Abstract
Background and Aim
Bacterial infection is involved in the progression of many gastrointestinal diseases, including those of pancreas; however, how and which bacteria colonize in pancreatic juice and tissue have yet to be elucidated. Recently, we reported that Enterococcus faecalis exists in the pancreatic juice and tissues of patients with chronic pancreatic disease. Here, we investigated the survival of E. faecalis in duodenal juice with different pH conditions.
Methods
Pancreatic juice samples from 62 patients with cancers of the duodeno‐pancreato‐biliary region were evaluated for the presence of E. faecalis. 16S ribosomal RNA polymerase chain reaction and 16S‐based metagenome analyses were performed to determine the bacterial composition. The survival of E. faecalis in various pancreatic juice conditions was evaluated.
Results
Of 62 samples, 27% (17/62) were positive for Enterococcus spp., among which 71% (12/17) contained E. faecalis. Enterococcus spp. showed the highest fitness for survival in alkaline pancreatic juice among various bacterial species. The microbiome of pancreatic juice from patients with pancreatic and bile duct cancer showed diversity, but Enterococcus spp. were enriched among duodenal tumors and intraductal papillary mucinous neoplasms.
Conclusions
Alkalinity is one of the important factors for the selective survival of E. faecalis among microbiota. E. faecalis can colonize the pancreatic duct when the pancreatic juice condition is altered.
Keywords: Enterococcus faecalis, microbiota, pancreatic cancer, pancreatic juice
Enterococcus spp. have higher fitness to survive in pancreatic juice. Enterococcus spp. can colonize pancreatic juice at subphysiological pH levels. Enterococcus spp. are enriched in the pancreatic juice of patients with intact ductal flow.
Introduction
Pancreatic juice, or pancreatic fluid, in healthy individuals is thought to be sterile due to its antibacterial activity toward a large spectrum of bacteria. 1 , 2 In a normal healthy situation, both the alkalinity of the fluid and small‐molecule peptides in the fluid protect the pancreas against ascending bacterial infection from duodenal microbiota. 2 However, bacterial infection is present in the pancreatic juice of patients with chronic pancreatic disease, including chronic pancreatitis 3 , 4 , 5 , 6 and pancreatic cancer. 3 , 5 , 7 Although bacterial infection in pancreatic juice has been regarded as a complication that affects the severity and therapeutic course of pancreatitis, the role of bacteria in pancreatic cancer development, carcinogenesis, and treatment is being investigated. 8 However, the mechanism by which bacteria become able to colonize in pancreatic juices, as well as the type of bacteria involved, has yet to be elucidated.
Enterococcus faecalis is a gram‐positive commensal bacterium that is part of the normal gut microbiota in healthy individuals. 9 E. faecalis and Enterococcus faecium, the most abundant species of the Enterococcus genus in humans, have recently emerged as a cause of nosocomial infection, in particular urinary tract infections. 10 We previously demonstrated the presence of Enterococcus spp. in pancreatic juice obtained from patients with cancers of the duodeno‐pancreato‐biliary region. Additionally, we showed an increase in serum antibodies against E. faecalis capsular polysaccharide in patients with chronic pancreatitis and pancreatic cancer, as well as the possible involvement of E. faecalis in the progression of pancreatic diseases. 5 In this previous report, the presence of E. faecalis in pancreatic juice was evaluated using a genetic and immunohistological approach; however, we have not confirmed the colonization of live E. faecalis in pancreatic juice. Here, we isolated live E. faecalis from pancreatic juice samples of patients with duodeno‐pancreato‐biliary disease. Additionally, we evaluated the effect of pancreatic juice pH levels on the survival of E. faecalis in pancreatic juice from the duodenum.
Methods
Pancreatic juice and tissue
The ethical committee at Osaka University Hospital approved the study protocol (protocol IDs 14107 and 15212), and written informed consent was obtained from each participant. Pancreatic juice was collected after pancreatectomy from the drainage tube from 62 patients, including 34 patients with pancreatic cancer and 28 with duodenal or bile duct cancer. None of the patients underwent previous sphincterotomy. There were no cases of distal pancreatectomy. Only clear colorless pancreatic juice was used to exclude samples with apparent retrograde contamination. All samples were collected at Osaka University Hospital, National Hospital Organization Kure Medical Center or Osaka Police Hospital and kept frozen at −80°C or refrigerated at 4°C until use.
Detection of bacterial DNA in pancreatic juice and bile by polymerase chain reaction analysis
Pancreatic juice was used directly as the template for polymerase chain reaction (PCR). PCR amplification was performed in 20‐μL reactions containing forward and reverse primers specific for the bacterial 16S ribosomal RNA (rRNA) gene. 11 The primers used in this study and temperature profiles for PCR are listed in Table S1, Supporting information.
