Abstract
Inflammation plays an important role in the outcome of patients with cystic fibrosis (CF). It may develop due to cystic fibrosis transmembrane conductance regulator protein dysfunction, pancreatic insufficiency, or prolonged pulmonary infection. Fecal calprotectin (FC) has been used as a noninvasive method to detect inflammation. Therefore, the aim of the current meta-analysis was to investigate the relationship between FC and phenotype severity in patients with CF. In this study, searches were conducted in PubMed, Science Direct, Scopus, and Embase databases up to August 2021 using terms such as “cystic fibrosis,” “intestine,” “calprotectin,” and “inflammation.” Only articles published in English and human studies were selected. The primary outcome was the level of FC in patients with CF. The secondary outcome was the relationship between FC and clinical severity. Statistical analysis was performed using Comprehensive Meta-Analysis software. Of the initial 303 references, only six articles met the inclusion criteria. The mean (95% confidence interval [CI]) level of FC was 256.5 mg/dL (114.1-398.9). FC levels were significantly associated with pancreatic insufficiency (mean, 243.02; 95% CI, 74.3 to 411.6; p=0.005; I2=0), pulmonary function (r=–0.39; 95% CI, –0.58 to –0.15; p=0.002; I2=60%), body mass index (r=–0.514; 95% CI, 0.26 to 0.69; p<0.001; I2=0%), and Pseudomonas colonization (mean, 174.77; 95% CI, 12.5 to 337.02; p=0.035; I2=71%). While FC is a reliable noninvasive marker for detecting gastrointestinal inflammation, it is also correlated with the severity of the disease in patients with CF.
Keywords: Cystic fibrosis, Calprotectin, Inflammation, Exocrine pancreatic insufficiency
INTRODUCTION
Cystic fibrosis (CF) is an inherited disorder caused by dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) protein [1], leading to progressive damage to the lungs, digestive system, and other organs in the body. In addition to CFTR dysfunction, inflammation also plays a key role in disease outcomes. Inflammation may develop due to internal dysfunction of the CFTR protein or external factors, such as prolonged infection [2]. Studies have indicated that the dysfunction of several inflammatory responses is related to CFTR deficiency, including innate and acquired immune dysregulation, signaling defects in various transcription factors (e.g., nuclear factor κB-dependent pathways), and changes in toll-like receptor responses [3,4].
In addition to CFTR dysfunction, pancreatic insufficiency [5] and chronic pulmonary disease [3] can independently prompt and perpetuate inflammation in individuals with CF. Furthermore, pancreatic insufficiency without the involvement of the CFTR protein is associated with changes in the paracellular space structure of the intestine or increased transit time, which could lead to increased intestinal permeability and a predisposition to intestinal inflammation [6]. In contrast, recurrent bronchopulmonary infections with Staphylococcus aureus, Pseudomonas aeruginosa, or several other pathogens lead to chronic airway and systemic inflammation and respiratory insufficiency [3]. Inflammation is classified as systemic, pulmonary, or intestinal inflammation [7].
Numerous proteins contribute to inflammation. Recent work has evaluated various markers in the setting of CF with delineation of inflammation in individuals with CF. Calprotectin is considered the most important fecal biomarker in CF. Calprotectin, which is formed by a combination of S100A8 and S100A9 proteins, regulates the inflammatory process and acts as an antibacterial and proliferative factor [8]. It can be detected in serum, sputum, and stool. Calprotectin is released into the intestine by granulocytes and monocytes [9]. FC testing is characterized by high sensitivity (95-100%) and variable specificity (44-93%) [10].
Several factors affect the concentration of calprotectin [11]. Irreversible factors include body mass index (BMI), pancreatic insufficiency, or pulmonary function. Furthermore, reversible factors such as drugs that are routinely prescribed to patients with CF (e.g., proton-pump inhibitors, pancreatic enzyme replacement therapy [PERT], and antibiotics) may also affect FC levels [10,12]. Moreover, patients with severe phenotypes (e.g., pancreatic insufficiency) and severe pulmonary diseases have been reported to have higher FC levels. Meanwhile, patients with severe CF (e.g., F508del) are at a higher risk of pancreatic exocrine insufficiency, malnutrition, and severe pulmonary diseases [13].
