Abstract
Sympathectomy of arteries has been adopted for the treatment of peripheral arterial disease and Raynaud's disease. However, the exact route for sympathetic axons to reach peripheral arteries awaits further investigation that could pave the way for development of new surgical strategies. In this study, saphenous neurovascular bundles from 10 neonatal Sprague‐Dawley rats first were harvested for whole‐mount immunostaining to show sympathetic innervation pattern of the artery. Secondly, 40 Sprague‐Dawley male rats weighing 350 to 400 g were assigned to five groups, receiving either sham, perivascular sympathectomy, nerve‐artery separation, nerve transection in the saphenous neurovascular bundle, or lumbar sympathectomy surgery that removes the lumbar sympathetic trunks. Immediately after surgery, the arterial perfusion and diameter were measured using laser speckling contrast imaging, and 1 week later the saphenous neurovascular bundles were harvested for immunostaining using antibodies against TH, neuron‐specific β‐tubulin (Tuj 1), and α‐SMA to show the presence or absence of the TH‐immuopositive staining in the adventitia. The differences among the five groups were determined using one‐way analysis of variance (ANOVA). We found that an average of 2.8 ± 0.8 branches with a diameter of 4.8 ± 1.2 μm derived from the saphenous nerve that morphed into a primary and a secondary sympathetic trunk for innervation of the saphenous artery. Nerve‐artery separation, nerve transection, and lumbar sympathectomy could eradicate TH‐immunopositive staining of the artery, resulting, respectively, in a 12%, 36%, and 59% increase in diameter (P < .05), and a 52%, 63%, and 201% increase in perfusion compared with sham surgery (P < .01). In contrast, perivascular sympathectomy did not have a significant impact on the TH‐immunopositive staining, the diameter, and perfusion of the distal part of the artery (P > .05). We conclude that the sympathetic innervation of an artery derives from segmental branches given off from its accompanying nerve. Nerve‐artery disconnection is a theoretic option in sympathectomy of an artery.
Keywords: arterial innervation, peripheral arterial disease, Raynaud's disease, sympathectomy, sympathetic nerve
1. INTRODUCTION
Clinically, disruption of sympathetic innervation of arteries has been mainly applied for the treatment of peripheral arterial diseases (PAD) 1 , 2 and Raynaud's disease. 3 The most common cause of PAD is atherosclerosis, 4 which has an estimated incidence of 3% to 10%. 5 Thirty two percent of People with PAD often present with muscle pain (intermittent claudication) in the leg from mild exertion, such as walk, or decreased blood flow to the legs that can be painful even at rest. 6 Conservative measures such as the use of antiplatelet and vasodilating agents, and exercise regimens are first attempted. Otherwise, endovascular procedures and bypass surgery are tried, which can result in decreasing the need of eventual amputation that will occur in 1% to 2% of patients. 7 And in patients who are not suitable to the endovascular or the bypass procedures because of the site and extent of the disease, 5 lumbar sympathectomy that can lower the vascular tone of the lower limb is an alternative choice. 8 In a clinical trial, cotton reported that lumbar sympathectomy can result in pain relief over long‐term follow‐up.
Raynaud's disease is a common disorder related to the exaggerated response of the sympathetic nerve system to cold exposure that exhibits triphasic colour change, ulceration, and infection in the extremities, mainly the fingers. 9 , 10 , 11 Population‐based studies suggest that the prevalence of the disorder is 4% to 9% in women and 3% to 6% in men. 12 , 13 , 14 , 15 Aside from conservative treatments such as warming, smoking cessation, and intake of calcium channel blocker, 9 , 10 , 11 a surgical approach named perivascular sympathectomy is often adopted. 16 , 17 Pace reported in a clinical trial that extended digital perivascular sympathectomy was reported to be 78% effective in healing digital ulcers. 18
Both lumbar sympathectomy and perivascular sympathectomy are designed to interrupt the sympathetic nerves that innervate the peripheral arteries. However, one question still lingers is the exact route for the sympathetic fibres to reach the peripheral arteries. As well documented, 19 two kinds of neurons are involved in the transmission of signals in the sympathetic system: pre‐ganglionic and post‐ganglionic. The pre‐ganglionic neurons, located at T1 to L2‐to‐L3 segments of the spinal cord, send axons to a ganglion, often one of the paravertebral ganglia, where they synapse with a post‐ganglionic neuron. The axons of the post‐ganglionic neurons can hitchhike large arteries, for example, the external and internal carotid arteries, following these arteries to innervate the organs and structures in the neck and head. Therefore, one possible route for the arteries in the limbs receiving its sympathetic innervation is the distal extension of the sympathetic nerve plexuses hitchhiking large arteries all the way to fingers or toes. However, this pattern of centripetal distribution is widely disputed, as sympathectomy on the proximal part of digital arteries does not result in sympathectomy of the digital part in monkeys. 20 Therefore, morphological details regarding how the axons of the post‐ganglionic neurons reach and innervate the arteries in the limbs still await further investigation. In a previous study in rabbits, we observed that severance of the sural nerve would augment the perfusion of the proximally based sural neurovascular flap in the leg. 21 Therefore, we hypothesise that within a neurovascular bundle, the artery must receive its innervation from the accompanying nerve, and transection of the nerve or severance of all connections between the nerve and artery would lead to sympathectomy of the artery.
