Neurosci. 2020;70:1471–1483. doi: 10.1007/s12031-020-01639-z. [DOI] [PubMed] [Google Scholar]
- 2.Khani P., Nasri F., Khani Chamani F., Saeidi F., Sadri Nahand J., Tabibkhooei A., Mirzaei H. Genetic and epigenetic contribution to astrocytic gliomas pathogenesis. J. Neurochem. 2019;148:188–203. doi: 10.1111/jnc.14616. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso J.C.L., Talkenberger K., Seifert M., Klink B., Hawkins-Daarud A., Swanson K.R., Hatzikirou H., Deutsch A. The biology and mathematical modelling of glioma invasion: a review. J. R. Soc. Interface. 2017;14:20170490. doi: 10.1098/rsif.2017.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G., Qin Z., Chen Z., Xie L., Wang R., Zhao H. Tumor microenvironment in treatment of glioma. Open Med. 2017;12:247–251. doi: 10.1515/med-2017-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Sanctis V., Mazzarella G., Osti M.F., Valeriani M., Alfó M., Salvati M., Banelli E., Tombolini V., Enrici R.M. Radiotherapy and sequential temozolomide compared with radiotherapy with concomitant and sequential temozolomide in the treatment of newly diagnosed glioblastoma multiforme. Anticancer Drugs. 2006;17:969–975. doi: 10.1097/01.cad.0000224446.31577.df. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R., Taillibert S., Kanner A., Read W., Steinberg D., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. Jama. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendell J.T., Olson E.N. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 9.Spizzo R., Almeida M.I., Colombatti A., Calin G.A. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashemian S.M., Pourhanifeh M.H., Fadaei S., Velayati A.A., Mirzaei H., Hamblin M.R. Non-coding RNAs and exosomes: their role in the pathogenesis of sepsis. Mol. Ther. Nucleic Acids. 2020;21:51–74. doi: 10.1016/j.omtn.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahimian N., Razavi Z.S., Aslanbeigi F., Mirkhabbaz A.M., Piroozmand H., Shahrzad M.K., Hamblin M.R., Mirzaei H. Non-coding RNAs related to angiogenesis in gynecological cancer. Gynecol. Oncol. 2021;161:896–912. doi: 10.1016/j.ygyno.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Mahjoubin-Tehran M., Rezaei S., Jesmani A., Birang N., Morshedi K., Khanbabaei H., Khan H., Piranviseh A., Nejati M., Aschner M., et al. New epigenetic players in stroke pathogenesis: from non-coding RNAs to exosomal non-coding RNAs. Biomed. Pharmacother. 2021;140:111753. doi: 10.1016/j.biopha.2021.111753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dashti F., Mirazimi S.M.A., Rabiei N., Fathazam R., Rabiei N., Piroozmand H., Vosough M., Rahimian N., Hamblin M.R., Mirzaei H. The role of non-coding RNAs in chemotherapy for gastrointestinal cancers. Mol. Ther. Nucleic Acids. 2021;26:892–926. doi: 10.1016/j.omtn.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolha L., Ravnik-Glavač M., Glavač D. Long noncoding RNAs as biomarkers in cancer. Dis. Markers. 2017;2017:7243968. doi: 10.1155/2017/7243968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shabaninejad Z., Yousefi F., Movahedpour A., Ghasemi Y., Dokanehiifard S., Rezaei S., Aryan R., Savardashtaki A., Mirzaei H. Electrochemical-based biosensors for microRNA detection: nanotechnology comes into view. Anal. Biochem. 2019;581:113349. doi: 10.1016/j.ab.2019.113349. [DOI] [PubMed] [Google Scholar]
- 16.Wu K., Jiang Y., Zhou W., Zhang B., Li Y., Xie F., Zhang J., Wang X., Yan M., Xu Q., et al. Long noncoding RNA RC3H2 facilitates cell proliferation and invasion by targeting MicroRNA-101-3p/EZH2 Axis in OSCC. Mol. Ther. Nucleic Acids. 2020;20:97–110. doi: 10.1016/j.omtn.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon J.H., Abdelmohsen K., Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayoumi A.S., Sayed A., Broskova Z., Teoh J.P., Wilson J., Su H., Tang Y.L., Kim I.M. Crosstalk between long noncoding RNAs and MicroRNAs in Health and disease. Int. J. Mol. Sci. 2016;17:356. doi: 10.3390/ijms17030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H., Shen J., Hodges T.R., Song R., Fuller G.N., Heimberger A.B. Serum microRNA profiling in patients with glioblastoma: a survival analysis. Mol. Cancer. 2017;16:59. doi: 10.1186/s12943-017-0628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H., Chen X., Hu Y., Shi Z., Zhou Q., Zheng J., Wang Y. Long non-coding RNA expression profile in cervical cancer tissues. Oncol. Lett. 2017;14:1379–1386. doi: 10.3892/ol.2017.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene J., Baird A.M., Brady L., Lim M., Gray S.G., Mcdermott R., Finn S.P. Circular RNAs: biogenesis, function and role in human diseases. Front. Mol. Biosci. 2017;4:38. doi: 10.3389/fmolb.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzman J., Chen R.E., Olsen M.N., Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szabo L., Morey R., Palpant N.J., Wang P.L., Afari N., Jiang C., Parast M.M., Murry C.E., Laurent L.C., Salzman J. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome Biol. 2015;16:126. doi: 10.1186/s13059-015-0690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shang Q., Yang Z., Jia R., Ge S. The novel roles of circRNAs in human cancer. Mol. Cancer. 2019;18:6. doi: 10.1186/s12943-018-0934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu S., Wang J., He Y., Meng N., Yan G.R. Peptides/proteins encoded by non-coding RNA: a novel resource bank for drug targets and biomarkers. Front. Pharmacol. 2018;9:1295. doi: 10.3389/fphar.2018.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., Schuman E. Circular RNAs in brain and other tissues: a functional enigma. Trends Neurosci. 2016;39:597–604. doi: 10.1016/j.tins.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Tamtaji O.R., Behnam M., Pourattar M.A., Hamblin M.R., Mahjoubin-Tehran M., Mirzaei H., Asemi Z. PIWI-interacting RNAs and PIWI proteins in glioma: molecular pathogenesis and role as biomarkers. Cell Commun. Signal. 2020;18:168. doi: 10.1186/s12964-020-00657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X., Pan Y., Fang Y., Zhang J., Xie M., Yang F., Yu T., Ma P., Li W., Shu Y. The biogenesis and functions of piRNAs in human diseases. Mol. Ther. Nucleic Acids. 2020;21:108–120. doi: 10.1016/j.omtn.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hira V.V., Ploegmakers K.J., Grevers F., Verbovšek U., Silvestre-Roig C., Aronica E., Tigchelaar W., Turnšek T.L., Molenaar R.J., Van Noorden C.J. CD133+ and Nestin+ glioma stem-like cells reside around CD31+ arterioles in niches that express SDF-1α, CXCR4, osteopontin and cathepsin K. J. Histochem. Cytochem. 2015;63:481–493. doi: 10.1369/0022155415581689. [DOI] [PubMed] [Google Scholar]
- 30.Verbovšek U., Van Noorden C.J., Lah T.T. Complexity of cancer protease biology: cathepsin K expression and function in cancer progression. Semin. Cancer Biol. 2015;35:71–84. doi: 10.1016/j.semcancer.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Almog N., Ma L., Raychowdhury R., Schwager C., Erber R., Short S., Hlatky L., Vajkoczy P., Huber P.E., Folkman J., et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 32.Hira V.V.V., Breznik B., Vittori M., Loncq De Jong A., Mlakar J., Oostra R.J., Khurshed M., Molenaar R.J., Lah T., Van Noorden C.J.F. Similarities between stem cell niches in glioblastoma and bone marrow: rays of hope for novel treatment strategies. J. Histochem. Cytochem. 2020;68:33–57. doi: 10.1369/0022155419878416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hossain A., Gumin J., Gao F., Figueroa J., Shinojima N., Takezaki T., Priebe W., Villarreal D., Kang S.G., Joyce C., et al. Mesenchymal stem cells isolated from human gliomas increase proliferation and maintain stemness of glioma stem cells through the IL-6/gp130/STAT3 pathway. Stem Cells. 2015;33:2400–2415. doi: 10.1002/stem.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minata M., Audia A., Shi J., Lu S., Bernstock J., Pavlyukov M.S., Das A., Kim S.H., Shin Y.J., Lee Y., et al. Phenotypic plasticity of invasive edge glioma stem-like cells in response to ionizing radiation. Cell Rep. 2019;26:1893–1905.e1897. doi: 10.1016/j.celrep.2019.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plaks V., Kong N., Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhat K.P.L., Balasubramaniyan V., Vaillant B., Ezhilarasan R., Hummelink K., Hollingsworth F., Wani K., Heathcock L., James J.D., Goodman L.D., et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behnan J., Finocchiaro G., Hanna G. The landscape of the mesenchymal signature in brain tumours. Brain. 2019;142:847–866. doi: 10.1093/brain/awz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halliday J., Helmy K., Pattwell S.S., Pitter K.L., Laplant Q., Ozawa T., Holland E.C. In vivo radiation response of proneural glioma characterized by protective p53 transcriptional program and proneural-mesenchymal shift. Proc. Natl. Acad. Sci. U S A. 2014;111:5248–5253. doi: 10.1073/pnas.1321014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anghileri E., Castiglione M., Nunziata R., Boffano C., Nazzi V., Acerbi F., Finocchiaro G., Eoli M. Extraneural metastases in glioblastoma patients: two cases with YKL-40-positive glioblastomas and a meta-analysis of the literature. Neurosurg. Rev. 2016;39:37–45. doi: 10.1007/s10143-015-0656-9. discussion 45-36. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan J.P., Nahed B.V., Madden M.W., Oliveira S.M., Springer S., Bhere D., Chi A.S., Wakimoto H., Rothenberg S.M., Sequist L.V., et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 2014;4:1299–1309. doi: 10.1158/2159-8290.CD-14-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skvortsova I., Debbage P., Kumar V., Skvortsov S. Radiation resistance: cancer stem cells (CSCs) and their enigmatic pro-survival signaling. Semin. Cancer Biol. 2015;35:39–44. doi: 10.1016/j.semcancer.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Del Pozo Martin Y., Park D., Ramachandran A., Ombrato L., Calvo F., Chakravarty P., Spencer-Dene B., Derzsi S., Hill C.S., Sahai E., et al. Mesenchymal cancer cell-stroma crosstalk promotes niche activation, epithelial reversion, and metastatic colonization. Cell Rep. 2015;13:2456–2469. doi: 10.1016/j.celrep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y., Dong Q., Li J., Zhang K., Qin J., Zhao J., Sun Q., Wang Z., Wartmann T., Jauch K.W., et al. Targeting cancer stem cells and their niche: perspectives for future therapeutic targets and strategies. Semin. Cancer Biol. 2018;53:139–155. doi: 10.1016/j.semcancer.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Lewis G.D., Rivera A.L., Tremont-Lukats I.W., Ballester-Fuentes L.Y., Zhang Y.J., Teh B.S. GBM skin metastasis: a case report and review of the literature. CNS Oncol. 2017;6:203–209. doi: 10.2217/cns-2016-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narayan A., Jallo G., Huisman T.A. Extracranial, peritoneal seeding of primary malignant brain tumors through ventriculo-peritoneal shunts in children: case report and review of the literature. Neuroradiol J. 2015;28:536–539. doi: 10.1177/1971400915609348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houston S.C., Crocker I.R., Brat D.J., Olson J.J. Extraneural metastatic glioblastoma after interstitial brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:831–836. doi: 10.1016/s0360-3016(00)00662-3. [DOI] [PubMed] [Google Scholar]
- 47.Bissell M.J. Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch. A rose by any other name? Am. J. Pathol. 1999;155:675–679. doi: 10.1016/S0002-9440(10)65164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carvalho J., Barbosa C.C.L., Feher O., Maldaun M.V.C., Camargo V.P., Moraes F.Y., Marta G.N. Systemic dissemination of glioblastoma: literature review. Rev. Assoc. Med. Bras. 2019;65:460–468. doi: 10.1590/1806-9282.65.3.460. [DOI] [PubMed] [Google Scholar]
- 49.Mehlen P., Puisieux A. Metastasis: a question of life or death. Nat. Rev. Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 50.Lehtinen M.K., Zappaterra M.W., Chen X., Yang Y.J., Hill A.D., Lun M., Maynard T., Gonzalez D., Kim S., Ye P., et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louis D.N., Perry A., Reifenberger G., Von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 52.Müller C., Holtschmidt J., Auer M., Heitzer E., Lamszus K., Schulte A., Matschke J., Langer-Freitag S., Gasch C., Stoupiec M., et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 2014;6:247ra101. doi: 10.1126/scitranslmed.3009095. [DOI] [PubMed] [Google Scholar]
