Summary
There is a bidirectional transplacental cell trafficking between mother and fetus during pregnancy in placental mammals. The presence and persistence of fetal cells in maternal tissues are known as fetal microchimerism (FMc). FMc has high multilineage potential with a great ability to differentiate and functionally integrate into maternal tissue. FMc has been found in various maternal tissues in animal models and humans. Its permanence in the maternal body up to decades after delivery suggests it might play an essential role in maternal pathophysiology. Studying the presence, localization, and characteristics of FMc in maternal tissues is key to understanding its impact on the woman’s body. Here we comprehensively review the existence of FMc in different species and organs and tissues, aiming to better characterize their possible role in human health and disease. We also highlight several methodological considerations that would optimize the detection, quantification, and functional determination of FMc.
Subject areas: Developmental biology
Graphical abstract
Developmental biology
Introduction
Feto-maternal microchimerism
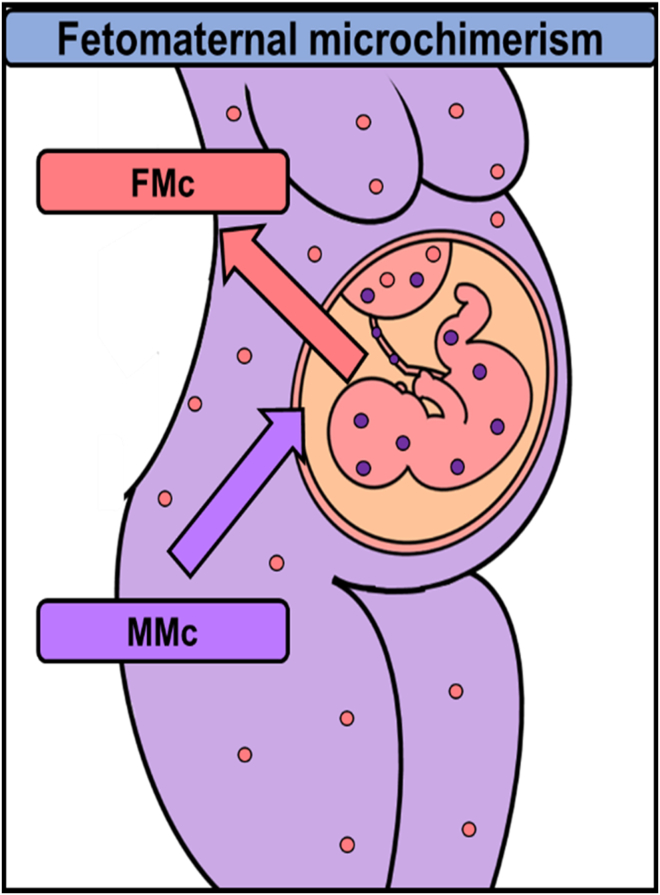
During pregnancy, there is a physiological exchange between the mother and her fetus through the placenta (Boddy et al., 2015; Pellicer Martínez and Bonilla Musoles, 2007;Lo et al., 2000). This transplacental traffic can be unidirectional or bidirectional. The unidirectional transfer involves nutrients, water, electrolytes, oxygen, hormones, and immunoglobulins in the mother-fetus direction and carbon dioxide and catabolism products in the fetus-mother direction (Pellicer Martínez and Bonilla Musoles, 2007; Lo et al., 2000; Chan et al., 2012). Bidirectional traffic consists of maternal-fetal exchanges of cells and genetic material such as DNA (Chan et al., 2012; Kolialexi et al., 2004; Lissauer et al., 2009; Lo et al., 1997,2000). This transplacental maternal-fetal cell transfer (MFCT), which has only been identified in uterine mammals (Boddy et al., 2015; Lo et al., 2000), is asymmetric, with a higher fetal cell trafficking to the maternal body (Lo et al., 2000) (Figure 1).
Figure 1.
Maternal-fetal cell transfer between mother and fetus in placental mammals
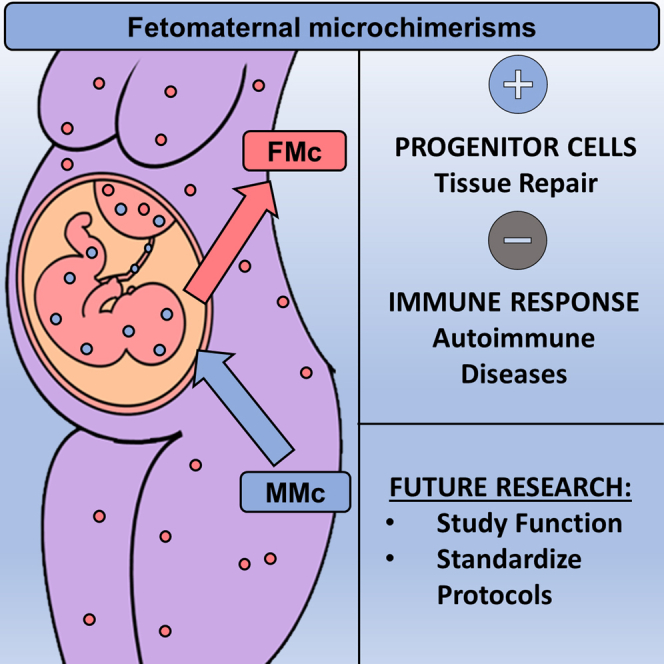
Fetal cells (pink circles) traffic into and set up in the maternal organism (FMc). Maternal cells (purple circles) also traffic into and remain in the fetal body (MMc).
Microchimerism is defined as a small population of cells that originate from another individual and are therefore genetically distinct from the host individual's cells (Müller et al., 2015). During pregnancy, there are two types of feto-maternal microchimerisms: fetal microchimerism (FMc) and maternal microchimerism (MMc). FMc is defined as the presence and persistence of fetal cells in maternal tissues, while MMc is defined as the presence and maintenance of maternal cells in fetal tissues (Bloch et al., 2013; Gadi et al., 2008; Gammill and Harrington, 2017;Shree et al., 2019) (Figure 2). FMc has been the subject of study since the early 1900s. In 1893, Schmorl first identified fetal cells in the lungs of women with eclampsia. Later, during the 60s and 70s, other studies pointed to fetal hematopoietic cells (monocytes, Natural Killer cells, T or B lymphocytes) in healthy and sick women (Klintschar et al., 2006). In 1981, Liegeois et al. located fetal cells in murine maternal tissues, prompting research on FMc (Liégeois et al., 1981).
Figure 2.
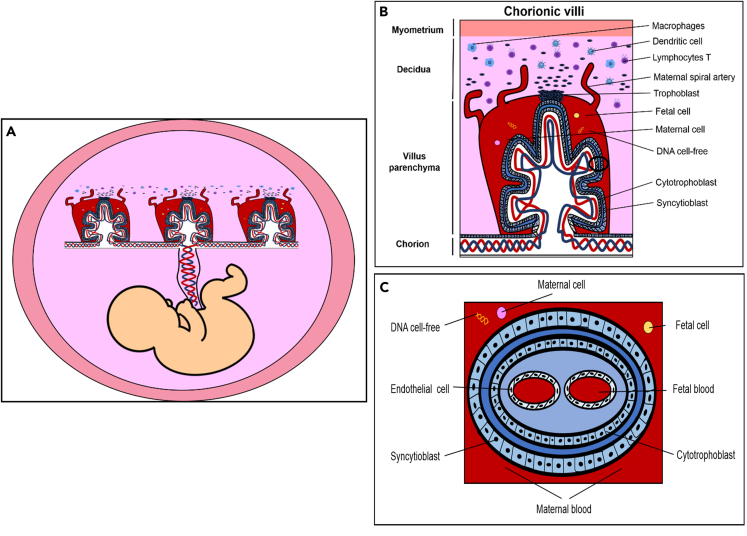
Schematic of human placenta
View from tissues to cells.
(A) Representation of the fetus with umbilical cord and components.
(B) Detail of chorionic villi where the maternal blood vessels are invaded by trophoblasts, causing an immune response of macrophages, dendritic cells, and T lymphocytes. In the chorionic villus, there is an exchange of fetal cells, maternal cells, and DNA free cell.
(C) Detail of the cross-sectional anatomy of the chorionic villi and different cells layers and components. These structures allow the transfer of nutrients, gases, and waste substances between mother and fetus through the umbilical cord.
This review focuses on FMc and trafficking of fetal cells to the maternal body and analyzes its potential role in maternal physiology (Lo et al., 2000).
An evolutionary perspective of FMc
Evolutionarily, FMc has been argued to be associated with cooperation and conflict of interest between males and females and between mother and her fetus (Apari and Rózsa 2009; Boddy et al., 2015; Boyon et al., 2011; Dawe et al., 2007; Haig 2014). On the one hand, some studies suggest that the transfer of fetal cells carries conflicting interests between males and females, providing them an adaptive and selective advantage (Apari and Rózsa 2009; Boyon et al.,2011). On the other hand, other investigations suggest that feto-maternal exchange leads to cooperation between mother and fetus (Boddy et al., 2015; Dawe et al., 2007; Haig 2014). The FMc could guarantee fetal survival and improve maternal health, thus increasing the fitness of both (Dawe et al., 2007). However, other studies suggest that fetal cells entail a conflict of interest between both since these cells could modify postnatal maternal physiology, inducing changes in lactation, thermoregulation, maternal affection, and neural plasticity, increasing only fetal fitness (Boddy et al., 2015; Haig 2014; Barba-Müller et al., 2019).
Detection and characterization of FMC in animal and human maternal tissues
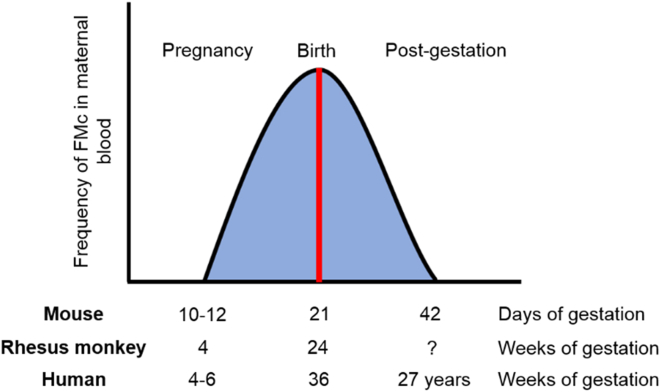
FMc has been mainly studied in humans, rodents, and rhesus monkeys (Jimenez and Tarantal 2003a,2003b; Jimenez et al., 2005; Khosrotehrani et al., 2005). Feto-maternal cell trafficking has been described to start between the fourth and sixth week of gestation in humans (Ariga et al., 2001), the 11th day in rodents (Yutaka et al.,2009) and the fourth week in rhesus monkeys and increases exponentially throughout the gestational period, reaching its maximum level in the days before childbirth (Jimenez and Tarantal 2003a,2003b; Jimenez et al., 2005; Kara et al., 2012; Klonisch and Drouin 2009; Vernochet et al., 2007). Most fetal cells gradually disappear from the blood circulation during the first weeks post-gestation (Kolialexi et al., 2004; Jimenez and Tarantal 2003a,2003b; Jimenez et al., 2005; Ariga et al., 2001; Lambert and Nelson, 2003; Nelson, 2002a, 2002b), being eliminated by the maternal immune system (Kolialexi et al., 2004; Ariga et al., 2001; Srivatsa et al., 2001). But a small proportion of fetal cells have been found integrated into maternal tissues up to three decades after delivery in humans (Chan and Nelson 2013) and up to 8 months in rodents (Dawe et al., 2007) (Figure 3).
Figure 3.
Graph of maternal-fetal cell transfer and presence during pregnancy
(A–C)During pregnancy, fetal cell trafficking to the mother starts at 10–12 days gestation in rodents, at 4 weeks in Rhesus monkey and at 4–6 weeks in humans. As the pregnancy advances there is a significant increase, reaching its maximum value at delivery at 21 days gestation in rodents (A), at 24 weeks in Rhesus monkey (B), and 36 weeks in humans (C). The chimeric cells remain up to 42 days in the maternal body in rodents, not known in Rhesus monkey and up to 27 years in humans. The types of cells being transferred from fetus to mother are stem cells and differentiated cells. The degree of cell differentiation could determine its function, being stem cells responsible for tissue repair and regeneration, and differentiated cells triggering maternal and fetal immune responses (Fujiki et al., 2008).
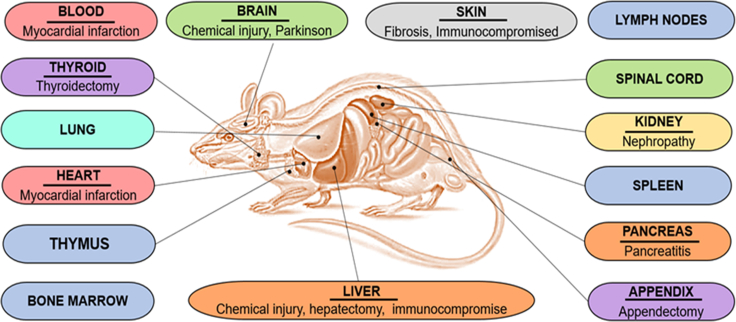
Fetal cells cross the placental barrier and enter the maternal circulation, where they can survive, migrate, and integrate into different maternal tissues (Zeng et al., 2010). In animal models, FMc has been detected in various organs such as the bone marrow (Fujiki et al., 2008,2009; Khosrotehrani et al., 2008; Pritchard et al., 2012), pituitary gland (Jimenez et al., 2005), skin (Jimenez et al., 2005; Aractingi et al., 2002), appendix (Santos et al., 2008), liver (Beksac et al., 2020; Fujiki et al., 2008,2009; Jimenez et al., 2005; Khosrotehrani et al., 2008; Wang et al., 2004), brain (Dawe et al., 2007; Zeng et al., 2010; Tan et al., 2005), lung (Fujiki et al., 2008,2009; Pritchard et al., 2012), heart (Fujiki et al., 2008,2009; Jimenez et al., 2005; Kara et al., 2012), spinal cord (Zhang et al., 2014), suprarenal gland (Jimenez et al., 2005), kidney (Fujiki et al., 2008, 2009; Wang et al., 2004), spleen (Fujiki et al., 2008, 2009; Jimenez et al., 2005; Khosrotehrani et al., 2008), thyroid (Imaizumi et al., 2002; Jimenez et al., 2005), lymph nodes (Jimenez et al., 2005; Nguyen Huu et al., 2006), thymus (Fujiki et al., 2008, 2009; Khosrotehrani et al., 2008) and pancreas (Vojdani et al., 2018) (Figure 4).