We hypothesized that FC could be a reliable biomarker in combination with or instead of CFTR mutation analysis for the symptomatic deterioration of patients with CF. The present study aimed to investigate the association between inflammatory markers and phenotype severity in patients with CF.
METHODS
Search strategy and selection criteria
This systematic review and meta-analysis were conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for reporting meta-analyses [14]. The literature review was initiated by searching databases such as PubMed, Science Direct, Scopus, and Embase for studies published until August 2021. The targeted studies were focused on assessing the intestinal inflammatory markers in patients with CF, and keywords, such as “cystic fibrosis,” “intestine,” “calprotectin,” and “inflammation,” were used in the search strategy. The full search strategies used for each database are described in the appendix (Supplementary Table 1). In addition, the reference lists of the retrieved articles were searched for other eligible studies.
The inclusion criteria were the reporting of the correlations (correlation coefficient) between inflammatory markers and symptom severity based on BMI, pulmonary function tests (e.g., forced expiratory volume in one second [FEV1]), Pseudomonas colonization, and pancreatic insufficiency, and differences between patient subgroups (normal vs. severe phenotypes) with the mean and standard deviation (SD) of each group. Only studies published in English and those conducted on human subjects were selected. Although the mentioned criteria were only met by observational studies in this regard, one randomized clinical trial was also retrieved, reporting the baseline FC of the study groups.
The exclusion criteria were abstracts, comments, review, posters, editorial review, and studies with no accessible full-text.
In the selection of the related studies, no restrictions were considered regarding age, gender, comorbidities, CF gene mutation type, and study duration/location. The search results from the database were then combined. Two researchers (S.T. and M.K.R.) screened the titles and abstracts of potentially eligible articles based on this criterion, and duplicate studies were removed using Endnote (version 7; Thomson Reuters, Toronto, ON, Canada) and manually. When the eligibility criteria were met based on the above-mentioned criteria, full-text articles were retrieved for quality assessment. At this stage, conference papers and abstracts without accessible full text were also excluded. Conflicting opinions were resolved by a third reviewer.
Quality assessment
The Newcastle–Ottawa scale was used to assess the quality of the assessment in the selected studies by two reviewers independently in three subcategories: selection (maximum of five stars), comparability (maximum of two stars), and outcome (maximum of three stars). Finally, studies with a rating of ≥7 were considered high quality [15]. The risk of bias was assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [16].
Data extraction
Data extraction was performed by two researchers (S.T. and M.K.R.) using a predefined data extraction form prepared in Excel 2007 (Microsoft, Redmond, WA, USA). The form contained data on study characteristics (study design, year of publication, and corresponding author), settings (location and period), population characteristics (sample size, age, and sex of patients), methodology (type of inflammatory markers and instruments), and outcomes (correlations or differences between phenotypes and intestinal inflammatory markers) (Table 1).
Table 1. Characteristics of included studies.
| Author | Year | Study | Place | Sex (M/F) | Age (yr) | Mutation | Calprotectin level (μg/g) | Instrument for calprotectin evaluation | Calprotectin level in PI vs. PS (μg/g) |
|---|---|---|---|---|---|---|---|---|---|
| Adriaanse et al. [18] | 2015 | Cross-sectional study | Canada | 27/22 | 0.7–46.3 | F508del homozygous: 29 | 596.33±581.48 | Enzyme-linked immunoassay (EliA calprotectin assay, Phadia, Sweden) | PI: 659.68±589.5; PS: 114±87.29; p=0.031 |
| F508del heterozygous: 16 | |||||||||
| Werlin et al. [20] | 2010 | Cross-sectional study | Israel | 27/14 | 10–36 | F508del homozygous: 0 | 187.33±274 | Enzyme-linked immunoassay (Calprest, Eurospital, Italy) | PI: 257.7±257.6 mg/g; PS: 23.2±23.2 mg/g; p=0.011 |
| F508del heterozygous: 16 | |||||||||
| Parisi et al. [10] | 2017 | Cross-sectional study | Italy | 29/25 | 6–31 | Not report | 598.7±277.5 | Immunosorbent assay kit (RIDASCREEN® Calprotectin - G09036) | PI: 665.5±271.7; PS: 417.1±197.1; p<0.01 |
| Dhaliwal et al. [19] | 2015 | Cross-sectional study | Australia | 16/12 | 0–18 | F508del homozygous: 18 | 94.3±100.6 | PhiCal kit (Calpro, San Diego, CA) | PI: 110.4±108.3; PS: 35.4±9.4 mg/kg; p=0.008 |
| F508del heterozygous: 10 |
Values are presented as number only or mean±standard deviation.
