Nephrotic syndrome after vaccination against influenza, measles, hepatitis B, or pneumococci is described as a very rare event (1, 2). There are currently no indications for an increased risk of kidney injury after COVID-19 vaccination in the safety report of the Paul Ehrlich Institute from 19 August 2021. A large-scale study on the BNT162b2 vaccine (Comirnaty, BioNTech/Pfizer) shows that COVID-19 vaccination has a clear overall positive benefit–risk ratio with respect to kidney injury (3). Furthermore, people vaccinated with BNT162b2 did not present increased kidney injury as compared to unvaccinated people (3). However, there are increasing reports in the international literature of persons with nephrotic syndrome a few days after COVID-19 vaccination (1, 2, 4). Here, three new cases from Germany are described for the first time.
Table. Summary of cases, with laboratory values during the course.
| Age/sex | Medical history | SARS-CoV-2 vaccine | Begin of symptoms | Laboratory values | Therapy/ results |
| Case 1 78 yr/male |
Normal kidney function Comorbidities: arterial hypertension, coronary heart disease, hyperlipoproteinemia, COPD, and allergies |
Comirnaty, mRNA, BioNTech/Pfizer | A few days after first vaccine: edema, weight + 7 kg | Cr normal | Diuretics for suspected cardiac disorder; second vaccination after 6 weeks; reappearance of edema Re-exposure |
| Relapse: edema a few days after second vaccination | New-onset NS, proteinuria 14 g/d, UPCR 12.2 g/g, Alb 1 480 mg/dL, UACR 14.6 g/g, Cr 1.4 mg/dL, Chol 244, LDL 170, TG 159 mg/dL, Biopsy: MCD |
Prednisolone 80 mg/d partial remission, after 3 weeks: proteinuria: 0.95 g/d, UACR 0.5 g/g, Alb 3 300 mg/dL, Cr normal |
|||
| Case 2 31 yr/female |
Normal kidney function, healthy Comorbidities: lipedema |
COVID-19 Vaccine Janssen, Vektor, Johnson & Johnson | Edema directly after first vaccination; next day,weight increase + 8 kg | New-onset NS, proteinuria: 15 g/d, U PCR 15.0 g/g, Alb 1 070 mg/dL, max. U PCR 22.6 g/g, Cr 0.55 mg/dL, Chol 589 mg/dL, Biopsy: MCD |
Prednisolone 250 mg 3 days, and then 70 mg/d, starting on 10th day, 35 mg; rituximab 1g initial + 14 days complette remission, after 2 weeks: Alb 3730 mg/dL, UPCR 0.09 g/g, Cr normal, after 24 and 52 days: Alb 3 170 mg/dL, Chol 276, LDL 178 mg/dL |
| Case 3 20 yr/female |
Normal kidney function, healthy Vegan diet |
Comirnaty, mRNA, BioNTech/Pfizer |
Edema about 5 days after first vaccination | New-onset NS, proteinuria: UPCR 10.3 g/g, Alb 2 120 mg/dL, Cr 0.47 mg/dL, Chol 566, LDL 350, TG 302 mg/dL, Biopsy: FSGS |
Prednisolone 60 mg/Tag partial remission, after 10 days: proteinuria: UPCR 3.6 g/g, Alb 2 280 mg/dL; after 28 days: proteinuria: UPCR 5.5 g/g; Alb 2340 mg/dL, Cr normal, Chol 450, TG 230 mg/dL |
Alb, serum albumin (norm 3 400–4 800 mg/dL); Chol, total cholesterol in mg/dL; COPD, chronic obstructive pulmonary disease; Cr, S-creatinine; FSGS, focal segmental glomerulosclerosis; LDL, LDL cholesterol in mg/dL; MCD, minimal change disease (glomerulopathy); NS, nephrotic syndrome; TG, triglycerides in mg/dL; UACR, urine albumin–creatinine ratio (norm <0.03 g/g Cr); UPCR, urine protein–creatinine ratio (normal < 0.1 g/g Cr)
Acknowledgments
Translated from the original German by Dr. Veronica A. Raker.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
Case 1
A 78-year-old retired man noticed weight gain and edema four days after the first vaccination with Comirnaty. Based on a finding of normal S-creatinine levels, inpatient therapy was carried out for suspected cardiac decompensation. 14 days after a homologous second vaccination, the patient was again admitted for inpatient treatment for weight gain, leg edema, and pleural effusions. Based on a clinical picture of nephrotic syndrome with protein deficiency, edema and hyperlipoproteinemia, a kidney biopsy was carried out, which led to the diagnosis of minimal change disease (MCD)/glomerulopathy (Figure 1). Findings of tests for antineutrophil cytoplasmic antibodies (ANCA) and antiglomerular basement membrane antibodies (AGBM-Ab) were negative. Clinical improvement was observed following prednisolone, diuretics, and anticoagulation administration; after three weeks, the patient showed reduced proteinuria and a 14-kg weight loss, with partial remission. Kidney function was not impaired. The patient lives independently at home and to date has not contracted COVID-19.
