Abstract
Mycoplasma gallisepticum (MG) is a worldwide ruined bacteria affecting different avian species, causing severe economic losses. Consequently, the current research sought to detect the incidence of MG among different commercial broiler, layer chickens and turkey farms, and environmental litter samples in different Egyptian governorates (Damietta, Giza, El-Qalyobia, El-Sharqia, and El-Behera) from January 2019 to December 2020. Four hundred samples (infraorbital sinus aspirates, tracheal swabs, serum from diseased birds, and organ samples; lung tissues, air sacs and tracheal bifurcation from freshly dead birds), and environmental samples (litter) were collected for MG isolation. Samples were subjected to phenotypic and molecular identification. Positive bacteriological samples were subjected for molecular identification using polymerase chain reaction (PCR) test to detect MG, then sequencing for PCR amplicon of mgc2 gene. Out of 332 samples subjected for bacteriological examination, 206 were bacteriologically positive for MG with an incidence of 62%. The highest incidence of MG was detected in turkey farms at a rate of 83%, followed by broiler chicken farms, layer chicken farms and litter samples at a percentage of 70, 40, and 40, respectively. The highest prevalence of MG in chickens and turkey was recorded during the winter and autumn seasons. Molecular identification of MG isolates revealed that 85% of isolates were positive for mgc2 gene using PCR. The Four sequenced strains in this study are closely related and placed in one group with the vaccine strain 6/85 and ts11 strain.
Key words: chickens, chronic respiratory disease, infectious sinusitis, Mycoplasma gallisepticum, Mgc2 gene
INTRODUCTION
The Egyptian poultry industry is a lucrative investment opportunity since the domestic market for poultry meat consumption is large and has significant growth potential due to population increase and per capita consumption growth, driven by the country's improving economy (Shatokhin et al., 2017; Abdelnour et al., 2020a,b; Sheiha et al., 2020). Mycoplasma is a major pathogen that threatens the poultry industry and causes severe economic losses worldwide (Eissa et al., 2014; Marouf et al., 2020; Yadav et al., 2021).
Mycoplasmas are small prokaryotes that lack a cell wall, but they are surrounded by 3 layers of the plasma membrane, 300 to 800 nm in diameter. It can be detected in humans and animals, with an optimum growth temperature of 37°C with the ability to replicate outside of host cells (Brown et al., 2007; Abdel Halium et al., 2019; Abo Elyazeed et al., 2020; Sawicka et al., 2020). Mycoplasma gallisepticum is the most virulent and economically significant bacterial respiratory infection of poultry. The Office International des Epizootics (OIE) has declared the infection with MG as a notifiable disease (OIE, 2004). MG is a serious problem in the poultry field worldwide, causing chronic respiratory disease (CRD) in the broiler, layer, breeder flocks, and infectious sinusitis in turkeys. It also harms egg production (Eid et al., 2019; Lysnyansky et al., 2005). In addition, MG infection also exaggerates viral infections and secondary bacterial complications and adversely affects the efficacy of avian viral vaccines (Fathy et al., 2017). Diseased chickens with CRD show signs of respiratory symptoms like sneezing, rales, coughing, conjunctivitis, lacrimation, exudation from the nostrils, increased carcass condemnation and embryonic mortality, and reduced egg production, hatchability, feed conversion rate, and weight gain (Marouf et al., 2020).
Environmental samples constitute a continuous source for MG infection in poultry houses; strict environmental hygienic measures should be adopted to overcome this problem (Marois et al., 2002). Diagnosis of avian mycoplasmosis based on isolation, identification, antibodies detection, and polymerase chain reaction (PCR). Culturing pathogenic avian mycoplasmas organisms is difficult, slow-growing, relatively fastidious, and might require up to 3 wk for detectable growth. Therefore, the rapid serum plate agglutination test (SPA) and hemagglutination inhibition test (HI) tests have been used routinely. Because the SPA test is rapid, relatively less costly, and sensitive, it has been widely used as a primitive screening test for serological monitoring of poultry flocks (Kleven et al., 1998). Problems of low sensitivity, cross-reactions, and nonspecific reactions were encountered with rapid SPA and HI tests (Ewing et al., 1996; Hemeg et al., 2020). Molecular biology techniques such as PCR and Random amplified polymorphic DNA (RAPD) or the Arbitrarily PCR (AP-PCR) were applied for detection and identification of Mycoplasmas (Fan et al., 1995). This study aimed to repertoire the mycoplasmal picture of chicken and turkey flocks in many Egyptian governorates. The study shows MG at the phenotypic and genotypic level and environmental shedding, facilitating the vaccine used decision in farms.
MATERIALS AND METHODS
Animal Ethics
All bird samples and related procedures were ethically approved by the Animal Ethics Committee of the Faculty of Veterinary Medicine, Cairo University, Egypt. (Vet.CU-IACUC) with approval number (Vet.CU-10102019093).
Samples Collection
Investigated broiler and layer farms suffered from respiratory manifestations and drop in egg production in layer farms while turkey farms suffered from infectious sinusitis. All investigated farms were reared birds in a deep litter system.
Four hundred samples of diseased birds and freshly dead (broiler and layer chickens suffered from CRD and complicated chronic respiratory disease [CCRD] as well as diseased turkey suffered from swelling in infraorbital sinus in addition to litter samples) were collected at different ages and different seasons from Damietta, Giza, El-Qalyobia, El-Sharqia and El-Behera Governorates between January 2019 and December 2020. The number and type of collected samples are summarized in Table 1.
Table 1.
