Abstract
Caregivers of patients with multimorbidity are important for improving patient outcomes. This descriptive study examines health status and burden of 22 caregivers of patients with multimorbidity discharged from the hospital who were enrolled in a self-management intervention study. Caregivers did not receive an intervention. Factors that increased caregiver burden were financial issues, caring for others (e.g., family members), and home obligations. Caregivers averaged between 2 and 3 chronic conditions themselves. Perceived caregiver burden remained unchanged over time for the caregiver whether the patient was in the intervention or the usual care group. We recommend rigorous research with larger samples to better understand the caregiver role, needed resources and potential interventions to mitigate caregiver burden in the multimorbid population during and after care transitions. Longitudinal studies that include assessment and interventions for the caregivers of patients with multimorbidity are needed.
Keywords: Caregiver, informal caregiver, multimorbidity, burden, multiple chronic conditions
Introduction
The prevalence of multimorbidity, having two or more chronic diseases, (World Health Organization, 2015) is estimated near 50% among older adults (Xu et al., 2017). As multimorbidity continues to rise in the aging American population, health-related decline and limited financial resources often necessitate an increased need for family or friends to provide care in the home. These informal caregivers of patients with multimorbidity face challenges that are especially burdensome as a result of having to provide care related to two or more chronic illnesses. Although traditionally patient focused, multimorbidity research increasingly acknowledges the important but arduous task of caring for those with multiple chronic conditions (Alemi et al., 2016; Duggleby et al., 2016; Mason et al., 2016; Nordin et al., 2018).
Providing care for multimorbid patients can be especially burdensome for informal caregivers (Riffin et al., 2019). Poor communication among providers, multifaceted responsibilities, psychosocial burden, and difficulty accessing respite services were themes identified in a literature review that examined experiences and support needs of informal caregivers (Price et al., 2020). Other studies identify demands specific to caregivers of multimorbid including multiple provider appointments, the stress of managing medications, and uncertainty in disease and symptom management that may contribute to detriments in mental health and give way to depression or anxiety (Denno et al., 2013; Mason et al., 2016). Multimorbid caregiver exigencies may be further compounded by their own physical or mental health issues, but many studies focus on caregiver burden and omit variables such as caregiver health status or quality of life (Nordin et al., 2018).
Complexities of caring for a loved one with multimorbidity require significant stamina and often results in considerable burden that may negatively impact caregivers’ quality of life and health (El-Jawahri et al., 2017; Farzi et al., 2019). Spousal caregivers have reported more psychological symptoms (depression and stress) than non-spousal caregivers (Oldenkamp et al., 2016; Pinquart & Sörensen, 2011) underscoring the need to address spousal caregivers’ psychosocial well-being. Increased caregiver burden is directly related to increased anxiety and depression (Denno et al., 2013). Likewise, decreased caregiver burden was associated with positive mental health in those caring for the multimorbid (Duggleby et al., 2016). Caregivers also report physical symptoms such as fatigue, weakness and weight loss (Choi & Seo, 2019). Furthermore, reallocation of time and resources may result in difficulty maintaining relationships which can manifest additional caregiver distress (Choi & Seo, 2019). Understanding how caregiver burden effects health outcomes is essential to supporting needs of caregivers for the multimorbid.
Exploring the health status of the patient and caregiver dyad may be of interest following a hospitalization. Discharge from acute care results in numerous changes that must be coordinated by the caregiver. In the case of a care recipient with multiple chronic conditions the care needs are greater and result in significantly more time being spent on caregiving activities (Lebrec et al., 2016). Another limitation among literature relates to examination of longitudinal changes in caregiver burden (Price et al., 2020). Assessing caregiver’s needs over time should not be overlooked. Extra family caregiver support directly after hospitalizations is needed to increase caregivers’ sense of control and social support (Hwang et al., 2011).