16S ribosome metagenome analysis of bacteria in duodenal and pancreatic juices
16S rRNA metagenome analysis was performed at the Genome Information Research Center in Research Institute for Microbial Diseases, Osaka University. In brief, bacterial DNA was extracted from pancreatic juice or duodenal juice samples using a DNeasy PowerSoil Pro kit (Qiagen, Venlo, Netherlands). Each library was prepared according to the Illumina 16S Metagenomic Sequencing Library Preparation Guide with the following primer sets targeting the V1–V2 region of 16S rRNA genes: 27Fmod, 5′‐AGRGTTTGATCMTGGCTCAG‐3′; and 338R, 5′‐TGCTGCCTCCCGTAGGAGT‐3′. For the amplicons, 251‐bp paired‐end sequencing was performed on a MiSeq system (Illumina, San Diego, CA, USA) using a MiSeq Reagent v2 500 cycle kit. The paired‐end sequences obtained were merged, filtered, and denoised using DADA2 (https://github.com/benjjneb/dada2). Taxonomic assignment was performed using the QIIME2 feature‐classifier plugin with the Greengenes 13_8 database. The QIIME2 pipeline, version 2020.2, was used as the bioinformatics environment for the processing of all relevant raw sequencing data (https://qiime2.org).
Bacterial culture and isolation of E. faecalis
To isolate live bacteria, pancreatic juice was cultured for 24 h at 37°C either with brain‐heart infusion (BHI) agar (3.7% BBL Brain Heart Infusion [BD, Franklin Lakes, NJ, USA], 1.35% Bacto Agar [BD] in H2O) or Enterococcosel agar (5.6% BBL Enterococcosel Agar [BD] in H2O) for the purpose of culturing entire bacterial spectra or selecting Enterococcus spp., respectively.
To isolate E. faecalis, colonies grown on Enterococcosel agar were isolated and inoculated in Colombia media (3.5% Difco Columbia broth [BD] in H2O). A portion of each bacterial culture was subjected for PCR analysis to detect E. faecalis‐specific 16S rRNA using the specific primer set listed in Table S1. Isolated bacterial cultures confirmed to be E. faecalis were frozen as glycerol stocks until further use.
Survival of duodenal bacterial flora in pancreatic juice
Aseptic pancreatic juice was selected by PCR‐based screening with a nonspecific 16S rRNA primer set (Table S1). Duodenal juice was mixed with either aseptic pancreatic juice or sterile phosphate‐buffered saline (PBS) at a ratio of 1:49 and incubated at 37°C for 24 h. Then, part of the mixture was analyzed for the constituents by 16S rRNA metagenome analysis, as described above. A separate portion of the mixture was spread onto Enterococcosel agar and cultured for 24 h at 37°C.
Bacterial culture in various conditions of pancreatic juice
Colorimetric pH measurement was performed for 12 independent pancreatic juice samples by applying 1 μL sample onto MColorpHast pH strips (Merck Millipore, Burlington, MA, USA). To adjust the pH of pancreatic juice, 4 μL NaHCO3 (1 M) was added to 46 μL pancreatic juice. After the adjustment, colorimetric measurement was completed to confirm the pH was within the physiological range (9.0–9.5). Each pancreatic juice sample (50 μL) before and after pH adjustment was inoculated in Enterococcosel agar and incubated for 24 h. After 24 h cultivation, each plate was assigned to one of five groups according to the number of colonies.
To evaluate the fitness in various pancreatic juices, E. faecalis cultured in Columbia medium was adjusted to OD 690 of 1.0 and diluted to 1:10−6 in pancreatic juice from three different patients wherein no bacterium was detected. After incubating at 37°C overnight, 10 μL culture was spread onto BHI agar and further cultured for 24 h.
Statistical analyses
Statistical analyses were performed using either the Wilcoxon rank‐sum test or Student's t‐test. A P value less than 0.05 was considered statistically significant.
Results
Enterococcus spp. are enriched in pancreatic juice among duodenal bacterial flora
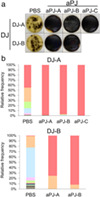
To evaluate the ability of Enterococcus spp. to survive in pancreatic juice, duodenal juices containing bacterial flora were cultured in aseptic pancreatic juice samples for 24 h. The enrichment of Enterococcus spp. was visualized by an expansion of colonies on Enterococcosel agar, an Enterococcus spp. selective agar plate (Fig. 1a). Additionally, 16S rRNA metagenome analysis confirmed the enrichment of Enterococcus spp. (Fig. 1b, c). These results suggest that Enterococcus spp. have a higher potential to survive in pancreatic juice among duodenal bacterial flora, which is the most likely source of bacterial colonization in bile and pancreatic juices.
Figure 1.
