Abstract
Skin ageing is associated with various structural alterations including a decreased strength of the dermo‐epidermal adhesion increasing the risk for shear type injuries (skin tears). Topical applications of basic skin care products seem to reduce skin tear incidence. The suction blister method leads to the artificial and controlled separation of dermis and epidermis. Therefore, time to blister formation may be used as outcome measuring the strength of dermo‐epidermal adhesion. We conducted an exploratory, randomised, controlled trial with a split‐body design on forearms in healthy female subjects (n = 12; mean age 70.3 [SD 2.1] years). Forearms assigned to the intervention were treated twice daily with petrolatum for 8 weeks. Suction blisters were induced on forearms after 4 and 8 weeks and time to blister formation was measured. Stratum corneum and epidermal hydration were measured and epidermal thickness was assessed via optical coherence tomography. Time to blistering was longer and stratum corneum as well as epidermal hydration was consistently higher in intervention skin areas. We conclude that topical application of basic skin care products may improve mechanical adhesion of the dermo‐epidermal junction and that the parameter “time to blistering” is a suitable outcome to measure dermo‐epidermal adhesion strength in clinical research.
Keywords: prevention, skin care, skin integrity, skin tears, suction blister
1. INTRODUCTION
The world population is growing and the life expectancy has risen continuously in developed and developing countries. One consequence is an increase of age associated disabilities and diseases. 1 The process of ageing involves numerous structural and functional changes including the skin. Due to its ageing‐related loss of functional capacity, the skin becomes more susceptible to develop adverse skin conditions and dermatological diseases (eg, xerosis cutis and itch, fungal infections, skin cancer and dermatitis). 2 , 3 , 4
Clinically very relevant age‐related structural changes can be observed at the dermo‐epidermal junction (DEJ). 5 The DEJ forms the interface between the lower layer of the epidermis and the top layer of the dermis and consists of a complex structural network of interacting proteins which mediate adhesion of these two very different tissue types. 6 Furthermore, it allows the exchange and transport of nutrients as well as various molecules between the non‐perfused epidermis and the perfused dermis. 7 On the epidermal site of this network, the basal keratinocytes are interlinked to the basement membrane via hemidesmosomes and anchoring filaments. The interconnection of the basement membrane to the collagen meshwork of the dermis is mediated by anchoring fibrils. 8 In addition to these specific molecular characteristics of the DEJ, it has also a characteristic three‐dimensional structure. It is described as finger‐like projections of rete ridges (epidermal protrusion down into the dermis) and upwardly protruding dermal papillae from the dermis into epidermis. 9 This interdigitation is important in order to provide the skin with structural integrity as well as mechanical stability. 6 A consequence of intrinsic skin ageing, besides a gradual disorganisation of the anchoring system, 10 is a significant thinning and flattening of the DEJ, 8 caused by the retraction of rete ridges 11 as well as a reduction of dermal papillae. 12 These age‐related structural changes beyond the 6th decade of life 13 leading to a flattened appearance of the DEJ and are associated with a reduced contact surface area and therefore less adhesion. 11 The more fragile dermo‐epidermal interface in aged skin makes it more prone to bulla formation and trauma and less resistant to shearing forces, potentially leading to shear‐type injuries such as skin tears. 6 , 13 , 14 , 15 , 16
The prevalence of skin tears in aged care settings is 3% to 22%. 17 , 18 , 19 , 20 Empirical evidence indicates that basic skin care strategies may help to prevent skin tear development. 21 For example, Carville et al 22 showed that the application of a moisturiser twice a day reduced the skin tear incidence in residents living in aged care facilities by almost 50% compared to the control group. These study results led to the recommendation that topical leave‐on products should be applied in long‐term care as one component of a skin tear prevention program. 23 However, the underlying mode of action is unclear. Topically applied basic skin care products such as petrolatum, waxes and comparable lipophilic substances exhibit physical and chemical effects on and in the uppermost skin layers (eg, the stratum corneum [SC]). Petrolatum is one of the most effective moisturisers. 24 By forming an occlusive layer, the transepidermal water loss (TEWL) is reduced with a simultaneous increase of the stratum corneum hydration (SCH). 24 , 25 , 26 , 27 Furthermore, the application of petrolatum causes an increase of the epidermal thickness by swelling of the SC. 28 However, effects on the DEJ have not been described and it is unclear whether basic skin care products actually increase the strength of the DEJ.
Despite a wide range of invasive and non‐invasive methods to measure structural and functional properties of the skin, approaches to quantify the dermo‐epidermal adhesion strength directly are less established. One proposed approach to measure this parameter in vivo is the artificial induction of suction blisters (SBs). 10
The first documented artificial mechanical separation of epidermis and dermis along the DEJ was described in 1887 by Unna. 29 Kiistala and Mustakallio 30 , 31 developed this method further by using suction cups and applying a constant negative pressure on the skin to create SBs. Through the application of constant negative pressure, interstitial fluid accumulates between the dermis and epidermis and hemidesmosomes detach from the basement membrane. 31 , 32 Initially, multiple tiny sub‐epidermal vesicles arise which coalesce to form eventually a single cavity. 30 This process leads to a complete dermal‐epidermal separation and results in a macroscopically visible cavity filled with suction blister fluid. Today the SB technique is widely used in dermatological research for studying morphological, physiological, or pharmacological phenomena 33 , 34 and the creation of standardised wounds allows to study wound healing. 35 , 36 This method is also widely used in medical practice for epidermal grafting to treat various skin conditions. 37 , 38
Based on the results of a systematic review about SBs in dermatology, 15 the parameter “blistering time” was proposed as a measure of the dermo‐epidermal adhesion in skin research recently. 10 The blistering time can be defined as the time period from the start of suction until the appearance of visible vesicles. Among other factors, empirical evidence suggests associations between blistering time and age 15 or smoking status. 39 , 40 Therefore, the parameter “blistering time” reflects the strength of the dermo‐epidermal adhesion and may be regarded as a clinically relevant parameter reflecting the mechanical integrity and resistance of the DEJ. 10 , 15 However, this proposed outcome has never been used in clinical research so far even though there is evidence supporting its usefulness. 10
Based on the observation that basic topical leave‐on products reduce the risk for skin tear development and that the parameter “time to blistering” is related to the dermo‐epidermal adhesion strengths, the objective of this study was to investigate, whether there is an association between a basic skin care intervention and the adhesion strength of the DEJ.