Figure 4.
Fetal microchimerism (FMc) presence in rodent organs and associated diseases
Representation of the different rodent organs where FMc have been identified and the associated diseases where FMc have been localized.
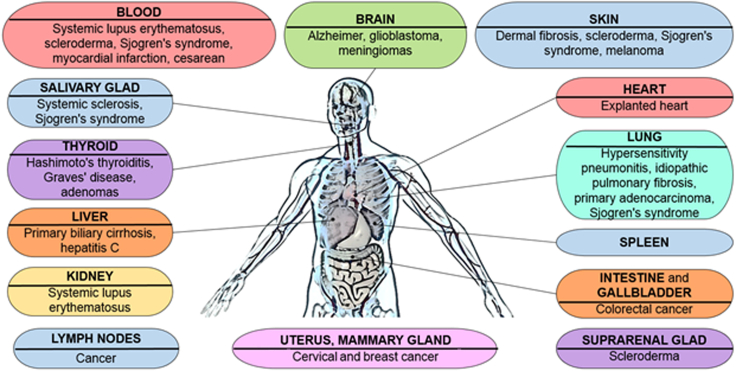
In humans, FMc has been found in various organs such as skin (Huerta Sil and Medrano Ramírez, 2006), spleen (Koopmans et al., 2005), liver (Koopmans et al., 2005; Khosrotehrani et al., 2004; Stevens et al., 2004), brain (Broestl et al., 2018; Chan and Nelson 2013; Chan et al., 2012), lung (Fugazzola et al., 2011; Bustos et al., 2007; Koopmans et al., 2008), heart (Bayes-Genis et al., 2005; Fett 2011), kidney (Kremer Hovinga et al., 2006; Koopmans et al., 2008), breast (Dubernard et al., 2008; Broestl et al., 2018; Fugazzola et al., 2011; Gadi et al., 2008; Nemescu et al., 2016), suprarenal glands (Johnson et al., 2001), thyroid (Ando et al., 2002; Cirello et al., 2008; Fugazzola et al., 2011; Khosrotehrani et al., 2004; Klintschar et al., 2006; Koopmans et al., 2008; Pritchard et al., 2012), lymph nodes (Khosrotehrani et al., 2004; Koopmans et al., 2008), salivary glands (Aractingi et al., 2002; Carlucci et al., 2003; Endo et al., 2002; Evans et al., 1999; Kuroki et al., 2002), uterus (Bhat et al., 2019), gallbladder (Khosrotehrani and Bianchi 2005) and intestine (Khosrotehrani and Bianchi 2005) (Figure 5).
Figure 5.
Fetal microchimerism (FMc) presence in human organs and associated diseases
Representation of the different organs where FMc have been localized in humans and the diseases associated.
Once FMc has been established in the maternal organs, they differentiate into cell lines thanks to their high multiline capacity and phenotypic plasticity (Mahmood and O’Donoghue 2014). Cardiomyocytes (Bayes-Genis et al., 2005; Fett 2011; Kara et al., 2012), endothelial cells (Bianchi et al., 1996; Fujiki et al., 2009,2008; Jimenez et al., 2005; Kara et al., 2012; Koopmans et al., 2005; Kremer Hovinga et al., 2006; Price et al., 1991), neurons (Bianchi 2000; Zhang et al., 2014), glial cells (Tan et al., 2005), epithelial cells (Cha et al., 2003; Dubernard et al., 2008; Khosrotehrani et al., 2004; Renné et al., 2004), dendritic cells (Lambert et al., 2001), hepatocytes (Khosrotehrani et al., 2004) macrophages and lymphocytes (Artlett et al., 2002; Cha et al., 2003; Evans et al., 1999; Khosrotehrani et al., 2004,2008; Koopmans et al., 2005; Kremer Hovinga et al., 2006; Koopmans et al., 2008; Lambert et al., 2001; Renné et al., 2004; Santos et al., 2008) of fetal origin have been identified in humans and animal models.
FMC function
It has also been described a high proportion of FMc in maternal and placental pathologies (Lambert 2007), gestational complications such as fetal aneuploidies (Down syndrome), pre-eclampsia, premature births, or miscarriages (Boddy et al., 2015) and placental anomalies (Khosrotehrani and Bianchi 2005). FMc is also present in cancers such as colorectal cancer (Kamper-Jørgensen et al., 2012), breast cancer (Dubernard et al., 2008; Fugazzola et al., 2011; Ye et al., 2010), thyroid cancer (Ye et al., 2010; Dubernard et al., 2008; Fugazzola et al., 2011), melanoma (Fugazzola et al., 2011; Hui and Bianchi 2017), cervix cancer (Cha et al., 2003), lung cancer (O’Donoghue et al., 2008; Fugazzola et al., 2011), bladder cancer (Korkmaz et al., 2015), pancreatic cancer (Vojdani et al., 2018) or lymphoma (Klonisch and Drouin 2009) being located mainly in the maternal tumor region. FMc may have conflicting roles, not mutually exclusive. These functions could be beneficial, harmful, or neutral for maternal pathophysiology (O’Donoghue et al., 2008).
On the one hand, FMc may have a beneficial (protective and regenerative) role in maternal health, participating in tissue repair and regeneration, cell replacement, and maternal homeostatic maintenance (Boddy et al., 2015). For instance, FMc has been found in inflamed maternal tissues, suggesting they are involved in angiogenic and healing processes (Nguyen Huu et al., 2009). In lung cancer, primiparous and multiparous women have a better prognosis than nulliparous women and men, suggesting that FMc could suppress maternal tumor development (Fugazzola et al., 2011; Hallum et al., 2020). FMc presence is also related to a lower risk of breast cancer (Broestl et al., 2018; Fugazzola et al., 2011; Ye et al., 2010) and bladder cancer (Nguyen Huu et al., 2009).
In contrast, FMc may have a detrimental role in maternal health. FMc was found in many post-pregnant women with autoimmune diseases (Lambert et al., 2001; Nelson, 2002a, 2002b). They were considered alloimmune diseases since the embryo is a semi-allogenic organism capable of being rejected by the maternal immune system (Bianchi 2000,2004). Subsequently, other studies have reported a higher proportion of fetal cells in women with cancer (specifically colon cancer) (Kamper-Jørgensen et al., 2012), women with autoimmune diseases such as systemic sclerosis (Artlett et al., 1998,2002; Burastero et al., 2003; Evans et al., 1999; Fugazzola et al., 2011; Gannagé et al., 2002; Johnson et al., 2001; Lambert et al., 2001; Nelson 1996,1999; Scaletti et al., 2002; Ye et al., 2010) rheumatoid arthritis (Adams et al., 2007; Yan et al., 2006; Fugazzola et al., 2011) or Sjögren syndrome (Fugazzola et al., 2011; Giacomelli et al., 2002; Miyashita et al., 2000) and with non-autoimmune pathologies such as hepatitis C (Boddy et al., 2015; Fugazzola et al., 2011; Johnson et al., 2002; Stelzer et al., 2015).
Other studies, however, suggest that FMc may have a possible neutral function (Boddy et al., 2015) and would be a by-product or remnant of pregnancy lacking biological value (Boddy et al., 2015; Lambert and Nelson 2003; Nelson, 2002a, 2002b).
FMC presence in different tissues
FMc in the cardiovascular system
In rodents, fetal cells enter the maternal circulation between 10 and 12 days of gestation and remain until 42 days postpartum (PPD) (Vernochet et al., 2007). In mice (Mus musculus) in which myocardial infarction (MI) was induced during pregnancy (on the 11th day of gestation), there was a higher percentage of fetal cells in the maternal blood and the maternal heart after MI (Post-MI) than before MI (Pre-MI) analyzed by FACS, qPCR, and IHC for the eGFP transgene detection. 50% of eGFP+ cells (1.5% of 3% of eGFP+ cells) expressed the cardiac marker α-actinin in infarcted hearts analyzed by IHC (Kara et al., 2012).
FMc cells persisted 1 and 2 weeks after MI and were preferentially located in the infarcted region, suggesting their participation in maternal tissue repair. One week after inducing MI, FMc began to differentiate toward cardiac lineages, expressing markers of immature cardiomyocytes (Nkx2.5, 80% of eGFP+cells), endothelial cells (CD31, 46% of eGFP+cells), and different stem and progenitor cell markers, including Sca-1 (21% of eGFP+cells), CDX2 (38% of eGFP+cells), c-kit (25% of eGFP+cells), CD34 (15% of eGFP+cells), Sox2 (24% of eGFP+cells), Islet1 (3% of eGFP+cells), Pou5f1 (2% of eGFP+cells) and Nanog (3% of eGFP+cells).
Three weeks after inducing MI, the fetal cells differentiated into cardiac cells, expressing markers of mature cardiomyocytes, smooth muscle cells, and endothelial cells. 50% of these cells expressed α-actinin suggesting that FMc differentiates into cardiomyocytes in the injured heart. These data suggested the FMC contribution to maternal angiogenesis, repair, and tissue regeneration (Ye et al., 2010). Previously, fetal cells were found in vascular tissues and expressed cell adhesion and migration markers such as ITGAM, ITGB1, and glycoprotein CD44. Fetal cells were identified in cardiac tissues and expressed the endothelial marker CD105 or ENG (Fujiki et al., 2008, 2009). Christner et al. also found FMc in blood in control mice and a dermal fibrosis model induced by intraperitoneal injection of vinyl chloride, increasing the FMc number almost 50-fold (Christner et al., 2000). These authors performed the PCR to amplify the H-2Kb gene.
In rhesus monkeys (Macaca mulatta), FMc was detected in pregnant females' blood using RT-PCR assay for SRY and TSPY detection (Jimenez and Tarantal 2003a,2003b;Jimenez et al., 2005).
Similarly, in humans, fetal cells cross the placental barrier and enter into the maternal circulation between the fourth and sixth week of gestation, increasing significantly during pregnancy (n = 72) at 30 days and 50 days of gestation with an increase of fetal DNA, reaching up to 2.7% of the total DNA in the third period of gestation (Jimenez and Tarantal 2003b). FMc expressing CD34 was also found in skin, thyroid, liver, pituitary, adrenal glands, spleen, and lymph nodes of all females with male offspring up to 3 years postpartum. In the heart, more than 50% of FMc analyzed expressed CD34, suggesting its pluripotential capability (Jimenez et al., 2005).
In humans (Homo sapiens), Bianchi et al. detected fetal cells in maternal blood up to 27 years postpartum by Y chromosome PCR amplification and FACS analysis. These expressed CD34 marker and the hematopoietic marker CD38 (Bianchi et al., 1996), confirming the multilineage capacity of these cells. Later, Artlett et al. found a higher frequency of FMc in the blood of women with systemic sclerosis (82.9% of cases) compared to controls (63.6% of cases) by MACS and qPCR analysis of Y chromosome sequences. These cells expressed the lymphocyte markers CD4 and CD8, which could indicate that they were T lymphocytes, contributing to the pathogenesis of the disease (Artlett et al., 2002). Shree et al. also detected fetal cells in this tissue and identified a higher proportion of FMc in the blood of women who had delivered by cesarean section compared to those whose delivery had been vaginal (Shree et al., 2019), which could postulate the hypothesis about its beneficial role in maternal health. These authors performed PCR analysis targeting fetus-specific polymorphism. Also, Bayes-Genis et al. identified fetal cells in the explanted heart of two women with heart disease by FISH and RT-PCR analysis for SRY detection and IHC for alpha-actinin detection. These cells represented only 0.25% and expressed α-actinin (Bayes-Genis et al., 2005; Fett 2011). The presence of FMc in maternal blood is lower than expected, detecting a minimal number of fetal cells ranging from 0.08% (Tan et al., 2005) (46) to 0.00006% (Fujiki et al., 2008, 2009).
FMc in the nervous system
FMc has also been found in the human brain (Chan et al., 2012) and murine spinal cord (Zhang et al., 2014).
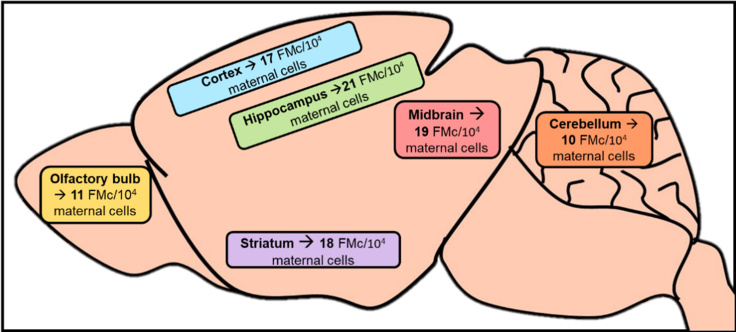
In murine models, Tan et al. analyzed by qPCR, FISH for the Y chromosome detection and IHC for eGFP+cells, the presence of FMc up to 62 PPD in females’ brains with an injury induced by intraventricular injection of NMDA during gestation. A higher proportion of FMc was found in brains at 31 PPD. Cells differentiated into neurons, oligodendrocytes, astrocytes, and perivascular macrophages, integrating functionally into maternal neural circuits (Tan et al., 2005). Brains of female mice with Parkinson’s disease induced by intraventricular injection of 6-OHDA showed a higher proportion of fetal cells at 7 PPD in olfactory bulbs, cerebral cortex, striatum, hippocampus, midbrain, cerebellum and mainly in the hippocampus (62.5% of cases) (Figure 6). These cells, concentrated in the CA1 region, both 7 and 60 PPD (Zeng et al., 2010) and differentiated into neurons and glial cells. In Parkinson’s disease mouse model, 60 and 100% of cells were eGFP + fetal cells at 7 and 60 PPD. In women with Alzheimer’s disease, 29% of cells were FMc, while in brain tumors, the percentages were 48% (meningioma) and 78% (glioblastoma) (Chan et al., 2012).