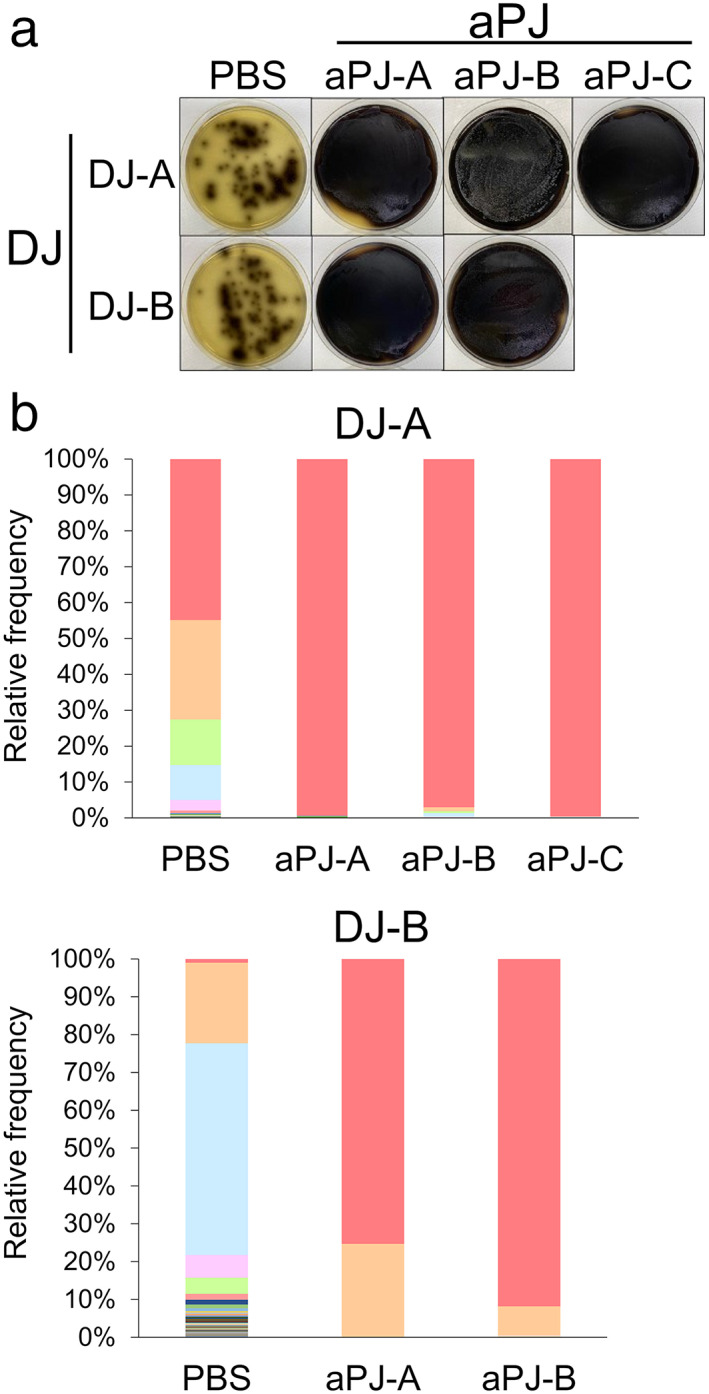
Enterococcus spp. have higher fitness to survive in pancreatic juice. (a) Visual agar plate assay to detect the abundance of Enterococcus spp. in a mixture of duodenal juice (DJ) and aseptic pancreatic juice (aPJ) at a 1:49 volume ratio. The DJ/aPJ mixture was incubated for 24 h before plating on Enterococcosel agar. Phosphate‐buffered saline was used as a control instead of aPJ. (b) Metagenome analysis of the DJ/aPJ mixture. After incubation, the samples were subjected to 16S rRNA metagenome analysis without selection on the agar plate. Each colored bar indicates the relative frequency of indicated bacterial species, which are listed on the right panel. ( ), Enterococcus spp.; (
), Enterococcus spp.; ( ), Enterobacteriaceae; (
), Enterobacteriaceae; ( ), Veillonella; (
), Veillonella; ( ), Bifidobacterium animalis; (
), Bifidobacterium animalis; ( ), Klebsiella; (
), Klebsiella; ( ), Enterococcus spp.; (
), Enterococcus spp.; ( ), Enterocuccus casseliflavus; (
), Enterocuccus casseliflavus; ( ), Bacteroides spp.; (
), Bacteroides spp.; ( ), Enterobacteriaceae; (
), Enterobacteriaceae; ( ), Fusobacterium spp. aPJ‐A, aseptic pancreatic juice from ampullary cancer; aPJ‐B, aseptic pancreatic juice from pancreatic neuroendocrine tumor; aPJ‐C, aseptic pancreatic juice from duodenal cancer; PBS, phosphate‐buffered saline.
), Fusobacterium spp. aPJ‐A, aseptic pancreatic juice from ampullary cancer; aPJ‐B, aseptic pancreatic juice from pancreatic neuroendocrine tumor; aPJ‐C, aseptic pancreatic juice from duodenal cancer; PBS, phosphate‐buffered saline.
