Abstract
Optimal treatment of full‐thickness skin injuries requires dermal and epidermal replacement. To spare donor dermis, dermal substitutes can be used ahead of split‐thickness skin graft (STSG) application. However, this two‐stage procedure requires an additional general anaesthetic, often prolongs hospitalisation, and increases outpatient services. Although a few case series have described successful single‐stage reconstructions, with application of both STSG and dermal substitute at the index operation, we have little understanding of how the physical characteristics of dermal substitutes affects the success of a single‐stage procedure. Here, we evaluated several dermal substitutes to optimise single‐stage skin replacement in a preclinical porcine model. A porcine full‐thickness excisional wound model was used to evaluate the following dermal substitutes: autologous dermal graft (ADG; thicknesses 0.15‐0.60 mm), Integra (0.4‐0.8 mm), Alloderm (0.9‐1.6 mm), and chitosan‐based hydrogel (0.1‐0.2 mm). After excision, each wound was treated with either a dermal substitute followed by STSG or STSG alone (control). Endpoints included graft take at postoperative days (PODs) 7 and 14, wound closure at POD 28, and wound contracture from POD 28‐120. Graft take was highest in the STSG alone and hydrogel groups at POD 14 (86.9% ± 19.5% and 81.3% ± 12.3%, respectively; P < .001). There were no differences in graft take at POD 7 or in wound closure at POD 28, though highest rates of wound closure were seen in the STSG alone and hydrogel groups (93.6% ± 9.1% and 99.8% ± 0.5%, respectively). ADG‐treated wounds demonstrated the least amount of wound contracture at each time point. Increase dermal substitute thickness was associated with worse percent graft take at PODs 14 and 28 (Spearman ρ of −0.50 and −0.45, respectively; P < .001). In this preclinical single‐stage skin reconstruction model, thinner ADG and hydrogel dermal substitutes outperformed thicker dermal substitutes. Both substitute thickness and composition affect treatment success. Further preclinical and clinical studies to optimise this treatment modality are warranted.
Keywords: contraction, dermal substitutes, graft take, single‐stage
1. INTRODUCTION
Full‐thickness injuries to the skin in the setting of burn, trauma, or surgery can be challenging to manage. Although early treatment of these wounds typically involves removal of non‐viable tissues, 1 damage to the deep dermal layers often necessitates transfer of donor dermis and epidermis for skin repair. 2 Skin replacement in the form of a skin graft can replace both dermis and epidermis but transferring more dermis means greater donor site morbidity. Conversely, sequential skin replacement using a dermal substitute followed by a skin graft potentially decreases donor‐site morbidity while maintaining the quality achieved by a thicker skin graft. The resultant two‐stage procedure involves achieving an adequate wound bed and placing a dermal substitute to replace missing dermis, with the second stage of definitive graft placement delayed for 1 to 4 weeks to allow for wound bed preparation. 3 , 4 , 5 This two‐stage procedure has historically resulted in graft survival of up to approximately 87% to 92% in burn patients 6 , 7 and 87% to 95% in patients with chronic non‐healing wounds or large soft tissue defects 8 , 9 as well as satisfactory functional outcomes. 10 , 11
Despite these excellent results, the two‐stage procedure is not without limitations. First, an extra procedure and an extra general anaesthetic is required. Second, the wound remains open in the interceding period as the skin substitute increases vascularity and granulates, which places the wound site at risk for possible infection. 7 Finally, delaying wound closure also prolongs hospital stays in most cases, necessitates multiple additional outpatient clinic visits at the very least, and prolongs time to physical therapy or return to work. Therefore, there remains a need for a single‐stage method for grafting deeper injuries that can accomplish similar degrees of graft survival and long‐term outcomes. A single operation utilising a dermal substitute would also obviate the need for the 1 to 4‐week delay and would spare donor dermis.
Although several clinical case series have described single‐stage techniques with varying degrees of success, 12 , 13 , 14 , 15 , 16 a comprehensive analysis of the various dermal substitutes used clinically based on their physical qualities for wound bed preparation in the context of a single‐stage procedure is lacking. 17 Since dermal substitutes differ in thickness, density, and composition, it is difficult to compare them without taking those factors into account. Furthermore, there is no gold standard dermal substitute to which other substitutes should be compared. In this preclinical study, we assessed the viability of split‐thickness skin grafts (STSGs) applied with a dermal substitute to full‐thickness excisional wounds in a single‐stage fashion using a swine model.
2. MATERIALS AND METHODS
2.1. Animals
Research was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council. The facility's Institutional Animal Care and Use Committee approved all research conducted in this study. The facility where this research was conducted is fully accredited by the AAALAC.
