Abstract
Case series
Patients: 7
Final Diagnosis: COVID-19 disease
Symptoms: Fever • dypspnea
Medication: Standard of care Clinical
Procedure: C-reactive protein apheresis
Specialty: Immunology • Infectious Diseases
Objective:
Unusual clinical course
Background:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced pneumonia is a disease with high mortality and, still, no effective treatment. Excessively elevated C-reactive protein (CRP) plasma levels inversely correlate with prognosis. As CRP, via complement and macrophage activation, can cause organ damage in COVID-19, we have recently introduced selective CRP apheresis as a potentially effective treatment. Now, we report on the first patients with severe SARS-CoV-2-induced pneumonia treated within the “C-reactive protein Apheresis in COVID” (CACOV) registry.
Case Reports:
Seven sequential hospitalized patients with documented COVID-19, strongly elevated CRP plasma levels, and respiratory failure were treated by selective CRP apheresis in addition to standard therapy after having given their informed consent for inclusion in the CACOV registry. We performed 2-8 CRP apheresis sessions via either peripheral or central venous access depending on clinical course and CRP plasma levels. CRP apheresis, in COVID-19, reduced CRP plasma levels by approximately 50–90%, and it was thus highly effective, feasible, and safe. Despite severe radiological lung involvement in all our patients, only 2 patients finally required intubation, and none required extracorporeal membrane oxygenation (ECMO). All 7 patients were discharged from our 2 hospitals in good clinical condition.
Conclusions:
Selective CRP apheresis, starting early after patient admission, may be an effective treatment of SARS-CoV-2-induced pneumonia. SARS-COV-2 can cause organ damage and multiple organ failure predominantly by an excessive CRP-mediated autoimmune response of the ancient innate immune system. Further registry data and randomized trials are needed.
Keywords: Blood Component Removal, C-Reactive Protein, COVID-19
Background
C-reactive protein (CRP), the prototype human acute-phase protein, has been considered as a marker of inflammation since it was first described in 1930 by Tillet and Francis [1]. Although CRP, like antibodies, is known to activate the complement system via the classical pathway [2] and macrophages via Fcγ-receptors [3,4], it was always thought to be only a marker of inflammation rather than an active contributor to disease. The idea that CRP, which appeared very early in evolution [5], was probably the first antibody molecule in the evolution of the immune system and may thus cause autoimmune reactions [6] seemed obvious but was nonetheless rejected by the scientific and medical community for a long time. Recently however, with the occurrence of SARS-CoV-2-induced disease COVID-19 and with the observation that highly elevated CRP plasma levels correlate with worse prognosis in this disease [7,8], the following questions were asked:
Why are CRP plasma levels in COVID-19 atypically high compared to other viral diseases?
Why do such highly elevated CRP plasma levels significantly correlate with worse outcome?
Is it just a coincidence that drugs with at least some (limited) therapeutic success in COVID-19 treatment, like tocilizumab [9] or dexamethasone [10], lower CRP plasma levels?
With the introduction of CRP apheresis [11–14], a specific extracorporeal procedure lowering CRP plasma levels selectively, quickly, and without severe side effects, such questions can now be answered. We have recently introduced selective CRP apheresis as a potential treatment of severe SARS-CoV-2-induced pneumonia [15–17]. CRP apheresis is performed using a highly selective CRP adsorber developed by Pentracor GmbH [17]. After publishing 3 case reports describing individual healing attempts [16,18–19], we have initiated the “C-reactive protein Apheresis in COVID” (CACOV) registry to treat COVID-19 disease by selective CRP apheresis within a reputable scientific framework for Health Services Research. In parallel, the randomized “C-reactive protein Apheresis for Attenuation of Pulmonary, Myocardial and/or Kidney Injury in COVID-19” (CAPMYKCO) trial (https://clinicaltrials.gov/ct2/show/NCT04898062) was initiated.
Here, we report on the first 7 sequential COVID-19 patients with severe SARS-CoV-2-induced pneumonia treated by highly selective CRP apheresis at our study center in the Kempten and Immenstadt hospitals, Bavaria, Germany within the CACOV registry.