2. MATERIALS AND METHODS
2.1. Trial design and setting
An exploratory, randomised controlled clinical trial (RCT) with a split‐body design (left versus right volar forearm) was conducted in 2018 at the Clinical Research Centre for Hair and Skin Science at the Charité‐Universitätsmedizin Berlin, Germany. The split‐body design allows an intra‐individual comparison of investigational sites and minimises inter‐individual biological variation. The trial was approved by the local ethics committee of the Charité‐Universitätsmedizin Berlin (EA1/060/18) and it was registered at clinicaltrials.gov (NCT03625167). No important changes were made after trial commencement.
2.2. Participants
Healthy female volunteers were invited to participate when meeting the following inclusion criteria: (a) Age between 65 and 85 years, (b) Caucasian with phototype I to III according to Fitzpatrick classification, (c) body mass index (BMI) between 20 and 28 kg/m2, (d) non‐smoker of at least 1 year, (e) absence of skin diseases, scars or tattoos at the skin areas of interest. For this exploratory trial, females were included only to reduce biological variability. Written informed consent was obtained from all participants before inclusion.
Major exclusion criteria among others were (a) known or suspected defect of healing, (b) any skin affection which may interfere with the trial assessment (eg, urticaria, psoriasis or scars on investigational areas), (c) any acute or chronic pathology that may interfere with the trial conduct, (d) diabetes mellitus or history or establishment of diabetes or pre‐diabetes, (e) use of topical or systemic treatment on the investigational areas within the past 4 weeks that would interfere with assessment, and/or investigational treatments (f) any known hyper‐sensibility to one of the compounds of the investigational product.
2.3. Interventions
All included subjects were instructed to apply petrolatum (Vaseline, white Ph.Eur., Fagron GmbH & Co. KG, Barsbüttel, Germany) to the randomly assigned interventional volar forearm. A member of the study team demonstrated the correct amount and application of the product at the baseline visit (pea‐sized amount, equal to 0.6‐0.7 g). During the trial, the skin care product was applied by the subjects at home. Adherence to the intervention was checked by weighing the petrolatum tubes at week 4 and week 8. Applications took place twice daily (morning and evening) for 4 and 8 weeks, respectively. This was performed after washing or showering to allow the product to stay on the skin during day or night. The other forearm remained untreated (control arm). During the trial, additional skin care products on the investigational skin areas were not allowed. Furthermore, the subjects were instructed not to have sun exposure or UV‐light sessions, use any topical drugs or cosmetic products on both arms (except usual cleaning products) or have any physical treatments on the investigational areas.
2.4. Outcomes
No distinctions were made between primary and secondary outcomes due to the exploratory nature of the trial. No changes to trial outcomes were made after trial commencement.
Outcomes for all investigational areas were the blistering time, SCH, epidermal hydration, and epidermal thickness on both forearms. The outcome “blistering time” was defined as (a) time to first vesicles (period of time until the appearance of the first macroscopically visible vesicles) and (b) time to full blister (period of time until the appearance of a full blister covering the entire area to which the suction pressure was applied). A full blister can result by expansion of a single initial vesicle or by multiple coalescent vesicles. The blistering time was measured in minutes.
SCH was measured with the Corneometer CM 825 (Courage + Khazaka, Cologne, Germany). The measurement is based on the difference in the dielectric constant of water and other substances and measures the water content in the SC. 41 Values are expressed in arbitrary units (AU) and range from 0 to 120, with higher values indicating higher SCH. Absolute measurement errors of SCH measurements in terms of upper and lower limits of agreement are expected to be +4 AU and − 4 AU 42 , 43 and reliability coefficients exceed 0.9. 42 , 43 Therefore, comparisons of means within and between groups are justified. 44 Values above 40 AU may be considered as “normal” SCH, values <40 AU are regarded as sign for dry skin. 45
Epidermal hydration was measured with MoistureMeterEpiD (Delfin Technologies Ltd, Kuopio, Finland). The measured dielectric constant values are proportional to the water content in the epidermal tissue and are expressed as percentage of tissue water (0%‐100%; 0.5 mm measurement depth). Reliability of epidermal hydration measurements are also very high. 42
The skin surface temperature of investigational areas was measured with the Skin‐Thermometer ST 500 (Courage+Khazaka, Cologne, Germany) and expressed in °C. Empirical evidence suggests high reliability of skin surface temperature measurements. 46 All physiological skin measurements are expressed as means of duplicate measurements per investigational area.
Epidermal thickness was measured with optical coherence tomography (Thorlabs, Lübeck, Germany), according to the methods described by Trojahn et al. 47 Epidermal thickness was expressed in μm.
Before any measurements or the induction of suction blisters, the study volunteers had to acclimatise for 30 minutes at 40% to 60% relative humidity and at a temperature of 20 to 22°C with having both forearms uncovered. The non‐invasive biophysical measurements on both volar forearms including SCH, epidermal hydration, and epidermal thickness were conducted at baseline visit as well as at week 2, 4, 6, and 8. At week 4 and 8, the skin surface temperature was measured additionally before induction of suction blisters.
