Abstract
The study aims to estimate the cost‐effectiveness of superabsorbent wound dressings compared to the standard‐of‐care (SoC) dressings mix for treatment of patients with moderate‐to‐highly exuding leg ulcers in the German healthcare settings. A model‐based cost‐effectiveness analysis was conducted from the German statutory health insurance perspective, following German specific and international recommendations of good research practice. An individual‐level (microsimulation) state‐transition model has been used with a cycle length of 1 week and time horizon of 6 months. Several comprehensive systematic reviews were conducted to inform all model inputs, including clinical parameters, efficacy, quality of life, resources utilisation, and cost inputs. In addition, primary data from two clinical trials were used. Based on this cost‐effectiveness analysis, using superabsorbent wound dressings instead of the SoC dressings of patients with moderate‐to‐highly exuding leg ulcers in Germany can lead to an improved healing rate of 2.57% (benefit ratio 1.08), improved health‐related quality of life of 0.152 quality‐adjusted life weeks, and total direct cost savings of €771 per patient in 6 months. Robustness of results was confirmed in sensitivity and scenario analyses.
Keywords: cost‐effectiveness, Germany, leg ulcers, superabsorbent wound dressings, wound dressings
1. INTRODUCTION
Hard‐to‐heal ulcers (chronic or complex wounds), defined as wounds with a duration of 3 weeks or longer, 1 generate a substantially high economical and humanistic burden in Germany, as well as in European Union and globally. Germany reported more than 4 million chronic wounds being treated annually at a substantial cost. 2 , 3 The mean annual cost of a chronic ulcer in Germany was estimated as of €9060, of which direct costs (total diagnostic and treatment costs for statutory health insurances) are €8288 and indirect costs (patient out‐of‐pocket payments) are €772. 2 , 4
Similar findings are reported in other countries. According to the National Health Service, the United Kingdom is treating around 2.2 million acute or chronic wounds annually with the total cost of care estimated as high as €4.5 to €5.3 billion. 5 Comparable calculations from the United States based on Medicare alone estimate the cost for all wound types to US $28.1 to $96.8 billion, 6 while Canada is reporting an expenditure of $509 million just on diabetic foot ulcers. 7 Interestingly, general findings from cost‐of‐illness studies are reporting lower costs but higher prevalence of venous leg ulcers when compared with diabetic foot ulcers. A systematic review of all identified cost‐of‐illness studies worldwide reports the annual costs of US $44200, $15400, and $11000 for diabetic foot, pressure ulcers, and venous leg ulcers, respectively. 7 The mean annual per wound cost in Germany of venous leg ulcers is estimated as €6905 and arterial ulcers €10 241 with higher costs among females and those younger than the median age of the study cohort (74 years). 2 All cost‐of‐illness studies report similar cost breakdowns according to which the main drivers of cost are not wound‐specific treatments such as dressings or devices but the cost‐of‐care provision. In Germany, the cost of dressings is merely around 14% of total annual cost, 2 while a similar estimate (13.9%) is reported for the United Kingdom. 5 Therefore, it is of critical importance for any cost analyses for wound dressings not to limit their scope to the cost of wound dressings per se but also include cost variations in patient care needs (eg, capturing the difference between the treatment of infected wound/wound [non]responding to treatment).
There are limited findings of highly accurate cost estimates specific for moderate‐to‐highly exuding leg ulcers. Current recommendations suggest superabsorbers as a first‐line treatment for moderate‐to‐highly exuding leg ulcers, together with alginates, hydrofibres, foams, and hydropolymers. 8 , 9 Superabsorbers have the highest capacity for retention of fluids. They can be used under compression, while specific types of polyacrylate superabsorber (SAP) can reduce inhibitors of wound healing, including matrix metalloprotease activity. 8 , 9 The newest generation of these superabsorbers feature silicone layers/border, which reduce the risk of skin damage and minimise pain during dressing changes. However, currently, there is a complete lack of evidence concerning the assessment of the relation of benefits to costs for care with first‐ and second‐line treatment of moderate‐to‐highly exuding leg ulcers in Germany.
Therefore, this study aims to estimate the cost‐effectiveness of treating patients with moderate‐to‐highly exuding leg ulcers with SAP vs standard of care (SoC), which is represented by the assortment of standard dressing options composed of other superabsorbers and guideline‐recommended dressings. The premise of the analysis is to test the counterfactual scenario in which current dressing mix will be replaced entirely with SAP. At the same time, all other systemic and local treatments, wound, and patient characteristics will remain the same.
2. METHODS
An economic evaluation was conducted following the current recommendations “General Methods for the Assessment of the Relation of Benefits to Costs”, issued by Quality and Efficiency in Health Care (IQWiG), 10 and international good modelling practice guidelines. 11 All findings are reported per the general international recommendations of CHEERS checklist, 12 and specific recommendations of the IQWiG guidelines mentioned earlier. The economic study design was a cost‐effectiveness analysis.
