Abstract
Objectives:
Adjuvant medications including proton pump inhibitors (PPI), antibiotics (trimethoprim/sulfamethoxazole, TMP-SMX), and inhaled corticosteroids (ICS) may be prescribed in conjunction with surgery for idiopathic subglottic stenosis (iSGS). We describe adjuvant medication use patterns with endoscopic dilation (ED) or endoscopic resection with medical treatment (ERMT) and evaluate impact on treatment outcomes.
Methods:
North American Airway Collaborative data were interrogated to determine if patients received adjuvant medications(s). Primary outcome was time to recurrent operation, evaluated using Kaplan-Meier curves and Cox regression analysis. Secondary outcomes of change in peak expiratory flow (PEF) and clinical chronic obstructive pulmonary disease questionnaire (CCQ) score over 12 months were compared.
Results:
61/129 patients undergoing ED received PPI (47%), and 10/143 patients undergoing ED received ICS (7%). TMP-SMX was used by 87/115 patients (76%) undergoing EMRT. PPI use in the ED group did not affect time to recurrence (HR=1.00, 95% CI: 0.53–1.88; p=0.99) or 12-month change in PEF (L/min) (median (IQR), 12.0 (10.7–12.2) vs. 8.7 (−5.1–24.9); p=0.59), but was associated with 12-month change in CCQ score (−0.05 (−0.97–0.75) vs. −0.50 (−1.60–0.20); p=0.04). ICS did not affect outcome measures. TMP-SMX use in ERMT did not affect time to recurrence (HR=0.842, 95% CI: 0.2345, 3.023; p=0.79), PEF at 12 months (75 (68–89) vs. 81 (68–89); p=0.92), or 12-month change in CCQ score (0.20 (−1.05–0.47) vs. −0.30 (−1.00–0.10); p=0.45).
Conclusion:
There is no standard practice for prescribing adjuvant medications. These data do not support that adjuvant medication use prolongs time to recurrence or increases PEF. Patients with iSGS and GERD may experience some symptom benefit with PPI use.
Keywords: Idiopathic subglottic stenosis, endoscopic dilation, endoscopic resection with medical therapy, proton pump inhibitor, inhaled corticosteroid, trimethoprim-sulfamethoxazole, adjuvant medical treatment
INTRODUCTION
Idiopathic subglottic stenosis (iSGS) is a fibroinflammatory disease leading to airway narrowing and dyspnea, occurring primarily in adult Caucasian females.1 Subepithelial mucosal thickening is associated with an inflammatory cellular infiltrate, fibroblast proliferation, and abnormal collagen deposition.2 Management is principally surgical, with both endoscopic and open approaches described.3–5 There is a tendency for disease to recur, particularly after endoscopic treatment. Accordingly, efforts at prolonging the interval between operations have been made, in part through the use of adjuvant medications.
Proton pump inhibitors (PPIs) have been used due to a potential role of gastroesophageal reflux disease (GERD) contributing to or perpetuating the inflammation and mucosal injury associated with iSGS. A potential causative role was proposed in 1994 based on the improvement in response to surgical intervention after medical management of GERD in six patients.6 Dual probe pH monitoring studies have demonstrated abnormal findings in approximately half of iSGS patients7 and pepsin, a marker of extraesophageal reflux, has also been identified in histologic specimens.8 However, there has been some mixed evidence regarding a potential role of GERD, as its presence was not associated with symptomatic recurrence of iSGS in patients undergoing endoscopic resection with medical treatment (ERMT),9 and use of PPIs alone did not affect the relative risk of stenosis recurrence.3 There has been a recent concern for potential adverse effects of long-term PPI use, including increased risk of osteoporotic fractures, gastrointestinal infection, and chronic kidney disease.10–12 While the precise relationship between PPI use and these effects is not known at this time, avoiding PPI use in patients for whom it is not required is desirable.