This sub-study provided an opportunity to explore the health status and quality of life of a patient/caregiver dyad and examine caregiver burden over time following a recent patient hospitalization. Caregivers of the multimorbid patients enrolled in a care transition intervention were invited to participate in this descriptive study. Caregivers did not receive an intervention. Understanding more about the demands and needs of caregiving over time for a patient with multimorbidity following a recent hospitalization is a gap that must be addressed to provide caregiver support and lessen the burden of caregiving. Therefore, the purpose of this descriptive study was to examine health status and demographics of patients and caregivers at baseline and to describe caregivers and their burden caring for patients with multimorbidity in the home setting following hospital discharge. Specifically, this project addressed the following aims:
Describe baseline and 60-day characteristics (demographic and health status) of caregivers and patients with who participated in a care transition self-management intervention.
Compare perceived caregiver burden between caregivers of patients receiving the intervention compared to patients receiving usual care on time demand of tasks for caregivers; difficulty of task of caregiving; and outside resources utilized by caregivers.
Describe health status measures (EQ-5D and PROMIS) between caregivers of patients receiving the intervention compared to caregivers of patients receiving usual care at baseline and 60 days after the intervention.
Methods and Measures
Study Design
Caregivers in this exploratory descriptive study were identified by the patient enrolled in the primary care transition study. No specific intervention was designed for the caregiver; however, they were asked to be available and part of the patient intervention. The primary study for this analysis was a repeated measure stratified randomized controlled pilot study. The intervention was designed to encourage self-management by promoting patient activation based on level of cognition. Patients were enrolled in the study prior to discharge from the acute hospital setting. Results of the cost-effectiveness of the self-management care transition interventions, in addition to a thorough description of the intervention and methods used are reported in the primary article (Zimmerman et al., 2017). Though the purpose of the primary study was not related to the caregiver or caregiving, we recognized the importance of gaining more knowledge of caregiver burden and health status when caring for the multimorbid patient. Due to the descriptive nature of our aims, we were not looking for effect differences therefore a power analysis to determine sample size was not done.
Sample
The sample included 22 caregivers who were adults and understood English. Thirteen caregivers were for patients in the intervention group and nine in the usual care group. Institution review board approval was obtained prior to initiating the study. The caregiver was a spouse, partner, friend, or family member who defined themselves as such and resided in the same home as the patient in the intervention study. Eligible caregivers were identified by the patient and present in the room when the patient enrolled in the intervention study. Once the patient agreed to participate, the caregiver was invited to participate in this caregiver study. While there were 90 potential caregivers who lived with patients enrolled in the study, most caregivers were not available in the patient room and therefore unable to consent. Twenty-seven caregivers signed consents, 22 were actively involved in the study. If the patient dropped out of the study or did not meet inclusion or exclusion criteria (e.g., discharged somewhere other than home), the caregiver also was dropped from the study. Reasons for and numbers of refusals to participate was not collected.
Measures
Caregiver burden. Oberst Caregiving Burden Scale (OCBS) is a two-dimensional 15-item scale that addresses time and difficulty of performing each of the items measuring tasks to be performed. Caregivers rate their level of time and then difficulty using a 5-point Likert scale from 1 (none) to a 5 (a great deal) with higher scores indicate more time and difficulty (Bakas et al., 2004; Oberst et al., 1989). Scores were calculated by averaging across the 15 items of the time and difficulty subscales, thus achieving an average score between 1 and 5 for each subscale. Summed scores were obtained by multiplying mean scores by 15 (which was used to compare results against other studies using OCBS, with possible range of 15–75). Evidence of construct validity and internal consistency reliability have been documented (Chung et al., 2016). Cronbach’s alpha for this instrument has been reported at .90 and .94 for OCBS time and difficulty scale respectively, (Bakas et al., 2004) and for this study the Cronbach’s alpha at baseline was .93 and .93, respectively.