2.1. Benefit assessment
As the current state of evidence concerning benefit‐harm analyses of SAP is not based on robust head‐to‐head comparison from randomised controlled trials, we conducted an early health technology assessment (HTA) using a health economic model to estimate the cost‐effectiveness. Health economic modelling is one of the most commonly used methods in early HTAs to support product development of medical products as wound dressings. 13 Early HTA should guide all relevant stakeholders, including clinicians, in their decisions during the period when definitive conclusions through robust randomised clinical trials are not available. However, conclusions from early assessments should be considered with all limitations that will be discussed in this article. For that reason, IQWiG suggests using terminology as a potential benefit or indication of (additional) benefit. 10
For the benefit assessment, we have merged two cohorts of published clinical studies on SAP dressings with patient populations presenting with moderate–to‐highly exuding leg ulcers. 14 , 15 The total number of patients with reported wound size at the end of the 2‐week follow‐up period was 84. Baseline patient characteristics are presented in Table 1. Some of the baseline patient characteristics have a small number of missing values that were imputed with multiple imputations of mean values in base case analysis. To check the potential effect on the results, several other imputation methods were tested in the scenario analyses. The clinical trial dataset has a small number of missing values. The base case analysis uses a multiple imputation of mean values. Several other imputation models were used including regression imputation and k‐nearest‐neighbour (k‐NN) without impact on results of the cost‐effectiveness analysis.
TABLE 1.
Characteristics of the baseline cohort
| Variable | Obs (MV) | Mean | SE | 95% CI | |
|---|---|---|---|---|---|
| LB | UB | ||||
| Patient characteristics | |||||
| Age | 78 (6) | 72.79 | 1.46 | 69.88 | 75.69 |
| Gender, males (%) | 83 (1) | 86.90 | 0.08 | 71.55 | 100 |
| Wound characteristics | |||||
| Number of wounds | 84 (0) | 1 | 0 | — | — |
| Duration of wounds (months) | 78 (6) | 15.09 | 3.39 | 8.34 | 21.84 |
| Wound size SoC (mm2): baseline | 84 (0) | 5764.74 | 2565.70 | 661.67 | 10 867.81 |
| Wound size SoC (mm2): after 2 weeks | 84 (0) | 3578.06 | 832.20 | 1922.84 | 5233.281 |
Abbreviations: CI, confidence interval; LB, lower bound; MV, missing values; Obs, number of observations; SE, standard error; UP, upper bound.
To increase the stability of the health economic model, we have randomly sampled from the available patient profiles (n = 84) to generate 1000 patients per arm using the patient characteristics of the available patients.
2.2. Selection of comparators
The interventions evaluated in this analysis are mix of two SAP dressings (Zetuvit Plus Silicone/Zetuvit Plus Silicone Border, manufacturer HARTMANN GROUP, Germany). To ensure a fair comparison, the dressings mix in clinical trials for SAP dressings 14 , 15 (at the baseline before switching to SAP) are used as an appropriate SoC dressings mix. The SoC was composed of other superabsorbers (29%), antimicrobials (26%), foams (20%), alginates (5%), and other dressings (19%). The composition of dressing mix is mainly in line with treatment recommendations and suitable to reproduce real‐world conditions in the treatment of patients with moderate‐to‐highly exuding leg ulcers. This selection of comparators is in line with IQWiG recommendations that comparators should be “all therapeutic alternatives relevant in a particular therapeutic area.” As with the current evidence base, there was no possibility to compare all the SoC products separately, and the comparison is made using SAP as the intervention arm and SoC dressings mix as a comparator arm.
2.3. Relevant outcomes
Keeping in mind that dressings are local treatment that cannot affect survival, the primary clinical outcome in this evaluation was the healing rate. Health‐related quality of life (HRQoL) was used as a secondary outcome measure using aggregated quality‐adjusted life weeks (QALWs). Utility values per health state are depicted in Table 2.
TABLE 2.
Utility values per health state
| Utilities per health state | Value | References |
|---|---|---|
| Healed (UHS1) | 1.000 | 16 |
| Unhealed grade 1: progressing (UHS2) | 0.730 | |
| Unhealed grade 1: static (UHS3) | 0.640 | |
| Unhealed grade 1: deteriorating (UHS4) | 0.640 | Assumption based on Reference 16 |
| Unhealed grade 2: severe (UHS5) | 0.610 | Assumption based on Reference 17 |
| Death (D) | 0 | NA |
Abbreviations: D, Death; NA, not applicable; UHS1, healed; UHS2, unhealed grade 1: progressing; UHS3, unhealed grade 1: static; UHS4, unhealed grade 1: deteriorating; UHS5, unhealed grade 2: severe.
Both outcomes measures are reported on the benefit axis of the efficiency frontier graph, in accordance with IQWiG recommendations. The concept of the efficiency frontier is an IQWiG/German‐specific method that is an extension of the standardised measure known as the incremental cost‐effectiveness ratio (ICER) commonly used in health economics. 16 However, ICER aims to provide decision‐makers with the measure for rational use of resources across all therapeutic areas while IQWiG/Germany considers optimization of resource utilisation only in a single therapeutic area and without an explicit willingness‐to‐pay threshold. 17 Therefore, the definition of the efficiency frontier plot is “plot compares the therapeutic benefit of available interventions within a given therapeutic area with the outcome‐related net costs of these interventions”. 17
IQWiG does not require or strongly recommended the use of QALYs. Furthermore, health economic evaluations and efficiency frontiers in Germany are recommended to be specific for indications or therapeutic areas and not conducted across indications. However, in cases where the quality of life is considerably affected by the underlying disease and there is a need for the quantification of multiple harms using QALYS is the most straightforward approach. 10
We have conducted a systematic search for the appropriate HRQoL inputs for Germany, which is measured with a generic instrument that can be used to generate utilities. Additionally, a search for a disease‐specific instrument that could be mapped using instruments such as EQ‐5D was also undertaken. However, we did not identify any appropriate study that could be used to inform the quality‐of‐life measure. Therefore, utility values per health state from the UK have been used.
