Abstract
Background and Purpose
This study aimed to identify the epidemiological features of Guillain-Barré syndrome (GBS) in the Korean population.
Methods
Patients with GBS were defined as those who were hospitalized with a primary diagnostic code of G61.0 on the Korean Classification of Disease in a department of neurology, rehabilitation medicine, or pediatrics. We evaluated the incidence and prevalence of GBS as well as physical disability, mortality, and cause of death in patients with GBS from 2002 to 2018 in the Korean population using the Korean National Health Insurance Service database.
Results
We identified 11,146 patients with GBS. The ratio of males to females was 1.48. The age-adjusted incidence rate per 100,000 persons increased steadily from 0.84 in 2002 to 1.68 in 2018, as did the age-adjusted prevalence rate per 100,000 persons, from 0.77 to 15.62. The incidence and prevalence of GBS increased with age, peaking at 70–79 years. Among 10,114 patients without physical disability at the time of GBS being diagnosed, 502 (5.0%) patients had moderate disability and 526 (5.2%) had severe disability by the end of the study period. A total of 1,221 (11.0%) patients with GBS died during the mean follow-up period of 17 years (2002–2019). There were 144 (1.3%) in-hospital deaths.
Conclusions
This was the first nationwide epidemiological study of patients with GBS covering the entire population including patients of all ages in the Republic of Korea. We have revealed the seasonality of admissions, disability, and long-term mortality rates in patients with GBS.
Keywords: Guillain-Barré syndrome, incidence, disability, mortality, cause of death
INTRODUCTION
Guillain-Barré syndrome (GBS) is the most common immune-mediated neuropathy, and is characterized by rapidly evolving muscle weakness, sensory loss, and hyporeflexia. The etiology of GBS remains unclear, but it is considered that an immune-mediated process with molecular mimicry plays an important role in the generation of autoimmune antibodies and the activation of inflammatory cells.1 The main clinical feature of GBS is progressive symmetric weakness of the limb muscles within 28 days, followed by clinical plateau. Approximately two-thirds of patients have a prodromal illness at from 3 days to 6 weeks before the onset of GBS.2 The diagnosis of GBS is based on typical clinical features, electrodiagnostic testing, antiganglioside antibodies, and elevated levels of protein in the cerebrospinal fluid. Intravenous immunoglobulin (IVIG) and plasmapheresis have proven to be effective treatments for GBS.
The incidence of GBS has been reported to be between 0.59 and 2.35 per 100,000 persons worldwide,3,4,5,6 while the reported mortality rate of GBS has ranged from 3% to 13%.7 The most common causes of death in patients with GBS are respiratory and cardiovascular complications.7 Although GBS is a common and life-threatening disease, there have been few epidemiological studies of GBS in Republic of Korea.8,9 Moreover, those studies were restricted to the association of GBS with influenza vaccination and with the elderly in the Korean population.8,9
The Republic of Korea has a single public medical insurance system, the National Health Insurance Service (NHIS), which covered approximately 52 million Korean citizens in 2018.10 All health insurance claims information is integrated into the NHIS database in the Republic of Korea. This claims database includes information on healthcare utilization throughout the country, including demographic characteristics, dates of hospital visits, admissions, and discharges; principal diagnoses based on the Korean Standard Classification of Disease (KCD), which is a modified version of the International Classification of Diseases, 10th edition (ICD-10); diagnostic procedures; medical and surgical procedures; prescriptions filled; and healthcare expenditure.11 The comprehensive nature of the NHIS database has resulted in it being used to determine the incidence and prevalence of neuromuscular disease in the Korean population.10,12
This study performed a nationwide population-based investigation of the incidence, prevalence, physical disability, mortality, and causes of death of GBS in the Korean population using the NHIS database.
METHODS
Developing an operational diagnosis of GBS
An appropriate operational definition for GBS was produced by evaluating the accuracy of various diagnostic criteria, including diagnostic codes, nerve conduction studies (NCSs), hospitalization, and treatment departments. Patients diagnosed with inflammatory polyneuropathy at Gangnam Severance Hospital between January 2018 and December 2019 were selected based on the KCD diagnostic codes for GBS (G61.0), serum neuropathy (G61.1), other inflammatory polyneuropathies (G61.8), and inflammatory polyneuropathy, unspecified (G61.9). Forty-four of the 146 patients identified with these diagnostic codes were identified as having GBS complying with the National Institute of Neurological and Communicative Disorders and Stroke Criteria.13 Two of these patients had Miller Fisher syndrome, which is cranial-dominant GBS. There were 102 patients with other diseases, comprising 47 with cervical disc disorder with myelopathy, unspecified cervical region (KCD code M50.0); 14 with chronic inflammatory demyelinating polyneuritis (G61.81); 10 with inflammatory polyneuropathy, unspecified (G61.9); 10 with malingering (Z76.5); 9 with diplopia (H53.2); 4 with multifocal motor neuropathy (G61.82); 4 with hereditary motor and sensory neuropathy (G60.0); and 4 with polyneuropathy, unspecified (G62.9).
Supplementary Table 1 (in the online-only Data Supplement) presented the accuracy of the operational definitions of GBS based on their sensitivity, specificity, positive predictive value, and negative predictive value. We finally decided upon an operational definition of GBS as hospitalized cases with the KCD diagnostic code for GBS (G61.0) treated in a specific department: neurology, pediatrics, or rehabilitation medicine.
Study population
We searched for all patients meeting the final operational definition of GBS in the NHIS database from January 2002 to December 2018. Data collected from patients with GBS included age, sex, medical-visit records, hospital type, income level, physical disability, death information, and the use of specific therapies including IVIG, plasmapheresis, and tracheostomy. Income levels were classified into the following three categories: 1) low (poorest 30% of the population), 2) middle (31%–70% of the population), and 3) high (richest 30% of the population). We defined hospitalization for ≥30 days as a long hospitalization period and hospitalization for <30 days as a short hospitalization period. Hospital type was divided into referral hospitals and nonreferral hospitals.
Incidence and prevalence
The crude incidence rate of GBS was calculated as the number of newly identified patients with GBS at the end of each year (from 2002 to 2018) divided by the total population in that year, as obtained from the Korean Statistical Information Service of Statistics Korea (http://kosis.kr/). The crude prevalence rate was calculated as the total number of patients with GBS at the end of each year divided by the total population in that year. In addition, age-adjusted incidence and prevalence were calculated using the world standard population, as determined by the World Health Organization for 2000–2025.14
Long-term physical disability
We evaluated long-term disability in patients with GBS who did not have previous physical disability using the NHIS database. The degree of physical disability was divided into six levels from index 1 (the most-severe disability) to index 6 (less-severe disability). Physical disability levels were reclassified into two categories: 1) moderate physical disability for indexes 4–6 and 2) severe physical disability for indexes 1–3. We then compared long-term physical disability according to age, sex, income level, hospitalization period, IVIG, hospital type, plasmapheresis, and tracheostomy.