Health Related Quality of Life. EQ-5D Well-being Index consists of a weighted sum of five dimensions by rating the amount of difficulty with five items: mobility, self-care, usual-activities, pain/discomfort, and anxiety/depression. This scale ranges from none, slight, moderate, and severe problems to being unable to perform the task which provided a simple descriptive profile. It also has one item visual analog scale (VAS) that rates health on a scale of 0 to 100 (100 being the best health) for a single index value for health status. The EQ-5D has been reviewed for validity and reliability and approved for use. Intraclass correlation for the EQ-5D VAS score was .82 for reliability and responsiveness as reported for the acute coronary syndrome population (Schweikert, 2006). The tool has been validated in several chronic populations and in several different countries (Berg et al., 2015; Jia et al., 2014; Schweikert, 2006).
Health Status. PROMIS 29 Profile measure was administered to assess caregiver self-reported mental, physical and social health status. PROMIS 29 is a collection of four-item short forms assessing physical functioning, anxiety, depression, fatigue, pain interference, sleep disturbance, ability to participate in social roles or activities, and a single pain intensity item. Each item has five responses ranging in value from 1 to 5 except for the pain intensity that ranges from a 0 to 10 value. The lowest score is 4 and highest possible is 20. Higher scores in the anxiety, depression, fatigue, pain interference, and sleep disturbance indicate worse health status, and lower scores in the physical and social functioning domains indicate worse health status. Cronbach’s alphas for the PROMIS-29 subscales ranged from .88 to .94 (Cella et al., 2010). PROMIS has been validated in a variety of clinical settings in the US general population and in several chronic disease populations (Cella et al., 2010; Goswami et al., 2019).
Procedures
After explanation of the study and written consent, baseline questionnaires including demographic data, OCBS, EQ-5D, and the PROMIS 29 were administered and repeated via telephone 60 days after initiation of the patient intervention. No specific intervention strategies were given to the caregiver; however, they were present at the discharge teaching and encouraged to be present during the care transition patient intervention. The project director of the primary study trained research assistants in administering caregiver questionnaires by demonstration and teach back. Fidelity checks were completed at least once with each research assistant during a 60-day follow up telephone call.
Data Analysis
Descriptive statistics (i.e., mean, standard deviation, and/or frequency distributions and percentages) were conducted on all variables. For aim 1, we used descriptive statistics and t-tests to compare baseline and 60-day characteristics on demographic and health status measures of caregivers and patients. For aim 2, Mann-Whitney U statistics were used to compare caregiver burden and health status measures between caregivers of patients receiving the intervention compared to caregivers of patients receiving usual care at baseline and 60 days. Descriptive statistics were used for aim 3.
Results
A total of 22 caregivers were included in this study. Patients were, on average, 67 (SD = 10.8) years old while their caregivers averaged 61 (SD = 9.8) years old. All patients and caregivers were Caucasian, and 68% were female. Most caregivers (91%) were spouses of the patient, others were family members. Half, n = 11, of the caregivers reported they were currently working however, patients were even working more hours (28 hr/week) at baseline than the caregiver (23.78 hr/week). Hypertension (n = 8, 31%), depression (n = 5, 19%), and diabetes (n = 4, 15%) were the most common caregiver chronic diseases. Most caregivers and patients had private insurance and over one third were on Medicare. There were only two significant differences (p ≤ .05) in demographic variables between patients and caregivers (see Table 1). Patients had significantly more comorbidities and a greater interference in their ability to do non-job type activities such as housekeeping and shopping compared to caregivers.
Table 1.
Comparison of Baseline Demographic Characteristics and Health Status Measures of Patients and Caregivers.