Sample's repertoire (type of samples, bird status, type, and number of examined of specimens).
| Samples | Examined birds and environmental samples | Bird status | Type of specimens | No. of examined specimens | |
|---|---|---|---|---|---|
| Broiler (100) | 50 | Diseased chicken | Blood (serum) | 50 | |
| Tracheal swab | 50 | ||||
| 50 | Dead chicken | Organs | Lung | 50 | |
| Tracheal bifurcation | 50 | ||||
| Air sacs | 50 | ||||
| Layer (36) | 18 | Diseased chicken | Blood (serum) | 18 | |
| Tracheal swab | 18 | ||||
| 18 | Dead chicken |
Organs | Lung | 18 | |
| Tracheal bifurcation | 18 | ||||
| Air sacs | 18 | ||||
| Turkey(30) | 30 | Diseased | Infraorbital sinus aspirates | 30 | |
| Environmental samples (30) | 10 | Broiler farms | Litter | 10 | |
| 10 | Layer farms | Litter | 10 | ||
| 10 | Turkey | Litter | 10 | ||
| Total | 400 | ||||
Microbiological Studies
The samples were subjected to bacterial isolation and identification by samples inoculated into Frey's broth and incubated at 37°C with 5 to 10% Co2 and humidity for 48 h following the standard procedures of Sabry and Ahmed (1975). Then, a loopful from each incubated sample, were streaked on pleuropneumonia-like organisms (PPLO) agar by drop technique and then incubated at 37°C with 5 to 10% Co2 with humidity for 10 to 14 d and examined under dissecting microscope for characteristic fried egg appearance of Mycoplasma colonies. Bio-typing of the purified colonies based on digitonin sensitivity test to distinguish between Mycoplasma and Acholeplasma isolates were adopted according to Edward and Freundt (1973). Glucose fermentation test was performed according to Ernq and Stipkovitls (1972), arginine hydrolysis, film and spot formation tests were performed. The growth inhibition test was formed to identify mycoplasmas isolate depending on serology. The serum samples were analyzed by serum agglutination plate based on Sabry and Ahmed (1975).
To develop a pure culture, one fried egg-shaped colony was chosen and inserted into a broth medium, along with the agar block. Purified isolates were kept at −20°C in the form of agar blocks.
Molecular Identification
DNA from isolated colonies was purified using QI Aquick PCR Product extraction kit. (Qiagen Inc. Valencia, CA). Oligonucleotide primer encoding mgc2 gene (Mgc2 F: 3’CGC AAT TTG GTC CTA ATC CCC AAC A’5; Mgc2 R: 5’ TAA ACC CAC CTC CAG CTT TAT TTC C’3) of 300 bp was specific for MG (Lysnyansky et al., 2005). The reaction mixture (total volume of 50 µL) was 5 µL of 10X PCR reaction buffer (Promega), 5 µL 1 m MdNTP mix (Sigma), 4 µL of 25 Mm Mgcl2, 5 µL DNA Taq polymerase, 1 µL containing 5 Mm of each primer, 5 µL of sample DNA. Then 0.25 mL Go-Taq polymerase (Promega) was added and the mixture was completed by sterile distilled water. The PCR protocol was repeated 40 cycles as listed by Ferguson et al. 2005; (Denaturation 94°C for 30 s, Annealing 55°C for 30 s, Extension 72°C for 1 min and Final extension 72°C for 5 min). Aliquots of amplified products (10 µL) were electrophoresed through 1% agarose gel and DNA was visualized by Ultraviolet transilluminator after ethidium bromide staining then photographed (Sambrook et al., 1989) using a digital camera (Acer CR-5130, Nanjing, China).
Sequence Analysis
On an applied biosystems 3130 automated DNA Sequencer, a purified RT-PCR product was sequenced in both forward and reverse directions (ABI, 3130). Using a PerkinElmer/Applied Biosystems, Foster City, CA, Bigdye Terminator V3.1 cycle sequencing kit (Cat. No. 4336817).
To determine sequence identity to Gen Bank accessions, a BLAST analysis (Basic Local Alignment Search Tool) was used first (Altschul et al., 1990). The sequence reaction was carried out according to the manufacturer's instructions.
Phylogenetic Analysis
The CLUSTAL W multiple sequence alignment program, version 1.83 of Meg Align module of Laser gene DNA Star software Pairwise, designed by Thompson et al. (1994), was used to compare sequences. To determine nucleotide and amino acid sequence similarities and linkages, the Meg Align module of the Laser gene DNA Star program was used to perform sequence alignments and phylogenetic analyses of the aligned sequences for the gene.
RESULTS
Clinical, Postmortem Investigations and Phenotypic Analysis
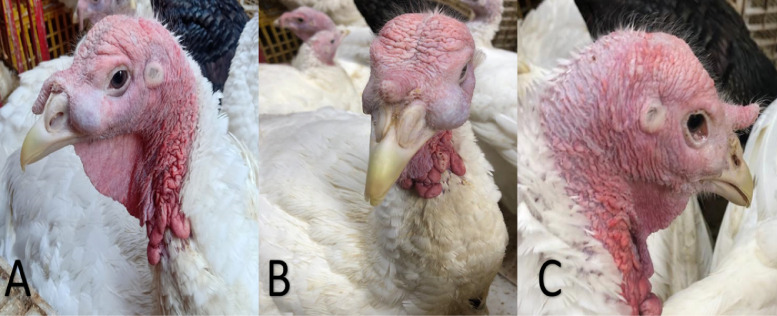
Investigated chicken farms suffered from respiratory manifestations such as conjunctivitis, cough, sneezing, gasping, rales, nasal discharge, ruffled feather, sleepy appearance, inappetence with general depression, and drop in egg production with the presence of eggshell abnormalities in layer flocks. Examined turkey farms suffered from infectious sinusitis with unilateral or bilateral swelling in infraorbital sinuses, conjunctivitis as seen in Figure 1 with nasal discharge, ruffled feathers, and general depression.