2.5. Suction blister induction
A vacuum pump (Hico‐Rapidovac 761, Hirtz, Cologne, Germany) was used to produce a constant negative pressure at – 200 mmHg. The pump was connected to a main tube in order to transmit the negative pressure to the skin. The main tube was subdivided to obtain multiple ends to ensure that the same negative pressure was applied to all investigational areas at the same time. At the end of each tube, an upside‐down‐positioned disposable syringe with a diameter of 8 mm was attached. The tip of the syringe was attached airtight to the tube and the plunger was removed. The resulting 8 mm diameter cavity was placed on the test area to aspirate the skin. Due to the suction, the skin is pulled into the syringe and appears in a dome‐like structure. After the blister fully developed, the syringes were removed, the blister fluid was punctured and a dressing was applied.
2.6. Sample size
Due to the explorative character of this pilot trial, a formal sample size estimation was not performed. Following the recommendation by Julious, 48 it was planned to include n = 12 female subjects.
2.7. Randomization and blinding
A two‐step randomization was applied in this trial. The first step was a simple computer‐generated randomization table with 1:1 allocation to the treatment volar forearm (right vs left). Sequentially numbered sealed opaque envelopes containing the assignment to the treatment volar forearm were prepared and used. After a participant was included and the baseline skin measurements were conducted, the study personnel opened the next numbered envelope in chronological order.
In the second step of randomization, for every participant a second sealed opaque envelope was prepared containing the order in which the suction blisters were induced on week 4 and week 8 (upper area A/C first vs lower area B/D first) (see Figure 1). The order was based on a computer‐generated 1:1 randomization. The envelope was opened by the study assistant at week 4 before the first induction of SBs.
FIGURE 1.
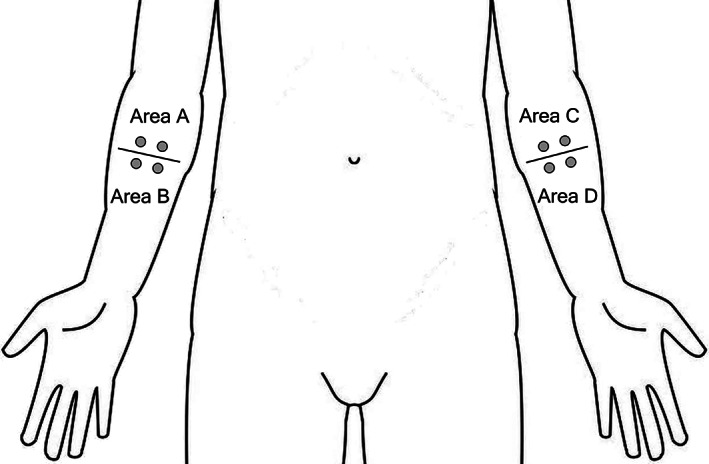
Schematic representation of investigational areas. Each of the indicated areas (A, B, C, and D) contain two suction blister induction sites (depicted as grey filled circles)
Both randomization lists were created by the data manager and the envelopes were prepared by another staff member, neither of whom was involved in any other of the study‐related procedures.
Due to the nature of the intervention, neither the investigators nor the participants were blinded.
2.8. Statistical analysis
Demographic characteristics were described using mean and spread estimates. For the outcomes medians and the 25% to 75% interquartile ranges (IQR) were calculated for the total sample, for each group, and for group differences. Grouped boxplots were used to describe values of SCH, epidermal hydration, and epidermal thickness on intervention and control sites at week 0, 2, 4, 6, and 8. Because of the exploratory design, no statistical hypothesis testing was conducted. Statistical analysis was performed using IBM SPSS Statistics 25.
3. RESULTS
3.1. Participant flow
In total, 18 subjects were screened for eligibility and 17 healthy female subjects were included. One forearm of all included subjects was randomly allocated to the intervention and the other forearm served as control arm. It was planned to conduct the study in 12 subjects but until week 6 in five subjects major protocol violations have occurred. All of them applied additional skin care products to one or both of their forearms. Therefore, these subjects were replaced by another five subjects. A detailed description of the participant flow is shown in Figure 2.
FIGURE 2.
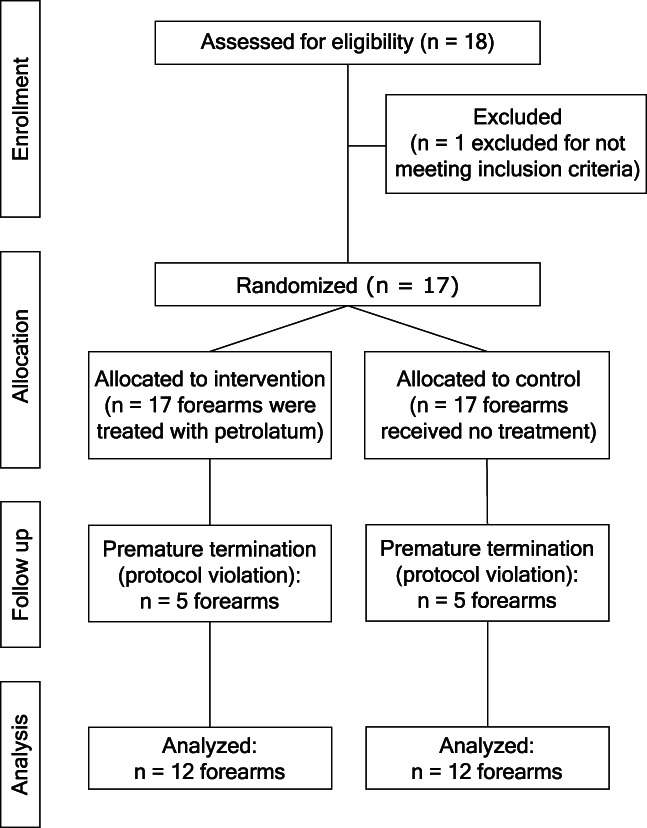
Flowchart outlining the participant flow during the trial
3.2. Recruitment
The recruitment period was from July 2018 to October 2018. The study stopped after the regular study termination of 12 subjects.
