Abstract
The purpose of this study was to elucidate the functions of estrogen and two estrogen receptors (ERs; ERα and ERβ) in the myoregeneration process and morphogenesis. Cardiotoxin (CTX) was injected into the tibialis anterior (TA) muscles of ovariectomized (OVX) mice to induce muscle injury, and subsequent myoregeneration was morphologically assessed. The diameter of regenerated myotubes in OVX mice was significantly smaller than that in intact mice at all time points of measurement. OVX mice also showed lower muscle recovery rates and slower speeds than did intact mice. ER protein levels showed a predominance of ERβ over ERα in both intact and OVX states. The ERβ level was increased significantly at 7 days after CTX injection in OVX mice and remained at a high level until 14 days. In addition, continuous administration of E2 to OVX mice in which muscle injury was induced resulted in a significantly larger diameter of regenerated myotubes than that in mice that did not receive estrogen. The results indicate that estrogen is an essential factor in the myoregeneration process since estrogen depletion delayed myoregeneration in injured muscles and administration of estrogen under the condition of a low estrogen status rescued delayed myoregeneration. The results strongly suggested that ERβ may be a factor that promotes myoregeneration more than does ERα.
Keywords: cardiotoxin injection, estrogen, myoregeneration, ovariectomized mice
Estrogen, which is mainly secreted by the ovaries, has been shown to play an important role in the maintenance of locomotive function [2, 6, 18, 23] as well as the regulation of reproductive organ function [36]. It has long been thought that estrogen is deeply involved in the regulation of locomotive organs (such as bones and skeletal muscles) [15, 30, 32]. It is well known that estrogen suppresses osteoblast-mediated osteoclast activation and functions as a factor in the maintenance of bone mass [22]. As well as bone loss, estrogen deficiency also induces qualitative and quantitative loss of muscle mass, a condition known as sarcopenia or frailty.
Estrogen function is exerted through two receptors, estrogen receptor (ER) α and ERβ [1, 8, 9, 13, 19], and it has been shown that both types of ER are distributed in mouse skeletal muscle [33, 34]. In a previous study, we found that hypoplasia of myofibers was induced in hypoestrogenic ER KO mice. Furthermore, injury to the muscle tissue of ER KO mice significantly delayed the regeneration of myotubes in ERβ KO mice compared to that in ERα KO, suggesting functional differences in ERα and ERβ [4]. A deficiency of estrogen has been suggested to interfere with the maintenance of satellite cells (skeletal muscle stem cells) and delay skeletal muscle repair after skeletal muscle injury [7]. Additionally, muscle injury-induced activation of satellite cells is less effective at a low level of estrogen [10]. It has been proposed that estrogen administration is important for maintaining musculoskeletal function in menopause women [12, 37, 43]. Thus, estrogen administration is expected to improve muscle function and its activity [14, 24]. The distribution of ERs in muscle tissue is thought to indicate that estrogen exerts some function in the tissue but the function of estrogen in each process of proliferation, differentiation, and regeneration of skeletal muscle cells remains unclear.
The purpose of this study was to clarify the roles of estrogen and ERs in the process of myoregeneration. For that purpose, we morphologically examined the process of myoregeneration in low estrogen status (ovariectomized: OVX) mice and investigated changes in ER protein levels after OVX and muscle injury induced by injection of cardiotoxin (CTX). Incidentally, CTX, an amphiphilic peptide derived from cobra venom, specifically inhibits protein kinase C and induces an increase in intracellular calcium ion concentration. Excessive influx of calcium ions into myofibers induces mitochondrial and cytoskeletal degeneration and production of reactive oxygen species, leading to myofiber degeneration and necrosis. [27, 38, 41, 46]. Furthermore, by regularly administering estrogen to OVX mice injected with CTX, we morphologically analyzed the improvement and promotion levels of muscle repair and attempted to evaluate the function of estrogen in the myoegeneration process.
MATERIALS AND METHODS
Animals
The animal care and experimental design were approved by the Animal Research Committee, Tottori University, Japan (approval number 18-T-37). Female C57BL/6JJcl mice were obtained from CLEA Japan (Tokyo, Japan) at 8 weeks of age for experimental myoregeneration in a low estrogen status. Additionally, female mice (C57BL/6) for experimental myoregeneration after estrogen administration were obtained by self-mating. All of the mice were maintained under a temperature-controlled (23 ± 1.0°C) room condition with a 12-hr light/12-hr dark cycle. The mice were freely fed food (CLEA Rodent Diet CE-2, CLEA Japan) and water.
Creation of low estrogen status mice and confirmation of low estrogen level
Low estrogen status mice were created by OVX. Medetomidine (Kyoritsu Seiyaku, Tokyo, Japan), midazolam (Maruishi, Osaka, Japan) and butorphanol (Meiji Seika, Tokyo, Japan) [20] were intraperitoneally administered to female mice (8 weeks old). To confirm the low estrogen level of OVX mice, intact female mice (controls, 8 weeks old) and the mice at 4 weeks after OVX treatment (12 weeks old) were anesthetized and blood was collected by tail laceration. The blood was immediately centrifuged for 10 min and serum was collected. The collected serum was frozen and stored at −30°C until use. Serum estradiol (E2) levels were measured by an enzyme-linked immunosorbent assay (Cayman Chemical, Ann Arbor, MI, USA). The experiment was conducted after confirming that serum E2 concentration in OVX mice was significantly lower than that in intact mice.