Antibiotics including trimethoprim-sulfamethoxazole (TMP-SMX) and macrolides such as azithromycin have also been used as adjunctive medications in the treatment of iSGS. TMP-SMX is used for the treatment of subglottic stenosis related to granulomatous polyangiitis13 and macrolides have anti-inflammatory effects that can reduce pro-inflammatory cytokine production.14 Further, alteration of the normal upper airway microbiome may be a relevant contributor to the pathophysiology of iSGS. Mycobacterium and Moraxellaceae species were recently identified within mucosal specimens from iSGS patients15–16 and antibacterial treatment may be helpful in eliminating pathogenic upper airway bacteria.
Lastly, inhaled corticosteroids have been employed due to anti-inflammatory effects. While use of these medications makes intuitive sense, the amount of medication actually deposited at the subglottis is uncertain. Frank-Ito and Cohen conducted a modeling simulation and found that only 2.6% of orally inhaled drug particles are deposited at the subglottis, with up to 2.4% deposited at the glottis.17
Despite theoretical promise, usage of these adjuvant medications is based on somewhat limited retrospective data. Further, current prescribing patterns for adjuvant medications after endoscopic surgery for iSGS are not well described. In this study, data from the North American Airway Collaborative (NoAAC), a prospective multi-institutional cohort of iSGS patients, were analyzed to identify practice patterns for two treatment groups (endoscopic dilation (ED) and endoscopic resection with medical treatment (EMRT)) to determine the effect of adjuvant medications on a primary outcome measure of inter-dilation interval as well as secondary outcome measures of peak expiratory flow (PEF) and clinical chronic obstructive pulmonary disease questionnaire (CCQ) scores. We hypothesized that prescribing pattern would vary across patients and between surgical approaches. We also hypothesized that use of adjuvant medications would increase the time to the subsequent operation (primary outcome) and produce improvements in peak expiratory flow and patient-reported breathing complaints (secondary outcomes).
METHODS
The study was approved by the Vanderbilt University Medical Center Institutional Review Board and utilized data from the iSGS1000 patient cohort,18 a prospective multi-institutional cohort of 1000 iSGS patients established in 2014 by the North American Airway Collaborative [NoAAC]) housed within the NoAAC data coordinating center.
Participants
General inclusion criteria for the multi-institutional cohort include age >18 years and presence of an obstructive airway lesion involving the subglottis.18 Exclusion criteria include history of intubation or tracheotomy within two years prior to symptoms, positive antinuclear cytoplasmic antibody titer, presence of a vasculitis or collagen vascular disease, and history of prior traumatic laryngotracheal injury, neck radiation, and laryngotracheal thermal or caustic injury.18
Additional inclusion and exclusion criteria were applied for this study. To be included, patients had to have undergone either ED or ERMT, and had confirmed they were or were not taking adjuvant medications. Patients provided a binary (yes or no) response regarding whether or not they were taking a given adjuvant medication within the first six months from date of procedure. Patients treated with cricotracheal resection were excluded, as the focus of this study was on impact of adjuvant medications following endoscopic intervention. Cricotracheal resection represents an entirely different approach, with removal of the visibly affected upper airway segment. Accordingly, adjuvant medication use may have less of an impact compared to patient and technical details of the operation itself, which are more difficult to evaluate across multiple institutions. With endoscopic interventions, the affected portion of the airway and, presumably, the disease process remain in place and adjuvant medication may represent one method of treating residual disease. Patients undergoing in-office serial intralesional steroid injections were also excluded. Data from this later group will be reported separately.
Study Protocol
The study followed a pre-specified protocol.18 At enrollment, patients completed baseline demographics and a series of patient-reported outcome measures (PROMs) evaluating constructs affected by the disease and its treatment. In addition, disease-specific data were abstracted from medical records. Symptomatic patients then underwent standard of care treatment at their respective medical centers. Patients underwent an “index” or most proximate operation (if last treatment predated study inception), defined as time zero (T0). Time to next operation, termed time to recurrence (TTR), was then evaluated, and compared between groups using Kaplan-Meier curve survival analysis over the study period. Percentage of patients undergoing repeat operation within one year of the index operation was determined.