Case Reports
The CACOV Registry
The CACOV registry is a prospective single-arm patient registry (https://clinicaltrials.gov/ct2/about-studies/glossary) to investigate the reduction of C-reactive protein (CRP) by selective C-reactive protein apheresis in patients with COVID-19 and highly elevated CRP plasma levels (https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00024376). Although the CACOV registry formally is an all-comer registry with no substantial exclusion criteria for patients with documented COVID-19 and elevated CRP plasma levels, we have decided at our study center to include only those patients with severe SARS-CoV-2-induced pneumonia and signs of respiratory failure. Whereas the CAPMYKCO trial (see above) is a randomized pilot trial with a control group that is not treated by selective CRP apheresis, the CACOV registry allows treatment of every patient who might benefit from the highly selective extracorporeal procedure.
Patient Characteristics
Patient characteristics are summarized in Table 1. Age (range 37–60 years), concomitant diseases (eg, arterial hypertension and obesity), duration of symptoms before hospital admission (0–21 days), hospital length of stay (7–21 days), ventilation therapy (non-invasive/invasive), and PCR and mutation analysis of throat smear are depicted. Although all the patients had concomitant diseases known to increase mortality risk in COVID-19, and although all the patients required either non-invasive oxygen supply or invasive ventilation therapy, none of them died and all were discharged from our hospitals Immenstadt and Kempten after 7–21 days with no need for respiratory support, able to care for themselves, and in good clinical condition.
Table 1.
Patient characteristics.
| Patient | Age | Sex | Concomitant diseases | Duration of symptoms before hospital admission (d) | Oxygen supply, intubation, ventilation duration (d) | Hospital length of stay (d) | PCR, C(t), mutation analysis | Antibiotic therapy | Further issues |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | F | Immune deficiency, multiple sclerosis | ca. 21 | 5 (NIV) | 7 | SARS-CoV-2-VOC, C(t) 28.2, B.1.1.7 (Alpha) | .. | COVID reactivation under Rituximab therapy |
| 2 | 60 | M | Arterial hypertension, coronary heart disease, obesity, DM type II | 2 | 16 (NIV) | 15 | SARS-CoV-2-VOC, C(t) 25.8, B.1.617.2 (Delta) | .. | .. |
| 3 | 57 | M | Arterial hypertension, emphysema | 11 | 9 (NIV) | 9 | SARS-CoV-2-VOC, C(t) 31.5, B.1.617.2 (Delta) | From d 1 to d 6 | .. |
| 4 | 54 | M | Arterial hypertension, obesity | 9 | 10 (NIV) | 9 | SARS-CoV-2, no mutation analysis | .. | .. |
| 5 | 57 | F | Coronary heart disease, ischemic cardiomyopathy, allergic diathesis | 7 | 4 (intubation), 7 (NIV) | 21 (15-21: social reasons) | SARS-CoV-2-VOC, C(t) 25.2, B.1.617.2 (Delta) | From d 11 to d 17 | Secondary bacterial pneumonia (from d 11 onwards) |
| 6 | 58 | M | .. | 0 | 8 (Intubation), 12 (NIV) | 21 | SARS-CoV-2-VOC, C(t) 19.9, B.1.617.2 (Delta) | From d 3 to d 8 and from d 9 to d 21 | Pneumocystis jirovecii pneumonia, anxious depressive adjustment disorder under COVID pneumonia |
| 7 | 43 | M | Sleep apnoea syndrome, arterial hypertension | 8 | 8 (NIV) | 8 | SARS-CoV-2-VOC, C(t) 28.2, B.1.617.2 (Delta) | .. | .. |
Table shows age, sex, concomitant diseases, respiratory supply, hospital length of stay, PCR, antibiotic therapy and further issues in our 7 patients. All patients had documented SARS-CoV-2-induced pneumonia and respiratory failure. DM – diabetes mellitus; C(t) – crossing threshold; PCR – polymerase chain reaction; SARS-CoV-2-VOC – severe-acute-respiratory-syndrome-coronavirus-2-variant of concern).