Figure 6.
Brain areas with fetal microchimerism (FMc) in a Parkinson'sdisease murine model
FMc has been found in the olfactory bulb, cortex, striatum, hippocampus, midbrain and cerebellum of mice brains with Parkinson’s disease. The most significant number of eGFP + fetal cells were detected in the hippocampus.
A higher frequency of FMc was detected in brains without injury than the injured ones at 60 ppd. Thus, FMc might have a reparative action on the brain participating in angiogenesis and neurogenesis in mouse models (Zeng et al., 2010).
FMC was detected in the murine spinal cord by PCR and IHC for eGFP+cells and found a higher percentage of chimeric cells in mice that were pregnant three times than in mice having gone through a single pregnancy (20% of cases, 1 of 5 females). These cells remained up to 3 weeks after delivery, but their frequency was relatively low at 1–3 GFP+ cells per 40 sections, detecting 1 fetal cell per 105 maternal cells. They expressed the neural marker NeuN, demonstrating that they can migrate to the spinal cord and differentiate into functional neurons.
Human brains with and without Alzheimer’s disease were analyzed, finding FMc in 35%of the analyzed cases (64 of the 183 samples). There was a higher FMc proportion in healthy ones (46% of cases, 30 of the 65 samples) than in brains with neurological disease (29% of cases, 34 of the 118 samples). These authors performed qPCR for the DYS14 detection. FMc was mainly localized in the medulla (88% of cases, 7 of 8 samples), amygdala (50% of cases, 1 of 2 samples), spinal cord (50% of cases, 1 of 2 samples), temporal lobe (46% of cases, 12 of 26 samples), cingulate gyrus (42% of cases, 11 of 26 samples), parietal lobe (41% of cases, 7 of 17 samples) and the hippocampus (35% of cases, 7 of 20 samples). A lower FMc percentage was found in the frontal lobe (0% of cases, 0 of 3 samples), putamen (0% of cases, 0 of 4 samples), caudate (11% of cases, 1 of 9 samples), cerebellum (13% of cases, 2 of 16 samples), occipital lobe (20% of cases, 7 of 17 samples) or the pons (31% of cases, 11 of 35 samples) (Figure 6) (Chan et al., 2012; Chan and Nelson 2013).
FMc was also in women's brains with glioblastomas (78% of cases, 25 of 32 tumors analyzed) and meningiomas (48% of cases, 12 of 32 tumors analyzed) (Broestl et al., 2018).
FMc in the respiratory system
Fujiki et al. detected FMc in murine lungs by FACS and RT-PCR amplification for eGFP that expressed cell adhesion and migration markers such as ITGAM, Integrin Beta1, CD44, CD34, CD105, ENG, PECA, PTPRC, CXCR4 (Fujiki et al., 2008, 2009). Pritchard et al. analyzed the presence and survival of FMc after delivery and 3 months postpartum. These cells remained alive and divided after 17–18 days postnatal and persisted up to 3 months postpartum, decreasing their number on the days after delivery (Pritchard et al., 2012).
In humans, FMc was initially identified in women with eclampsia (Bustos et al., 2007). Bustos et al. studied the relationship between FMc and maternal hypersensitivity pneumonitis (HP) by PCR of TSPY gene. They detected a high percentage of FMc in bronchoalveolar lavage fluid (64% of cases) and blood (33% of cases, 34 of 103 women) of women with HP (20% of cases, 2 of 10 women). Women with idiopathic pulmonary fibrosis (IPF) presented a lower percentage (10% of cases, 3 of 30 women) and controls shown 16% of cases, 7 of 43 women. FMc were present only in five lungs of women with HP; they localized in inflammatory regions and expressed the macrophage marker CD14+ and T lymphocyte markers (CD8+ or CD3+, CD4+) (Bustos et al., 2007). Fetal cells were also found by FISH for Y chromosome, in the alveolar septum of 10 out of 38 lungs analyzed (36% of cases) (Koopmans et al., 2008). Women lung tumors presented a higher number of FMc (48.5 cells per tissue section) than healthy women (0 cells per tissue section) and were concentrated in the tumoral region (Fugazzola et al., 2011; O’Donoghue et al., 2008).
FMc in the excretory system
In animals, Wang et al. detected FMc by FACS and IHC for eGFP+ cells, in the tubular basement membrane of kidneys in a model of nephropathy induced by gentamicin (Wang et al., 2004). Subsequently, Fujiki et al. also identified FMc in this tissue by FACS and RT-PCR for eGFP, which expressed ITGB1 and PECAM (Fujiki et al., 2008, 2009). Kajbafzadeh et al. also found these cells in injured kidneys by PCR and IHC of eGFP, at 3 months postpartum that localized in the renal blood vessels and the injured kidney area, which led them to propose a role for FMc in maternal angiogenesis and kidney repair (Kajbafzadeh et al., 2018).
Koopmanset al. found fetal cells in the kidney from women with systemic lupus erythematosus (SLE, 26% of cases, 13 out 51 samples) and controls (55% of cases. 27 out of 49 samples) by FISH and IHC for the Y chromosome. These cells localized in the renal glomeruli and the renal tubules. They expressed the hematopoietic precursor marker CD34 and the lymphocyte marker CD3, suggesting they could be potential functional T lymphocytes (Kremer Hovinga et al., 2006).
FMc in the reproductive system
In human uterus, Cha et al. found fetal cells in tumors of women with cervical cancer (50% of cases, 4 of the 8 samples) with a frequency of 4.4 cells per million maternal cells, but not in controls (0% of cases, 0 of the 2 samples) by Y chromosome FISH. These cells expressed the epithelial marker cytokeratin (24% of chimeric cells, 9 of 37 cells) or the lymphocyte marker CD45 (44% of chimeric cells, 8 of 18 cells) (Cha et al., 2003; Fugazzola et al., 2011). Using FISH and PCR for the Y chromosome, Khosrotehrani et al. also identified fetal cells in three women that expressed cytokeratin (between 20% and 56% of chimeric cells) or CD45 (between 30% and 55% of chimeric cells (Khosrotehrani et al., 2004). The authors suggested an FMc protective role in maternal health (Cha et al., 2003; Fugazzola et al., 2011; Khosrotehrani et al., 2004; Khosrotehrani and Bianchi 2005).
In human breast tissue, Gadi et al. detected the presence of FMc in the blood of women with breast cancer (14% of cases, 5 of the 35 samples) and in healthy women (43% of cases, 20 of the 47 samples) by qPCR for DYS14, One year later, Gadi et al. also detected the presence of FMc in the blood of women with breast cancer (26% of cases, 14 of the 54 samples) and in healthy women (56% of cases, 25 of the 45 samples) by qPCR for the Y chromosome, establishing a possible association between the presence of fetal cells and a lower probability of developing breast cancer (Gadi et al., 2008; Fugazzola et al., 2011). Dubernard et al. also identified fetal cells in 9 out of the ten mammary carcinomas analyzed (90% of cases), detecting 36.5 fetal cells per million maternal cells) by Y chromosome FISH. These cells expressed cytokeratin marker (22% of chimeric cells) or the mesenchymal marker vimentin (16% of chimeric cells) (Fugazzola et al., 2011; Dubernard et al., 2008). Later, Kamper-Jørgensenet al. found FMc in the blood of healthy women (70% of cases, 190 of the 272 healthy women analyzed) compared with the blood of women who developed breast cancer (40% of cases, 36 of the 89 women studied). These authors performed the qPCR for sex determining region Y chromosome (DYS14) to find these cells and they associated the presence of these cells with a 70% reduction in the probability of developing breast cancer (Kamper-Jørgensen et al., 2012). Nemescu et al. also detected fetal cells in mammary carcinomas. These cells were located up to 5.5 times more frequently in the tumor region (100% of cases) than in healthy tissues (64% of cases) (Nemescu et al., 2016). These authors amplified by qPCR analysis for the sex determining region Y chromosome (SRY) to find these cells.
Hallum et al. found a lower frequency of circulating FMC in women blood samples with ovarian cancer (46% of cases, 40 women of 87) compared to controls (66% of cases, 333 of 505 women). These authors used PCR analysis for detecting the Y chromosome to localize these cells and also detected a lower mean shrinkage of chimeric cells from patients’ blood (2.84 cells per 106 maternal cells) as opposed to healthy women (3.23 cells per 106 maternal cells). In agreement with the results obtained in breast cancer, women with chimeric cells had a lower risk rate of ovarian cancer compared to women who not had these cells, which confirms the hypothesis about the beneficial role of FMc in maternal health (Hallum et al., 2020).
FMc in the digestive system
In murine models, Wang et al. detected fetal cells in livers injured by ethanol consumption; they used FACS and IHC for the eGFP sequence to find these cells. These cells expressed albumin, suggesting that they could be functional hepatocytes capable of contributing to liver regeneration (Wang et al., 2004). Khosrotehrani et al. analyzed the presence of FMc in this organ 4 and 8 weeks after inducing a lesion. They found a higher proportion of fetal cells in livers at eight weeks (69% of cases) compared to 4 weeks (20% of cases) after inducing chemical injury, compared with partial hepatectomy. Khosrotehrani et al. found that 4% of FMc in the liver expressed the hepatic marker heppar-1 and could be functional hepatocytes (Khosrotehrani et al., 2004).
As in other organs, these cells expressed the cell adhesion and migration markers ITGAM, ITGB1, the glycoproteins CD44 and CD34, the endothelial markers PECAM, ENG, and the lymphocyte marker PTPRC (Fujiki et al., 2008, 2009); this was detected by eGFP FACS analysis and RT-PCR amplification FMc were identified in controls and immunocompromised animals treated with cyclosporine. A higher proportion of fetal cells were found using at four and especially at 12 months postpartum in both groups, with a slightly higher percentage IHC for eGFP detection, in immunocompromised livers (Beksac et al., 2020). Women with primary biliary cirrhosis and hepatitis C had FMc in the liver Koopmans et al. also identified FMc in 7 of the 64 livers analyzed in women with sons (21%), concluding that these cells could be functional hepatocytes (Koopmans et al., 2005).
In the murine pancreas, Vojdani et al. identified 20% of FMc after inducing acute pancreatitis by caerulein injection, using IHC and PCR for eGFP detection, located in the acini and around the blood vessels. Females with a history of pregnancy showed an improvement in the pathology, suggesting a beneficial role of FMc in maternal pathophysiology (Vojdani et al., 2018).
In the liver of Rhesus monkey females, most FMc was found 3 years postpartum using RT-PCR analysis for SRY detection and the specific protein Y genes (TSPY). As in other tissues, these cells expressed the precursor marker CD34, indicating that they might contribute to different lineages (Jimenez et al., 2005).
In the gallbladder and the intestine of two women, FMc was localized and expressed cytokeratin (20%–56% of the FMc) or CD45 (30%–55% of the FMC) (Khosrotehrani and Bianchi 2005; Khosrotehrani et al., 2004).
Steve et al. also found fetal cells in the liver of women suffering from primary biliary cirrhosis and hepatitis C by FISH and IHC for the male-chromosome-specific sequence. These cells expressed CAM-5.2 (149). In addition, Johnson et al. detected FMc in the liver tissue of a woman with chronic hepatitis C by Y chromosome PCR and FISH (Johnson et al., 2002). Subsequently, Koopmans et al. also identified FMc in 7 of the 64 human livers analyzed in women with sons (21% of cases) by PCR and FISH for SRY and concluded that these cells could be functional hepatocytes (Koopmans et al., 2005). This study also identified fetal cells in 90% of the 67 women who developed colorectal cancer, linking the presence of these cells to a four times higher chance of developing colon cancer (Koopmans et al., 2005; Kamper-Jørgensen et al., 2012).
FMc in the endocrine system
Pregnant mice immunized with Tg (46% of cases, 12 of the 26 samples) presented a higher frequency of FMc in the thyroid compared to non-immunized ones (20% of cases, 2 of the 10 samples) by PCR and ELISA for SRY and IHC for eGFP+cells. These cells expressed CD45, CD8, CD4, and CD11c cell markers, suggesting they could be T lymphocytes or dendritic cells (Imaizumi et al., 2002).
In female Rhesus monkeys, more than 50% frequency of FMc was found in the thyroid, pituitary gland, and suprarenal glands after 3 years postpartum by RT-PCR analysis of SRY and TSPY. These cells expressed CD34 and could be a source of progenitor cells, supporting that FMc contributes to different lineages (Jimenez et al., 2005).
FM was detected in 12 thyroids of 20women with thyroiditis (63% of cases, 1–165 fetal cells for tissue section), presented FMc were not present in healthy women (0% of cases, 0 of 8 samples) by FISH of Y chromosome (Srivatsa et al., 2001). Ando et al. observed FMc only in thyroid biopsies from women with Graves' disease (19%, 5 out of 27 samples) by PCR and ELISA of SRY (Ando et al., 2002). A high proportion of fetal cells were present in the thyroid of women with Hashimoto’s thyroiditis (60% of cases, 15 of 25 samples) and Graves’ disease (40% of cases, 6 of 15 samples). These cells expressed epithelial or lymphocyte markers CD45, CD20, and CD3 (Renné et al., 2004. Another study described FMc by RT-PCR of DYS14, in all thyroids of 21 women with Hashimoto’s thyroiditis (100% of cases) with a frequency of 15–4900 chimeric cells for every 100,000 maternal cells (Klintschar et al., 2006). Women with papillary thyroid cancer presented FMc in the thyroid (48% of cases, 19 out of 40 samples), compared to controls (25% of cases, 5 of 20 samples) with a higher number in tumors of women with cancer (11.1 cells per million maternal cells) than in controls (0–3 cells per million maternal cells). This study was performed using immuno-FISH and IHC for Y chromosome. Fetal cells were located in the tumoral region and expressed the leukocyte marker CD45 (13% of chimeric cells) and the epithelial marker thyroglobulin (66% of chimeric cells) (Fugazzola et al., 2011; Cirello et al., 2008).