Healthy pancreatic juice is thought to be aseptic, and this is explained in part by its alkaline condition. 2 To evaluate the pH level of pancreatic juice in disease and its correlation with bacterial colonization, pancreatic juice samples from 62 patients were analyzed. Of 62 samples, 45% (28/62) were positive for bacterial culture, of which 61% (17/28) were positive for Enterococcus spp. (Fig. 2a). Among Enterococcus spp., 71% (12/17) contained E. faecalis (Fig. 2a). The pH of pancreatic juice varied from 7.5 to 9.5, with the pH of most samples lower than that of normal pancreatic juice (Fig. 2b–d). Interestingly, the pH of pancreatic juice in which bacteria were detected was significantly lower than that of aseptic pancreatic juice (Fig. 2b). Despite the correlation between lower pH and general bacterial colonization in pancreatic juice, the detection of Enterococcus spp. and E. faecalis was not significantly correlated with a lower pancreatic juice pH (Fig. 2c,d). These results support the potential of Enterococcus spp., including E. faecalis, to survive in pancreatic juice.
Figure 2.
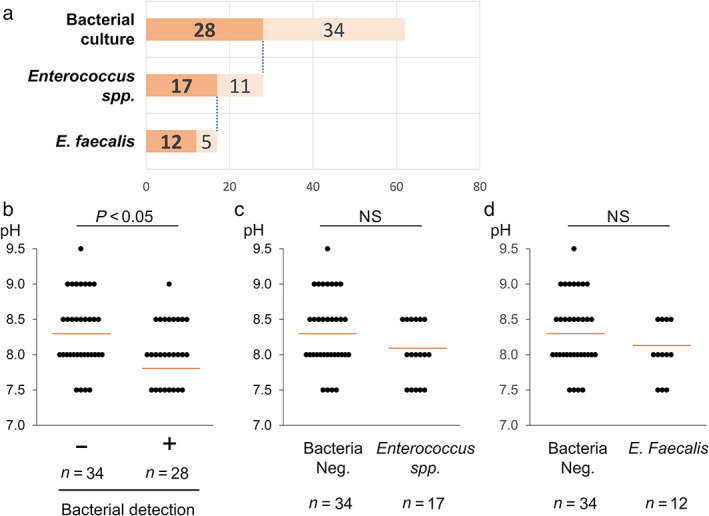
Enterococcus spp. can survive in pancreatic juice with a higher pH than other bacteria. (a) Chart showing the detection of Enterococcus faecalis from 62 pancreatic juice samples. ( ), Positive; (
), Positive; ( ), negative. (b–d) Comparison of the pH level in pancreatic juice samples with negative versus positive culture for any bacteria (b), Enterococcus spp. (c), and E. faecalis (d). The number of samples assigned for each group is indicated below the bars. Red lines designate the average in each group. Wilcoxon rank‐sum test was used for the statistical analysis.
), negative. (b–d) Comparison of the pH level in pancreatic juice samples with negative versus positive culture for any bacteria (b), Enterococcus spp. (c), and E. faecalis (d). The number of samples assigned for each group is indicated below the bars. Red lines designate the average in each group. Wilcoxon rank‐sum test was used for the statistical analysis.
Enterococcus spp., including E. faecalis, can colonize pancreatic juice at subphysiological pH levels
Although Enterococcus spp. have higher survival potential in pancreatic juice, bacteria were not detected in any of the pancreatic juice samples with a pH from 9 to 9.5 with only one exception. This is consistent with the common recognition that pancreatic juice is sterile in healthy patients due to its alkaline condition. This leads to the hypothesis that the slightly lowered pH of pancreatic juice, which can occur in pathogenic situations such as chronic pancreatitis, can be a trigger that allows colonization of Enterococcus spp., including E. faecalis.
To investigate this hypothesis, we evaluated the fitness of E. faecalis in pancreatic juice at different pH levels. E. faecalis strains isolated from 12 different pancreatic juice samples were cultured in pancreatic juice samples from pancreatic cancer patients with a pH of 9.5, 9.0, or 8.5, and colony formation capacity was measured. Our results showed that survival of all 12 strains was suppressed in pancreatic juice with a pH 9.5, whereas 5 in 12 and 11 in 11 strains survived in pancreatic juice with a pH 9.0 and 8.5, respectively (Fig. 3a, Figure S1). To further support the correlation between bacterial fitness in pancreatic juice and its pH, bacterial culture was completed for pancreatic juice samples with increased pH within the physiological range (pH, 9.0–9.5). pH levels were adjusted by the addition of NaHCO3 to the pancreatic juice samples. Consistent with the above results, colony formation was suppressed in 75% (9/12) of the pancreatic juice samples compared with the nonadjusted culture (Fig. 3b, Figure S2). These results indicate that Enterococcus spp. can survive pancreatic juice at subphysiological pH levels.
Figure 3.