2.4. Study perspective and time horizon
The economic evaluation was conducted from the perspective of the community of citizens insured in the Statutory health insurance (SHI), as recommended by IQWiG guidelines and German law (§ 35b 1 SGB V). 18 , 19 In scenario analysis, we conducted additional analysis from the societal perspective. We used only German‐specific sources for cost data inputs expressed in Euros, and therefore no currency conversion was needed. Costs from older studies were adjusted for inflation for the value in 2020 using German consumer price index. 20
As the dressings are used for local treatment of the wound and not for the systematic treatment of the underlying disease, a lifelong time horizon was not considered appropriate in this setting. Considering the natural history of a chronic wound, we deemed that a 6‐month time horizon is the most suitable. Of note, we did not model wound recurrence in the same localization due to lack of data, where a more appropriate time horizon would have been 1 year.
2.5. Estimations of costs
To ensure the best available German‐specific resource utilisation and cost inputs, a systematic review was conducted in Medline via the PubMed and CRD databases. The details and results of the systematic search are presented in the supplementary file Part II: Resource use and cost analyses. Combining results from a systematic search and Diagnosis Related Groups (DRG) analysis, we have estimated the cost per wound health state as depicted in Table 3 and Table 4.
TABLE 3.
Direct costs per ulcer health state
| Direct medical costs per health state | Estimated costs (€) | Range for OWSA | Parameters for PSA* (distribution) |
|---|---|---|---|
| UHS1 | 10 | ±20% | (gamma) |
| UHS2 | 147 | ±20% | (gamma) |
| UHS3 | 169 | ±20% | (gamma) |
| UHS4 | 269 | ±20% | (gamma) |
| UHS5 | 1076 | ±20% | (gamma) |
Abbreviations: OWSA, one‐way sensitivity analysis, PSA, probabilistic sensitivity analysis; UHS, ulcer health state.
*Calculated by method of moments.
TABLE 4.
Indirect costs per ulcer health state
| Indirect medical costs per health state | Estimated costs (€) | Range for OWSA | Parameters for PSA* (distribution) |
|---|---|---|---|
| UHS1 | 1 | ±20% | (gamma) |
| UHS2 | 14 | ±20% | (gamma) |
| UHS3 | 16 | ±20% | (gamma) |
| UHS4 | 25 | ±20% | (gamma) |
| UHS5 | 99 | ±20% | (gamma) |
Abbreviations: OWSA, one‐way sensitivity analysis, PSA, probabilistic sensitivity analysis; UHS, ulcer health state.
*Calculated by method of moments.
Additionally, the fraction of dressings with an associated number of dressing changes per week is depicted in Table 5. Instead of using predefined and fixed dressing sizes and associated costs, we have used a range of dressings from each type and matched appropriate dressing sizes with the wound size of every patient in the model. All used dressings and costs are presented in the Part II supplementary material. The dressing received in the SoC arm follows the size of the cohort fraction of particular dressing type (Table 5).
TABLE 5.
Dressing‐specific resource use and associated costs (per week)
| Product (dressing type) | Fraction of cohort | Source | N of dressing changes per week | Source |
|---|---|---|---|---|
| Intervention | ||||
| Zetuvit plus silicone | 100% | 14, 15 | 2.80 | 32 |
| Comparator (standard of care) | ||||
| Other superabsorbents | 36% | 14, 15 | 2.80 | 32 |
| Antimicrobials | 30% | 4.00 | ||
| Foams | 20% | 4.00 | 32 | |
| Alginates | 9% | 4.00 | ||
| Other dressings | 5% | 4.00 |
Note: Parameters in one‐way sensitivity analysis varied ±20%. Following distributions were used in probabilistic sensitivity analysis: fraction of cohort: Dirichlet distribution, N of dressing changes: normal distribution.
2.6. Modelling
We have followed the steps recommended by IQWiG guidelines for developing a health economic model. 10 The modelling approach was selected according to the nature of the research problem. Chronic ulcers are notably heterogeneous conditions and can exhibit many diverse characteristics that can affect wound healing. Those factors are related to different domains such as sociodemographic factors (gender, age, socio‐economic status, etc.), main underlying disease that increases the risk of wound occurrence (diabetes, venous insufficiency, hypertension), comorbidities (the considerable spectrum of chronic and degenerative disease), the effect of previous related intervention(s) (venous surgery, compression therapy, etc.), patient status (mobility, cognitive status, etc.), wound characteristics (size, depth, duration, colour, infection, etc.), and laboratory parameters (Hba1C, glucose, etc.). It is recommended that research problems with such underlying heterogeneity should be modelled with an individual‐level state‐transition model (microsimulation). 11
Therefore, we used an individual‐level state‐transition model 11 to track patients including their characteristics allowing for individual values of model parameters. We started the modelling process by generating two identical patients according to the inputs from Table 1.