2. MATERIALS AND METHODS
The study consists of two parts. The first part, using whole‐mount immunostaining, was devoted to morphological observation of whether there are sympathetic branches deriving from the saphenous nerve that innervate the accompanying artery. The second part was designed to functionally verify the morphological observation of the first part in 15 adult rats, employing surgical manipulations to observe whether sympathectomy on the saphenous artery was achieved based on the hemodynamic change of the saphenous artery immediately after surgery, and on the presence or absence of sympathetic fibres on the arterial adventitia 1 week after surgery (Figure 1). All procedures involved in this study were approved by the Ethics Committee of Fujian Medical University and followed the National Institute of Health Guidelines for Care and Use of Laboratory animals.
FIGURE 1.

Experimental design. The study was divided into two parts: morphologic observation and functional verification. In the part of panoramic morphologic observation, saphenous neurovascular bundles from 10 neonatal rats were harvested for whole‐mount immunostaining to observe the source of sympathetic innervation to the saphenous artery. Without the need for sectioning before staining, a panoramic picture of sympathetic innervation of the artery could be observed. In the part of surgical manipulations for verification, 40 rats were evenly assigned into five groups. A, Control group: only exposure of the saphenous neurovascular bundle was performed; B, perivascular sympathectomy: the small rectangle represents stripping of the adventitia for about 3 mm at the root of the saphenous artery; C, nerve‐artery disconnection: the green dotted line indicates severance of connections between the saphenous nerve and artery; D, nerve transection: the cross indicates transection of the saphenous nerve at the root; E, the two crosses indicate removal of the bilateral lumbar sympathetic trunks. Immediately after surgery, laser speckle contrast imaging (LSCI) was adopted for measurement of the inner diameter and perfusion intensity of the saphenous artery to determine whether haemodynamic changes were initiated by the surgical manipulations. One week later, the neurovascular bundles about 1.5 cm away from the root of the nerve indicated by the large rectangles were harvested, and conventional cryosection and immunostaining were performed to observe the presence or absence of immunopositive TH staining on the adventitia of the saphenous artery to evaluate the efficacy of sympathectomy
2.1. Whole‐mount immunofluorescent staining of saphenous neurovascular bundles
Ten Sprague‐Dawley rats 1 week after birth were euthanised and the saphenous neurovascular bundles were harvested and placed in 4% paraformaldehyde for 30 minutes at room temperature. After fixation, the bundles were washed in PBS and incubated in blocking buffer (5% normal goat serum, 2% Triton X‐100, 5% bovine serum albumin in PBS) for 1 hour at room temperature. The tissues were then stained with primary antibodies, rabbit anti‐tyrosine hydroxylase antibody (TH; 1:500; Ab152; Chemicon), a marker of sympathetic neurons, and mouse anti‐α‐smooth muscle actin antibody conjugated with cy‐3 (α‐SMA; 1:1000; C6198; Sigma), a marker of vascular smooth muscle, overnight at 4C. After rinsing in washing buffer (PBS with 0.2% Triton X‐100) for 45 minutes, the bundles were incubated with goat anti‐rabbit IgG(H + L) Alexa 488 (1:500; A1103; Thermo Fisher Scientific) for 1 hour at room temperature. After rinsing in washing buffer (PBS with 0.2% Triton X‐100) for 45 minutes, the bundles were mounted using mounting medium, and observed and photographed using a confocal microscope (Leica TCS SP8).
2.2. Surgical manipulations for sympathectomy of saphenous artery
Forty male Sprague‐Dawley rats, weighing 350 to 400 g, were used in this part and were evenly divided into five groups with eight rats in each group. In the control group, no further surgical manipulation was taken after exposure of the saphenous neurovascular bundles. In the perivascular sympathectomy group, microsurgical forceps were used to tease away the adventitia at the root of the saphenous arteries for about 3 mm. In the nerve‐artery disconnection group, the vascular sheath was first opened, and the saphenous nerve and artery were then slightly separated with microsurgical forceps. Then, a pair of microsurgical scissors were inserted into the space between the nerve and artery and cut all possible connections. In the nerve‐transection group, the saphenous nerve was transected at the root. In the lumbar sympathectomy group, bilateral lumbar sympathetic trunks were removed with the procedures described by Hweidi. 22 Briefly, a longitudinal incision from the xiphoid process to the pubic symphysis was first made along the abdominal skin and the linea alba to open the abdominal cavity. Upon entering the abdomen, the intestine, spleen, liver, and stomach were retracted to the right after freeing the posterior peritoneal attachments. A blunt dissection was carried out to peel off the peritoneal covering of the posterior abdominal wall to explore deeper into the gutter between the psoas major muscles lying over the lumbar vertebrae (Figure 2). All manipulations were performed under a stereomicroscope at ×2 magnification. The rats that underwent lumbar sympathectomy were relatively weak after surgery, and they were kept at a temperature of 28 °C until they regained mobility.