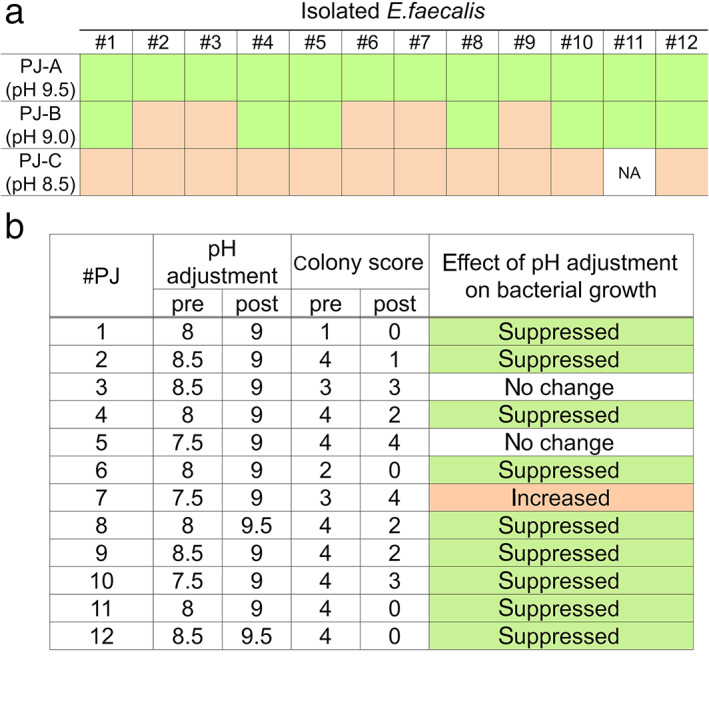
Enterococcus spp. grown in pancreatic juice with a pH above physiological limit. (a) Visual agar plate assay to detect the fitness of isolated Enterococcus faecalis to pancreatic juice with different pH levels. Twelve isolated E. faecalis samples were incubated with each pancreatic juice sample and plated on brain‐heart infusion agar. The changes in bacterial growth compared with the control (phosphate‐buffered saline) were shown. See Figure S1 for the original images of the agar plates. ( ), Suppressed growth in pancreatic juice (PJ); (
), Suppressed growth in pancreatic juice (PJ); ( ), increased growth in PJ. (b) Visual agar plate assay to detect the fitness of Enterococcus spp. in pancreatic juice. The 12 pancreatic juice samples, with or without adjusted pH levels, were plated on Enterococcosel agar. Bacterial growth was scored according to number of colonies, as shown in Figure S2. NA, non‐assessable.
), increased growth in PJ. (b) Visual agar plate assay to detect the fitness of Enterococcus spp. in pancreatic juice. The 12 pancreatic juice samples, with or without adjusted pH levels, were plated on Enterococcosel agar. Bacterial growth was scored according to number of colonies, as shown in Figure S2. NA, non‐assessable.
Enterococcus spp. are enriched in the pancreatic juice of diseased pancreas with intact pancreatic and biliary ductal flow, but not with cancer in the pancreatobiliary region
Although Enterococcus spp. and E. faecalis were enriched in pancreatic juice samples compared with other bacterial species, bacterial flora was not enriched for Enterococcus spp. in the samples of pancreatic cancer patients (Figure S3) when compared with matched duodenal juice samples. To understand this discrepancy, we analyzed the relationship between the frequency of Enterococcus spp. and the disease type for each case. Metagenome analysis was performed on pancreatic juice samples from 11 patients who had undergone pancreatoduodenectomy: five for pancreatic or bile duct cancers, two for intraductal papillary mucinous neoplasia (IPMN), and four for duodenal tumors. The pancreatic juice samples from five pancreatobiliary cancer patients exhibited a limited frequency of Enterococcus spp., whereas those from IPMN and duodenal tumor patients exhibited clear enrichment of Enterococcus spp. (Fig. 4a). Interestingly, the pH of each group (five samples from pancreatobiliary cancers vs six samples from the others) was not statistically different (Fig. 4b; P > 0.05 by Wilcoxon rank‐sum test), indicating the presence of factors in addition to the pH level allowing bacterial species other than Enterococcus spp. to colonize pancreatic juice.
Figure 4.