From that point, the model had two arms with identical patients with the only difference being the dressing received, which was SAP in arm 1 and SoC dressing mix in arm 2. The patient was entered into the model in ulcer health state 3 (UHS3) as defined by costing methodology. In line with recommendations, the modelling process follows the natural history of disease/condition. The models flow and the structure is depicted in Figure 1 (details described in the Supplementary file, PART II: Resource use and cost analyses [Estimation of costs]).Transition probabilities, defined as a probability that patient will “transit” from one ulcer health state to another, are informed by the best available evidence from the literature (Table 6). We assumed that the dressings are local wound treatments and do not affect survival. Mortality due to other causes was derived from the German life tables using age‐ and gender‐specific inputs for every patient. 21
FIGURE 1.

Model flow and structure
TABLE 6.
Transition probabilities for ulcer health states
| Transition probability per ulcer health state | Model input value | References |
|---|---|---|
| From “UHS2” to “UHS1” | 0.0250 | 32 |
| From “UHS3” to “UHS2” | Patient specific | Risk prediction clinical trials data |
| From “UHS3” to “UHS1” | 0.0188 | 33 |
| From “UHS3” to “UHS4” | 0.0188 | 34 |
| From “UHS3” to “UHS5” | 0.0170 | 33 |
| From “UHS4” to “UHS5” | 0.0040 | 35 |
| From “UHS5” to “UHS3” | 0.8000 | 34 |
| From any UHS to death | Age and gender specific | 36 |
Abbreviation: UHS, ulcer health state.
To inform transition probabilities, Cochrane Wounds reviews were assessed and the systematic review by Norman et al. was identified as a most relevant for derivation of transition probabilities. Relevant parameters from control arms were extracted from studies included in Norman et al's systematic review, and in the following step they were transformed to transition probabilities with cycle length of 1 week (Supplementary material PART IV: Other, Table 19, Table 20).
Both arms will have the same transition through health states apart from the transition from “UHS3” to “UHS2” (transition from health state “static ulcer” towards health state “ulcer progressing towards healing”). This transition was informed for both arms separately using the previously developed and validated risk prediction model. The risk prediction model was nested in the health state UHS3 to predict how many patients will stay at that health state and how many will transit toward UHS2 based on the following predictors: age, gender, number of wounds, the log of the duration of the wound in months, the log of wound size in mm2, and wound grade. 22 The SAP arm was informed based on the outcomes of two clinical studies, and the SoC arm was based on baseline values from the same clinical studies. 14 , 15
Method for derivation of transition probabilities in adequate metrics for model cycle length is described in the derivation of transition Supplementary material PART IV: Other.
2.7. Sensitivity analyses
Following both IQWiG and international guidelines, 10 , 23 we conducted sensitivity analyses to quantify uncertainty around model results and in addition scenario analyses. Two types of sensitivity analyses were conducted: deterministic one‐way sensitivity analysis (OWSA) and multivariate probabilistic (Monte Carlo) sensitivity analysis (PSA). In OWSA, we changed the value of one variable to a lower bound (lower confidence interval limit or −20% of used point estimate) and to an upper bound (upper confidence interval limit or +20% of used point estimate) while keeping all others fixed to check the impact on results. This procedure was repeated for all variables and presented graphically in the form of the Tornado diagram. In the PSA, instead of changing one parameter value at a time, all variables were changed at once according to their plausible values by random sampling from their distributions. This procedure was repeated 5000 times, and the results are presented as a scatter plot over the entire efficiency frontier. Distribution for parameters was selected according to current recommendations of good modelling practice, 23 and parameters for distributions were constructed using methods of moments. 24 Used distributions and parameters are presented in Supplementary material PART IV: Other, Table 21.
Detailed description of the scenario analyses is reported in the Supplementary file, Part IV: Other.
3. RESULTS
Based on the modelling analysis, 6 months of using SAP instead of SoC dressings mix in patients with moderate‐to‐highly exuding leg ulcers in Germany will lead to an improved healing rate of 2.57% (benefit ratio 1.08) associated with 0.152 QALWs improvements in quality of life and total direct cost reduction of €771 per patient and 6 months (Table 7).
TABLE 7.
Results of cost‐effectiveness analysis
| SAP | SoC | Incremental difference | RR (HR) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (€) | QALWs | HR | Cost (€) | QALWs | HR | Costs (€) | HR | QALWs | |
| 4528 | 17.229 | 34.27% | 5299 | 17.077 | 31.70% | −770.96 | 2.57% | 0.152 | 1.08 |
Abbreviations: HR, healing rate; RR, risk ratio; SAP, polyacrylate superabsorbers; SoC, standard of care; QALW, quality‐adjusted life weeks.
As reported in Table 8, after 6 months, 34.27% of patients will have wound closure when treated with SAP, compared to 31.70% when treated with SoC. Although the quality of life is only slightly improved among the groups, the cost savings are €771 per single average patient. A more detailed breakdown of those results is provided in Table 8.
TABLE 8.
