Abstract
Chronic venous insufficiency is a chronic disease of the venous system with a prevalence of 25% to 40% in females and 10% to 20% in males. Venous leg ulcers (VLUs) result from venous insufficiency. VLUs have a prevalence of 0.18% to 1% with a 1‐year recurrence of 25% to 50%, bearing significant socioeconomic burden. It is therefore important for regular assessment and monitoring of VLUs to prevent worsening. Our study aims to assess the intra‐ and inter‐rater reliability of a machine learning‐based handheld 3‐dimensional infrared wound imaging device (WoundAide [WA] imaging system, Konica Minolta Inc, Tokyo, Japan) compared with traditional measurements by trained wound nurse. This is a prospective cross‐sectional study on 52 patients with VLUs from September 2019 to January 2021 using three WA imaging systems. Baseline patient profile and clinical demographics were collected. Basic wound parameters (length, width and area) were collected for both traditional measurements and measurements taken by the WA imaging systems. Intra‐ and inter‐rater reliability was analysed using intra‐class correlation statistics. A total of 222 wound images from 52 patients were assessed. There is excellent intra‐rater reliability of the WA imaging system on three different image captures of the same wound (intra‐rater reliability ranging 0.978‐0.992). In addition, there is excellent inter‐rater reliability between the three WA imaging systems for length (0.987), width (0.990) and area (0.995). Good inter‐rater reliability for length and width (range 0.875‐0.900) and excellent inter‐rater reliability (range 0.932‐0.950) were obtained between wound nurse measurement and each of the WA imaging system. In conclusion, high intra‐ and inter‐rater reliability was obtained for the WA imaging systems. We also obtained high inter‐rater reliability of WA measurements against traditional wound measurement. The WA imaging system is a useful clinical adjunct in the monitoring of VLU wound documentation.
Keywords: chronic venous insufficiency, machine learning, venous leg ulcer, wound healing, wound imaging
1. INTRODUCTION
Chronic venous insufficiency (CVI) is a debilitating chronic disease of the venous system commonly affecting the lower limbs, with a prevalence of 25% to 40% in females and 10% to 20% in males. 1 , 2 Mild to moderate CVI may manifest itself as varicose veins, swelling, lipodermatosclerosis associated with itch, burning sensation or pain, hence impacting on patients' quality of life. 3 More severe forms of CVI may present itself as venous leg ulcers (VLUs), or in rare cases, have malignant transformation into Marjolin's ulcer. 4 , 5
VLUs have a prevalence of 0.18% to 1% with a 1‐year recurrence of 25% to 50%, bearing significant socioeconomic burden. 6 , 7 , 8 Management of VLUs is hence important to reduce morbidity; similar to other chronic wounds, management of VLUs requires multidisciplinary team of doctors, nurses, and allied health professionals for successful management. In majority of VLUs where the primary aetiology is venous hypertension, compression is the mainstay treatment option. 9 Wound care, consisting of wound assessment, monitoring, and management, is another important facet of the management of VLU. Traditionally, wound assessment and monitoring are performed by specialised and trained wound nurses for patients with stable VLUs in specialist outpatient clinics. With advancements in technology, there are to‐date several commercially available wound assessment or monitoring systems available for monitoring of chronic wounds. 10
Our study group performed a recent systematic review in 2020 on existing wound imaging modalities available. 11 Wound monitoring and imaging systems help in standard practice by being contactless (reducing risk of infection) and time‐effective; some features include easy trending of results, allow easy integration into electronic health record systems, and remote monitoring of wounds through images uploaded by their patients. 12 , 13 , 14 , 15 However, we concluded the following: (a) a paucity of existing studies evaluating the efficacy of wound assessment and imaging systems; (b) bias in existing studies evaluating the effectiveness of wound imaging systems with lack of sample size calculation. 11 Majority of existing commercially available wound monitoring systems have not been reviewed in the literature on measurement accuracy. This may be of greater significance in VLUs which occur predominantly in gaiter regions with sloping edges and often circumferential in shape, hence rendering greater difficulty in measurement of ulcer size. This study aims to add on to existing literature on commercially available wound imaging systems, by clinically validating a 3‐dimensional (3‐D) enabled machine learning‐based imaging system (WoundAide (WA) imaging system, Konica Minolta Inc, Tokyo, Japan) against traditional wound assessment measurements in patients with VLUs.