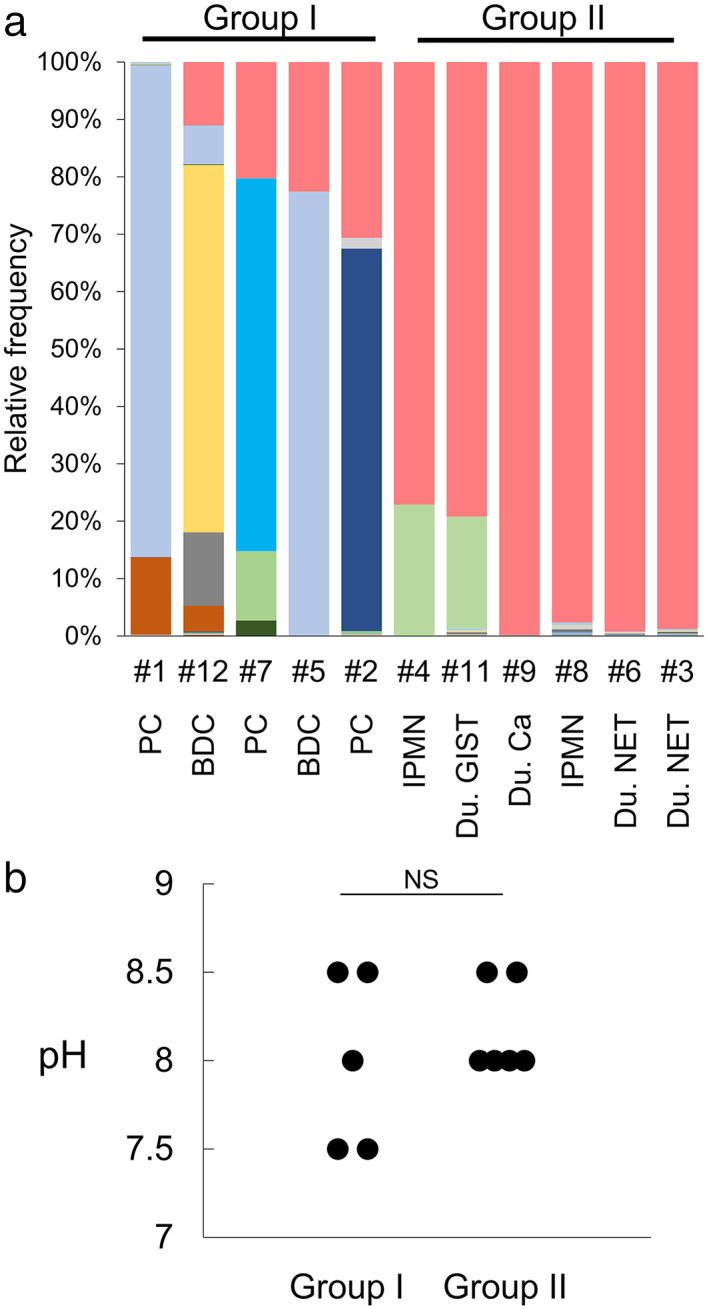
Microbiota in pancreatic juice is diverse in patients with pancreatobiliary cancer but is highly enriched with Enterococcus spp. in patients with duodenal region tumors and IPMN. (a) Metagenome analysis of 11 pancreatic juice samples. 16S rRNA metagenome analysis was performed on pancreatic juice samples from the indicated cases. Each colored bar indicates the relative frequency of the indicated bacterial species, which are listed on the right panel. ( ), Enterococcus spp.; (
), Enterococcus spp.; ( ), Enterobacteriaceae; (
), Enterobacteriaceae; ( ), Stenotrophomonas spp.; (
), Stenotrophomonas spp.; ( ), Stenotrophomonas spp.; (
), Stenotrophomonas spp.; ( ), Pseudomonas spp.; (
), Pseudomonas spp.; ( ), Aeromonas caviae; (
), Aeromonas caviae; ( ), Klebsiella oxytoca; (
), Klebsiella oxytoca; ( ), Escherichia coli; (
), Escherichia coli; ( ), Stenotrophomonas geniculata; (
), Stenotrophomonas geniculata; ( ), Enterococcaceae. (b) Comparison of pH levels in pancreatic juice samples of patients with pancreatobiliary cancer versus those with duodenal tumors and IPMN. Wilcoxon rank‐sum test was used for the statistical analysis. BDC, bile duct cancer; Du, duodenal‐; IPMN, intraductal papillary mucinous neoplasia; PC, pancreatic cancer.
), Enterococcaceae. (b) Comparison of pH levels in pancreatic juice samples of patients with pancreatobiliary cancer versus those with duodenal tumors and IPMN. Wilcoxon rank‐sum test was used for the statistical analysis. BDC, bile duct cancer; Du, duodenal‐; IPMN, intraductal papillary mucinous neoplasia; PC, pancreatic cancer.
Discussion
In the present study, we showed Enterococcus spp. have a higher potential to survive and colonize pancreatic juice than other bacteria. This ability of Enterococcus spp. to endure the antibacterial activity of pancreatic juice is, at least in part, due to its higher tolerance of the alkalinity of pancreatic juice than other bacteria.