Death information
Participant deaths up to December 31, 2019 were ascertained using the NHIS database based on information derived from the Resident Register of the Republic of Korea. Information on causes of death from January 2002 to December 2018 were obtained from Statistics Korea and based on death certificates, most of which were confirmed by physicians. Causes of death were classified according to the relevant ICD-10 codes, as in a previous study.10 The standardized mortality ratios (SMRs) for all-cause and cause-specific mortality, along with their 95% confidence intervals (CIs), were determined relative to the general Korean population for 2002–2018. The SMR was calculated as the number of observed deaths in patients with GBS divided by the expected number of deaths in the general population as determined from the data recorded by Statistics Korea. We also analyzed in-hospital deaths, which were defined as death occurring during hospitalization until up to 7 days after discharge.
The survival rate and survival time from the initial diagnosis of patients with GBS were analyzed using the Cox proportional-hazards model.15 The starting point for these analyses was defined as the date of the initial diagnosis. The end point was defined as either the date of death or December 31, 2019, for those who survived until study termination. We analyzed survival curves affected by factors including age at diagnosis, sex, income level, hospital type, hospitalization period, IVIG, plasmapheresis, and tracheostomy using the Cox proportional-hazards model.
Statistical analysis
All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA). All p-values were two-sided, and p<0.05 was considered statistically significant. Cox regression analysis was used to examine the associations of patient characteristics (age, sex, income level, hospital type, hospitalization period, IVIG, plasmapheresis, and tracheostomy) with physical disability and mortality in patients with GBS.
Ethical considerations
This study was approved by the Institutional Review Board of Gangnam Severance Hospital (approval number: 2020-0438), which waived the requirement to obtain informed consent because all of the data obtained from the NHIS included all personal information anonymized using a strict confidentiality protocol.
RESULTS
Incidence and clinical characteristics of GBS
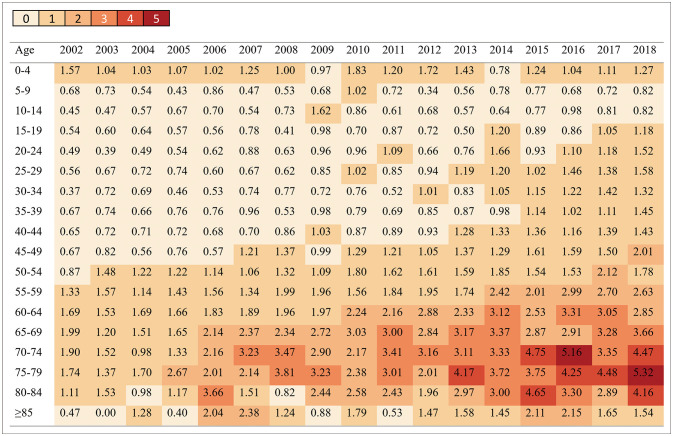
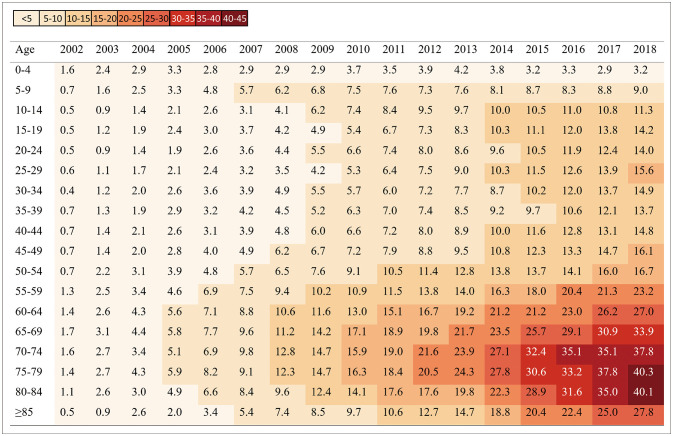
We identified 11,146 patients fulfilling the operational definition for GBS during the 17-year study period (2002–2018). The ratio of males to females was 1.48. Table 1 summarizes the incidence and prevalence of GBS. The age-adjusted incidence rate per 100,000 persons increased steadily from 0.84 in 2002 to 1.68 in 2018, as did the age-adjusted prevalence rate per 100,000 persons, from 0.77 to 15.62. The incidence and prevalence of GBS increased with age and peaked at 70-79 years (Figs. 1 and 2). The number of GBS admissions was higher in summer (3,152 cases, 28%) and spring (2,949 cases, 26%) than in winter (2,694 cases, 24%) and autumn (2,351 cases, 21%) (Supplementary Fig. 1 in the online-only Data Supplement). The 11,146 patients comprised 2,955 (27%), 3,354 (30%), and 3,805 (34%) with low, middle, and high incomes, respectively, and 7,032 patients (63%) who were treated at the referral hospitals. A total of 2,190 (20%) patients had a long hospitalization period. During hospitalization, 4,420 (40%) and 165 (1%) patients were treated with IVIG and plasmapheresis, respectively, while 497 patients (4%) underwent tracheostomy.
Table 1. Annual incidence and prevalence rates of Guillain-Barré syndrome per 100,000 persons at the end of each year in Korea.