| Patients N = 22 |
Caregivers N = 22 |
Test statistic | |
|---|---|---|---|
| M (SD) | M (SD) | ||
| Demographics | |||
| Age | 66.68 (10.79) | 60.95 (9.78) | t = 1.45 |
| Years of education | 14.23 (3.28) | 14.39 (2.79) | t = .174 |
| Total number of comorbidities | 6.46 (2.41) Range = 4–12 |
2.31 (2.44) Range = 0–10 |
t =5.677** |
| Number of hours worked | 28.00 (21.89) | 23.78 (12.18) | t = −.506 |
| Interference in ability to do daily non-work activities (e.g., housecleaning or shopping) | 5.76 (3.27) | 2.65 (2.94) | t = 3.055* |
| Patient activation | 61.35 (13.82) | 65.07 (12.76) | t = .928 |
| f (%) | f (%) | ||
| Gender | |||
| Female | 8 (36%) | 15 (68%) | X2 = .3367 |
| Male | 14 (64%) | 7 (32%) | |
| Race | |||
| Caucasian | 22 | 22 | |
| Employment | |||
| Yes | 9 (41%) | 11 (50%) | |
| No | 13 (59%) | 11 (50%) | |
| Marital Status | |||
| Married | 20 (91%) | ||
| Not married | 2 (9%) | ||
| Caregiver relationship to patient | |||
| Spouse | 20 (91%) | ||
| Family member | 2 (9%) | ||
| Health status measures | |||
| EQ-5D index | .685 (.203) | .865 (.086) | t = 3.818* |
| EQ-5D VAS | 65.90 (18.59) | 80.57 (13.17) | t = 2.95* |
| Anxiety | 54.81 (7.39) | 52.86 (7.13) | t = −.880 |
| Depression | 55.13 (8.83) | 49.24 (6.27) | t = −1.658 |
| Fatigue | 58.63 (8.23) | 49.15 (4.74) | t = −4.599** |
| Physical function | 39.77 (7.22) | 50.41 (7.42) | t = 4.762** |
| Pain interference | 59.23 (10.86) | 49.45 (7.55) | t = −3.276* |
| Sleep disturbance | 54.83 (6.33) | 48.26 (6.39) | t = −3.386* |
| Ability to participate in social roles and activity | 41.64 (6.61) | 49.98 (4.90) | t = 4.064** |
p < .01. **p < .001.
While this study was not designed or powered to test for differences between caregivers and patients, some interesting trends were noted when comparing results for these two groups. Caregivers as compared to patients reported better health related quality of life (EQ-5D scores), higher functioning and increased social life, and less fatigue, pain, and sleep disturbances. Depression and anxiety were two measures of health status that showed caregivers and patients being more similar in score responses. See Table 1 for results for each health status measure.
Using Mann-Whitney U, there were no significant differences on the OCBS between caregivers in the intervention and usual care group in the time spent or with the difficulty of the caregiving at baseline or 60 days, see Table 2. Time spent and difficulty at baseline were close to indicating differences, the caregivers with patients in the intervention group had higher median scores on both measures compared to the caregiver of patients in the usual care group. Median number of comorbidities of the caregiver were not different by group, with a median of 2 (range 0–10) in the intervention group of caregivers and 1 (range 0–5) in the usual care group of caregivers, see Table 2.
Table 2.
Time and Difficulty of Caregiving Tasks Reported by Caregiver at Baseline and 60 days After Patient’s Hospital Discharge.
| OCBS | Caregivers of intervention patients n = 13 median | Caregivers of usual care patients n = 9 median | Mann-Whitney U statistic (N) | z | p value |
|---|---|---|---|---|---|
| Time spent baseline | 31.5 (12) | 28 (9) | 45.50 (21) | −.605 | .554 |
| Time spent 60 days | 32 (11) | 23 (6) | 18.50 (17) | −1.459 | .149 |
| Difficulty baseline | 22 (13) | 20 (9) | 52.50 (22) | −.402 | .695 |
| Difficulty 60 days | 19 (11) | 15.5 (6) | 17.00 (17) | −1.633 | .122 |
Note. N = 22.
In relation to other resources commonly used by caregivers for patients in both the intervention and usual care group was other family members (intervention = 6, usual care = 5) and friends (intervention = 2, usual care = 2). Individual caregivers also were supported by neighbors and adult day care programs. One caregiver in the intervention group used the American Association for the Blind for support. Church and hospice were used by a caregiver in the usual care group. When specifically asked about other factors that increased burden in caregivers, financial issues (loss of second paycheck, one vehicle), caring for additional individuals (elderly parents, in-laws), and other obligations at home (housecleaning, yardwork) were reported by both groups.