Figure 1.
(A, B) Adult turkey showing unilateral swelling in infraorbital sinus, (C) adult turkey showing severe conjunctivitis.
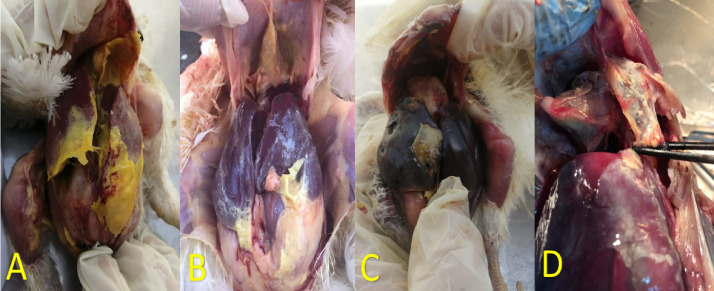
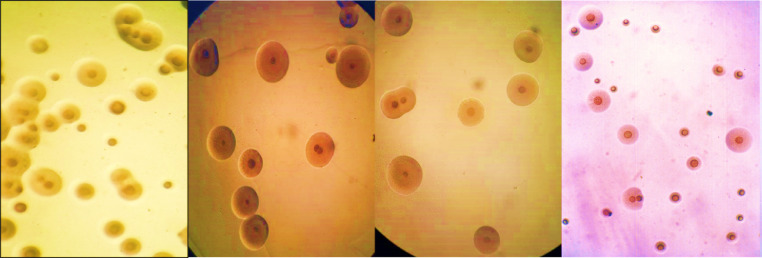
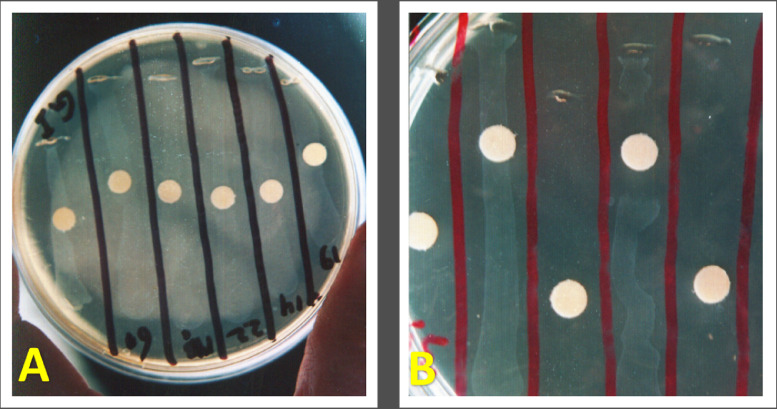
The postmortem examination of freshly dead birds revealed the presence of variable degree (foamy to caseous) air saculitis, pericarditis, perihepatitis, general serositis as seen in Figure 2. Out of 332, only 206 samples appeared fried egg appearance with depressed center colonies with cultivation ratio of (62%) as seen in Figure 3. Bio-typing profile reveals a ratio of 95% (196/206) were digitonin sensitive as seen in Figure 4, glucose positive, arginine negative and form Film and spot.
Figure 2.
(A, B) Postmortem of freshly dead broiler chickens showing caseous pericarditis, perihepatitis and air saculitis. (C) Postmortem of freshly dead layer chicken showing air saculitis, pericarditis and perihepatitis (CCRD).
Figure 3.
Microscopical appearance of M. gallisepticum with characteristic fried egg appearance with depressed center colonies.
Figure 4.
(A) Growth inhibition test against M. gallisepticum antiserum. (B) Digitonin sensitivity test.
The incidence of MG infection between bacteriologically examined samples was 62%. The highest incidence was detected in turkey farms 83%, followed by chicken broiler farms 70%, then chicken layer arms 40%, and 40% from litter samples, as summarized in Table 2.
Table 2.
Incidence of Mycoplasma gallisepticum (MG) infection.
| Type of sample | No. of bacteriologically examined samples | No. of MG positive samples | Incidence of positive samples |
|---|---|---|---|
| Broiler | 200 | 140 | 70% |
| Layer | 72 | 29 | 40% |
| Turkey | 30 | 25 | 83% |
| Litter | 30 | 12 | 40% |
| Total | 332 | 206 | 62% |
Serological Analysis
At the examination, all serum samples were agglutinated at a ratio of 100% using a slide agglutination plate (SAP). The suspected colonies were serologically investigated using a growth inhibition test against specific MG antisera, which showed a ratio 95% (196/206), as seen in Figure 4.
Molecular Analysis
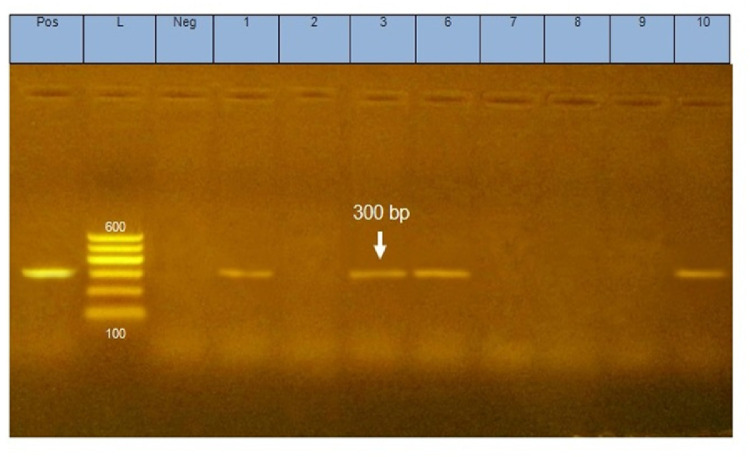
PCR characterized 206 suspected colonies by using MG primer (mgc2 gene). One hundred and seventy-five colonies had amplified fragments at 300 bp with a ratio of 85%, as shown in Figure 5.