Experimental myoregeneration in low estrogen status
Four weeks after OVX treatment, 50 μl of 10 μM CTX (Latoxan, Portes lès Valence, France) was injected into the right tibialis anterior (TA) muscles of mice (C57BL/6JJcl) using a syringe with an injection needle (29G 1/2 insulin syringe, Nippon Becton Dickinson, Fukushima, Japan) (OVX/CTX group). We also set up an Intact/CTX group in which mice were not treated with OVX and were injected with CTX. In each group, TA muscles were sampled on 3, 7, 10 and 14 days after CTX injection (D3, D7, D10 and D14) (n=4 at each time point). The left TA muscle on D3 was analyzed as a non-injured (control) group. All mice were sacrificed by cervical dislocation under anesthesia with isoflurane (MSD Animal Health, Osaka, Japan). The collected TA muscles were divided into two parts: 1) the proximal part was used for ER protein analysis (Western blotting) and was kept at −80°C until use and 2) the distal part was fixed with 10% neutral buffer formalin (NBF; Wako, Osaka, Japan) and used for histological analysis.
Experimental myoregeneration after estrogen administration
Four weeks after OVX (12 weeks of age), mice (C57BL/6) were injected CTX into right TA muscle and randomly divided into two groups. One group of mice received regular E2 (Wako) dissolved in dimethyl sulfoxide (DMSO; Wako) as a vehicle and the other group of mice received only DMSO. In the former group, E2 was continuously administered intraperitoneally every 4 days until the 28th day (D28) after CTX injection. The concentration of E2 per administration was 0.8 μg/body weight of 100 g, and it was dissolved in 50 μl DMSO [35]. In the latter group, only the same amount of DMSO was administered each time. TA muscles on both legs (left and right) were collected at D28. The right TA muscles injected with CTX were designated as OVX/CTX/E2 and OVX/CTX based on the respective treatments. The left TA muscles that were not injected with CTX were similarly designated as OVX/E2 and OVX. These four types of TA muscles with different treatments were fixed with 10% NBF and then analyzed histologically.
Evaluation of myoregeneration
The 10% NBF-fixed TA muscles were embedded in paraffin. Sections were then stained with hematoxylin and eosin (HE) for histological analysis. For evaluation of myoregeneration, the minor axis diameters (smallest diameters) of myotubes were measured using image analysis software (ImageJ; v1.46r, National Institutes of Health, Bethesda, MD, USA). The diameter of the minor axis of myotubes per animal was measured at a magnification of 200 times [26].
Evaluation of muscle recovery
Evaluation of the recovery ratio (%) of muscle injury was based on the method of Chaiyasing et al. [4]. That is, the average diameter of newly formed myotubes with a central nucleus in both the Intact/CTX and OVX/CTX groups was divided by the average diameter of myofibers in the non-injured group (control). The recovery ratio (%) of OVX mice for comparison with intact mice was calculated by the average diameter of myofibers or newly formed myotubes with a central nucleus divided by the average diameter in intact mice at each time point. The repair speeds (µm/day) in the first period (D7 to D10), second period (D10 to D14) and total period (D7 to D14) of CTX-injected mice were determined by the method described previously [4].
Western blotting
After TA muscle protein extraction by using a protein extraction kit (Cosmobio, Tokyo, Japan), protein concentration was determined by the bicinchoninic acid method (Thermo Fisher Scientific, Rockford, IL, USA). Proteins were electrophoresed on polyacrylamide gels (Life Technologies, Carlsbad, CA, USA) and transferred to a nitrocellulose membrane (Life Technologies). After blocking in 5% w/v non-fat dry milk for 1 hr, the membrane was incubated for 1 hr at room temperature (RT) with rabbit monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2,000; Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal anti-ERα antibody (1:1,000, Sigma, St. Louis, MO, USA), and mouse monoclonal anti-ERβ antibody (1:400, Novus Biological, Centennial, CO, USA) as the primary antibody. The membrane was washed in Tris-buffered saline with 0.1% Tween 20 (TBS-T) and incubated for 1 hr at RT with goat anti-rabbit IgG-peroxidase (1:20,000 for GAPDH, 1:5,000 for ERα, Sera Care Life Sciences, Milford, MA, USA) or goat anti-mouse IgG-peroxidase (1:1,000 for ERβ, R&D Systems, Minneapolis, MN, USA) as the secondary antibody. After washing five times in TBS-T and incubation in immunodetection reagent (Bio-Rad, Hercules, CA, USA), protein levels were visualized using a Western blotting image membrane scanner (LI-COR Biosciences, Lincoln, NE, USA) and densitometric values were determined with Image Studio software (ver 4.0; LI-COR Biosciences). Protein levels of ERs were calculated on the basis of GAPDH.
Statistical analysis
The E2 concentration in serum was analyzed by an unpaired Student’s t-test. Histometric and ER protein intensity data were analyzed by one-way ANOVA with Bonferroni’s post-hoc test. All data are expressed as average ± standard deviation. Analyses were performed with Excel Statistics 2016 for Windows (ver. 3.21; SSRI, Tokyo, Japan). Statistical significance was defined as P <0.05.
RESULTS
Serum estrogen level in OVX mice
Serum E2 level at 4 weeks after OVX treatment was significantly lower than that in the intact group, confirming that the OVX mice used in the myoregeneration experiment were in a low estrogen status (Fig. 1).