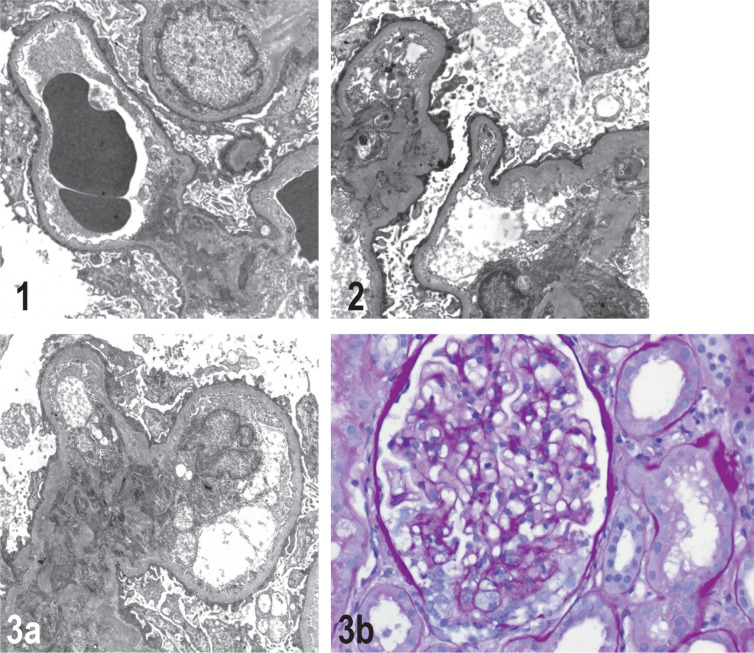
Figures 1–3.
Histopathological findings in kidneys
Electron microscopy (EM × 5000): primary podocytopathy of the minimal change Glomerulopathy (MCD) type, glomerular basement membranes surrounded by plate-like, fused, partially degenerative changes and long, clumpy podocyte foot processes. No evidence of immune complex or complement-mediated glomerulonephritis or amyloidosis; no osmiophilic deposits or fibrillary deposits. 3b: Podocytopathy of primary (or possibly secondary after vaccination) podocytopathic focal segmental glomerulosclerosis (FSGS). Light microscopy (LM × 40), periodic acid–Schiff (PAS) staining: glomerulus incisions with small segmental scleroses, partly with foam cell deposits. The remaining glomeruli showed a slight mesangial cell and matrix proliferation. Numerous tubules were ectastic and enlarged, with signs of protein storage.
Case 2
A 31-year-old woman presented six days after vaccination with the Janssen COVID-19 vaccine with shortness of breath, leg edema, and weight gain. Immediately after vaccination, she experienced syncope with orthostatic dysregulation. That evening, the patient noticed tension in her legs, and the following day, she experienced leg edema and noticeable foamy urine.