Eight female pigs (Sus scrofa domestica—Yorkshire) approximately 4 to 5 months of age were selected for use. Veterinary staff from our research institute administered and maintained anaesthesia and analgesia in accordance with standard‐of‐care protocols. Briefly, each pig was induced using intramuscular injection of tiletamine‐zolazepam (Telazol, 4‐6 mg/kg) and inhaled isoflurane via facemask. The airway was secured with endotracheal intubation and the pig was maintained throughout surgery with 1% to 3% isoflurane in 100% oxygen. Baseline pain control was given through subcutaneous injection of buprenorphine HCl sustained release (0.12‐0.24 mg/kg) 24 hours prior to surgery and continued for up to 72 hours following surgery if needed for further pain control. For wound assessments after the initial surgery, each pig was sedated with intramuscular ketamine (10‐25 mg/kg) and maintained throughout the assessment with mask anaesthesia (1%‐5% isoflurane in 100% oxygen). Upon completion of the experiment, each animal was humanely euthanised under anaesthesia with FatalPlusIV (0.2 mL/kg).
2.2. Surgeries
Each animal was mechanically shaved and 6‐cm‐diameter circles were tattooed on the bilateral dorsolateral aspects of each animal. Allotting for 4 cm spacing between sites and 2 cm distance from the spine, a total of 10 locations were used per animal. Two additional 6‐cm circular areas were identified and tattooed on each pig to serve as a control site for normal skin growth during the course of the experiment (growth control). Full‐thickness excisional wounds down to fascia were then created at each of the planned sites inside the tattoo, excluding the growth control (Figure 1A). Autologous STSGs were harvested with a pneumatic dermatome (Zimmer Surgical Inc., Dover, OH) adjusted to 12 of 1000 in. (approximately 0.3 mm) thickness to mimic an intermediate‐thickness STSG. After wound excision, treatments were randomised by location to control for anatomic positional effects and then applied. STSGs were then cut to fit, applied to each wound, and secured with 3‐0 nylon sutures (Figure 1B). Bolsters of Xeroform and cotton gauze were then created to further secure the grafts and ensure uniform contact with the wound bed (Figure 1C). Bolsters and dorsum of the animals were then covered with Ioban (3M, Maplewood, MN), a fabric vest, and stockinet to provide additional stabilisation.
FIGURE 1.
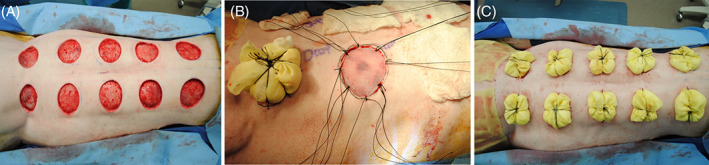
Depiction of the porcine full‐thickness excisional model used in these experiments. On the date of surgery, full‐thickness wounds were created using sharp excision (A). Wounds were then dressed with a dermal substitute and split‐thickness skin graft (STSG) or STSG alone (B), with grafts secured to wound edges using suture. Wounds were then bolstered using Xeroform and gauze for protection (C)
2.3. Treatment arms
To determine the dermal substitute that resulted in optimal graft take after a single‐stage procedure, multiple dermal substitutes of different thicknesses were compared. The dermal substitutes chosen for use in this study are as follows: Integra (Integra LifeSciences; Plainsboro Township, NJ), Alloderm RTM (BioHorizons Implant Systems; Birmingham, Alabama), and autologous dermal graft (ADG). An additional treatment arm utilising hydrogel (see below for hydrogel construction techniques) was included as a means of assessing the impact of the interposition of a thin avascular material between the graft and tissue bed. Hydrogels were not intended to serve as true dermal substitutes as they lack the structural components of a substitute that can impact wound contracture and healing. The purpose of the hydrogel was to interpose an avascular layer of known thickness between the graft and wound bed to attempt to assess the independent impact of substitute thickness (rather than thickness and structural composition) on graft take and wound healing. Thicknesses of each of these substitutes and number of wounds treated with each substitute can be found in Table 1.
TABLE 1.
Description of treatments by substitute thickness and count
| Comparison of dermal substitutes | n | Thickness (mm) | Mean thickness (mm) |
|---|---|---|---|
| None (STSG alone) | 8 | 0.0 | 0 |
| ADG | 23 | 0.3 | 0.3 |
| Alloderm | 7 | 0.9 to 1.6 | 1.25 |
| Integra 0.4 mm | 6 | 0.4 | 0.4 |
| Integra 0.8 mm | 8 | 0.8 | 0.8 |
| Hydrogel | 8 | 0.1 to 0.2 | 0.15 |
| Comparison of ADG thicknesses | n | ||
|---|---|---|---|
| None (STSG alone) | 2 | ||
| 0.15 mm | 4 | ||
| 0.30 mm | 8 | ||
| 0.45 mm | 2 | ||
| 0.60 mm | 2 |
Abbreviations: ADG, acellular dermal graft; STSG, split‐thickness skin graft.