Fig. 1.
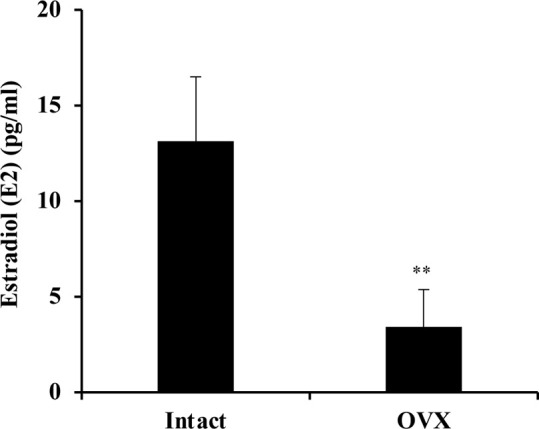
Estradiol (E2) concentrations in serum of ovariectomized (OVX) mice and intact mice as controls. n=6 in the OVX group and n=3 in the Intact group, Data are expressed as average ± standard deviation (SD), ** indicates significant difference between the two group, P<0.01, unpaired Student’s t-test.
Myoregeneration in a low estrogen status
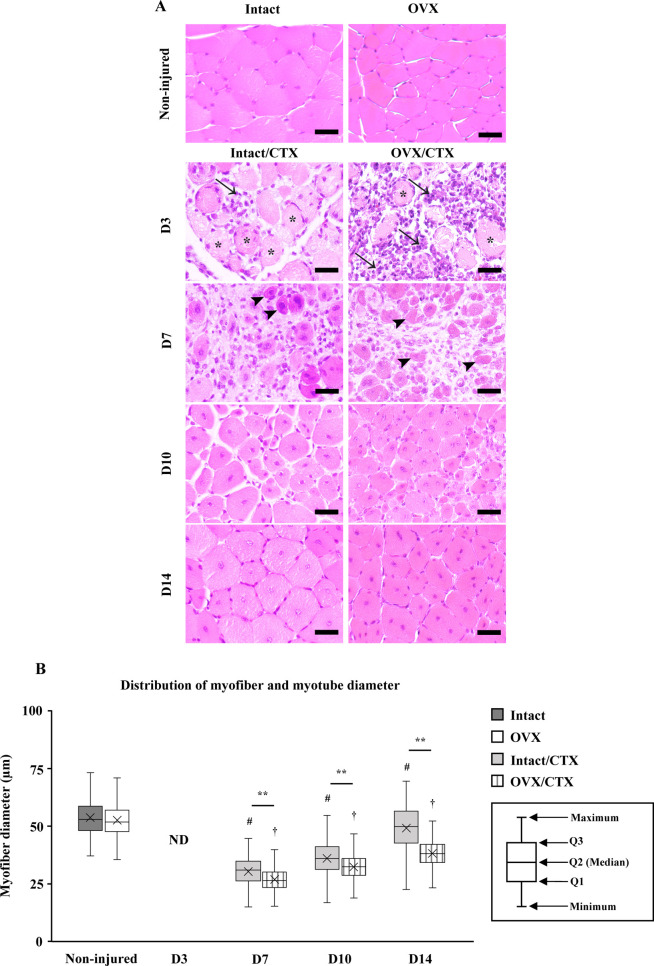
On D3 after CTX injection, a large amount of cell infiltration into the muscle tissue was observed. Muscle degeneration was more severe in the OVX/CTX group than in the Intact/CTX group. On D7, new eosin-stained myofibers with a central nucleus appeared in both groups. In addition, cells with less cytoplasm, which are clearly different from myotubes, were distributed in the inter-myotube space. These newly formed myotubes had a nearly circular cross section in mice in the Intact/CTX group, but many myotubes in mice in the OVX/CTX group showed a distorted shape rather than a circular shape. On D10, myotubes of various sizes with central nuclei were observed in both the Intact/CTX and OVX/CTX groups. On D14, the myotubes had begun to form a polygonal structure with multiple nuclei distributed around the myotubes, resembling non-injured muscles, but they still had a central nucleus. On D10 and D14, myotubes with large diameters were observed more frequently in the Intact/CTX group than in the OVX/CTX group (Fig. 2A).
Fig. 2.
(A) Morphological changes of tibialis anterior (TA) muscles in the Intact/Cardiotoxin (CTX) group compared with those in the ovariectomized (OVX)/CTX group at days 3, 7, 10 and 14 (D3, D7, D10 and D14) after CTX injection. Arrows indicate cell infiltration. Asterisks indicate myofiber degeneration. Arrowheads indicate newly formed myotubes, scale bar: 50 µm. (B) Comparison of the diameters of regenerated myotubes at different time points after CTX injection between the Intact/CTX group and OVX/CTX group. The boxes represent the distribution of diameters between first quartile (Q1) and third quartile (Q3). The horizontal line between Q1 and Q3 represents the median (Q2) of regenerated myotubes in each group. Outliers are not shown in the graph. ×indicates average diameter of regenerated myotubes in each group, ** indicates significant difference between groups, # indicates significant difference from intact groups (non-injured), † indicates significant difference from OVX groups (non-injured), P<0.01. ND, not detected.