Comparison of the caregivers means by patient group (intervention vs. usual care) on EQ-5D index (M = 0.878, SD = 0.083; M = 0.876, SD = 0.074, respectively) and EQ-5D VAS (M = 79.27, SD = 16.33; M = 84.00, SD = 9.62, respectively) were similar at baseline and showed negligible differences at 60 days. Figure 1 depicts group means on the PROMIS-29 subscales near the population mean and negligible differences between groups at 60 days.
Figure 1.
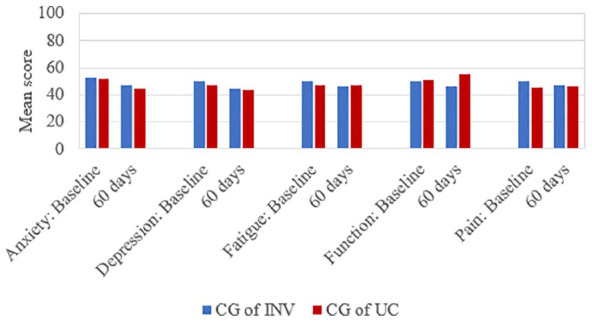
Promis-29 baseline and 60 days: caregivers of patients in intervention (CG of INV) or usual care (CG of UC) group.
Discussion
Study findings are consistent with existing research related to caregiver burden of multimorbid and suggest implications for future research and nursing practice. As one might expect, the caregivers reported fewer comorbidities as compared to patients. Caregivers had between 2 and 3 chronic conditions on average, with one caregiver having as many as 10 comorbidities. We assumed that caregivers would be “healthier” than the patient they were caring for, however, sometimes the caregiver also has many comorbidities, as was the case in our study.
Perceived caregiver burden remained unchanged in both the intervention and usual care groups. This finding aligns with results from a systematic review performed by (de Bruin et al., 2012) that observed no change in caregiver burden despite a comprehensive care program implemented for patients with multimorbidity and their caregivers. Additionally, it should be noted that the intervention in this study was directed at the patient with multimorbidity, not the caregiver. Additional study of this population of caregivers of multimorbid patient is needed.
Caregiving burden is difficult to compare across studies because of the heterogeneity of populations and assessment tools (Nordin et al., 2018). When compared to studies that utilized the same OCBS, caregivers of patients with heart failure (Chung et al., 2016) and stroke (Ganapathy et al., 2015) both reported higher levels of burden than participants in our study. Higher median scores of time spent and difficulty at baseline for caregivers in the intervention group may favor a simpler, less time-consuming self-management intervention. However, without statistical significance or explanation for increased time demands or difficulty, this observation is recognized as speculative. Research participation itself may account for the variation in scores. Though intervention studies are intended to improve processes and health outcomes for patients and caregivers, participation itself is time and energy consuming and may unintentionally contribute in a negative manner. This potential sequela advocates for caregiver assessment as an important component of a transitional care program. Subsequently, resources that caregivers have available to them to provide support is important to consider.
Caregivers in our study reported that managing finances, caring for others, and home obligations contributed to caregiver burden. In a similar manner, Price et al. (2020) describe multifaceted responsibilities that create challenges for caregivers of patients with multimorbidity. Consideration of baseline responsibilities are thus important to consider when assessing needs for caregivers of patients with multimorbidity.
As patients reported the ability to perform fewer household tasks, caregivers reported increased home obligations, these results may suggest some burden during care transition could arise from home obligations being shifted from patient to caregiver. Insightful discharge planning may help alleviate caregiver burden by assessing anticipated needs to replace the usual home responsibilities of patients with resources such as housekeeping, grocery delivery, or lawn care services.