2.4. Hydrogel preparation
The hydrogels used in this study were constructed using a chitosan solution at our facility. Briefly, an aqueous 2.5% (w/v) chitosan solution was prepared by dissolving low molecular weight chitosan (Sigma, St. Louis; viscosity 20.0 cps) in 0.1 M acetic acid. This solution was then dialyzed extensively against 0.01 M acetic acid for 2 days. The dialyzed chitosan solution was lyophilised to dryness and redissolved in sterilised 0.05 M acetic acid at a final concentration of 3% (w/v) (pH 5.8) and used to prepare the gels. Hydrogels were prepared by mixing 850 μL of this solution with 150 μL of sterilised 1 M dibasic potassium orthophosphate (KH2PO4, Sigma). Hydrogels were placed in a 37°C incubator for 30 minutes to initiate gelation and then frozen at −80°C overnight. The frozen gels were then lyophilised for 48 hours to complete dryness. Thickness varied but was typically between 0.10 and 0.20 mm at the time of application onto the wound bed.
2.5. Study design
Prior to comparing dermal substitutes directly, we assessed varying thicknesses of ADG in the single‐stage model. The purpose of this pilot experiment was to identify an autologous “baseline” to serve as an additional benchmark to which non‐autologous substitutes could be compared. An initial pilot experiment using two animals was performed to identify the ideal ADG thickness to use. In this pilot, STSGs (thickness of 0.12 in.) were harvested from each animal using a standard dermatome (Zimmer, Warsaw, Indiana). Next, sequential tangential excisions of the underlying autologous dermis were performed at a variety of thicknesses: 0.15, 0.3, 0.45, and 0.60 mm. One wound on each animal (n = 2) was treated with STSG alone without a dermal substitute to serve as a control. This pilot was carried out for 28 days before animals were euthanised. The primary outcomes of this study were graft take and wound closure (see below). In selecting the optimal ADG thickness, consideration was given to both the outcomes of this pilot study and also the thickness of the other dermal substitutes to be tested. Closely matching the thickness of the ADG to the thicknesses of the other dermal substitutes would help more directly compare the impact of the composition of the substitute on the outcomes measured.
After identifying the optimal ADG thickness, the main experiment comparing the dermal substitutes and hydrogel listed above was conducted using six additional swine. To provide more robust study power, the STSG, ADG, and growth control data taken from the pilot study were included in the overall analysis as well. Two thicknesses of Integra were tested separately: 0.4 and 0.8 mm. Alloderm thicknesses ranged from 0.9 to 1.6 mm (per product description) but were not independently measured prior to application. Hydrogel thicknesses were as described previously: 0.10‐0.20 mm. In this study, the animals were followed for 120 days. Wound assessments were performed on postoperative days (POD) 7, 14, 28, 60, 90, and 120. Assessments included high‐resolution digital photographs of each wound (Nikon D3000+AF‐S Nikkor objective) and planimetry measurements (SilhouetteStar from Aranz Medical; Christchurch, New Zealand). A schematic detailing the study timeline could be found in Supplemental Figure 1.
2.6. Percent graft take
SilhouetteStar images were collected during each assessment from each wound and traced with SilhouetteConnect software to determine total wound area and area of graft take (assessed as area of healthy‐appearing STSG). Percent graft take for each time point was then calculated as area of graft take divided by total wound area (both measured from the same assessment time point) multiplied by 100.
2.7. Wound contraction
The “contraction index” of each wound was taken at each time point and normalised to both the initial size of the wound at day 7 and to the growth control in the following fashion: ([day X wound area]/[day X growth control area])/([day 7 wound area]/[day 7 growth control area]). Controlling at each time point for the change in growth control allowed us to isolate the change in wound size secondary to contracture alone. The structure of this variable is such that higher contraction index percentages indicate less contraction, while lower contraction index percentages indicate more contraction.
2.8. Statistical analyses
All data analysis was conducted using SPSS v 24. (IBM; Armonk, New York). Continuous variables were reported as mean and SD. Treatment groups were compared using an analysis of variance (ANOVA) test at each measured time point. Pair‐wise comparisons were performed using the Bonferroni method. No longitudinal “over time” comparisons between treatment groups were performed, though such comparisons were performed within the same treatment group. Associations between dermal substitute thickness and outcome variables were performed using Spearman's correlation. As certain dermal substitutes were available in a “range” of thicknesses, the mean thickness was calculated as the mean value of that range (Table 1). Significance for all tests was set at P < .05.