FIGURE 2.

Presence of lumbar sympathetic trunks in the groove between the bilateral psoas majors. The arrowheads denote the usual four pairs of sympathetic ganglia that are removed during surgery. The first pair can be found underneath the crura of diaphragm, and the last pair can be found underneath the forking site of the abdominal aorta. In this study, all four pairs of the ganglia were removed. The arrow denotes the abdominal aorta and the inferior vena cava that are retracted aside to expose the lumbar sympathetic trunk. The two stars denote the psoas majors
2.3. Measurement of haemodynamics of saphenous artery after surgical manipulations
For the three rats in the nerve‐artery disconnection group, because opening of the vascular sheath and separation of the saphenous nerve and artery would induce obvious vasospasm, the rats were put aside for 1‐hour recovery after surgical separation before being placed under the probe for imaging. Immediately after surgical manipulations, rats in the other four groups were placed under a laser speckle contrast imaging system (LSCI, RFLSI Pro, Reward) for measurement of the diameter and the perfusion intensity of the saphenous artery. LSCI is based on the laser speckle pattern that arises when laser light illuminates a surface. 23 , 24 If the object is moved or contains moving particles (e.g., blood cells), the interfering beam changes and produces a dynamic speckle pattern. With a camera and a computer, the speckle pattern can be visualised and processed to form an image that can reflect the perfusion of a vessel. 25 The imaging system in this study uses a 785 nm, 90 mW laser diode and acquires images at 9 ms exposure time and a distance of 110 mm. A high spatial resolution mode calculating the blood flow index through LSTCA (temporal algorithm) is used. 26 The relative value of perfusion through a transverse section of a vessel can be calculated using the formula: perfusion = diameter × perfusion intensity. 27
2.4. Immunostaining for sympathetic fibres after surgical manipulations
The 40 rats were kept alive for a week, and then euthanised. The saphenous neurovascular bundles were harvested, fixed overnight in 4% paraformaldehyde, and dehydrated in 30% sucrose for a week. The middle segment of the bundles (about 1.5 cm distal to the root) underwent routine cryosection and immunofluorescent staining. The primary antibodies used included the anti‐TH antibody and anti‐α‐SMA antibody as mentioned above, and the mouse anti‐neuron‐specific Class III β‐tubulin antibody (Tuj1; 1:500; MMS‐435P; Covance; pan‐axonal marker). The secondary antibodies used included goat anti‐rabbit IgG(H + L) Alexa 488 (1:500; A1103; Thermo Fisher), and goat anti‐mouse IgG(H + L) Alexa 647 (1:500; A0468; Beyotime). After staining, photomicrographs were taken using a confocal microscope (Leica TCS SP8). TH/α‐SMA ratio was calculated by dividing the immunopositive area of TH staining around the saphenous vessels (measured using adobe photoshop CS5) over that of α‐SMA in the vessels as an indicator of the density of the sympathetic fibres around the vessels. Likewise, TH/β‐tubulin ratio was calculated by dividing the immunopositive area of TH over that of β‐tubulin in the saphenous nerve as an indicator of the density of sympathetic fibres within the saphenous nerve. The staining results were all analysed by an observer blinded to group assignment.
2.5. Statistical analysis
One‐way ANOVA was adopted to examine the difference of the inner diameter and perfusion among the control, perivascular sympathectomy, nerve‐artery disconnection, nerve‐transection, and lumbar sympathectomy groups with Fisher's least significant difference (LSD) adopted for post hoc comparisons. TH/α‐SMA ratio of the saphenous artery was compared between the control and perivascular sympathectomy groups using Student's t‐test. Statistical analyses were conducted using SPSS 19 and Graphpad prism 5. A P value lesser than .05 was considered statistically significant.
3. RESULTS
3.1. Innervation of accompanying vessels by segmental sympathetic branches deriving from saphenous nerve
Within the trunk of the nerve, curvy TH‐immunopositive axons indicative of sympathetic fibres could be observed. At various points, sympathetic fibres in bundles, named as segmental sympathetic branches, leave the nerve trunk. These segmental branches, 2.8 ± 0.8 in number and 4.8 ± 1.2 μm in diameter, travelled alongside the artery, communicated with each other, forming a primary, sympathetic trunk with a diameter of 8.9 ± 0.6 μm between the nerve and artery. The primary sympathetic trunk gave off numerous minute filaments to the adventitia of the saphenous artery. Aside from the minute filaments, the primary sympathetic trunk sent out transverse branches with a diameter of 7.4 ± 1.9 μm and at an interval of 0.55 ± 0.1 mm that travelled across the artery, bifurcating into ascending and descending sub‐branches that communicate with each other to form a secondary sympathetic trunk between the artery and vein with a diameter of 6.6 ± 1.6 μm. The secondary sympathetic trunk gave off small filaments to both the adventitia of the artery and vein. Compared with the dense sympathetic network over the arterial adventitia, the sympathetic network over the venous adventitia was relatively scarce (Figure 3).