Overview of included studies with their design, number of patients, genetic mutations, fecal calprotectin (FC) level, instrument analyzer for FC levels, correlations (correlation-coefficient) between calprotectin and BMI, FEV1, pseudomonas colonization, and pancreatic insufficiency and differences between patient subgroups (normal vs. severe phenotypes) with the mean and standard deviation of each group.
PI: pancreatic insufficiency, PS: pancreatic sufficiency, BMI: body mass index, W/H: weight to height ratio, PA: pseudomonas aeruginosa.
In this review, the corresponding authors of the selected studies were consulted for additional information necessary.
Outcomes
The primary outcome measure was the level of FC in patients with CF. Secondary outcomes included correlations between inflammatory markers and clinical signs and symptoms, which indicated the clinical severity of CF based on factors such as BMI, pulmonary function test, Pseudomonas colonization, and gastrointestinal involvement with pancreatic insufficiency.
Statistical analysis
The Comprehensive Meta-Analysis software (CMA version 2.2.064; Biostat, Englewood, NJ, USA) was used to determine the effect estimates (r and 95% confidence intervals [CIs]) for the correlations between BMI, pulmonary function test (FEV1), and FC in patients with CF. In addition, software was applied to calculate the random effects estimates (SD and 95% CIs) for differences in mean FC levels between patient subgroups with and without pancreatic deficiency and Pseudomonas colonization. The heterogeneity of the retrieved studies was determined by I2, with a value of 0% indicating no heterogeneity, 25% showing low heterogeneity, 50% indicating moderate heterogeneity, and 75% showing high heterogeneity [17]. The random-effects model was also used to estimate the pulled effect when the I2 value was higher than 50%. Publication bias was examined through the Egger regression intercept and visual inspection of the funnel plots.
Role of the funding source
This study had no funding source and the corresponding author had full access to all data, as well as the final responsibility of submitting for publication.
RESULTS
Data of the studies
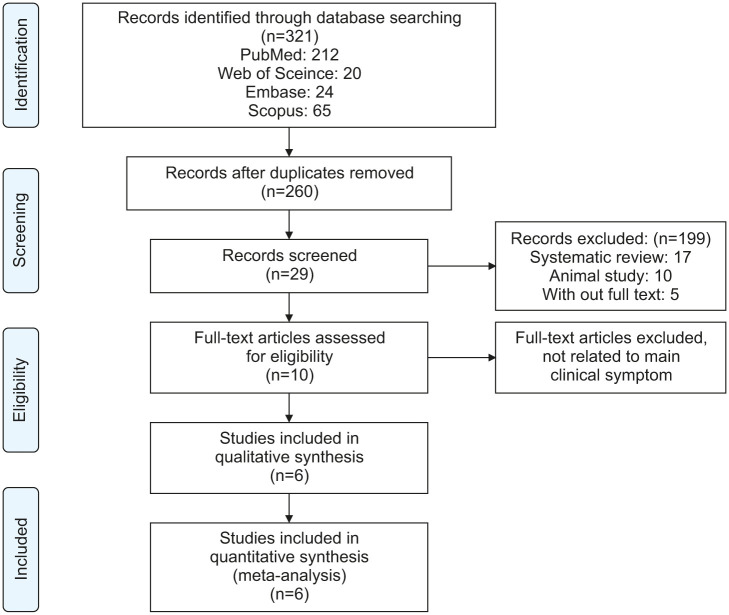
Of the 303 initial references, the abstracts of 246 articles were reviewed, and 29 full-text articles were retrieved (Fig. 1). For the systematic review, data from six articles were used. Five of the studies were cross-sectional in design. One was a randomized clinical trial, in which case the baseline data of the investigation were used.