3. RESULTS
3.1. ADG thickness pilot study
Percent graft take at day 14, percent of wound closure achieved at day 28, and contracture at day 28 are presented in Figure 2. At day 14 (Figure 2A), there was no significant difference in percent graft take between any of the treatment groups (overall P = .67), though mean percent take did appear to decrease as ADG thickness increased (0.15 mm: 90.9% ± 8.0%; 0.30 mm: 84.5% ± 24.6%; 0.45 mm: 75.3% ± 34.9%; and 0.60 mm: 72.6% ± 30.7%). Similarly, while there were no statistically significant differences between treatment groups in wound closure at day 28 (Figure 2B; P = .81), mean percent closure decreased with increasing ADG thickness (0.15 mm: 75.1% ± 29.9%; 0.30 mm: 53.4% ± 41.2%; 0.45 mm: 46.8% ± 66.2%; and 0.60 mm: 46.6% ± 65.9%). Cumulatively, ADG thicknesses of 0.15 and 0.30 mm performed most similarly to the STSG control. Of these two thicknesses, 0.30 mm ADG thickness was selected over the 0.15 mm ADG for continued use in the study as the planned thicknesses of the other dermal substitutes were all 0.4 mm or greater and matching thickness as closely as possible would help attribute any differences in outcomes to graft composition rather than graft thickness alone.
FIGURE 2.
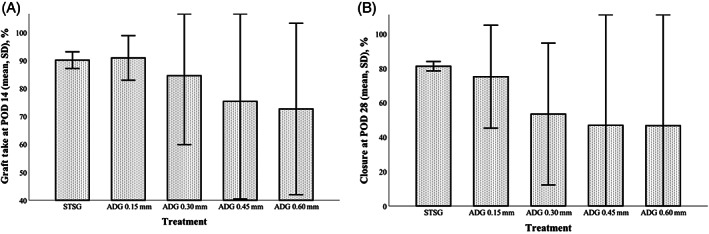
Results of the autologous dermal graft (ADG) pilot study. (A) Graft take at postoperative day (POD) 14; (B) and wound closure at POD 28. Thinner ADG constructs appeared to most closely approximate results seen from the split‐thickness skin graft (STSG) controls. Graphs represent mean and SD
3.2. Graft take and wound closure
Graft take was assessed at day 7 and day 14 (Figure 3A). There were no differences between groups in percent graft take at POD 7 (P = .63). STSG alone (n = 8) had the highest percent take (81.8% ± 15.4%), followed by hydrogel (n = 8; 79.5% ± 10.3%) and Integra 0.4 mm (n = 6; 77.9% ± 8.8%). However, there was a significant difference overall in percent take at POD 14 (P < .001), with both STSG alone and hydrogel demonstrating higher percent graft take (86.9% ± 19.5% and 81.3% ± 12.3%, respectively) when compared to Alloderm (n = 7; 32.4% ± 24.4%, P = .001 and P = .006, respectively) and Integra 0.8 mm (n = 8; 42.4% ± 20.5%, P = .012 and P = .045, respectively). In addition, Integra 0.4 mm (77.4% ± 27.1%) demonstrated higher percent graft take than Alloderm (P = .031).
FIGURE 3.
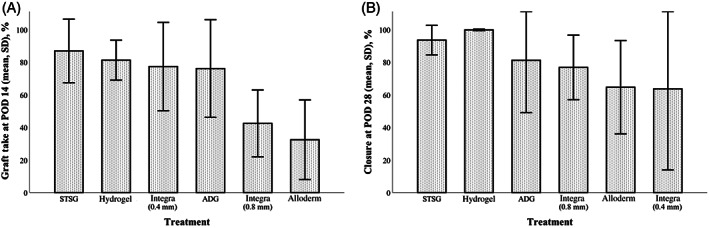
Comparison of dermal substitutes with regard to graft take at postoperative day 14 (A) and wound closure at POD 28 (B). Autologous dermal graft (ADG) and hydrogel constructs most closely approximated graft take and closure rates seen in the split‐thickness skin graft (STSG) control group. Graphs represent mean and SD
Despite differences in graft take at day 14, there were no differences across groups in percentage wound closure at day 28 (P = .10; Figure 3B). The highest rates of wound closure were seen in the STSG alone (93.6% ± 9.1%) and hydrogel (99.8% ± 0.5%) groups, while the lowest rates of wound closure were seen in the Integra 0.4 mm (63.7% ± 49.7%) and Alloderm (64.7% ± 28.7%) groups.
3.3. Wound contracture
The contraction index of each treatment arm was compared at POD 28, 60, 90, and 120 (Figure 4). Higher contraction indices indicated less wound contraction while lower contraction indices indicated more extensive wound contraction (as these percentages represented the area of the wound at the time measured relative to the area of the wound at baseline). There were significant differences between groups at day 28 (P = .001) and day 60 (P = .007). At POD 28, ADG (n = 23) had a significantly higher contraction index (72.3% ± 12.9%) than Integra 0.8 mm (55.6% ± 11.0%, P = .001) and hydrogel (61.3% ± 9.6%, P = .034). At day 60, ADG maintained a significantly higher contraction index (ie, less wound contraction overall) relative to Integra 0.8 mm (60.6% ± 15.0% vs 40.9% ± 10.0%, P = .005) though not hydrogel (P = .36). At day 120, the greatest degree of wound contraction (ie, lowest contraction index scores) was seen in the Alloderm (42.4% ± 14.7%) and Integra 0.8 mm (38.5% ± 16.8%) groups. The ADG group demonstrated the least amount of wound contraction (contraction index of 58.0% ± 16.2%).