FIGURE 3.

Whole‐mount staining of a saphenous neurovascular bundle. 1, 2, and 3 represent the saphenous nerve, artery, and vein, respectively; 4 and 5 indicate the primary and secondary sympathetic trunks formed by the segmental sympathetic branches. Note the dense sympathetic network on the adventitia of the saphenous artery in comparison to the scarce one on the adventitia of the saphenous vein; the arrowheads denote the segmental sympathetic branches given off from the nerve to the artery; the arrows denote the transverse branches given off from the primary sympathetic trunk that cross the artery to form the secondary sympathetic trunk. The scale bar represents 500 μm
3.2. Increase of diameter and perfusion of saphenous artery after surgical manipulations except for perivascular sympathectomy
Significant difference of the inner diameter and perfusion of the saphenous artery among the five groups could be found (P < .001) (Figure 4). The inner diameter of the artery was 261 ± 52 μm, 291 ± 33 μm, and 339 ± 33 μm in the nerve‐artery disconnection, nerve‐transection, and lumbar sympathectomy groups, which were 12%, 36%, and 59% larger than 213 ± 11 μm in the control group (P = .028, .001, and .001 for the control group vs the nerve‐artery disconnection, nerve‐transection, and lumbar sympathectomy groups, respectively). Moreover, the inner diameter of the saphenous artery in the lumbar sympathectomy was 30% and 16% larger than that in the nerve‐artery disconnection and nerve‐transection groups (P < .001 and P = .027 for the lumbar sympathectomy group vs the nerve‐artery disconnection and nerve‐transection groups, respectively). In contrast, no significant difference of the inner diameter could be found between the control and perivascular sympathectomy groups (P = .895).
FIGURE 4.

Speckling images of the saphenous vessels after surgical manipulations and the statistical bar charts. The green arrowhead denotes the saphenous arteries; the red arrowheads denote the saphenous veins. A, B, C, D, and E represent the perfusion images of the saphenous vessels collected using LSCI from the control, perivascular sympathectomy, nerve‐artery disconnection, nerve‐transection, and lumbar sympathectomy groups, respectively. In the nerve‐artery disconnection group, because of the reason that the nerve and artery should be slightly separated by pinching using microsurgical forceps before spring scissors could be inserted to disrupt connections between the nerve and artery, a slightly undulated surface could be observed on the saphenous artery. The diameter of the saphenous artery in this group was calculated by averaging the inner calibre measured at the dented site and that measured not at the dented site. The arrow indicates the saphenous nerve that was retracted aside after manipulation. The scare bar represents 0.5 cm. F statistical comparisons of the inner diameter among the five groups. There were significant differences among the five groups regarding both the inner calibre and perfusion (P < .001). * indicates that significant differences of the inner diameter and perfusion of the saphenous artery could be found compared with those of the saphenous artery in the control group (P < .05). ∆ indicates that no significant difference of the inner diameter and perfusion of the saphenous artery could be found between the control and perivascular sympathectomy groups (P > .05). # indicates the inner diameter and perfusion of the saphenous artery in the lumbar sympathectomy were significantly larger than that those of the saphenous artery in the nerve‐artery disconnection and nerve‐transection groups (P < .05)
The perfusion of the saphenous artery was 227 ± 17 PU μm, 233 ± 31 PU μm, 344 ± 65 PU μm, 371 ± 47 PU μm, and 457 ± 68 PU μm in the control, perivascular sympathectomy, nerve‐artery disconnection, nerve‐transection, and lumbar sympathectomy groups, respectively. The perfusion of the artery was 344 ± 65 PU μm, 371 ± 47 PU μm, and 457 ± 68 PU μm in the nerve‐artery disconnection, nerve‐transection, and lumbar sympathectomy groups, which was 52%, 63%, and 201% higher than 227 ± 17 PU μm in the control group (P < .001 for the control group vs the other three groups). Moreover, perfusion of the saphenous artery in the lumbar sympathectomy was 33% and 23% larger than that in the nerve‐artery disconnection and nerve‐transection groups (P < .001 and P = .008 for the lumbar sympathectomy group vs the nerve‐artery disconnection and nerve‐transection groups, respectively). In contrast, no significant difference of the inner diameter could be found between the control and perivascular sympathectomy groups (P = .602). The data regarding the diameter and perfusion are listed in Table 1.
TABLE 1.