Fig. 1. A flow diagram of the screening and eligibility process.
The clinical trial conducted by Adriaanse et al. [18] aimed to evaluate the association between FC, pancreatic insufficiency, Pseudomonas colonization, pulmonary function tests, and BMI Z score. In another study, Parisi et al. [10] reported correlations of pulmonary insufficiency, Pseudomonas colonization, and pulmonary function with FC levels.
Furthermore, the findings of Dhaliwal et al. [19] indicated the association of pancreatic insufficiency, pulmonary function tests, and BMI Z scores with FC levels. Finally, Werlin et al. [20] investigated the association between BMI, pancreatic insufficiency, and FC levels. The studies conducted by Stallings et al. [21] and de Freitas et al. [22] confirmed the associations of pulmonary function tests and BMI with the FC level separately. Each of the six reviewed studies was of high technical quality (≥7-9 scores based on the Newcastle–Ottawa scale) (Supplementary Table 2).
The risk of bias based on the ROBINS-I tool was low to moderate (Supplementary Table 3).
Data of the participants
The reviewed studies were conducted on 229 participants, including 121 males and 108 females within the age range of 0-61 years.
Primary outcome
The primary outcome was to determine the mean (95% CI) level of FC in patients with CF, and the meta-analysis indicated that this index was 256.5 mg/dL (114.1 to 398.9) (Supplementary Fig. 1).
Secondary outcome
The secondary outcome was the confirmed association of the phenotype that shows the disease severity in patients with CF, such as pancreatic insufficiency, pulmonary function tests, underweight status, and Pseudomonas colonization with FC.
Pancreatic insufficiency and FC level
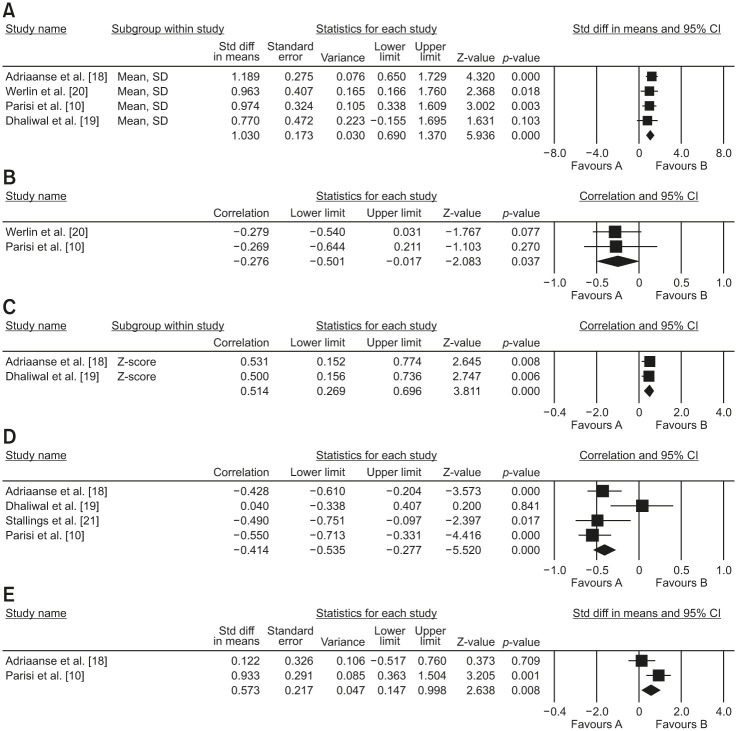
Four of the included studies provided data on these variables. In a meta-analysis of four cross-sectional studies, median FC was significantly associated with pancreatic insufficiency in patients with CF (mean, 243.02; 95% CI, 74.3 to 411.6; p=0.005; I2=0). A funnel plot was also evaluated for publication bias. No publication bias was detected by Egger’s test (t=3.26; p=0.08).