FIGURE 4.
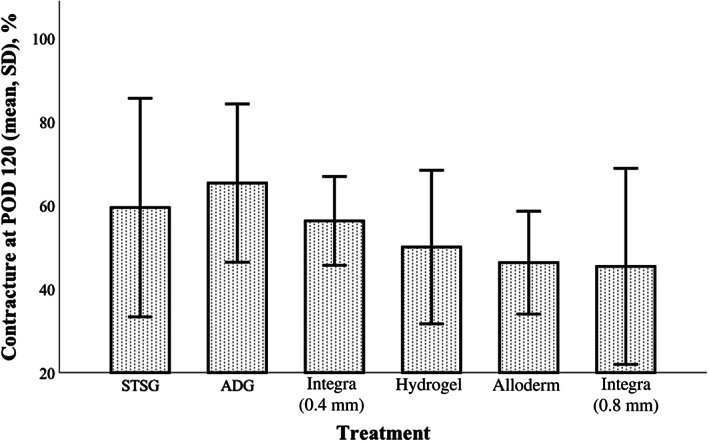
Comparison of dermal substitutes with regard to wound contraction. Higher contraction index scores represent less contraction (100% = no wound contraction occurred). Autologous dermal graft (ADG) and hydrogel again most closely resembled split‐thickness skin graft (STSG). Graphs represent mean and SD
3.4. Correlations between thickness and wound healing
Correlations were made between the mean thickness of each treatment used and the outcome variables collected in this study (Table 2). No correlation was seen between contraction index and the thickness of treatment deployed. Percent take at POD 14 and wound closure at POD 28 both demonstrated moderate correlations with mean thickness (−0.50 and −0.45, respectively; both P < .001), with increasing thickness associated with worse percent graft take and wound closure.
TABLE 2.
Correlations between outcomes measured and mean thickness of dermal substitute
| Outcome correlated | Spearman's ρ | P‐value |
|---|---|---|
| Percent take, POD 7 | − 0.12 | .34 |
| Percent take, POD 14 | − 0.5 | <.001 |
| Percent wound closure, POD 28 | − 0.45 | <.001 |
| Contraction index, POD 28 | − 0.2 | .13 |
| Contraction index, POD 60 | − 0.28 | .06 |
| Contraction index, POD 90 | − 0.24 | .10 |
| Contraction index, POD 120 | − 0.24 | .10 |
Abbreviation: POD, postoperative day.
4. DISCUSSION
Our study examined various dermal substitutes in a single‐stage skin reconstruction procedure using an excisional wound swine model. The control treatment for this experiment was STSG placed directly onto a recently excised wound, with the expectation that rates of graft take and wound closure in this group would be close to 100% given that the wound bed was vascular. ADGs of various thicknesses were used as a gold standard dermis to which other commercially available dermal substitutes could be compared. We found that thinner ADG thicknesses placed on the wound bed beneath the STSG performed similarly to STSG alone, while thicker ADG substitutes demonstrated worse graft take. Among the various treatments applied, ADG, hydrogel, and Integra 0.4 mm showed the highest rates of graft take at POD 14, and ADG and hydrogel showed highest rates of wound closure at POD 28. The ADG and hydrogel treatment groups also showed the lowest rates of wound contraction by POD 120. Finally, there were moderate inverse correlations between the dermal substitute thickness and percent graft take at day 14 and percent wound closure at day 28, though substitute thickness did not correlate with wound contraction measured at any timepoint or with initial percent take at POD 7. ADG was the thinnest dermal substitute and hydrogel was the thinnest treatment overall that were used, so these correlation findings are likely driven by the performance of these two substitutes at POD 14 and 28. Cumulatively, our data suggest that single‐stage procedures may be viable in the setting of a thinner allogeneic dermal substitute or a dermal substitute containing autologous tissue.
The single‐stage procedure hinges on the ability of a graft to survive during a period of relative ischaemia. Our study evaluated two variables that affected time to revascularisation of the graft: dermal substitute thickness and the underlying regenerative potential of the substitute. Prior studies have demonstrated that the vascularisation of an acellular dermal substitute (with minimal inherent regenerative potential) is substantially slower than the neovascularisation of an STSG. 18 Using microscopy, Greenwood et al assessed blood flow through Integra applied to a wound bed in anticipation of a two‐stage procedure and found that convincing blood flow through the dermal substitute was not present until day 23, at which time an STSG was applied. 19 Similarly, Fourman et al used laser Doppler imaging and indocyanine green angiography to show that perfusion peaked at 21 days after Integra placement on a full‐thickness wound. 20 Our data specifically evaluating Integra as a dermal substitute in a single‐stage procedure reflect this slow time to neovascularisation as thinner Integra (0.4 mm) had more graft take at POD 14 as compared to thicker Integra (0.8 mm). While we did not measure rates of revascularisation, tissue survival and wound coverage are reasonable surrogates for this endpoint and our data are therefore consistent with prior findings.