- 53.Ma Q., Long W., Xing C., Chu J., Luo M., Wang H.Y., Liu Q., Wang R.F. Cancer stem cells and immunosuppressive microenvironment in glioma. Front. Immunol. 2018;9:2924. doi: 10.3389/fimmu.2018.02924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otvos B., Silver D.J., Mulkearns-Hubert E.E., Alvarado A.G., Turaga S.M., Sorensen M.D., Rayman P., Flavahan W.A., Hale J.S., Stoltz K., et al. Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells. 2016;34:2026–2039. doi: 10.1002/stem.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weidenfeld K., Barkan D. EMT and stemness in tumor dormancy and outgrowth: are they intertwined processes? Front Oncol. 2018;8:381. doi: 10.3389/fonc.2018.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarado A.G., Thiagarajan P.S., Mulkearns-Hubert E.E., Silver D.J., Hale J.S., Alban T.J., Turaga S.M., Jarrar A., Reizes O., Longworth M.S., et al. Glioblastoma cancer stem cells evade innate immune suppression of self-renewal through reduced TLR4 expression. Cell Stem Cell. 2017;20:450–461.e454. doi: 10.1016/j.stem.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozlowska A.K., Topchyan P., Kaur K., Tseng H.C., Teruel A., Hiraga T., Jewett A. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J. Cancer. 2017;8:537–554. doi: 10.7150/jca.15989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lun M., Lok E., Gautam S., Wu E., Wong E.T. The natural history of extracranial metastasis from glioblastoma multiforme. J. Neurooncol. 2011;105:261–273. doi: 10.1007/s11060-011-0575-8. [DOI] [PubMed] [Google Scholar]
- 59.Pietschmann S., Von Bueren A.O., Kerber M.J., Baumert B.G., Kortmann R.D., Müller K. An individual patient data meta-analysis on characteristics, treatments and outcomes of glioblastoma/gliosarcoma patients with metastases outside of the central nervous system. PLoS One. 2015;10:e0121592. doi: 10.1371/journal.pone.0121592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunha M., Maldaun M.V.C. Metastasis from glioblastoma multiforme: a meta-analysis. Rev. Assoc. Med. Bras. 2019;65:424–433. doi: 10.1590/1806-9282.65.3.424. [DOI] [PubMed] [Google Scholar]
- 61.Pàez-Ribes M., Allen E., Hudock J., Takeda T., Okuyama H., Viñals F., Inoue M., Bergers G., Hanahan D., Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang W.G., Sanders A.J., Katoh M., Ungefroren H., Gieseler F., Prince M., Thompson S.K., Zollo M., Spano D., Dhawan P., et al. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015;35:244–275. doi: 10.1016/j.semcancer.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Cooper L.A., Gutman D.A., Chisolm C., Appin C., Kong J., Rong Y., Kurc T., Van Meir E.G., Saltz J.H., Moreno C.S., et al. The tumor microenvironment strongly impacts master transcriptional regulators and gene expression class of glioblastoma. Am. J. Pathol. 2012;180:2108–2119. doi: 10.1016/j.ajpath.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwadate Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol. Lett. 2016;11:1615–1620. doi: 10.3892/ol.2016.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pareja F., Macleod D., Shu C., Crary J.F., Canoll P.D., Ross A.H., Siegelin M.D. PI3K and Bcl-2 inhibition primes glioblastoma cells to apoptosis through downregulation of Mcl-1 and Phospho-BAD. Mol. Cancer Res. 2014;12:987–1001. doi: 10.1158/1541-7786.MCR-13-0650. [DOI] [PubMed] [Google Scholar]
- 66.Foster D.A., Yellen P., Xu L., Saqcena M. Regulation of G1 cell cycle progression: distinguishing the restriction point from a nutrient-sensing cell growth checkpoint(s) Genes Cancer. 2010;1:1124–1131. doi: 10.1177/1947601910392989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao N.B., He Y.F., Li X.Q., Wang K., Wang R.L. The role of miRNA and lncRNA in gastric cancer. Oncotarget. 2017;8:81572–81582. doi: 10.18632/oncotarget.19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao T., Rajasingh S., Samanta S., Dawn B., Bittel D.C., Rajasingh J. Biology and clinical relevance of noncoding sno/scaRNAs. Trends Cardiovasc. Med. 2018;28:81–90. doi: 10.1016/j.tcm.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jha P., Agrawal R., Pathak P., Kumar A., Purkait S., Mallik S., Suri V., Chand Sharma M., Gupta D., Suri A., et al. Genome-wide small noncoding RNA profiling of pediatric high-grade gliomas reveals deregulation of several miRNAs, identifies downregulation of snoRNA cluster HBII-52 and delineates H3F3A and TP53 mutant-specific miRNAs and snoRNAs. Int. J. Cancer. 2015;137:2343–2353. doi: 10.1002/ijc.29610. [DOI] [PubMed] [Google Scholar]
- 70.Xu B., Ye M.H., Lv S.G., Wang Q.X., Wu M.J., Xiao B., Kang C.S., Zhu X.G. SNORD47, a box C/D snoRNA, suppresses tumorigenesis in glioblastoma. Oncotarget. 2017;8:43953–43966. doi: 10.18632/oncotarget.16693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L., Han L., Wei J., Zhang K., Shi Z., Duan R., Li S., Zhou X., Pu P., Zhang J., et al. SNORD76, a box C/D snoRNA, acts as a tumor suppressor in glioblastoma. Sci. Rep. 2015;5:8588. doi: 10.1038/srep08588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng R., Yao Q., Ren C., Liu Y., Yang H., Xie G., Du S., Yang K., Yuan Y. Upregulation of long noncoding RNA small nucleolar RNA host gene 18 promotes radioresistance of glioma by repressing semaphorin 5A. Int. J. Radiat. Oncol. Biol. Phys. 2016;96:877–887. doi: 10.1016/j.ijrobp.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 73.Wang Q., Li Q., Zhou P., Deng D., Xue L., Shao N., Peng Y., Zhi F. Upregulation of the long non-coding RNA SNHG1 predicts poor prognosis, promotes cell proliferation and invasion, and reduces apoptosis in glioma. Biomed. Pharmacother. 2017;91:906–911. doi: 10.1016/j.biopha.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 74.Ronchetti D., Mosca L., Cutrona G., Tuana G., Gentile M., Fabris S., Agnelli L., Ciceri G., Matis S., Massucco C., et al. Small nucleolar RNAs as new biomarkers in chronic lymphocytic leukemia. BMC Med. Genomics. 2013;6:27. doi: 10.1186/1755-8794-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ronchetti D., Todoerti K., Tuana G., Agnelli L., Mosca L., Lionetti M., Fabris S., Colapietro P., Miozzo M., Ferrarini M., et al. The expression pattern of small nucleolar and small Cajal body-specific RNAs characterizes distinct molecular subtypes of multiple myeloma. Blood Cancer J. 2012;2:e96. doi: 10.1038/bcj.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong Y., He D., Peng Z., Peng W., Shi W., Wang J., Li B., Zhang C., Duan C. Circular RNAs in cancer: an emerging key player. J. Hematol. Oncol. 2017;10:2. doi: 10.1186/s13045-016-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Enuka Y., Lauriola M., Feldman M.E., Sas-Chen A., Ulitsky I., Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370–1383. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng J., Liu X., Xue Y., Gong W., Ma J., Xi Z., Que Z., Liu Y. TTBK2 circular RNA promotes glioma malignancy by regulating miR-217/HNF1β/Derlin-1 pathway. J. Hematol. Oncol. 2017;10:52. doi: 10.1186/s13045-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y., Gao X., Zhang M., Yan S., Sun C., Xiao F., Huang N., Yang X., Zhao K., Zhou H., et al. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J. Natl. Cancer Inst. 2018;110:304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ren Q., Li H., Wang X. The circular RNA ZNF292 alleviates OGD-induced injury in H9c2 cells via targeting BNIP3. Cell Cycle. 2019;18:3365–3377. doi: 10.1080/15384101.2019.1676585. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Yang P., Qiu Z., Jiang Y., Dong L., Yang W., Gu C., Li G., Zhu Y. Silencing of cZNF292 circular RNA suppresses human glioma tube formation via the Wnt/β-catenin signaling pathway. Oncotarget. 2016;7:63449–63455. doi: 10.18632/oncotarget.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farazi T.A., Juranek S.A., Tuschl T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development. 2008;135:1201–1214. doi: 10.1242/dev.005629. [DOI] [PubMed] [Google Scholar]
- 83.Lu Y., Zhang K., Li C., Yao Y., Tao D., Liu Y., Zhang S., Ma Y. Piwil2 suppresses p53 by inducing phosphorylation of signal transducer and activator of transcription 3 in tumor cells. PLoS One. 2012;7:e30999. doi: 10.1371/journal.pone.0030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhong F., Zhou N., Wu K., Guo Y., Tan W., Zhang H., Zhang X., Geng G., Pan T., Luo H., et al. A SnoRNA-derived piRNA interacts with human interleukin-4 pre-mRNA and induces its decay in nuclear exosomes. Nucleic Acids Res. 2015;43:10474–10491. doi: 10.1093/nar/gkv954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li C., Zhou X., Chen J., Lu Y., Sun Q., Tao D., Hu W., Zheng X., Bian S., Liu Y., et al. PIWIL1 destabilizes microtubule by suppressing phosphorylation at Ser16 and RLIM-mediated degradation of Stathmin1. Oncotarget. 2015;6:27794–27804. doi: 10.18632/oncotarget.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mani S.R., Megosh H., Lin H. PIWI proteins are essential for early Drosophila embryogenesis. Dev. Biol. 2014;385:340–349. doi: 10.1016/j.ydbio.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan H., Liao H., Zhao L., Lu Y., Jiang S., Tao D., Liu Y., Ma Y. HILI destabilizes microtubules by suppressing phosphorylation and Gigaxonin-mediated degradation of TBCB. Sci. Rep. 2017;7:46376. doi: 10.1038/srep46376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du W.W., Yang W., Xuan J., Gupta S., Krylov S.N., Ma X., Yang Q., Yang B.B. Reciprocal regulation of miRNAs and piRNAs in embryonic development. Cell Death Differ. 2016;23:1458–1470. doi: 10.1038/cdd.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He X., Chen X., Zhang X., Duan X., Pan T., Hu Q., Zhang Y., Zhong F., Liu J., Zhang H., et al. An Lnc RNA (GAS5)/SnoRNA-derived piRNA induces activation of TRAIL gene by site-specifically recruiting MLL/COMPASS-like complexes. Nucleic Acids Res. 2015;43:3712–3725. doi: 10.1093/nar/gkv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou N., Liu C., Wang X., Mao Q., Jin Q., Li P. Downregulated SASH1 expression indicates poor clinical prognosis in gastric cancer. Hum. Pathol. 2018;74:83–91. doi: 10.1016/j.humpath.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 91.O’brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Losko M., Kotlinowski J., Jura J. Long noncoding RNAs in metabolic syndrome related disorders. Mediators Inflamm. 2016;2016:5365209. doi: 10.1155/2016/5365209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez I., Munita R., Agirre E., Dittmer T.A., Gysling K., Misteli T., Luco R.F. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat. Struct. Mol. Biol. 2015;22:370–376. doi: 10.1038/nsmb.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romero-Barrios N., Legascue M.F., Benhamed M., Ariel F., Crespi M. Splicing regulation by long noncoding RNAs. Nucleic Acids Res. 2018;46:2169–2184. doi: 10.1093/nar/gky095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vicens Q., Westhof E. Biogenesis of circular RNAs. Cell. 2014;159:13–14. doi: 10.1016/j.cell.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Starke S., Jost I., Rossbach O., Schneider T., Schreiner S., Hung L.H., Bindereif A. Exon circularization requires canonical splice signals. Cell Rep. 2015;10:103–111. doi: 10.1016/j.celrep.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X.O., Dong R., Zhang Y., Zhang J.L., Luo Z., Zhang J., Chen L.L., Yang L. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26:1277–1287. doi: 10.1101/gr.202895.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 99.Ye C.Y., Chen L., Liu C., Zhu Q.H., Fan L. Widespread noncoding circular RNAs in plants. New Phytol. 2015;208:88–95. doi: 10.1111/nph.13585. [DOI] [PubMed] [Google Scholar]
- 100.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y., Jiang F., Xiong Y., Cheng X., Qiu Z., Song R. LncRNA TTN-AS1 sponges miR-376a-3p to promote colorectal cancer progression via upregulating KLF15. Life Sci. 2020;244:116936. doi: 10.1016/j.lfs.2019.116936. [DOI] [PubMed] [Google Scholar]
- 102.Herr I., Sähr H., Zhao Z., Yin L., Omlor G., Lehner B., Fellenberg J. MiR-127 and miR-376a act as tumor suppressors by in vivo targeting of COA1 and PDIA6 in giant cell tumor of bone. Cancer Lett. 2017;409:49–55. doi: 10.1016/j.canlet.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 103.Moore D.L., Blackmore M.G., Hu Y., Kaestner K.H., Bixby J.L., Lemmon V.P., Goldberg J.L. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng Z., Zou X., Jin Y., Gao S., Lv J., Li B., Cui R. The role of KLF(4) in Alzheimer's disease. Front. Cell Neurosci. 2018;12:325. doi: 10.3389/fncel.2018.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y., Song W., Wang L., Rane M.J., Han F., Cai L. Multiple roles of KLF15 in the heart: underlying mechanisms and therapeutic implications. J. Mol. Cell. Cardiol. 2019;129:193–196. doi: 10.1016/j.yjmcc.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 106.Chen L., Jing S.Y., Liu N., Han S., Yang Y.K., Han M.Y., Yan C.X. MiR-376a-3p alleviates the development of glioma through negatively regulating KLF15. Eur. Rev. Med. Pharmacol. Sci. 2020;24:11666–11674. doi: 10.26355/eurrev_202011_23811. [DOI] [PubMed] [Google Scholar]