Data regarding the inner calibre and perfusion of the saphenous artery in the five groups
| Control | Perivascular sympathectomy | Nerve‐artery separation | Nerve transection | Lumbar sympathectomy | |
|---|---|---|---|---|---|
| Inner diameter (μm) | 213 ± 11 | 217 ± 36 | 261 ± 52 | 291 ± 33 | 339 ± 33 |
| Perfusion (PU mm) | 227 ± 17 | 233 ± 31 | 344 ± 65 | 371 ± 47 | 457 ± 68 |
3.3. Disappearance of immunopositive TH staining in adventitia after surgical manipulations except perivascular sympathectomy
In the control group, the TH/α‐SMA ratio of the saphenous artery was 0.22 ± 0.03 compared with 0.07 ± 0.01 of the saphenous vein. In the saphenous nerve, sympathetic axons were evenly scattered among other non‐adrenergic axons, with a TH/TH/β‐tubulin ratio of 0.61 ± 0.11. In the perivascular sympathectomy group, sympathetic fibres only disappeared at the site where the adventitial stripping was performed while remained rich in the adventitia about 1.5 cm distal, with a TH/ α‐SMA ratio of 0.21 ± 0.03, not significantly different from that of the control group (P = .569). In the nerve‐artery disconnection group, the sympathetic fibres on the adventitia of the artery completely disappeared. In the nerve‐transection group, all axons underwent degeneration in the nerve, and sympathetic fibres on the adventitia of the artery completely disappeared. In the lumbar sympathectomy group, sympathetic fibres in the nerve and the adventitia of the artery disappeared, whereas other non‐adrenergic axons still abounded in the nerve (Figure 5).
FIGURE 5.
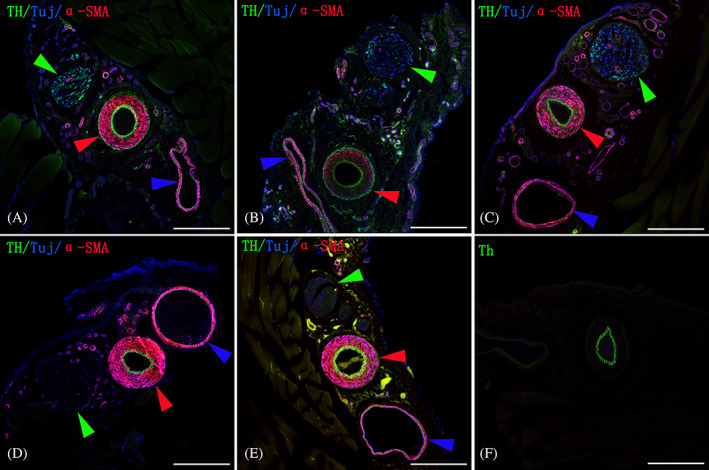
TH, Tuj1, and α‐SMA‐immunostaining for sympathetic axons (green), pan‐axons (blue), and smooth muscle cells (red) of cross sections of the saphenous neurovascular bundles from the five groups. The green, red, and blue arrowheads denote the saphenous nerve, artery, and vein, respectively. A, a representative image from the control group. Note the evenly scattered distribution of sympathetic fibres among other non‐adrenergic axons in the saphenous nerve; dense and scarce sympathetic network could also be observed, respectively, over the adventitia of the saphenous artery and vein; B, after perivascular sympathectomy, the sympathetic fibres in the adventitia about 1.5 cm distal to the surgical site were not obviously affected. C, after disruption of the connections between the nerve and artery, the nerve was not affected, whereas the sympathetic fibres disappeared in the adventitia; D, after transection of the saphenous nerve at the root, all axons in the nerve underwent degeneration, and the sympathetic fibres in the adventitia disappeared; E, after lumbar sympathectomy, sympathetic fibres disappeared in both the nerve and the adventitia, whereas other non‐adrenergic axons in the nerve still existed; F. the strong green staining of the tunica intima is a consistent artefact that manifests even when no primary or secondary antibodies are added when a 488 nm laser line is used. The scale bar represents 250 μm
4. DISCUSSION
The outcomes of the four surgical manipulations demonstrated that transection of the nerve at the root or severance of connections between the nerve and artery could both result in disappearance of sympathetic fibres in the adventitia, corroborating the morphological observation that sympathetic innervation of the saphenous artery derived from the saphenous nerve. Removal of the lumbar sympathetic trunks resulted in simultaneous disappearance of sympathetic fibres in the nerve and the adventitia, revealing that sympathetic fibres within the saphenous nerve and the adventitia of the saphenous artery both derive from the lumbar sympathetic trunks. Thus, a complete pathway for sympathetic axons to reach a peripheral artery can be formulated as follows: the axons of post‐ganglionic neurons return to the spinal nerves as grey ramus communicates, and then travel as part of the spinal nerves to the limbs, giving off segmental branches to innervate the arteries during their courses (Figure 6).
FIGURE 6.