Our data comparing various thicknesses of ADG suggested that both variables, thickness and substitute composition, must be accounted for independently when considering the relationship between rate of revascularisation and graft survival. One reason we believe ADG performed so well in our experiment was that the revascularisation process for ADG was inherently different from other dermal substitutes. 18 , 21 , 22 , 23 Since ADGs contained intact blood vessels, revascularisation through an ADG required two separate inosculation events (one at the wound bed‐ADG interface and the other at the ADG‐STSG interface) but no new vessel growth. Acellular substitutes, conversely, would require the regrowth of blood vessels through the entirety of the substitute while the graft survived on imbibition from the wound bed or inosculation from the lateral wound edges. As inosculation of grafts occurs much more rapidly than neovascularisation, 19 we expected revascularisation of the STSG placed over ADG to occur more rapidly than an STSG placed over an acellular matrix. However, analysis of our ADG pilot suggested that this assumption of healing may not be accurate. Thicker ADGs were associated with worse graft take than thinner ADGs, despite the fact that the thickness of the ADG should have no impact on the inosculation events at either interface. A theoretical benefit to thicker ADGs would be improved contraction rates during the maturation phase of wound healing, but our pilot study did not carry the ADG comparison study past POD 28. Therefore, we are unable to definitely determine an “optimal” thickness of ADG to use, but our data does demonstrate that both substitute thickness and composition can impact the success of the overlying STSG in a single‐stage procedure.
Clinical case series thus far support the use of thinner substitutes for single‐stage procedures. Rudnicki et al recently reported the results of a case series of 13 sites subjected to a single‐stage procedure in which an STSG meshed in a 1:1 ratio was applied over a perforated single‐layer Integra wound matrix (0.4 mm in 11 cases, 0.8 mm in two cases). Of these wounds, 10 of 13 were secondary to contraction release while 3 of 13 were secondary to the primary excision of an acute burn. The authors reported a mean graft take of 86.2% and functional acceptable outcomes. 14 Similarly, Gabriel et al applied a 1:1.5 meshed STSG to a perforated single‐layer Integra wound matrix in 20 consecutive patients with soft tissue defects secondary to trauma or wide debridement of an ulcer or tumour. Here, the authors reported an excellent mean graft take of 98.3% and average time to graft take of 5.6 days. 24 Importantly, both groups reported that negative pressure wound dressings (NPWDs) were applied to the surface of each STSG to help prevent the accumulation of seroma or hematoma formation. In our study, each wound was bolstered but no NPWD was applied. NPWD has been demonstrated to improve rates of endothelial cell migration within acellular dermal matrices, resulting in improved dermal regeneration when compared to the use of an acellular dermal matrix alone. 25 Thus, the difference between the results of these clinical series utilising Integra and our results with Integra may be at least partially due to the application of the NPWD.
As the chitosan‐based hydrogels used in this study carried no intrinsic properties to facilitate wound bed development or revascularisation, the relatively positive outcomes seen with applying an STSG to the hydrogel are likely entirely secondary to the thin and porous nature of the hydrogel construct. A potential limitation to the use of the gels as designed in our study is the relative fragility of the construct, particularly in the context of our study design. Each gel was covered with an STSG but then protected with a bolster, which was secured to the surrounding skin. The use of a thicker chitosan construct was not possible as the force of the bolster would compress the gel, causing extrusion from the wound edges and a likely irregular layer beneath the graft. However, a significant potential benefit to using chitosan‐based gels is that these gels can be used as vehicles to modulate the wound bed. Multiple groups have demonstrated that these fabricated chitosan gels can be successfully impregnated with antibiotics, 26 growth factors, 27 exosome elements, 28 and stem cells, 29 all of which can act on the wound bed and assist with wound healing and angiogenesis. Future studies to determine optimal delivery methods and hydrogel composition are certainly warranted, but this field represents a promising alternative to the conventional acellular dermal substitutes currently employed in clinical practice.