Family, friends, and neighbors were identified by caregivers as commonly sought out resources suggesting high-risk patients with multimorbidity may utilize a network of informal caregivers rather than just one primary person to assist with needs following hospital discharge. Further investigation of multiple non-resident caregivers and the roles they play during care transitions is needed. Accessing respite services is important to support the needs or caregivers (Price et al., 2020). Caregivers in our study identify options of seeking out adult day programs or church members to help relieve burden of caring for patients with multimorbidity.
We anticipated that quality of life and general health status would be impacted by the level of burden these caregivers were experiencing caring for the multimorbid patient, however, in our study that was not the case. Additional research with larger sample sizes is needed to better understand the caregiver role, needed resources and potential interventions to mitigate caregiver burden of the multimorbid during and after care transitions.
Limitations
Limitations in this study were primarily related to self-report of participants, small sample size, and generalizability issues. Our primary purpose was not the caregiver subset and recruitment of caregivers present in the room during patient enrollment resulted in a smaller number of caregivers. Caregivers for this study were also required to reside with the patient which may have excluded informal caregivers such as neighbors or friends that may assist with cares. In addition, only one caregiver was enrolled per patient although multiple caregivers may be involved in a patient’s everyday care.
This study was part of a transitional care intervention study for patients, and the results may have poor generalizability. As an exploratory study, strong conclusions cannot be drawn, and results need validated in larger study with the primary focus on the caregiver and patient/caregiver dyad. In addition, the scope of caregiver assistance was not well-described, particularly regarding specific caregiving tasks that contributed to burden and whether caregivers of the patients with multimorbidity and low cognition required additional caregiving attention during hospital to home transitions.
Conclusion
Caregivers have major chronic diseases themselves as shown in this study. Caregiver comorbidities and the need for self-care place additional burden on an already burdened situation. Determining caregiver comorbidities, areas of need, strategies for successful implementation, and accessible resources for caregivers of patients must be considered. Caregivers relied most heavily on other family members for assistance with caregiving activities. Respite care and adult day programs to mitigate fatigue and allow time for self-care by the caregiver could be explored. Some states have adopted programs that allow family caregivers to receive pay for caregiving services to relieve some of the financial burden, exploration of such programs may be of great benefit. Social supports in the form of neighbors or church members may provide additional assistance such as helping with transportation to help alleviate caregiver burden.
In addition to perceived burden, we agree with other authors that the general well-being of those caring for individuals with multiple health problems is important to consider (Duggleby et al., 2016; Price et al., 2020). Patients and caregivers may both experience similar degrees of anxiety and depression, so mental health should be considered when implementing a transitional care program. Physical health status and quality of life are also variables that deserve additional inquiry (Nordin et al., 2018). Discovering ways to improve experiences of caregivers may help preserve the vital role of individuals providing care to increasingly complex multimorbid patients. Longitudinal approaches are desirable, as care needs may change over time.