Later in 2010, Cirello et al. found fetal cells in 6 of the 19 samples of women with papillary thyroid cancer analyzed (32% of cases, 2.1 to 6.9 cells/section) by FISH and PCR for the sex determining region gene (SRY). These authors also detected these cells in the blood of women with papillary thyroid cancer (49.1% of cases, 28 of the 57 samples) and in controls (77.6% of cases, 38 of the 49 samples), finding a greater number of fetal cells in tumor tissues than in the blood of women with thyroid cancer (Cirello et al., 2010). Santos et al. found FMc in the appendix of 8 women who had undergone an appendectomy during pregnancy by Y chromosome FISH and IHC. These cells were located mainly in inflamed areas, expressed CD3 and they could be lymphocytes (Santos et al., 2008).
Women with multinodular goiters who underwent a partial thyroidectomy presented FMc in the thyroid. These cells expressed cytokeratin (between 14% and 60%) or CD45 (67%) and were located mainly in the thyroid follicles and healthy tissues (114 out of 150 fetal cells) that surrounded the adenoma (36 out of 150 fetal cells) (Khosrotehrani et al., 2004; Khosrotehrani and Bianchi 2005). FMc was also found in 10% of mothers’ spleens, confirming its contribution to the immune system (Rijnink et al., 2015). Moreover, FMc was observed in 8 of the 44 maternal thyroids analyzed (18%) in the thyroid follicles. They also found FMc in one of the seven lymph nodes analyzed (14%), which could be lymphocytes (Koopmans et al., 2008).
FMc was found in the suprarenal glands of women with systemic sclerosis by FISH of Y Chromosome (Jonson et al., 2001). In some studies, FMc was present in salivary glands (36% of cases, 10 out of 28 samples) of women with Sjögren syndrome by FISH and PCR assays of Y chromosome (Kuroki et al., 2002; Carlucci et al., 2003; Endo et al., 2002; Giacomelli et al., 2002). Subsequently, Endo, Negishi & Ishikawa, moreover, discovered fetal cells of salivary glands and these cells were in a greater frequency of women with Sjögren’s syndrome (55% of cases, 11 of 20 samples) compared to controls (13% of cases, 1 of 8 samples). For finding these cells, the authors performed FISH and PCR analysis for the Y-chromosome-specific sex determination region (SRY) (Endo et al., 2002;Carlucci et al., 2003). In contrast, other authors found FMc in the salivary glands of women with scleroderma (45%, 5 of 11 samples), but not in women suffering from Sjögren’s syndrome (0% of cases, 0 of 16 samples) using PCR assays for SRY (Giacomelli et al., 2002; Carlucci et al., 2003).
FMc in the integumentary system
Fetal cells have been identified in the maternal skin in both animal models (Christner et al., 2000; Nguyen Huu et al., 2006,2008) and humans (Nguyen Huu et al., 2006; Koopmans et al., 2008).
Nguyen Huu et al. detected fetal cells in the mouse tumors with precancerous skin lesions (75% of cases, 9 of the 12 samples) but they did not find these cells in the controls (0% of cases, 0 of the 12 samples) by Y chromosome FISH and IHC for the eGFP+. The frequency of FMc detected was 90 fetal cells per million maternal cells in tumor tissues and 0 fetal cells per million cells in controls. These cells located in the tumor expressed VWF (36% of cases, 12 of the 33 microchimeric cells) participating in the maternal blood vasculature, CD45 (17% of cases, 5 of the 30 microchimeric cells) or cytokeratin (10% of cases, 4 of 41 microchimeric cells). However, the fetal cells did not express keratin or alpha-smooth, showing that it was not keratocytes (Nguyen Huu et al., 2008).
Later, Beksac et al. analyzed the presence of fetal cells in the skin of control mice injected with saline and in immunocompromised mice injected with cyclosporine at 7 and 15 days of gestation and found them at 4, 12 and 24 months postpartum using IHC and IF. They found a higher proportion of FMc at 4 and 12 months postpartum (especially at 12 months) both in controls and in immunosuppressed patients, with a slightly higher percentage of fetal cells located in the epithelial tissue of immunocompromised mice (Beksac et al., 2020). Using PCR for amplifying H-2Kb gene FMc was also found in the maternal epithelial tissue of control mice and a higher proportion in mice with dermal fibrosis induced with vinyl chloride (Christner et al., 2000).
In mice, FMc was detected in 13 of 23 melanomas removed during pregnancy (56% of cases) but not in controls (0% of cases, 0 of 23 samples) analyzed by PCR. These cells expressed VWF (56% of cells, 18 of 32 fetal cells detected), the lymphatic vessel marker LYVE-1 (37% of cells, 7 of 19 fetal cells) or CD45 (20% of cells, 3 of 15 fetal cells) and were located in the lymphatic vascular ring surrounding the tumor (Aractingi et al., 2002). They were more abundant in tumorigenic samples (292 FMc per million maternal cells, 62%) than in controls (12%).
In female Rhesus monkeys’ skin, more than 50% frequency of chimeric cells expressing CD34 3 years postpartum analyzed by RT-PCR detection of SRY and TSPY, suggesting the multilineage potential of FMc again. However, as in the liver, a small percentage of these cells were differentiated, in this case, to keratinocytes.
Koopmans et al. found fetal cells in 3 of the 21 human samples analyzed (14% of cases) by Y chromosome FISH. These cells were located in the epidermis and could be keratinocytes (98). Huuet al. also detected these cells in 10 of the 16 melanomas (63% of cases) removed during pregnancy compared to controls (13% of cases, 1 of 8 samples) analyzed by PCR to amplified for DNA specific sequence. The results presented a higher proportion of these cells in the tumorigenic samples (62% of cells, 292 fetal cells per million maternal cells) compared to controls (12% of cells, 2 fetal cells per million maternal cells). These cells expressed CD31 and CD34 markers (71% of chimeric cells, in 20 of the 28 fetal cells detected) or CD35 marker (20% of chimeric cells, in 2 of the 10 fetal cells detected), located in the dermis, suggesting a possible protective or carcinogenic function in the mother (Lee et al., 2010;Nguyen Huu et al., 2006).
FMc in the immune system and autoimmune diseases
In murine models, FMc was found in 31% of bone marrows analyzed (4 of 13), 39% of thymuses analyzed (5 of 13), 15% of spleens analyzed (2 of 13) at 14–17 days of gestation, and 4–6 weeks postpartum. These authors used FACS, MACS, OCR, IHC and Western Blot for eGFP. FMc of bone marrow and the spleen expressed IgM and CD19, indicating that they could be B lymphocyte precursor cells. FMc localized in the thymus and spleen expressed CD3, CD4, and CD8, suggesting that they could be functional T lymphocyte precursor cells with antigen-specific allogenic activity (T cell receptors+ (TCR+) expression) (Fujiki et al., 2008,2009;Khosrotehrani et al., 2008). The presence and survival of FMc in bone marrow were studied after delivery and at 3 months postpartum using eGFP FACS and RT-PCR and they expressed ITGAM, ENG and PTPRC. Pritchard et al. analyzed the presence and survival of FMc in bone marrow after delivery and at 3 months postpartum by q-PCR and FACS for the eGFP transgene. These authors discovered that these cells remain alive after 17–18 days after delivery and persisted up to 3 months after delivery, significantly decreasing their number in the days after delivery, mainly detecting dead fetal cells (Pritchard et al., 2012). Huu et al. identified FMc in 5 of the six metastatic lymph nodes analyzed (83.3% of cases); 50% of these cells expressed CD45 marker (4 of 8 fetal cells) (Nguyen Huu et al., 2009).
In monkeys, there was 50% less frequency of chimeric cells in the lymph node and spleen of females 3 years postpartum by RT-PCR analysis for SRY and TSPY. These cells expressed CD34 and could be a source of stem cells, suggesting their capacity to contribute to different lineages (Price et al., 1991).
In human tissues, in the spleen and the lymph nodes of two women FMc was found by PCR, FISH and IHC for Y chromosome sequence, and 90% expressed CD45 marker.
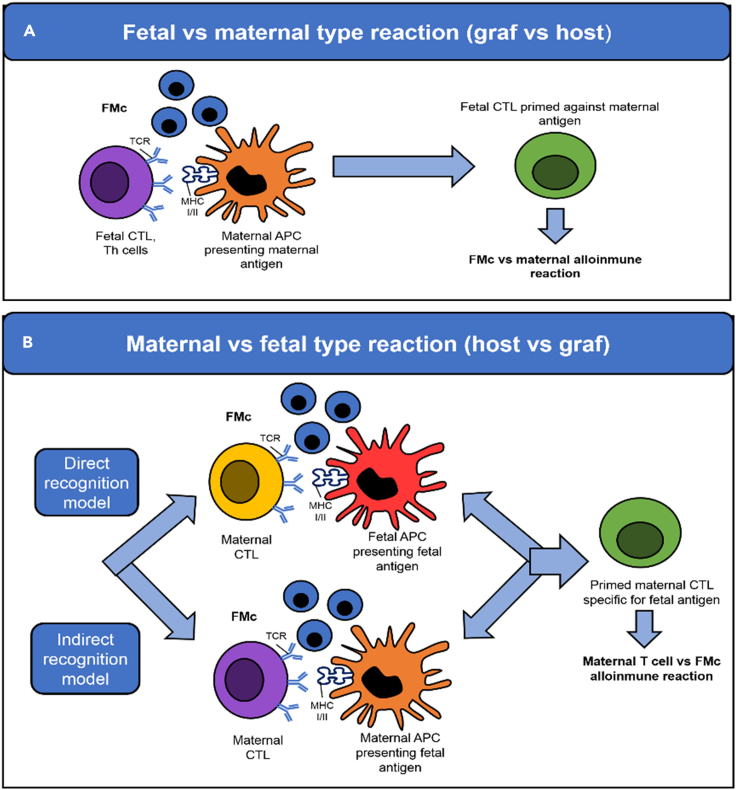
The possible relationship between FMc and autoimmune diseases due to the high frequency of post-pregnant women suffering from this type of pathology has been suggested. FMc has been found in women with systemic sclerosis or scleroderma (Artlett et al., 1998,2002; Burastero et al., 2003; Evans et al., 1999; Fugazzola et al., 2011; Gannagé et al., 2002; Lambert et al., 2001; Johnson et al., 2001; Nelson et al., 1998; Nelson 1999; Scaletti et al., 2002; Ye et al., 2010), rheumatoid arthritis (Adams et al., 2007; Fugazzola et al., 2011; Yan et al., 2006; Ye et al., 2010), systemic lupus erythematosus (Abbud Filho et al., 2002; Fugazzola et al., 2011; Gannagé et al., 2002; Kremer Hovinga et al., 2006; Mosca et al., 2003; Stevens 2006; Ye et al., 2010), Sjögren syndrome (Fett 2011; Fugazzola et al., 2011; Giacomelli et al., 2002; Kremer Hovinga et al., 2006; Miyashita et al., 2000), primary biliary cirrhosis (Corpechot et al., 2000; Fanning et al., 2000; Jones 2000; Renné et al., 2004; Tanaka et al., 1999; Yan et al., 2006), autoimmune thyroiditis (Klonisch and Drouin 2009; Renné et al., 2004; Srivatsa et al., 2001; Ye et al., 2010) such as Grave’s disease or hyperthyroidism (Ando et al., 2002; Christner et al., 2000) and Hashimoto or hypothyroidism (Fugazzola et al., 2011). A possibility that supports this hypothesis is that FMc may trigger graft-versus-host disease (GvHD), activating and regulating maternal immune responses, and could even participate in inducing the immune tolerance necessary to continue the pregnancy. However, the exact mechanisms are unknown (Ando et al., 2002; Fugazzola et al., 2011; Lissauer et al., 2009). Fetal cells could differentiate into active T lymphocytes, developing an autoimmune response against the maternal tissues (graft-versus-host reaction). This response involves cytotoxic or auxiliary fetal T lymphocytes in the presence of FMc: fetal versus maternal reaction (Figure 7A) or maternal versus fetal reaction (Figure 7B) where fetal antigen presenting-cells (APC) present FMc (direct recognition model) or maternal APC present FMc (indirect recognition model) (Table 1).
Figure 7.
The maternal immune response against fetal microchimerism
(A) Fetal cells can differentiate into active T lymphocytes and react against the maternal cells.
(B) Alternatively, maternal T lymphocytes can react against fetal cells either directly, when the fetal microchimerism (FMc) act as a fetal antigen presenting-cells (APC), or indirectly when FMc antigens are presented by maternal APC. CTL, Cytotoxic T Lymphocyte; TCR, T cell receptors; MHC, major histocompatibility complex; Th, T helper cell.
Table 1.