Figure 5.
Agarose gel electrophoresis showing amplification fragments of 300 bp of MG (mgc2 gene). Lanes 1,3,6,10 are field isolates of M. gallisepticum; positive control (M. gallisepticum S6) and negative control (Escherichia coli). L shows a 100–600 bp ladder.
DNA Sequencing Result
Sequencing of mgc2 gene was conducted in both directions and a consensus sequence of 300 bp was used for nucleotides and deduced amino acid analysis. The original sequence was trimmed to remove indefinite nucleotides. Sequences usually exist at the beginning of the sequencing reaction. Four mgc2 sequences were submitted to GenBank database where obtained the accession numbers; MG496039 (2032138 seq 1), MG356828 (2032742 seq1), MG356829 (2058726 seq1), and MG356830 (2058734 seq1). Identification of homologies between nucleotide and amino acid sequences of the Egyptian MG strains and other strains published on GenBank was made using BLAST 2.0 and PSI-BLAST search programs (National Center for Biotechnology Information “NCBI” http://www.ncbi.nlm.nih.gov/), respectively. The obtained nucleotide sequences and the deduced amino acid of Egyptian MG strains and other strains published on GenBank were made using the BioEdit sequence alignment editor, ClustalW software for multiple sequence alignment, Clusta lV and Meg Align, DNA STAR, Laser gene, Version 7.1.0. The phylogenetic trees were constructed using MegAlign for tree reconstruction of sequences by the Neighbor-joining method based on ClustalW. MegAlign calculated sequence divergence and identity percent. The histological character of the mgc2 protein sequence was identified by Protean (DNASTAR, Lasergene, Version 7.1.0, Germany) by measuring the antigenicity index.
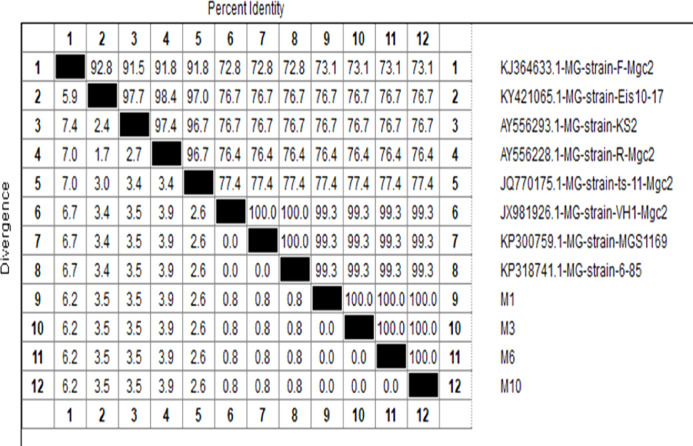
The four samples showed 73.1% maximum identity to the MG - strain - F - Mgc2 strain (Accession no. KJ364633), 76.7% maximum identity to the MG – strain – Eis 10 – 17 (Accession no. KY421065) and MG – strain - KS2 (Accession no. AY556293), 76.4% maximum identity to the MG – strain – R – Mgc2 (Accession no. AY556228), 77.4 % maximum identity to the MG – strain – ts - 11 – Mgc2 (Accession no. JQ770175) and 99.3% maximum identity to the MG – strain – VH1 – Mgc2 (Accession no. JX981926), MG – strain – MGS 1169(Accession no. KP300759) and MG – strain – 6 - 85(Accession no. KP318741).
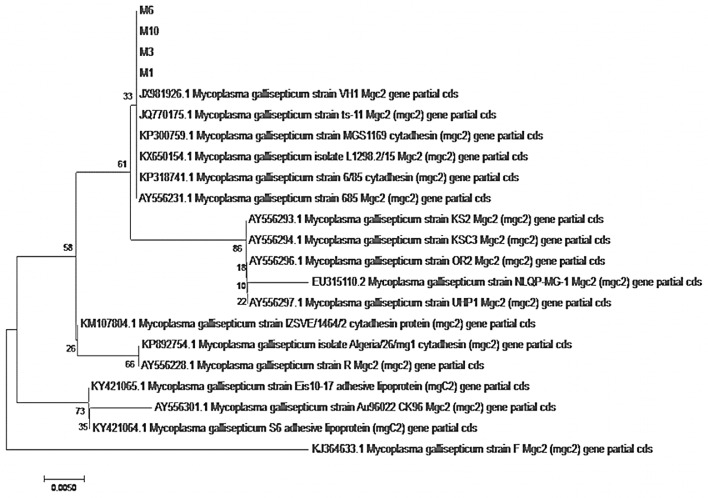
The four sequenced isolates in this study are closely related and placed in one group with the vaccine strain 6/85 and ts11 strain. They are distinct from other field isolates from Egypt and other countries from Ks2 strain and far from the recently identified isolates from the Middle East from R strain and the original vaccine strain F as represented in Figure 6, Figure 7.
Figure 6.
Sequence distance between the samples.