3.3. Baseline data
Demographic characteristics are shown in Table 1.
TABLE 1.
Sample characteristics at baseline
| Age (y) mean (SD); median (IQR) | 70.3 (2.1); 70.5 (69.0‐72.5) |
|---|---|
| BMI (kg/m2) mean (SD); median (IQR) | 26.0 (2.2); 26.5 (24.3‐28.0) |
| Skin phototype | |
| II | 3 |
| III | 9 |
| Body temperature (°C) mean (SD); median (IQR) | 36.3 (0.2); 36.3 (36.2‐36.4) |
| Blood pressure (mmHg) mean (SD); median (IQR) | |
| Systolic | 127 (16); 124 (117‐140) |
| Diastolic | 83 (9); 83 (76‐92) |
Abbreviation: IQR, interquartile ranges.
3.4. Outcomes and estimation
Table 2 shows the medians and IQRs for time to first vesicles as well as time to full blisters in minutes after 4 and 8 weeks on interventional and control skin areas. The median time to blistering was 3 to 7 minutes longer on the intervention compared to the control arms. After week 4, the median time to fist vesicles was 3 minutes and after 8 weeks treatment 7 minutes longer on intervention sites. In both, week 4 and 8, the median time to full blister development was 6 minutes longer at intervention sites compared to control sites. At the beginning and during the induction, no drop or other changes in suction pressure were observed during the procedure at any induction sites of all subjects.
TABLE 2.
Medians and IQR of time to first vesicles and time to full blisters in minutes (min)
| Time to first vesicles (min) (IQR) | Time to full blister [min] (IQR) | |||||
|---|---|---|---|---|---|---|
| Intervention | Control | Difference | Intervention | Control | Difference | |
| Week 4 | 43 (29‐69) | 39 (25‐76) | 3 (−8 to 10) | 63 (53‐94) | 54 (45‐71) a | 6 (−9 to 15) |
| Week 8 | 47 (32‐69) a | 27 (24‐60) a | 7 (−2 to 17) | 65 (49‐75) a | 62 (42‐73) a | 6 (−6 to 12) |
Abbreviation: IQR, interquartile ranges.
No development of second blister in one subject.
The boxplots in Figure 3A display the values of SCH for intervention and control measurements in all subjects at week 0, 2, 4, 6, and 8. At baseline, the median SCH was higher in the intervention compared to the control skin areas. During the course of the study, there seemed to be a decline in SCH in both groups, but the median differences persisted. The boxplots indicate relatively few outliers which are mainly restricted to subject S05 and S06. While subject S05 showed relatively high SCH values at intervention sites, subject S06 had low SCH values at intervention and control sites at the beginning of the study.
FIGURE 3.
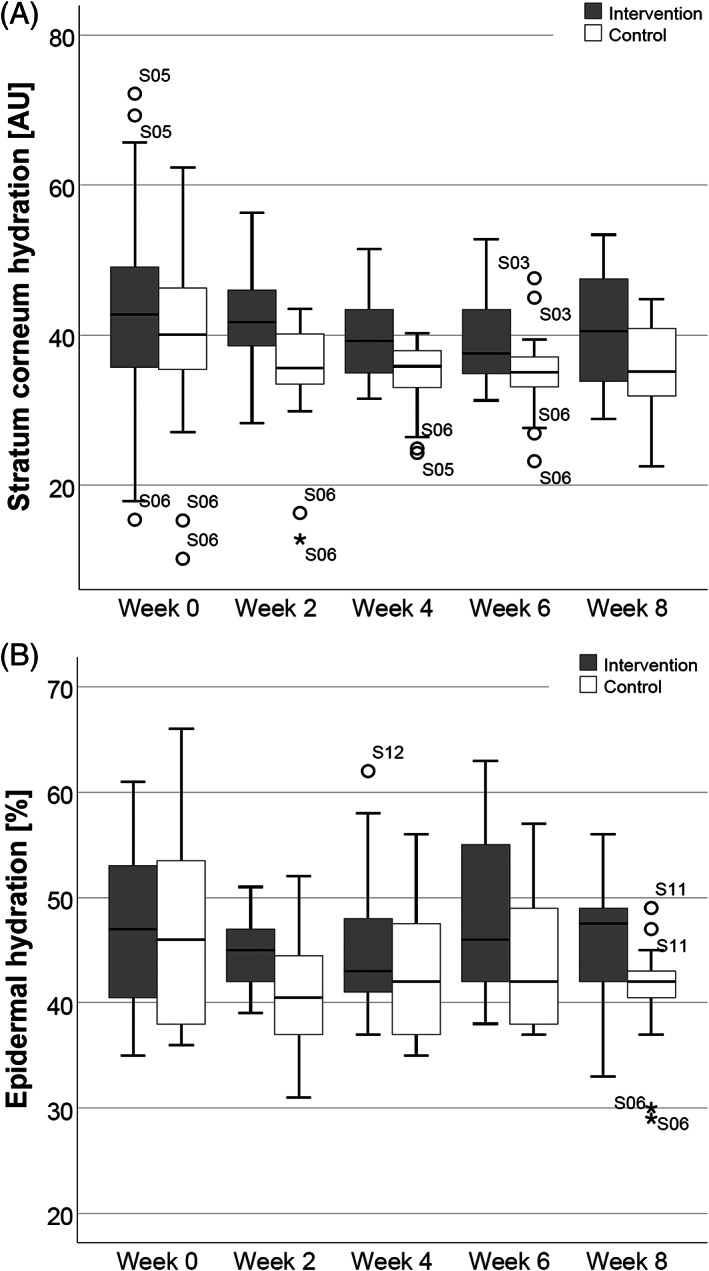
Boxplot representation of stratum corneum hydration (SCH) and epidermal hydration. SCH values expressed in arbitrary units (AU), A, and epidermal hydration values expressed as percentage of tissue water (%), B, all measurements performed as duplicates in all subjects on intervention and control arms at week 0, 2, 4, 6, and 8
In contrast to measured SCH values, baseline epidermal hydration was similar in both arms and a difference developed over time. Measured epidermal hydration values for all time points, subjects, and sites are shown as boxplots in Figure 3B. Table 3 summarises the median SCH and epidermal hydration values as well as median differences between groups over the course of the trial.