- 107.Ren F., Su H., Jiang H., Chen Y. Overexpression of miR-623 suppresses progression of hepatocellular carcinoma via regulating the PI3K/Akt signaling pathway by targeting XRCC5. J. Cell. Biochem. 2020;121:213–223. doi: 10.1002/jcb.29117. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y., Peng S., Cen H., Lin Y., Huang C., Chen Y., Shan H., Su Y., Zeng L. MicroRNA hsa-miR-623 directly suppresses MMP1 and attenuates IL-8-induced metastasis in pancreatic cancer. Int. J. Oncol. 2019;55:142–156. doi: 10.3892/ijo.2019.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luo H., Xu R., Chen B., Dong S., Zhou F., Yu T., Xu G., Zhang J., Wang Y., You Y. MicroRNA-940 inhibits glioma cells proliferation and cell cycle progression by targeting CKS1. Am. J. Transl. Res. 2019;11:4851–4865. [PMC free article] [PubMed] [Google Scholar]
- 110.Xu R., Zhou F., Yu T., Xu G., Zhang J., Wang Y., Zhao L., Liu N. MicroRNA-940 inhibits epithelial-mesenchymal transition of glioma cells via targeting ZEB2. Am. J. Transl. Res. 2019;11:7351–7363. [PMC free article] [PubMed] [Google Scholar]
- 111.Wang T., Zhang Y., Cui B., Wang M., Li Y., Gao K. miR-4530 inhibits the malignant biological behaviors of human glioma cells by directly targeting RTEL1. Acta Biochim. Biophys. Sin. 2020;52:1394–1403. doi: 10.1093/abbs/gmaa126. [DOI] [PubMed] [Google Scholar]
- 112.Cui D., Wang K., Liu Y., Gao J., Cui J. MicroRNA-623 inhibits epithelial-mesenchymal transition to attenuate glioma proliferation by targeting TRIM44. Onco Targets Ther. 2020;13:9291–9303. doi: 10.2147/OTT.S250497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y., Yang L., Liao F., Wang W., Wang Z.F. MiR-450a-5p strengthens the drug sensitivity of gefitinib in glioma chemotherapy via regulating autophagy by targeting EGFR. Oncogene. 2020;39:6190–6202. doi: 10.1038/s41388-020-01422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peng H., Qin C., Zhang C., Su J., Xiao Q., Xiao Y., Xiao K., Liu Q. circCPA4 acts as a prognostic factor and regulates the proliferation and metastasis of glioma. J. Cell. Mol. Med. 2019;23:6658–6665. doi: 10.1111/jcmm.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J., Xu S., Xu J., Li Y., Zhang J., Zhang J., Lu X. miR-767-5p inhibits glioma proliferation and metastasis by targeting SUZ12. Oncol. Rep. 2019;42:55–66. doi: 10.3892/or.2019.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lv Q.L., Zhu H.T., Li H.M., Cheng X.H., Zhou H.H., Chen S.H. Down-regulation of miRNA-320c promotes tumor growth and metastasis and predicts poor prognosis in human glioma. Brain Res. Bull. 2018;139:125–132. doi: 10.1016/j.brainresbull.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 117.Chen Y., Li Z.H., Liu X., Liu G.X., Yang H.M., Wu P.F. Reduced expression of miR-3653 in glioma and its correlations with clinical progression and patient survival. Eur. Rev. Med. Pharmacol. Sci. 2019;23:6596–6601. doi: 10.26355/eurrev_201908_18547. [DOI] [PubMed] [Google Scholar]
- 118.Wu H., Liu L., Zhu J.M. MiR-93-5p inhibited proliferation and metastasis of glioma cells by targeting MMP2. Eur. Rev. Med. Pharmacol. Sci. 2019;23:9517–9524. doi: 10.26355/eurrev_201911_19446. [DOI] [PubMed] [Google Scholar]
- 119.Ji C.X., Fan Y.H., Xu F., Lv S.G., Ye M.H., Wu M.J., Zhu X.G., Wu L. MicroRNA-375 inhibits glioma cell proliferation and migration by downregulating RWDD3 in vitro. Oncol. Rep. 2018;39:825–1834. doi: 10.3892/or.2020.7785. [DOI] [PubMed] [Google Scholar]
- 120.Liu X., Wang S., Yuan A., Yuan X., Liu B. MicroRNA-140 represses glioma growth and metastasis by directly targeting ADAM9. Oncol. Rep. 2016;36:2329–2338. doi: 10.3892/or.2016.5007. [DOI] [PubMed] [Google Scholar]
- 121.Gulinaer A.J., Ju A.N., Gao M., Luo Y., Bo Y.L. Over-expression of miR-187 inhibited cell proliferation and metastasis of glioma via down-regulating SMAD1. Eur. Rev. Med. Pharmacol. Sci. 2019;23:10908–10917. doi: 10.26355/eurrev_201912_19794. [DOI] [PubMed] [Google Scholar]
- 122.Peng T., Zhang S., Li W., Fu S., Luan Y., Zuo L. MicroRNA-141 inhibits glioma cells growth and metastasis by targeting TGF-β2. Am. J. Transl. Res. 2016;8:3513–3521. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Li J., Yuan J., Yuan X., Zhao J., Zhang Z., Weng L., Liu J. MicroRNA-200b inhibits the growth and metastasis of glioma cells via targeting ZEB2. Int. J. Oncol. 2016;48:541–550. doi: 10.3892/ijo.2015.3267. [DOI] [PubMed] [Google Scholar]
- 124.Yang Y., Liu X., Cheng L., Li L., Wei Z., Wang Z., Han G., Wan X., Wang Z., Zhang J., et al. Tumor suppressor microRNA-138 suppresses low-grade glioma development and metastasis via regulating IGF2BP2. Onco Targets Ther. 2020;13:2247–2260. doi: 10.2147/OTT.S232795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou H., Zhang Y., Lai Y., Xu C., Cheng Y. Circ_101064 regulates the proliferation, invasion and migration of glioma cells through miR-154-5p/PIWIL1 axis. Biochem. Biophys. Res. Commun. 2020;523:608–614. doi: 10.1016/j.bbrc.2019.12.096. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y.H., Li B., Meng F.G., Qiu L. MiR-508-5p is a prognostic marker and inhibits cell proliferation and migration in glioma. Eur. Rev. Med. Pharmacol. Sci. 2017;21:76–81. [PubMed] [Google Scholar]
- 127.Thunshelle C., Yin R., Chen Q., Hamblin M.R. Current advances in 5-aminolevulinic acid mediated photodynamic therapy. Curr. Dermatol. Rep. 2016;5:179–190. doi: 10.1007/s13671-016-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li L., Shao M.Y., Zou S.C., Xiao Z.F., Chen Z.C. MiR-101-3p inhibits EMT to attenuate proliferation and metastasis in glioblastoma by targeting TRIM44. J. Neurooncol. 2019;141:19–30. doi: 10.1007/s11060-018-2973-7. [DOI] [PubMed] [Google Scholar]
- 129.Shi H.Z., Wang D., Sun X.N., Sheng L. MicroRNA-378 acts as a prognosis marker and inhibits cell migration, invasion and epithelial-mesenchymal transition in human glioma by targeting IRG1. Eur. Rev. Med. Pharmacol. Sci. 2018;22:3837–3846. doi: 10.26355/eurrev_201806_15268. [DOI] [PubMed] [Google Scholar]
- 130.Liu J., Jiang J., Hui X., Wang W., Fang D., Ding L. Mir-758-5p suppresses glioblastoma proliferation, migration and invasion by targeting ZBTB20. Cell. Physiol. Biochem. 2018;48:2074–2083. doi: 10.1159/000492545. [DOI] [PubMed] [Google Scholar]
- 131.Liu C., Liang S., Xiao S., Lin Q., Chen X., Wu Y., Fu J. MicroRNA-27b inhibits Spry2 expression and promotes cell invasion in glioma U251 cells. Oncol. Lett. 2015;9:1393–1397. doi: 10.3892/ol.2015.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao C., Wang X.B., Zhang Y.H., Zhou Y.M., Yin Q., Yao W.C. MicroRNA-424 inhibits cell migration, invasion and epithelial-mesenchymal transition in human glioma by targeting KIF23 and functions as a novel prognostic predictor. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6369–6378. doi: 10.26355/eurrev_201810_16049. [DOI] [PubMed] [Google Scholar]
- 133.Wang X., Sun S., Tong X., Ma Q., Di H., Fu T., Sun Z., Cai Y., Fan W., Wu Q., et al. MiRNA-154-5p inhibits cell proliferation and metastasis by targeting PIWIL1 in glioblastoma. Brain Res. 2017;1676:69–76. doi: 10.1016/j.brainres.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 134.Wang H., Li X.T., Wu C., Wu Z.W., Li Y.Y., Yang T.Q., Chen G.L., Xie X.S., Huang Y.L., Du Z.W., et al. miR-132 can inhibit glioma cells invasion and migration by target MMP16 in vitro. Onco Targets Ther. 2015;8:3211–3218. doi: 10.2147/OTT.S79282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Peng B., Hu S., Jun Q., Luo D., Zhang X., Zhao H., Li D. MicroRNA-200b targets CREB1 and suppresses cell growth in human malignant glioma. Mol. Cell. Biochem. 2013;379:51–58. doi: 10.1007/s11010-013-1626-6. [DOI] [PubMed] [Google Scholar]
- 136.Pan D.S., Cao P., Li J.J., Fan D., Song Z.Q. MicroRNA-374b inhibits migration and invasion of glioma cells by targeting EGFR. Eur. Rev. Med. Pharmacol. Sci. 2019;23:4254–4263. doi: 10.26355/eurrev_201905_17930. [DOI] [PubMed] [Google Scholar]
- 137.Zhang R., Luo H., Wang S., Chen Z., Hua L., Wang H.W., Chen W., Yuan Y., Zhou X., Li D., et al. MiR-622 suppresses proliferation, invasion and migration by directly targeting activating transcription factor 2 in glioma cells. J. Neurooncol. 2015;121:63–72. doi: 10.1007/s11060-014-1607-y. [DOI] [PubMed] [Google Scholar]
- 138.Zhao Y., Huang W., Kim T.M., Jung Y., Menon L.G., Xing H., Li H., Carroll R.S., Park P.J., Yang H.W., et al. MicroRNA-29a activates a multi-component growth and invasion program in glioblastoma. J. Exp. Clin. Cancer Res. 2019;38:36. doi: 10.1186/s13046-019-1026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang H., Tang C., Na M., Ma W., Jiang Z., Gu Y., Ma G., Ge H., Shen H., Lin Z. miR-422a inhibits glioma proliferation and invasion by targeting IGF1 and IGF1R. Oncol. Res. 2017;25:187–194. doi: 10.3727/096504016X14732772150389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shi L., Yuan Y., Li H.Y. MicroRNA-139-3p suppresses growth and metastasis of glioblastoma via inhibition of NIN1/RPNI2 binding protein 1 homolog. Eur. Rev. Med. Pharmacol. Sci. 2019;23:4264–4274. doi: 10.26355/eurrev_201905_17931. [DOI] [PubMed] [Google Scholar]
- 141.Meng Y., Shang F.R., Zhu Y.L. MiR-491 functions as a tumor suppressor through Wnt3a/β-catenin signaling in the development of glioma. Eur. Rev. Med. Pharmacol. Sci. 2019;23:10899–10907. doi: 10.26355/eurrev_201912_19793. [DOI] [PubMed] [Google Scholar]
- 142.Yan L., Cai K., Sun K., Gui J., Liang J. MiR-1290 promotes proliferation, migration, and invasion of glioma cells by targeting LHX6. J. Cell. Physiol. 2018;233:621–6629. doi: 10.1002/jcp.26381. [DOI] [PubMed] [Google Scholar]
- 143.Wang W., Dai L.X., Zhang S., Yang Y., Yan N., Fan P., Dai L., Tian H.W., Cheng L., Zhang X.M., et al. Regulation of epidermal growth factor receptor signaling by plasmid-based microRNA-7 inhibits human malignant gliomas growth and metastasis in vivo. Neoplasma. 2013;60:74–283. doi: 10.4149/neo_2013_036. [DOI] [PubMed] [Google Scholar]
- 144.Wu X., Hu C., Long C., Zhai X., Liang P., Yu Z. MicroRNA-351 promotes the proliferation and invasion of glioma cells through downregulation of NAIF1. J. Mol. Neurosci. 2020;70:1493–1499. doi: 10.1007/s12031-020-01582-z. [DOI] [PubMed] [Google Scholar]
- 145.Tan Z., Jia J., Jiang Y. MiR-150-3p targets SP1 and suppresses the growth of glioma cells. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180019. BSR20180019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li C., Liu Z., Yang K., Chen X., Zeng Y., Liu J., Li Z., Liu Y. miR-133b inhibits glioma cell proliferation and invasion by targeting Sirt1. Oncotarget. 2016;7:36247–36254. doi: 10.18632/oncotarget.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jian Y., Xu C.H., Li Y.P., Tang B., Xie S.H., Zeng E.M. Down-regulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by up-regulating RECK. Biosci. Rep. 2019;39 doi: 10.1042/BSR20182226. BSR20182226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 148.Liu Z.Q., Ren J.J., Zhao J.L., Zang J., Long Q.F., Du J.J., Jia X.T., Gu N.B., Di Z.L., Qian Y.H., et al. MicroRNA-144 represses gliomas progression and elevates susceptibility to Temozolomide by targeting CAV2 and FGF7. Sci. Rep. 2020;10:4155. doi: 10.1038/s41598-020-60218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y., Wu Z., Li L., Xie M. miR-30a inhibits glioma progression and stem cell-like properties by repression of Wnt5a. Oncol. Rep. 2017;38:1156–1162. doi: 10.3892/or.2017.5728. [DOI] [PubMed] [Google Scholar]
- 150.Yang F., Wang W., Zhou C., Xi W., Yuan L., Chen X., Li Y., Yang A., Zhang J., Wang T. MiR-221/222 promote human glioma cell invasion and angiogenesis by targeting TIMP2. Tumour Biol. 2015;36:3763–3773. doi: 10.1007/s13277-014-3017-3. [DOI] [PubMed] [Google Scholar]
- 151.Wang Y., Lin Y. Hsa-mir-127 impairs survival of patients with glioma and promotes proliferation, migration and invasion of cancerous cells by modulating replication initiator 1. Neuroreport. 2018;29:1166–1173. doi: 10.1097/WNR.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 152.Yang J., Fan B., Zhao Y., Fang J. MicroRNA-202 inhibits cell proliferation, migration and invasion of glioma by directly targeting metadherin. Oncol. Rep. 2017;38:1670–1678. doi: 10.3892/or.2017.5815. [DOI] [PubMed] [Google Scholar]
- 153.Jiang B., Li M., Ji F., Nie Y. MicroRNA-219 exerts a tumor suppressive role in glioma via targeting Sal-like protein 4. Exp. Ther. Med. 2017;14:6213–6221. doi: 10.3892/etm.2017.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xia Z., Liu F., Zhang J., Liu L. Decreased expression of MiRNA-204-5p contributes to glioma progression and promotes glioma cell growth, migration and invasion. PLoS One. 2015;10:e0132399. doi: 10.1371/journal.pone.0132399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ding L., Wang L., Guo F. microRNA-188 acts as a tumour suppressor in glioma by directly targeting the IGF2BP2 gene. Mol. Med. Rep. 2017;16:7124–7130. doi: 10.3892/mmr.2017.7433. [DOI] [PubMed] [Google Scholar]