| Year | General population | Incidence | Prevalence | ||||
|---|---|---|---|---|---|---|---|
| n | Crude rate (95% CI) | Age-adjusted rate* (95% CI) | n | Crude rate (95% CI) | Age-adjusted rate* (95% CI) | ||
| 2002 | 48,125,745 | 391 | 0.81 (0.73–0.89) | 0.84 (0.75–0.92) | 370 | 0.77 (0.69–0.85) | 0.77 (0.71–0.87) |
| 2003 | 48,308,386 | 410 | 0.85 (0.77–0.93) | 0.84 (0.76–0.93) | 760 | 1.57 (1.46–1.69) | 1.59 (1.47–1.70) |
| 2004 | 48,485,314 | 392 | 0.81 (0.73–0.89) | 0.81 (0.73–0.89) | 1,130 | 2.33 (2.19–2.47) | 2.33 (2.20–2.47) |
| 2005 | 48,683,040 | 419 | 0.86 (0.78–0.94) | 0.85 (0.77–0.93) | 1,517 | 3.12 (2.96–3.27) | 3.07 (2.92–3.23) |
| 2006 | 48,887,027 | 500 | 1.02 (0.93–1.11) | 0.98 (0.89–1.06) | 1966 | 4.02 (3.84–4.20) | 3.89 (3.72–4.07) |
| 2007 | 49,130,354 | 527 | 1.07 (0.98–1.16) | 1.03 (0.94–1.12) | 2,440 | 4.97 (4.77–5.16) | 4.78 (4.59–4.97) |
| 2008 | 49,404,648 | 548 | 1.11 (1.01–1.20) | 1.03 (0.94–1.12) | 2,941 | 5.95 (5.74–6.17) | 5.64 (5.44–5.85) |
| 2009 | 49,656,756 | 630 | 1.27 (1.17–1.37) | 1.21 (1.12–1.31) | 3,496 | 7.04 (6.80–7.27) | 6.62 (6.40–6.84) |
| 2010 | 49,879,812 | 658 | 1.32 (1.22–1.42) | 1.27 (1.17–1.37) | 4,075 | 8.17 (7.91–8.42) | 7.64 (7.40–7.87) |
| 2011 | 50,111,476 | 645 | 1.29 (1.19–1.39) | 1.18 (1.09–1.27) | 4,627 | 9.23 (8.97–9.50) | 8.51 (8.27–8.76) |
| 2012 | 50,345,325 | 669 | 1.33 (1.23–1.43) | 1.20 (1.11–1.29) | 5,198 | 10.32 (10.04–10.61) | 9.40 (9.14–9.65) |
| 2013 | 50,558,952 | 720 | 1.42 (1.32–1.53) | 1.25 (1.16–1.34) | 5,804 | 11.48 (11.18–11.78) | 10.31 (10.04–10.57) |
| 2014 | 50,763,158 | 851 | 1.68 (1.56–1.79) | 1.45 (1.36–1.55) | 6,560 | 12.92 (12.61–13.24) | 11.42 (11.15–11.70) |
| 2015 | 50,951,719 | 849 | 1.67 (1.55–1.78) | 1.42 (1.32–1.52) | 7,290 | 14.31 (13.98–14.64) | 12.44 (12.15–12.73) |
| 2016 | 51,112,972 | 937 | 1.83 (1.72–1.95) | 1.52 (1.43–1.62) | 8,076 | 15.80 (15.46–16.14) | 13.52 (13.23–13.82) |
| 2017 | 51,230,704 | 947 | 1.85 (1.73–1.97) | 1.54 (1.44–1.63) | 8,886 | 17.35 (16.98–17.71) | 14.60 (14.30–14.91) |
| 2018 | 51,300,880 | 1,053 | 2.05 (1.93–2.18) | 1.68 (1.58–1.78) | 9,743 | 18.99 (18.61–19.37) | 15.62 (15.31–15.93) |
*Age-adjusted rate (per 100,000 persons) using the World Health Organization (2000–2025) world standard population.14
CI, confidence interval.
Fig. 1. Incidence of Guillain-Barré syndrome per 100,000 persons by age in Korea, 2002–2018.
Fig. 2. Prevalence of Guillain-Barré syndrome per 100,000 persons by age in Korea, 2002–2018.
Long-term physical disability
We evaluated physical disability in 10,114 patients with GBS who did not have a physical disability recorded before the onset of GBS in the NHIS database (Table 2 and Supplementary Table 2 [in the online-only Data Supplement]). A total of 1,028 (10.2%) patients had physical disabilities after their GBS diagnosis. The disability registration occurred at a median of 0.6 years after the GBS diagnosis. There were 502 (5.0%) patients with moderate physical disability and 526 (5.2%) with severe physical disability. Table 2 indicates that increasing age, long hospitalization period, IVIG, plasmapheresis, and tracheostomy were associated with a higher risk of disability in GBS patients.
Table 2. Independent predictors of mortality and disability in patients with Guillain-Barré syndrome.
| Characteristics | Mortality (n=11,146) | Disability* (n=10,144) | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p | ||
| Age (yr) | |||||||
| Each 10-year increase | 2.02 | 1.95–2.10 | <0.001 | 1.10 | 1.04–1.12 | <0.001 | |
| Sex | |||||||
| Female | 0.55 | 0.49–0.61 | <0.001 | 0.88 | 0.78–1.00 | 0.053 | |
| Male | 1.00 | Reference | 1.00 | Reference | |||
| Income level† | |||||||
| Low | 1.36 | 1.21–1.53 | <0.001 | 1.05 | 0.90–1.22 | 0.535 | |
| Middle | 1.11 | 0.98–1.26 | 0.103 | 1.00 | 0.86–1.16 | 0.968 | |
| High | 1.00 | Reference | 1.00 | Reference | |||
| Hospital type | |||||||
| Referral hospital | 0.83 | 0.75–0.91 | <0.001 | 0.94 | 0.83–1.06 | 0.305 | |
| Nonreferral hospital | 1.00 | Reference | 1.00 | Reference | |||
| Hospitalization period | |||||||
| ≥30 days | 0.99 | 0.88–1.12 | 0.906 | 3.36 | 2.92–3.87 | <0.001 | |
| <30 days | 1.00 | Reference | 1.00 | Reference | |||
| IVIG | |||||||
| Yes | 1.21 | 1.08–1.34 | 0.001 | 1.46 | 1.28–1.68 | <0.001 | |
| No | 1.00 | Reference | 1.00 | Reference | |||
| Plasmapheresis | |||||||
| Yes | 1.02 | 0.69–1.50 | 0.933 | 1.92 | 1.46–2.54 | <0.001 | |
| No | 1.00 | Reference | 1.00 | Reference | |||
| Tracheostomy | |||||||
| Yes | 1.92 | 1.61–2.30 | <0.001 | 2.85 | 2.41–3.38 | <0.001 | |
| No | 1.00 | Reference | 1.00 | Reference | |||
*GBS patients who had no physical disability at the time of GBS diagnosis; †Income levels were classified into three categories: 1) low (poorest 30% of the population), 2) middle (31%–70% of the population), and 3) high (richest 30% of the population).
CI, confidence interval; GBS, Guillain-Barré syndrome; IVIG, intravenous immunoglobulin.
SMR and causes of death
A total of 1,403 (12.6%) patients with GBS (927 males and 476 females) died during the 17 year study period (2002–2018). The median follow-up duration was 6.2 years (interquartile range: 3.0–10.7 years) after the first diagnosis. There were 144 (1.3%) in-hospital deaths, the main cause of which was GBS (n=86, 60%), followed by acute myocardial infarction (n=6, 4%) and pneumonia (n=3, 2%). Additionally, 552 (5.0%) patients had died within 1 year after being diagnosed with GBS. The SMR decreased as each year passed after the diagnosis (Supplementary Table 3 in the online-only Data Supplement). The SMR was 5.63 (95% CI: 5.17–6.12) within 1 year after the diagnosis, 3.99 (3.70–4.30) within 2 years after the diagnosis, 3.16 (2.93–3.39) within 3 years after the diagnosis, 2.83 (2.63–3.03) within 4 years after the diagnosis, and 2.64 (2.46–2.83) within 5 years after the diagnosis.