Previous studies have reported that E. faecalis can survive in experimental alkaline conditions with pH as high as 11.5. 12 , 13 This alkaline tolerance has been of interest in particular in the dental field because E. faecalis has been isolated from failed endodontic cases, possibly due to its resistance to calcium hydroxide, which has antibacterial activity derived from strong alkalinity. 12 However, pancreatic juice is an alkaline fluid; its pH normally ranges from 8.3 to 8.6 and can be as high as 9.0 due to bicarbonate. 14 , 15 , 16 Therefore, the weak alkalinity of pancreatic juice alone is insufficient to explain the different antibacterial activity against E. faecalis compared with that of most other bacteria, which are alkaline sensitive. Nevertheless, E. faecalis fitness decreased in pancreatic juice at pH levels of 8.5 or higher in our experiments. This could be due to other antibacterial peptides in pancreatic juice, wherein the pH level is critical for their optimal antibacterial activity. 2 Thus, the mechanism of bacterial colonization in pancreatic juice is hypothesized to contain the following components: (i) triggering events (e.g. slight changes in the pH or antibacterial peptide level of the pancreatic juice, or a change in E. faecalis strain) initiate the colonization of E. faecalis; (ii) E. faecalis colonization in pancreatic juice induces further changes to the exocrine function of the pancreas; (iii) decreased exocrine function leads to reduced antibacterial activity in pancreatic juice; and (iv) these events eventually allow colonization of non‐Enterococcus bacteria. Indeed, our results also showed enrichment of Enterococcus spp. in samples from patients with duodenal tumors, which are supposed to lack chronic pancreatic inflammation. However, the pancreatic juice of patients with pancreatic cancer and bile duct cancer, which may be accompanied by chronic pancreatic inflammation, 17 showed a highly heterogeneous bacterial composition. Clinically, stenting for obstructive jaundice could be an additional factor that promotes heterogeneous bacterial colonization.
Recently, more studies have investigated the effect of the microbiota on cancer. 8 , 18 Patients with pancreatic cancer exhibit an abundant intratumoral microbiome; some species can induce immunosuppression to promote the progression of pancreatic ductal adenocarcinoma (PDAC) 19 as well as basal‐like subtype pf PDAC. 20 Additionally, the fungal microbiome can promote pancreatic oncogenesis by driving the complement cascade through the activation of mannose‐binding lectin. 21 Bacteria can influence not only carcinogenesis and cancer progression but also the therapeutic response. Gammaproteobacteria might contribute to gemcitabine resistance in PDAC. 22 Although the effect of E. faecalis on pancreatic cancer is not clear, a possible effect on colorectal cancer can be seen in several reports. However, whether it is tumor promoting or protecting is controversial. 23 Our previous report showed that chronic inflammation and fibrosis exist in the adjacent tissue of pancreatic cancer 17 and that E. faecalis resides in pancreatic cancer tissue of clinical samples. 5 These results suggest that chronic pancreatitis, accelerated by infection, may be involved in the pathogenesis of pancreatic cancer. Additionally, as indicated in this present study, E. faecalis can initiate translocation of the microbiome to the pancreas by virtue of its higher survivability in pancreatic juice than that of other bacteria. These results warrant additional future investigation to elucidate the involvement of E. faecalis in chronic pancreatitis as well as pancreatic cancer.
Supporting information
Figure S1. Original images for the visual agar plate assay used in Figure 3a. Twelve isolated E. faecalis (#1–#12) were incubated with each pancreatic juice (PJ A‐C) and plated on BHI agar plates. The changes in the bacterial growth compared to control (CTRL) was judged and described in Figure 3a. Unspecified green colony grew in the culture of #1, 3, and 11 with PJ C, which was initially characterized as aseptic, suggesting the presence of unspecified bacteria below detectable level in original PJ C. #11 culture in PJ C was judged as non‐assessable (NA) due to the contamination, while colonies of #1 and #3 culture in PJ C were clearly increased.
Figure S2. A legend for the scoring used in the Figure 3b. Bacterial growth was scored according to the colony number as indicated.
Figure S3. Metagenome analysis of the paired duodenal and pancreatic juice from five pancreatic cancer patients. Each colored bar indicates the relative frequency of indicated bacterial species, which are listed on the right panel. D, duodenal juice; P, pancreatic juice.
Table S1. Supplementary table.
Acknowledgments
We thank the next‐generation sequencing core facility of the Genome Information Research Center at the Research Institute for Microbial Diseases of Osaka University for the support in DNA sequencing and data analysis. The manuscript was edited by BioScience Writers, LLC (4008 Law St., Houston, TX, USA) with funds from the Japan Society for the Promotion of Science's Grant‐in‐Aid for Scientific Research (No. 19H03562).
Saki Itoyama, Emika Noda, and Shinji Takamatsu contributed equally to this study.
Declaration of conflict of interest: The authors have no potential conflicts relevant to the manuscript.
Financial support: This study was supported by Princess Takamatsu Cancer Research Fund and a KAKENHI grant (19H03562) from the Japan Society for the Promotion of Science.
Contributor Information
Hirofumi Akita, Email: hirofumi.akita@oici.jp.
Eiji Miyoshi, Email: emiyoshi@sahs.med.osaka-u.ac.jp.