Schematic drawing of the pathway for sympathetic fibres to reach and innervate a peripheral artery. As illustrated, the lower centre of the sympathetic nervous system is located in the intermediolateral nucleus of the grey matter of the spinal cord. The axons leaving the intermediolateral nucleus (pre‐ganglionic neurons) are named pre‐ganglionic fibres, which travel in the anterior root of the spinal nerve and course to the paravertebral sympathetic trunks through the white rami communicante. In the paravertebral trunks, the pre‐ganglionic fibres can synapse with post‐ganglionic neurons, whose axons, named post‐ganglionic fibres, return to spinal nerves through the grey rami communicante, and then travel with the spinal nerves to the peripheral nerves, such as the femoral nerve and radial nerve, exiting the peripheral nerves as segmental sympathetic branches, which then morph into a primary and secondary sympathetic trunk that send off filaments to form sympathetic networks that are dense over the artery and scarce over the vein
The concept of periarterial sympathectomy was first suggested by Leriche more than a century ago, who, however, noted that the early vasodilation effect was later lost, prompting anatomists to cast doubt on the existence of long centripetal sympathetic nerves running along the arteries. 28 In order to improve the efficacy of sympathectomy, proximal sympathectomy at the spinal cord level was then proposed. Barcroft and Walker demonstrated an early 6‐fold increase in circulation with proximal cervicothoracic sympathectomy, 29 but this also had a disappointing long‐term result, because of brachial plexus not receiving its rami communicans exclusively from the cervical sympathetic trunk. 30 Distal periarterial sympathectomy was then described by Flatt in 1980 30 for patients with refractory vasospastic disease of the hand because of these discouraging results. He proposed that distal periarterial sympathectomy would be more effective by removing 3 to 4 mm of adventitia from the proper digital arteries at their bifurcation from the common volar artery, theorising sympathetic response would be ablated on the finger's distal vessels. Flatt's approach belongs to limited periarterial sympathectomy confined only to short segments of digital arteries. Modified from it, extended periarterial sympathectomy with better efficacy has been proposed, which entails sympathectomy, to various extents, of the whole length of the digital arteries, superficial palmar arch, and the distal segments of the radial and ulnar arteries. 31 , 32 , 33 , 34 , 35
Our results showed that removal of the arterial adventitia at the root of the saphenous artery did not have a significant impact on the density of sympathetic fibres on the adventitia as well as on the haemodynamics of the distal part of the artery. Although previous publications using vessels from rabbits, rats, and monkeys have already suggested that limited proximal sympathectomy of a vessel could not eradicate the sympathetic nerves in the distal part, the exact underlying reason was only speculated. 17 , 20 With this study, we can now confidently conclude that the sympathetic nerves on the adventitia of the distal part are preserved simply because the segmental sympathetic branches are not disrupted.
In order to achieve widespread removal of sympathetic innervation of an artery, severance of all connections between the artery and the nerve in company or just transection of the nerve at the root can be options. Raynaud's disease often affects human digits, 36 , 37 , 38 which are mainly supplied by proper digital arteries that are accompanied by proper digital nerves. Therefore, aside from wide‐ranged perivascular sympathectomy, transecting the proper digital nerve at the root or severance of connections between the digital artery and nerve are two other theoretically feasible options. These two surgical procedures are much less time‐consuming and technique‐challenging than whole‐length perivascular sympathectomy. And between the two, nerve‐artery disconnection is a more reasonable choice, because it poses no risk of causing sensational deficit as that could be following nerve transection (Figure 7).
FIGURE 7.

Disconnection between the digital artery and nerve is a theoretic way to achieve sympathectomy of the digital artery. In clinic practice, the vascular sheath shrouding the proper digital vessels and nerves can be opened first, and then scissors could be used conveniently to transect all connections between the proper digital artery and nerve. The same operation can be applied to the upper‐order artery and nerve
Although lumbar sympathectomy is already used as a clinical surgical approach, experimental research studies in animals showing the extent of augmentation in perfusion it can bring to the peripheral arteries is rare. In this study, we demonstrated that the inner diameter and perfusion of the saphenous artery could reach about 1.6‐fold and 2‐fold as much as those in the control group. Furthermore, the boost in the inner diameter and perfusion initiated by lumbar sympathectomy is even more profound than that initiated by nerve transection or nerve‐artery disconnection. It might be explained by lumbar sympathectomy triggering a more wide‐ranged disruption to the vascular tone and the circulation from the level of diaphragm down. The results further validate the clinical application of lumbar sympathectomy in a circumstance when conservative treatments fail, and endovascular procedures and bypass surgery are impossible.
A weakness of this study is that the long‐term impact on the vascular function initiated by the various ways of sympathectomy is unexplored. Also, the outcomes of the study obtained in rodents should be verified in large animals before being translated to clinical practice.