Primary treatments were endoscopic dilation (ED) and endoscopic resection with adjuvant medical therapy (ERMT). Cricotracheal resection was outside the scope of this study as noted above. ED could be performed with rigid instruments or controlled radial expansion devices (e.g., balloon dilation).18 ERMT consisted of injection of steroid within the stenosis, CO2 laser-based resection of portions of the stenosis with preservation of intervening mucosa, avoidance of dilation, and application of mitomycin-C.3
Patients completed an electronic health status survey every three months after enrollment. The electronic data capture system generated a scheduled automated query that solicited data on prespecified adjuvant medication use. Medications specifically queried were proton pump inhibitors (PPI), inhaled corticosteroids (ICS), trimethoprim-sulfamethoxazole (TMP-SMX), and macrolide antibiotics (ML). The status check was tracked which patients had undergone any interval interventions to treat their iSGS (operative or clinic-based). If a patient underwent a subsequent operative intervention, that time would be noted. Patients undergoing clinic-based interventions (serial steroid injections) were excluded from this study. For patients undergoing ERMT, specific adverse events and tolerance of TMP-SMX were queried as a component of the initial study. The degree of compliance with concomitant ICS and PPI was not reported for all patients. Accordingly, multivariate analyses considering potential interactions between medications could not be performed.
Patients completed the CCQ every 6 months. The CCQ is a 10-item psychophysical scale developed for monitoring of chronic obstructive pulmonary disease that has been previously validated for use in adult patients with laryngotracheal stenosis.19 Scores range from 0 to 6 with a reported minimal clinically important difference of 0.4.20 Peak expiratory flow (PEF) was measured on a handheld device and self-reported results were recorded by participants on mobile device software. PEF is a reliable indicator of disease progression and good predictor of receipt of surgical intervention in patients with iSGS.21
Outcomes and statistical analysis
The primary outcome measure was time to recurrent operation (TTR) defined as days from T0 to recurrent operation (TR). Kaplan-Meier curves were created for patients receiving and not receiving a given adjuvant medication, and log-rank tests were used to compare differences between curves. Hazard ratios with 95% confidence intervals were calculated using the Cox proportional hazard model. Hazard ratios were included to allow for comparison across the range of TTR values among participants and were interpreted to reflect the probability ratio of a participant receiving an adjuvant medication undergoing a recurrent operation versus a participant not receiving an adjuvant medication undergoing a recurrent operation.
Secondary outcome measures included PEF and CCQ score. Proportion of patients taking each adjuvant medication within each surgical group is presented descriptively. Wilcoxon rank sum tests or signed rank tests were used to evaluate for differences in change in PEF and CCQ score over 12 months between groups (i.e., score at 12 months minus the baseline score) for patients undergoing ED. The 12-month change in CCQ score was also used for the ERMT group; however, as only two patients undergoing ERMT had baseline PEF scores, the PEF at 12 months was used rather than a change over 12 months. Medians and interquartile ranges were used to summarize continuous data. Two-sided P values less than or equal to 0.05 were considered statistically significant. Alpha corrections were not performed as each statistical test evaluated a different set of variables (treatment group, adjuvant medication, outcome measure). Analyses were performed using SPSS software, version 17.0 (IBM Corp., Armonk, NY), and R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Participants
Data for 656 patients were reviewed. Within this group, data on adjuvant medication use were available for 143 patients undergoing ED (median (IQR) age: 48 years (42–57); 99% female) and 115 patients undergoing ERMT (age: 56 years (48–63); 99% female). Within the ED group, 73 patients received ICS, PPI, or macrolide antibiotic while 68 did not receive any of these; 2 did not receive ICS and macrolide antibiotic and had unknown status for PPI. There were no patients in the ED group taking TMP-SMX. Ten patients received ICS, 61 received PPI, and only 2 patients received macrolide antibiotic. None of these patients received more than one adjuvant medication. All 115 patients meeting inclusion criteria who underwent ERMT were prescribed an ICS, PPI, and TMP-SMX.3,22 However, 28 patients did not tolerate TMP-SMX, leaving 87 patients who received TMP-SMX. As noted above, specific compliance data regarding ICS and PPI were not available for this group. Given the available data, analysis of the primary and secondary outcome measures focused on the effect of PPIs and ICS within the ED group and the effect of TMP-SMX within the ERMT group. Data are presented for macrolide antibiotics within the ED group but due to significant group size imbalance, comparative statistical analyses were not performed.