TABLE 3.
Medians and IQR of stratum corneum hydration expressed in AU and epidermal hydration expressed as percentage of local tissue water (%)
| Stratum corneum hydration (AU) (IQR) | Epidermal hydration (%) (IQR) | |||||
|---|---|---|---|---|---|---|
| Intervention | Control | Difference | Intervention | Control | Difference | |
| Week 0 | 43 (35‐49) | 40 (35‐47) | 4 (−1 to 9) | 47 (39‐54) | 46 (38‐54) | 0 (−2 to 1) |
| Week 2 | 41 (40‐46) | 36 (34‐40) | 6 (4‐11) | 46 (42‐48) | 41 (37‐46) | 5 (2‐7) |
| Week 4 | 39 (36‐44) | 36 (33‐39) | 5 (0‐8) | 43 (42‐49) | 41 (38‐48) | 3 (0‐4) |
| Week 6 | 38 (35‐43) | 36 (33‐37) | 4 (1‐7) | 46 (42‐58) | 42 (38‐50) | 4 (2‐6) |
| Week 8 | 41 (33‐48) | 35 (32‐41) | 5 (1‐8) | 47 (42‐50) | 42 (40‐43) | 6 (1‐9) |
Abbreviations: AU, arbitrary units; IQR, interquartile ranges.
Figure 4 represents measured values of epidermal thickness on week 0, 2, 4, 6, and 8 of intervention and control sites of all subjects. Measurement values of epidermal thickness ranged from 44 to 112 μm with median values of 67 to 76 μm across both groups and all time points. Median epidermal thickness values and median differences between groups at week 0, 4 and 8 are shown in Table 4. Measurements on both, intervention and control sites, showed some fluctuation over the course of the study without a group‐related tendency. The majority of the few observed outliers were seen in subject S02.
FIGURE 4.
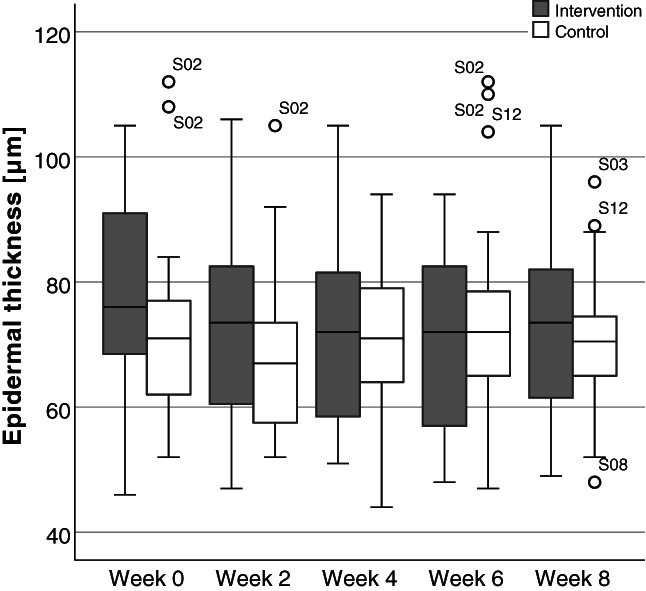
Boxplot representation of epidermal thickness. Epidermal thickness values expressed in micrometres (μm). Standardised thickness measurements based on duplicate optical coherence tomography images for all subjects on intervention and control arms at week 0, 2, 4, 6, and 8
TABLE 4.
Median epidermal thickness and IQR expressed in micrometres (μm)
| Epidermal thickness (μm) (IQR) | |||
|---|---|---|---|
| Intervention | Control | Difference | |
| Week 0 | 78 (65‐86) | 68 (63‐80) | 3 (−5 to 11) |
| Week 4 | 68 (60‐84) | 72 (63‐79) | 4 (−13 to 14) |
| Week 8 | 75 (62‐79) | 71 (65‐77) | 4 (−9 to 14) |
Abbreviation: IQR, interquartile ranges.
3.5. Harms
No harms or unintended effects were observed during the trial.
4. DISCUSSION
The overall aim of the present trial was to investigate the effect of the daily application of a basic skin care intervention on the structural strength of the DEJ measured by the novel outcome time to blistering.
Baseline values of SCH were comparable to previous research in aged individuals. 49 , 50 , 51 , 52 , 53 , 54 Despite baseline differences, the forearms assigned to the intervention group consistently had higher SCH values compared to control forearms. There were no baseline differences regarding epidermal hydration but during treatment, it was higher on intervention arms compared to the control arm. Taken together, this supports the hydrating effect of petrolatum. 24 , 25 , 26 , 27
Values of epidermal thickness were also comparable with previously reported results. 47 During the trial, no significant changes in epidermal thickness were seen in the intervention or control arms. Minor variations of epidermal thickness were observed in both groups. According to highly standardised measurements of the epidermal thickness, a range between 49 and 113 μm is reported for arms. 55 Our estimates are similar, ranging from 44 to 112 μm with only slight biological variations between measurement time points. Therefore, we assume that the hydrating effect of petrolatum on the epidermal thickness within the period of the trial was too low to cause a measurable epidermal thickness increase.