A comparison of the diameters of myofibers in intact and OVX mice in non-injured controls showed that the average value in OVX mice was slightly lower than that in intact mice, but there was no significant difference between the two groups. On D3, the diameter of myofibers could not be measured due to collapse of the myofibers. The OVX/CTX group had smaller myotube diameters than those in the Intact/CTX group at all time points, D7, D10 and D14. Throughout the 14-day experimental period after CTX injection, the diameter of regenerated myotubes increased in both groups. However, the diameter in the OVX/CTX group was always significantly smaller than that in the Intact/CTX group throughout the experimental period. The myotube diameters at D14 were 49.1 ± 2.72 µm in the Intact/CTX group and 38.1 ± 0.39 µm in the OVX/CTX group (Fig. 2B).
These diameters at D14 were 91.4 ± 5.06% and 72.7 ± 0.74%, respectively, of the diameter of myotubes in the non-injured control mice. In both groups, the ratio (%) of the average diameter of myotubes to non-injured controls increased with the passage of days. However, the ratio in the OVX/CTX group was significantly lower than that in the Intact/CTX group (Table 1). The ratios (%) of the average diameters of myofibers and myotubes in OVX mice to those in intact mice at each time point are shown in Table 2. OVX/CTX mice showed significantly lower ratios than those in non-injured mice at all time points. In addition, the ratios on D14 in OVX/CTX mice were significantly lower than the ratios on D7 and D10. Table 3 shows the repair speed of regenerated myotubes during a period of 7 days (first half period: D7 to D10, second half period: D10 to D14) after CTX injection. In the first half period, there was no significant difference between the two groups, but the OVX/CTX group showed a significantly lower speed than that in the Intact/CTX group in the second half period and throughout the whole period (D7 to D14). In the second half period, the Intact/CTX group had the highest repair speed, while the OVX/CTX group had the lowest, which was about 2.26-times lower than that in the Intact/CTX group.
Table 1. Ratio (%) of the average diameter of myotubes to non-injured controls.
| Treatment | Time post injury | ||
|---|---|---|---|
| D7 | D10 | D14 | |
| Intact/CTX | 56.3 ± 2.98 | 67.0 ± 3.01 | 91.4 ± 5.06 |
| OVX/CTX | 51.2 ± 2.56* | 61.6 ± 3.91* | 72.7 ± 0.74** |
Data are shown as average ratio ± standard deviation. * and ** indicate significant difference from Intact/CTX mice, P<0.05 and P<0.01, respectively. D, day; CTX, cardiotoxin; OVX, ovariectomized.
Table 2. Ratio (%) of the average diameter of myofibers and myotubes in ovariectomized mice to those in intact mice.
| OVX (non-injured) | OVX/CTX | ||
|---|---|---|---|
| D7 | D10 | D14 | |
| 97.6 ± 2.59 | 88.8 ± 4.44** | 89.7 ± 5.69* | 77.6 ± 0.79**, †, ‡ |
Data are shown as average ratio ± standard deviation. * and ** indicate significant difference from non-injured, P<0.05 and P<0.01, respectively. † indicates significant difference from D7, P<0.01. ‡ indicates significant difference from D10, P<0.01. OVX, ovariectomized; CTX, cardiotoxin; D, day.
Table 3. Repair speed (µm/day) of regenerated myotubes at several duration periods of days after cardiotoxin injury.
| Treatment | Duration period | ||
|---|---|---|---|
| D7 to D10 | D10 to D14 | D7 to D14 | |
| Intact/CTX | 1.91 ± 0.54 | 3.28 ± 0.68 | 2.69 ± 0.39 |
| OVX/CTX | 1.83 ± 0.68 | 1.45 ± 0.10** | 1.61 ± 0.06** |
Data are shown as repair speed ± standard deviation. ** indicates significant difference from Intact/CTX mice, P<0.01. D, day; CTX, cardiotoxin; OVX, ovariectomized.
Evaluation of ER proteins in a low estrogen status
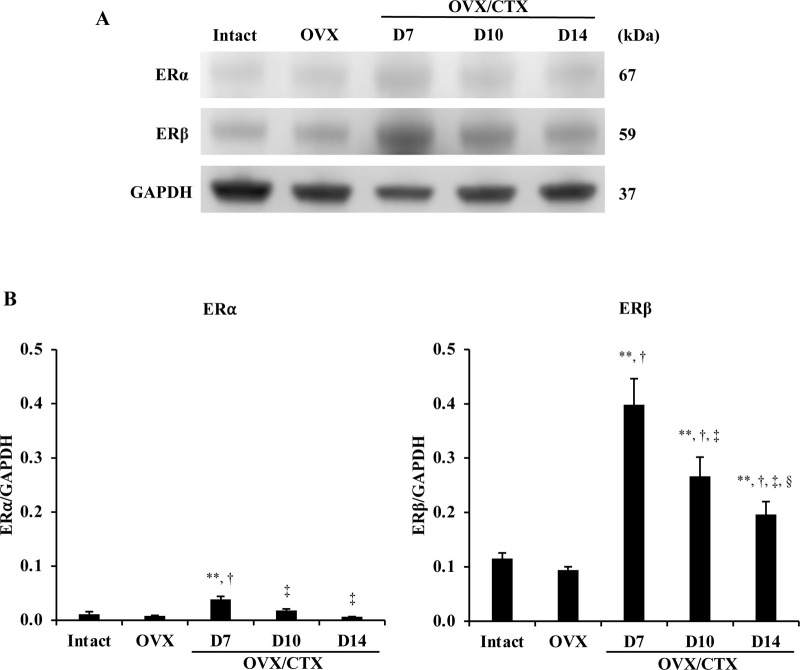
Changes in ER (ERα and ERβ) proteins after injection of CTX into OVX mice (OVX/CTX) were evaluated by Western blotting. The protein levels (band intensity ratios) of the two ERs that are reportedly distributed in skeletal muscle showed a predominance of ERβ over ERα in all samples. However, when the amounts of each receptor protein were compared in intact and OVX mice, there was no significant difference between the two groups. On D7, the samples of OVX/CTX mice showed a sharp increase in values in both ERs compared to those in intact and OVX mice. After that, the values of both ERs decreased over time, but the values of ERβ remained significantly higher at D14 than those in intact and OVX mice (Fig. 3). The intensity of the GAPDH band at D7 in OVX/CTX mice was lower than that of the other samples. In this experiment, the proteins extracted from each sample were adjusted to the same concentration (15 μg/15 μl/lane) and electrophoresis was repeated, and the same results were obtained in each case.