Proton pump inhibitor
Overall, PPI were used as adjuvant therapy in 61 of 129 (47%) patients in the ED group. GERD diagnosis rates differed between groups (65% versus 9%; p<0.001). There was no difference in TTR (HR=1.00, 95% CI: 0.53 to 1.88; p=0.99; Figure 1) or change in 12-month peak expiratory flow between those not on PPI and those on a PPI (no PPI: median (IQR), 12.0 L/min (10.7–12.2) versus PPI: 8.7 (−5.1– 24.9); p=0.59; Table 1), respectively. PPI use was associated with the change in 12-month CCQ score (no PPI: −0.05 (−0.97 to 0.75) vs. PPI: −0.50 (−1.60 to 0.20); p=0.044; Table 1).
Figure 1.

Kaplan-Meier curve demonstrating recurrence of disease over time in patients undergoing endoscopic dilation with or without taking adjuvant proton pump inhibitor. There was no difference in time to recurrence.
Table 1.
Summary Data for Secondary Outcome Measures of 12-Month Change in Peak Expiratory Flow and Clinical COPD Questionnaire for Patients Undergoing Endoscopic Dilation.
| Medication | Endoscopic Dilation | Results | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| N | Median (IQR) | N | Median (IQR) | ||
| 12-mo change in peak expiratory flow (L/min) | |||||
| Proton pump inhibitor | 61 | 8.7 (−5.1, 24.9) | 68 | 12.0 (10.7, 12.2) | P = .59 |
| Inhaled corticosteroid | 10 | 28.3 (25.7, 31.0) | 133 | 11.4 (3.9, 21.2) | P = .21 |
| 12-mo change in clinical COPD questionnaire | |||||
| Proton pump inhibitor | 61 | −0.50 (−1.60, 0.20) | 68 | −0.05 (−0.97, 0.75) | P = .044 |
| Inhaled corticosteroid | 10 | −2.05 (−2.43, 0.48) | 133 | −0.30 (−1.40, 0.50) | P = .28 |
IQR = interquartile range.
Inhaled corticosteroid
Ten patients (7%) within the ED group received ICS versus 133 that did not. There was no between group difference in TTR (HR=1.37, 95% CI: 0.49 to 3.83; p=0.55; Figure 2), change in PEF (no ICS: median (IQR), 11.4 (3.9 to 21.2) L/min vs. ICS: 28.3 (25.7 to 31.0); p=0.21; Table 2), or CCQ score (no ICS: −0.30 (−1.40 to 0.50) vs. ICS −2.05 (−2.43 to 0.48); p=0.28; Table 2) at 12 months.
Figure 2.

Kaplan-Meier curve demonstrating recurrence of disease over time in patients undergoing endoscopic dilation with or without taking adjuvant inhaled corticosteroid. There was no difference in time to recurrence.
Table 2.