Fig. 2A Mean difference between fecal calprotectin levels in patients with CF with pancreatic insufficient and pancreatic sufficient.
Fig. 2. Mean difference between fecal calprotectin levels in patients with pancreatic sufficient and insufficient with CF (A). Correlation between BMI (B) and BMI Z-score (C) with fecal calprotectin in patients with CF, correlation between pulmonary function test and fecal calprotectin in patients with CF (D). Mean difference between fecal calprotectin levels in patients with CF with or without pseudomonas colonization (E).
CF: cystic fibrosis, BMI: body mass index, CI: confidence interval.
Supplementary Fig. 2A Funnel plot of included studies to evaluate the relationship between fecal calprotectin levels in patients with CF with pancreatic insufficient and pancreatic sufficient.
Anthropometric status and FC level
A meta-analysis of two cross-sectional studies with anthropometry data indicated that the median FC level was inversely associated with BMI (r=–0.276; 95% CI, –0.501 to 0.017; p=0.03; I2=0%). A similar pattern of this association was also observed in the meta-analysis of two cross-sectional studies between the median FC and BMI Z score (r=0.514; 95% CI, 0.26 to 0.69; p<0.001; I2=0%).
Fig. 2B Correlation between BMI and BMI Z score with fecal calprotectin in patients with CF.
Pulmonary function test and FC level
In a meta-analysis of four cross-sectional studies, medium FC was inversely associated with FEV1 (r=–0.39; 95% CI, –0.58 to –0.15; p=0.002; I2=60%). A funnel plot was used to assess publication bias. No publication bias was detected by the Eggers test (t=0.74; p=0.53).
Fig. 2C Correlation between the pulmonary function test and fecal calprotectin in patients with CF.
Supplementary Fig. 2B Funnel plot of included studies to evaluate the relationship between pulmonary function test and fecal calprotectin in patients with CF.
Pseudomonas colonization and FC level
In the meta-analysis of two cross-sectional studies, the median FC value was significantly associated with Pseudomonas colonization in patients with CF (mean, 174.77; 95% CI, 12.5 to 337.02; p=0.035; I2=71%).
Fig. 2D Mean difference between fecal calprotectin levels in patients with CF with and without Pseudomonas colonization.
DISCUSSION
The results of this meta-analysis indicated higher levels of FC in individuals with CF; overall, significant correlations were also observed between FC and the factors that characterize a more severe phenotype in patients with CF. Higher FC values were observed in patients with pancreatic insufficiency, colonization with Pseudomonas, reduced lung function, and poor growth.
Several individual studies have evaluated the association between FC and these factors. For example, Parisi et al. [10] have reported higher FC levels in patients with severe phenotypes, such as airway colonization by P. aeruginosa, predicted FEV1<50%, pancreatic insufficiency, and underweight status. Similarly, the findings of Ellemunter et al. [12] have shown two-fold higher levels of FC in patients with CF with pancreatic insufficient function compared with those with normal pancreatic function. In contrast, Garg et al. [23] observed no significant difference in FC values according to pancreatic status in a group of children with CF aged <10 years of age.
Conflicting results have been proposed regarding the extent of inflammation in patients with pancreatic insufficiency [24]. However, a positive association between calprotectin and pancreatic insufficiency has been reported infrequently [18,20,25]. The positive correlation could be attributed to the development of altered intestinal permeability (leaky gut) in patients with pancreatic insufficiency [6,20]. Reduced bicarbonate secretion in patients with pancreatic insufficiency leads to lower intestinal pH, which affects the integrity of the small intestine and alters mucin secretion and mucus properties. These mechanisms lead to bacterial colonization and alteration of the normal gut microbiota. Furthermore, increased penetration of commensal bacteria into the intestinal mucosa contributes to chronic intestinal inflammation [20,26]. The current meta-analysis indicated high levels of calprotectin in patients with CF and pancreatic insufficiency. Therefore, it could be inferred that significant inflammation occurs in patients with CF, and such cases require special attention for anti-inflammatory therapy, while the evaluation of FC is also recommended to follow the outcomes of treatment.