Fig. 3.
(A) Western blot analysis of estrogen receptor (ER) α and ERβ in the tibialis anterior (TA) muscles of intact mice, ovariectomized (OVX) mice and OVX mice at 7, 10 and 14 days (D7, D10 and D14) after cardiotoxin (CTX) injection (OVX/CTX). (B) Bar graph shows the band intensity ratios in ERα and ERβ. Data are expressed as average ± standard deviation, n=3/group, ** indicates significant differences from intact mice, † indicates significant differences from OVX mice, ‡ indicates significant differences from D7, P<0.01, § indicates significant differences from D10, P<0.05.
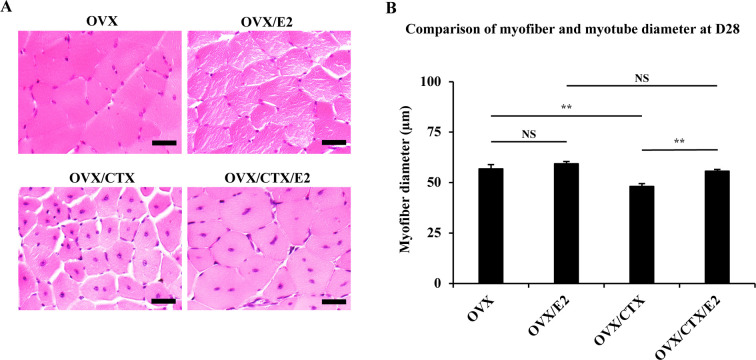
Myoregeneration after estrogen administration
Myofibers in the OVX and OVX/E2 groups, unaffected by CTX-induced muscle injury, maintained a polygonal cross-section with peripheral nuclei. On the other hand, in the OVX/CTX and OVX/CTX/E2 groups, myotubes with a central nucleus were still observed at D28 after CTX injection. In addition, most of the myotubes in the OVX/CTX group had a clearly smaller diameter than those in the other three groups (Fig. 4A). The average diameters of myofibers in the OVX and OVX/E2 groups were 56.8 ± 2.13 μm and 59.3 ± 1.09 μm, respectively, and there was no significant difference between the two groups. On the other hand, the OVX/CTX group, which had muscle injury under the condition of a low estrogen status, had a significantly low value of 48.1 ± 1.40 µm, but the diameter of myotubes in the OVX/CTX/E2 group, which was continuously administered E2, was 55.7 ± 0.85 µm. This value was not significantly different from OVX/E2 group (Fig. 4B).
Fig. 4.
(A) Morphological changes of tibialis anterior (TA) muscles in the ovariectomized (OVX), OVX/estradiol (E2), OVX/cardiotoxin (CTX) and OVX/CTX/E2 groups at day 28 (D28) after CTX injection, scale bars: 50 µm. (B) Comparison of the diameters of myofibers and regenerated myotubes in the OVX, OVX/E2, OVX/CTX and OVX/CTX/E2 groups. Data are expressed as average ± standard deviation, ** indicates significant difference between groups, P<0.01. NS, not significant.
DISCUSSION
The diameters of regenerated myotubes in OVX mice after CTX injection (OVX/CTX) were significantly smaller than those in intact mice after CTX injection (Intact/CTX) at all time points (D7, D10, and D14). McHale et al. [31] also reported that cross-sectional area (CSA) of regenerated myotubes in OVX mice at 14 days after CTX injection was smaller than that in intact mice. Most of the satellite cells, which play an important role in the myoregeneration process, remain in a quiescent status in healthy or uninjured muscle tissue. However, the muscle injury caused by mechanical loading or chemical injection activates satellite cells from a quiescent state to a state of proliferation, leading to the myoregeneration process [5, 28, 39, 42, 45]. In this study, the ratio and speed of muscle repair after CTX injection differed depending on the estrogen concentration. The myoregeneration ratio was generally low in OVX mice compared to that in intact mice (Table 1). In addition, the ratio of myotube diameter in OVX/CTX mice to that in Intact/CTX mice was lowest at D14 (Table 2). The repair speed of regenerated myotubes in OVX/CTX mice was significantly slower than that in Intact/CTX mice throughout the period of D7-D14, and the decrease in speed was remarkable in the second half period (D10 to D14). Several studies have shown that estrogen is deeply involved in apoptosis avoidance and cell proliferation via ERs [11, 17, 21, 40, 44]. Therefore, estrogen deficiency is directly linked to the loss of satellite cells [7]. The significant difference in the second half period may be due to an insufficient number of satellite cells required to maintain a smooth myoregeneration process. The results strongly suggest that muscle injury in a low estrogen status induces satellite cell loss, followed by delayed differentiation into myoblasts, resulting in delayed myoregeneration.