Breakdown of results per health state
| Health state | SAP | SoC | Difference | |
|---|---|---|---|---|
| QALWs per health state at the end of the modelling period | HS1 | 4.59 | 4.19 | 0.40 |
| HS2 | 3.31 | 3.24 | 0.07 | |
| HS3 | 7.24 | 7.50 | −0.26 | |
| HS4 | 1.94 | 1.99 | −0.05 | |
| HS5 | 0.15 | 0.16 | −0.01 | |
| Incidence per health state at the end of the modelling period | HS1 | 0.34 | 0.32 | 0.03 |
| HS2 | 0.25 | 0.25 | 0.00 | |
| HS3 | 0.19 | 0.20 | −0.01 | |
| HS4 | 0.19 | 0.20 | −0.01 | |
| HS5 | 0.01 | 0.01 | 0.00 | |
| Cost per health state at the end of the modelling period | HS1 | 204.56 | 301.26 | −96.70 |
| HS2 | 784.67 | 837.36 | −52.68 | |
| HS3 | 2337.66 | 2817.35 | −479.69 | |
| HS4 | 924.66 | 1048.37 | −123.71 | |
| HS5 | 276.41 | 294.58 | −18.17 |
Abbreviations: SAP, polyacrylate superabsorbers; SoC, standard of care; QALW, quality‐adjusted life weeks.
Furthermore, results are reported in the form of two efficacy frontiers as recommended by IQWiG guidelines. According to these guides, the vertical axis should reflect the value of the health benefits on a cardinal scale. Therefore, for one graph, we selected healing rate and for the other QALWs (Figures 2 and 3). The horizontal line reflects total expected net costs per patient during the time horizon of 6 months. In both assessments, SAP leads to higher benefits with fewer costs. Any therapeutic solution that is placed on the graph below the line crossing SAP dot is less effective (in terms of QALWs or healing rate). And all technologies that are placed on the graph right from the vertical line that crosses the SAP dot are more expensive.
FIGURE 2.

Efficacy frontier healing rate to cost
FIGURE 3.

Efficacy frontier QALWs to cost. QALWs, quality‐adjusted life weeks
3.1. Sensitivity analysis
One‐way deterministic sensitivity analysis results are presented in the tornado diagram. Although all variables included in the model are used for sensitivity analysis, we are presenting the 10 most influential parameters on incremental cost, healing rate, and QALWs (Figure 4).
FIGURE 4.

Tornado diagrams
Probabilistic sensitivity analysis demonstrates that SAP is a cost‐saving option and leads to higher benefits in 100% of iterations, as depicted in Figure 5.
FIGURE 5.
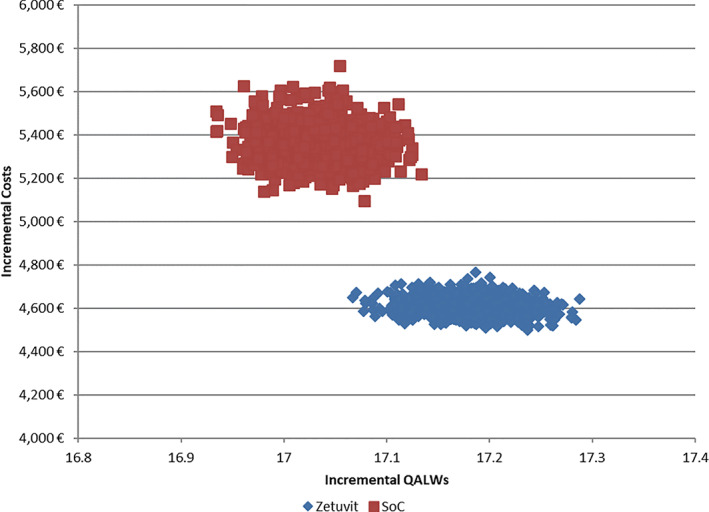
Probabilistic efficacy frontiers
Detailed breakdown of probabilistic sensitive analysis results (Monte Carlo simulation with 5000 iterations) are presented in Table 9.
TABLE 9.
Probabilistic sensitivity analysis results
| SAP | SoC | Incremental Cost (€) | Incremental QALYs | |||
|---|---|---|---|---|---|---|
| Cost (€) | QALYs | Cost (€) | QALYs | |||
| Means | 4608 | 17.180 | 5364 | 17.033 | −755 | 0.147 |
| Medians | 4632 | 17.177 | 5408 | 17.034 | −776 | 0.143 |
Abbreviations: SAP, polyacrylate superabsorber; SoC, standard of care; QALW, quality‐adjusted life weeks.
3.2. Model validation
We applied two types of validation: internal and cross‐models validation. 25 As a part of internal validation, we applied a standardised quality checklist combined with an analysis of extremes to identify and correct all technical errors (bugs) in the model programing.
For cross‐model validation, we used all recent cost studies from Germany identified by our systematic literature search to compare our model's SoC arm projected costs vs the cost of treatment determined by other models or research over the 6 months. We have selected the SoC arm because there are no other research studies that target SAP in Germany. The results of this exercise are presented in Table 10.
TABLE 10.
Cross‐model validation: cost of leg ulcer treatment in Germany
| Study | Total cost (€) | Time horizon | Costing year | Inflated to 2020 (€) | Cost estimation for 6 months (€) |
|---|---|---|---|---|---|
| This model | 4891 | 6 mo | 2020 | 8701 | 4350 |
| Augustin et al 5 | 8287.55 | 1 y | 2012 | 10 846 | 5423 |
| Purwins et al 6 | 9569 | 1 y | 2006 | 4066 | 2033 |
| Droeschel et al 7 | 3977.44 | 1 y | 2017 | 3697 | 5354 |
| Guest et al 8 | 2654.02 | 18 wk | 2002 | 1406 | 4583 |
| Augustin et al 6 | 1335.51 | 8 wk | 2015 | 8701 | 4350 |
As previously commented, all studies reported mainly similar costs for 6 months treatment of leg ulcers, demonstrating that our model is projecting the cost in expected ranges for Germany.