- 156.Qin Y., Chen W., Liu B., Zhou L., Deng L., Niu W., Bao D., Cheng C., Li D., Liu S., et al. MiR-200c inhibits the tumor progression of glioma via targeting moesin. Theranostics. 2017;7:1663–1673. doi: 10.7150/thno.17886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun J.Y., Xiao W.Z., Wang F., Wang Y.Q., Zhu Y.H., Wu Y.F., Miao Z.L., Lin Y.C. MicroRNA-320 inhibits cell proliferation in glioma by targeting E2F1. Mol. Med. Rep. 2015;12:2355–2359. doi: 10.3892/mmr.2015.3657. [DOI] [PubMed] [Google Scholar]
- 158.Tian W., Wu W., Li X., Rui X., Wu Y. MiRNA-139-3p inhibits the proliferation, invasion, and migration of human glioma cells by targeting MDA-9/syntenin. Biochem. Biophys. Res. Commun. 2019;508:295–301. doi: 10.1016/j.bbrc.2018.11.144. [DOI] [PubMed] [Google Scholar]
- 159.Que T., Song Y., Liu Z., Zheng S., Long H., Li Z., Liu Y., Wang G., Liu Y., Zhou J., et al. Decreased miRNA-637 is an unfavorable prognosis marker and promotes glioma cell growth, migration and invasion via direct targeting Akt1. Oncogene. 2015;34:4952–4963. doi: 10.1038/onc.2014.419. [DOI] [PubMed] [Google Scholar]
- 160.Li Y., Wu Y., Sun Z., Wang R., Ma D. MicroRNA-376a inhibits cell proliferation and invasion in glioblastoma multiforme by directly targeting specificity protein 1. Mol. Med. Rep. 2018;17:1583–1590. doi: 10.3892/mmr.2017.8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pang H., Zheng Y., Zhao Y., Xiu X., Wang J. miR-590-3p suppresses cancer cell migration, invasion and epithelial-mesenchymal transition in glioblastoma multiforme by targeting ZEB1 and ZEB2. Biochem. Biophys. Res. Commun. 2015;468:739–745. doi: 10.1016/j.bbrc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 162.Han Y., Wu Z., Wu T., Huang Y., Cheng Z., Li X., Sun T., Xie X., Zhou Y., Du Z. Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by downregulation of MMP2 and inactivation of ERK/MAPK signaling. Cell Death Dis. 2016;7:e2123. doi: 10.1038/cddis.2015.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen Z., Cheng Q., Ma Z., Xi H., Peng R., Jiang B. Overexpression of RKIP inhibits cell invasion in glioma cell lines through upregulation of miR-98. Biomed. Res. Int. 2013;2013:695179. doi: 10.1155/2013/695179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu M., Wang Y., Zhou S., Xu J., Li J., Tao R., Zhu Y. MicroRNA-370 suppresses the progression and proliferation of human astrocytoma and glioblastoma by negatively regulating β-catenin and causing activation of FOXO3a. Exp. Ther. Med. 2018;15:1093–1098. doi: 10.3892/etm.2017.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yue S., Wang L., Zhang H., Min Y., Lou Y., Sun H., Jiang Y., Zhang W., Liang A., Guo Y., et al. miR-139-5p suppresses cancer cell migration and invasion through targeting ZEB1 and ZEB2 in GBM. Tumour Biol. 2015;36:6741–6749. doi: 10.1007/s13277-015-3372-8. [DOI] [PubMed] [Google Scholar]
- 166.Wang H., Zhi H., Ma D., Li T. MiR-217 promoted the proliferation and invasion of glioblastoma by repressing YWHAG. Cytokine. 2017;92:93–102. doi: 10.1016/j.cyto.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 167.Pan Y., Liang W., Zhao X., Liu L., Qing Y., Li Y. miR-548b inhibits the proliferation and invasion of malignant gliomas by targeting metastasis tumor-associated protein-2. Neuroreport. 2016;27:1266–1273. doi: 10.1097/WNR.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y., Zhu Y., Chen J., Wang Y., Sherwood M.E., Murray C.K., Vrahas M.S., Hooper D.C., Hamblin M.R., Dai T. Antimicrobial blue light inactivation of Candida albicans: in vitro and in vivo studies. Virulence. 2016;7:536–545. doi: 10.1080/21505594.2016.1155015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Y., Ma X., Wang Y., Li G. miR-489 inhibits proliferation, cell cycle progression and induces apoptosis of glioma cells via targeting SPIN1-mediated PI3K/AKT pathway. Biomed. Pharmacother. 2017;93:435–443. doi: 10.1016/j.biopha.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 170.Ma C., Wei F., Xia H., Liu H., Dong X., Zhang Y., Luo Q., Liu Y., Li Y. MicroRNA-10b mediates TGF-β1-regulated glioblastoma proliferation, migration and epithelial-mesenchymal transition. Int. J. Oncol. 2017;50:1739–1748. doi: 10.3892/ijo.2017.3947. [DOI] [PubMed] [Google Scholar]
- 171.Wang Z., Wang B., Shi Y., Xu C., Xiao H.L., Ma L.N., Xu S.L., Yang L., Wang Q.L., Dang W.Q., et al. Oncogenic miR-20a and miR-106a enhance the invasiveness of human glioma stem cells by directly targeting TIMP-2. Oncogene. 2015;34:1407–1419. doi: 10.1038/onc.2014.75. [DOI] [PubMed] [Google Scholar]
- 172.Xia H., Qi Y., Ng S.S., Chen X., Li D., Chen S., Ge R., Jiang S., Li G., Chen Y., et al. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–165. doi: 10.1016/j.brainres.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 173.Pal D., Mukhopadhyay D., Ramaiah M.J., Sarma P., Bhadra U., Bhadra M.P. Regulation of cell proliferation and migration by miR-203 via GAS41/miR-10b Axis in human glioblastoma cells. PLoS One. 2016;11:e0159092. doi: 10.1371/journal.pone.0159092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mao J., Zhang M., Zhong M., Zhang Y., Lv K. MicroRNA-204, a direct negative regulator of ezrin gene expression, inhibits glioma cell migration and invasion. Mol. Cell. Biochem. 2014;396:117–128. doi: 10.1007/s11010-014-2148-6. [DOI] [PubMed] [Google Scholar]
- 175.Wang R.J., Li J.W., Bao B.H., Wu H.C., Du Z.H., Su J.L., Zhang M.H., Liang H.Q. MicroRNA-873 (miRNA-873) inhibits glioblastoma tumorigenesis and metastasis by suppressing the expression of IGF2BP1. J. Biol. Chem. 2015;290:8938–8948. doi: 10.1074/jbc.M114.624700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cheng Z.X., Song Y.X., Wang Z.Y., Wang Y., Dong Y. miR-144-3p serves as a tumor suppressor by targeting FZD7 and predicts the prognosis of human glioblastoma. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4079–4086. [PubMed] [Google Scholar]
- 177.Zhao W.H., Wu S.Q., Zhang Y.D. Downregulation of miR-124 promotes the growth and invasiveness of glioblastoma cells involving upregulation of PPP1R13L. Int. J. Mol. Med. 2013;32:101–107. doi: 10.3892/ijmm.2013.1365. [DOI] [PubMed] [Google Scholar]
- 178.Wu X., Hu C., Long C., Zhai X., Liang P., Yu Z. Correction to: MicroRNA-351 promotes the proliferation and invasion of glioma cells through downregulation of NAIF1. J. Mol. Neurosci. 2020;70:1500. doi: 10.1007/s12031-020-01655-z. [DOI] [PubMed] [Google Scholar]
- 179.Ben-Hamo R., Zilberberg A., Cohen H., Efroni S. hsa-miR-9 controls the mobility behavior of glioblastoma cells via regulation of MAPK14 signaling elements. Oncotarget. 2016;7:23170–23181. doi: 10.18632/oncotarget.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang J., Chen C., Yan X., Wang P. The role of miR-382-5p in glioma cell proliferation, migration and invasion. Onco Targets Ther. 2019;12:4993–5002. doi: 10.2147/OTT.S196322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ong C.A., Shannon N.B., Ross-Innes C.S., O'donovan M., Rueda O.M., Hu D.E., Kettunen M.I., Walker C.E., Noorani A., Hardwick R.H., et al. Amplification of TRIM44: pairing a prognostic target with potential therapeutic strategy. J. Natl. Cancer Inst. 2014;106:dju050. doi: 10.1093/jnci/dju050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li C.G., Hu H., Yang X.J., Huang C.Q., Yu X.Q. TRIM44 promotes colorectal cancer proliferation, migration, and invasion through the Akt/mTOR signaling pathway. Onco Targets Ther. 2019;12:10693–10701. doi: 10.2147/OTT.S228637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen Z., Lin T.C., Bi X., Lu G., Dawson B.C., Miranda R., Medeiros L.J., Mcniece I., Mccarty N. TRIM44 promotes quiescent multiple myeloma cell occupancy and survival in the osteoblastic niche via HIF-1α stabilization. Leukemia. 2019;33:469–486. doi: 10.1038/s41375-018-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou X., Yang Y., Ma P., Wang N., Yang D., Tu Q., Sun B., Xiang T., Zhao X., Hou Z., et al. TRIM44 is indispensable for glioma cell proliferation and cell cycle progression through AKT/p21/p27 signaling pathway. J. Neurooncol. 2019;145:211–222. doi: 10.1007/s11060-019-03301-0. [DOI] [PubMed] [Google Scholar]
- 185.Xiong D., Jin C., Ye X., Qiu B., Jianjun X., Zhu S., Xiang L., Wu H., Yongbing W. TRIM44 promotes human esophageal cancer progression via the AKT/mTOR pathway. Cancer Sci. 2018;109:3080–3092. doi: 10.1111/cas.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou Z., Liu Y., Ma M., Chang L. Knockdown of TRIM44 inhibits the proliferation and invasion in papillary thyroid cancer cells through suppressing the Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2017;96:98–103. doi: 10.1016/j.biopha.2017.09.132. [DOI] [PubMed] [Google Scholar]
- 187.Liang S., Gong X., Zhang G., Huang G., Lu Y., Li Y. MicroRNA-140 regulates cell growth and invasion in pancreatic duct adenocarcinoma by targeting iASPP. Acta Biochim. Biophys. Sin. 2016;48:174–181. doi: 10.1093/abbs/gmv127. [DOI] [PubMed] [Google Scholar]
- 188.Kong X.M., Zhang G.H., Huo Y.K., Zhao X.H., Cao D.W., Guo S.F., Li A.M., Zhang X.R. MicroRNA-140-3p inhibits proliferation, migration and invasion of lung cancer cells by targeting ATP6AP2. Int. J. Clin. Exp. Pathol. 2015;8:12845–12852. [PMC free article] [PubMed] [Google Scholar]
- 189.Dong W., Yao C., Teng X., Chai J., Yang X., Li B. MiR-140-3p suppressed cell growth and invasion by downregulating the expression of ATP8A1 in non-small cell lung cancer. Tumour Biol. 2016;37:2973–2985. doi: 10.1007/s13277-015-3452-9. [DOI] [PubMed] [Google Scholar]
- 190.Zhang W., Zou C., Pan L., Xu Y., Qi W., Ma G., Hou Y., Jiang P. MicroRNA-140-5p inhibits the progression of colorectal cancer by targeting VEGFA. Cell. Physiol. Biochem. 2015;37:1123–1133. doi: 10.1159/000430237. [DOI] [PubMed] [Google Scholar]
- 191.Lan H., Chen W., He G., Yang S. miR-140-5p inhibits ovarian cancer growth partially by repression of PDGFRA. Biomed. Pharmacother. 2015;75:117–122. doi: 10.1016/j.biopha.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 192.Li W., Jiang G., Zhou J., Wang H., Gong Z., Zhang Z., Min K., Zhu H., Tan Y. Down-regulation of miR-140 induces EMT and promotes invasion by targeting slug in esophageal cancer. Cell. Physiol. Biochem. 2014;34:1466–1476. doi: 10.1159/000366351. [DOI] [PubMed] [Google Scholar]
- 193.Kai Y., Peng W., Ling W., Jiebing H., Zhuan B. Reciprocal effects between microRNA-140-5p and ADAM10 suppress migration and invasion of human tongue cancer cells. Biochem. Biophys. Res. Commun. 2014;448:308–314. doi: 10.1016/j.bbrc.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 194.Zou M.X., Huang W., Wang X.B., Lv G.H., Li J., Deng Y.W. Identification of miR-140-3p as a marker associated with poor prognosis in spinal chordoma. Int. J. Clin. Exp. Pathol. 2014;7:4877–4885. [PMC free article] [PubMed] [Google Scholar]
- 195.Güllü G., Peker I., Haholu A., Eren F., Küçükodaci Z., Güleç B., Baloglu H., Erzik C., Özer A., Akkiprik M. Clinical significance of miR-140-5p and miR-193b expression in patients with breast cancer and relationship to IGFBP5. Genet. Mol. Biol. 2015;38:21–29. doi: 10.1590/S1415-475738120140167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang H., Fang F., Chang R., Yang L. MicroRNA-140-5p suppresses tumor growth and metastasis by targeting transforming growth factor β receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology. 2013;58:205–217. doi: 10.1002/hep.26315. [DOI] [PubMed] [Google Scholar]
- 197.Yuan Y., Shen Y., Xue L., Fan H. miR-140 suppresses tumor growth and metastasis of non-small cell lung cancer by targeting insulin-like growth factor 1 receptor. PLoS One. 2013;8:e73604. doi: 10.1371/journal.pone.0073604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Blobel C.P. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 199.Guaiquil V., Swendeman S., Yoshida T., Chavala S., Campochiaro P.A., Blobel C.P. ADAM9 is involved in pathological retinal neovascularization. Mol. Cell. Biol. 2009;29:2694–2703. doi: 10.1128/MCB.01460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Duffy M.J., Mckiernan E., O'donovan N., Mcgowan P.M. Role of ADAMs in cancer formation and progression. Clin. Cancer Res. 2009;15:1140–1144. doi: 10.1158/1078-0432.CCR-08-1585. [DOI] [PubMed] [Google Scholar]
- 201.Fritzsche F.R., Wassermann K., Jung M., Tölle A., Kristiansen I., Lein M., Johannsen M., Dietel M., Jung K., Kristiansen G. ADAM9 is highly expressed in renal cell cancer and is associated with tumour progression. BMC Cancer. 2008;8:179. doi: 10.1186/1471-2407-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fritzsche F.R., Jung M., Tölle A., Wild P., Hartmann A., Wassermann K., Rabien A., Lein M., Dietel M., Pilarsky C., et al. ADAM9 expression is a significant and independent prognostic marker of PSA relapse in prostate cancer. Eur. Urol. 2008;54:1097–1106. doi: 10.1016/j.eururo.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 203.O'shea C., Mckie N., Buggy Y., Duggan C., Hill A.D., Mcdermott E., O'higgins N., Duffy M.J. Expression of ADAM-9 mRNA and protein in human breast cancer. Int. J. Cancer. 2003;105:754–761. doi: 10.1002/ijc.11161. [DOI] [PubMed] [Google Scholar]