Protein levels of the two ERs (ERα and ERβ) in TA muscles of mice showed a predominance of ERβ over ERα in both intact and OVX mouse samples. The expression of both ERα and ERβ has been reported in skeletal muscle in many animal species [19, 33, 34], but ERβ is thought to be the major isoform in mice [34]. In this experiment, injection of CTX into OVX mice (OVX/CTX) significantly increased ERβ production at D7, followed by a gradual decrease while maintaining high levels compared to those in OVX mice. Serum E2 levels increase in the early stages of trauma in adult human patients. This phenomenon suggests that estrogen may play an important role in the protection of traumatic organs [3, 16]. Continuous measurement of serum E2 levels after CTX injection into TA muscles of healthy (non-OVX-injection) mice showed a significant increase in E2 levels 1–10 days (peaking on the 7th day) after CTX injection and a sharp decline after the 15th day [25]. Unfortunately, serum E2 levels in OVX mice after CTX injection were not measured in our experiments. However, it is reasonable to assume that mice lacking ovaries, the main source of E2 in the body, have low E2 levels. We thought that the reason for the increase in ERβ level in OVX/CTX mice at D7 to D14 may be the cellular response to low E2 levels in the body. It is considered that the expression of ERβ in injured muscle tissue was upregulated to maintain the signal input from E2, which has a strong impact on myoregeneration. However, when considering this result, attention should also be paid to the OVX/CTX band intensity at D7 of the Western blot shown in Fig. 3. In the OVX/CTX tissue image of D7 in Fig. 2A, newly generated myotubes are scattered and necrotic cell/tissue fragments remain in the space between the myotubes. Also, some infiltrating cells are observed. Therefore, abundant cell/tissue fragments within regenerated muscle tissue, secreted proteins from infiltrating cells, and the extracellular matrix may have affected the intensity of the GAPDH band. It will be necessary to consider the effects of these proteins on the ER band. Further experimentation is needed to clarify the details of these remaining questions.
In this study, we investigated the effect of a low E2 status on muscle recovery. In a previous study in which E2 was administered to OVX rats with hindlimb suspension-induced disuse atrophy of skeletal muscle, the soleus muscle CSA recovered as in intact rats [29]. This suggests that estrogen is an essential factor for rescue of muscle recovery in a low E2 status. Being consistent with the results of that study, we found that the diameter of regenerated myotubes in CTX-injured mice continuously treated with estrogen (OVX/CTX/E2 in Fig. 4) was significantly larger than that in OVX/CTX mice. We also showed that the diameter of myofibers in OVX/E2 mice was not affected by estrogen administration. It can be said that estrogen is a factor that acts when satellite cells are in a proliferated or differentiated state after muscle damage.
In conclusion, our study provided evidence that estrogen is an essential factor for the maintenance of satellite cell proliferation and differentiation in the smooth progression of myoregeneration. Further elucidation of the mechanism of the myoregeneration process will enable the establishment of new strategies for maintaining female muscle function by targeting the estrogen-ERβ pathway.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Acknowledgments
This work was supported by JSPS Grant-in-Aid for Scientific Research (B) Grant Number JP17H03934.
REFERENCES
- 1.Baltgalvis K. A., Greising S. M., Warren G. L., Lowe D. A.2010. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One 5: e10164. doi: 10.1371/journal.pone.0010164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown M.2008. Skeletal muscle and bone: effect of sex steroids and aging. Adv. Physiol. Educ. 32: 120–126. doi: 10.1152/advan.90111.2008 [DOI] [PubMed] [Google Scholar]
- 3.Brown C. M., Suzuki S., Jelks K. A., Wise P. M.2009. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin. Reprod. Med. 27: 240–249. doi: 10.1055/s-0029-1216277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaiyasing R., Ishikawa T., Warita K., Hosaka Y. Z.2021. Absence of estrogen receptors delays myoregeneration and leads to intermuscular adipogenesis in a low estrogen status: Morphological comparisons in estrogen receptor alpha and beta knock out mice. J. Vet. Med. Sci. 83: 1022–1030. doi: 10.1292/jvms.20-0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chargé S. B., Rudnicki M. A.2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84: 209–238. doi: 10.1152/physrev.00019.2003 [DOI] [PubMed] [Google Scholar]