In conclusions, our study provides novel anatomic knowledge about how a peripheral artery is innervated by segmental sympathetic branches emanated from a nerve morphing into a primary and a secondary sympathetic trunk. Also, we demonstrate that nerve‐artery disconnection is a theoretic option for wide‐ranged sympathectomy of a peripheral artery.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ACKNOWLEDGEMENTS
We are grateful to the technical assistance from Lin Ling of Experimental center of Fujian Medical University. We are deeply grateful for the funding from Fujian natural science foundation (2020 J01625), Hunan natural science foundation (2019JJ40010).
Xie Y, Fang F, Lin P, Zhang Z, Zhuang Y. Segmental branches emanating from saphenous nerve morphing into sympathetic trunks for innervation of saphenous artery and its clinical implication for arterial sympathectomy. Int Wound J. 2022;19(2):294–304. 10.1111/iwj.13630
Yun Xie and Fang Fang made the same contribution to this study, and should be considered as co‐first author.
Funding information Fujian Natural Science Foundation, Grant/Award Number: 2020J01625; Hunan Natural Science Foundation, Grant/Award Number: 2019JJ40010
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Sen I, Agarwal S, Tharyan P, Forster R. Lumbar sympathectomy versus prostanoids for critical limb ischaemia due to non‐reconstructable peripheral arterial disease. Cochrane Database Syst Rev. 2018;4:CD009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karanth VK, Karanth TK, Karanth L. Lumbar sympathectomy techniques for critical lower limb ischaemia due to non‐reconstructable peripheral arterial disease. Cochrane Database Syst Rev. 2016;12:CD011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murata K, Omokawa S, Kobata Y, Tanaka Y, Yajima H, Tamai S. Long‐term follow‐up of periarterial sympathectomy for chronic digital ischaemia. J Hand Surg Eur Vol. 2012;37:788‐793. [DOI] [PubMed] [Google Scholar]
- 4. Kullo IJ, Rooke TW. Clinical practice. Peripheral artery disease. N Engl J Med. 2016;374:861‐871. [DOI] [PubMed] [Google Scholar]
- 5. Norgren L, Hiatt WR, Dormandy JA, et al. Inter‐society consensus for the management of peripheral arterial disease (tasc ii). Eur J Vasc Endovasc Surg: Off J Eur Soc Vasc Surg. 2007;33(Suppl 1):S1‐S75. [DOI] [PubMed] [Google Scholar]
- 6. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599‐1606. [DOI] [PubMed] [Google Scholar]
- 7. Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham study. J Am Geriatr Soc. 1985;33:13‐18. [DOI] [PubMed] [Google Scholar]
- 8. Tay VK, Fitridge R, Tie ML. Computed tomography fluoroscopy‐guided chemical lumbar sympathectomy: simple, safe and effective. Aust Radiol. 2002;46:163‐166. [DOI] [PubMed] [Google Scholar]
- 9. Wilgis EFS. Evaluation and treatment of chronic digital ischemia. Ann Surg. 1981;193:693‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller LM, Morgan RF. Vasospastic disorders. Etiology, recognition, and treatment. Hand Clin. 1993;9:171‐187. [PubMed] [Google Scholar]
- 11. Cappelli L, Wigley FM. Management of Raynaud phenomenon and digital ulcers in scleroderma. Rheum Dis Clin North Am. 2015;41:419‐438. [DOI] [PubMed] [Google Scholar]
- 12. Weinrich MC, Maricq HR, Keil JE, McGregor AR, Diat F. Prevalence of Raynaud phenomenon in the adult population of South Carolina. J Clin Epidemiol. 1990;43:1343‐1349. [DOI] [PubMed] [Google Scholar]
- 13. O'Keeffe ST, Tsapatsaris NP, Beetham WP Jr. Color chart assisted diagnosis of Raynaud's phenomenon in an unselected hospital employee population. J Rheumatol. 1992;19:1415‐1417. [PubMed] [Google Scholar]
- 14. Fraenkel L, Zhang Y, Chaisson CE, et al. Different factors influencing the expression of Raynaud's phenomenon in men and women. Arthritis Rheum. 1999;42:306‐310. [DOI] [PubMed] [Google Scholar]
- 15. Gelber AC, Wigley FM, Stallings RY, et al. Symptoms of Raynaud's phenomenon in an inner‐city African‐American community: prevalence and self‐reported cardiovascular comorbidity. J Clin Epidemiol. 1999;52:441‐446. [DOI] [PubMed] [Google Scholar]
- 16. Waris T, Kaarela O, Lasanen L, Junila J, Ruuskanen M, Kyösola K. Perivascular sympathectomy does not remove adrenergic nerves from distal vessels. The effect of various denervations on the rat saphenous bundle: a histochemical study. J Surg Res. 1991;51:303‐309. [DOI] [PubMed] [Google Scholar]