- 204.Tao K., Qian N., Tang Y., Ti Z., Song W., Cao D., Dou K. Increased expression of a disintegrin and metalloprotease-9 in hepatocellular carcinoma: implications for tumor progression and prognosis. Jpn. J. Clin. Oncol. 2010;40:645–651. doi: 10.1093/jjco/hyq030. [DOI] [PubMed] [Google Scholar]
- 205.Grützmann R., Lüttges J., Sipos B., Ammerpohl O., Dobrowolski F., Alldinger I., Kersting S., Ockert D., Koch R., Kalthoff H., et al. ADAM9 expression in pancreatic cancer is associated with tumour type and is a prognostic factor in ductal adenocarcinoma. Br. J. Cancer. 2004;90:1053–1058. doi: 10.1038/sj.bjc.6601645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim Y.H., Shin E.K., Kim D.H., Lee H.H., Park J.H., Kim J.K. Antiangiogenic effect of licochalcone A. Biochem. Pharmacol. 2010;80:1152–1159. doi: 10.1016/j.bcp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 207.Chen C.M., Hsieh Y.H., Hwang J.M., Jan H.J., Hsieh S.C., Lin S.H., Lai C.Y. Fisetin suppresses ADAM9 expression and inhibits invasion of glioma cancer cells through increased phosphorylation of ERK1/2. Tumour Biol. 2015;36:3407–3415. doi: 10.1007/s13277-014-2975-9. [DOI] [PubMed] [Google Scholar]
- 208.Qiu T., Zhou X., Wang J., Du Y., Xu J., Huang Z., Zhu W., Shu Y., Liu P. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Lett. 2014;588:1168–1177. doi: 10.1016/j.febslet.2014.02.054. [DOI] [PubMed] [Google Scholar]
- 209.Chen X.N., Wang K.F., Xu Z.Q., Li S.J., Liu Q., Fu D.H., Wang X., Wu B. MiR-133b regulates bladder cancer cell proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer Cell Int. 2014;14:70. doi: 10.1186/s12935-014-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Crawford M., Batte K., Yu L., Wu X., Nuovo G.J., Marsh C.B., Otterson G.A., Nana-Sinkam S.P. MicroRNA 133B targets pro-survival molecules MCL-1 and BCL2L2 in lung cancer. Biochem. Biophys. Res. Commun. 2009;388:483–489. doi: 10.1016/j.bbrc.2009.07.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hu G., Chen D., Li X., Yang K., Wang H., Wu W. miR-133b regulates the MET proto-oncogene and inhibits the growth of colorectal cancer cells in vitro and in vivo. Cancer Biol. Ther. 2010;10:190–197. doi: 10.4161/cbt.10.2.12186. [DOI] [PubMed] [Google Scholar]
- 212.Wang J., Li Y., Jiang C. MiR-133b contributes to arsenic-induced apoptosis in U251 glioma cells by targeting the hERG channel. J. Mol. Neurosci. 2015;55:985–994. doi: 10.1007/s12031-014-0455-8. [DOI] [PubMed] [Google Scholar]
- 213.Wu Y., Meng X., Huang C., Li J. Emerging role of silent information regulator 1 (SIRT1) in hepatocellular carcinoma: a potential therapeutic target. Tumour Biol. 2015;36:4063–4074. doi: 10.1007/s13277-015-3488-x. [DOI] [PubMed] [Google Scholar]
- 214.Qiu G., Li X., Che X., Wei C., He S., Lu J., Jia Z., Pang K., Fan L. SIRT1 is a regulator of autophagy: implications in gastric cancer progression and treatment. FEBS Lett. 2015;589:2034–2042. doi: 10.1016/j.febslet.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 215.Li L., Bhatia R. Role of SIRT1 in the growth and regulation of normal hematopoietic and leukemia stem cells. Curr. Opin. Hematol. 2015;22:324–329. doi: 10.1097/MOH.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cetrullo S., D'adamo S., Tantini B., Borzi R.M., Flamigni F. mTOR, AMPK, and Sirt1: key players in metabolic stress management. Crit. Rev. Eukaryot. Gene Expr. 2015;25:59–75. doi: 10.1615/critreveukaryotgeneexpr.2015012975. [DOI] [PubMed] [Google Scholar]
- 217.Ramis M.R., Esteban S., Miralles A., Tan D.X., Reiter R.J. Caloric restriction, resveratrol and melatonin: role of SIRT1 and implications for aging and related-diseases. Mech. Ageing Dev. 2015;146-148:28–41. doi: 10.1016/j.mad.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 218.Sasaki T. Age-associated weight gain, leptin, and SIRT1: a possible role for hypothalamic SIRT1 in the prevention of weight gain and aging through modulation of leptin sensitivity. Front. Endocrinol. 2015;6:109. doi: 10.3389/fendo.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Park S.Y., Lee S.W., Kim H.Y., Lee S.Y., Lee W.S., Hong K.W., Kim C.D. Suppression of RANKL-induced osteoclast differentiation by cilostazol via SIRT1-induced RANK inhibition. Biochim. Biophys. Acta. 2015;1852:2137–2144. doi: 10.1016/j.bbadis.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 220.Marampon F., Gravina G.L., Festuccia C., Popov V.M., Colapietro A., Sanità P., Musio D., De Felice F., Lenzi A., Jannini E.A., et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Invest. 2016;39:411–422. doi: 10.1007/s40618-015-0381-9. [DOI] [PubMed] [Google Scholar]
- 221.Kim H., Lee K.H., Park I.A., Chung Y.R., Im S.A., Noh D.Y., Han W., Moon H.G., Jung Y.Y., Ryu H.S. Expression of SIRT1 and apoptosis-related proteins is predictive for lymph node metastasis and disease-free survival in luminal A breast cancer. Virchows Arch. 2015;467:563–570. doi: 10.1007/s00428-015-1815-7. [DOI] [PubMed] [Google Scholar]
- 222.Zhang X., Chen S., Cheng M., Cao F., Cheng Y. The expression and correlation of SIRT1 and Phospho-SIRT1 in colorectal cancer. Int. J. Clin. Exp. Med. 2015;8:809–817. [PMC free article] [PubMed] [Google Scholar]
- 223.Lu J., Zhang L., Chen X., Lu Q., Yang Y., Liu J., Ma X. SIRT1 counteracted the activation of STAT3 and NF-κB to repress the gastric cancer growth. Int. J. Clin. Exp. Med. 2014;7:5050–5058. [PMC free article] [PubMed] [Google Scholar]
- 224.Qu Y., Zhang J., Wu S., Li B., Liu S., Cheng J. SIRT1 promotes proliferation and inhibits apoptosis of human malignant glioma cell lines. Neurosci. Lett. 2012;525:168–172. doi: 10.1016/j.neulet.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 225.Chang C.J., Hsu C.C., Yung M.C., Chen K.Y., Tzao C., Wu W.F., Chou H.Y., Lee Y.Y., Lu K.H., Chiou S.H., et al. Enhanced radiosensitivity and radiation-induced apoptosis in glioma CD133-positive cells by knockdown of SirT1 expression. Biochem. Biophys. Res. Commun. 2009;380:236–242. doi: 10.1016/j.bbrc.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 226.Lee J.S., Park J.R., Kwon O.S., Lee T.H., Nakano I., Miyoshi H., Chun K.H., Park M.J., Lee H.J., Kim S.U., et al. SIRT1 is required for oncogenic transformation of neural stem cells and for the survival of "cancer cells with neural stemness" in a p53-dependent manner. Neuro Oncol. 2015;17:95–106. doi: 10.1093/neuonc/nou145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wan L., Zhang L., Fan K., Wang J. MiR-27b targets LIMK1 to inhibit growth and invasion of NSCLC cells. Mol. Cell. Biochem. 2014;390:85–91. doi: 10.1007/s11010-013-1959-1. [DOI] [PubMed] [Google Scholar]
- 228.Ye J., Wu X., Wu D., Wu P., Ni C., Zhang Z., Chen Z., Qiu F., Xu J., Huang J. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS One. 2013;8:e60687. doi: 10.1371/journal.pone.0060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ishteiwy R.A., Ward T.M., Dykxhoorn D.M., Burnstein K.L. The microRNA -23b/-27b cluster suppresses the metastatic phenotype of castration-resistant prostate cancer cells. PLoS One. 2012;7:e52106. doi: 10.1371/journal.pone.0052106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lee J.J., Drakaki A., Iliopoulos D., Struhl K. MiR-27b targets PPARγ to inhibit growth, tumor progression and the inflammatory response in neuroblastoma cells. Oncogene. 2012;31:3818–3825. doi: 10.1038/onc.2011.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jin L., Wessely O., Marcusson E.G., Ivan C., Calin G.A., Alahari S.K. Prooncogenic factors miR-23b and miR-27b are regulated by Her2/Neu, EGF, and TNF-α in breast cancer. Cancer Res. 2013;73:2884–2896. doi: 10.1158/0008-5472.CAN-12-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cabrita M.A., Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. doi: 10.1007/s10456-008-9089-1. [DOI] [PubMed] [Google Scholar]
- 233.Mei Y., Bian C., Li J., Du Z., Zhou H., Yang Z., Zhao R.C. miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J. Cell. Biochem. 2013;114:1374–1384. doi: 10.1002/jcb.24479. [DOI] [PubMed] [Google Scholar]
- 234.Wang C., Delogu S., Ho C., Lee S.A., Gui B., Jiang L., Ladu S., Cigliano A., Dombrowski F., Evert M., et al. Inactivation of Spry2 accelerates AKT-driven hepatocarcinogenesis via activation of MAPK and PKM2 pathways. J. Hepatol. 2012;57:577–583. doi: 10.1016/j.jhep.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kwak H.J., Kim Y.J., Chun K.R., Woo Y.M., Park S.J., Jeong J.A., Jo S.H., Kim T.H., Min H.S., Chae J.S., et al. Downregulation of Spry2 by miR-21 triggers malignancy in human gliomas. Oncogene. 2011;30:2433–2442. doi: 10.1038/onc.2010.620. [DOI] [PubMed] [Google Scholar]
- 236.Xu T.P., Wang W.Y., Ma P., Shuai Y., Zhao K., Wang Y.F., Li W., Xia R., Chen W.M., Zhang E.B., et al. Upregulation of the long noncoding RNA FOXD2-AS1 promotes carcinogenesis by epigenetically silencing EphB3 through EZH2 and LSD1, and predicts poor prognosis in gastric cancer. Oncogene. 2018;37:5020–5036. doi: 10.1038/s41388-018-0308-y. [DOI] [PubMed] [Google Scholar]
- 237.Rong L., Zhao R., Lu J. Highly expressed long non-coding RNA FOXD2-AS1 promotes non-small cell lung cancer progression via Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2017;484:586–591. doi: 10.1016/j.bbrc.2017.01.141. [DOI] [PubMed] [Google Scholar]
- 238.Yuan Y., Liu W., Zhang Y., Zhang Y., Sun S. CircRNA circ_0026344 as a prognostic biomarker suppresses colorectal cancer progression via microRNA-21 and microRNA-31. Biochem. Biophys. Res. Commun. 2018;503:870–875. doi: 10.1016/j.bbrc.2018.06.089. [DOI] [PubMed] [Google Scholar]
- 239.Yang X., Duan B., Zhou X. Long non-coding RNA FOXD2-AS1 functions as a tumor promoter in colorectal cancer by regulating EMT and Notch signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017;21:3586–3591. [PubMed] [Google Scholar]
- 240.Liu Z., Zhou W., Lin C., Wang X., Zhang X., Zhang Y., Yang R., Chen W., Cao W. Dysregulation of FOXD2-AS1 promotes cell proliferation and migration and predicts poor prognosis in oral squamous cell carcinoma: a study based on TCGA data. Aging. 2020;13:2379–2396. doi: 10.18632/aging.202268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ni W., Xia Y., Bi Y., Wen F., Hu D., Luo L. FoxD2-AS1 promotes glioma progression by regulating miR-185-5P/HMGA2 axis and PI3K/AKT signaling pathway. Aging. 2019;11:1427–1439. doi: 10.18632/aging.101843. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 242.Zhao J., Zeng X.B., Zhang H.Y., Xiang J.W., Liu Y.S. Long non-coding RNA FOXD2-AS1 promotes cell proliferation, metastasis and EMT in glioma by sponging miR-506-5p. Open Med. 2020;15:921–931. doi: 10.1515/med-2020-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Palmieri G., Paliogiannis P., Sini M.C., Manca A., Palomba G., Doneddu V., Tanda F., Pascale M.R., Cossu A. Long non-coding RNA CASC2 in human cancer. Crit. Rev. Oncol. Hematol. 2017;111:31–38. doi: 10.1016/j.critrevonc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 244.Wang P., Liu Y.H., Yao Y.L., Li Z., Li Z.Q., Ma J., Xue Y.X. Long non-coding RNA CASC2 suppresses malignancy in human gliomas by miR-21. Cell Signal. 2015;27:275–282. doi: 10.1016/j.cellsig.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 245.Wang X., Yan Y., Zhang C., Wei W., Ai X., Pang Y., Bian Y. Upregulation of lncRNA PlncRNA-1 indicates the poor prognosis and promotes glioma progression by activation of Notch signal pathway. Biomed. Pharmacother. 2018;103:216–221. doi: 10.1016/j.biopha.2018.03.150. [DOI] [PubMed] [Google Scholar]
- 246.Wang R., Li Y., Zhu G., Tian B., Zeng W., Yang Y., Li Z. Long noncoding RNA CASC2 predicts the prognosis of glioma patients and functions as a suppressor for gliomas by suppressing Wnt/β-catenin signaling pathway. Neuropsychiatr. Dis. Treat. 2017;13:1805–1813. doi: 10.2147/NDT.S137171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Komatsu S., Ichikawa D., Takeshita H., Morimura R., Hirajima S., Tsujiura M., Kawaguchi T., Miyamae M., Nagata H., Konishi H., et al. Circulating miR-18a: a sensitive cancer screening biomarker in human cancer. In Vivo. 2014;28:293–297. [PubMed] [Google Scholar]
- 248.Song Y., Wang P., Zhao W., Yao Y., Liu X., Ma J., Xue Y., Liu Y. MiR-18a regulates the proliferation, migration and invasion of human glioblastoma cell by targeting neogenin. Exp. Cell Res. 2014;324:54–64. doi: 10.1016/j.yexcr.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 249.Jiang Y., Zhou J., Zhao J., Hou D., Zhang H., Li L., Zou D., Hu J., Zhang Y., Jing Z. MiR-18a-downregulated RORA inhibits the proliferation and tumorigenesis of glioma using the TNF-α-mediated NF-κB signaling pathway. EBioMedicine. 2020;52:102651. doi: 10.1016/j.ebiom.2020.102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang J., Qin C., Zhong C., Wen Y., Ke S., Liao B.O. Long non-coding RNA CASC2 targeting miR-18a suppresses glioblastoma cell growth, metastasis and EMT in vitro and in vivo. J. Biosci. 2020;45:1–14. [PubMed] [Google Scholar]