- 6.Chidi-Ogbolu N., Baar K.2019. Effect of estrogen on musculoskeletal performance and injury risk. Front. Physiol. 9: 1834. doi: 10.3389/fphys.2018.01834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins B. C., Arpke R. W., Larson A. A., Baumann C. W., Xie N., Cabelka C. A., Nash N. L., Juppi H. K., Laakkonen E. K., Sipilä S., Kovanen V., Spangenburg E. E., Kyba M., Lowe D. A.2019. Estrogen regulates the satellite cell compartment in females. Cell Rep. 28: 368–381.e6. doi: 10.1016/j.celrep.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diel P.2014. The role of the estrogen receptor in skeletal muscle mass homeostasis and regeneration. Acta Physiol. (Oxf.) 212: 14–16. doi: 10.1111/apha.12341 [DOI] [PubMed] [Google Scholar]
- 9.Ekenros L., Papoutsi Z., Fridén C., Dahlman Wright K., Lindén Hirschberg A.2017. Expression of sex steroid hormone receptors in human skeletal muscle during the menstrual cycle. Acta Physiol. (Oxf.) 219: 486–493. doi: 10.1111/apha.12757 [DOI] [PubMed] [Google Scholar]
- 10.Enns D. L., Tiidus P. M.2008. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol (1985) 104: 347–353. doi: 10.1152/japplphysiol.00128.2007 [DOI] [PubMed] [Google Scholar]
- 11.Galluzzo P., Rastelli C., Bulzomi P., Acconcia F., Pallottini V., Marino M.2009. 17β-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-α-mediated signals. Am. J. Physiol. Cell Physiol. 297: C1249–C1262. doi: 10.1152/ajpcell.00188.2009 [DOI] [PubMed] [Google Scholar]
- 12.Geraci A., Calvani R., Ferri E., Marzetti E., Arosio B., Cesari M.2021. Sarcopenia and menopause: the role of estradiol. Front. Endocrinol. (Lausanne) 12: 682012. doi: 10.3389/fendo.2021.682012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorres B. K., Bomhoff G. L., Gupte A. A., Geiger P. C.2011. Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J Appl Physiol (1985) 110: 1046–1053. doi: 10.1152/japplphysiol.00541.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greising S. M., Baltgalvis K. A., Lowe D. A., Warren G. L.2009. Hormone therapy and skeletal muscle strength: a meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 64: 1071–1081. doi: 10.1093/gerona/glp082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greising S. M., Baltgalvis K. A., Kosir A. M., Moran A. L., Warren G. L., Lowe D. A.2011. Estradiol’s beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol (1985) 110: 109–115. doi: 10.1152/japplphysiol.00852.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groswasser Z.2001. Gender and traumatic brain injury. J. Neurosurg. 94: 862–864. [DOI] [PubMed] [Google Scholar]
- 17.Haines M., McKinley-Barnard S. K., Andre T. L., Gann J. J., Hwang P. S., Willoughby D. S.2018. Skeletal muscle estrogen receptor activation in response to eccentric exercise up-regulates myogenic-related gene expression independent of differing serum estradiol levels occurring during the human menstrual cycle. J. Sports Sci. Med. 17: 31–39. [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda K., Horie-Inoue K., Inoue S.2019. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J. Steroid Biochem. Mol. Biol. 191: 105375. doi: 10.1016/j.jsbmb.2019.105375 [DOI] [PubMed] [Google Scholar]
- 19.Kalbe C., Mau M., Wollenhaupt K., Rehfeldt C.2007. Evidence for estrogen receptor α and β expression in skeletal muscle of pigs. Histochem. Cell Biol. 127: 95–107. doi: 10.1007/s00418-006-0224-z [DOI] [PubMed] [Google Scholar]
- 20.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 21.Kitajima Y., Ono Y.2016. Estrogens maintain skeletal muscle and satellite cell functions. J. Endocrinol. 229: 267–275. doi: 10.1530/JOE-15-0476 [DOI] [PubMed] [Google Scholar]
- 22.Koike T., Mikami T., Shida M., Habuchi O., Kitagawa H.2015. Chondroitin sulfate-E mediates estrogen-induced osteoanabolism. Sci. Rep. 5: 8994. doi: 10.1038/srep08994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosir A. M., Mader T. L., Greising A. G., Novotny S. A., Baltgalvis K. A., Lowe D. A.2015. Influence of ovarian hormones on strength loss in healthy and dystrophic female mice. Med. Sci. Sports Exerc. 47: 1177–1187. doi: 10.1249/MSS.0000000000000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le G., Novotny S. A., Mader T. L., Greising S. M., Chan S. S. K., Kyba M., Lowe D. A., Warren G. L.2018. A moderate oestradiol level enhances neutrophil number and activity in muscle after traumatic injury but strength recovery is accelerated. J. Physiol. 596: 4665–4680. doi: 10.1113/JP276432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Z. H., Huang T., Xiao J. W., Gu R. C., Ouyang J., Wu G., Liao H.2019. Estrogen signaling effects on muscle-specific immune responses through controlling the recruitment and function of macrophages and T cells. Skelet. Muscle 9: 20. doi: 10.1186/s13395-019-0205-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahdy M. A., Lei H. Y., Wakamatsu J., Hosaka Y. Z., Nishimura T.2015. Comparative study of muscle regeneration following cardiotoxin and glycerol injury. Ann. Anat. 202: 18–27. doi: 10.1016/j.aanat.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 27.Mahdy M. A., Warita K., Hosaka Y. Z.2016. Early ultrastructural events of skeletal muscle damage following cardiotoxin-induced injury and glycerol-induced injury. Micron 91: 29–40. doi: 10.1016/j.micron.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 28.Mann C. J., Perdiguero E., Kharraz Y., Aguilar S., Pessina P., Serrano A. L., Muñoz-Cánoves P.2011. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 1: 21. doi: 10.1186/2044-5040-1-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClung J. M., Davis J. M., Wilson M. A., Goldsmith E. C., Carson J. A.2006. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol (1985) 100: 2012–2023. doi: 10.1152/japplphysiol.01583.2005 [DOI] [PubMed] [Google Scholar]