Table 3 and Supplementary Table 4 (in the online-only Data Supplement) list the causes of death in patients with GBS. The main cause of death for patients with GBS was neurological diseases (SMR: 19.7, 95% CI: 17.8–21.8). Of 381 patients who died from neurological diseases, 298 (78%) died from inflammatory polyneuropathy including GBS (Supplementary Table 5 in the online-only Data Supplement). Causes of death other than colon cancer and mental disorders were also significantly more common in patients with GBS than in the general population.
Table 3. Causes of death among patients with Guillain-Barré syndrome in Korea, 2002–2018.
| Diseases | Observed deaths | Expected deaths | SMR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Total | Male | Female | |
| Total | 1,403 | 927 | 476 | 710.5 | 458.8 | 251.8 | 1.97 (1.87–2.08) | 2.02 (1.89–2.15) | 1.89 (1.72–2.07) |
| Neoplasms (C00–D48) | 302 | 225 | 77 | 210.9 | 151.5 | 59.3 | 1.43 (1.28–1.60) | 1.48 (1.30–1.69) | 1.30 (1.02–1.62) |
| Endocrine, nutritional, and metabolic diseases (E00–E90) | 59 | 30 | 29 | 33.9 | 19.8 | 14.1 | 1.74 (1.33–2.25) | 1.52 (1.02–2.17) | 2.05 (1.37–2.95) |
| Diseases of the nervous system (G00–G99) | 381 | 226 | 155 | 19.3 | 10.4 | 8.9 | 19.75 (17.8–21.8) | 21.77 (19.0–24.8) | 17.39 (14.8–20.4) |
| Diseases of the circulatory system (I00–I99) | 198 | 121 | 77 | 165.4 | 94.9 | 70.5 | 1.20 (1.04–1.38) | 1.27 (1.06–1.52) | 1.09 (0.86–1.37) |
| Diseases of the respiratory system (J00–J99) | 111 | 74 | 37 | 64.3 | 44.3 | 20.0 | 1.73 (1.42–2.08) | 1.67 (1.31–2.10) | 1.85 (1.30–2.55) |
| External causes of morbidity and mortality (V01–Y98) | 90 | 70 | 20 | 67.1 | 50.3 | 16.9 | 1.34 (1.08–1.65) | 1.39 (1.09–1.76) | 1.19 (0.72–1.83) |
| Others (A00–B99, D50–89, F00–F99, H00–H95, K00–K93, L00–L99, M00–M99, N00–N99, O00–O99, P00–P96, Q00–Q99, R00–R99) | 262 | 181 | 81 | 149.7 | 87.6 | 62.1 | 1.75 (1.54–1.98) | 2.07 (1.78–2.39) | 1.31 (1.04–1.62) |
CI, confidence interval; SMR, standardized mortality ratio.
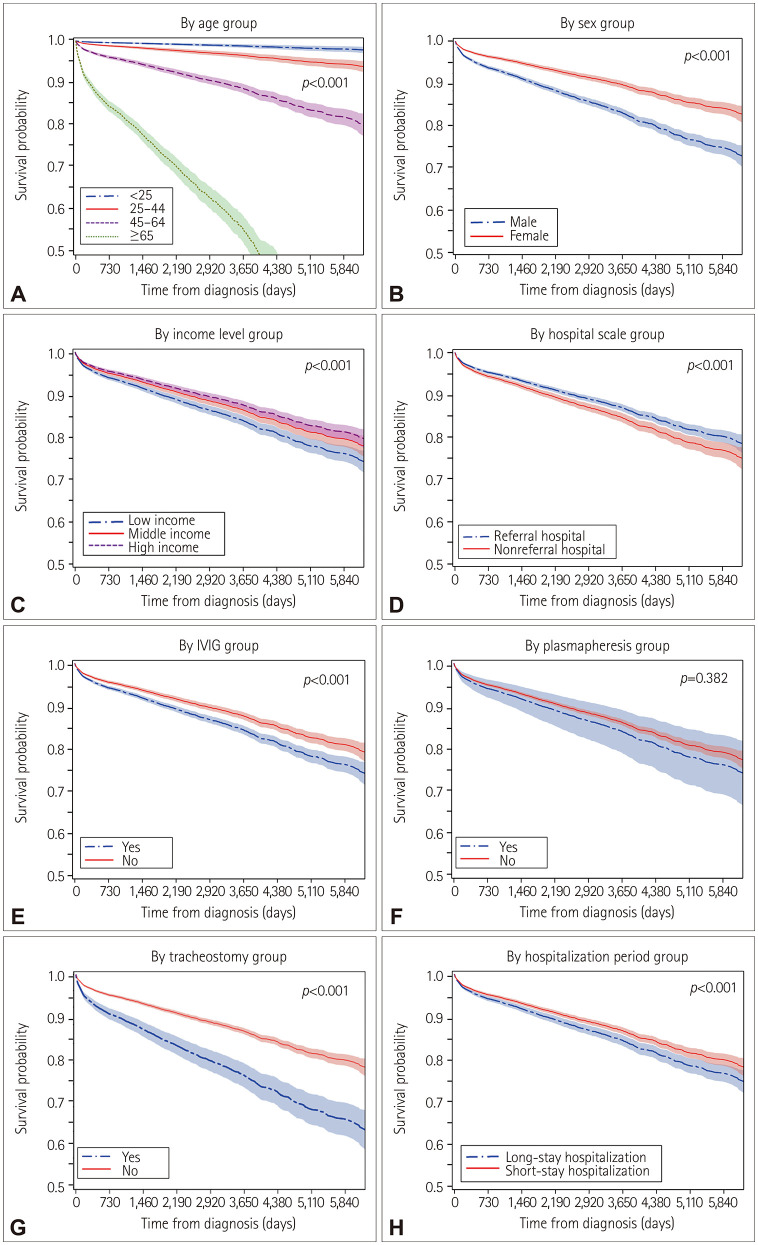
Comparing survival times between groups according to age, sex, income level, hospitalization period, hospital type, IVIG, plasmapheresis, and tracheostomy revealed that the mean survival time was significantly shorter among the elderly, males, patients with a low income, patients treated at a nonreferral hospital, patients treated with IVIG and tracheostomy, and patients with a long hospitalization period (all p<0.001) (Fig. 3). However, survival time was not significantly related to plasmapheresis (p=0.382). Cox regression analysis showed that increasing age, male sex, low income, admission to a nonreferral hospital, and treatment with IVIG and tracheostomy were associated with increased mortality in GBS patients (Table 2).