- 251.Gutschner T., Hämmerle M., Diederichs S. MALAT1 -- a paradigm for long noncoding RNA function in cancer. J. Mol. Med. 2013;91:791–801. doi: 10.1007/s00109-013-1028-y. [DOI] [PubMed] [Google Scholar]
- 252.Wang X., Li M., Wang Z., Han S., Tang X., Ge Y., Zhou L., Zhou C., Yuan Q., Yang M. Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. J. Biol. Chem. 2015;290:3925–3935. doi: 10.1074/jbc.M114.596866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Okugawa Y., Toiyama Y., Hur K., Toden S., Saigusa S., Tanaka K., Inoue Y., Mohri Y., Kusunoki M., Boland C.R., et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35:2731–2739. doi: 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liu W.T., Lu X., Tang G.H., Ren J.J., Liao W.J., Ge P.L., Huang J.F. LncRNAs expression signatures of hepatocellular carcinoma revealed by microarray. World J. Gastroenterol. 2014;20:6314–6321. doi: 10.3748/wjg.v20.i20.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gutschner T., Hämmerle M., Eissmann M., Hsu J., Kim Y., Hung G., Revenko A., Arun G., Stentrup M., Gross M., et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ma K.X., Wang H.J., Li X.R., Li T., Su G., Yang P., Wu J.W. Long noncoding RNA MALAT1 associates with the malignant status and poor prognosis in glioma. Tumour Biol. 2015;36:3355–3359. doi: 10.1007/s13277-014-2969-7. [DOI] [PubMed] [Google Scholar]
- 257.Frische E.W., Zwartkruis F.J. Rap1, a mercenary among the Ras-like GTPases. Dev. Biol. 2010;340:1–9. doi: 10.1016/j.ydbio.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 258.Guo H., Hu X., Ge S., Qian G., Zhang J. Regulation of RAP1B by miR-139 suppresses human colorectal carcinoma cell proliferation. Int. J. Biochem. Cell Biol. 2012;44:1465–1472. doi: 10.1016/j.biocel.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 259.Peng H., Luo J., Hao H., Hu J., Xie S.K., Ren D., Rao B. MicroRNA-100 regulates SW620 colorectal cancer cell proliferation and invasion by targeting RAP1B. Oncol. Rep. 2014;31:2055–2062. doi: 10.3892/or.2014.3075. [DOI] [PubMed] [Google Scholar]
- 260.Lin K.T., Yeh Y.M., Chuang C.M., Yang S.Y., Chang J.W., Sun S.P., Wang Y.S., Chao K.C., Wang L.H. Glucocorticoids mediate induction of microRNA-708 to suppress ovarian cancer metastasis through targeting Rap1B. Nat. Commun. 2015;6:5917. doi: 10.1038/ncomms6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Li Z., Xu C., Ding B., Gao M., Wei X., Ji N. Long non-coding RNA MALAT1 promotes proliferation and suppresses apoptosis of glioma cells through derepressing Rap1B by sponging miR-101. J. Neurooncol. 2017;134:19–28. doi: 10.1007/s11060-017-2498-5. [DOI] [PubMed] [Google Scholar]
- 262.Liu K., Liu Y., Mo W., Qiu R., Wang X., Wu J.Y., He R. MiR-124 regulates early neurogenesis in the optic vesicle and forebrain, targeting NeuroD1. Nucleic Acids Res. 2011;39:2869–2879. doi: 10.1093/nar/gkq904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cheng L.C., Pastrana E., Tavazoie M., Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hu C.B., Li Q.L., Hu J.F., Zhang Q., Xie J.P., Deng L. miR-124 inhibits growth and invasion of gastric cancer by targeting ROCK1. Asian Pac. J. Cancer Prev. 2014;15:6543–6546. doi: 10.7314/apjcp.2014.15.16.6543. [DOI] [PubMed] [Google Scholar]
- 265.Zhang W., Mao Y.Q., Wang H., Yin W.J., Zhu S.X., Wang W.C. MiR-124 suppresses cell motility and adhesion by targeting talin 1 in prostate cancer cells. Cancer Cell Int. 2015;15:49. doi: 10.1186/s12935-015-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sun Y., Ai X., Shen S., Lu S. NF-κB-mediated miR-124 suppresses metastasis of non-small-cell lung cancer by targeting MYO10. Oncotarget. 2015;6:8244–8254. doi: 10.18632/oncotarget.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wan H.Y., Li Q.Q., Zhang Y., Tian W., Li Y.N., Liu M., Li X., Tang H. MiR-124 represses vasculogenic mimicry and cell motility by targeting amotL1 in cervical cancer cells. Cancer Lett. 2014;355:148–158. doi: 10.1016/j.canlet.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 268.Feng T., Shao F., Wu Q., Zhang X., Xu D., Qian K., Xie Y., Wang S., Xu N., Wang Y., et al. miR-124 downregulation leads to breast cancer progression via LncRNA-MALAT1 regulation and CDK4/E2F1 signal activation. Oncotarget. 2016;7:16205–16216. doi: 10.18632/oncotarget.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wan Makhtar W.R., Browne G., Karountzos A., Stevens C., Alghamdi Y., Bottrill A.R., Mistry S., Smith E., Bushel M., Pringle J.H., et al. Short stretches of rare codons regulate translation of the transcription factor ZEB2 in cancer cells. Oncogene. 2017;36:6640–6648. doi: 10.1038/onc.2017.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Huang X.-Y., Huang Z.-L., Xu Y.-H., Zheng Q., Chen Z., Song W., Zhou J., Tang Z.-Y., Huang X.-Y. Comprehensive circular RNA profiling reveals the regulatory role of the circRNA-100338/miR-141-3p pathway in hepatitis B-related hepatocellular carcinoma. Sci. Rep. 2017;7:5428. doi: 10.1038/s41598-017-05432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Prislei S., Martinelli E., Zannoni G.F., Petrillo M., Filippetti F., Mariani M., Mozzetti S., Raspaglio G., Scambia G., Ferlini C. Role and prognostic significance of the epithelial-mesenchymal transition factor ZEB2 in ovarian cancer. Oncotarget. 2015;6:18966–18979. doi: 10.18632/oncotarget.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Qi S., Song Y., Peng Y., Wang H., Long H., Yu X., Li Z., Fang L., Wu A., Luo W., et al. ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma. PLoS One. 2012;7:e38842. doi: 10.1371/journal.pone.0038842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Guan H., Liang W., Xie Z., Li H., Liu J., Liu L., Xiu L., Li Y. Down-regulation of miR-144 promotes thyroid cancer cell invasion by targeting ZEB1 and ZEB2. Endocrine. 2015;48:566–574. doi: 10.1007/s12020-014-0326-7. [DOI] [PubMed] [Google Scholar]
- 274.You J., Li Y., Fang N., Liu B., Zu L., Chang R., Li X., Zhou Q. MiR-132 suppresses the migration and invasion of lung cancer cells via targeting the EMT regulator ZEB2. PLoS One. 2014;9:e91827. doi: 10.1371/journal.pone.0091827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Guo F., Cogdell D., Hu L., Yang D., Sood A.K., Xue F., Zhang W. MiR-101 suppresses the epithelial-to-mesenchymal transition by targeting ZEB1 and ZEB2 in ovarian carcinoma. Oncol. Rep. 2014;31:2021–2028. doi: 10.3892/or.2014.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Wu S.M., Ai H.W., Zhang D.Y., Han X.Q., Pan Q., Luo F.L., Zhang X.L. MiR-141 targets ZEB2 to suppress HCC progression. Tumour Biol. 2014;35:9993–9997. doi: 10.1007/s13277-014-2299-9. [DOI] [PubMed] [Google Scholar]
- 277.Xiao H., Tang K., Liu P., Chen K., Hu J., Zeng J., Xiao W., Yu G., Yao W., Zhou H., et al. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell kidney carcinoma. Oncotarget. 2015;6:38005–38015. doi: 10.18632/oncotarget.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Cheng H., Zhao H., Xiao X., Huang Q., Zeng W., Tian B., Ma T., Lu D., Jin Y., Li Y. Long non-coding RNA MALAT1 upregulates ZEB2 expression to promote malignant progression of glioma by attenuating miR-124. Mol. Neurobiol. 2021;58:1006–1016. doi: 10.1007/s12035-020-02165-0. [DOI] [PubMed] [Google Scholar]
- 279.Wu Y., Wang W., Jiang W., Yao J., Zhang D. An investigation of obesity susceptibility genes in Northern Han Chinese by targeted resequencing. Medicine. 2017;96:e6117. doi: 10.1097/MD.0000000000006117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Min K.W., Davila S., Zealy R.W., Lloyd L.T., Lee I.Y., Lee R., Roh K.H., Jung A., Jemielity J., Choi E.J., et al. eIF4E phosphorylation by MST1 reduces translation of a subset of mRNAs, but increases lncRNA translation. Biochim. Biophys. Acta Gene Regul. Mech. 2017;1860:761–772. doi: 10.1016/j.bbagrm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 281.Sun F., Yu M., Yu J., Liu Z., Zhou X., Liu Y., Ge X., Gao H., Li M., Jiang X., et al. miR-338-3p functions as a tumor suppressor in gastric cancer by targeting PTP1B. Cell Death Dis. 2018;9:522. doi: 10.1038/s41419-018-0611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nie H., Li J., Yang X.M., Cao Q.Z., Feng M.X., Xue F., Wei L., Qin W., Gu J., Xia Q., et al. Mineralocorticoid receptor suppresses cancer progression and the Warburg effect by modulating the miR-338-3p-PKLR axis in hepatocellular carcinoma. Hepatology. 2015;62:1145–1159. doi: 10.1002/hep.27940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Han B., Meng X., Chen H., Chen L., Liu X., Wang H., Liu D., Gao F., Lin L., Ming J., et al. Epigenetic silencing of miR-338 facilitates glioblastoma progression by de-repressing the pyruvate kinase M2-β-catenin axis. Aging. 2017;9:1885–1897. doi: 10.18632/aging.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shi J., Hu N., Li J., Zeng Z., Mo L., Sun J., Wu M., Hu Y. Unique expression signatures of circular RNAs in response to DNA tumor virus SV40 infection. Oncotarget. 2017;8:98609. doi: 10.18632/oncotarget.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Luan W., Wang Y., Chen X., Shi Y., Wang J., Zhang J., Qian J., Li R., Tao T., Wei W., et al. PKM2 promotes glucose metabolism and cell growth in gliomas through a mechanism involving a let-7a/c-Myc/hnRNPA1 feedback loop. Oncotarget. 2015;6:13006–13018. doi: 10.18632/oncotarget.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Liang J., Cao R., Zhang Y., Xia Y., Zheng Y., Li X., Wang L., Yang W., Lu Z. PKM2 dephosphorylation by Cdc25A promotes the Warburg effect and tumorigenesis. Nat. Commun. 2016;7:12431. doi: 10.1038/ncomms12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liu X., Zhu Q., Guo Y., Xiao Z., Hu L., Xu Q. LncRNA LINC00689 promotes the growth, metastasis and glycolysis of glioma cells by targeting miR-338-3p/PKM2 axis. Biomed. Pharmacother. 2019;117:109069. doi: 10.1016/j.biopha.2019.109069. [DOI] [PubMed] [Google Scholar]
- 288.Guan N., Wang R., Feng X., Li C., Guo W. Long non-coding RNA NBAT1 inhibits the progression of glioma through the miR-21/SOX7 axis. Oncol. Lett. 2020;20:3024–3034. doi: 10.3892/ol.2020.11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang L., Cao Y., Kou X., Che L., Zhou X., Chen G., Zhao J. Long non-coding RNA HCG11 suppresses the growth of glioma by cooperating with the miR-4425/MTA3 axis. J. Gene Med. 2019;21:e3074. doi: 10.1002/jgm.3074. [DOI] [PubMed] [Google Scholar]
- 290.Zhang S., Guo W. Long non-coding RNA MEG3 suppresses the growth of glioma cells by regulating the miR-96-5p/MTSS1 signaling pathway. Mol. Med. Rep. 2019;20:4215–4225. doi: 10.3892/mmr.2019.10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yang J.X., Sun Y., Gao L., Meng Q., Yang B.Y. Long non-coding RNA DANCR facilitates glioma malignancy by sponging miR-33a-5p. Neoplasma. 2018;65:790–798. doi: 10.4149/neo_2018_170724N498. [DOI] [PubMed] [Google Scholar]
- 292.Perez-Laguna V., Gilaberte Y., Millan-Lou M.I., Agut M., Nonell S., Rezusta A., Hamblin M.R. A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: a systematic review. Photochem. Photobiol. Sci. 2019;18:1020–1029. doi: 10.1039/c8pp00534f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhang S., Wang W., Liu G., Xie S., Li Q., Li Y., Lin Z. Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis. Biomed. Pharmacother. 2017;95:711–720. doi: 10.1016/j.biopha.2017.08.133. [DOI] [PubMed] [Google Scholar]
- 294.Wu Y.J., Yang Q.S., Chen H., Wang J.T., Wang W.B., Zhou L. Long non-coding RNA CASC19 promotes glioma progression by modulating the miR-454-3p/RAB5A axis and is associated with unfavorable MRI features. Oncol Rep. 2020;45:728–737. doi: 10.3892/or.2020.7876. [DOI] [PubMed] [Google Scholar]
- 295.Cheng H., Zhao H., Xiao X., Huang Q., Zeng W., Tian B., Ma T., Lu D., Jin Y., Li Y. Long non-coding RNA MALAT1 upregulates ZEB2 expression to promote malignant progression of glioma by attenuating miR-124. Mol. Neurobiol. 2021;58:1006–1016. doi: 10.1007/s12035-020-02165-0. [DOI] [PubMed] [Google Scholar]
- 296.Cao W., Liu B., Ma H. Long non-coding RNA GHET1 promotes viability, migration and invasion of glioma cell line U251 by down-regulation of miR-216a. Eur. Rev. Med. Pharmacol. Sci. 2019;23:1591–1599. doi: 10.26355/eurrev_201902_17118. [DOI] [PubMed] [Google Scholar]