Computed Tomography (CT) Scans
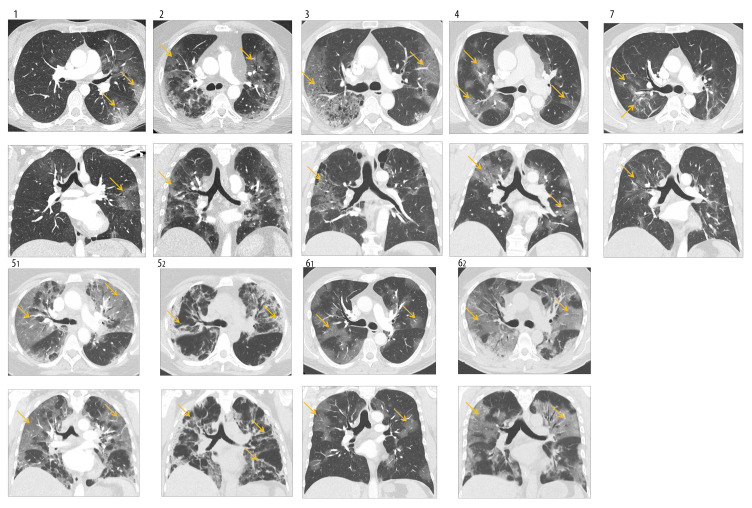
Figure 1 shows chest computed tomography (CT) scans (axial/coronal) of all patients on admission. In each patient, the typical bilateral infiltrates and the beginning of fibrosing alveolitis are observable. Control chest CT scan of patient number 5 on day 11 and of patient number 6 on day 8 shows peripheral lung consolidation interpreted as bacterial superinfection by our local radiologist.
Figure 1.
(1–7) CT scans during hospital stay. Chest CT scans (Contrast spiral-CT; axial [above] and coronal [below] plane) on admission showed bilateral infiltrates, predominant basal distribution, and development of fibrosing alveolitis in all patients (yellow arrows). Patients 5 (and 6) received a second CT scan (52/62) on day 11 (and 8), showing peripheral lung consolidation interpreted as bacterial superinfection by our local radiologist.
CRP Plasma Levels
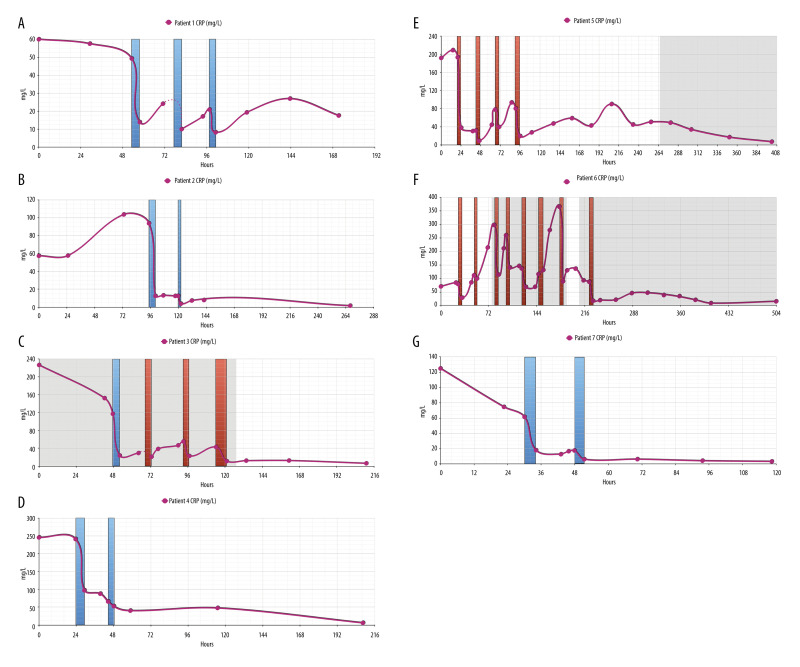
Figure 2 summarizes the courses of CRP plasma levels in each patient. CRP levels were elevated on admission (57.24–245.36 mg/L). In patients 1, 3, and 7 we observed a (slight) decrease in CRP plasma levels during the first 24 h of hospital stay. As we treated every patient who had documented COVID-19 and requiring oxygen supply by 6 mg/d dexamethasone (which is known to decrease CRP plasma levels itself), the initial decrease in CRP levels may indeed be considered as steroid-induced. Due to CRP apheresis via either peripheral (blue columns) or central (red columns) venous access, CRP levels markedly dropped and further declined later on. Similar to the results in our second case report [18], a later small CRP peak (7.79–47.39 mg/L) on days 6–7 remained clinically inapparent in patients 1–4. In contrast, patients 5 and 6 acquired ventilator-associated pneumonia (Staphylococcus aureus and Pneumocystis jirovecii, respectively) with a second rise in CRP levels, as well as a rise in PCT levels (Figure 3) and leukocyte count on days 9 and 14, respectively. These 2 patients received antibiotic therapy with piperacillin-tazobactam (and cotrimoxazole, respectively), with a resulting clinical improvement and a normalization of CRP plasma levels after 3 and 6 (piperacillin-tazobactam)/12 (cotrimoxazole) days of antibiotic treatment, respectively. Possible interference between CRP apheresis and bacterial infection is discussed below.