Suction blister time for both, the formation of first vesicles as well as the formation of complete blisters, took longer at the intervention than at control sites. The difference ranged from 3 to 7 minutes at all time points and skin areas. This finding suggests that the treatment increased the dermo‐epidermal adhesion. The underlying mechanism of this finding is not fully understood so far but is in line with the reduction of skin tear incidence due to topical applications. 21 , 22 The treatment with petrolatum increased the SCH and epidermal hydration which may also affect the DEJ. It is well known that in addition to hydrating effects basic topical treatments such as petrolatum change the entire epidermal structure, differentiation, and function. 27 , 56 Maybe these changes also increase the resistance against mechanical loads such as suction.
In addition, trial results indicate that the suction blister model is a suitable technique to investigate the effect of interventions or exposures on the strength of the dermo‐epidermal adhesion. Because suction blister creation is time‐consuming and invasive, it is unlikely that it can be widely applied. However, in skin research it is crucial to measure functional capacities and reserves 57 , 58 in addition to the many widely applied static non‐invasive measures. Because this method has only a minimally invasive character and causes no or minimal pain or discomfort and collapsed SBs heal without scarring, we consider this approach as safe and reasonable. Despite existing evidence that this method seems to be a useful and direct approach to quantify the dermo‐epidermal adhesion strength, it is surprisingly not used for this purpose in clinical research so far. 10 We see the potential of the parameter “blistering time” together with other well‐established parameters for a better and more comprehensive understanding of the skin and its changes in structure/function over the life course. However, we are aware that the establishment of this outcome in clinical research requires a high degree of standardisation of the conduct and analysis to enable interpretation and comparability of different studies. The lack of a standardised guideline could also be a possible reason why this approach is not used in clinical research.
4.1. Limitations
This exploratory trial had a small sample size and served to provide first empirical evidence about possible effects on the mechanical adhesion strength of the DEJ. Therefore, results should be regarded as descriptive and hypothesis generating. Due to the small sample size, we included only female subjects to reduce the group variance. SCH was measured in AU. This may limit comparisons with other skin hydration measures. Petrolatum was chosen because of its simple composition, safety, and well‐known properties. However, compared to specific mixtures of hydrating ingredients the hydrating effect of petrolatum is lower. 59 , 60 Therefore, the use of other skin care products should be considered in future trials. Risk group‐specific factors should be also taken into account, for example, elderly subjects with dry skin because they may benefit in particular from topical skin care products.
4.2. Conclusions
Topical skin care products reduce the incidence of shear type injuries (skin tears) in aged populations, but the underlying mechanism is unclear. We hypothesise that topical applications of basic skin care products increase the hydration and change the structure and function of the entire epidermis and the DEJ. The parameter “time to blistering” is a suitable outcome to measure the dermo‐epidermal adhesion strength in clinical skin research.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
El Genedy‐Kalyoncu M, Richter C, Surber C, Blume‐Peytavi U, Kottner J. The effect of a basic skin care product on the structural strength of the dermo‐epidermal junction: An exploratory, randomised, controlled split‐body trial. Int Wound J. 2022;19(2):426–435. 10.1111/iwj.13643
Funding information Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Charité – Universitätsmedizin Berlin
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. World Health Organization (WHO). World report on ageing and health. 2015.
- 2. Chang AL, Wong JW, Endo JO, Norman RA. Geriatric dermatology review: major changes in skin function in older patients and their contribution to common clinical challenges. J Am Med Dir Assoc. 2013;14(10):724‐730. [DOI] [PubMed] [Google Scholar]
- 3. Kottner J, Lichterfeld A, Blume‐Peytavi U, Kuhlmey A. Skin health promotion in the elderly. Z Gerontol Geriatr. 2015;48(3):231‐236. [DOI] [PubMed] [Google Scholar]
- 4. Hahnel E, Lichterfeld A, Blume‐Peytavi U, Kottner J. The epidemiology of skin conditions in the aged: a systematic review. J Tissue Viability. 2017;26(1):20‐28. [DOI] [PubMed] [Google Scholar]
- 5. Farage MA, Miller KW, Maibach HI. Degenerative Changes in Aging Skin. In: Farage MA, Miller KW, Maibach HI, eds. Textbook of Aging Skin. Berlin, Heidelberg: Springer; 2010. [Google Scholar]
- 6. Langton AK, Halai P, Griffiths CE, Sherratt MJ, Watson RE. The impact of intrinsic ageing on the protein composition of the dermal‐epidermal junction. Mech Ageing Dev. 2016;156:14‐16. [DOI] [PubMed] [Google Scholar]
- 7. Briggaman RA, Wheeler CE Jr. The epidermal‐dermal junction. J Invest Dermatol. 1975;65(1):71‐84. [DOI] [PubMed] [Google Scholar]