There are a number of important limitations to our study. First, our model is an imperfect replica of the clinical situation in which a single‐stage procedure might be employed. The excisional wounds were created and grafted on the same day in a sterile fashion, with STSG placed directly onto a vascularised wound bed. As evidenced in our data, the STSG control performed extremely well despite lacking the majority of a dermal layer. A more appropriate comparison would be to apply a full‐thickness skin graft (FTSG), STSG, and STSG with dermal substitute to wound beds of borderline viability, but this technique would be nearly impossible to standardise. Additionally, the standard of care for management of these wounds in practice remains a two‐stage procedure. Although this study assessed the feasibility of a single‐stage procedure, inclusion of a two‐stage study group in any study directly comparing outcomes following single‐stage reconstruction to the standard of care would be mandatory. Second, and related to the first point, our study utilised excisional injuries rather than burn injuries. The purpose of this was to standardise the depth of injury and remaining healing potential of the wound bed. Sequential burns, even when using a standardised model, could have introduced variable zones of coagulation or stasis that impacted healing. Therefore, our study may not be immediately generalisable to a burn setting, and further studies using validated burn models are warranted. Third, as this was a pilot study intent on gathering preliminary data, our sample size and statistical power are low. Further studies using more well‐powered models are indicated, particularly to test thinner dermal substitutes. Finally, our endpoints included only measurements of graft take and wound contraction and did not include assessments of functionality. Although we would expect functionality to be similar across all groups given that the same depth of STSG was used, future studies should investigate functionality comparisons between wounds grafted with FTSG vs the STSG/dermal substitute combination.
In conclusion, this study demonstrated that a single‐stage method that utilises dermal substitutes in the treatment of deep skin injuries is feasible. Our use of various dermal substitutes in a single‐stage procedure using a full‐thickness excisional wound model in swine demonstrated that the thinner ADG and hydrogel substitutes outperformed thicker dermal substitutes. Although this likely reflects the relatively minimal distance between the wound bed and STSG (in the case of the hydrogel) and both thickness and physiologic composition (ADG), both thickness and composition likely contribute to the success of a single‐stage procedure. Further preclinical and clinical studies are warranted to optimise the type and thickness of dermal substitute employed, identify the optimal wound that would benefit from a single‐stage procedure, and determine additional adjuncts (such as NPWD therapy or the application of exogenous nutrients) to improve performance of single‐stage procedures.
CONFLICT OF INTERESTS
The authors declare no potential conflict of interest. The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, DoD, or the U.S. Government.
Supporting information
Figure S1 Supporting Information
Kemp Bohan PM, Cooper LE, Fletcher JL, et al. Impact of dermal matrix thickness on split‐thickness skin graft survival and wound contraction in a single‐stage procedure. Int Wound J. 2022;19(2):370–379. 10.1111/iwj.13637
Funding information US Army Institute of Surgical Research, Grant/Award Number: G_002_2015_USAISR
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cope O, Langohr JL, Moore FD, Webster RC. Expeditious care of full‐thickness burn wounds by surgical excision and grafting. Ann Surg. 1947;125(1):1‐22. [PMC free article] [PubMed] [Google Scholar]
- 2. Navsaria HA, Kangesu T, Manek S, Green CJ, Leigh IM. An animal model to study the significance of dermis for grafting cultured keratinocytes on full thickness wounds. Burns. 1994;20(suppl 1):S57‐S60. [DOI] [PubMed] [Google Scholar]
- 3. Moiemen NS, Vlachou E, Staiano JJ, Thawy Y, Frame JD. Reconstructive surgery with Integra dermal regeneration template: histologic study, clinical evaluation, and current practice. Plast Reconstr Surg. 2006;117(suppl 7):160s‐174s. [DOI] [PubMed] [Google Scholar]
- 4. Stern R, McPherson M, Longaker MT. Histologic study of artificial skin used in the treatment of full‐thickness thermal injury. J Burn Care Rehabil. 1990;11(1):7‐13. [DOI] [PubMed] [Google Scholar]
- 5. Moiemen NS, Staiano JJ, Ojeh NO, Thway Y, Frame JD. Reconstructive surgery with a dermal regeneration template: clinical and histologic study. Plast Reconstr Surg. 2001;108(1):93‐103. [DOI] [PubMed] [Google Scholar]
- 6. Heimbach DM, Warden GD, Luterman A, et al. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24(1):42‐48. [DOI] [PubMed] [Google Scholar]