Nephrotic syndrome was diagnosed based on proteinuria with hypoalbuminemia, hypercholesterolemia, significantly reduced immunoglobulin G (IgG), and antithrombin III levels with normal S-creatinine; kidney biopsy confirmed podocytopathy (MCD form) (Figure 2). This was followed by a course of infection complications and proteinuria at a maximum of 22 g/g Cr and a weight gain of 15 kg. A calculated administration of antibiotics, immunoglobulin, and prednisolone was initiated. Rituximab induction was administered and repeated after 14 days, to avoid possible side effects of long-term steroid therapy. Anticoagulation and diuretics therapy led to reduced proteinuria, and the prednisolone dose was halved on day 24; the patient was discharged on day 26 with a weight loss of 22 kg. On day 52, the patient was in complete remission with remaining mild hyperlipoproteinemia. To date, she has not contracted COVID-19.
Case 3
A 20-year-old woman had generalized edema five days after the first vaccination with Comirnaty. Forty days later, the patient was admitted to the hospital due to increasing edema with nephrotic syndrome, with pronounced proteinuria, normal S-creatinine, hyperlipidemia, and eumorphic hematuria as well as vitamin D deficiency. She did not exhibit leukocyturia, had normal levels of complement factors C3 and C4, and tested negative for anti-nuclear antibodies (ANA) and ANCA. As a vegan, she supplemented with iron and vitamin B12. Two months before the vaccination, she had normal-range levels of S-albumin and cholesterol. Renal biopsy revealed podocytopathy with podocytopathic focal segmental glomerulosclerosis (FSGS) (Figure 3).
Prednisolone therapy, diuretic and lipid-lowering therapy, and vitamin D substitution were started; after ten days, the patient showed a weight loss of 5 kg with reduced proteinuria. 68 days after vaccination, the patient was in partial remission with persisting proteinuria and hyperlipoproteinemia. To date, the patient has not contracted COVID-19.
Discussion
In this report, we describe two female patients who presented with nephrotic syndrome in MCD and FSGS a few days after the first vaccination with the vaccine Janssen or Comirnaty, and a male patient with MCD after re-exposure to homologous Comirnaty vaccination. While COVID-19 vaccination has an overall positive benefit–risk profile, the literature reports various glomerular diseases connected to the COVID-19 vaccines approved in Germany, with more than half of the cases occurring a few days after vaccination (2, 5). Cases of MCD mostly occurred around seven days after the first vaccination, while cases of IgA nephritis, vasculitis, and AGBM-Ab occurred only after the second vaccination with an mRNA vaccine (2, 4). The case 1 described here as well as a published case from France suggest that a homologous second vaccination with Comirnaty can intensify the course of the disease; in contrast, in another case treated with prednisolone and tacrolimus, a homologous second vaccination with Comirnaty was tolerated without relapse and with a good immune response (4, 5).
Two other patients with steroid-dependent MCD who relapsed after a first vaccination with Vaxzevria were given a heterologous second vaccination (vaccine not mentioned) and treated with prednisolone; they showed no further relapse (4).
Conclusions
Immediately or a few days after the COVID-19 vaccination, foamy urine and edema can occur with renal protein loss. In these cases, patients should be screened for proteinuria and, if the finding is positive, a nephrological work-up should be carried out. Therapy with prednisolone can prevent a complicated course and lead to remission.
References
- 1.Lim JH, Han MH, Kim YJ, et al. New-onset nephrotic syndrome after Janssen COVID-19 vaccination: a case report and literature review. J Korean Med Sci. 2021;36 doi: 10.3346/jkms.2021.36.e218. e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bomback AS, Kudose S, D’Agati VD. De novo and relapsing glomerular diseases after COVID-19 vaccination: What do we know so far? Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.06.004. doi: 10.1053/j.ajkd.2021.06.004 (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110475. doi: 10.1056/NEJMoa2110475 (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izzedine H, Bonilla M, Jhaveri KD. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: true association or circumstantial? Nephrol Dial Transplant. 2021;36:1565–1569. doi: 10.1093/ndt/gfab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kervella D, Jacquemont L, Chapelet-Debout A, Deltombe C, Ville S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. 2021;100:457–458. doi: 10.1016/j.kint.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]