- 297.Xiang J., Guo S., Jiang S., Xu Y., Li J., Li L., Xiang J. Silencing of long non-coding RNA MALAT1 promotes apoptosis of glioma cells. J. Korean Med. Sci. 2016;31:688–694. doi: 10.3346/jkms.2016.31.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Jia P., Cai H., Liu X., Chen J., Ma J., Wang P., Liu Y., Zheng J., Xue Y. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381:359–369. doi: 10.1016/j.canlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 299.Cheng Z., Li Z., Ma K., Li X., Tian N., Duan J., Xiao X., Wang Y. Long non-coding RNA XIST promotes glioma tumorigenicity and angiogenesis by acting as a molecular sponge of miR-429. J. Cancer. 2017;8:4106–4116. doi: 10.7150/jca.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Chen D., Yu X. Long noncoding RNA TSLNC8 suppresses cell proliferation and metastasis and promotes cell apoptosis in human glioma. Mol. Med. Rep. 2018;18:5536–5544. doi: 10.3892/mmr.2018.9609. [DOI] [PubMed] [Google Scholar]
- 301.Zheng Y., Miao Y., Xie J., Lin Y., Yao Q., Cai J., Yang X. Long noncoding RNA lysophospholipase-like 1-2 as ceRNA modulates glioma metastasis by regulating miR-217/YWHAG. Am. J. Transl. Res. 2020;12:4204–4215. [PMC free article] [PubMed] [Google Scholar]
- 302.Abedi-Gaballu F., Dehghan G., Ghaffari M., Yekta R., Abbaspour-Ravasjani S., Baradaran B., Dolatabadi J.E.N., Hamblin M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today. 2018;12:177–190. doi: 10.1016/j.apmt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Xu W., Hu G.Q., Da Costa C., Tang J.H., Li Q.R., Du L., Pan Y.W., Lv S.Q. Long noncoding RNA UBE2R2-AS1 promotes glioma cell apoptosis via targeting the miR-877-3p/TLR4 axis. Onco Targets Ther. 2019;12:3467–3480. doi: 10.2147/OTT.S201732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Dong H., Cao W., Xue J. Long noncoding FOXD2-AS1 is activated by CREB1 and promotes cell proliferation and metastasis in glioma by sponging miR-185 through targeting AKT1. Biochem. Biophys. Res. Commun. 2019;508:1074–1081. doi: 10.1016/j.bbrc.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 305.Zheng R., Yao Q., Li X., Xu B. Long noncoding ribonucleic acid SNHG18 promotes glioma cell motility via disruption of α-enolase nucleocytoplasmic transport. Front. Genet. 2019;10:1140. doi: 10.3389/fgene.2019.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Xiao S., Wang R., Wu X., Liu W., Ma S. The long noncoding RNA TP73-AS1 interacted with miR-124 to modulate glioma growth by targeting inhibitor of apoptosis-stimulating protein of p53. DNA Cell Biol. 2018;37:117–125. doi: 10.1089/dna.2017.3941. [DOI] [PubMed] [Google Scholar]
- 307.Liu H., Lv Z., Guo E. Knockdown of long noncoding RNA SPRY4-IT1 suppresses glioma cell proliferation, metastasis and epithelial-mesenchymal transition. Int. J. Clin. Exp. Pathol. 2015;8:9140–9146. [PMC free article] [PubMed] [Google Scholar]
- 308.Liu Q., Yu W., Zhu S., Cheng K., Xu H., Lv Y., Long X., Ma L., Huang J., Sun S., et al. Long noncoding RNA GAS5 regulates the proliferation, migration, and invasion of glioma cells by negatively regulating miR-18a-5p. J. Cell. Physiol. 2018;234:757–768. doi: 10.1002/jcp.26889. [DOI] [PubMed] [Google Scholar]
- 309.Meng L., Ma P., Cai R., Guan Q., Wang M., Jin B. Long noncoding RNA ZEB1-AS1 promotes the tumorigenesis of glioma cancer cells by modulating the miR-200c/141-ZEB1 axis. Am. J. Transl. Res. 2018;10:3395–3412. [PMC free article] [PubMed] [Google Scholar]
- 310.Wang S.J., Wang H., Zhao C.D., Li R. Long noncoding RNA LINC01426 promotes glioma progression through PI3K/AKT signaling pathway and serves as a prognostic biomarker. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6358–6368. doi: 10.26355/eurrev_201810_16047. [DOI] [PubMed] [Google Scholar]
- 311.Wu D.M., Wang S., Wen X., Han X.R., Wang Y.J., Fan S.H., Zhang Z.F., Shan Q., Lu J., Zheng Y.L. Long noncoding RNA nuclear enriched abundant transcript 1 impacts cell proliferation, invasion, and migration of glioma through regulating miR-139-5p/CDK6. J. Cell. Physiol. 2019;234:5972–5987. doi: 10.1002/jcp.27093. [DOI] [PubMed] [Google Scholar]
- 312.Liao K., Lin Y., Gao W., Xiao Z., Medina R., Dmitriev P., Cui J., Zhuang Z., Zhao X., Qiu Y., et al. Blocking lncRNA MALAT1/miR-199a/ZHX1 Axis inhibits glioblastoma proliferation and progression. Mol. Ther. Nucleic Acids. 2019;18:388–399. doi: 10.1016/j.omtn.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ding H., Cui L., Wang C. Long noncoding RNA LIFR-AS1 suppresses proliferation, migration and invasion and promotes apoptosis through modulating miR-4262/NF-κB pathway in glioma. Neurol. Res. 2020;43:210–219. doi: 10.1080/01616412.2020.1836465. [DOI] [PubMed] [Google Scholar]
- 314.Voce D.J., Bernal G.M., Wu L., Crawley C.D., Zhang W., Mansour N.M., Cahill K.E., Szymura S.J., Uppal A., Raleigh D.R., et al. Temozolomide treatment induces lncRNA MALAT1 in an NF-κB and p53 codependent manner in glioblastoma. Cancer Res. 2019;79:2536–2548. doi: 10.1158/0008-5472.CAN-18-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Luo C., Quan Z., Zhong B., Zhang M., Zhou B., Wang S., Luo X., Tang C. lncRNA XIST promotes glioma proliferation and metastasis through miR-133a/SOX4. Exp. Ther. Med. 2020;19:1641–1648. doi: 10.3892/etm.2020.8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Xu C.H., Xiao L.M., Liu Y., Chen L.K., Zheng S.Y., Zeng E.M., Li D.H. The lncRNA HOXA11-AS promotes glioma cell growth and metastasis by targeting miR-130a-5p/HMGB2. Eur. Rev. Med. Pharmacol. Sci. 2019;23:241–252. doi: 10.26355/eurrev_201901_16770. [DOI] [PubMed] [Google Scholar]
- 317.Cao S., Wang Y., Li J., Lv M., Niu H., Tian Y. Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by suppressing miR-155 expression and activating FBXW7 function. Am. J. Cancer Res. 2016;6:2561–2574. [PMC free article] [PubMed] [Google Scholar]
- 318.Wu Y., Liang S., Xu B., Zhang R., Zhu M., Zhou W., Zhang S., Guo J., Xu L., Zhu H. Long noncoding RNA eosinophil granule ontogeny transcript inhibits cell proliferation and migration and promotes cell apoptosis in human glioma. Exp. Ther. Med. 2017;14:3817–3823. doi: 10.3892/etm.2017.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Chen Y., Zhao F., Cui D., Jiang R., Chen J., Huang Q., Shi J. HOXD-AS1/miR-130a sponge regulates glioma development by targeting E2F8. Int. J. Cancer. 2018;142:2313–2322. doi: 10.1002/ijc.31262. [DOI] [PubMed] [Google Scholar]
- 320.Huang W., Shi Y., Han B., Wang Q., Zhang B., Qi C., Liu F. LncRNA GAS5-AS1 inhibits glioma proliferation, migration, and invasion via miR-106b-5p/TUSC2 axis. Hum. Cell. 2020;33:416–426. doi: 10.1007/s13577-020-00331-z. [DOI] [PubMed] [Google Scholar]
- 321.Lv Z.H., Wang Z.Y., Li Z.Y. LncRNA PVT1 aggravates the progression of glioma via downregulating UPF1. Eur. Rev. Med. Pharmacol. Sci. 2019;23:8956–8963. doi: 10.26355/eurrev_201910_19294. [DOI] [PubMed] [Google Scholar]
- 322.Wang Z.-C., Huang F.-Z., Xu H.-B., Sun J.-C., Wang C.-F. MicroRNA-137 inhibits autophagy and chemosensitizes pancreatic cancer cells by targeting ATG5. Int. J. Biochem. Cell Biol. 2019;111:63–71. doi: 10.1016/j.biocel.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 323.Jia Y., Lin R., Jin H., Si L., Jian W., Yu Q., Yang S. MicroRNA-34 suppresses proliferation of human ovarian cancer cells by triggering autophagy and apoptosis and inhibits cell invasion by targeting Notch 1. Biochimie. 2019;160:193–199. doi: 10.1016/j.biochi.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 324.Qin W.X., Shi Y., Zhu D., Li Y.P., Chen Y.H., Cui J., Cui G.Y., Pan J.X., Ren Z.Y. EZH2-mediated H3K27me3 enrichment on the lncRNA MEG3 promoter regulates the growth and metastasis of glioma cells by regulating miR-21-3p. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3204–3214. doi: 10.26355/eurrev_202003_20687. [DOI] [PubMed] [Google Scholar]
- 325.Cheng C., Dong Y., Ru X., Xia Y., Ji Y. LncRNA ANCR promotes glioma cells invasion, migration, proliferation and inhibits apoptosis via interacting with EZH2 and repressing PTEN expression. Cancer Gene Ther. 2020;28:1025–1034. doi: 10.1038/s41417-020-00263-8. [DOI] [PubMed] [Google Scholar]
- 326.Zhang N., Shuai K., Cheng J., Yang W., Kan Z. LncRNA linc01116 prometes glioma cell migration and invasion by modulation of radixin targeted by miR-31. Int. J. Clin. Exp. Pathol. 2019;12:1078–1086. [PMC free article] [PubMed] [Google Scholar]
- 327.Liu B., Cao W., Ma H. Knockdown of lncRNA LSINCT5 suppresses growth and metastasis of human glioma cells via up-regulating miR-451. Artif. Cells Nanomed. Biotechnol. 2019;47:2507–2515. doi: 10.1080/21691401.2019.1626404. [DOI] [PubMed] [Google Scholar]
- 328.Yang J., Gan X., Tan B., Wang J., Chen Y. Corticotropin-releasing factor suppresses glioma progression by upregulation of long non-coding RNA-p21. Life Sci. 2019;216:92–100. doi: 10.1016/j.lfs.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 329.Zhang T., Wang F., Liao Y., Yuan L., Zhang B. LncRNA AWPPH promotes the invasion and migration of glioma cells through the upregulation of HIF1α. Oncol. Lett. 2019;18:6781–6786. doi: 10.3892/ol.2019.11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zheng D., Che D., Lin F., Wang X., Lu L., Chen J., Xu X. LncRNA MACC1-AS1/MACC1 enhances the progression of glioma via regulating metabolic plasticity. Cell Cycle. 2020;19:2286–2297. doi: 10.1080/15384101.2020.1795595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 331.Zhou Q., Liu Z.Z., Wu H., Kuang W.L. LncRNA H19 promotes cell proliferation, migration, and angiogenesis of glioma by regulating Wnt5a/β-catenin pathway via targeting miR-342. Cell Mol Neurobiol. 2020 doi: 10.1007/s10571-020-00995-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ren S., Xu Y. AC016405.3, a novel long noncoding RNA, acts as a tumor suppressor through modulation of TET2 by microRNA-19a-5p sponging in glioblastoma. Cancer Sci. 2019;110:1621–1632. doi: 10.1111/cas.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mao Y., Shen G., Su Z., Du J., Xu F., Yu Y. RAD21 inhibited transcription of tumor suppressor MIR4697HG and led to glioma tumorigenesis. Biomed. Pharmacother. 2020;123:109759. doi: 10.1016/j.biopha.2019.109759. [DOI] [PubMed] [Google Scholar]
- 334.Li F., Shen Z.Z., Xiao C.M., Sha Q.K. YY1-mediated up-regulation of lncRNA LINC00466 facilitates glioma progression via miR-508/CHEK1. J. Gene Med. 2021;23:e3287. doi: 10.1002/jgm.3287. [DOI] [PubMed] [Google Scholar]
- 335.Kong F., Yan Y., Deng J., Zhu Y., Li Y., Li H., Wang Y. LncRNA SNHG16 promotes proliferation, migration, and invasion of glioma cells through regulating the miR-490/PCBP2 axis. Cancer Biother. Radiopharm. 2020 doi: 10.1089/cbr.2019.3535. [DOI] [PubMed] [Google Scholar]
- 336.Argadal O.G., Mutlu M., Ak Aksoy S., Kocaeli H., Tunca B., Civan M.N., Egeli U., Cecener G., Bekar A., Taskapilioglu M.O., et al. Long noncoding RNA MALAT1 may be a prognostic biomarker in IDH1/2 wild-type primary glioblastomas. Bosn J. Basic Med. Sci. 2020;20:63–69. doi: 10.17305/bjbms.2019.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Alcon-Giner C., Dalby M.J., Caim S., Ketskemety J., Shaw A., Sim K., Lawson M.A., Kiu R., Leclaire C., Chalklen L. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: an observational study. Cell Rep. Med. 2020;1:100077. doi: 10.1016/j.xcrm.2020.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Chen H.H., Zong J., Wang S.J. LncRNA GAPLINC promotes the growth and metastasis of glioblastoma by sponging miR-331-3p. Eur. Rev. Med. Pharmacol. Sci. 2019;23:262–270. doi: 10.26355/eurrev_201901_16772. [DOI] [PubMed] [Google Scholar]
- 339.Zhao H., Peng R., Liu Q., Liu D., Du P., Yuan J., Peng G., Liao Y. The lncRNA H19 interacts with miR-140 to modulate glioma growth by targeting iASPP. Arch. Biochem. Biophys. 2016;610:1–7. doi: 10.1016/j.abb.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 340.Zhang Y., Li Y., Wang J., Lei P. Long non-coding RNA ferritin heavy polypeptide 1 pseudogene 3 controls glioma cell proliferation and apoptosis via regulation of the microRNA-224-5p/tumor protein D52 axis. Mol. Med. Rep. 2018;18:4239–4246. doi: 10.3892/mmr.2018.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Xu C., He T., Li Z., Liu H., Ding B. Regulation of HOXA11-AS/miR-214-3p/EZH2 axis on the growth, migration and invasion of glioma cells. Biomed. Pharmacother. 2017;95:1504–1513. doi: 10.1016/j.biopha.2017.08.097. [DOI] [PubMed] [Google Scholar]
- 342.Ren J., Yang Y., Xue J., Xi Z., Hu L., Pan S.J., Sun Q. Long noncoding RNA SNHG7 promotes the progression and growth of glioblastoma via inhibition of miR-5095. Biochem. Biophys. Res. Commun. 2018;496:712–718. doi: 10.1016/j.bbrc.2018.01.109. [DOI] [PubMed] [Google Scholar]
- 343.He Z., Wang Y., Huang G., Wang Q., Zhao D., Chen L. The lncRNA UCA1 interacts with miR-182 to modulate glioma proliferation and migration by targeting iASPP. Arch. Biochem. Biophys. 2017;623–624:1–8. doi: 10.1016/j.abb.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 344.Wu D.M., Han X.R., Wen X., Wang S., Wang Y.J., Shen M., Fan S.H., Zhuang J., Zhang Z.F., Shan Q., et al. Long non-coding RNA LINC01260 inhibits the proliferation, migration and invasion of spinal cord glioma cells by targeting CARD11 via the NF-κB signaling pathway. Cell. Physiol. Biochem. 2018;48:1563–1578. doi: 10.1159/000492279. [DOI] [PubMed] [Google Scholar]