Fig. 3. Comparison of survival curves from the time of diagnosis to the time of death in patients with Guillain-Barré syndrome reveals significant differences among groups defined by age at diagnosis (A), sex (B), income level (C), hospital type (D), IVIG (E), plasmapheresis (F), tracheostomy (G), and hospitalization period (H) (2002–2018). The mean survival time was significantly shorter in the elderly, males, patients with a low income, patients treated at a nonreferral hospital, patients treated with IVIG and tracheostomy, and patients with a long hospitalization period (log-rank test, p<0.05). IVIG, intravenous immunoglobulin.
DISCUSSION
This study is the first to assess the incidence and prevalence of GBS in the entire Korean population including patients of all ages, as well as the relationships of these metrics with sex, income level, physical disability, mortality, and causes of death. Although there were restrictions in the clinical information regarding GBS in the NHIS database, the accuracy of the operational diagnosis was confirmed as being high since it was based on medical records at a tertiary hospital.
We identified that the age-adjusted incidence and prevalence of GBS in 2018 were 1.68 and 15.62 per 100,000 persons, respectively, in the Korean population. This incidence rate is consistent with those found in other countries (0.59-2.35 per 100,000 persons) (Table 4).3,4 We found that the incidence of GBS was higher in elderly and male patients, which is also a frequent finding in many countries worldwide; however, the biological reasons are unclear.6,16,17,18 The incidence of GBS gradually increased as each year passed during the study period, which is consistent with the results obtained in a Taiwanese population.18 Additionally, we found that the incidence of GBS was not influenced by influenza or influenza vaccination. The incidence of GBS in 2009, when there was an influenza A (H1N1) outbreak, was similar to that in 2008 and 2010, which was also consistent with previous results.9,19 Our results showed that the incidence of GBS peaked in late spring and summer, which is similar to results obtained in northern China.20,21,22 This may be due to the high proportion of acute motor axonal neuropathies in the Korean and Chinese populations, which is caused by prodromal infection (including Campylobacter jejuni infection) in the summer. However, studies of the seasonality of GBS have produced highly heterogeneous results, especially in Western countries, with some studies showing a winter peak,23,24,25 others showing a peak in late summer and autumn,26,27 and still others not finding any seasonal variation.5,28,29
Table 4. Comparison of the incidence rates of GBS in various countries.
| Study location | Data source | Diagnostic criteria | Period | Age (yr) | Cases | Incidence per 100,000 | Reference |
|---|---|---|---|---|---|---|---|
| Victoria, Australia | Medical record systems at teaching hospitals | Not reported | 1980–1984 | ≥15 | 110 | 0.90 | Storey et al.39 |
| Western Australia | Case record search for GBS, polyneuritis, or polyneuropathy codes | Asbury criteria | 1980–1985 | All | 109 | 1.35 | Hankey40 |
| UK | Population-based study using a general-practice research database | Codes for GBS or infective neuritis | 1992–2000 | All | 228 | 1.33 | Hughes et al.41 |
| Southeast England | A voluntary reporting scheme, hospital activity analysis, a contemporary research database, and death certificates | Asbury and Cornblath criteria | 1993 & 1994 | All | 79 | 1.20 | Rees et al.32 |
| Denmark | Danish national patient registry | ICD-8 code for polyradiculitis acuta and ICD-10 code for GBS | 1987–2016 | ≥16 | 2,319 | 1.77 | Levison et al.42 |
| Germany | Nationwide administrative database from reimbursement scheme implementation | ICD-10 code G61.0 | 2003–2005 | All | 4,349 | 1.60–1.89 | Lehmann et al.43 |
| Southwest Greece | Medical records at two referral hospitals | NINCDS | 1989–2001 | All | 105 | 0.99 | Chroni et al.29 |
| USA | Nationwide inpatient sample database | ICD-9-CM code for GBS (357.0) | 2000–2004 | ≥18 | 4,954 | 1.65–1.79 | Alshekhlee et al.36 |
| USA | Vaccine Safety Datalink | ICD-9 code for GBS (357.0) | 2000–2009 | All | 1,619 | 1.72 | Shui et al.16 |
| Finland | Hospital discharge database | Asbury criteria and Poser criteria | 1981–1986 | All | 247 | 0.84 | Kinnunen et al.44 |
| Finland | Finnish Care Register for Health Care | ICD-10 code G61.0 | 2004–2014 | All | 989 | 1.70 | Sipilä et al.45 |
| Italy | Italian network for the study of GBS | Asbury criteria | 2010 & 2011 | ≥18 | 365 | 1.84 | Benedetti et al.46 |
| Lombardy, Italy | Prospective hospital-based survey, in which patients were interviewed and atypical cases were discussed | NINCDS | 1994 & 1995 | All | 109 | 0.92 | Beghi et al.47 |
| Piemonte and Valle d’Aosta, Italy | Piemonte and Valle d’Aosta Register for Guillain-Barré Syndrome | ICD-9 codes 357.0, 357.8, and 357.9 | 1995 & 1996 | All | 120 | 1.36 | Chiò et al.48 |
| Taiwan | National Health Insurance Research Database | ICD-9-CM code for GBS (357.0) | 1997–2011 | All | 5,998 | 1.52–2.31 | Huang et al.18 |
| Jiangsu province, China | Survey | NINCDS | 2008–2010 | All | 441 | 0.59 | Chen et al.5 |
| Western Norway | Hospital records | NINCDS | 1957–1982 | All | 109 | 1.19 | Larsen et al.49 |
| Serbia | Medical records at five tertiary healthcare centers in Serbia | Brighton criteria | 2009–2018 | ≥18 | 640 | 1.07 | Stojanov et al.38 |
| Spain | Retrospective review of national health service | NINCDS | 1985–1997 | >19 | 337 | 0.86 | Cuadrado et al.50 |
| Sweden | Hospital Inpatient Register of the National Board of Health and Welfare in Sweden | ICD-9 code for GBS (357A) | 1978–1993 | All | 2,257 | 1.77 | Jiang et al.28 |
| Quebec and Ontario, Canada | Hospital discharge databases | ICD-9 codes 357.0, 357.8, 357.9, and 375.0 | 1983–1989 | All | 2,333 | 1.51–1.78 | McLean et al.51 |
| Chile | Department of Statistics and Health Information of the Chilean Ministry of Health for all public and private health providers in Chile | ICD-10 code for GBS (G61.0) | 2001–2012 | All | 4,158 | 1.61–2.35 | Rivera-Lillo et al.6 |
| Korea | Medical records at 28 randomly selected hospitals | Brighton criteria | 2008–2010 | All | 245 | 0.63–0.87 | Kim et al.9 |
| Korea | Korean National Health Insurance Service claims data | Hospitalized cases with ICD-10 code for GBS (G61.0) | 2014–2016 | ≥65 | 320 | 4.14–4.16 | Lee et al.8 |
GBS, Guillain-Barré syndrome; ICD, International Classification of Diseases; NINCDS, National Institute of Neurological and Communicative Disorders and Stroke Criteria.