4. DISCUSSION
We have already described the magnitude of resource use caused by a chronic wound, which has become more extensive over the years due to the growth of the most affected population (elderly). Even some of the presented cost is only part of the cost of wound treatments. Disproportionally, a large fraction of health care spending going towards the treatment of chronic wounds using the current approach, especially on dressing pricing, is based on too few parameters. For example, they may consider the changes in the number of dressings, while not taking into account overall and long‐term changes in treatment pathways that contribute to much higher cost and patient‐related outcomes. In such a situation, the new dressings are not assessed by value addition but rather through short‐term resource use benefits. This only generates the illusion of savings when long‐term consequences are overseen. Therefore, we do believe that the HTA process for wound care products is necessary for such conditions as well as gradually switching to value‐based procurement, for example, the Most Economically Advantageous Tender (MEAT), introduced by the European Parliament for public procurement that is considering the values of the product and not just the price. 26 However, for such an evolution, there is a need to better define reference cases in terms of both clinical trials and economic evaluations. Standardisation of the methods and outcome needs to take place so that different wound care technologies can be indirectly compared in terms of their benefits, harms, and costs. Current practice, especially with the different methods approach in health economics, prohibits robust comparisons, and it is not pragmatic to expect that all technologies will eventually be compared head‐to‐head in randomised control trials. As mentioned, health economics studies should focus on the complete patient journey, for example, wound dressing rather than point‐of‐care economics focusing only on nursing time and the number of dressings changes. Those outcomes alone fail to capture full oversight on much higher additional costs driven by changes in the natural history of diseases, for example, the difference between a healed static wound and a wound with complications.
Moreover, by reviewing the cost breakdown of any of the appropriate DRGs for hospital stays due to the wound treatment, it has become evident that many other factors are considerably important drivers of spending while wound dressing costs remain only small fraction. However, the selection of a proper dressing can modify many other important and more significant drivers, for example, re‐hospitalizations or reduced days of hospital stay. We tried to overcome this by modelling the natural history of the chronic wound following a cost methodology recommended by Harding et al. 27 We would suggest that other attempts to model the cost‐effectiveness of dressings product should follow this approach. However, there is a need that further studies should estimate transition probabilities with more precision and, if possible, from one source. In that sense, utilisation of real‐world evidence should be considered more frequently. One of the sources for Germany is certainly The German Chronic Wound Register. 28 Apart from this evidence, other databases should be explored more comprehensibly to determine the feasibility for wound care research, including but not limited to IMS Disease Analyser or The German Pharmacoepidemiological Research Database. 29 However, real‐world data require advanced and proper analytical handling and further dissemination of causal inference methods for observational data in wound research is needed.
When comparing our study with other published health economic evidence in Germany, we have experienced some of the issues with methodology standardisation discussed earlier. 2 , 4 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 To make a proper validation of our model projections, we have reviewed the overall cost of treatment of the most similar patient across different studies in Germany. Almost all studies project the cost of treatment of exuding leg ulcers in the range between €4000 to €5000. However, even in the same group of patients, SoCs are different due to the selection of comparator or time lag between different studies. Although some health economics studies aim to compare one wound dressing vs others (even the particular size of dressings), in our opinion, this is of little value for decision‐makers. For example, in the practice, wound care is conducted using a large number of different products where dressing size and properties are selected depending on size and other characteristics of the wound itself. Therefore, comparing two dressings of a particular size is a more theoretical exercise with a limited possibility for extrapolations of those results in real‐world practice. We tried to overcome that by comparing SAP product lines (two products Zetuvit Plus Silicone/Zetuvit Plus Silicone Border) in all available sizes vs the mix of dressings of all possible sizes, which is used for the treatment of patients with moderate‐to‐highly exuding leg ulcers. Therefore, patient wound characteristics (exuding leg ulcers) and its size are modelled with appropriate dressings in terms of size and properties. This approach is much closer to real‐world practice and should be of higher utility for decision‐makers. However, this approach requires more advanced modelling (microsimulation), but remaining mindful of the heterogeneity of the chronic wound's patients, we believe that this method, despite its complexity, is the most appropriate. One more difference in our approach is that we tried to apply more German‐specific methods for conducting and reporting results of health economic study, according to IQWiG methods guide rather than the prevailing international approach using a willingness‐to‐pay threshold. 17 The primary motivation for this is to make the analysis more useful for decision‐making in German settings.