As multimorbidity continues to rise in the ageing population, family members of the patients with multimorbidity will most likely be called upon to serve in the multi-faceted role of informal caregiver. Understanding the burden of caregiving along with the necessary support to provide care and to remain healthy to continue as a caregiver will be paramount. Additional research is needed to better understand caregiving burden and the necessary resources and interventions to mitigate the burden of caregiving.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Partial funding was obtained from Blue Cross and Blue Shield of Nebraska
ORCID iDs: Myra Schmaderer  https://orcid.org/0000-0003-1003-0871
https://orcid.org/0000-0003-1003-0871
Courtney Loecker  https://orcid.org/0000-0002-2754-5233
https://orcid.org/0000-0002-2754-5233
References
- Alemi F., Levy C. R., Kheirbek R. E. (2016). The multimorbidity index: A tool for assessing the prognosis of patients from their history of illness. EGEMS (Washington, DC), 4(1), 1235–1235. 10.13063/2327-9214.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas T., Austin J. K., Jessup S. L., Williams L. S., Oberst M. T. (2004). Time and difficulty of tasks provided by family caregivers of stroke survivors. The Journal of Neuroscience Nursing: Journal of the American Association of Neuroscience Nurses, 36(2), 95–106. 10.1097/01376517-200404000-00007 [DOI] [PubMed] [Google Scholar]
- Berg J., Lindgren P., Mejhert M., Edner M., Dahlström U., Kahan T. (2015). Determinants of utility based on the EuroQol five-dimensional questionnaire in patients with chronic heart failure and their change over time: Results from the swedish heart failure registry. Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research, 18(4), 439–448. 10.1016/j.jval.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., Amtmann D., Bode R., Buysse D., Choi S., Cook K., Devellis R., DeWalt D., Fries J., Gershon R., Hahn E. A., Lai J., Pilkonis P., Revicki D., . . . Hays R. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Seo J. (2019). Analysis of caregiver burden in palliative care: An integrated review. Nursing Forum, 54(2), 280–290. 10.1111/nuf.12328 [DOI] [PubMed] [Google Scholar]
- Chung M. L., Lennie T. A., Mudd-Martin G., Dunbar S. B., Pressler S. J., Moser D. K. (2016). Depressive symptoms in patients with heart failure negatively affect family caregiver outcomes and quality of life. European Journal of Cardiovascular Nursing: Journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology, 15(1), 30–38. 10.1177/1474515114535329 [DOI] [PubMed] [Google Scholar]
- de Bruin S. R., Versnel N., Lemmens L. C., Molema C. C. M., Schellevis F. G., Nijpels G., Baan C. A. (2012). Comprehensive care programs for patients with multiple chronic conditions: A systematic literature review. Elsevier Scientific Publishers. 10.1016/j.healthpol.2012.06.006 [DOI] [PubMed] [Google Scholar]
- Denno M. S., Gillard P. J., Graham G. D., DiBonaventura M. D., Goren A., Varon S. F., Zorowitz R. (2013). Anxiety and depression associated with caregiver burden in caregivers of stroke survivors with spasticity. Archives of Physical Medicine and Rehabilitation, 94(9), 1731–1736. 10.1016/j.apmr.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Duggleby W., Williams A., Ghosh S., Moquin H., Ploeg J., Markle-Reid M., Peacock S. (2016). Factors influencing changes in health related quality of life of caregivers of persons with multiple chronic conditions. Health and Quality of Life Outcomes, 14(1), 81–81. 10.1186/s12955-016-0486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Jawahri A., Greer J. A., Pirl W. F., Park E. R., Jackson V. A., Back A. L., Kamdar M., Jacobsen J., Chittenden E., Rinaldi S., Gallagher E., Eusebio J., Fishman S., VanDusen H., Li Z., Muzikansky A., Temel J. S. (2017). Effects of early integrated palliative care on caregivers of patients with lung and gastrointestinal cancer: A randomized clinical trial. The Oncologist, 22(12), 1528–1534. 10.1634/theoncologist.2017-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzi S., Farzi S., Moladoost A., Ehsani M., Shahriari M., Moieni M. (2019). Caring burden and quality of life of family caregivers in patients undergoing hemodialysis: A descriptive-analytic study. International Journal of Community Based Nursing and Midwifery, 7(2), 88–96. 10.30476/IJCBNM.2019.44888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V., Graham G. D., DiBonaventura M. D., Gillard P. J., Goren A., Zorowitz R. D. (2015). Caregiver burden, productivity loss, and indirect costs associated with caring for patients with poststroke spasticity. Clinical Interventions in Aging, 10, 1793–1802. 10.2147/CIA.S91123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S., Peipert B. J., Mongelli M. N., Kurumety S. K., Helenowski I. B., Yount S. E., Sturgeon C. (2019). Clinical factors associated with worse quality-of-life scores in united states thyroid cancer survivors. Surgery, 166(1), 69–74. 10.1016/j.surg.2019.01.034 [DOI] [PubMed] [Google Scholar]