The association between pulmonary function and intestinal inflammation is an interesting and controversial subject. Although some studies have shown that calprotectin levels are inversely correlated with pulmonary function and exacerbation in patients with CF, no such correlation has been observed in other reports [10,12,18,19]. Although the main mechanism remains unclear, the role of the gut-lung axis could be highlighted in this regard as it alters the gut microbiota. Metabolites, such as short-chain fatty acids, have been associated with changes in immune responses, inflammation, and the development of pulmonary disease [27]. For example, the reduction of Parabacteroides could predict airway colonization by P. aeruginosa [28]. Furthermore, oral administration of probiotics to patients with CF decreases the frequency of exacerbations [29]. In addition to altered intestinal pH in patients with CF secondary to the CFTR gene mutation, bicarbonate secretion may impair pulmonary function and give rise to lung protein dysfunction while also increasing mucin viscosity. This phenomenon affects bacterial clearance in the lungs and alters the lung microbiome [30]. In the present study, a significant inverse correlation was observed between intestinal inflammation and pulmonary involvement, indicating a strong connection between the gut and lung.
Malnutrition (especially undernutrition) is a common complication in individuals with CF. Some of the factors that contribute to failure to thrive are inadequate calorie intake due to poor appetite, acute/chronic pulmonary infections, high energy intake, energy loss due to malabsorption by incorrect adjustment of enzyme dose, or abnormal pH to improve the effectiveness of PERT, small intestinal bacterial overgrowth, and intestinal dysbiosis [31,32]. Furthermore, studies have shown positive correlations between weight, height, and weight-for-height Z scores and FC levels [10,18,19]. However, no such associations were observed in other studies [22,33] as we detected an inverse correlation between intestinal inflammation and anthropometric assessment. Therefore, it was concluded that in addition to a sufficient dose of PERT and proper nutritional recommendations, strategies for the correction of inflammation may play a key role in weight gain in patients with CF.
Interestingly, it seems that the common pathway of all influential factors for calprotectin is the altered intestinal microbiota. The current findings suggest a significant inverse correlation between the richness of the CF microbiota (e.g., Bacteroides) and intestinal inflammation [34]. Moreover, the association of the intestinal microbiota with FEV1 and growth factors was confirmed in the current study [35], while the question whether intestinal dysbiosis is the main influential factor in altered FC levels or the confounding factor for this correlation remains unanswered. The main limitation of this study was that we were unable to evaluate the possible confounding effects of the intestinal microbiota on this association. To the best of our knowledge, this is the first study to report a pooled estimation of the association between FC and phenotype severity in patients with CF.
CONCLUSION
According to the results of this evaluation of the published literature, FC is a noninvasive marker that could be applicable for the detection of gastrointestinal inflammation and the assessment of the severity of the disease in patients with CF.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIALS
Search strategy result
The Newcastle–Ottawa scale for assessing the risk of bias in selected studies
Risk of bias based on ROBINS-I assessment tool
Mean, upper, and lower limit range of fecal calprotectin levels in cystic fibrosis patients.
(A) Funnel plot of including studies for evaluating the relationship between fecal calprotectin level in pancreatic insufficient and pancreatic sufficient cystic fibrosis (CF) patients. (B) Funnel plot of included studies for evaluating the relationship between pulmonary function test and fecal calprotectin in CF patients.