Figure 2.
(A–G) CRP levels during hospital stay. In each patient, CRP levels (reference range 0.00–5.00 mg/L) were highly elevated on admission. In patients 1, 3, and 7, they slightly decreased until the next day, most likely due to immediate prednisolone treatment. With each CRP apheresis session via peripheral (blue columns) or central venous access/Shaldon catheter (red columns), however, CRP levels markedly decreased in each patient and normalized during the hospital stay. Notably, CRP apheresis was continued during bacterial superinfection. Gray shadows: antibiotic treatment.
Figure 3.
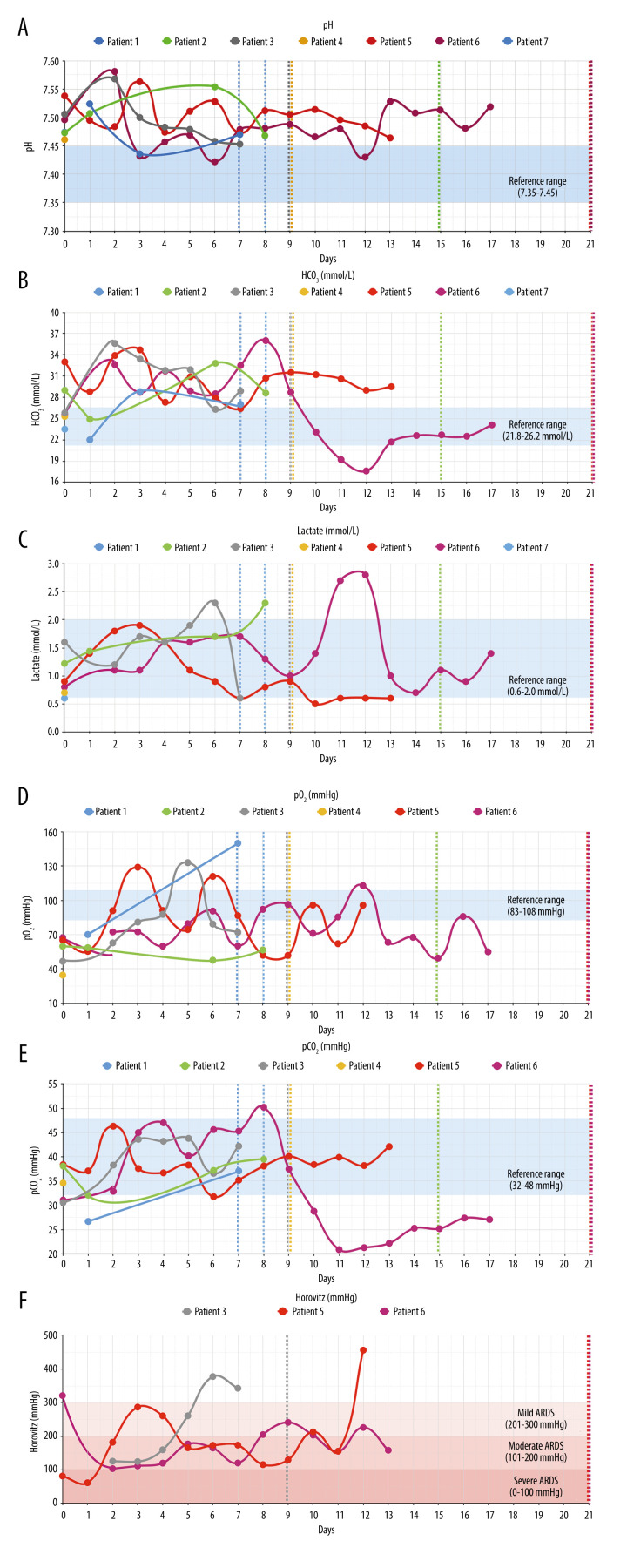
(A–F) Respiratory parameters. Figure shows respiratory parameters in all patients. All patients had respiratory/metabolic alkalosis (pH >7.45, HCO3 >26.2 mmol/L). Horovitz quotient was only determined on ICU in patients 3 (high flow oxygen therapy), 5, and 6 (intubation) and normalized in each case. Blue shadows: reference ranges. Dashed lines: patient discharge.