Figure 7.
Phylogenetic tree of the four sequenced samples.
Incidence of MG in Examined Samples
The incidence of MG isolation is summarized in Tables 2 and 3. Following all diagnostic technique in the study, 206 mycoplasmas showed fried egg appearance of MG while 175 isolates were confirmed by PCR (85%).
Table 3.
Incidence of Mycoplasma gallisepticum (MG) isolation.
| Items | Examined samples Number = 400 (68 sera + 332 samples for bacterial isolation) | Positive |
Negative |
||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| SPA test | Serum (68) | 68 | 100 | 0 | 0 |
| Bacterial isolation | Tracheal swabs (68) | 20 | 29.4 | 48 | 70.6 |
| Lung (68) | 54 | 79.4 | 14 | 20.6 | |
| Trachea bifurcation (68) | 46 | 67.4 | 22 | 32.6 | |
| Air sacs (68) | 49 | 72 | 19 | 28 | |
| Infraorbital sinus aspirates (30) | 25 | 83 | 5 | 17 | |
| Litter samples (30) | 12 | 40 | 18 | 60 | |
| Total | (332) samples used for bacterial isolation | 206 | 62 | 126 | 38 |
Through tracking complaints in farms, most of the samples were collected in winter (60%) and autumn (25%), followed by spring (10%) and finally summer (5%). MG was identified in Damietta, Giza, El-Sharqia, El-Behera and El-Qalyobia Governorates with ratio 41.2 (85/206), 40 (72/206), 10.6 (22/206), 7.2 (15/206), and 5.8% (12/206), respectively.
The highest isolation percentage of sample types was 83, 79.4, 72, 67.4, 40, and 29.4% from infraorbital sinus aspirates, lung, air sacs, tracheal bifurcation, litter samples, and tracheal swabs, respectively.
DISCUSSION
The poultry business is one of Egypt's most important agricultural industries, providing a significant portion of the country's animal protein supply (white meats and eggs) (Salem and Attia, 2021). The chicken industry is tied to other industries such as animal feed, medication, and veterinary inputs and provides animal protein for human use. Respiratory diseases represent a great threat to the poultry industry worldwide (Setta et al., 2018; El-Naggar et al., 2021). MG is the most pathogenic species within the genus Mycoplasma of the family Mycoplasmataceae that consider poultry devastating (Evans and Leigh 2008; Erfan and Marouf, 2019; Prabhu et al., 2021). The present investigation shows the Mycoplasma incidence in different Egyptian governorates based on classical and modern techniques.
Our results showed that the investigated chicken flocks showed variable signs of respiratory manifestations and that postmortem examination revealed variable degree air saculitis, pericarditis, perihepatitis, and serositis. Similar findings were recorded by Saif et al. (2003), Bharathi et al. (2018) and Emam et al. (2020). Our investigations showed that diseased turkeys suffered from unilateral or bilateral swelling in the infraorbital sinuses with conjunctivitis, mild respiratory manifestation, and general depression. Similar findings were recorded by Prabhu et al. (2021) in turkeys infected with MG in India. The Biotyping profile of MG reveals a ratio of 95% (digitonin sensitive, glucose positive, arginine negative and form Film and spot tests as indicated in Roussan et al. (2008). Serological tests are monitoring methods for MG infection in a flock. Serological analysis in work by SAP was positive with a ratio of 100%. These tests showed many cross-reactivities (Abdelmoumen and Roy, 1995; Eljakee et al., 2011; Osman et al., 2014; Abu-Seida et al., 2015). The suspected colonies were serologically examined by growth inhibition test against specific MG antisera and revealed a ratio of 95%, as mentioned by Osman et al. (2009).
From our results, the highest isolation percentage was 83% from infraorbital sinus aspirates, 79.4% from lung, 72% from air sacs, 67.4% from tracheal bifurcation, 40% from litter samples and 29.4% from tracheal swabs. Also, Mohamed (1997), Reda and El-Samie (2012), Emam et al. (2020) showed that the highest isolation percentage was from air sacs as 15.7, 23.3, and 21.5%, respectively.
From our finding, the lowest isolation percentage was from the tracheal swabs (29.4%). On the other hand, EI-Jakee et al. (2019) obtained 14 Mycoplasma isolates from tracheal swabs collected from 12 broiler breeder flocks. From our results, out of 30 litter samples from different sources (broiler, layer chickens and turkey litter), only 12 samples were positive for MG infection. We concluded that the litter is a continuous source of Mycoplasma infection, so it should be treated with appropriate disinfectants before getting rid of it to limit the spread of Mycoplasma. This finding agrees with Marois et al. (2002) reported as they were isolated and molecularly detected MG in environmental samples collected from turkey farms. From our results, the widespread prevalence of MG in Egypt increases in the winter and autumn seasons. It can be related to that the fall season is thought to favor microbial multiplication and excessive ammonia levels, poor ventilation accompanied with expanded poultry stocking capacity, and create a favorable environment for respiratory pathogens infection (Hassan et al., 2016). From our findings, the incidence of MG infection was 62%. The highest incidence was noticed in turkey farms, 83%, followed by chicken broiler farms, 70%, then chicken layer arms 40%, and 40% from litter samples. The incidence profiles of the study, as mentioned in the results, is closely nearly to the conclusion of Bharathi et al. (2018), Erfan and Marouf (2019), Emam et al. (2020), Marouf et al. (2020). On the other hand, the layer flocks are more prevalent in Osman et al. (2009) study.