Frequency of chimeric cells in women maternal tissues with autoimmune diseases and controls
| Disease | Tissue | Frequency of cases with FMc |
Other characteristics (cell markers) | Ref. | |
|---|---|---|---|---|---|
| Control | Disease | ||||
| Systemic sclerosis | Blood | 0.38 cells per 16 mL | 1.11 cells per 16 mL | Nelson et al. (1998) | |
| Blood and skin lesions | 4% | 46% | – | Artlett et al. (1998) | |
| Blood | 33% | 60% | CD3, CD19, CD14, CD56/16 | Evans et al. (1999) | |
| Blood and skin lesions | 64% | 83% | CD3, CD4, CD8 | Artlett et al. (2002) | |
| Blood | 7% | 0% | CD3, CD4, CD8 | Müller et al. (2015) | |
| Blood | 16% | 22% | Male offspring | Gannagé et al. (2002) | |
| Blood | 33% | 60% | No male offspring | Evans et al. (1999) | |
| Salivary glands | – | 45% | Ariga et al. (2001) | ||
| Autoimmune thyroiditis | Thyroid | 0% | 55% | 1–165 cells per slide, mature thyroid follicles | Srivatsa et al. (2001) |
| Thyroid | – | 19% | – | Ando et al. (2002) | |
| Thyroid | – | 40%–60% | CD45, CD20, CD3 | Renné et al. (2004) | |
| Thyroid | – | 100% | 15-4900 per 100,000 maternal cells | Klonisch and Drouin (2009) | |
| Systemic lupus erythematosus | Blood | 33% | 68% | – | Abbud Filho et al. (2002) |
| Blood | 16% | 26% | Male offspring | Gannagé et al. (2002) | |
| Blood | 23% | 0% | No male offspring | Gannagé et al. (2002) | |
| Blood | 50% | 50% | LES 2.4 cells per 100,000 maternal cells, control 2.5 cells per 100,000 maternal cells | Mosca et al. (2003) | |
| Kidney | 55% | 26% | CD34, CD3 | Kremer Hovinga et al. (2006) | |
| Sjogren's syndrome | Salivary glands | – | 36% | – | Kuroki et al. (2002) |
| Bronchoalveolar lavage fluid | – | 22% | |||
| Salivary glands | 13% | 55% | – | Endo et al. (2002) | |
| Blood | 25% | 33% | |||
| Primary biliary cirrhosis | Liver | 72% | 70% | – | Tanaka et al. (1999) |
| Liver | 32% | 33% | Corpechot et al. (2000) | ||
| Blood | 25% | 45% | – | ||
| Liver | 6% | 42% | CD45 | Fanning et al. (2000) | |
Systemic sclerosis
A possible relationship between systemic sclerosis or scleroderma (SSc) and FMc has been established by detecting a higher percentage of fetal cells in the blood of women with this pathology (1.11 chimeric cells per 16mL of blood) compared to healthy women (0.38 chimeric cells per 16mL of blood). Evans et al. found the same tendency detecting a higher number of fetal cells in SSc (60% of cases, 12 of 20 women) compared to controls (33% of cases, 16 of 48 women). For discovering these cells, they performed FACS and PCR for male-chromosome-specific sequence and HLA-specific sequence (Lambert et al., 2001; Nelson et al., 1998). FMc expressed lymphocyte markers such as CD3, CD19, CD14, and CD56/16 and persisted up to 38 years after delivery (Endo et al., 2002). Moreover, a higher frequency of fetal lymphocyte cells (CD3+) was found in the blood of women with SSc (46% of cases, 32 of 69 samples) compared with healthy women (4% of cases, 1 of 25 samples) and they found these cells in inflamed skin tissues of women with the disease (56% of cases, 11 of 19 samples) using PCR and FISH to detect Y chromosome (Hahn et al., 1998; Artlett et al., 1998). These cells expressed CD4+ and CD8+ lymphocyte markers and could act as functional T lymphocytes with allogeneic antigen (maternal) specific capacity. FMc could be involved in the pathogenesis of the disease (Artlett et al., 2002) by triggering a graft-versus-host reaction (Burastero et al., 2003; Johnson et al., 2001). FMc was found in women with SSc, mainly in the lungs, skin, spleen, lymph nodes, and adrenal glands tissues. However, they were absent in the pancreas or in samples of women who had died of non-autoimmune pathologies (Johnson et al., 2001). Other studies have found T cells of fetal origin in the blood and skin of 3 women with this pathology (18% chimeric cells, 7 T cells of 39 reactive clones) and 3 controls (9% chimeric cells, 1 T cells of 11 reactive clones) by Y chromosome FISH. These cells were autoreactive, producing high concentrations of interferon-gamma (IFNγ) and interleukin 4 (IL-4) when reacting with maternal antigens MHC (Major Histocompatibility Complex), which supports the hypothesis mentioned above (Scaletti et al., 2002). FMc was detected in other studies only in the blood of women with SSc (7% of cases, 3 of 43 samples) compared to controls (0% of cases, 0 of 30 samples) (Burastero et al., 2003). Another hypothesis considered for these studies could be that fetal cells are not the cause of maternal autoimmune diseases but could be one of the factors that increase the ability to develop these pathologies because they contribute to maternal inflammation and autoimmunity, as they are partially foreign cells (Lambert et al., 2001).
Autoimmune thyroiditis
Srivatsa et al. were the first researchers to identify fetal cells in thyroid biopsies of women with autoimmune thyroiditis or thyroid adenomas: (63% of cases, 12 of the 20 samples) compared to control thyroid biopsies, in which these cells were not detected (0% of cases, 0 of 8 samples). The molecular method they used to detect these cells was Y chromosome FISH. These cells stood out for having a frequency of 1–165 cells per slide and differentiated into mature thyroid follicles (Srivatsa et al., 2001). FMc was found later only in thyroid biopsies of women with Graves' disease (19% of cases, 5 of 27 samples) as opposed to female adenoma samples (0% of cases, 0 of 6 samples) using FISH for the Y chromosome detection (Ando et al., 2002). A higher proportion of FMc in thyroid biopsies of women with Hashimoto’s thyroiditis (60% samples) and Graves' disease (40% samples) than thyroid biopsies of women with follicular adenomas (22%) was found. These cells expressed epithelial or lymphocyte markers CD45, CD20 and CD3 (Renné et al., 2004). Other studies found FMc in all thyroid biopsies of the 21 women with Hashimoto's thyroiditis analyzed (100% of cases), with a low frequency of chimeric cells (15 of 4900 fetal cells for every 100.000 maternal cells, while it was absent in healthy women. These authors located fetal cells in 1 of the 18 nodular goiters analyzed(6% of cases), with a frequency of 182 fetal cells for every 100,000 maternal cells using RT-PCR analysis of DYS14 (Klonisch and Drouin 2009).
Systemic lupus erythematosus
A relationship between FMc and systemic lupus erythematosus has been described by PCR analysis. However, there is not a significant increase in circulating FMc in women with SLE (0% of cases, 0 of 20 samples) compared to controls (20% of cases, 8 of 41 samples) (Miyashita et al., 2000; Stevens 2006). A higher FMc number was detected in blood of women with SLE (68% women analyzed) than in healthy women (33% of cases, 6 of 28 women analyzed) confirmed by RT-PCR of SRY. They also discovered a higher frequency of FMc in women with older children, suggesting that these cells can multiply in maternal tissue (Stevens 2006; Abbud Filho et al., 2002). Similar results were obtained in kidney samples from women with SLE 26% of cases, 13 of 51 samples) compared to controls (55% of cases, 27 of 49 samples) detected by FISH and PCR of SRY. These cells were located in the renal glomeruli and expressed CD34 or CD3 (Kremer Hovinga et al., 2006). FMc were identified in the blood of healthy and sick women, with and without male offspring. In blood of women who had male offspring, a higher percentage of FMc was found in the blood of women with SLE (26% of cases, 8 of 50 samples) compared to samples from women with SSc (22% of cases, 12 of 47 samples) and with controls (16% of cases, 5 of 23 samples). Analyzing women without male children, women suffering from SSc (33% of cases, 5 of 15 samples) had a higher number of FMc than in women with SLE (23% of cases, 19 of 83 samples) and controls (0% of cases, 0 of 24 samples). Regarding the chimeric cells detected, their exact origin is unknown, and their existence is attributed to an incomplete pregnancy or a spontaneous abortion, being, therefore, fetal cells (56, 149, 163). Some authors, however, have not reproduced these results. Mosca et al. identified no significant differences in the frequency or the number of FMc in the blood of women with SLE (50% of cases, 11 of 22 samples) and healthy women (50% of cases, 12 of 24 samples). Regarding the number of fetal cells, 2.4 cells were detected for every 100,000 maternal cells in the blood of women with SLE and 2.5 cells for every 100,000 maternal cells in the samples of healthy women; the technique they used was the PCR analysis for the Y chromosome (Mosca et al., 2003).
Rheumatoid arthritis
In women with rheumatoid arthritis (RA), Yan et al. detected higher levels of fetal DNA in the third trimester of pregnancy in those women with an improvement in this disease by RT-PCR for the Y-chromosome-specific sequence and HLA sequence. They established a possible relationship between the DNA level and the pathogenicity of the disease in women suffering from RA during the gestational period (Yan et al., 2006). Later, Kanaan et al. studied the presence of FMc in the blood of women using q-PCR against the HLA-DRB1 allele that codes for DERAA. These authors detected a higher number of fetal cells in the blood of DERAA -/- women with RA (53% of cases) compared to healthy women (6% of cases). These cells activated CD4+ T lymphocytes against maternal joint antigens, increasing the autoimmunity of mothers with RA, which may contribute to the pathogenesis of the disease (Kanaan et al., 2019).
Sjogren’s syndrome
Not a significant increase in circulating FMc was found in women with Sjögren’s syndrome (SS) (33% of cases, 6 of 18 samples) compared to controls (20% of cases, 8 of 41 samples) by PCR assays (Leduc et al., 2009). FMc was not found either in the salivary glands of women with SS (0% of cases, 0 of 6 samples) nor controls (0% of cases, 0 of 10 samples) by FISH and PCR for SRY (Bustos et al., 2007; Carlucci et al., 2001; Gannagé et al., 2002; Tan et al., 2011). Kuroki et al. first identified FMc in salivary gland biopsies (36% of cases, 10 out of 28 samples) and bronchoalveolar lavage fluid samples (22% of cases, 2 out of 9 samples) from women with SS by FISH and PCR of Y chromosome. However, they did not detect these cells in blood samples (Kuroki et al., 2002). Endo et al. observed a higher frequency of fetal cells in biopsies of the salivary glands of women with SS (55% of cases, 11 of 20 samples) compared to controls (13% of cases, 1 of 8 samples). However, again they did not find significant differences in blood samples of these patients (33% of cases, 2 of 6 samples) as opposed to controls (25% of cases, 5 of 20 samples) (Endo et al., 2002). Finally, Aractingi et al. did not find FMc in biopsies of salivary glands of women with SS (0% of cases, 0 of 16 samples). Still, they did in women with scleroderma (45% of cases, 5 of 11 samples) using PCR of SRY, suggesting that these diseases have different pathogenesis (Aractingi et al., 2002).
Primary biliary cirrhosis
Tanaka et al. identified for the first time these cells in liver biopsies of women with primary biliary cirrhosis (PBC) (70% of cases, 26 of 37 samples) and controls (72% of cases, 28 of 39 samples), without presenting any significant difference of these cells’ frequency. The authors used Y chromosome PCR to locate chimeric cells in this tissue (Tanaka et al., 1999). Rubbia-Brandt et al. did not find fetal cells in liver biopsies of women with PBC (0%, 0 of 10 samples) or hepatitis C chronic (0%, 0 of 3 samples) by Y chromosome FISH (Rubbia-Brandt et al., 1999). Later, Corpechot et al. detected a higher percentage of FMc in the blood of with PBC (45% of cases, 9 of 20 samples) compared to controls (25%, 5 of 20 samples). However, they did not find significant differences between the liver tissues of women with this pathology (33% of cases, 5 of 15 samples) and the controls (32% of cases, 8 of 25 samples) by PCR analysis for the male-chromosome-specific sequence (DYS1) (41, 81). Finally, Fanning et al. found chimeric cells in the liver biopsy of women with PBC (42%, 8 of 19 samples), which expressed the lymphocyte marker CD45, while these cells were not detected in liver biopsies of women with chronic hepatitis C or alcoholic liver disease (0%, 0 of 20 samples). The authors used molecular PCR analysis for the male-chromosome sequence. It should be noted that they also located FMc in the blood of a healthy woman (6% of cases, 1 of 18 samples), with a frequency of 1 chimeric cell for every 106 maternal cells. These authors suggest that the chimeric cells could be involved in this pathology (Corpechot, C.2000, Jones, D.E. 2000).
Considerations and future lines
FMc has been found as undifferentiated or differentiated cells throughout the maternal tissues. The short-and long-term consequences of FMc in the maternal body have been subjected to mismatching results, leading to a current major controversy in the field. FMc has been associated with autoimmune tolerance and tissue repair but also with autoimmune diseases and cancer, raising the possibility that they exert multiple and opposing effects in a mother’s body. Also, one should be aware that different pregnancy and birth complications, including preeclampsia, antepartum hemorrhage, and cesarean sections, might alter the functional fate of FMc (Bianchi et al., 2001; Hahn et al., 2019; Shree et al., 2019). Functionality might also depend on the source tissue, its cellular environment (for instance, pro-vs. anti-inflammatory) and the timepoint analyzed (shortly after delivery, during late postpartum or at a later reproductive age). Animal models will continue to be very useful in controlling for these variables and establishing the mechanisms underlying FMc.
We also highlight the necessity of improving the tools for identifying and quantifying fetal FMc in the maternal body. Currently, the primary choice for detecting FMc which targets cells containing the male only Y chromosome can't distinguish fetal and maternal cells if the progeny is female and misdetect cells from previous miscarriages, non-viable male twins, or older siblings. In murine models, one solution would be to use molecular techniques against the eGFP gene, previously crossing eGFP-expressing transgenic males with females not expressing fluorescent proteins. In humans, a more recently developed technique targets paternally inherited fetal DNA sequences that the mother does not possess (i.e., the HLA-polymorphism or various single nucleotide polymorphisms). By obtaining the genotypes of the parents and the child, this technique can accurately detect FMc from both female and male fetuses and discern the cumulative effects of several pregnancies in multiparous mothers. If genotyping is not available, human studies should at least always control the number of pregnancies a participant has had, including elective terminations and miscarriages.
FMc is a promising source for non-invasive prenatal diagnostic tests, identification of gestational complications, selection of histocompatible donors, and tolerogenic induction in autoimmune diseases. Unraveling the modulators of FMC cells' fate and functionality will provide further insight into their therapeutic potential.
Conclusions
-
1.
During pregnancy, transplacental bidirectional cell trafficking occurs between a pregnant woman and her fetus. FMc during pregnancy and after childbirth exists in both animal models (rodents and rhesus monkeys) and humans. FMc proliferates and differentiates into specific phenotypes in maternal tissues, including cardiomyocytes, neurons, endothelial cells, hematopoietic cells, and lymphocytes. The functional role of these cells in maternal pathophysiology remains under debate.
-
2.
On the one hand, FMc could be a source of progenitor cells with a beneficial effect on the mother’s health by intervening in tissue repair, angiogenesis, or neurogenesis. On the other hand, FMc might have a detrimental function by activating the immune response and contributing to autoimmune diseases.
-
3.