Summary Data for Secondary Outcome Measures of 12-Month Peak Expiratory Flow and 12-Month Change in Clinical COPD Questionnaire for Patients Undergoing Endoscopic Dilation. Baseline Data Were Not Available for Peak Expiratory Flow, So These Values Represent the Measurement at 12 Months Rather Than a Change Over 12 Months.
| Medication | Endoscopic Resection With Medical Treatment | Results | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| N | Median (IQR) | N | Median (IQR) | ||
| 12-mo peak expiratory flow (L/min) | |||||
| TMP-SMX | 87 | 81 (66–90) | 28 | 75 (68–89) | P = .92 |
| 12-mo change in clinical COPD questionnaire | |||||
| TMP-SMX | 87 | −0.30 (−1.00, 0.10) | 28 | 0.20 (−1.05, 0.47) | P = .45 |
IQR = interquartile range; TMP-SMX = trimethoprim-sulfamethoxazole.
Antibiotics
Two patients within the ED group received macrolide antibiotics versus 141 that did not. Accordingly, no formal analyses were performed given the group size disparity.
Within the ERMT group, 87 received TMP-SMX and 28 did not. As noted above, only two subjects had baseline PEF scores. Thus, 12-month PEF rather than change in PEF at 12 months was used to compare groups. There was no difference between groups for TTR (HR=0.84, 95% CI: 0.23 to 3.02; p=0.79; figure 3), 12-month PEF (no TMP-SMX: 75 L/min (68 to 89) vs. TMP-SMX: 81 L/min (66 to 90); p=0.92), or change in 12-month CCQ score (no TMP-SMX: 0.20 (−1.05 to 0.47) versus TMP-SMX: −0.30 (−1.00 to 0.10); p=0.45).
Figure 3.
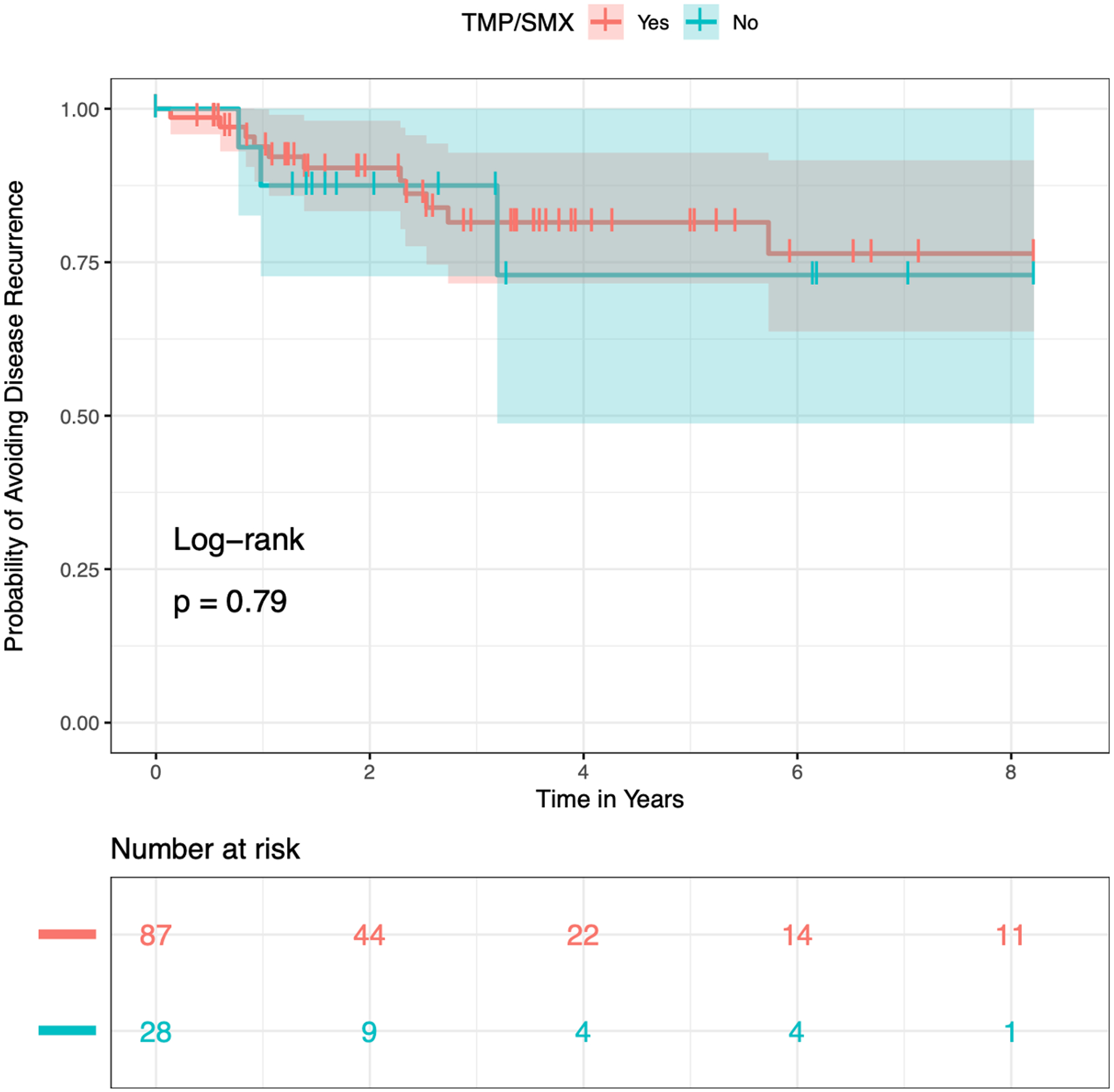
Kaplan-Meier curve demonstrating recurrence of disease over time in patients undergoing endoscopic resection with or without taking adjuvant trimethoprim/sulfamethoxazole. There was no difference in time to recurrence.
DISCUSSION
Idiopathic subglottic stenosis has notable impacts on patient quality of life,23 related both to symptom burden and the need for ongoing periodic operative interventions. Adjuvant medications designed to reduce symptoms and prolong the time between interventions are thus desirable. The multi-institutional design employed here allows for inclusion of a larger group of patients than has been evaluated previously.3,24