- Hwang B., Fleischmann K. E., Howie-Esquivel J., Stotts N. A., Dracup K. (2011). Caregiving for patients with heart failure: Impact on patients’ families. American Journal of Critical Care: An Official Publication, American Association of Critical-Care Nurses, 20(6), 431–441. 10.4037/ajcc2011472 [DOI] [PubMed] [Google Scholar]
- Jia Y, Cui F., Li L., Zhang D., Shang G., Wang F., Gong X., Sheng H., Wu Z., Miao N., Sun J., Shand L., Lv J. M, Yang F. (2014). Comparison between the EQ-5D-5L and the EQ-5D-3L in patients with hepatitis B. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 23(8), 2355–2363. 10.1007/s11136-014-0670-3 [DOI] [PubMed] [Google Scholar]
- Lebrec J., Ascher-Svanum H., Chen Y., Reed C., Kahle-Wrobleski K., Hake A., Raskin J., Naderali E., Schuster D., Heine R., Kendall D. (2016). Effect of diabetes on caregiver burden in an observational study of individuals with alzheimer’s disease. BMC Geriatrics, 16(1):93. 10.1186/s12877-016-0264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B., Nanton V., Epiphaniou E., Murray S. A., Donaldson A., Shipman C., Daveson B., Harding R., Higginson I., Munday D., Barclay S., Dale J., Kendall M., Worth A., Boyd K. (2016). ‘My body’s falling apart.’ understanding the experiences of patients with advanced multimorbidity to improve care: Serial interviews with patients and carers. BMJ Supportive & Palliative Care, 6(1), 60–65. 10.1136/bmjspcare-2013-000639 [DOI] [PubMed] [Google Scholar]
- Nordin A. A., Hairi F. M., Choo W. Y., Hairi N. N. (2018). Care recipient multimorbidity and health impacts on informal caregivers: A systematic review. The Gerontologist, 59(5), e611–e628. 10.1093/geront/gny072 [DOI] [PubMed] [Google Scholar]
- Oberst M. T., Thomas S. E., Gass K. A., Ward S. E. (1989). Caregiving demands and appraisal of stress among family caregivers. Cancer Nursing, 12(4), 209–215. [PubMed] [Google Scholar]
- Oldenkamp M., Hagedoorn M., Slaets J., Stolk R., Wittek R., Smidt N. (2016). Subjective burden among spousal and adult-child informal caregivers of older adults: Results from a longitudinal cohort study. BMC Geriatrics, 16(1), 208–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M., Sörensen S. (2011). Spouses, adult children, and children-in-law as caregivers of older adults: A meta-analytic comparison. Psychology and Aging, 26(1), 1–14. 10.1037/a0021863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. L., Surr C. A., Gough B., Ashley L. (2020). Experiences and support needs of informal caregivers of people with multimorbidity: A scoping literature review. Psychology & Health, 35(1), 36–69. 10.1080/08870446.2019.1626125 [DOI] [PubMed] [Google Scholar]
- Riffin C., Van Ness P. H., Wolff J. L., Fried T. (2019). Multifactorial examination of caregiver burden in a national sample of family and unpaid caregivers. Journal of the American Geriatrics Society, 67(2), 277–283. 10.1111/jgs.15664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikert B. (2006). Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart, 92(1), 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2015). World health report on aging and health. Retrieved from https://books.google.com/bookshl=en&lr=&id=n180DgAAQBAJ&oi=fnd&pg=PP1&ots=uSK2jqPWn4&sig=getDi_IkbbG9LTkJcQTLypn77tc#v=onepage&q&f=false
- Xu X., Mishra G. D., Jones M. (2017). Evidence on multimorbidity from definition to intervention: An overview of systematic reviews. Ageing Research Reviews, 37, 53–68. 10.1016/j.arr.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Zimmerman L., Wilson F. A., Schmaderer M. S., Struwe L., Pozehl B., Paulman A., Bratzke L. C., Moore K., Raetz L., George B. (2017). Cost-effectiveness of a care transition intervention among multimorbid patients. Western Journal of Nursing Research, 39(5), 622–642. 10.1177/0193945916673834 [DOI] [PubMed] [Google Scholar]