Here, we gathered and analyzed the results and advances obtained so far in the investigation of FMc in different organs, tissues, and species, examining and comparing multiple maternal diseases to understand the role of FMc in human health and disease (Table 2). These studies’ results are sometimes controversial due to the different techniques used to detect FMc and the technical variability of the methods. Therefore, the study of the FMc requires the standardization of specific protocols, the use of techniques that overcome the underestimation and misdetection of FMc, and an adequate selection of patients and controls.
-
4.
Future research on FMc should focus on clarifying their characteristics and, especially, their function. Understanding the peculiarities of FMc could be key to understanding the development of different pathologies, such as autoimmune diseases, and to develop effective and specific therapeutic strategies.
Table 2.
Presence of FMc in different maternal systems and organs in both animal models (rodents and rhesus monkeys) and humans, including the cell type they contribute to and the cell markers they express
| System | Organ | Species | Cell markers | Cell type | Diseases | Possible function |
|---|---|---|---|---|---|---|
| Cardio-vascular | Blood | Rodent | ITGAM, ITGB1, CD44, ENG | Hematopoietic cells | Myocardial infarction | Protective, tissue repair, regeneration |
| Monkey | CD34 | Hematopoietic stem cells or endothelial cells | – | Stem cells source | ||
| Human | CD34, CD38, CD4, CD8, | Hematopoietic cells, endothelial cell, lymphocytes T | Systemic lupus erythematosus, systemic sclerosis, Sjogren's syndrome, myocardial infarction, cesarean | Protective, tissue repair, regeneration, active immune response | ||
| Heart | Rodent | α-sarcomeric actin, α-actinin, Nkx2.5, CDX2, CD31, Sca-1, C-kit, CD34, Sox, Pou5f1, Nanog CD31, VE-cadherin, ENG | Mature and immature cardiomyocytes, endothelial cells, smooth muscle cells, trophoblasts, cardiac progenitor cells, hematopoietic stem cells, embryonic stem cells | Myocardial infarction | Protective, angiogenesis, tissue repair, regeneration, stem cells source | |
| Monkey | CD34 | Hematopoietic stem cells or endothelial cells | – | Stem cells source | ||
| Human | α-actinin | Cardiomyocytes | Explanted heart | Protective, angiogenesis, tissue repair regeneration | ||
| Nervous | Brain | Rodent | MAP2, NeuN, GFAP, NG2, CD45, β3-tubulin, DCX, nestin, PSA-NCAM, calbindin | Neuron, oliodendrocytes, astrocytes, macrophages | Lesion with NMDA, Parkinson | Protective, neurogenesis angiogenesis,, tissue repair regeneration, stem cells source |
| Human | – | – | Alzheimer, glioblastoma, meningiomas | Protective, tissue repair, regeneration | ||
| Spinal cord | Rodent | NeuN | Neuron | – | Protective, neurogenesis | |
| Respiratory | Lung | Rodent | ITGAM, ITGB1, CD44, CD34, CD105, ENG, PECAM, PTPRC, CXRC4 | Endothelial cells, lymphocytes, hematopoietic stem cells | – | Protective, tissue repair, regeneration, stem cells source, active immune response |
| Human | CD14, CD3, CD4, CD8 | Macrophages, lymphocytes T | Hypersensitivity pneumonitis, idiopathic pulmonary fibrosis, lung cancer, primary adenocarcinoma, Sjogren's syndrome | Active immune response, protective | ||
| Excretory | Kidney | Rodent | ITGB1, PECAM | Endothelial cells | Nephropathy | Protective, tissue repair, regeneration, |
| Human | CD34, CD3 | Hematopoietic stem cells, lymphocytes | Systemic lupus erythematosus | Active immune response | ||
| Reproductive | Uterus | Human | Cytokeratin, CD45 | Endothelial cells, lymphocytes | Cervical cancer | Protective, tissue repair, regeneration, active immune response, lower cancer risk |
| Breasts | Human | Cytokeratin, vimentin | Epithelial and mesenchymal cells | Mammary carcinoma | Protective, tissue repair, regeneration, stem cells source, lower cancer risk | |
| Ovary | Human | – | – | Ovarian cancer | Lower cancer risk | |
| Integumentary | Skin | Rodent | VWF, LYVE-1, CD45 | Endothelial cells, lymphocytes | Immunocompromised | Protective, tissue repair, regeneration, active immune response |
| Monkey | CD34 | Hematopoietic stem cells and endothelial cells | – | Stem cells source | ||
| Human | CD31, CD34, CD35 | Endothelial cells, lymphocytes | Dermal fibrosis, systemic sclerosis, Sjogren's syndrome, melanoma | Protective, tissue repair, regeneration, active immune response, carcinogenic | ||
| Digestive | Liver | Rodent | ITGAM, ITGB1, CD44, CD34, PECAM, ENG, PTPRC | Hepatocytes, endothelial cells, hematopoietic stem cells, lymphocytes | Chemical injury, hepatectomy, immunocompromised, | Protective, tissue repair, regeneration, stem cells source, active immune response |
| Monkey | CD34 | Hematopoietic stem cells or endothelial cells | – | Stem cells source | ||
| Human | Heppar-1, cytokeratin CAM-5.2 | Hepatocytes | Primary biliary cirrhosis, hepatitis C | Protective, tissue repair, regeneration | ||
| Pancreas | Rodent | – | – | Pancreatitis | – | |
| Intestine | Human | Cytokeratin, CD45 | Endothelial cells, lymphocytes | Colorectal cancer | Active immune response, carcinogenic, increased cancer risk | |
| Gallbladder | Human | Cytokeratin, CD45 | Endothelial cells, lymphocytes | – | Protective, tissue repair, regeneration, active immune response | |
| Immune | Bone marrow | Rodent | CD19, IgM, ITGAM, ENG, PTPRC | Endothelial cells, lymphocytes B | – | Activate immune response, |
| Thymus | Rodent | CD3, CD4, CD8, SLAMF1 | Lymphocytes T | – | Activate immune response | |
| Spleen | Rodent | CD19, IgM, ITGAM, CD44, PECAM, PTPRC | Endothelial cells, lymphocytes B | – | Protective, tissue repair, regeneration, active immune response | |
| Monkey | CD34 | Hematopoietic stem cells or endothelial cells | – | Stem cells source | ||
| Human | Cytokeratin, CD45 | Epithelial cells, Lymphocytes | – | Protective, tissue repair, regeneration, active immune response | ||
| Lymph nodes | Rodent | CD45 | Lymphocytes | – | Activate immune response | |
| Monkey | CD34 | Hematopoietic stem cells or endothelial cells | – | Stem cells source | ||
| Human | Cytokeratin, CD45 | Epithelial cells, Lymphocytes | Cancer | Activate immune response | ||
| Endocrine | Thyroid | Rodent | CD45, CD8, CD4, CD11c | Lymphocytes T, dendritic cells | Thyroidectomy | Activate immune response |
| Monkey | CD34 | Hematopoietic stem cells or endothelial cells | – | Stem cells source | ||
| Human | CD45, CD20, CD3, thyroglobulin | Endothelial cells, epithelial cells, lymphocytes | Hashimoto's thyroiditis and Graves' disease, thyroid adenomas, papillary thyroid cancer | Activate immune response, protective | ||
| Appendix | Human | CD3 | Lymphocytes | Appendectomy | Activate immune response | |
| Suprarenal glad | Human | – | – | Systemic sclerosis | Activate immune response | |
| Monkey | CD34 | Hematopoietic stem cells or endothelial cells | – | Stem cells source | ||
| Salivary glad | Human | – | – | Systemic sclerosis, Sjogren's syndrome | Activate immune response | |
| Pituitary glad | Monkey | CD34 | Hematopoietic stem cells or endothelial cells | – | Stem cells source |
Acknowledgments
This work was supported by Ministerio de Ciencia e Innovación project RTI2018-093952-B-100 and by Instituto de Salud Carlos III projects CP16/00096, PI18/00462 and PI17/00064, and co-funded by European Regional Development Fund (ERDF), A way to make Europe. MMG was funded by Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III, Predoctorales de Formación en Investigación en Salud (PFIS) (contract FI18/00255) and a predoctoral Fulbright grant. BC was supported by Consejería de Educación e Investigación, Comunidad de Madrid, co-funded by European Social Fund “Investing in your future” (PEJD-2019-PRE/BMD-16977). SC was funded by a Miguel Servet Type I research contract (CP16/00096). MM-G and SC were co-funded by European Social Fund Investing in your future. The project leading to these results has received funding from la Caixa Foundation under the project code LCF/PR/HR19/52160001, from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 883069), and from the Centro Nacional de Investigaciones Cardiovasculares (CNIC). The CNIC is supported by Instituto de Salud Carlos III (ISCIII), Ministerio de Ciencia e Innovación and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-050). We thank Vicente Llorente Úbeda for reading the manuscript.
Author contributions
BC, MMG, BGG and MP wrote the paper. SC, MD and MVG revised the document, obtained the funding, and coordinated the work.
Declaration of interests
The authors declare that they have no conflict of interest.
Contributor Information
Manuel Desco, Email: mdesco@hggm.es.
Susanna Carmona, Email: scarmona@hggm.es.
María Victoria Gómez-Gaviro, Email: vgomez@hggm.es.
References
- Abbud Filho M., Pavarino-Bertelli E.C., Alvarenga M.P.S., Fernandes I.M.M., Toledo R.A., Tajara E.H., Savoldi-Barbosa M., Goldmann G.H., Goloni-Bertollo E.M. Systemic lupus erythematosus and microchimerism in autoimmunity. Transplant. Proc. 2002;34:2951–2952. doi: 10.1016/s0041-1345(02)03501-7. [DOI] [PubMed] [Google Scholar]
- Adams K.M., Yan Z., M Stevens A., Nelson J.L. The changing maternal ‘Self’hypothesis: amechanism for maternal tolerance of the fetus. Placenta. 2007;28:378–382. doi: 10.1016/j.placenta.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Ando T., Imaizumi M., Graves P.N., Unger P., Davies T.F. Intrathyroidal fetal microchimerism in Graves’disease. J. Clin.Endocrinol.Metab. 2002;87:3315–3320. doi: 10.1210/jcem.87.7.8656. [DOI] [PubMed] [Google Scholar]
- Apari P., Rózsa L. The tripartite immune conflict in placentals and a hypothesis on Fetal-->maternal microchimerism. Med. Hypotheses. 2009;72:52–54. doi: 10.1016/j.mehy.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Aractingi S., Sibilia J., Meignin V., Launay D., Hachulla E., Le Danff C., Janin A., Mariette X. Presence of microchimerism in labial salivary glands in systemic sclerosis but not in Sjögren’s syndrome. Arthritis Rheum. 2002;46:1039–1043. doi: 10.1002/art.10137. [DOI] [PubMed] [Google Scholar]
- Ariga H., Ohto H., P Busch M., Imamura S., Watson R., Reed W., Lee T.H. Kinetics of fetal cellular and cell-free DNA in the maternal circulation duringand after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41:1524–1530. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- Artlett C.M., Smith J.B., Jimenez S.A. “Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. New Engl. J. Med. 1998;338:1186–1191. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- Artlett C.M., Cox L.A., Ramos R.C., Dennis T.N., Fortunato R.A., Hummers L.K., Jimenez S.A., B Smith J. Increasedmicrochimeric CD4+ T lymphocytes in peripheral blood from women with systemic sclerosis. Clin.Immunol. 2002;103:303–308. doi: 10.1006/clim.2002.5222. [DOI] [PubMed] [Google Scholar]
- Barba-Müller E., Craddock S., Carmona S., Hoekzema E. Brain plasticity in pregnancy and the postpartum period: links to maternalcaregiving and mental health. Arch. Women’s Ment. Health. 2019;22:289–299. doi: 10.1007/s00737-018-0889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes-Genis A., Bellosillo B., de la Calle O., Salido M., Roura S., Ristol F.S., Soler C., Martinez M., Espinet B., Serrano S., et al. Identification of male cardiomyocytes of extracardiac origin in the hearts of women with male progeny: male fetal cell microchimerism of the heart. J. Heart Lung Transplant. 2005;24:2179–2183. doi: 10.1016/j.healun.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Beksac M.S., Fadiloglu E., Cakar A.N., Gurbuz R.H., Atilla P., Onbasilar I., Beksac K., Katlan D.C., Mumusoglu S., Calis P., et al. Fetal cell microchimerism; normal and immunocompromised gestations in mice. Fetal Pediatr.Pathol. 2020;39:277–287. doi: 10.1080/15513815.2019.1651803. [DOI] [PubMed] [Google Scholar]
- Bhat M.A., Sharma J.B., Roy K.K., Sengupta J., Ghosh D. Genomic evidence of Y chromosome microchimerism in the endometrium during endometriosis and in cases of infertility. Reprod. Biol. Endocrinol. 2019;17:22. doi: 10.1186/s12958-019-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi D.W. Fetomaternal cell trafficking: anew cause of disease? Am. J. Med. Genet. 2000;91:22–28. doi: 10.1002/(sici)1096-8628(20000306)91:1<22::aid-ajmg4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bianchi D.W. Fetomaternal cell traffic, pregnancy-associated progenitor cells, and autoimmune disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2004;18:959–975. doi: 10.1016/j.bpobgyn.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Bianchi D.W., K Zickwolf G., J Weil G., Sylvester S., DeMaria M.A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc. Natl. Acad. Sci. USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi D.W., Farina A., Weber W., Delli-Bovi L.C., DeRiso M., Williams J.M., Klinger K.W. Significant fetal-maternal hemorrhage after termination of pregnancy: implications for development of fetal cell microchimerism. Am.J.Obstet.Gynecol. 2001;184:703–706. doi: 10.1067/mob.2001.111072. [DOI] [PubMed] [Google Scholar]