Similar to differences in operative approach across institutions,5 there are differences in adjuvant medication use. There was greater variability within the endoscopic dilation group, as may be expected given that approach is used at more institutions than endoscopic resection with medical treatment.
Notably, this study did not demonstrate an effect on time to recurrence or change in PEF for use of PPIs or inhaled corticosteroids as adjuvant medication in isolation for patients with iSGS undergoing endoscopic dilation, nor was there demonstrated efficacy for use of TMP/SMX in patients undergoing endoscopic resection. The only effect which was observed was a greater reduction in clinical COPD questionnaire score at 12 months for patients undergoing endoscopic dilation and receiving PPI. The difference in median change of 0.45 did exceed the reported minimal clinically important difference of 0.4.20 Predictably, there was a difference in GERD prevalence between those receiving and not receiving PPI (65% vs. 9%). Further, some of the items in the questionnaire may be relevant not only to those with respiratory impairment but also those with GERD, such as “In general, during the past week, how much of the time did you cough?” and “In general, during the past week, how much of the time did you produce phlegm?” The finding of a difference in clinical COPD questionnaire score but not time to recurrence or PEF may reflect an impact of PPI use on comorbid GERD rather than modification of iSGS severity, though data regarding responses to each individual question on the questionnaire were not available.
Data on the benefits of adjuvant medication use from prior studies are mixed. This study includes the largest cohort of patients with iSGS specifically investigating the impact of adjuvant medications. If there is a benefit with regular use of these medications that was not observed in this study, the potential effect size may be modest, particularly for endoscopic dilation.