The conventional, traditional methods for MG isolation and identification are laborious, time-consuming, less sensitive, and fail to detect Mycoplasma species from treated birds (Emam et al., 2020). Meanwhile, the PCR technique is a quick, sensitive, and accurate method for detecting MG from suspected cases (Gondal et al., 2015). From our investigations, 175 colonies had amplified fragments at 300 bp by PCR based on mgc2 gene as shown in Figures 6 and 7 with a ratio of 85% that nearly agree with those recorded by Heleili et al. (2012) and Bayatzadeh et al. (2011), Emam et al., 2020 who found the incidence of Mycoplasma was 60.33, 60, and 87.5%, respectively. The PCR has various advantages. However, it is constrained by the formation of contamination because of improper sample handling, which results in false-positive results (OIE. 2000). As a result, traditional cultural approaches should be used in parallel with PCR (Emam et al., 2020).
Sequencing of mgc2 gene for 4 isolates in this study are closely related to each other and placed in one group with the vaccine strain 6/85 and ts11 strain. These results are like those reported by Eissa et al., (2014), who stated mutations located in the mgc2 region led to changes in the antigenicity indices in this region of mgc2 protein compared to other published MG strains. Efforts should be intensified to reduce the negative impacts of mycoplasmosis in the poultry industry by adopting biosafety measures and effective vaccinations in the breeder flocks to minimize the vertical transmission of the disease (El-Naggar et al., 2021). It is also supposed to use some safe products such as herbal extracts (Abou-Kassem et al., 2021a), essential oil (Abd El-Hack et al., 2021a; Alagawany et al., 2021a; El-Tarabily et al., 2021), amino acids (Abou-Kassem et al., 2021b; Arif et al., 2021), phytogenic products (Abd El-Hack et al., 2021b,c; El-Shall et al., 2021; Reda et al., 2021), enzymes (Llamas-Moya et al., 2019), bioactive peptides (El-Saadony et al., 2021a,b), probiotics (Alagawany et al., 2021b; El-Saadony et al., 2021c), plant bioactive compounds (Abd El-Hack et al., 2021d; El-Saadony et al., 2021d), and prebiotics (Abd El-Hack et al., 2021e; Yaqoob et al., 2021), and biological synthesized nanoparticles (Abd El-Ghany et al., 2021; Abd El-Hack et al., 2021f) to raise the general health status of birds and improve their resistance to different diseases including mycoplasmosis.
CONCLUSIONS
Mycoplasma gallisepticum is a severe pathogen that threatens the poultry industry. The results confirmed the importance of applying the theory of prevention programs, hygienic disposal of farm wastes, effective, safe treatments, and vaccination for reducing MG infection in Egypt and saving this industry. The conventional isolation method for MG diagnosis is still the gold standard, even though it is time-intensive, and PCR is more rapid and accurate than conventional methods. Sequencing analysis suggested the use of strain 6/85 and ts11 strain in vaccines application.
Acknowledgments
ACKNOWLEDGMENTS
Authors thank their respective universities and institutes for their support. The current work was funded by Taif University Researchers Supporting Project number (TURSP - 2020/310), Taif University, Taif, Saudi Arabia.
Author contributions: Conceptualization, S.M., H.M.S. and M.A.K.; methodology, S.M., H.M.S. and M.A.K.; investigation, S.M., H.M.S. and M.A.K.; data curation, M.E.A.E-H. and M.T.E.-S.; writing—original draft preparation, M.E.A.E-H., A.M.E.-T. and M.T.E.-S.; writing—review and editing, M.A., A.M.E.-S. and M.E.A.E-H.; visualization, M.E.A.E-H. and M.T.E.-S. All authors have read and agreed to the published version of the manuscript.
DISCLOSURES
The authors declare no conflict of interests.
REFERENCES
- Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. J. 2021;77:1001–1025. [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2021;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., El-Tahan A.M., Khafaga A.F., Taha A.E., AbuQamar S.F., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives – a comprehensive review. Poult. Sci. 2021;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. Int. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S.…Abdel-Moneim A.M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abdel Halium M.M., Salib F.A., Marouf S.A., Abdel Massieh E.S. Isolation and molecular characterization of Mycoplasma spp. in sheep and goats in Egypt. Vet. World. 2019;12:664–670. doi: 10.14202/vetworld.2019.664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmoumen B., Roy R.S. Antigenic relatedness between seven avian Mycoplasma species as revealed by western blot analysis. Avian Dis. 1995;39:250–262. [PubMed] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al- Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abo Elyazeed H., Al-Atfeehy N.M., Abotaleb R., Sayed R., Marouf S. Preparation of ELISA and lateral flow kits for rapid diagnosis of Mycoplasma gallisepticum in poultry. Sci. Rep. 2020;10:9056. doi: 10.1038/s41598-020-65848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Abou-Kassem D.E., El-Abasy M.M., Al-Harbi M.S., Abol-Ela S., Salem H.M., El-Tahan A.M.…Ashour E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Seida A.M., Ahmed K.A., Torad F.A., Marouf S.A. Ultrasonographic and histopathological findings in rams with epididymo-orchitis caused by Brucella melitensis. Pak. Vet. J. 2015;35:456–460. [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arif M., Baty R.S., Althubaiti E.H., Ijaz M.T., Fayyaz M., Shafi M.E., Albaqami N.M., Alagawany M., Abd M.E., El-Hack A.E.Taha, Salem H.M., El-Tahan A.M., Elnesr S.S. The impact of betaine supplementation in quail diet on growth performance, blood chemistry, and carcass traits. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayatzadeh M.A., Pourbakhsh S.A., Homayounimehr A.R., Ashtari A., Abtin A.R. Application of culture and polymerase chain reaction (PCR) methods for isolation and identification of Mycoplasma synoviae on broiler chicken farms. Arch. Razi Inst. 2011;66:87–94. [Google Scholar]