- 17. Junila J, Kaarela O, Waris T. Failure of perivascular sympathectomy to remove adrenergic nerves from peripheral vessels of the rabbit ear skin. Scand J Plast Reconstr Surg Hand Surg. 1991;25:199‐202. [DOI] [PubMed] [Google Scholar]
- 18. Cotton LT, Cross FW. Lumbar sympathectomy for arterial disease. Br J Surg. 1985;72:678‐683. [DOI] [PubMed] [Google Scholar]
- 19. Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. Aalborg, Denmark: Elsevier Limited; 2016. [Google Scholar]
- 20. Kaarela O, Raatikainen T, Carlson S, Huopaniemi T, Waris T. Effect of perivascular sympathectomy on distal adrenergic innervation in the hands of monkeys. J Hand Surg. 1991;16:386‐388. [DOI] [PubMed] [Google Scholar]
- 21. Fang F, Zou W, Zhang Z, Zhang Q, Xie Y. Patterns of sural nerve innervation of the sural artery with implication for reconstructive surgery. J Surg Res. 2017;220:261‐267. [DOI] [PubMed] [Google Scholar]
- 22. Hweidi SA, Lee S, Wolf P. Effect of sympathectomy on microvascular anastomosis in the rat. Microsurgery. 1985;6:92‐96. [DOI] [PubMed] [Google Scholar]
- 23. Chen C, Zhang M, Yu N, Zhang W, Wang X. Heterogeneous features of keloids assessed by laser speckle contrast imaging: a cross‐sectional study. Lasers Surg Med. 2020. 10.1002/lsm.23331. [DOI] [PubMed] [Google Scholar]
- 24. Kelly A, Pai A, Lertsakdadet B, Choi B, Kelly KM. Microvascular effects of pulsed dye laser in combination with oxymetazoline. Lasers Surg Med. 2020;52:17‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ronn JH, Nerup N, Strandby RB, et al. Laser speckle contrast imaging and quantitative fluorescence angiography for perfusion assessment. Langenbecks Arch Surg. 2019;404:505‐515. [DOI] [PubMed] [Google Scholar]
- 26. Humeau‐Heurtier A, Marche P, Dubois S, Mahe G. Analysis of microvascular perfusion with multi‐dimensional complete ensemble empirical mode decomposition with adaptive noise algorithm: processing of laser speckle contrast images recorded in healthy subjects, at rest and during acetylcholine stimulation. Conference proceedings: Annual international conference of the IEEE engineering in medicine and biology society. IEEE engineering in medicine and biology society. Annu Conf. 2015;2015:7370‐7373. [DOI] [PubMed] [Google Scholar]
- 27. Ma Q, Liu D, Gong R, Chen S, Fang F, Zhuang Y. Mechanically induced vasospasm‐evaluation of spasmolytic efficacy of 10 pharmaceutical agents using laser speckle contrast imaging. Lasers Surg Med. 2020. 10.1002/lsm.23347. [DOI] [PubMed] [Google Scholar]
- 28. Pace CS, Merritt WH. Extended periarterial sympathectomy: evaluation of long‐term outcomes. Hand. 2018;13:395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barcroft H, Walker AJ. Return to tone in blood‐vessels of the upper limb after sympathectomy. Lancet. 1949;1:1035‐1039. [DOI] [PubMed] [Google Scholar]
- 30. Flatt AE. Digital artery sympathectomy. J Hand Surg Am. 1980;5:550‐556. [DOI] [PubMed] [Google Scholar]
- 31. Goddard N. Commentary on murata et al. long‐term follow‐up of periarterial sympathectomy for chronic digital ischaemia. J Hand Surg Eur Vol. 2012;37:794. [DOI] [PubMed] [Google Scholar]
- 32. Merritt WH. Role and rationale for extended periarterial sympathectomy in the management of severe Raynaud syndrome: techniques and results. Hand Clin. 2015;31:101‐120. [DOI] [PubMed] [Google Scholar]
- 33. McCall TE, Petersen DP, Wong LB. The use of digital artery sympathectomy as a salvage procedure for severe ischemia of Raynaud's disease and phenomenon. J Hand Surg Am. 1999;24:173‐177. [DOI] [PubMed] [Google Scholar]
- 34. Soberón JR Jr, Greengrass RA, Davis WE, Murray PM, Feinglass N. Intermediate‐term follow‐up of chronically ill patients with digital ischemia treated with peripheral digital sympathectomy. Rheumatol Int. 2016;36:301‐307. [DOI] [PubMed] [Google Scholar]
- 35. Letamendia A, López‐Román J, Bustamante‐Munguira J, Herreros J. Digital periarterial sympathectomy in the management of post‐traumatic Raynaud syndrome. J Vasc Surg. 2016;63:459‐465. [DOI] [PubMed] [Google Scholar]
- 36. Devgire V, Hughes M. Raynaud's phenomenon. Br J Hosp Med (London, England: 2005). 2019;80:658‐664. [DOI] [PubMed] [Google Scholar]
- 37. Temprano KK. A review of Raynaud's disease. Mo Med. 2016;113:123‐126. [PMC free article] [PubMed] [Google Scholar]
- 38. Valdovinos ST, Landry GJ. Raynaud syndrome. Tech Vasc Interv Radiol. 2014;17:241‐246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