References
- 1.Lubamba B, Dhooghe B, Noel S, Leal T. Cystic fibrosis: insight into CFTR pathophysiology and pharmacotherapy. Clin Biochem. 2012;45:1132–1144. doi: 10.1016/j.clinbiochem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Roesch EA, Nichols DP, Chmiel JF. Inflammation in cystic fibrosis: an update. Pediatr Pulmonol. 2018;53(S3):S30–S50. doi: 10.1002/ppul.24129. [DOI] [PubMed] [Google Scholar]
- 3.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros. 2015;14:419–430. doi: 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Belcher CN, Vij N. Protein processing and inflammatory signaling in cystic fibrosis: challenges and therapeutic strategies. Curr Mol Med. 2010;10:82–94. doi: 10.2174/156652410791065408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briars G. Intestinal inflammation in cystic fibrosis. Arch Dis Child. 2001;84:374–375. doi: 10.1136/adc.84.4.373c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack DR, Flick JA, Durie PR, Rosenstein BJ, Ellis LE, Perman JA. Correlation of intestinal lactulose permeability with exocrine pancreatic dysfunction. J Pediatr. 1992;120:696–701. doi: 10.1016/s0022-3476(05)80230-6. [DOI] [PubMed] [Google Scholar]
- 7.Taylor-Cousar JL, Von Kessel KA, Young R, Nichols DP. Potential of anti-inflammatory treatment for cystic fibrosis lung disease. J Inflamm Res. 2010;3:61–74. doi: 10.2147/jir.s8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rumman N, Sultan M, El-Chammas K, Goh V, Salzman N, Quintero D, et al. Calprotectin in cystic fibrosis. BMC Pediatr. 2014;14:133. doi: 10.1186/1471-2431-14-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjerke K, Halstensen TS, Jahnsen F, Pulford K, Brandtzaeg P. Distribution of macrophages and granulocytes expressing L1 protein (calprotectin) in human Peyer’s patches compared with normal ileal lamina propria and mesenteric lymph nodes. Gut. 1993;34:1357–1363. doi: 10.1136/gut.34.10.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parisi GF, Papale M, Rotolo N, Aloisio D, Tardino L, Scuderi MG, et al. Severe disease in Cystic Fibrosis and fecal calprotectin levels. Immunobiology. 2017;222:582–586. doi: 10.1016/j.imbio.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Schnapp Z, Hartman C, Livnat G, Shteinberg M, Elenberg Y. Decreased fecal calprotectin levels in cystic fibrosis patients after antibiotic treatment for respiratory exacerbation. J Pediatr Gastroenterol Nutr. 2019;68:282–284. doi: 10.1097/MPG.0000000000002197. [DOI] [PubMed] [Google Scholar]
- 12.Ellemunter H, Engelhardt A, Schüller K, Steinkamp G. Fecal calprotectin in cystic fibrosis and its relation to disease parameters: a longitudinal analysis for 12 years. J Pediatr Gastroenterol Nutr. 2017;65:438–442. doi: 10.1097/MPG.0000000000001544. [DOI] [PubMed] [Google Scholar]
- 13.Fraser-Pitt D, O’Neil D. Cystic fibrosis - a multiorgan protein misfolding disease. Future Sci OA. 2015;1:FSO57. doi: 10.4155/fso.15.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20:123–129. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 18.Adriaanse MP, van der Sande LJ, van den Neucker AM, Menheere PP, Dompeling E, Buurman WA, et al. Evidence for a cystic fibrosis enteropathy. PLoS One. 2015;10:e0138062. doi: 10.1371/journal.pone.0138062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhaliwal J, Leach S, Katz T, Nahidi L, Pang T, Lee JM, et al. Intestinal inflammation and impact on growth in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2015;60:521–526. doi: 10.1097/MPG.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 20.Werlin SL, Benuri-Silbiger I, Kerem E, Adler SN, Goldin E, Zimmerman J, et al. Evidence of intestinal inflammation in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;51:304–308. doi: 10.1097/MPG.0b013e3181d1b013. [DOI] [PubMed] [Google Scholar]