2. METHODS
This is a prospective cross‐sectional study on patients with VLUs from September 2019 to January 2021 in a single‐centre university‐affiliated tertiary hospital. Inclusion criteria were all patients aged 21 years and above with VLUs. Exclusion criteria were patients who were pregnant, breastfeeding, had leg ulcers of non‐venous origin such as primarily arterial ulcer or neuropathic ulcer, or did not have capacity to consent. Mixed arterio‐venous ulcers were included in the study. Our study was approved by a local institutional review board (National Healthcare Group Domain Specific Review Board Ref No: 2019/00566). Written consent was obtained for all patients included in the study with appropriate translations as required for non‐English speakers.
2.1. Study protocol
Our study protocol is shown in Figure 1. Patients were identified and recruited from both inpatient and outpatient settings. Baseline demographics and clinical profile were collected prior to the study. The approximate study duration for each patient is five visits or until complete resolution of the ulcer, whichever is earlier. All patients who were included in the study were subjected to a standardised VLU management pathway with standardised follow‐up. Participants did not require additional clinic visits for the purpose of this study. During each clinic visit, wound measurements were recorded traditionally by a trained specialised wound nurse and electronically by a dedicated research coordinator using the WA imaging system. Wound episode was defined as the wound images taken at each clinic visit. This implied that each wound episode will comprise nine wound images if three images were taken by each WA device (a total of three WA devices were used).
FIGURE 1.

Study protocol for participation recruitment and standardisation of process for wound measurement
2.2. WoundAide imaging system
The WA imaging system is a medical device consisting of a 3‐D depth perception‐enabled camera attached to a tablet device (iPad). The 3‐D camera uses a laser projector to cast structured light, precise pattern of thousands of invisible infrared dots onto targeted object. It then uses a frequency‐matched infrared camera to record pattern changes, thereby capturing the 3‐D data of the targeted object. The 3‐D camera is a class‐I laser product, it is safe under all conditions of normal use (without optical aids such as telescope or microscope). The WA imaging system permits automated detection of wound margins and measurement of wound dimensions using machine‐learning algorithms. Data output is captured and stored on the WA imaging system which can be remotely assessed using a secured web‐based portal system or uploaded directly into the hospital electronic medical records system to improve overall patient management.
2.3. Standardisation of ulcer measurement
For patients with multiple ulcers, an index ulcer was identified for the purpose of the study during the first clinic visit which will be monitored during subsequent clinic visits. Prior to consultation with a doctor for their regular clinic visit, patients were directed to a dedicated room with adequate lighting. Measurement of the index ulcer was performed in the room. All participants were positioned sitting on a chair with their feet overhanging. Wound measurements (length and width) were taken traditionally by a specialised trained wound nurse first, and subsequently taken using the WA imaging system by a dedicated research coordinator. Two trained specialised wound nurses were involved in the wound measurement, with standardisation of measurements performed prior to the conduct of the study: Tracing paper was placed over wound, followed by the use of sterile marker pen to outline the wound. The length (defined as the longest axis) and width (defined as longest axis perpendicular to the length) were measured using the traced wound (Figure 2). Area was calculated by multiplying length and width.
FIGURE 2.

Schematic diagram for the determination of length and width using traditional measurements by a trained wound nurse
After manual measurements were taken by the wound nurse, the wound nurse will leave the dedicated treatment room. A dedicated research coordinator will then enter the room to reduce bias to each other's findings. Digital photograph of the wound with a reference ruler was subsequently taken with a digital camera (Canon PowerShot G7X) by the dedicated research coordinator. The research coordinator then takes digital measurement of the wound with three separate WA imaging systems (version 1.5.0.10) on iPad (iPad Mini 4, iOS version 12.4.1). An optical zoom of ×1.0 was used to capture the images at approximately 40 cm from the wound (Figure 3). Three images of the same wound were taken from each iPad device. Each repeat image of the same wound involved repositioning of the research coordinator and the patient. This was repeated across the three different devices. The parameters measured (length, width and area) were automatically calculated based on the image boundaries determined by the imaging system. The application interface of the WA imaging system is shown in Figure 4. Automated boundary detection was difficult or vastly different from the actual wound boundaries in a small select group of wound images in view of (a) poor colour contrast with patients' skin tone, (b) were too small (<1 cm), or (c) were located in areas where there is large variation in skin contours (such as bony prominences on the malleolus). Manual adjustments were made to the wound boundary selection window in these circumstances.
FIGURE 3.