- 30.McCormick K. M., Burns K. L., Piccone C. M., Gosselin L. E., Brazeau G. A.2004. Effects of ovariectomy and estrogen on skeletal muscle function in growing rats. J. Muscle Res. Cell Motil. 25: 21–27. doi: 10.1023/B:JURE.0000021398.78327.39 [DOI] [PubMed] [Google Scholar]
- 31.McHale M. J., Sarwar Z. U., Cardenas D. P., Porter L., Salinas A. S., Michalek J. E., McManus L. M., Shireman P. K.2012. Increased fat deposition in injured skeletal muscle is regulated by sex-specific hormones. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302: R331–R339. doi: 10.1152/ajpregu.00427.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messier V., Rabasa-Lhoret R., Barbat-Artigas S., Elisha B., Karelis A. D., Aubertin-Leheudre M.2011. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 68: 331–336. doi: 10.1016/j.maturitas.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 33.Milanesi L., Russo de Boland A., Boland R.2008. Expression and localization of estrogen receptor alpha in the C2C12 murine skeletal muscle cell line. J. Cell. Biochem. 104: 1254–1273. doi: 10.1002/jcb.21706 [DOI] [PubMed] [Google Scholar]
- 34.Milanesi L., Vasconsuelo A., de Boland A. R., Boland R.2009. Expression and subcellular distribution of native estrogen receptor beta in murine C2C12 cells and skeletal muscle tissue. Steroids 74: 489–497. doi: 10.1016/j.steroids.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 35.Nagai S., Ikeda K., Horie-Inoue K., Shiba S., Nagasawa S., Takeda S., Inoue S.2016. Estrogen modulates exercise endurance along with mitochondrial uncoupling protein 3 downregulation in skeletal muscle of female mice. Biochem. Biophys. Res. Commun. 480: 758–764. doi: 10.1016/j.bbrc.2016.10.129 [DOI] [PubMed] [Google Scholar]
- 36.Nelson L. R., Bulun S. E.2001. Estrogen production and action. J. Am. Acad. Dermatol. 45 Suppl: S116–S124. doi: 10.1067/mjd.2001.117432 [DOI] [PubMed] [Google Scholar]
- 37.Pöllänen E., Sipilä S., Alen M., Ronkainen P. H., Ankarberg-Lindgren C., Puolakka J., Suominen H., Hämäläinen E., Turpeinen U., Konttinen Y. T., Kovanen V.2011. Differential influence of peripheral and systemic sex steroids on skeletal muscle quality in pre- and postmenopausal women. Aging Cell 10: 650–660. doi: 10.1111/j.1474-9726.2011.00701.x [DOI] [PubMed] [Google Scholar]
- 38.Raynor R. L., Zheng B., Kuo J. F.1991. Membrane interactions of amphiphilic polypeptides mastoparan, melittin, polymyxin B, and cardiotoxin. Differential inhibition of protein kinase C, Ca2+/calmodulin-dependent protein kinase II and synaptosomal membrane Na,K-ATPase, and Na+ pump and differentiation of HL60 cells. J. Biol. Chem. 266: 2753–2758. doi: 10.1016/S0021-9258(18)49909-7 [DOI] [PubMed] [Google Scholar]
- 39.Schmidt M., Schüler S. C., Hüttner S. S., von Eyss B., von Maltzahn J.2019. Adult stem cells at work: regenerating skeletal muscle. Cell. Mol. Life Sci. 76: 2559–2570. doi: 10.1007/s00018-019-03093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seko D., Fujita R., Kitajima Y., Nakamura K., Imai Y., Ono Y.2020. Estrogen receptor β controls muscle growth and regeneration in young female mice. Stem Cell Reports 15: 577–586. doi: 10.1016/j.stemcr.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suh B. C., Song S. K., Kim Y. K., Kim K. T.1996. Induction of cytosolic Ca2+ elevation mediated by Mas-7 occurs through membrane pore formation. J. Biol. Chem. 271: 32753–32759. doi: 10.1074/jbc.271.51.32753 [DOI] [PubMed] [Google Scholar]
- 42.Tidball J. G.2017. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 17: 165–178. doi: 10.1038/nri.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiidus P. M., Lowe D. A., Brown M.2013. Estrogen replacement and skeletal muscle: mechanisms and population health. J Appl Physiol (1985) 115: 569–578. doi: 10.1152/japplphysiol.00629.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velders M., Schleipen B., Fritzemeier K. H., Zierau O., Diel P.2012. Selective estrogen receptor-β activation stimulates skeletal muscle growth and regeneration. FASEB J. 26: 1909–1920. doi: 10.1096/fj.11-194779 [DOI] [PubMed] [Google Scholar]
- 45.Wada K., Katsuta S., Soya H.2008. Formation process and fate of the nuclear chain after injury in regenerated myofiber. Anat. Rec. (Hoboken) 291: 122–128. doi: 10.1002/ar.20626 [DOI] [PubMed] [Google Scholar]
- 46.Wang H. X., Lau S. Y., Huang S. J., Kwan C. Y., Wong T. M.1997. Cobra venom cardiotoxin induces perturbations of cytosolic calcium homeostasis and hypercontracture in adult rat ventricular myocytes. J. Mol. Cell. Cardiol. 29: 2759–2770. doi: 10.1006/jmcc.1997.0511 [DOI] [PubMed] [Google Scholar]