As all decision‐analytic modeling studies, our decision analysis has several limitations that are important to consider when interpreting results. As depicted already in the title, this is early‐stage health economic evaluation, referring to the fact that clinical benefits are not derived from mature randomised control trials but from observational before‐after analyses. Such data can suffer from inadequate control of unmeasured confounding variables, inability to control for temporal changes, and regression towards the mean. Therefore, causal inference and direction and magnitude of those biases are not possible to determine at the current stage, and there is a clear need for further research, since already depicted transition probabilities are sourced from different studies and fixed over time (time‐invariant). Although this is biasing both arms equally, there is a need for calculation of more precise and time‐dependent estimates. Modelling and simulation have inherent limitations in that they are the only approximations of reality, and therefore, some essential relations (known or unknown) can affect results. In general, there is also a need to model the recurrence of the wound as an essential aspect of wound natural history. However, due to the lack of available data, we did not include this vital health state in our model. Finally, some of the used sources including studies for HRQoL and clinical trials for SAP are conducted in the United Kingdom and maybe not representative for current German practice. Good correspondence of our cost estimates with other studies can be seen as a clear indicator that those limitations do not deviate results dramatically. However, decision‐makers and users should take them into account.
5. CONCLUSIONS
Based on this cost‐effectiveness analysis, using SAP instead of current dressings mix for the SoC of patients with moderate‐to‐highly exuding leg ulcers in Germany can lead to an improved healing rate of 2.57% (benefit ratio 1.08), improved HRQoL of 0.152 QALWs, and total direct cost savings for the statutory health insurance of €771 per patient during 6 months. Further research is needed to confirm the findings from current early‐stage health economic assessment.
CONFLICT OF INTERESTS
Vladica M. Velickovic and TG are employees of HARTMANN GROUP.
AUTHOR CONTRIBUTIONS
Vladica M. Velickovic researched literature, conceptualised the study, and conducted analyses. Vladica M. Velickovic and Thomas Godfrey wrote the first draft of the manuscript. Mate Szilcz, Zoran Milošević, and Uwe Siebert were involved in protocol development, concept, and method validations. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGEMENTS
We would like to thank Linder Jörg and Florian Botzenhardt for their fruitful feedbacks at early version of the manuscript.
Veličković VM, Szilcz M, Milošević Z, Godfrey T, Siebert U. Cost‐effectiveness analysis of superabsorbent wound dressings in patients with moderate‐to‐highly exuding leg ulcers in Germany. Int Wound J. 2022;19(2):447–459. 10.1111/iwj.13645
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta‐analysis of observational studies. Ann Epidemiol. 2019;29:8‐15. [DOI] [PubMed] [Google Scholar]
- 2. Augustin M, Brocatti LK, Rustenbach SJ, Schäfer I, Herberger K. Cost‐of‐illness of leg ulcers in the community. Int Wound J. 2014;11(3):283‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heyer K, Augustin M, Protz K, Herberger K, Spehr C, Rustenbach SJ. Effectiveness of advanced versus conventional wound dressings on healing of chronic wounds: systematic review and meta‐analysis. Dermatology. 2013;226(2):172‐184. [DOI] [PubMed] [Google Scholar]
- 4. Purwins S, Herberger K, Debus ES, et al. Cost‐of‐illness of chronic leg ulcers in Germany. Int Wound J. 2010;7(2):97‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5(12):e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 7. Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost‐of‐illness studies in chronic ulcers: a systematic review. J Wound Care. 2017;26(sup4):S4‐s14. [DOI] [PubMed] [Google Scholar]
- 8. Harding K, Carville K, Chadwick P, Moore Z, Nicodème M, Percival S, Romanelli M, Schultz G, Tariq G. WUWHS Consensus Document: Wound Exudate, effective assessment and management. 2019.
- 9. Wiegand C, Hipler U‐C, Elsner P, Tittelbach J. Clinical efficacy of dressings for treatment of heavily exuding chronic wounds. Chronic Wound Care Management Res. 2015;2:101. [Google Scholar]
- 10. Vincenzo Atella JC, de Pouvourville Gérard, Henry David, McGregor Maurice, McGuire Alistair, Nord Erik, Siebert Uwe. Methods for Assessment of the Relation of Benefits to Costs in the German Statutory Health Care System. 2008. https://www.iqwig.de/download/General_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf
- 11. Siebert U, Alagoz O, Bayoumi AM, et al. State‐transition modeling. Med Decis Making. 2012;32(5):690‐700. [DOI] [PubMed] [Google Scholar]
- 12. Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ: British Med J. 2013;346:1‐6. [DOI] [PubMed] [Google Scholar]
- 13. IJ MJ, Koffijberg H, Fenwick E, Krahn M. Emerging use of early health technology assessment in medical product development: a scoping review of the literature. Pharmacoeconomics. 2017;35(7):727‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atkin L, Barrett S, Chadwick P, et al. Evaluation of a superabsorbent wound dressing, patient and clinician perspective: a case series. J Wound Care. 2020;29(3):174‐182. [DOI] [PubMed] [Google Scholar]
- 15. Barrett S, Rippon M, Rogers AA. Treatment of 52 patients with a self‐adhesive siliconised superabsorbent dressing: a multicentre observational study. J Wound Care. 2020;29(6):340‐349. [DOI] [PubMed] [Google Scholar]
- 16. Caro J, Nord E, Siebert U, et al. The efficiency frontier approach to economic evaluation of health‐care interventions. Health Econ. 2010;19:1117‐1127. [DOI] [PubMed] [Google Scholar]
- 17. Sandmann FG, Mostardt S, Lhachimi SK, Gerber‐Grote A. The efficiency‐frontier approach for health economic evaluation versus cost‐effectiveness thresholds and internal reference pricing: combining the best of both worlds? Expert Rev Pharmacoecon Outcomes Res. 2018;18(5):475‐486. [DOI] [PubMed] [Google Scholar]
- 18. JJea Caro. Methods for Assessment of the Relation of Benefits to Costs in the German Statutory Health Care System. 2008. https://www.iqwig.de/download/General_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf
- 19. Sozialgesetzbuch (SGB) Fünftes Buch (V) ‐ Gesetzliche Krankenversicherung ‐ (Artikel 1 des Gesetzes v. 20. Dezember 1988, BGBl. I S. 2477) § 35b Kosten‐Nutzen‐Bewertung von Arzneimitteln. In: Verbraucherschutz BdJuf; 1988.