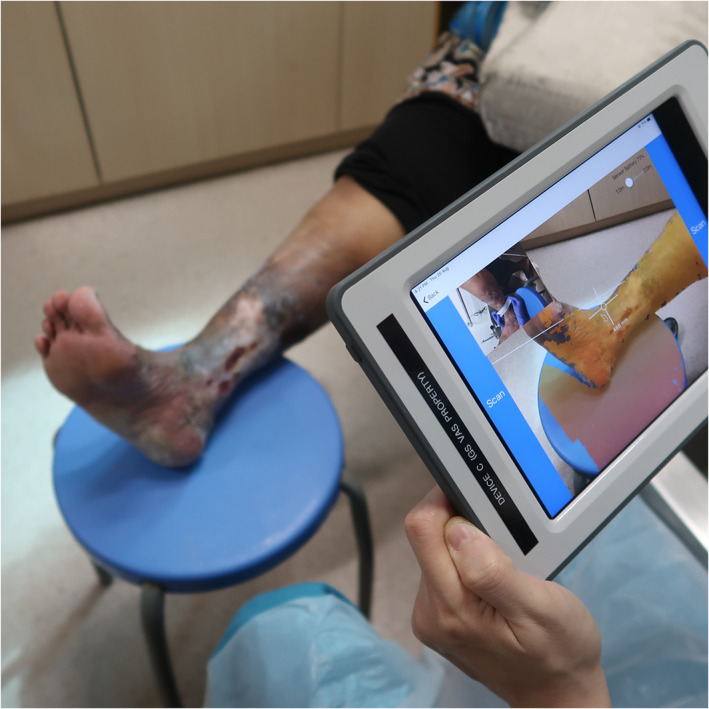
Usage of the WoundAide imaging system for the measurement of venous leg ulcer at approximately 40 cm from the wound with optical zoom of ×1.0
FIGURE 4.

Application interface of the WoundAide imaging system (Version 1.5.0.10) on an iPad (iPad Mini 4, iOS version 12.4.1)
2.4. Sample size calculation
Sample size was calculated as per number of wound images (defined as wound episode) rather than the number of subjects as this is a cross‐sectional study on wound imaging. Based on WA's internal validation and pilot analysis of 30 patients with 60 wound images, the baseline mean accuracy is 85% to 95%. Hence, assuming baseline correction (R0) at 0 and alternative correlation (R1) at 0.2, sample size required for one correlation test with power 90% and alpha 0.05 is 341 wound images.
2.5. Statistical analysis
All statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, III., USA). Statistical significance was determined by P < .05. Intra‐class correlation statistics (ICC) was used to analyse intra‐rater and inter‐rater reliability. 16 Intra‐rater reliability between measurements taken by the same WA device, and inter‐rater reliability between the wound nurse measurements and each WA device was analysed using two‐way mixed effects model, absolute agreement, and single measure. Inter‐rater reliability between the WA devices was analysed using two‐way random effects model, absolute agreement, and single measure. Two‐way random effects model was used for inter‐rater reliability between the WA devices for generalising our results for all existing WA devices in the market. 17
There is no standard definition or cut‐offs for ICC to determine the extent of reliability; we used the following definitions in our study: ICC <0.5 indicates poor reliability, 0.5 to 0.75 indicates moderate reliability, between 0.75 and 0.9 indicates good reliability and > 0.9 indicates excellent reliability. 18
3. RESULTS
3.1. Patient demographics and clinical profile
A total of 52 patients were included in the study, with a median age of 67 (interquartile range [IQR] 60.3‐75.8) and male predominance (n = 30, 57.7%). The commonest co‐morbidity was presence of peripheral vascular disease (n = 50, 86.2%). Forty‐six patients (88.5%) had previous history of skin and/or leg ulcers. Twenty‐five patients (48.1%) had varicose veins, and 16 (30.8%) had history of previous venous surgery. The commonest sites of ulcer were at the medial malleolus (n = 17, 32.7%) and the lateral malleolus (n = 15, 28.8%). The overall patient demographics is summarised in Table 1.
TABLE 1.