Our study found that 40% of patients received IVIG, whereas only 1% received plasmapheresis. We believe that the proportion of patients treated with IVIG is much higher than 40%, since patients with mild muscle weakness can be treated with IVIG via prescriptions that are not claimed from the NHIS. The use of IVIG can only be claimed in patients with GBS with the following conditions: 1) when walking is difficult without the help of others due to at least moderate muscle weakness in both legs or arms (Medical Research Council scale grades 0–3) or 2) having difficulties in respiration or swallowing related to aspiration. The proportion of patients receiving plasmapheresis was tiny since this is usually only applied to hemodynamically stable patients at major referral hospitals with the requisite equipment and trained medical staff.30 These findings are consistent with a previous report that IVIG is usually the treatment of choice in GBS due to its high availability and convenience.31
Few studies have investigated disabilities in patients with GBS. Our study found that about 10% of people had moderate-to-severe physical disabilities, and 90% had a good functional outcome during the long-term follow-up. The disability registration occurred at a median of 0.6 years after the GBS diagnosis, which was due to disability being recorded no earlier than 0.6 years after the onset of a disease. The rate of good functional outcomes found here is similar to previous results from southeast England (88%), northern Italy (91%), and Nepal (93%),32,33,34 but higher than those found in southwest Greece (75%) and Taiwan (80%).29,35
Our study revealed both short- and long-term mortality rates. The in-hospital mortality rate was 1.3% in the present study, which is lower than those found in the USA and Taiwan.18,36 The 1-year mortality rate was 5.0% in the present study, which is similar to previous results in southeast England (8%), Asian countries (6%), Taiwan (4%), and the Netherlands (3.9%),7,32,35,37 and better than the 6-month mortality rate of 9.7% found in Serbia.38 Our study also revealed the mortality rate over a very long mean observation period of 9.0 years after the diagnosis. Finally, the SMR of patients with GBS had decreased year by year after the diagnosis, but remained significantly high until 5 years after the diagnosis in the Korean population.
The rates of death in all categories were significantly higher in patients with GBS than in the general population, which means overall health was worse for patients with GBS. Among the causes of death, diseases of the nervous system (including GBS) were the most common underlying cause in the Republic of Korea, while other studies have often found immediate causes of death such as pneumonia in patients with GBS.7 This is due to causes of death frequently being recorded as underlying conditions in the Republic of Korea and many of the deaths due to conditions of the nervous system being ascribed to GBS in the Republic of Korea.
This study has identified that the independent predictors of physical disability are age, hospitalization period, IVIG, plasmapheresis, and tracheostomy in Korean patients with GBS. In contrast, the independent predictors of mortality are age, sex, income level, hospital type, the use of IVIG, and tracheostomy. These differences are probably due to mortality being associated with both the severity of GBS and the general factors contributing to mortality in the Korean population, which are being older, male sex, and lower income level (https://kosis.kr/eng/). The poor prognosis associated with treatment was probably due to the severity of GBS in the treated group. For example, the NHIS database contains only claimed prescriptions related to the use of IVIG in patients with severe GBS. Many patients with mild disability—both those treated with IVIG as an unclaimed prescription and those who were not treated—were included in the untreated group in our study. Therefore, the poor prognosis may be due to the disease severity rather than the use of IVIG.
Our study had critical limitations. First, the NHIS database does not provide detailed clinical information, such as on the presence of prodromal infection, NCS results, antiganglioside antibody profiles, or clinical severity. Therefore, we could not determine the subtype of GBS according to NCS results or antiganglioside antibody profiles. Second, considering our use of hospital data, the analyzed sample would have included a small number of patients with cranial-dominant GBS. Third, the proportion of patients treated with IVIG was probably underestimated due to the exclusion of prescriptions that were not claimed from the NHIS.
In summary, the incidence of GBS in Korean patients was 1.7 per 100,000 persons, with a higher incidence in the elderly, male individuals, and during the summer. Approximately 11% of the patients had physical disabilities after the diagnosis of GBS. The in-hospital and long-term (mean: 9 years) mortality rates were 1.3% and 11.0%, respectively.
In conclusion, this first nationwide epidemiological study of patients with GBS covering the entire population of all ages in the Republic of Korea has revealed the seasonality of admissions, disability, and long-term mortality of patients with GBS.
Footnotes
- Conceptualization: Sang-Wook Yi, Young-Chul Choi, Hyung Jun Park.
- Data curation: Sang-Wook Yi.
- Formal analysis: Sang-Wook Yi, Jung Hwan Lee, Ji-Man Hong, Hyung Jun Park.
- Funding acquisition: Hyung Jun Park.
- Supervision: Young-Chul Choi.
- Writing—original draft: Sang-Wook Yi, Hyung Jun Park.
- Writing—review & editing: Sang-Wook Yi, Hyung Jun Park.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2020R1I1A1A01068066).
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2022.18.1.48.
Accuracy of various operational definitions of GBS in Gangnam Severance Hospital
Physical disability in patients with GBS who had no disability at the time of GBS diagnosis
Annual mortality rates among patients with Guillain-Barré syndrome
Causes of death among patients with Guillain-Barré syndrome in the Republic of Korea, 2005–2018
Detailed causes of death in patients who died from neurological diseases
Total numbers of GBS admissions in Korea during 2002–2018 by month. GBS, Guillain-Barré syndrome.