- 345.Kang C.M., Hu Y.W., Nie Y., Zhao J.Y., Li S.F., Chu S., Li H.X., Huang Q.S., Qiu Y.R. Long non-coding RNA RP5-833A20.1 inhibits proliferation, metastasis and cell cycle progression by suppressing the expression of NFIA in U251 cells. Mol. Med. Rep. 2016;14:5288–5296. doi: 10.3892/mmr.2016.5854. [DOI] [PubMed] [Google Scholar]
- 346.Liang C., Yang Y., Guan J., Lv T., Qu S., Fu Q., Zhao H. LncRNA UCA1 sponges miR-204-5p to promote migration, invasion and epithelial-mesenchymal transition of glioma cells via upregulation of ZEB1. Pathol. Res. Pract. 2018;214:1474–1481. doi: 10.1016/j.prp.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 347.Meng X., Deng Y., Lv Z., Liu C., Guo Z., Li Y., Liu H., Xie B., Jin Z., Lin F., et al. LncRNA SNHG5 promotes proliferation of glioma by regulating miR-205-5p/ZEB2 Axis. Onco Targets Ther. 2019;12:11487–11496. doi: 10.2147/OTT.S228439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Han Y., Zhou L., Wu T., Huang Y., Cheng Z., Li X., Sun T., Zhou Y., Du Z. Downregulation of lncRNA-MALAT1 affects proliferation and the expression of stemness markers in glioma stem cell line SHG139S. Cell. Mol. Neurobiol. 2016;36:1097–1107. doi: 10.1007/s10571-015-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zhu H., Chen Z., Shen L., Tang T., Yang M., Zheng X. Long noncoding RNA LINC-PINT suppresses cell proliferation, invasion, and EMT by blocking Wnt/β-catenin signaling in glioblastoma. Front. Pharmacol. 2020;11:586653. doi: 10.3389/fphar.2020.586653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Qiu L., Xu H., Ji M., Shang D., Lu Z., Wu Y., Tu Z., Liu H. Circular RNAs in hepatocellular carcinoma: biomarkers, functions and mechanisms. Life Sci. 2019;231:116660. doi: 10.1016/j.lfs.2019.116660. [DOI] [PubMed] [Google Scholar]
- 351.Wang D., Yang S., Wang H., Wang J., Zhang Q., Zhou S., He Y., Zhang H., Deng F., Xu H., et al. The progress of circular RNAs in various tumors. Am. J. Transl. Res. 2018;10:1571–1582. [PMC free article] [PubMed] [Google Scholar]
- 352.Cui X.L., Wang X.D., Lin S.K., Miao C.M., Wu M., Wei J.G. Circular RNA circ_0067934 functions as an oncogene in glioma by targeting CSF1. Eur. Rev. Med. Pharmacol. Sci. 2019;23:8449–8455. doi: 10.26355/eurrev_201910_19157. [DOI] [PubMed] [Google Scholar]
- 353.Shi F., Shi Z., Zhao Y., Tian J. CircRNA hsa-circ-0014359 promotes glioma progression by regulating miR-153/PI3K signaling. Biochem. Biophys. Res. Commun. 2019;510:614–620. doi: 10.1016/j.bbrc.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 354.Zhang F., Mai S.R., Zhang L. Circ-ZNF264 promotes the growth of glioma cells by upregulating the expression of miR-4493 target gene apelin. J. Mol. Neurosci. 2019;69:75–82. doi: 10.1007/s12031-019-01334-8. [DOI] [PubMed] [Google Scholar]
- 355.Zhong Y., Du Y., Yang X., Mo Y., Fan C., Xiong F., Ren D., Ye X., Li C., Wang Y., et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol. Cancer. 2018;17:79. doi: 10.1186/s12943-018-0827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Qu Y., Zhu J., Liu J., Qi L. Circular RNA circ_0079593 indicates a poor prognosis and facilitates cell growth and invasion by sponging miR-182 and miR-433 in glioma. J. Cell. Biochem. 2019;120:18005–18013. doi: 10.1002/jcb.29103. [DOI] [PubMed] [Google Scholar]
- 357.Xing Y., Zha W.J., Li X.M., Li H., Gao F., Ye T., Du W.Q., Liu Y.C. Circular RNA circ-Foxo3 inhibits esophageal squamous cell cancer progression via the miR-23a/PTEN axis. J. Cell. Biochem. 2020;121:2595–2605. doi: 10.1002/jcb.29481. [DOI] [PubMed] [Google Scholar]
- 358.Nie J.H., Li T.X., Zhang X.Q., Liu J. Roles of non-coding RNAs in normal human brain development, brain tumor, and neuropsychiatric disorders. Noncoding RNA. 2019;5:36. doi: 10.3390/ncrna5020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Lei B., Huang Y., Zhou Z., Zhao Y., Thapa A.J., Li W., Cai W., Deng Y. Circular RNA hsa_circ_0076248 promotes oncogenesis of glioma by sponging miR-181a to modulate SIRT1 expression. J. Cell. Biochem. 2019;120:6698–6708. doi: 10.1002/jcb.27966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Jahanafrooz Z., Baradaran B., Mosafer J., Hashemzaei M., Rezaei T., Mokhtarzadeh A., Hamblin M.R. Comparison of DNA and mRNA vaccines against cancer. Drug Discov Today. 2019;25:552–560. doi: 10.1016/j.drudis.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Gao Z.G., Yang P., Huang J., Ding Y.Q. CircFBXW7 alleviates glioma progression through regulating miR-23a-3p/PTEN axis. Anat. Rec. 2021;304:279–290. doi: 10.1002/ar.24410. [DOI] [PubMed] [Google Scholar]
- 362.Sun S.Q., Ren L.J., Liu J., Wang P., Shan S.M. Sevoflurane inhibits migration and invasion of colorectal cancer cells by regulating microRNA-34a/ADAM10 axis. Neoplasma. 2019;66:887–895. doi: 10.4149/neo_2018_181213N962. [DOI] [PubMed] [Google Scholar]
- 363.Gao C., Shen J., Meng Z.X., He X.F. Sevoflurane inhibits glioma cells proliferation and metastasis through miRNA-124-3p/ROCK1 Axis. Pathol. Oncol. Res. 2020;26:947–954. doi: 10.1007/s12253-019-00597-1. [DOI] [PubMed] [Google Scholar]
- 364.Xie P., Wang Y., Liao Y., Han Q., Qiu Z., Chen Y., Zuo X. MicroRNA-628-5p inhibits cell proliferation in glioma by targeting DDX59. J. Cell. Biochem. 2019;120:17293–17302. doi: 10.1002/jcb.28991. [DOI] [PubMed] [Google Scholar]
- 365.Zheng K., Yang Q., Xie L., Qiu Z., Huang Y., Lin Y., Tu L., Cui C. Overexpression of MAGT1 is associated with aggressiveness and poor prognosis of colorectal cancer. Oncol. Lett. 2019;18:3857–3862. doi: 10.3892/ol.2019.10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Wang G., Li Y., Li J., Zhang D., Luo C., Zhang B., Sun X. microRNA-199a-5p suppresses glioma progression by inhibiting MAGT1. J. Cell. Biochem. 2019;120:15248–15254. doi: 10.1002/jcb.28791. [DOI] [PubMed] [Google Scholar]
- 367.Li H., Xia T., Guan Y., Yu Y. Sevoflurane regulates glioma progression by circ_0002755/miR-628-5p/MAGT1 Axis. Cancer Manag. Res. 2020;12:5085–5098. doi: 10.2147/CMAR.S242135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Sun H., Xi P., Sun Z., Wang Q., Zhu B., Zhou J., Jin H., Zheng W., Tang W., Cao H., et al. Circ-SFMBT2 promotes the proliferation of gastric cancer cells through sponging miR-182-5p to enhance CREB1 expression. Cancer Manag. Res. 2018;10:5725–5734. doi: 10.2147/CMAR.S172592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Xie F., Li Y., Wang M., Huang C., Tao D., Zheng F., Zhang H., Zeng F., Xiao X., Jiang G. Circular RNA BCRC-3 suppresses bladder cancer proliferation through miR-182-5p/p27 axis. Mol. Cancer. 2018;17:144. doi: 10.1186/s12943-018-0892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Jiang Q., Ren Y., Cheng J., Qin J. Effect of miR-182 targeting MTSS1 on the proliferation and metastasis of esophageal cancer. Int. J. Clin. Exp. Pathol. 2016;9:10871–10877. [Google Scholar]
- 371.Wang J., Li J., Shen J., Wang C., Yang L., Zhang X. MicroRNA-182 downregulates metastasis suppressor 1 and contributes to metastasis of hepatocellular carcinoma. BMC Cancer. 2012;12:227. doi: 10.1186/1471-2407-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Hirata H., Ueno K., Shahryari V., Deng G., Tanaka Y., Tabatabai Z.L., Hinoda Y., Dahiya R. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS One. 2013;8:e55502. doi: 10.1371/journal.pone.0055502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zhang S., Qin W., Yang S., Guan N., Sui X., Guo W. Circular RNA SFMBT2 inhibits the proliferation and metastasis of glioma cells through mir-182-5p/Mtss1 Pathway. Technol Cancer Res Treat. 2020;19 doi: 10.1177/1533033820945799. 1533033820945799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Hu D., Zhang Y. Circular RNA HIPK3 promotes glioma progression by binding to miR-124-3p. Gene. 2019;690:81–89. doi: 10.1016/j.gene.2018.11.073. [DOI] [PubMed] [Google Scholar]
- 375.Li Y., Xu J., Chen H., Bai J., Li S., Zhao Z., Shao T., Jiang T., Ren H., Kang C., et al. Comprehensive analysis of the functional microRNA-mRNA regulatory network identifies miRNA signatures associated with glioma malignant progression. Nucleic Acids Res. 2013;41:e203. doi: 10.1093/nar/gkt1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zhang Q., Lu D., Liu W., Ye S., Guo H., Liao T., Chen C. Effects of KIF2A on the prognosis of nasopharyngeal carcinoma and nasopharyngeal carcinoma cells. Oncol. Lett. 2019;18:2718–2723. doi: 10.3892/ol.2019.10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zhao P., Lan F., Zhang H., Zeng G., Liu D. Down-regulation of KIF2A inhibits gastric cancer cell invasion via suppressing MT1-MMP. Clin. Exp. Pharmacol. Physiol. 2018;45:1010–1018. doi: 10.1111/1440-1681.12974. [DOI] [PubMed] [Google Scholar]
- 378.Wang K., Lin C., Wang C., Shao Q., Gao W., Song B., Wang L., Song X., Qu X., Wei F. Silencing Kif2a induces apoptosis in squamous cell carcinoma of the oral tongue through inhibition of the PI3K/Akt signaling pathway. Mol. Med. Rep. 2014;9:273–278. doi: 10.3892/mmr.2013.1804. [DOI] [PubMed] [Google Scholar]
- 379.Zhang X., Ma C., Wang Q., Liu J., Tian M., Yuan Y., Li X., Qu X. Role of KIF2A in the progression and metastasis of human glioma. Mol. Med. Rep. 2016;13:1781–1787. doi: 10.3892/mmr.2015.4700. [DOI] [PubMed] [Google Scholar]
- 380.Yin H., Cui X. Knockdown of circHIPK3 facilitates temozolomide sensitivity in glioma by regulating cellular behaviors through miR-524-5p/KIF2A-mediated PI3K/AKT pathway. Cancer Biother Radiopharm. 2020;36:556–567. doi: 10.1089/cbr.2020.3575. [DOI] [PubMed] [Google Scholar]
- 381.Zhang S., Qin W., Yang S., Guan N., Sui X., Guo W. Circular RNA SFMBT2 inhibits the proliferation and metastasis of glioma cells through mir-182-5p/Mtss1 pathway. Technol. Cancer Res. Treat. 2020;19 doi: 10.1177/1533033820945799. 1533033820945799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wang R., Zhang S., Chen X., Li N., Li J., Jia R., Pan Y., Liang H. EIF4A3-induced circular RNA MMP9 (circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol. Cancer. 2018;17:166. doi: 10.1186/s12943-018-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Gao Z.G., Yang P., Huang J., Ding Y.Q. CircFBXW7 alleviates glioma progression through regulating miR-23a-3p/PTEN axis. Anat. Rec. 2021;304:279–290. doi: 10.1002/ar.24410. [DOI] [PubMed] [Google Scholar]
- 384.Xia L., Yi F., Zhai X., Zhang M. [Circular RNA homeodomain-interacting protein kinase 3 (circHIPK3) promotes growth and metastasis of glioma cells by sponging miR-124-3p] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36:609–615. [PubMed] [Google Scholar]
- 385.Yin K., Liu X. CircMMP1 promotes the progression of glioma through miR-433/HMGB3 axis in vitro and in vivo. IUBMB Life. 2020;72:2508–2524. doi: 10.1002/iub.2383. [DOI] [PubMed] [Google Scholar]
- 386.Liu Y., Li R., Wang X., Yang W. CircTTBK2 contributes to the progression of glioma through regulating miR-145-5p/CPEB4 Axis. Cancer Manag. Res. 2020;12:8183–8195. doi: 10.2147/CMAR.S263586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Song D., Ye L., Xu Z., Jin Y., Zhang L. CircRNA hsa_circ_0030018 regulates the development of glioma via regulating the miR-1297/RAB21 axis. Neoplasma. 2021;68:391–403. doi: 10.4149/neo_2020_200702N682. [DOI] [PubMed] [Google Scholar]
- 388.Hamblin M.R. How to write a good photobiomodulation article. Photobiomodul. Photomed. Laser Surg. 2019;37:325–326. doi: 10.1089/photob.2019.4648. [DOI] [PubMed] [Google Scholar]
- 389.Jin T., Liu M., Liu Y., Li Y., Xu Z., He H., Liu J., Zhang Y., Ke Y. Lcn2-derived circular RNA (hsa_circ_0088732) inhibits cell apoptosis and promotes EMT in glioma via the miR-661/RAB3D Axis. Front. Oncol. 2020;10:170. doi: 10.3389/fonc.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Chi G., Yang F., Xu D., Liu W. Silencing hsa_circ_PVT1 (circPVT1) suppresses the growth and metastasis of glioblastoma multiforme cells by up-regulation of miR-199a-5p. Artif. Cells Nanomed. Biotechnol. 2020;48:188–196. doi: 10.1080/21691401.2019.1699825. [DOI] [PubMed] [Google Scholar]
- 391.Yin D., Liu L., Shi Z., Zhang L., Yang Y. Ropivacaine inhibits cell proliferation, migration and invasion, whereas induces oxidative stress and cell apoptosis by circSCAF11/miR-145-5p Axis in glioma. Cancer Manag. Res. 2020;12:11145–11155. doi: 10.2147/CMAR.S274975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Xin J., Zhang X.Y., Sun D.K., Tian L.Q., Xu P. Up-regulated circular RNA hsa_circ_0067934 contributes to glioblastoma progression through activating PI3K-AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:3447–3454. doi: 10.26355/eurrev_201904_17709. [DOI] [PubMed] [Google Scholar]
- 393.Zhang G., Sun W., Zhu L., Feng Y., Wu L., Li T. Overexpressed circ_0029426 in glioblastoma forecasts unfavorable prognosis and promotes cell progression by sponging miR-197. J. Cell. Biochem. 2019;120:10295–10302. doi: 10.1002/jcb.28313. [DOI] [PubMed] [Google Scholar]