- 20. Consumer price index . 2020. https://www.destatis.de/EN/Themes/Economy/Prices/Consumer-Price-Index/_node.html
- 21. GENESIS‐ONLINE . Life Tables 2020. https://www-genesis.destatis.de/genesis/online?operation=statistic&levelindex=0&levelid=1609774358730&code=12621#abreadcrumb
- 22. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004;12(2):163‐168. [DOI] [PubMed] [Google Scholar]
- 23. Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR‐SMDM modeling good research practices task force working Group‐6. Med Decis Making. 2012;32(5):722‐732. [DOI] [PubMed] [Google Scholar]
- 24. Jesus J, Chandler RE. Estimating functions and the generalized method of moments. Interface Focus. 2011;1(6):871‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR‐SMDM modeling good research practices task Force‐7. Med Decis Making. 2012;32(5):733‐743. [DOI] [PubMed] [Google Scholar]
- 26. Lupi A. The Use of MEAT. Forum on the Competitiveness of the European Rail, Supply Industry, 1st Workshop on Trade and Procurement. Brussels: European Commission; 2017. [Google Scholar]
- 27. Harding K, Cutting K, Price P. The cost‐effectiveness of wound management protocols of care. Br J Nurs. 2000;9(19):S6‐S10. [DOI] [PubMed] [Google Scholar]
- 28. Droeschel D, Gutknecht M, Walzer S, Lindsay F, Shannon R, Augustin M. Eine probabilistische Kosteneffektivitätsanalyse einer azellulären synthetischen Matrix (ASM) als Ergänzung zur Standardversorgung venöser und gemischter Ulzera cruris in Deutschland auf Basis eines Discrete‐Event‐Simulations‐Modells. Gesundheitsökonomie Qualitätsmanagement. 2018;23(02):75‐87. [Google Scholar]
- 29. The German Pharmacoepidemiological Research Database (GePaRD). 2020; https://www.bips-institut.de/en/research/research-infrastructures/gepard.html
- 30. Augustin M, Herberger K, Kroeger K, Muenter KC, Goepel L, Rychlik R. Cost‐effectiveness of treating vascular leg ulcers with UrgoStart(®) and UrgoCell(®) contact. Int Wound J. 2016;13(1):82‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghatnekar O, Willis M, Persson U. Cost‐effectiveness of treating deep diabetic foot ulcers with Promogram in four European countries. J Wound Care. 2002;11:70‐74. [DOI] [PubMed] [Google Scholar]
- 32. Gueltzow M, Khalilpour P, Kolbe K, Zoellner Y. Budget impact of antimicrobial wound dressings in the treatment of venous leg ulcers in the German outpatient care sector: a budget impact analysis. J Mark Access Health Policy. 2018;6(1):1527654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guest JF, Ruiz FJ, Mihai A, Lehman A. Cost effectiveness of using carboxymethylcellulose dressing compared with gauze in the management of exuding venous leg ulcers in Germany and the USA. Curr Med Res Opin. 2005;21(1):81‐92. [DOI] [PubMed] [Google Scholar]
- 34. Guest JF, Sladkevicius E, Panca M. Cost‐effectiveness of using Polyheal compared with surgery in the management of chronic wounds with exposed bones and/or tendons due to trauma in France, Germany and the UK. Int Wound J. 2015;12(1):70‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hartmann M, Schulz D, Gundermann C, Norgauer J. Ökonomische Effekte der Standardisierung in der modernen Wundversorgung. Hautarzt. 2007;58(11):970‐975. [DOI] [PubMed] [Google Scholar]
- 36. Horch R, Nord D, Augustin M, Germann G, Leffler M, Dragu A. Economic aspects of surgical wound therapies. Der Chirurg; Zeitschrift für Alle Gebiete der Operativen Medizen. 2008;79:518‐525. [DOI] [PubMed] [Google Scholar]
- 37. Jünger M, Arnold A, Zuder D, Stahl HW, Heising S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse): a prospective, placebo controlled, double blind trial. Wound Repair Regen. 2008;16(4):480‐487. [DOI] [PubMed] [Google Scholar]
- 38. Rabe E, Pannier F. Societal costs of chronic venous disease in CEAP C4, C5, C6 disease. Phlebology. 2010;25(Suppl 1):64‐67. [DOI] [PubMed] [Google Scholar]
- 39. Ukat A, Konig M, Vanscheidt W, Münter KC. Short‐stretch versus multilayer compression for venous leg ulcers: a comparison of healing rates. J Wound Care. 2003;12(4):139‐143. [DOI] [PubMed] [Google Scholar]
- 40. Wolke R, Hennings D, Scheu P. Gesundheitsökonomische Evaluation in der Pflege. Z Gerontol Geriatr. 2007;40(3):158‐177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