References
- 1.Israeli E, Agmon-Levin N, Blank M, Chapman J, Shoenfeld Y. Guillain-Barré syndrome--a classical autoimmune disease triggered by infection or vaccination. Clin Rev Allergy Immunol. 2012;42:121–130. doi: 10.1007/s12016-010-8213-3. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs BC, Rothbarth PH, van der Meché FG, Herbrink P, Schmitz PI, de Klerk MA, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51:1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 3.McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 4.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Ma F, Zhang J, Chu X, Xu Y. Population incidence of Guillain-Barré syndrome in parts of China: three large populations in Jiangsu province, 2008–2010. Eur J Neurol. 2014;21:124–129. doi: 10.1111/ene.12265. [DOI] [PubMed] [Google Scholar]
- 6.Rivera-Lillo G, Torres-Castro R, Burgos PI, Varas-Díaz G, Vera-Uribe R, Puppo H, et al. Incidence of Guillain-Barré syndrome in Chile: a population-based study. J Peripher Nerv Syst. 2016;21:339–344. doi: 10.1111/jns.12182. [DOI] [PubMed] [Google Scholar]
- 7.van den Berg B, Bunschoten C, van Doorn PA, Jacobs BC. Mortality in Guillain-Barré syndrome. Neurology. 2013;80:1650–1654. doi: 10.1212/WNL.0b013e3182904fcc. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Kang HY, Jung SY, Lee YM. Incidence of Guillain-Barré syndrome is not associated with influenza vaccination in the elderly. Vaccines (Basel) 2020;8:431. doi: 10.3390/vaccines8030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim C, Rhie S, Suh M, Kang DR, Choi YJ, Bae GR, et al. Pandemic influenza A vaccination and incidence of Guillain–Barré syndrome in Korea. Vaccine. 2015;33:1815–1823. doi: 10.1016/j.vaccine.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Choi YC, Oh JW, Yi SW. Prevalence, mortality, and cause of death in Charcot-Marie-Tooth disease in Korea: a nationwide, population-based study. Neuroepidemiology. 2020;54:313–319. doi: 10.1159/000505815. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–728. doi: 10.3346/jkms.2017.32.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong JM, Choi YC, Shin S, Lee JH, Shin HY, Kim SM, et al. Prevalence and socioeconomic status of patients with genetic myopathy in Korea: a nationwide, population-based study. Neuroepidemiology. 2019;53:115–120. doi: 10.1159/000501102. [DOI] [PubMed] [Google Scholar]
- 13.Asbury AK, Arnason BG, Karp HR, McFarlin DE. Criteria for the diagnosis of Guillain-Barré syndrome. Ann Neurol. 1978;3:565–566. doi: 10.1002/ana.410030628. [DOI] [PubMed] [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER) Program. World (WHO 2000–2025) standard [Internet] Bethesda (MD): National Cancer Institute (NCI); [cited 2020 Dec 31]. Available from: https://seer.cancer.gov/stdpopulations/world.who.html. [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methol. 1972;34:187–202. [Google Scholar]
- 16.Shui IM, Rett MD, Weintraub E, Marcy M, Amato AA, Sheikh SI, et al. Guillain-Barré syndrome incidence in a large United States cohort (2000–2009) Neuroepidemiology. 2012;39:109–115. doi: 10.1159/000339248. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Q, Jiang GX, Fredrikson S, Link H, De Pedro-Cuesta J. Incidence of Guillain–Barré syndrome in Sweden 1996. Eur J Neurol. 2000;7:11–16. [PubMed] [Google Scholar]
- 18.Huang WC, Lu CL, Chen SC. A 15-year nationwide epidemiological analysis of Guillain-Barré syndrome in Taiwan. Neuroepidemiology. 2015;44:249–254. doi: 10.1159/000430917. [DOI] [PubMed] [Google Scholar]
- 19.Grave C, Boucheron P, Rudant J, Mikaeloff Y, Tubert-Bitter P, Escolano S, et al. Seasonal influenza vaccine and Guillain-Barré syndrome: a self-controlled case series study. Neurology. 2020;94:e2168–e2179. doi: 10.1212/WNL.0000000000009180. [DOI] [PubMed] [Google Scholar]
- 20.Ho TW, Mishu B, Li CY, Gao CY, Cornblath DR, Griffin JW, et al. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118:597–605. doi: 10.1093/brain/118.3.597. [DOI] [PubMed] [Google Scholar]
- 21.Baoxun Z, Yinchang Y, Huifen H, Xiuqin L. Acute polyradiculitis (Guillain-Barré syndrome): an epidemiological study of 156 cases observed in Beijing. Ann Neurol. 1981;9 Suppl:146–148. doi: 10.1002/ana.410090720. [DOI] [PubMed] [Google Scholar]
- 22.McKhann GM, Cornblath DR, Griffin JW, Ho TW, Li CY, Jiang Z, et al. Acute motor axonal neuropathy: a frequent cause of acute flaccid paralysis in China. Ann Neurol. 1993;33:333–342. doi: 10.1002/ana.410330402. [DOI] [PubMed] [Google Scholar]
- 23.Winner SJ, Evans JG. Age-specific incidence of Guillain-Barré syndrome in Oxfordshire. QJM. 1990;77:1297–1304. doi: 10.1093/qjmed/77.3.1297. [DOI] [PubMed] [Google Scholar]
- 24.Boucquey D, Sindic CJ, Lamy M, Delmée M, Tomasi JP, Laterre EC. Clinical and serological studies in a series of 45 patients with Guillain-Barré syndrome. J Neurol Sci. 1991;104:56–63. doi: 10.1016/0022-510x(91)90216-t. [DOI] [PubMed] [Google Scholar]
- 25.Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barré syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol. 2009;169:382–388. doi: 10.1093/aje/kwn310. [DOI] [PubMed] [Google Scholar]
- 26.Ramírez-Zamora M, Burgos-Ganuza CR, Alas-Valle DA, Vergara-Galán PE, Ortez-González CI. Guillain-Barre syndrome in the paediatric age: epidemiological, clinical and therapeutic profile in a hospital in El Salvador. Rev Neurol. 2009;48:292–296. [PubMed] [Google Scholar]
- 27.Dowling PC, Menonna JP, Cook SD. Guillain-Barré syndrome in greater New York-New Jersey. JAMA. 1977;238:317–318. [PubMed] [Google Scholar]
- 28.Jiang GX, Cheng Q, Link H, de Pedro-Cuesta J. Epidemiological features of Guillain-Barré syndrome in Sweden, 1978–93. J Neurol Neurosurg Psychiatry. 1997;62:447–453. doi: 10.1136/jnnp.62.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chroni E, Papapetropoulos S, Gioldasis G, Ellul J, Diamadopoulos N, Papapetropoulos T. Guillain–Barré syndrome in Greece: seasonality and other clinico-epidemiological features. Eur J Neurol. 2004;11:383–388. doi: 10.1111/j.1468-1331.2004.00799.x. [DOI] [PubMed] [Google Scholar]