- Bloch E.M., Jackman R.P., Lee T.-H., Busch M.P. Transfusion-associated microchimerism: the hybrid within. Transfus. Med. Rev. 2013;27:10–20. doi: 10.1016/j.tmrv.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy A.M., Fortunato A., Sayres M.W., Aktipis A. Fetal microchimerism and maternal health: areview and evolutionary analysis of cooperation and conflict beyond the womb. BioEssays News Rev. Mol. Cell Dev. Biol. 2015;37:1106–1118. doi: 10.1002/bies.201500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyon C., Collinet P., Boulanger L., Vinatier D. Is fetal microchimerism beneficial for the fetus or the mother. Gynecol. Obstet. Fertil. 2011;39:224–231. doi: 10.1016/j.gyobfe.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Broestl L., Rubin J.B., Dahiya S. Fetal microchimerism in human brain tumors. Brain Pathol. 2018;28:484–494. doi: 10.1111/bpa.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burastero S.E., Galbiati S., Vassallo A., Sabbadini M.G., Bellone M., Marchionni L., Smid M., Ferrero E., Ferrari A., Ferrari M., et al. Cellular microchimerism as a lifelong physiologic status parous women: immunologic basis its amplification patients systemic sclerosis. Arthritis Rheum. 2003;48:1109–1116. doi: 10.1002/art.10888. [DOI] [PubMed] [Google Scholar]
- Bustos M.L., Frías S., Ramos S., Estrada A., L Arreola J., Mendoza F., Gaxiola M., Salcedo M., Pardo A., Selman M. Local and circulating microchimerism is associated with hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 2007;176:90–95. doi: 10.1164/rccm.200608-1129OC. [DOI] [PubMed] [Google Scholar]
- Carlucci F., Priori R., Alessandri C., Valesini G., Stoppacciaro A. Y chromosome microchimerism in Sjögren’s syndrome. Ann. Rheum. Dis. 2001;60:1078–1079. doi: 10.1136/ard.60.11.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci F., Priori R., Valesini G. Microchimerism in Sjögren’s syndrome. Rheumatology. 2003;61:1039–1040. doi: 10.1093/rheumatology/keg105. [DOI] [PubMed] [Google Scholar]
- Cha D., Khosrotehrani K., Kim Y., Stroh H., Bianchi D.W., Johnson K.L. Cervical cancer and microchimerism. Obstet. Gynecol. 2003;102:774–781. doi: 10.1016/s0029-7844(03)00615-x. [DOI] [PubMed] [Google Scholar]
- Chan W.F.N., Nelson J.L. Microchimerism in the human brain: more questions than answers. Chimerism. 2013;4:32–33. doi: 10.4161/chim.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W.F.N., Gurnot C., J Montine T., Sonnen J.A., Guthrie K.A., Nelson J.L. Male microchimerism in the human female brain. PLoS One. 2012;7:e45592. doi: 10.1371/journal.pone.0045592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christner P.J., Artlett C.M., Conway R.F., Jiménez S.A. “Increased numbers of microchimeric cells of fetal origin are associated with dermal fibrosis in mice following injection of vinyl chloride. Arthritis Rheum. 2000;43:2598–2605. doi: 10.1002/1529-0131(200011)43:11<2598::AID-ANR30>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Cirello V., Recalcati M.P., Muzza M., Rossi S., Perrino M., Vicentini L., Beck-Peccoz P., Finelli P., Fugazzola L. Fetal cell microchimerism in papillary thyroid cancer: apossible role in tumor damage and tissue repair. Cancer Res. 2008;68:8482–8488. doi: 10.1158/0008-5472.CAN-08-0672. [DOI] [PubMed] [Google Scholar]
- Cirello V., Perrino M., Colombo C., Muzza M., Filopanti M., Vicentini L., Beck Peccoz P., Fugazzola L. Fetal cell microchimerism in papillary thyroid cancer: studies in peripheral blood and tissues. Int J Cancer. 2010;126:2874–2878. doi: 10.1002/ijc.24993. [DOI] [PubMed] [Google Scholar]
- Corpechot C., Barbu V., Chazouillères O., Poupon R. Fetal microchimerism in primary biliary cirrhosis. J. Hepatol. 2000;33:696–700. doi: 10.1016/s0168-8278(00)80298-6. [DOI] [PubMed] [Google Scholar]
- Dawe G.S., Tan X.W., Xiao Z.-C. Cell migration from baby to mother. Cell Adhes.Migr. 2007;1:19–27. [PMC free article] [PubMed] [Google Scholar]
- Dubernard G., Aractingi S., Oster M., Rouzier R., Mathieu M.-C., Uzan S., Khosrotehrani K. Breast cancer stroma frequently recruits fetal derived cells during pregnancy. Breast Cancer Res. 2008;10:R14. doi: 10.1186/bcr1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Negishi I., Ishikawa O. Possible contribution of microchimerism to the pathogenesis of Sjögren’s syndrome. Rheumatology. 2002;41:490–495. doi: 10.1093/rheumatology/41.5.490. [DOI] [PubMed] [Google Scholar]
- Evans P.C., Lambert N., Maloney S., Furst D.E., M Moore J.M., Nelson J.L. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93:2033–2037. [PubMed] [Google Scholar]
- Fanning P.A., Jonsson J.R., Clouston A.D., Edwards-Smith C., Balderson G.A., Macdonald G.A., Crawford D.H., Kerlin P., Powell L.W., Powell E.E. Detection of male DNA in the liver of female patients with primary biliary cirrhosis. J. Hepatol. 2000;33:690–695. doi: 10.1016/s0168-8278(00)80297-4. [DOI] [PubMed] [Google Scholar]
- Fett J.D. Fetal and maternal microchimerism: aboost for mom and baby? Int. J. Cardiol. 2011;47:347–348. doi: 10.1016/j.ijcard.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Fugazzola L., Cirello V., Beck-Peccoz P. Fetal microchimerism as an explanation of disease. Nat. Rev. Endocrinol. 2011;7:89–97. doi: 10.1038/nrendo.2010.216. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Johnson K.L., Tighiouart H., Peter I., Bianchi D.W. “Fetomaternal trafficking in the mouse increases as delivery approaches and is highest in the maternal lung. Biol. Reprod. 2008;79:841–848. doi: 10.1095/biolreprod.108.068973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Johnson K.L., Peter I., Tighiouart H., Bianchi D.W. Fetal cells in the pregnant mouse are diverse and express a variety of progenitor and differentiated cell markers. Biol. Reprod. 2009;81:26–32. doi: 10.1095/biolreprod.108.074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadi V.K., Malone K.E., Guthrie K.A., Porter P.L., Nelson J.L. Case-control study of fetal microchimerism and breast cancer. PLoS One. 2008;3:e1706. doi: 10.1371/journal.pone.0001706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill H.S., Harrington W.E. Microchimerism: defining and redefining the prepregnancy context - areview. Placenta. 2017;60:130–133. doi: 10.1016/j.placenta.2017.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannagé M., Amoura Z., Lantz O., Piette J.-C., Caillat-Zucman S. Feto-maternal microchimerism in connective tissue diseases. Eur. J. Immunol. 2002;32:3405–3413. doi: 10.1002/1521-4141(200212)32:12<3405::AID-IMMU3405>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Giacomelli R., Matucci-Cerinic M., Bombardieri S. Microchimerism in Sjögren’s syndrome. Ann. Rheum. Dis. 2002;61:1039–1040. doi: 10.1136/ard.61.12.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Sant R., Holzgreve W. Fetal cells in maternal blood: current and future perspectives. Mol. Hum. Reprod. 1998;4:515–521. doi: 10.1093/molehr/4.6.515. [DOI] [PubMed] [Google Scholar]
- Hahn S., Hasler P., Vokalova L., Van Breda S.V., Than N.G., Hoesli I.M., Lapaire O., Rossi S.W. Feto-maternal microchimerism: the pre-eclampsia conundrum. Front.Immunol. 2019;10:659. doi: 10.3389/fimmu.2019.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. Does microchimerism mediate kin conflicts? Chimerism. 2014;5:53–55. doi: 10.4161/chim.29122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallum S., Jakobsen M.A., Gerds T.A., Pinborg A., Tjønneland A., Kamper-Jørgensen M. Male origin microchimerism and ovarian cancer. Int. J. Epidemiol. 2020;50:87–94. doi: 10.1093/ije/dyaa019. [DOI] [PubMed] [Google Scholar]
- Huerta Sil G., Medrano Ramírez G.M. Fetal microchimerism in rheumatic diseases. Reumatol.Clin. 2006;2:202–209. doi: 10.1016/S1699-258X(06)73046-7. [DOI] [PubMed] [Google Scholar]
- Hui L., Bianchi D.W. Noninvasive prenatal DNA testing: the vanguard of genomic medicine. Annu. Rev. Med. 2017;68:459–472. doi: 10.1146/annurev-med-072115-033220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi M., Pritsker A., Unger P., Davies T.F. Intrathyroidal fetal microchimerism in pregnancy and postpartum. Endocrinology. 2002;143:247–253. doi: 10.1210/endo.143.1.8563. [DOI] [PubMed] [Google Scholar]
- Jimenez D.F., Tarantal A.F. Quantitative analysis of male fetal DNA in maternal serum of gravid rhesus monkeys (Macaca mulatta) Pediatr. Res. 2003;53:18–23. doi: 10.1203/00006450-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Jimenez D.F., Tarantal A.F. Fetal gender determination in early first trimester pregnancies of rhesus monkeys (Macaca mulatta) by fluorescent PCR analysis of maternal serum. J. Med. Primatol. 2003;32:315–319. doi: 10.1046/j.1600-0684.2003.00041. [DOI] [PubMed] [Google Scholar]
- Jimenez D.F., Leapley A.C., Lee C.I., Ultsch M.-N., Tarantal A.F. Fetal CD34+ cells in the maternal circulation and long-term microchimerism in rhesus monkeys (Macaca mulatta) Transplantation. 2005;79:142–146. doi: 10.1097/01.tp.0000144468.71962.aa. [DOI] [PubMed] [Google Scholar]
- Johnson K.L., Nelson J.L., Furst D.E., McSweeney P.A., Roberts D.J., Zhen D.K., Bianchi D.W. Fetal cell microchimerism in tissue from multiple sites in women with systemic sclerosis. Arthritis Rheum. 2001;44:1848–1854. doi: 10.1002/1529-0131(200108)44:8<1848::AID-ART323>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Johnson K.L., Samura O., Nelson J.L., McDonnell W.M., Bianchi D.W. Significant fetal cell microchimerism in a nontransfused woman with hepatitis C: evidence of long-term survival and expansion. Hepatology. 2002;36:1295–1297. doi: 10.1053/jhep.2002.35622. [DOI] [PubMed] [Google Scholar]
- Jones D.E. Fetal microchimerism: an aetiological factor in primary biliary cirrhosis? J. Hepatol. 2000;33:834–837. doi: 10.1016/s0168-8278(00)80317-7. [DOI] [PubMed] [Google Scholar]
- Kajbafzadeh A.-M., Sabetkish S., Sabetkish N. The role of fetal-maternal microchimerism as a natural-born healer in integrity improvement of maternal damaged kidney. Int. Braz J Urol. 2018;44:608–616. doi: 10.1590/S1677-5538.IBJU.2017.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper-Jørgensen M., Biggar R.J., Tjønneland A., Hjalgrim H., Kroman N., Rostgaard K., Stamper C.L., Olsen A., Andersen A.-M.N., Gadi V.K. Opposite effects of microchimerism on breast and colon cancer. Eur. J. Cancer. 2012;48:2227–2235. doi: 10.1016/j.ejca.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Kanaan S.B., Sensoy O., Yan Z., Gadi V.K., Richardson M.L., Nelson J.L. Immunogenicity of a rheumatoid arthritis protective sequence when acquired through microchimerism. Proc. Natl. Acad. Sci. U S A. 2019;116:201904779. doi: 10.1073/pnas.1904779116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara R.J., Bolli P., Matsunaga I., Tanweer O., Altman P., Chaudhry H.W. A mouse model for fetal maternal stem cell transfer during ischemic cardiac injury. Clin. Transl. Sci. 2012;5:321–328. doi: 10.1111/j.1752-8062.2012.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosrotehrani K., Bianchi D.W. Multi-lineage potential of fetal cells in maternal tissue: alegacy in reverse. J. Cell Sci. 2005;118:1559–1563. doi: 10.1242/jcs.02332. [DOI] [PubMed] [Google Scholar]
- Khosrotehrani K., Johnson K.L., Cha D.H., Salomon R.N., Bianchi D.W. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- Khosrotehrani K., Johnson K.L., Guégan S., Stroh H., Bianchi D.W. Natural history of fetal cell microchimerism during and following murine pregnancy. J. Reprod.Immunol. 2005;66:1–12. doi: 10.1016/j.jri.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Khosrotehrani K., Leduc M., Bachy V., Huu S.N., Oster M., Abbas A., Uzan S., Aractingi S. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and Bcells mothers. J. Immunol. 2008;180:889–897. doi: 10.4049/jimmunol.180.2.889. [DOI] [PubMed] [Google Scholar]
- Klintschar M., Immel U.-D., Kehlen A., Schwaiger P., Mustafa T., Mannweiler S., Regauer S., Kleiber M., Hoang-Vu C. Fetal microchimerism in Hashimoto’s thyroiditis: aquantitative approach. Eur. J.Endocrinol. 2006;154:237–241. doi: 10.1530/eje.1.02080. [DOI] [PubMed] [Google Scholar]
- Klonisch T., Drouin R. Fetal-maternal exchange multipotent stem/progenitor cells microchimerism in diagnosis and disease. Trends Mol. Med. 2009;15:510–518. doi: 10.1016/j.molmed.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Kolialexi A., Tsangaris G.T., Antsaklis A., Mavroua A. Rapid clearance of fetal cells from maternal circulation after delivery. Ann.N. Y. Acad. Sci. 2004;1022:113–118. doi: 10.1196/annals.1318.018. [DOI] [PubMed] [Google Scholar]