- 8. Le Varlet B, Chaudagne C, Saunois A, et al. Age‐related functional and structural changes in human dermo‐epidermal junction components. J Investig Dermatol Symp Proc. 1998;3(2):172‐179. [DOI] [PubMed] [Google Scholar]
- 9. Newton VL, Bradley RS, Seroul P, et al. Novel approaches to characterize age‐related remodelling of the dermal‐epidermal junction in 2D, 3D and in vivo. Skin Res Technol. 2017;23(2):131‐148. [DOI] [PubMed] [Google Scholar]
- 10. Kottner J, Vogt A. Dermoepidermal adhesion strength measurement using suction blisters. In: Maibach H, Osman N, eds. Cutaneous Biometrics. Cham: Springer; 2019. [Google Scholar]
- 11. Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;11(4):221‐235. [DOI] [PubMed] [Google Scholar]
- 12. Neerken S, Lucassen GW, Bisschop MA, Lenderink E, Nuijs TA. Characterization of age‐related effects in human skin: a comparative study that applies confocal laser scanning microscopy and optical coherence tomography. J Biomed Opt. 2004;9(2):274‐281. [DOI] [PubMed] [Google Scholar]
- 13. Hull MT, Warfel KA. Age‐related changes in the cutaneous basal lamina: scanning electron microscopic study. J Invest Dermatol. 1983;81(4):378‐380. [DOI] [PubMed] [Google Scholar]
- 14. Lavker RM, Zheng PS, Dong G. Aged skin: a study by light, transmission electron, and scanning electron microscopy. J Invest Dermatol. 1987;88(3 Suppl):44s‐51s. [DOI] [PubMed] [Google Scholar]
- 15. Hatje LK, Richter C, Blume‐Peytavi U, Kottner J. Blistering time as a parameter for the strength of dermoepidermal adhesion: a systematic review and meta‐analysis. Br J Dermatol. 2015;172(2):323‐330. [DOI] [PubMed] [Google Scholar]
- 16. Fenske NA, Lober CW. Skin changes of aging: pathological implications. Geriatrics. 1990;45(3):27‐35. [PubMed] [Google Scholar]
- 17. Skiveren J, Wahlers B, Bermark S. Prevalence of skin tears in the extremities among elderly residents at a nursing home in Denmark. J Wound Care. 2017;26(suppl 2):S32‐S36. [DOI] [PubMed] [Google Scholar]
- 18. Hahnel E, Blume‐Peytavi U, Trojahn C, Kottner J. Associations between skin barrier characteristics, skin conditions and health of aged nursing home residents: a multi‐center prevalence and correlational study. BMC Geriatr. 2017;17(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Tiggelen H, Van Damme N, Theys S, et al. The prevalence and associated factors of skin tears in Belgian nursing homes: a cross‐sectional observational study. J Tissue Viability. 2019;28(2):100‐106. [DOI] [PubMed] [Google Scholar]
- 20. Leblanc K, Christensen D, Cook J, Culhane B, Gutierrez O. Prevalence of skin tears in a long‐term care facility. J Wound Ostomy Continence Nurs. 2013;40(6):580‐584. [DOI] [PubMed] [Google Scholar]
- 21. Lichterfeld‐Kottner A, El Genedy M, Lahmann N, Blume‐Peytavi U, Büscher A, Kottner J. Maintaining skin integrity in the aged: a systematic review. Int J Nurs Stud. 2020;103:103509. [DOI] [PubMed] [Google Scholar]
- 22. Carville K, Leslie G, Osseiran‐Moisson R, Newall N, Lewin G. The effectiveness of a twice‐daily skin‐moisturising regimen for reducing the incidence of skin tears. Int Wound J. 2014;11(4):446‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LeBlanc K, Kozell K, Martins L, Forest‐Lalande L, Langlois M, Hill M. Is twice‐daily skin moisturizing more effective than routine care in the prevention of skin tears in the elderly population? J Wound Ostomy Continence Nurs. 2016;43(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 24. Draelos ZD. The science behind skin care: moisturizers. J Cosmet Dermatol. 2018;17(2):138‐144. [DOI] [PubMed] [Google Scholar]
- 25. Proksch E, Lachapelle JM. The management of dry skin with topical emollients‐recent perspectives. J Dtsch Dermatol Ges. 2005;3(10):768‐774. [DOI] [PubMed] [Google Scholar]
- 26. Lodén M. The increase in skin hydration after application of emollients with different amounts of lipids. Acta Derm Venereol. 1992;72(5):327‐330. [PubMed] [Google Scholar]
- 27. Ghadially R, Halkier‐Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26(3 Pt 2):387‐396. [DOI] [PubMed] [Google Scholar]
- 28. Choe C, Lademann J, Darvin ME. Analysis of human and porcine skin in vivo/ex vivo for penetration of selected oils by confocal Raman microscopy. Skin Pharmacol Physiol. 2015;28(6):318‐330. [DOI] [PubMed] [Google Scholar]
- 29. Unna P. Zur Anatomie der Blasenbildung an der menschlichen Haut. Vjschr Dermatol Syphil. 1878;5:3. [Google Scholar]
- 30. Kiistala U, Mustakallio KK. In‐vivo separation of epidermis by production of suction blisters. Lancet. 1964;2(7348):1444‐1445. [DOI] [PubMed] [Google Scholar]
- 31. Kiistala U, Mustakallio KK. Dermo‐epidermal separation with suction. Electron microscopic and histochemical study of initial events of blistering on human skin. J Invest Dermatol. 1967;48(5):466‐477. [PubMed] [Google Scholar]
- 32. Beerens EG, Slot JW, van der Leun JC. Rapid regeneration of the dermal‐epidermal junction after partial separation by vacuum: an electron microscopic study. J Invest Dermatol. 1975;65(6):513‐521. [DOI] [PubMed] [Google Scholar]
- 33. Lévy JJ, von Rosen J, Gassmüller J, Kleine Kuhlmann R, Lange L. Validation of an in vivo wound healing model for the quantification of pharmacological effects on epidermal regeneration. Dermatology. 1995;190(2):136‐141. [DOI] [PubMed] [Google Scholar]
- 34. Panoutsopoulou IG, Wendelschafer‐Crabb G, Hodges JS, Kennedy WR. Skin blister and skin biopsy to quantify epidermal nerves: a comparative study. Neurology. 2009;72(14):1205‐1210. [DOI] [PubMed] [Google Scholar]