Respiratory and Laboratory Parameters
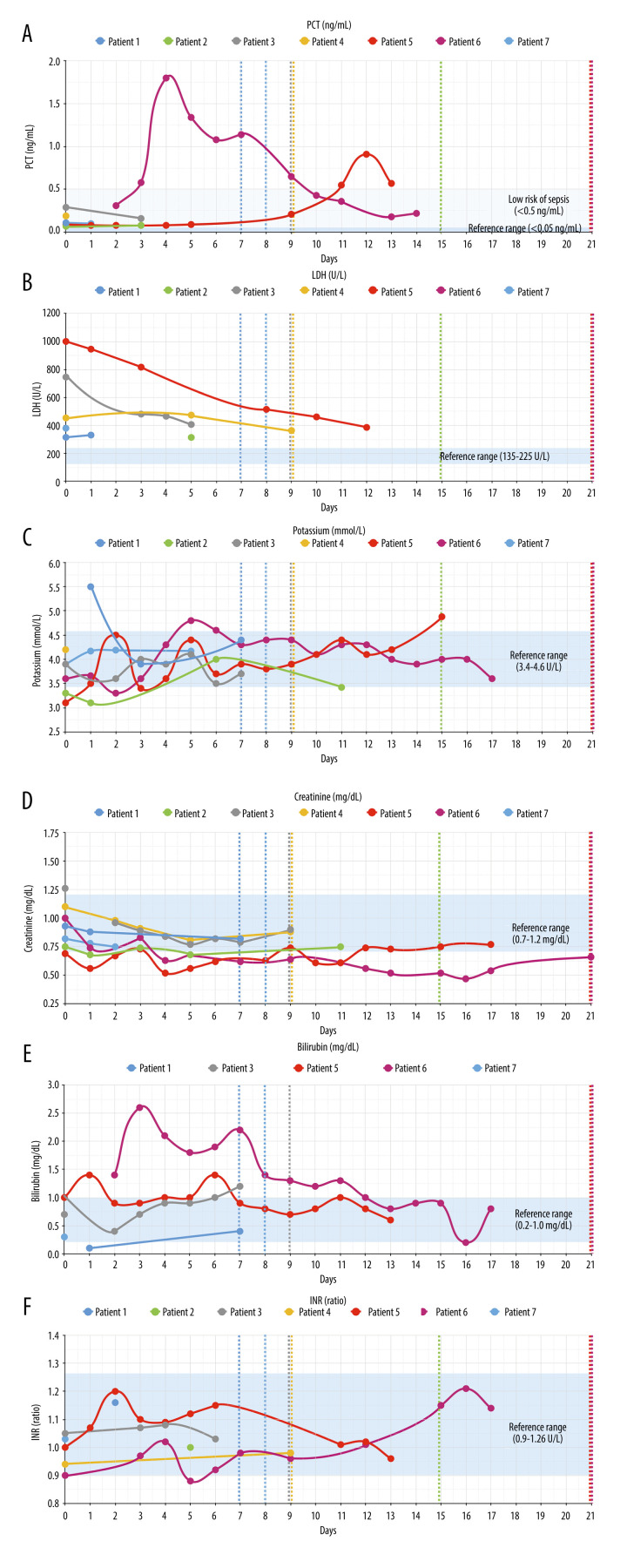
Figure 3 shows respiratory parameters in all patients and Figure 4 shows other laboratory results. With regard to respiratory parameters (Figure 3), all patients markedly improved during hospitalization. All patients had respiratory/metabolic alkalosis, which needs to be monitored carefully during apheresis [20]. Only patients 5 and 6 required intubation in the first evening after start of CRP apheresis. For patient 5, extracorporeal membrane oxygenation (ECMO) was discussed but proved to be unnecessary as the patient’s respiratory parameters markedly improved overnight. Regarding other laboratory parameters (Figure 4), the following issues were remarkable: None of the patients developed signs of multiple organ failure such as acute renal or hepatic failure or cardiac insufficiency, and none of them developed blood coagulation derailment. All the patients recovered relatively quickly, with the exception of patients 5 and 6, who developed bacterial super-infection and had to be treated by antibiotics. Patients 5 and 6 finally also recovered and were discharged from the hospital on day 21. Hospital discharge for patient 5, although clinically possible on day 15, was postponed due to social reasons.
Figure 4.
(A–F) Non-respiratory parameters. Figure shows laboratory parameters indicating organ function. Whereas all patients showed LDH elevation, none of them developed acute renal (creatinine) or hepatic (bilirubin) failure, and none of them developed blood coagulation (INR) derailment. Blue shadows: reference ranges. Dashed lines: patient discharge.
Discussion
SARS-CoV-2, following its invasion in pulmonary tissue with the resulting direct cytotoxic effects, is known to induce pro-inflammatory mediators such as high levels in cytokines as well as hypercoagulability and endothelial dysfunction in most fulminant COVID-19 courses [21]. These secondary autoimmune reactions may be causally linked to CRP. CRP, by activating complement and macrophages via Fcγ-receptors [2–4,21] and through its direct cytotoxic effects [22,23], can cause an excessive autoimmune reaction of ancient innate immunity and may thus be the central effector molecule in severe SARS-CoV-2-induced pneumonia and subsequent multiple organ failure. Based on this hypothesis, selective extracorporeal CRP apheresis may indeed be the therapy of choice to avoid severe courses of COVID-19 [16,18–19].
Recently, we treated 3 patients as individual healing attempts and published their case reports [16,18,19]. We then started the CACOV registry and now describe the first 7 sequential patients with respiratory failure and strongly elevated CRP plasma levels whose treatment and detailed analyses have been finalized up to the present day. Regarding these 7 patients, we observed the following issues:
First, in analogy to the initial 3 case reports [16,18,19], CRP apheresis effectively counteracted elevation of CRP plasma levels. CRP apheresis was begun very early after patient admission and was continued until CRP plasma levels remained below 50 mg/L for at least 72 h. Second, in each of the 7 patients, respiratory parameters markedly improved during the 2–8 CRP apheresis sessions. Third, all patients were discharged from the hospital in good clinical condition. Fourth, in COVID-19 patients, central venous access seems to be more practical than peripheral venous access for performing CRP apheresis. We sometimes observed low blood flow and clotting when using peripheral venous access. This is in contrast to our STEMI patients, who can be treated by CRP apheresis via peripheral access [12–14]. This clotting is probably due to the known hypercoagulability in COVID-19 [24], and may also be caused by hemodynamic centralization in these severely ill patients. Thus, we now use Shaldon catheters for CRP apheresis in our COVID patients. Of course, Shaldon catheter implantation is done under sterile conditions. Infection through lines is avoided by hygiene monitoring and surveillance as generally required for all these severely ill patients. Fifth, patients 3, 5, and 6 received antibiotic treatment (Table 1), patient 3 because of the suspicion of bacterial superinfection (elevated procalcitonin [PCT] levels but no positive blood culture), patient 5 because of documented secondary bacterial pneumonia (hospital-acquired pneumonia) and tracheal secretion positive for Staphylococcus aureus, patient 6 additionally because of documented Pneumocystis infection. Notably, CRP apheresis was continued despite bacterial superinfection. It is indeed important to note that this did not harm the patient. Sixth, patient 5 developed severe alkalosis which had to be corrected by acetazolamide treatment. Here, the known respiratory alkalosis in COVID-19 [20] as well as metabolic alkalosis related to SARS-CoV-2-induced renal abnormalities or even metabolic alkalosis related to apheresis and citrate application need to be carefully monitored. Notably, the latter is not observable when treating STEMI patients [12–14] because of their ability of hypoventilation and respiratory compensation. Obviously, hypoventilation is not an option for COVID-19 patients. Seventh, we did not observe any severe hypodynamic episodes during apheresis. Lastly, dexamethasone itself also lowered CRP plasma levels to a certain extent in patients 1, 3, and 7. This CRP-lowering capacity of dexamethasone may be the reason for its limited therapeutic success. Selective CRP apheresis, however, with an up to 87% reduction within hours, was obviously much more effective in lowering CRP plasma levels.