- 21.Stallings VA, Tindall AM, Mascarenhas MR, Maqbool A, Schall JI. Improved residual fat malabsorption and growth in children with cystic fibrosis treated with a novel oral structured lipid supplement: a randomized controlled trial. PLoS One. 2020;15:e0232685. doi: 10.1371/journal.pone.0232685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Freitas MB, Moreira EAM, Tomio C, Moreno YMF, Daltoe FP, Barbosa E, et al. Altered intestinal microbiota composition, antibiotic therapy and intestinal inflammation in children and adolescents with cystic fibrosis. PLoS One. 2018;13:e0198457. doi: 10.1371/journal.pone.0198457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg M, Leach ST, Coffey MJ, Katz T, Strachan R, Pang T, et al. Age-dependent variation of fecal calprotectin in cystic fibrosis and healthy children. J Cyst Fibros. 2017;16:631–636. doi: 10.1016/j.jcf.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Garg M, Leach ST, Pang T, Needham B, Coffey MJ, Katz T, et al. Age-related levels of fecal M2-pyruvate kinase in children with cystic fibrosis and healthy children 0 to 10years old. J Cyst Fibros. 2018;17:109–113. doi: 10.1016/j.jcf.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Więcek S, Woś H, Kordys-Darmolińska B, Sankiewicz-Szkółka M, Grzybowska-Chlebowczyk U. The concentration of calprotectin in the stools of children with diagnosed cystic fibrosis. Prz Gastroenterol. 2017;12:38–43. doi: 10.5114/pg.2016.58897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorsey J, Gonska T. Bacterial overgrowth, dysbiosis, inflammation, and dysmotility in the Cystic Fibrosis intestine. J Cyst Fibros. 2017;16(Suppl 2):S14–S23. doi: 10.1016/j.jcf.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Marsland BJ, Gollwitzer ES. Host-microorganism interactions in lung diseases. Nat Rev Immunol. 2014;14:827–835. doi: 10.1038/nri3769. [DOI] [PubMed] [Google Scholar]
- 28.Hoen AG, Li J, Moulton LA, O’Toole GA, Housman ML, Koestler DC, et al. Associations between gut microbial colonization in early life and respiratory outcomes in cystic fibrosis. J Pediatr. 2015;167:138–47.e1-3. doi: 10.1016/j.jpeds.2015.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson JL, Miles C, Tierney AC. Effect of probiotics on respiratory, gastrointestinal and nutritional outcomes in patients with cystic fibrosis: a systematic review. J Cyst Fibros. 2017;16:186–197. doi: 10.1016/j.jcf.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Héry-Arnaud G, Boutin S, Cuthbertson L, Elborn SJ, Tunney MM. The lung and gut microbiome: what has to be taken into consideration for cystic fibrosis? J Cyst Fibros. 2019;18:13–21. doi: 10.1016/j.jcf.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Sabharwal S. Gastrointestinal manifestations of cystic fibrosis. Gastroenterol Hepatol (N Y) 2016;12:43–47. [PMC free article] [PubMed] [Google Scholar]
- 32.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun. 2004;72:6040–6049. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werlin S, Benuri-Silbiger I, Cohen L, Kerem E, Aviram M, Bentur L, et al. Enteropathy – a new finding in cystic fibrosis. J Cyst Fibros. 2008;7(Suppl 2):S79. [Google Scholar]
- 34.Bruzzese E, Callegari ML, Raia V, Viscovo S, Scotto R, Ferrari S, et al. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: a randomised clinical trial. PLoS One. 2014;9:e87796. doi: 10.1371/journal.pone.0087796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coffey MJ, Nielsen S, Wemheuer B, Kaakoush NO, Garg M, Needham B, et al. Gut microbiota in children with cystic fibrosis: a taxonomic and functional dysbiosis. Sci Rep. 2019;9:18593. doi: 10.1038/s41598-019-55028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy result
The Newcastle–Ottawa scale for assessing the risk of bias in selected studies
Risk of bias based on ROBINS-I assessment tool
Mean, upper, and lower limit range of fecal calprotectin levels in cystic fibrosis patients.
(A) Funnel plot of including studies for evaluating the relationship between fecal calprotectin level in pancreatic insufficient and pancreatic sufficient cystic fibrosis (CF) patients. (B) Funnel plot of included studies for evaluating the relationship between pulmonary function test and fecal calprotectin in CF patients.