- Bharathi R., Karthik K., Mahaprabhu R., Manimaran K., Geetha T., Tensingh Gnanaraj P., Roy P.R. Outbreak and management of Mycoplasma gallisepticum infection in desi chicken and turkey flocks in an organized mixed farm. Comp. Clin. Pathol. 2018;27:621–625. [Google Scholar]
- Brown D.R., Whitcomb R.F., Bradbury J.M. Revised minimal standards for description of new species of the class Mollicutes (division Tenericutes) Int. J. Syst. Evol. 2007;57:2703–2719. doi: 10.1099/ijs.0.64722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward D.G.ff., Freundt E.A. Type strains of species of the order Mycoplasmatales, including designation of neotypes for Mycoplasma mycoides subsp. mycoides, Mycoplasma agalactiae subsp. agalactiae, and Mycoplasma arthritidis. Int. J. Syst. Evol. 1973;23:55–61. [Google Scholar]
- EI-Jakee J., Marouf S.H., Amin B.H., Hedia R.H. Characterization of Mycoplasmae isolated from chicken. Biosci. Res. 2019;16:1843–1853. [Google Scholar]
- Eissa S.I., Metwally A.M., Hashem Y.M., Khalifa R.A., Refai M.K. Molecular comparative analysis of Mycoplasma gallisepticum and vaccine strains in Egypt. Eur. J. Vet. Med. 2014;9:15–25. http://scik.org [Google Scholar]
- EL-Jakee J., Mohamed Kh.F., Marouf S.A. Preparation of autogenous bivalent vaccine for M. bovis and M. bovigenitalium in Egypt. Life Sci. 2011;8:338–343. [Google Scholar]
- El-Naggar M.S., Ibrahim H.M., Salem H.M., Marouf S. A novel locally prepared inactivated bivalent Mycoplasma vaccine for chicken flocks in Egypt. Adv. Anim. Vet. Sci. 2021;10:55–61. [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28:4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Hussein E.O.S., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20:762–776. [Google Scholar]
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A., Dhama K., Abdel-Latif H.M.R. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehia N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2021;37:1–23. [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., Tarabily El- K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2021 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emam M., Hashem Y.M., El-Hariri M., El-Jakee J. Detection and antibiotic resistance of Mycoplasma gallisepticum and Mycoplasma synoviae among chicken flocks in Egypt. Vet. World. 2020;13:1410–1416. doi: 10.14202/vetworld.2020.1410-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfan A.M., Marouf S. Cinnamon oil downregulates virulence genes of poultry respiratory bacterial agents and revealed significant bacterial inhibition: an in vitro perspective. Vet. World. 2019;12:1707–1715. doi: 10.14202/vetworld.2019.1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernq, H., Stipkovitls 1972. Cultural and biochemical studies of bovine Mycoplasma species or serogroups Abstract). Second Conference on the Taxonomy and Physiology of Mycoplasmas. Brno, Czechoslovakia (in the press).
- Evans J.D., Leigh S.A. Differentiation of Mycoplasma gallisepticum vaccine strains ts-11 and 6/85 from commonly used Mycoplasma gallisepticum challenge strains by PCR. Avian Dis. 2008;52:491–497. doi: 10.1637/8187-120307-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Ewing M.L., Kleven S.H., Brown M.B. Comparison of enzyme-linked immunosorbent assay and hemagglutination- inhibition for detection of antibody to Mycoplasma gallisepticum in commercial broiler, fair and exhibition, and experimentally infected birds. Avian Dis. 1996;40:13–22. [PubMed] [Google Scholar]
- Fan H.H., Kleven S.H., Jackwood M.W., Johansson K.E., Pettersson B., Levisohn S. Species identification of avian mycoplasmas by polymerase chain reaction and restriction fragment length polymorphism analysis. Avian Dis. 1995;39:398–407. [PubMed] [Google Scholar]
- Fathy M., El-safty M.M., El-Jakee J.K., ABbd-alla H.I., Mahmoud H. Study the effect of Mycoplasma contamination of eggs used in virus titration and efficacy of some live attenuated poultry viral vaccines. Asian J. Pharm. Clin. Res. 2017;10:216–222. [Google Scholar]
- Ferguson N.M., Hepp D., Sun S., Ikuta N., Levisohn S., Kleven S.H., Garcia M. Use of molecular diversity of Mycoplasma gallisepticum by gene-targeted sequencing (GTS) and random amplified polymorphic DNA (RAPD) analysis for epidemiological studies. Microbiol. 2005;151:1883–1893. doi: 10.1099/mic.0.27642-0. [DOI] [PubMed] [Google Scholar]
- Gondal M.A., Rabbani M., Muhammad K., Yaqub T., Babar M.E., Sheikh A.A., Khan M.I. Characterization of Mycoplasma gallisepticum isolated from commercial poultry flocks. J. Anim. Plant Sci. 2015;25:108–113. [Google Scholar]