Clinical profile of patients included in the study
| Number of patients (n = 52) | |
|---|---|
| Age, median (IQR) | 67 (60.3‐75.8) |
| Gender, male, n (%) | 30 (57.7) |
| Co‐morbidities, n (%) | |
| Diabetes mellitus | 18 (34.6) |
| Hypertension | 31 (59.6) |
| Heart disease | 9 (17.3) |
| Kidney disease | 7 (13.5) |
| Cerebrovascular accident | 5 (9.6) |
| Peripheral vascular disease | 50 (96.2) |
| Varicose veins | 25 (48.1) |
| Previous venous surgery | 16 (30.8) |
| Previous skin/leg ulcers | 46 (88.5) |
| Location of ulcer, n (%) | |
| Calf | 3 (5.8) |
| Shin | 12 (23.1) |
| Medial gaiter | 2 (3.8) |
| Medial malleolus | 17 (32.7) |
| Lateral malleolus | 15 (28.8) |
| Dorsum of foot | 3 (5.8) |
| Wound parameters, median (IQR) | |
| Length, cm | 3.10 (2.00‐5.88) |
| Width, cm | 2.35 (1.28‐4.00) |
| Area, cm2 | 6.65 (2.10‐11.50) |
Abbreviations: IQR, interquartile range.
3.2. Wound episodes
A total of 222 wound episodes with resulting 1621 wound images were analysed in this study. Three hundred seventy‐seven wound images (exclusive of the above count) were not included in this analysis in view of missing data: patients have yet to attend their subsequent follow‐up, defaulted their appointment, had complete resolution of ulcer or due to unavailability of a particular WA device during the clinic visit. Clinical characteristics of each wound episode is summarised in Table 2. Table 3 summarises the intra‐rater reliability of the wound images taken by the same WA device. We obtained excellent intra‐rater for length (intra‐rater reliability ranging 0.978‐0.989), width (intra‐rater reliability ranging 0.978‐0.980), and area (intra‐rater reliability ranging 0.990‐0.992).
TABLE 2.
Baseline characteristics of all included wound episodes
| Number of wound episodes (n = 222) | |
|---|---|
| Age, median (IQR) | 67 (60.8‐74) |
| Gender, male, n (%) | 137 (61.7) |
| Co‐morbidities | |
| Diabetes mellitus | 76 (34.2) |
| Hypertension | 126 (56.8) |
| Heart disease | 39 (17.6) |
| Kidney disease | 27 (12.2) |
| Cerebrovascular accident | 24 (10.8) |
| Peripheral vascular disease | 214 (96.4) |
| Varicose veins | 104 (46.8) |
| Previous venous surgery | 68 (30.6) |
| Previous skin/leg ulcers | 199 (89.6) |
| Location of ulcer | |
| Calf | 13 (5.9) |
| Shin | 51 (23.0) |
| Medial gaiter | 8 (3.6) |
| Medial malleolus | 72 (32.4) |
| Lateral malleolus | 64 (28.8) |
| Dorsum of foot | 14 (6.3) |
| Wound parameters, median (IQR) | |
| Length, cm | 3.00 (1.90‐5.03) |
| Width, cm | 2.20 (1.10‐3.50) |
| Area, cm2 | 5.00 (1.68‐11.00) |
Abbreviations: IQR, interquartile range.
TABLE 3.
Intra‐rater reliability of the same WoundAide device on three different images obtained from the same wound
| Measurements, mean ± SD | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image 1 | Image 2 | Image 3 | Intra‐rater reliability (95% CI) | P value | |||||||||||
| Length | Width | Area | Length | Width | Area | Length | Width | Area | Length | Width | Area | Length | Width | Area | |
| Device 1 | 3.43 ± 2.55 | 2.49 ± 1.73 | 9.06 ± 12.69 | 3.45 ± 2.49 | 2.49 ± 1.69 | 9.00 ± 12.27 | 3.44 ± 2.52 | 2.49 ± 1.74 | 9.17 ± 12.80 | 0.979 (0.974, 0.983) | 0.978 (0.972, 0.982) | 0.990 (0.987, 0.992) | <.001 | <.001 | <.001 |
| Device 2 | 3.34 ± 2.43 | 2.47 ± 1.69 | 8.88 ± 12.55 | 3.34 ± 2.43 | 2.45 ± 1.69 | 8.86 ± 12.30 | 3.30 ± 2.40 | 2.46 ± 1.76 | 8.83 ± 12.53 | 0.978 (0.973, 0.983) | 0.979 (0.973, 0.983) | 0.990 (0.988, 0.992) | <.001 | <.001 | <.001 |
| Device 3 | 3.45 ± 2.52 | 2.48 ± 1.70 | 9.18 ± 12.90 | 3.42 ± 2.54 | 2.49 ± 1.75 | 9.32 ± 13.08 | 3.42 ± 2.52 | 2.48 ± 1.74 | 9.19 ± 12.76 | 0.989 (0.986, 0.991) | 0.980 (0.975, 0.984) | 0.992 (0.990, 0.994) | <.001 | <.001 | <.001 |
Note: Length and breadth are expressed in cm. Area was expressed in cm2.