- Koopmans M., Kremer Hovinga I.C.L., Baelde H.J., Fernandes R.J., de Heer E., Bruijn J.A., Bajema I.M. Chimerism in Kidneys, Livers and Hearts of Normal Women: Implications for Transplantation Studies. Am. J. Transplant. 2005;5:1495–1502. doi: 10.1111/j.1600-6143.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Koopmans M., Kremer Hovinga I.C.L., Baelde H.J., Harvey M.S., de Heer E., Bruijn J.A., Bajema I.M. Chimerism occurs inthyroid, lung skin lymph nodes women sons. J. Reprod.Immunol. 2008;78:68–75. doi: 10.1016/j.jri.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Korkmaz D.T., Demirhan O., Abat D., Demirberk B., Tunç E., Kuleci S. Microchimericcells, sex chromosome aneuploidies and cancer. Pathol.Oncol. Res. 2015;21:1157–1165. doi: 10.1007/s12253-015-9934-7. [DOI] [PubMed] [Google Scholar]
- Kremer Hovinga I.C.L., Koopmans M., Baelde H.J., van der Wal A.M., Sijpkens Y.W.J., de Heer E., Bruijn J.A., Bajema I.M. Chimerism occurs twice as often in lupus nephritis as in normal kidneys. Arthritis Rheum. 2006;54:2944–2950. doi: 10.1002/art.22038. [DOI] [PubMed] [Google Scholar]
- Kuroki M., Okayama A., Nakamura S., Sasaki T., Murai K., Shiba R., Shinohara M., Tsubouchi H. Detection of maternal-fetal microchimerism in the inflammatory lesions of patients with Sjögren’s syndrome. Ann. Rheum. Dis. 2002;61:1041–1046. doi: 10.1136/ard.61.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N.C. Microchimericcells: guardians or actors of immunity in scleroderma? Rheumatology. 2007;46:382–383. doi: 10.1093/rheumatology/kel370. [DOI] [PubMed] [Google Scholar]
- Lambert N., Nelson J.L. Microchimerism in autoimmune disease: more questions than answers? Autoimmun. Rev. 2003;2:133–139. doi: 10.1016/s1568-9972(02)00149-0. [DOI] [PubMed] [Google Scholar]
- Lambert N.C., Stevens A.M., Tylee T.S., Erickson T.D., Furst D.E., Nelson J.L. From the simple detection of microchimerism in patients with autoimmune diseases to its implication in pathogenesis. Ann.N. Y. Acad. Sci. 2001;945:164–171. doi: 10.1111/j.1749-6632.2001.tb03881.x. [DOI] [PubMed] [Google Scholar]
- Leduc M., Aractingi S., Khosrotehrani K. Fetal-cell microchimerism, lymphopoiesis, and autoimmunity. Arch. Immunol.Ther. Exp. 2009;57:325–329. doi: 10.1007/s00005-009-0044-7. [DOI] [PubMed] [Google Scholar]
- Lee E.S.M., Bou-Gharios G., Seppanen E., Khosrotehrani K., Fisk N.M. Fetal stem cell microchimerism: natural-born healers or killers? Mol. Hum.Reprod. 2010;16:869–878. doi: 10.1093/molehr/gaq067. [DOI] [PubMed] [Google Scholar]
- Liégeois A., Gaillard M.C., Ouvre E., Lewin D. Microchimerism in pregnant mice. Transplant. Proc. 1981;13:1250–1252. [PubMed] [Google Scholar]
- Lissauer D.M., Piper K.P., Moss P.A.H., Kilby M.D. Fetal microchimerism: the cellular and immunological legacy of pregnancy. Expert Rev. Mol. Med. 2009;11:e33. doi: 10.1017/S1462399409001264. [DOI] [PubMed] [Google Scholar]
- Lo Y.M., Corbetta N., Chamberlain P.F., Rai V., Sargent I.L., Redman C.W., Wainscoat J.S. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- Lo Y.M., Lau T.K., Chan L.Y., Leung T.N., Chang A.M. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin. Chem. 2000;46:1301–1309. [PubMed] [Google Scholar]
- Mahmood U., O’Donoghue K. Microchimericfetal cells play a role in maternal wound healing after pregnancy. Chimerism. 2014;5:40–52. doi: 10.4161/chim.28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y., Ono M., Ono M., Ueki H., Kurasawa K. Ychromosome microchimerism in rheumatic autoimmune disease. Ann. Rheum. Dis. 2000;59:654. doi: 10.1136/ard.59.8.654b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca M., Curcio M., Lapi S., Valentini G., D’Angelo S., Rizzo G., Bombardieri S. Correlations of Y chromosome microchimerism with disease activity in patients with SLE: analysis of preliminary data. Ann. Rheum. Dis. 2003;62:651–654. doi: 10.1136/ard.62.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A.C., Jakobsen M.A., Barington T., Arthur Vaag A., Grunnet L.G., Olsen S.F., Kamper-Jørgensen M. Microchimerism of male origin in a cohort of Danish girls. Chimerism. 2015;6:65–71. doi: 10.1080/19381956.2016.1218583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J.L. Maternal-fetal immunology and autoimmune disease: is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 1996;39:191–194. doi: 10.1002/art.1780390203. [DOI] [PubMed] [Google Scholar]
- Nelson J.L. Microchimerism and scleroderma. Curr.Rheumatol. Rep. 1999;1:15–21. doi: 10.1007/s11926-999-0019-z. [DOI] [PubMed] [Google Scholar]
- Nelson J.L. Microchimerism and human autoimmune diseases. Lupus. 2002;11:651–654. doi: 10.1191/0961203302lu271oa. [DOI] [PubMed] [Google Scholar]
- Nelson J.L. Microchimerism: incidental byproduct of pregnancy or active participant in human health? Trends Mol. Med. 2002;8:109–113. doi: 10.1016/s1471-4914(01)02269-9. [DOI] [PubMed] [Google Scholar]
- Nelson J.L., Furst D.E., Maloney S., Gooley T., Evans P.C., Smith A., Bean M.A., Ober C., Bianchi D.W. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- Nemescu D., G Ursu R., Nemescu E.R., Negura L. Heterogeneous distribution of fetal microchimerism in local breast cancer environment. PLoS One. 2016;11:e0147675. doi: 10.1371/journal.pone.0147675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Huu S., Dubernard G., Aractingi S., Khosrotehrani K. Feto-maternal cell trafficking: atransfer of pregnancy associated progenitor cells. Stem Cell Rev. 2006;2:111–116. doi: 10.1007/s12015-006-0017-8. [DOI] [PubMed] [Google Scholar]
- Nguyen Huu S., Khosrotehrani K., Oster M., Moguelet P., Espié M.-J., Aractingi S. Early phase of maternal skin carcinogenesis recruits long-term engrafted fetal cells. Int. J. Cancer. 2008;123:2512–2517. doi: 10.1002/ijc.23819. [DOI] [PubMed] [Google Scholar]
- Nguyen Huu S., Oster M., Avril M.-F., Boitier F., Mortier L., Richard M.-A., Kerob D., Maubec E., Souteyrand P., Moguelet P., et al. Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy. Am. J. Pathol. 2009;174:630–637. doi: 10.2353/ajpath.2009.080566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue K., Sultan H.A., Al-Allaf F.A., Anderson J.R., Wyatt-Ashmead J., Fisk N.M. Microchimericfetal cells cluster at sites of tissue injury in lung decades after pregnancy. Reprod.Biomed. 2008;16:382–390. doi: 10.1016/s1472-6483(10)60600-1. [DOI] [PubMed] [Google Scholar]
- Pellicer Martínez A., Bonilla Musoles F.M. 1st ed. Editorial Médica Panamericana S.A; 2007. Obstetricia, Reproducción Y Ginecología Básicas. [Google Scholar]
- Price J.O., Elias S., Wachtel S.S., Klinger K., Dockter M., Tharapel A., P Shulman L., P Phillips O., Meyers C.M., Shook D. Prenatal diagnosis with fetal cells isolated from maternal blood by multiparameter flow cytometry. Am. J. Obstet. Gynecol. 1991;165:1731–1737. doi: 10.1016/0002-9378(91)90024-l. [DOI] [PubMed] [Google Scholar]
- Pritchard S., Peter I., L Johnson K., W Bianchi D. The natural history of fetal cells in postpartum murine maternal lung and bone marrow: atwo-stage phenomenon. Chimerism. 2012;3:59–64. doi: 10.4161/chim.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renné C., Lopez E.R., Steimle-Grauer S.A., Ziolkowski P., Pani M.A., Luther C., Holzer K., Encke A., Wahl R.A., Bechstein W.O., et al. Thyroid fetal male microchimerisms in mothers with thyroid disorders: presence of Y-chromosomal immunofluorescence in thyroid-infiltrating lymphocytes is more prevalent in Hashimoto’s thyroiditis and graves’ disease than in follicular adenomas. J. Clin.Endocrinol.Metab. 2004;89:5810–5814. doi: 10.1210/jc.2004-1049. [DOI] [PubMed] [Google Scholar]
- Rijnink E.C., Penning M.E., Wolterbeek R., Wilhelmus S., Zandbergen M., van Duinen S.G., Schutte J., Bruijn J.A., Bajema I.M. Tissue microchimerism is increased during pregnancy: a human autopsy study. Mol. Hum.Reprod. 2015;21:857–864. doi: 10.1093/molehr/gav047. [DOI] [PubMed] [Google Scholar]
- Rubbia-Brandt L., Philippeaux M.M., Chavez S., Mentha G., Borisch B., Hadengue A. FISH for Y chromosome in women with primary biliary cirrhosis: lack of evidence for leukocyte microchimerism. Hepatology. 1999;30:821–822. doi: 10.1002/hep.510300322. [DOI] [PubMed] [Google Scholar]
- Santos M.A., O'Donoghue K., Wyatt-Ashmead J., M Fisk N. Fetal cells in the maternal appendix: a marker of inflammation or fetal tissue repair? Hum.Reprod. 2008;23:2319–2325. doi: 10.1093/humrep/den261. [DOI] [PubMed] [Google Scholar]
- Scaletti C., Vultaggio A., Bonifacio S., Emmi L., Torricelli F., Maggi E., Romagnani S., Piccinni M.-P. Th2-oriented profile of male offspring T cells present in women with systemic sclerosisand reactive with matern major histocompatibility complex antigens. Arthritis Rheum. 2002;46:445–450. doi: 10.1002/art.10049. [DOI] [PubMed] [Google Scholar]
- Shree R., Harrington W.E., Kanaan S.B., Forsyth A., Cousin E., Lopez A., Nelson J.L., Gammill H.S. Fetal microchimerism by mode of delivery: a prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2019;126:24–31. doi: 10.1111/1471-0528.15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsa B., Srivatsa S., Johnson K.L., Samura O., Lee S.L., Bianchi D.W. “Microchimerism of presumed fetal origin in thyroid specimens from women: acase-control study. Lancet. 2001;358:2034–2038. doi: 10.1016/S0140-6736(01)07099-4. [DOI] [PubMed] [Google Scholar]
- Stelzer I.A., Thiele K., Solano M.E. Maternal microchimerism: lessons learned from murine models. J. Reprod.Immunol. 2015;108:12–25. doi: 10.1016/j.jri.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Stevens A.M. Microchimeric cells in systemic lupus erythematosus: targets or innocent bystanders? Lupus. 2006;15:820–826. doi: 10.1177/0961203306070068. [DOI] [PubMed] [Google Scholar]
- Stevens A.M., Michael McDonnell W., Mullarkey M.E., Pang J.M., Leisenring W., Nelson J.L. Liver biopsies from human females contain male hepatocytes in the absence of transplantation. Lab. Invest.J. Tech. Methods Pathol. 2004;84:1603–1609. doi: 10.1038/labinvest.3700193. [DOI] [PubMed] [Google Scholar]
- Tan X.-W., Liao H., Sun L., Okabe M., Xiao Z.-C., Dawe G.S. Fetal microchimerism in the maternal mouse brain: anovel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells. 2005;23:1443–1452. doi: 10.1634/stemcells.2004-0169. [DOI] [PubMed] [Google Scholar]
- Tan K.H., X Zeng X., Sasajala P., Yeo A., Udolph G. Fetomaternal microchimerism: some answers and many new questions. Chimerism. 2011;2:16–18. doi: 10.4161/chim.2.1.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Lindor K., Gish R., Batts K., Shiratori Y., Omata M., Nelson J.L., Ansari A., Coppel R., Newsome M., et al. Fetal microchimerism alone does not contribute to the induction of primary biliary cirrhosis. Hepatology. 1999;30:833–838. doi: 10.1002/hep.510300410. [DOI] [PubMed] [Google Scholar]
- Vernochet C., M Caucheteux S., Kanellopoulos-Langevin C. Bi-directional cell trafficking between mother and fetus in mouse placenta. Placenta. 2007;28:639–649. doi: 10.1016/j.placenta.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Vojdani Z., Bagheri J., Talaei-Khozani T., Azarpira N., Salmannjad M., Farrokhi A. Fetal microchimerism in mouse caerulein-induced pancreatitis model. Iran. J. Basic Med. Sci. 2018;21:889–895. doi: 10.22038/IJBMS.2018.26976.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Iwatani H., Ito T., Horimoto N., Yamato M., Matsui I., Imai E., Hori M. Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochem.Biophys. Res. Commun. 2004;325:961–967. doi: 10.1016/j.bbrc.2004.10.105. [DOI] [PubMed] [Google Scholar]
- Yan Z., Lambert N.C., Ostensen M., Adams K.M., Guthrie K.A., Nelson J.L. Prospective study of fetal DNA in serum and disease activity during pregnancy in women with inflammatory arthritis. Arthritis Rheum. 2006;54:2069–2073. doi: 10.1002/art.21966. [DOI] [PubMed] [Google Scholar]
- Ye Y., Van Zyl B., Hellmich C., Gillespie K.M. Microchimerism: covert genetics? Int. J. Mol. Epidemiol.Genet. 2010;1:350–357. [PMC free article] [PubMed] [Google Scholar]
- Yutaka F., Johnson K.L., Peter I., Tighiouart H., Bianchi D.W. Fetal cells in the pregnant mouse are diverse and express a variety of progenitor and differentiated cell markers. Biol. Reprod. 2009;81:26–32. doi: 10.1095/biolreprod.108.074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.X., Tan K.H., Yeo A., Sasajala P., Tan X., C Xiao Z., Dawe G., Udolph G. Pregnancy-Associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem CellsDev. 2010;19:1819–1830. doi: 10.1089/scd.2010.0046. [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhao Y., Li X.-M., Kong J. “Fetal cell microchimerism in the maternal mouse spinal cord. Neurosci. Bull. 2014;30:81–89. doi: 10.1007/s12264-013-1392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]