- Hassan K.E., Salama A.S., Shany A.A., Al-Hussien M.D., El-Sawah A.A., El-Kady M.F. Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poult. Sci. 2016;95:1271–1280. doi: 10.3382/ps/pew068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heleili N., Ayachi A., Mamache B., Chelihi A.J. Seroprevalence of Mycoplasma synoviae and Mycoplasma gallisepticum at Batna commercial poultry farms in Algeria. Vet. World. 2012;5:709–712. [Google Scholar]
- Hemeg H.A., Moussa I.M., Ibrahim S., Dawoud T.M., Alhaji J.H., Mubarak A.S., Kabli S.A., Alsubki R.A., Tawfik A.M., Marouf S. Antimicrobial effect of different herbal plant extracts against different microbial population Saudi. J. Biol. Sci. 2020;27:3221–3227. doi: 10.1016/j.sjbs.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven S.H., Swayne D.E., Glisson J.R., Jackwood M.W., Pearson J.E., Reed W.M. 4th ed. American Association of Avian Pathologists; Kennett Square, PA: 1998. Pages 74–80 in A Laboratory Manual for the Isolation and Identification of Avian Pathogens. [Google Scholar]
- Llamas-Moya S., Girdler C.P., Shalash S.M.M., Atta A.M., Gharib H.B., Morsy E.A., Salim H., Awaad M.H.H., Elmenawey M. Effect of a multicarbohydrase containing a-galactosidase enzyme on the performance, carcass yield, and humoral immunity of broilers fed corn–soybean meal–based diets of varying energy density. J. Appl. Poult. Res. 2019;29:142–151. [Google Scholar]
- Lysnyansky I., Garcia M., Levisohn S. Use of mgc2-polymerase chain reaction-restriction fragment length polymorphism for rapid differentiation between field isolates and vaccine strains of Mycoplasma gallisepticum in Israel. Avian Dis. 2005;49:238–245. doi: 10.1637/7285-10020R. [DOI] [PubMed] [Google Scholar]
- Marois C., Dufour-Gesbert F., Kempf I. Polymerase chain reaction for detection of Mycoplasma gallisepticumin environmental samples. Avian Pathol. 2002;31:163–168. doi: 10.1080/03079450120118658. [DOI] [PubMed] [Google Scholar]
- Marouf S., Moussa I.M., Salem H., Sedeik M., Elbestawy A.H.A., Hemeg T.M., Mubarakb D.A.S., Mahmouda H., Alsubki R.A., Bahkali A.H. A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: phenotypic and genotypic characterization. J. King Saud Univ. Sci. 2020;32:2263–2268. [Google Scholar]
- Mohamed, Z. R. 1997. Some studies on Mycoplasma gallisepticum in broiler chickens in Egypt. M.V.Sc. Thesis. (Poultry and Rabbit Diseases) Faculty of Veterinary Medicine, Zagazig University, Egypt.
- OIE, 2004. Avian mycoplasmosis. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 5th ed.
- Osman K.M., Aly M.M., Amin Z.M., Hasan B.S. Mycoplasma gallisepticum: an emerging challenge to the poultry industry in Egypt. Rev. Sci. Tech. 2009;28:1015–1023. doi: 10.20506/rst.28.3.1940. [DOI] [PubMed] [Google Scholar]
- Osman K.M., Marouf S.H., Mehana O.A., AlAtfeehy N. Salmonella enterica serotypes isolated from squabs reveal multidrug resistance and a distinct pathogenicity gene repertoire. Rev. Sci. Tech. 2014;33:997–1006. doi: 10.20506/rst.33.3.2336. [DOI] [PubMed] [Google Scholar]
- Prabhu M., Malmarugan S., Sweetline Anne N., Parthiban S., Balakrishnan G., Johnson Rajeswar J. Detection of Mycoplasma gallisepticum infection in Turkey and chicken farms of Tamilnadu, India. Int. J. Curr. Microbiol. App. Sci. 2021;10:3151–3158. [Google Scholar]
- Reda F., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda L.M., El-Samie L.A. Some studies on the diagnosis of Mycoplasma gallisepticum in chicken. Nat. Sci. 2012;10:247–251. [Google Scholar]
- Roussan D.A., Haddad R., Khawaldeh G. Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult. Sci. 2008;87:444–448. doi: 10.3382/ps.2007-00415. [DOI] [PubMed] [Google Scholar]
- Sabry M.Z., Ahmed A.A. Evaluation of media and cultural procedure for the primary isolation of Mycoplasma from female genitalia of farm animals. J. Egypt. Vet. Med. Assoc. 1975;35:18–36. [Google Scholar]
- Saif Y.M.H, Barnes J., Glisson J.R., Fadly A.M., McDougald L.R., Swayne D.E. 11th ed. Iowa State University Press; Ames, IA: 2003. Pages 719–745 in Diseases of Poultry. [Google Scholar]
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect Sci. 2021;41:1–6. doi: 10.1007/s42690-021-00492-w. [DOI] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual (No. Ed. 2) [Google Scholar]
- Sawicka A., Durkalec M., Tomczyk G., Kursa O. Occurrence of Mycoplasma gallisepticum in wild birds: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setta A., Salem H.M., Elhady M., El-Hussieny A., Arafa A.S. Molecular and genetic characterization of infectious bronchitis viruses isolated from commercial chicken flocks in Egypt between 2014 and 2016. J. World Poult. Res. 2018;8:01–07. [Google Scholar]
- Shatokhin Y., El Gammal M., Prikhodko D. FAO; 2017. Pages 1-43 in Broiler Poultry Industry: Investment Challenges and Opportunities. [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd-El-Hack M.E., Khafaga A.F., Metwally K.A.…El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav J.P., Tomar P., Singh Y., Khurana S.K. Insights on Mycoplasma gallisepticum and Mycoplasma synoviae infection in poultry: a systematic review. Anim. Biotechnol. 2021;10:1–10. doi: 10.1080/10495398.2021.1908316. [DOI] [PubMed] [Google Scholar]
- Yaqoob M., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]