- 30.Shahar E. Current therapeutic options in severe Guillain-Barré syndrome. Clin Neuropharmacol. 2006;29:45–51. doi: 10.1097/00002826-200601000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Hughes RAC, Wijdicks EFM, Barohn R, Benson E, Cornblath DR, Hahn AF, et al. Practice parameter: immunotherapy for Guillain–Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736–740. doi: 10.1212/wnl.61.6.736. [DOI] [PubMed] [Google Scholar]
- 32.Rees JH, Thompson RD, Smeeton NC, Hughes RA. Epidemiological study of Guillain-Barré syndrome in south east England. J Neurol Neurosurg Psychiatry. 1998;64:74–77. doi: 10.1136/jnnp.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bersano A, Carpo M, Allaria S, Franciotta D, Citterio A, Nobile-Orazio E. Long term disability and social status change after Guillain-Barré syndrome. J Neurol. 2006;253:214–218. doi: 10.1007/s00415-005-0958-x. [DOI] [PubMed] [Google Scholar]
- 34.Bhagat SK, Sidhant S, Bhatta M, Ghimire A, Shah B. Clinical profile, functional outcome, and mortality of Guillain-Barre syndrome: a five-year tertiary care experience from Nepal. Neurol Res Int. 2019;2019:3867946. doi: 10.1155/2019/3867946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng BC, Chang WN, Chen JB, Chee EC, Huang CR, Lu CH, et al. Long-term prognosis for Guillain-Barré syndrome: evaluation of prognostic factors and clinical experience of automated double filtration plasmapheresis. J Clin Apher. 2003;18:175–180. doi: 10.1002/jca.10066. [DOI] [PubMed] [Google Scholar]
- 36.Alshekhlee A, Hussain Z, Sultan B, Katirji B. Guillain–Barré syndrome: incidence and mortality rates in US hospitals. Neurology. 2008;70:1608–1613. doi: 10.1212/01.wnl.0000310983.38724.d4. [DOI] [PubMed] [Google Scholar]
- 37.Wong AHY, Umapathi T, Shahrizaila N, Chan YC, Kokubun N, Fong MK, et al. The value of comparing mortality of Guillain–Barré syndrome across different regions. J Neurol Sci. 2014;344:60–62. doi: 10.1016/j.jns.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Stojanov A, Berisavac I, Bozovic I, Arsenijevic M, Lukic-Rajic S, Petrovic M, et al. Incidence and mortality rates of Guillain-Barré syndrome in Serbia. J Peripher Nerv Syst. 2020;25:350–355. doi: 10.1111/jns.12412. [DOI] [PubMed] [Google Scholar]
- 39.Storey E, Cook M, Peppard R, Newton-John H, Byrne E. Guillain-Barré syndrome and related conditions in Victorian teaching hospitals 1980–84. Aust N Z J Med. 1989;19:687–693. doi: 10.1111/j.1445-5994.1989.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 40.Hankey GJ. Guillain–Barré syndrome in Western Australia, 1980–1985. Med J Aust. 1987;146:130–133. doi: 10.5694/j.1326-5377.1987.tb120153.x. [DOI] [PubMed] [Google Scholar]
- 41.Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med. 2006;166:1301–1304. doi: 10.1001/archinte.166.12.1301. [DOI] [PubMed] [Google Scholar]
- 42.Levison LS, Thomsen RW, Christensen DH, Mellemkjær T, Sindrup SH, Andersen H. Guillain-Barré syndrome in Denmark: validation of diagnostic codes and a population-based nationwide study of the incidence in a 30-year period. Clin Epidemiol. 2019;11:275–283. doi: 10.2147/CLEP.S199839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann HC, Köhne A, Meyer zu Hörste G, Kieseier BC. Incidence of Guillain-Barré syndrome in Germany. J Peripher Nerv Syst. 2007;12:285. doi: 10.1111/j.1529-8027.2007.00150.x. [DOI] [PubMed] [Google Scholar]
- 44.Kinnunen E, Junttila O, Haukka J, Hovi T. Nationwide oral poliovirus vaccination campaign and the incidence of Guillain-Barré syndrome. Am J Epidemiol. 1998;147:69–73. doi: 10.1093/oxfordjournals.aje.a009369. [DOI] [PubMed] [Google Scholar]
- 45.Sipilä JOT, Soilu-Hänninen M, Ruuskanen JO, Rautava P, Kytö V. Epidemiology of Guillain-Barré syndrome in Finland 2004–2014. J Peripher Nerv Syst. 2017;22:440–445. doi: 10.1111/jns.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benedetti MD, Pugliatti M, D’Alessandro R, Beghi E, Chiò A, Logroscino G, et al. A multicentric prospective incidence study of Guillain-Barré syndrome in Italy. The ITANG study. Neuroepidemiology. 2015;45:90–99. doi: 10.1159/000438752. [DOI] [PubMed] [Google Scholar]
- 47.Beghi E, Bogliun G the Italian GBS Study Group. The Guillain-Barré syndrome (GBS) Ital J Neurol Sci. 1996;17:355–361. doi: 10.1007/BF01999898. [DOI] [PubMed] [Google Scholar]
- 48.Chiò A, Cocito D, Leone M, Giordana MT, Mora G, Mutani R. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology. 2003;60:1146–1150. doi: 10.1212/01.wnl.0000055091.96905.d0. [DOI] [PubMed] [Google Scholar]
- 49.Larsen JP, Kvale G, Nyland H. Epidemiology of the Guillain-Barré syndrome in the county of Hordaland, Western Norway. Acta Neurol Scand. 1985;71:43–47. doi: 10.1111/j.1600-0404.1985.tb03165.x. [DOI] [PubMed] [Google Scholar]
- 50.Cuadrado JI, de Pedro-Cuesta J, Ara JR, Cemillán CA, Díaz M, Duarte J, et al. Guillain-Barré syndrome in Spain, 1985–1997: epidemiological and public health views. Eur Neurol. 2001;46:83–91. doi: 10.1159/000050769. [DOI] [PubMed] [Google Scholar]
- 51.McLean M, Duclos P, Jacob P, Humphreys P. Incidence of Guillain-Barré syndrome in Ontario and Quebec, 1983–1989, using hospital service databases. Epidemiology. 1994:443–448. doi: 10.1097/00001648-199407000-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Accuracy of various operational definitions of GBS in Gangnam Severance Hospital
Physical disability in patients with GBS who had no disability at the time of GBS diagnosis
Annual mortality rates among patients with Guillain-Barré syndrome
Causes of death among patients with Guillain-Barré syndrome in the Republic of Korea, 2005–2018
Detailed causes of death in patients who died from neurological diseases
Total numbers of GBS admissions in Korea during 2002–2018 by month. GBS, Guillain-Barré syndrome.
Data Availability Statement
All data generated or analyzed during the study are included in this published article (and its supplementary information files).