- 35. Kottner J, Hillmann K, Fimmel S, Seité S, Blume‐Peytavi U. Characterisation of epidermal regeneration in vivo: a 60‐day follow‐up study. J Wound Care. 2013;22(8):395‐400. [DOI] [PubMed] [Google Scholar]
- 36. Czaika V, Alborova A, Richter H, et al. Comparison of transepidermal water loss and laser scanning microscopy measurements to assess their value in the characterization of cutaneous barrier defects. Skin Pharmacol Physiol. 2012;25(1):39‐46. [DOI] [PubMed] [Google Scholar]
- 37. Gupta S, Shroff S. Modified technique of suction blistering for epidermal grafting in vitiligo. Int J Dermatol. 1999;38(4):306‐309. [DOI] [PubMed] [Google Scholar]
- 38. Costanzo U, Streit M, Braathen LR. Autologous suction blister grafting for chronic leg ulcers. J Eur Acad Dermatol Venereol. 2008;22(1):7‐10. [DOI] [PubMed] [Google Scholar]
- 39. Sørensen LT, Zillmer R, Agren M, Ladelund S, Karlsmark T, Gottrup F. Effect of smoking, abstention, and nicotine patch on epidermal healing and collagenase in skin transudate. Wound Repair Regen. 2009;17(3):347‐353. [DOI] [PubMed] [Google Scholar]
- 40. Knuutinen A, Kokkonen N, Risteli J, et al. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br J Dermatol. 2002;146(4):588‐594. [DOI] [PubMed] [Google Scholar]
- 41. du Plessis J, Stefaniak A, Eloff F, et al. International guidelines for the in vivo assessment of skin properties in non‐clinical settings: part 2. Transepidermal water loss and skin hydration. Skin Res Technol. 2013;19(3):265‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kottner J, Blume‐Peytavi U. Reliability and agreement of instrumental skin barrier measurements in clinical pressure ulcer prevention research. Int Wound J. 2021;18(5):716‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anthonissen M, Daly D, Peeters R, et al. Reliability of repeated measurements on post‐burn scars with corneometer CM 825(®). Skin Res Technol. 2015;21(3):302‐312. [DOI] [PubMed] [Google Scholar]
- 44. Kottner J, Audigé L, Brorson S, et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64(1):96‐106. [DOI] [PubMed] [Google Scholar]
- 45. Heinrich U, Koop U, Leneveu‐Duchemin MC, et al. Multicentre comparison of skin hydration in terms of physical‐, physiological‐ and product‐dependent parameters by the capacitive method (Corneometer CM 825). Int J Cosmet Sci. 2003;25(1–2):45‐53. [DOI] [PubMed] [Google Scholar]
- 46. Elban F, Hahnel E, Blume‐Peytavi U, Kottner J. Reliability and agreement of skin barrier measurements in a geriatric care setting. J Tissue Viability. 2020;29(4):269‐276. [DOI] [PubMed] [Google Scholar]
- 47. Trojahn C, Dobos G, Richter C, Blume‐Peytavi U, Kottner J. Measuring skin aging using optical coherence tomography in vivo: a validation study. J Biomed Opt. 2015;20(4):045003. [DOI] [PubMed] [Google Scholar]
- 48. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287‐291. [Google Scholar]
- 49. Akdeniz M, Boeing H, Müller‐Werdan U, et al. Effect of fluid intake on hydration status and skin barrier characteristics in geriatric patients: an explorative study. Skin Pharmacol Physiol. 2018;31(3):155‐162. [DOI] [PubMed] [Google Scholar]
- 50. Hahnel E, Blume‐Peytavi U, Trojahn C, et al. The effectiveness of standardized skin care regimens on skin dryness in nursing home residents: a randomized controlled parallel‐group pragmatic trial. Int J Nurs Stud. 2017;70:1‐10. [DOI] [PubMed] [Google Scholar]
- 51. Sato N, Kitahara T, Fujimura T. Age‐related changes of stratum corneum functions of skin on the trunk and the limbs. Skin Pharmacol Physiol. 2014;27(4):181. [DOI] [PubMed] [Google Scholar]
- 52. Luebberding S, Krueger N, Kerscher M. Skin physiology in men and women: in vivo evaluation of 300 people including TEWL, SC hydration, sebum content and skin surface pH. Int J Cosmet Sci. 2013;35(5):477‐483. [DOI] [PubMed] [Google Scholar]
- 53. Carvalho PRS, Sumita JM, Soares JLM, Sanudo A, Bagatin E. Forearm skin aging: characterization by instrumental measurements. Int J Cosmet Sci. 2017;39(5):564‐571. [DOI] [PubMed] [Google Scholar]
- 54. Kottner J, Ludriksone L, Garcia Bartels N, Blume‐Peytavi U. Do repeated skin barrier measurements influence each other's results? An explorative study. Skin Pharmacol Physiol. 2014;27(2):90‐96. [DOI] [PubMed] [Google Scholar]
- 55. Xu H, Fonseca M, Wolner Z, et al. Reference values for skin microanatomy: a systematic review and meta‐analysis of ex vivo studies. J Am Acad Dermatol. 2017;77(6):1133‐44.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this "inert" moisturizer. J Allergy Clin Immunol. 2016;137(4):1091‐1102. [DOI] [PubMed] [Google Scholar]
- 57. Kottner J, Beeckman D, Vogt A, Blume‐Peytavi U. Skin health and integrity. In: Gefen A, ed. Innovations and Emerging Technologies in Wound Care. London, United Kingdom: Academic Press; 2020:183‐196. [Google Scholar]
- 58. Ghadially R, Brown BE, Sequeira‐Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest. 1995;95(5):2281‐2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Izumi R, Negi O, Suzuki T, et al. Efficacy of an emollient containing diethylene glycol/dilinoleic acid copolymer for the treatment of dry skin and pruritus in patients with senile xerosis. J Cosmet Dermatol. 2017;16(4):e37‐e41. [DOI] [PubMed] [Google Scholar]
- 60. Vaillant L, Georgescou G, Rivollier C, Delarue A. Combined effects of glycerol and petrolatum in an emollient cream: a randomized, double‐blind, crossover study in healthy volunteers with dry skin. J Cosmet Dermatol. 2020;19(6):1399‐1403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