- 7. Muangman P, Deubner H, Honari S, et al. Correlation of clinical outcome of integra application with microbiologic and pathological biopsies. J Trauma. 2006;61(5):1212‐1217. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SA, Beers RJ, Lentz CW. Use of acellular dermal replacement in reconstruction of nonhealing lower extremity wounds. J Burn Care Res. 2011;32(1):124‐128. [DOI] [PubMed] [Google Scholar]
- 9. Richardson MA, Lange JP, Jordan JR. Reconstruction of full‐thickness scalp defects using a dermal regeneration template. JAMA Facial Plast Surg. 2016;18(1):62‐67. [DOI] [PubMed] [Google Scholar]
- 10. Frame JD, Still J, Lakhel‐LeCoadou A, et al. Use of dermal regeneration template in contracture release procedures: a multicenter evaluation. Plast Reconstr Surg. 2004;113(5):1330‐1338. [DOI] [PubMed] [Google Scholar]
- 11. Branski LK, Herndon DN, Pereira C, et al. Longitudinal assessment of Integra in primary burn management: a randomized pediatric clinical trial. Crit Care Med. 2007;35(11):2615‐2623. [DOI] [PubMed] [Google Scholar]
- 12. Burd A, Wong PS. One‐stage Integra reconstruction in head and neck defects. J Plast Reconstr Aesthet Surg. 2010;63(3):404‐409. [DOI] [PubMed] [Google Scholar]
- 13. Koenen W, Felcht M, Vockenroth K, Sassmann G, Goerdt S, Faulhaber J. One‐stage reconstruction of deep facial defects with a single layer dermal regeneration template. J Eur Acad Dermatol Venereol. 2011;25(7):788‐793. [DOI] [PubMed] [Google Scholar]
- 14. Rudnicki PA, Purt B, True D, Siordia H, Lohmeier S, Chan RK. Single‐stage composite skin reconstruction using a dermal regeneration template. Plast Reconstr Surg Glob Open. 2020;8(2):e2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demiri E, Papaconstantinou A, Dionyssiou D, Dionyssopoulos A, Kaidoglou K, Efstratiou I. Reconstruction of skin avulsion injuries of the upper extremity with integra(®) dermal regeneration template and skin grafts in a single‐stage procedure. Arch Orthop Trauma Surg. 2013;133(11):1521‐1526. [DOI] [PubMed] [Google Scholar]
- 16. Lv Z, Yu L, Wang Q, Jia R, Ding W, Shen Y. Dermal regeneration template and vacuum sealing drainage for treatment of traumatic degloving injuries of upper extremity in a single‐stage procedure. ANZ J Surg. 2019;89(7–8):950‐954. [DOI] [PubMed] [Google Scholar]
- 17. Widjaja W, Tan J, Maitz PKM. Efficacy of dermal substitute on deep dermal to full thickness burn injury: a systematic review. ANZ J Surg. 2017;87(6):446‐452. [DOI] [PubMed] [Google Scholar]
- 18. Frueh FS, Menger MD, Lindenblatt N, Giovanoli P, Laschke MW. Current and emerging vascularization strategies in skin tissue engineering. Crit Rev Biotechnol. 2017;37(5):613‐625. [DOI] [PubMed] [Google Scholar]
- 19. Greenwood J, Amjadi M, Dearman B, Mackie I. Real‐time demonstration of split skin graft inosculation and integra dermal matrix neovascularization using confocal laser scanning microscopy. Eplasty. 2009;9:e33. [PMC free article] [PubMed] [Google Scholar]
- 20. Fourman MS, Phillips BT, Fritz JR, et al. Laser‐assisted indocyanine green dye angiography accurately predicts the split‐thickness graft timing of integra artificial dermis. Ann Plast Surg. 2014;73(2):150‐155. [DOI] [PubMed] [Google Scholar]
- 21. Frueh FS, Später T, Körbel C, et al. Prevascularization of dermal substitutes with adipose tissue‐derived microvascular fragments enhances early skin grafting. Sci Rep. 2018;8(1):10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meruane MA, Rojas M, Marcelain K. The use of adipose tissue‐derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast Reconstr Surg. 2012;130(1):53‐63. [DOI] [PubMed] [Google Scholar]
- 23. Qi Y, Dong Z, Chu H, et al. Denatured acellular dermal matrix seeded with bone marrow mesenchymal stem cells for wound healing in mice. Burns. 2019;45(7):1685‐1694. [DOI] [PubMed] [Google Scholar]
- 24. Gabriel A, Wong W, Gupta S. Single‐stage reconstruction for soft tissue defects: a case series. Ostomy Wound Manage. 2012;58(6):30‐32. [PubMed] [Google Scholar]
- 25. González Alaña I, Torrero López JV, Martín Playá P, Gabilondo Zubizarreta FJ. Combined use of negative pressure wound therapy and Integra® to treat complex defects in lower extremities after burns. Ann Burns Fire Disasters. 2013;26(2):90‐93. [PMC free article] [PubMed] [Google Scholar]
- 26. Liang Y, Chen B, Li M, He J, Yin Z, Guo B. Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromolecules. 2020;21(5):1841‐1852. [DOI] [PubMed] [Google Scholar]
- 27. Li Q, Cui J, Huang H, et al. IGF‐1C domain‐modified chitosan hydrogel accelerates cutaneous wound healing by promoting angiogenesis. Future Med Chem. 2020;12(13):1239‐1251. [DOI] [PubMed] [Google Scholar]
- 28. Nooshabadi VT, Khanmohamadi M, Valipour E, et al. Impact of exosome‐loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J Biomed Mater Res A. 2020;108(11):2138‐2149. [DOI] [PubMed] [Google Scholar]
- 29. Alapure BV, Lu Y, He M, et al. Accelerate healing of severe burn wounds by mouse bone marrow mesenchymal stem cell‐seeded biodegradable hydrogel scaffold synthesized from arginine‐based poly(ester amide) and chitosan. Stem Cells Dev. 2018;27(23):1605‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