It is important to note that, up to the present day, multiple therapeutic apheresis methods have been attempted and are a category 3 recommendation in the therapeutic apheresis guideline only [25]. CRP apheresis, however, highly selectively targeting a potential key molecule in SARS-CoV-2-induced organ damage, may probably turn out to be more successful.
We would finally like to emphasize that this case series of the first 7 patients treated at our study center within the CACOV registry is no conclusive proof that CRP apheresis is the therapy of choice in severe SARS-CoV-2-induced pneumonia. The latter can only be proven by randomized controlled trials, as for example the CAPMYKCO trial (please see above). It is, however, important to note that our clinical observations support the concept that CRP apheresis is effective in treating COVID-19; therefore, randomized trials may have to be thoroughly supervised by ethics committees, steering committees, and interim analyses. CRP apheresis is highly effective and highly selective [17]. Thus, any clinical observation made in our patients may potentially be related to the depletion of CRP. Importantly, further registry data and, even more important, randomized clinical trials are urgently needed.
Conclusions
Selective CRP apheresis, starting early after patient admission, may potentially be an effective treatment of SARS-CoV-2-induced pneumonia. SARS-COV-2 can cause organ damage and multiple organ failure predominantly by an excessive CRP-mediated autoimmune response of the ancient innate immune system. Further registry data and randomized clinical trials are needed.
Acknowledgments
We gratefully acknowledge Charlene Edge, Pentracor GmbH, Germany, Kerstin Rziha, Medical Care Center Kempten, Germany, and our study nurses, Jacqueline Fiedler and Tina Kienle, for organizing and performing CRP apheresis with great commitment and empathy. We gratefully acknowledge Dr. Patrizia Brunner, Pentracor GmbH, for proof reading and substantial improvement of the manuscript. Finally, we gratefully acknowledge the doctors and nurses of hospitals Immenstadt and Kempten, Bavaria, Germany, for treating the patients with all their patience and empathy.
Footnotes
Role of Funding Source
Clinic Association Allgaeu advocated the CACOV Registry. German Health Insurance Companies covered the costs for CRP apheresis within the CACOV registry. Clinic Association Allgaeu and German Health Insurance Companies did not influence the study design, data collection and analysis, interpretation of data, or writing of this report.