Table 4 summarises the inter‐rater reliability between the three WA devices; we obtained excellent inter‐rater reliability between the three WA devices for length (inter‐rater reliability 0.987 [95% CI: 0.983‐0.990, P < .001]), width (inter‐rater reliability 0.990 [95% CI: 0.988‐0.992, P < .001]), and area (inter‐rater reliability 0.995 [95% CI: 0.994‐0.996, P < .001]).
TABLE 4.
Inter‐rater reliability of the three different WoundAide device on the first image, second image and third image and the average measurements across all three images taken on each device
| Measurements, mean ± SD | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Device 1 | Device 2 | Device 3 | Inter‐rater reliability | P value | |||||||||||
| Length | Width | Area | Length | Width | Area | Length | Width | Area | Length | Width | Area | Length | Width | Area | |
| Image | 3.45 ± 2.50 | 2.50 ± 1.71 | 9.13 ± 12.56 | 3.36 ± 2.44 | 2.47 ± 1.69 | 8.99 ± 12.48 | 3.49 ± 2.57 | 2.52 ± 1.74 | 9.50 ± 13.23 | 0.987 (0.983, 0.990) | 0.990 (0.988, 0.992) | 0.995 (0.994, 0.996) | <.001 | <.001 | <.001 |
Note: Length and breadth are expressed in cm. Area was expressed in cm2.
Tables 5, 6, and 7 summarise the inter‐rater reliability between the trained wound nurse and each of the WA devices. We obtained good inter‐rater reliability for length (range 0.875‐0.889) and width (range 0.891‐0.900) and excellent inter‐rater reliability for area (range 0.932‐0.950).
TABLE 5.
Inter‐rater reliability between wound nurse and WoundAide device 1 for the corresponding image
| Measurements, mean ± SD | Inter‐rater reliability | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Width | Area | Length | Width | Area | Length | Width | Area | |
| Device 1 | 3.43 ± 2.49 | 2.49 ± 1.70 | 9.03 ± 12.47 | 0.882 (0.829, 0.916) | 0.893 (0.860, 0.918) | 0.936 (0.899, 0.957) | <.001 | <.001 | <.001 |
| Wound nurse | 3.86 ± 2.82 | 2.67 ± 1.94 | 10.95 ± 16.36 | ||||||
Note: Length and breadth are expressed in cm. Area was expressed in cm2.
TABLE 6.
Inter‐rater reliability between wound nurse and WoundAide device 2 for the corresponding image
| Measurements, mean ± SD | Inter‐rater reliability | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Width | Area | Length | Width | Area | Length | Width | Area | |
| Device 2 | 3.36 ± 2.44 | 2.47 ± 1.69 | 8.99 ± 12.48 | 0.875 (0.802, 0.917) | 0.891 (0.854, 0.919) | 0.932 (0.890, 0.956) | <.001 | <.001 | <.001 |
| Wound nurse | 3.88 ± 2.83 | 2.69 ± 1.95 | 11.07 ± 16.48 | ||||||
Note: Length and breadth are expressed in cm. Area was expressed in cm2.
TABLE 7.
Inter‐rater reliability between wound nurse and WoundAide device 3 for the corresponding image
| Measurements, mean ± SD | Inter‐rater reliability | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Width | Area | Length | Width | Area | Length | Width | Area | |
| Device 3 | 3.47 ± 2.55 | 2.51 ± 1.73 | 9.39 ± 13.14 | 0.889 (0.844, 0.920) | 0.900 (0.870, 0.923) | 0.950 (0.926, 0.965) | <.001 | <.001 | <.001 |
| Wound nurse | 3.86 ± 2.82 | 2.67 ± 1.94 | 10.95 ± 16.36 | ||||||
Note: Length and breadth are expressed in cm. Area was expressed in cm2.
4. DISCUSSION
Developing technology in wound imaging systems increases their relevance in clinical care. Features such as automated wound measurements, analysis of wound bed and easy transmission of data into hospital electronic medical records improves the efficiency in the complex management of VLUs. The integration of 3‐D sensors and machine learning algorithms allows the modification of commercially available electronic devices such as the iPad to be used as an adjunct in the clinical setting. This study demonstrated high intra‐rater reliability between devices and inter‐rater reliability between the WA imaging system and traditional wound measurements by wound nurse.