Maldanado et al. performed a single institution review of recurrence rates in patients undergoing endoscopic resection for iSGS, including an analysis of the impact of adjuvant medications.3 This included 17 patients not receiving adjuvant medications, 19 on PPI alone, 25 receiving PPI and inhaled corticosteroids, and 49 receiving PPI, inhaled corticosteroids, and TMP-SMX. Those patients receiving PPI alone or PPI with inhaled corticosteroids had recurrence rates very similar to the group not receiving adjuvant medications. The group receiving all three appeared to have a reduced risk though this was not statistically significant. Importantly, the current study evaluated only the impact of TMP-SMX and insufficient clinical information was available to evaluate impact of multiple adjuvant medications taken simultaneously. Interestingly, no effect was observed for taking TMP-SMX. Thus, in conjunction with the findings from the study by Maldonado et al., it remains plausible that a synergistic effect exists when taking all three adjuvant medications, rather than the benefit being primarily due to TMP-SMX.
The findings of this study do not imply that adjuvant medications should not be used in this patient group, but rather that there is not support for their indiscriminate use. Patients with regurgitation and pyrosis will still benefit from PPIs, and patients with true dynamic obstructive lower airway disease as demonstrated on pulmonary function tests will still benefit from inhaled corticosteroids. However, use of these medications in the setting of iSGS and the absence of other comorbid factors is not expected to affect time to recurrence. Importantly, however, there may be a selection bias present, with incomplete understanding regarding which subtle clinical characteristics may predict benefit from a given adjuvant medication.
Limitations to this study deserve note. The multi-institutional design adds inherent heterogeneity to operative approach, patient selection for receiving and continuing on a given adjuvant medication, and decision-making on when to perform the next operation. This added variability may result in an inability to observe more subtle differences in the outcome measures related to use of adjuvant medication. We feel the generalizability and increased sample size afforded by the multi-institutional approach, particularly when studying a rare disease such as iSGS, offset this limitation. While the overall sample size was large considering the disease prevalence, confirmation that a participant in the overall study was taking or not taking each adjuvant medication was not always available. Thus, some sub-group sizes were particularly small, precluding meaningful analyses for those medications (e.g., macrolide antibiotics) or analysis of potential interactions in those patients receiving multiple adjuvant medications. This is further reflected by the large confidence intervals observed for the analysis on inhaled corticosteroid use in the endoscopic dilation group, for which only ten patients were included in the medication group. Though the group sizes were unbalanced, the data were included to allow for inclusion in potential future meta-analyses. Lastly, we did not include participants receiving serial office-based intralesional steroid injections, as this is a different type of intervention (serial procedures performed in the clinic rather than adjuvant medications taken by a patient at home) and will be the focus of a separate study.
CONCLUSION
There is variability in patterns of adjuvant medication use across different institutions caring for patients with idiopathic subglottic stenosis. Use of adjuvant proton pump inhibitors or inhaled steroids for patients undergoing endoscopic dilation and use of trimethoprim/sulfamethoxazole for patients undergoing endoscopic resection does not change time to the next operation or peak expiratory flow. There may be an effect on dyspnea-related symptoms for those patients with iSGS and comorbid GERD undergoing endoscopic dilation. If adjuvant medications are prescribed, it should be on a patient-specific basis.
ACKNOWLEDGEMENTS
This work was funded by grant 1409-22214 from the Patient Centered Outcomes Research Institute. Dr. Gelbard is also supported by grant R01HL146401-01 from the NIH National Heart, Lung, and Blood Institute. Dr. Francis is also supported by grant R01CA251566-01 from the National Cancer Institute, NIH and R21016724-01 from the National Institute for Deafness and Communication Disorders, NIH.
Footnotes
Conflicts of Interest: None.
Level of evidence: level 4.