Ethics Committee Approval
Eth-28/21 (Aerztekammer Berlin), mb21035 (Ethikkommission der Bayerischen Landesärztekammer). All patients gave their written informed consent.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52(4):561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112(6):2135–47. [PubMed] [Google Scholar]
- 3.Bharadwaj D, Stein MP, Volzer M, et al. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J Exp Med. 1999;190(4):585–90. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolov DE, Rocker C, Hombach V, et al. Ultrasensitive confocal fluorescence microscopy of C-reactive protein interacting with FcgammaRIIa. Arterioscler Thromb Vasc Biol. 2004;24(12):2372–77. doi: 10.1161/01.ATV.0000147407.17137.02. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen NY, Suzuki A, Cheng SM, et al. Isolation and characterization of Limulus C-reactive protein genes. J Biol Chem. 1986;261(22):10450–55. [PubMed] [Google Scholar]
- 6.Zimmermann O, Li K, Zaczkiewicz M, et al. C-reactive protein in human atherogenesis: Facts and fiction. Mediators Inflamm. 2014;2014:561428. doi: 10.1155/2014/561428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepys MB. C-reactive protein predicts outcome in COVID-19: Is it also a therapeutic target? Eur Heart J. 2021;42(23):2280–83. doi: 10.1093/eurheartj/ehab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smilowitz NR, Kunichoff D, Garshick M, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42(23):2270–79. doi: 10.1093/eurheartj/ehaa1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas IO, Brau N, Waters M, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021;384(16):1503–16. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheriff A, Schindler R, Vogt B, et al. Selective apheresis of C-reactive protein: A new therapeutic option in myocardial infarction? J Clin Apher. 2015;30(1):15–21. doi: 10.1002/jca.21344. [DOI] [PubMed] [Google Scholar]
- 12.Ries W, Sheriff A, Heigl F, Zimmermann O, et al. “First in man”: Case report of selective C-reactive protein apheresis in a patient with acute ST segment elevation myocardial infarction. Case Rep Cardiol. 2018;2018:4767105. doi: 10.1155/2018/4767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ries W, Heigl F, Garlichs C, et al. Selective C-reactive protein-apheresis in patients. Ther Apher Dial. 2019;23(6):570–74. doi: 10.1111/1744-9987.12804. [DOI] [PubMed] [Google Scholar]
- 14.Ries W, Torzewski J, Heigl F, et al. C-reactive protein apheresis as anti-inflammatory therapy in acute myocardial infarction: Results of the CAMI-1 study. Front Cardiovasc Med. 2021;8(155):591714. doi: 10.3389/fcvm.2021.591714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayser S, Kunze R, Sheriff A. Selective C-reactive protein apheresis for COVID-19 patients suffering from organ damage. Ther Apher Dial. 2021;25(2):251–52. doi: 10.1111/1744-9987.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torzewski J, Heigl F, Zimmermann O, et al. First-in-man: Case report of selective C-reactive protein apheresis in a patient with SARS-CoV-2 infection. Am J Case Rep. 2020;21:e925020. doi: 10.12659/AJCR.925020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattecka S, Brunner P, Hähnel B, et al. PentraSorb C-reactive protein: Characterization of the selective C-reactive protein adsorber resin. Ther Apher Dial. 2019;23(5):474–81. doi: 10.1111/1744-9987.12796. [DOI] [PubMed] [Google Scholar]
- 18.Ringel J, Ramlow A, Bock C, Sheriff A. Case report: C-reactive protein apheresis in a patient with COVID-19 and fulminant CRP increase. Front Immunol. 2021;12(3140):708101. doi: 10.3389/fimmu.2021.708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torzewski J, Zimmermann O, Kayser S, et al. Successful treatment of a 39-year-old COVID-19 patient with respiratory failure by selective C-reactive protein apheresis. Am J Case Rep. 2021;22:e932964. doi: 10.12659/AJCR.932964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Wang G, Zhang Q, et al. Association between respiratory alkalosis and the prognosis of COVID-19 patients. Front Med. 2021;8(478):564635. doi: 10.3389/fmed.2021.564635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Liu S, Liu J, et al. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Temming AR, Tammes Buirs M, Bentlage AEH, et al. C-reactive protein enhances IgG-mediated cellular destruction through IgG-Fc receptors in vitro. Front Immunol. 2021;12:594773. doi: 10.3389/fimmu.2021.594773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheriff A, Kayser S, Brunner P, Vogt B. C-reactive protein triggers cell death in ischemic cells. Front Immunol. 2021;12(273):630430. doi: 10.3389/fimmu.2021.630430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tufano A, Rendina D, Abate V, et al. Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: A systematic review with meta-analysis. J Clin Med. 2021;10(21):4925. doi: 10.3390/jcm10214925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 2019;34(3):171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]