Our study group recently summarised existing literature (till March 2020) on the use of wound assessment, imaging, and monitoring systems in diabetic foot ulcers (DFUs). 11 Unlike DFUs which are secondary to arterial insufficiency and/or neuropathy, VLUs are most commonly caused by venous hypertension secondary to CVI or post‐thrombotic syndrome. 19 Characteristics of VLUs are also different from DFUs; VLUs are usually located at the gaiter region or malleolus, shallow, irregular in shape, and have sloping edges. 20 Location of VLUs on bony prominences with contours and the presence of sloping edges with irregular shape may increase difficulty in the automated detection of wound edges. Locally, a pilot study by Khong et al in 2017 demonstrated high reliability of WA on VLUs, with intraclass correlation coefficient of >0.95 for length, width, and area across all measuring devices. 21 The study also demonstrated similar reliability of WA with existing imaging systems, WoundZoom (WoundZoom, Inc., Wisconsin, United States) and Visitrak (Smith & Nephew, London, United Kingdom). Unfortunately, the study was a pilot study which evaluated only 10 venous wounds and readers are unable to draw conclusive interpretations from the study. Our current study adds on to the previous study by Khong et al with excellent intra‐rater reliability (ICC >0.900) for each of the WA imaging system and inter‐rater reliability between each of the WA imaging systems.
Foltynski et al examined the accuracy of various commercially available wound monitoring systems, Visitrak (Smith & Nephew, London, United Kingdom) device, the SilhouetteMobile device (ARANZ Medical Ltd., Christchurch, New Zealand), and the TeleDiaFoS system (Nalecz Institute of Biocybernetics and Biomedical Engineering, Warsaw, Poland) demonstrated low overall relative errors of 6.8%, 2.3%, and 2.1%, respectively. 22 The overall relative errors were significantly lower (relative error for traditional method = 13.3%, P < .001) compared with traditional measurement using the elliptical method (area of the wound A was calculated using the formula for an ellipse, where A = ¼ [π × l × w], where l is the longest length, and w is the longest width perpendicular to l). The overestimation of wound measurements using standard measurements has been previously demonstrated; Rogers et al showed that standard measurements in ruler (where area = length × width) overestimated area by up to 41%, and Chan et al demonstrated that traditional measurements for wound area were 13.4% to 25.2% higher compared with measurements using the CARES4WOUNDS system, an artificial intelligence‐enabled wound imaging mobile application (Tetsuyu, Singapore). 23 , 24 Our study is concordant with available literature; we showed an overestimation in wound area by 21.3% to 39.9% compared with the WA measurements. Our recent study on the use of the CARES4WOUNDS system also showed at least good inter‐rater reliability (ICC 0.872‐0.932) compared with traditional wound measurements. This is shown in our current study (ICC 0.932‐0.950).
Unlike some mobile applications which use the native camera system of the handheld device, the WA imaging system requires the installation of a 3‐D camera sensor to serve its purpose of depth perception and automated wound detection. While this may lead to inconvenience in preparation of the equipment prior to obtaining wound measurements, the precision of the WA imaging system is not limited by the development of tablet companies. The use of machine learning algorithms, similar to existing wound imaging systems, allow the continued improvement in detection of wound boundaries and better characterisation of wound bed, especially over bony prominences or in a circumferential fashion.
The high intra‐ and inter‐rater reliability demonstrated in our study for the WA imaging system is a stepping stone for its use in application practice. Compared with traditional wound measurements which require tracing of wound, followed by manual measurement of length and width and calculation of wound area, this tedious task may be substituted by wound imaging systems which permit quick image capture and calculation of wound parameters, reducing unnecessary time spent. Our institution previously showed that there were 5791 clinic consults locally in our institution from 2013 to 2017 with an average cost of USD$92 per episode. 8 Reduction in time taken during wound assessment may reduce waiting times and result in cost savings. 25 Au et al compared the use of Swift app (Swift Medical, Ontario, Canada), a mobile application for wound management, to standard measurements using ruler; they showed that the use of Swift app (including the time for wound measurement and documentation) was significantly faster (57% faster) compared with traditional ruler measurements (Swift app 30.77 ± 5.21 seconds vs ruler measurement 48.17 ± 7.81 seconds, P < .001). 26 This is an important finding as wound monitoring includes assessment of the wound parameters, wound bed characteristics, as well as documentation/trending of wound parameters to observe for the extent of healing. It is likely that wound applications will be faster in measurement compared with traditional measurements as boundaries and parameters are automatically calculated; hence, combined time taken should be taken into account, for which Au et al showed almost 100% faster for Swift app in terms of measurement time, and 57% faster when taking into account measurement and documentation time. Unfortunately, apart from wound parameters, there are other aspects of wound assessment, which include characterisation of the wound, such as determining the extent of granulation tissue, slough, and the extent of exudates or discharge. These are important features which determine subsequent management. 27 A recent study in 2021 by Howell et al showed reliability of their artificial intelligence‐based software in quantification of the extent of granulation tissue using 199 clinical photographs. 28 The use of wound‐imaging devices to monitor wound bed characteristics is promising and should be studied in future studies.