REFERENCES
- 1.Gelbard A, Francis DO, Sandulache VC, et al. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope 2015; 125(5):1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mark EJ, Meng F, Kradin RL, Mathisen DJ, Matsubara O. Idiopathic tracheal stenosis: A clinicopathologic study of 63 cases and comparison of the pathology with chondromalacia. Am J Surg Pathol 2008; 32:1138–43. [DOI] [PubMed] [Google Scholar]
- 3.Maldonado F, Loiselle A, DePew ZS, et al. Idiopathic subglottic stenosis: An evolving therapeutic algorithm. Laryngoscope 2014; 124:498–503. [DOI] [PubMed] [Google Scholar]
- 4.Axtell AL, Mathisen DJ. Idiopathic subglottic stenosis: techniques and results. Ann Cardiothorac Surg 2018; 7(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope 2016; 126:1390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindal JR, Milbrath MM, Hogan WJ, et al. Gastroesophageal reflux disease as a lukely cause of “idiopathic” subglottic stenosis. Ann Otol Rhinol Laryngol 1994; 103(3):186–91. [DOI] [PubMed] [Google Scholar]
- 7.Fang H, Codipilly DC, Ravi K, et al. Gastroesophageal reflux characteristics and patterns in patients with idiopathic subglottic stenosis. Gastroenterol Res Prac 2018. Jun 11;2018:8563697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumin JH, Johnston N. Evidence of extraesophageal reflux in idiopathic subglottic stenosis. Laryngoscope 2011; 121:1266–73. [DOI] [PubMed] [Google Scholar]
- 9.Menapace DC, Ekbom DC, Larson DP, et al. Evaluating the association of clinical factors with symptomatic recurrence of idiopathic subglottic stenosis. JAMA Otolaryngol Head Neck Surg 2019. Jun 1; 145(6):524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired Clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol 2016; 37(12):1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf 2019; 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG. Trimethroprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med 1996; 335:16–20. [DOI] [PubMed] [Google Scholar]
- 14.Black PN. Anti-inflammatory effects of macrolide antibiotics. Eur Resp J 1997; 10:971–2. [DOI] [PubMed] [Google Scholar]
- 15.Gelbard A, Katsantonis NG, Mizuta M, et al. Molecular analysis of idiopathic subglottic stenosis for Mycobacterium species. Laryngoscope 2017; 127:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillel AT, Tang SS, Carlos C, et al. Laryngotracheal microbiota in adult laryngotracheal stenosis. mSphere 2019. May 1; 4(3):e00211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank-Ito DO, Cohen SM. Orally inhaled drug particle transport in computerized models of laryngotracheal stenosis. Otolaryngol Head Neck Surg 2020. Oct 13; 1945992820959674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelbard A, Shyr Y, Berry L, et al. Treatment options in idiopathic subglottic stenosis: Protocol for a prospective international multicentre pragmatic trial. BMJ Open 2018; 8:e022243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nouraei SAR, Randhawa PS, Koury EF, et al. Validation of the clinical COPD questionnaire as a psychophysical outcome measure in adult laryngotracheal stenosis. Clin Otolaryngol 2009; 34:343–8. [DOI] [PubMed] [Google Scholar]
- 20.Kon SSC, Dilaver D, Mittal M, et al. The clinical COPD questionnaire: response to pulmonary rehabilitation and minimal clinically important difference. Thorax 2014. Sep; 69(9):793–8. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter DJ, Ferrante S, Bakos SR, Clary MS, Gelbard AH, Daniero JJ. Utility of routine spirometry measures for surveillance of idiopathic subglottic stenosis. JAMA Otolaryngol Head Neck Surg 2019. Jan 1; 145(1):21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekbom DC, Bayan SL, Goates AJ, Kasperbauer JL. Endoscopic wedge excisions with CO2 laser for subglottic stenosis. Laryngoscope 2020. Aug 21. [DOI] [PubMed] [Google Scholar]
- 23.Noud M, Hovis K, Gelbard A, et al. Patient-reported outcome measures in upper airway-related dyspnea: A systematic review. JAMA Otolaryngol Head Neck Surg 2017. Aug; 143(8):824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouveris H, Karaiskaki N, Koutsimpelas D, Chongolwatana C, Mann W. Eur Arch Otorhinolaryngol 2013. Mar; 270(3):989–93. [DOI] [PubMed] [Google Scholar]