The strength of our study is the large sample size of 1621 wound images compared with existing studies. We included baseline clinical characteristics of the study population and location of the ulcers. We also included multiple parameters in wound assessment (length, width and area) in the wound imaging system which is not present in some of the existing reviews of wound imaging products. We also had a clear study protocol starting from the recruitment of patients to their follow‐up. Wound measurement was also standardised; only one research coordinator was involved in the digital image capture using the WA devices.
Our study however has its limitations. We also did not include qualitative characteristics of the wound (such as the extent of granulation tissue formation, presence of exudates, eschar and edema) as the main aim of our study was to examine the measurement accuracy of the WA imaging system. Time was not assessed to determine the efficiency of the WA imaging system compared with traditional measurements. We were also unable to have a single dedicated wound nurse to be involved in the traditional measurements in view of the large sample size and time required. However, we limited the number of nurses to two and standardised the method of measurement.
5. CONCLUSION
Our study demonstrates excellent inter‐ and intra‐rater reliability of the measurements taken by the WA imaging systems against traditional wound assessment by a trained wound nurse. The WA imaging system serves as a useful adjunct in the monitoring of VLUs. Technical limitations in small select group of patients limit the accuracy of the detection of wound boundaries, in which those cases, require manual adjustments. Further large‐scale prospective studies should be carried out to assess other features of the wound monitoring system, such as wound characterisation, to better maximise its use as a clinical adjunct in the monitoring of VLUs.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
Ban Wei CHUA, Yu Xuan LI, Eric KWAN, Gautam KUMAR SINGH, Fu Song CHIAM and Julious GLORI from Konica Minolta (Singapore), for technical assistance in WA device optimization during the course of the study.
Chan KS, Liang S, Cho YT, et al. Clinical validation of a machine‐learning‐based handheld 3‐dimensional infrared wound imaging device in venous leg ulcers. Int Wound J. 2022;19(2):436–446. 10.1111/iwj.13644
Funding information Agency for Science, Technology and Research (A*STAR): Industry Alignment Fund ‐ Pre‐Positioning; Programme (IAF‐PP), Grant/Award Number: H17/01/a0/004 and H19/01/a0/0Y9; Konica Minolta Inc (Tokyo, Japan) ‐ Investigator‐initiated
DATA AVAILABILITY STATEMENT
The research data is not publicly available. Special requests may be made to the corresponding author for request of data.
REFERENCES
- 1. Bergan JJ, Schmid‐Schönbein GW, Smith PDC, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(5):488‐498. [DOI] [PubMed] [Google Scholar]
- 2. Fowkes F. Prevalence and risk factors for chronic venous insufficiency. Acta Phlebol. 2000;1:69‐78. [Google Scholar]
- 3. Duque MI, Yosipovitch G, Chan YH, Smith R, Levy P. Itch, pain, and burning sensation are common symptoms in mild to moderate chronic venous insufficiency with an impact on quality of life. J Am Acad Dermatol. 2005;53(3):503‐507. [DOI] [PubMed] [Google Scholar]
- 4. Beebe HG, Bergan JJ, Bergqvist D, et al. Classification and grading of chronic venous disease in the lower limbs: a consensus statement. Vasc Surg. 1996;30(1):5‐11. [DOI] [PubMed] [Google Scholar]
- 5. Bazaliński D, Przybek‐Mita J, Barańska B, Więch P. Marjolin's ulcer in chronic wounds–review of available literature. Contemp Oncol. 2017;21(3):197‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cornwall JV, Doré CJ, Lewis JD. Leg ulcers: epidemiology and aetiology. Br J Surg. 1986;73(9):693‐696. [DOI] [PubMed] [Google Scholar]
- 7. Franks PJ, Oldroyd MI, Dickson D, Sharp EJ, Moffatt CJ. Risk factors for leg ulcer recurrence: a randomized trial of two types of compression stocking. Age Ageing. 1995;24(6):490‐494. [DOI] [PubMed] [Google Scholar]
- 8. Lo ZJ, Lim X, Eng D, et al. Clinical and economic burden of wound care in the tropics: a 5‐year institutional population health review. Int Wound J. 2020;17:790‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blair SD, Wright D, Backhouse CM, Riddle E, McCollum CN. Sustained compression and healing of chronic venous ulcers. Br Med J. 1988;297(6657):1159‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WoundSource . Wound Assessment & Monitoring Systems. Kestrel Health Information. Published 2020. Accessed 07 Apr 2020. https://www.woundsource.com/product-category/wound-assessment-documentation/wound-assessment-monitoring-systems
- 11. Chan KS, Lo ZJ. Wound assessment, imaging and monitoring systems in diabetic foot ulcers: a systematic review. Int Wound J. 2020;17(6):1909‐1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raizman R, Dunham D, Lindvere‐Teene L, et al. Use of a bacterial fluorescence imaging device: wound measurement, bacterial detection and targeted debridement. J Wound Care. 2019;28(12):824‐834. [DOI] [PubMed] [Google Scholar]
- 13. Papazoglou ES, Neidrauer M, Zubkov L, Weingarten MS, Pourrezaei K. Noninvasive assessment of diabetic foot ulcers with diffuse photon density wave methodology: pilot human study. J Biomed Opt. 2009;14(6):064032. [DOI] [PubMed] [Google Scholar]
- 14. Laji K, Kumar J, Bishop J, Page M. Locally developed digital image archive for diabetic foot clinic: a DGH experience. Pract Diab Int. 2001;18(7):231‐234. [Google Scholar]
- 15. Quan SY, Lazarus GS, Kohli AR, Kapoor R, Margolis DJ. Digital imaging of wounds: are measurements reproducible among observers? Int J Low Extrem Wounds. 2007;6(4):245‐248. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Tang W, Chen G, Lu Y, Feng C, Tu XM. Correlation and agreement: overview and clarification of competing concepts and measures. Shanghai Arch Psychiatry. 2016;28(2):115‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koo TK, Li MY. A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Vol 892. NJ: Pearson/Prentice Hall Upper Saddle River; 2009. [Google Scholar]
- 19. Simon DA, Dix FP, McCollum CN. Management of venous leg ulcers. BMJ. 2004;328(7452):1358‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vasudevan B. Venous leg ulcers: pathophysiology and classification. Indian Dermatol Online J. 2014;5(3):366‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khong P, Yeo M, Goh C. Evaluating an iPad app in measuring wound dimension: a pilot study. J Wound Care. 2017;26(12):752‐760. [DOI] [PubMed] [Google Scholar]
- 22. Foltynski P, Ladyzynski P, Sabalinska S, Wojcicki JM. Accuracy and precision of selected wound area measurement methods in diabetic foot ulceration. Diab Technol Therap. 2013;15(8):712‐721. [DOI] [PubMed] [Google Scholar]
- 23. Rogers LC, Bevilacqua NJ, Armstrong DG, Andros G. Digital planimetry results in more accurate wound measurements: a comparison to standard ruler measurements. J Diabetes Sci Technol. 2010;4(4):799‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chan KS, Chan YM, Tan AHM, et al. Clinical validation of an artificial intelligence‐enabled wound imaging mobile application in diabetic foot ulcers. Int Wound J. 2022;19(1):114‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bailey NT. A study of queues and appointment systems in hospital out‐patient departments, with special reference to waiting‐times. J R Stat Soc B Methodol. 1952;14(2):185‐199. [Google Scholar]
- 26. Au Y, Beland B, Anderson JA, Sasseville D, Wang SC. Time‐saving comparison of wound measurement between the ruler method and the swift skin and wound app. J Cutan Med Surg. 2019;23(2):226‐228. [DOI] [PubMed] [Google Scholar]
- 27. Keast DH, Bowering CK, Evans AW, Mackean GL, Burrows C, D'Souza L. Contents: MEASURE: a proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen. 2004;12:s1‐s17. [DOI] [PubMed] [Google Scholar]
- 28. Howell RS, Liu HH, Khan AA, et al. Development of a method for clinical evaluation of artificial intelligence–based digital wound assessment tools. JAMA Netw Open. 2021;4(5):e217234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data is not publicly available. Special requests may be made to the corresponding author for request of data.