References
- 1. Day AA, Gibbs WM. The action of pancreatic juice on bacteria. J Infect Dis. 1930; 46: 26–30. [Google Scholar]
- 2. Rubinstein E, Mark Z, Haspel J et al. Antibacterial activity of the pancreatic fluid. Gastroenterology. 1985; 88: 927–32. [DOI] [PubMed] [Google Scholar]
- 3. Gregg JA. Detection of bacterial infection of the pancreatic ducts in patients with pancreatitis and pancreatic cancer during endoscopic cannulation of the pancreatic duct. Gastroenterology. 1977; 73: 1005–7. [PubMed] [Google Scholar]
- 4. Parida SK, Pottakkat B, Raja K, Vijayahari R, Padma Lakshmi C. Bacteriological profile of pancreatic juice in patients with chronic pancreatitis. J. Pancreas. 2014; 15: 475–7. [DOI] [PubMed] [Google Scholar]
- 5. Maekawa T, Fukaya R, Takamatsu S et al. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Commun. 2018; 506: 962–9. [DOI] [PubMed] [Google Scholar]
- 6. Frost F, Weiss FU, Sendler M et al. The gut microbiome in patients with chronic pancreatitis is characterized by significant dysbiosis and overgrowth by opportunistic pathogens. Clin. Transl. Gastroenterol. 2020; 11: e00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kikuyama M, Sato T, Kurokami T, Ota Y, Yokoi Y. Pancreatic juice culture in acute pancreatitis and other pancreatic disorders. J. Pancreas. 2016; 17: 241–6. [Google Scholar]
- 8. McAllister F, Khan MAW, Helmink B, Wargo JA. The tumor microbiome in pancreatic cancer: bacteria and beyond. Cancer Cell. 2019; 36: 577–9. [DOI] [PubMed] [Google Scholar]
- 9. Silva N, Igrejas G, Gonçalves A, Poeta P. Commensal gut bacteria: distribution of Enterococcus species and prevalence of Escherichia coli phylogenetic groups in animals and humans in Portugal. Ann. Microbiol. 2012; 62: 449–59. [Google Scholar]
- 10. Kau AL, Martin SM, Lyon W, Hayes E, Caparon MG, Hultgren SJ. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 2005; 73: 2461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herlemann DP, Labrenz M, Jurgens K, Bertilsson S, Waniek JJ, Andersson AF. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011; 5: 1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakajo K, Komori R, Ishikawa S et al. Resistance to acidic and alkaline environments in the endodontic pathogen Enterococcus faecalis . Oral Microbiol. Immunol. 2006; 21: 283–8. [DOI] [PubMed] [Google Scholar]
- 13. Weckwerth PH, Zapata RO, Vivan RR, Tanomaru M, Maliza AGA, Duarte MAH. In vitro alkaline pH resistance of Enterococcus faecalis . Braz. Dent. J. 2013; 24: 474–6. [DOI] [PubMed] [Google Scholar]
- 14. Jones KK. A comparison of the buffer value of bile and pancreatic juice secreted simultaneously. Proc. Soc. Exp. Biol. Med. 1931; 28: 567–8. [Google Scholar]
- 15. Hoerner MT. The buffer capacity of the pancreatic juice. Am. J. Dig. Dis. Nutr. 1935; 2: 300–2. [Google Scholar]
- 16. Takeshima T, Adler M, Nacchiero M, Rudick J, Dreiling DA. Effects of duodenal alkalinization on pancreatic secretion. Am. J. Gastroenterol. 1977; 67: 54–62. [PubMed] [Google Scholar]
- 17. Tomita Y, Azuma K, Nonaka Y et al. Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas. 2014; 43: 1032–41. [DOI] [PubMed] [Google Scholar]
- 18. Sethi V, Vitiello GA, Saxena D, Miller G, Dudeja V. The role of the microbiome in immunologic development and its implication for pancreatic cancer immunotherapy. Gastroenterology. 2019; 156: 2097–115.e2. [DOI] [PubMed] [Google Scholar]
- 19. Pushalkar S, Hundeyin M, Daley D et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018; 8: 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo W, Zhang Y, Guo S et al. Tumor microbiome contributes to an aggressive phenotype in the basal‐like subtype of pancreatic cancer. Commun. Biol. 2021; 4: 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aykut B, Pushalkar S, Chen R et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019; 574: 264–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geller LT, Barzily‐Rokni M, Danino T et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017; 357: 1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Almeida CV, Taddei A, Amedei A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap. Adv. Gastroenterol. 2018; 11: 1756284818783606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Original images for the visual agar plate assay used in Figure 3a. Twelve isolated E. faecalis (#1–#12) were incubated with each pancreatic juice (PJ A‐C) and plated on BHI agar plates. The changes in the bacterial growth compared to control (CTRL) was judged and described in Figure 3a. Unspecified green colony grew in the culture of #1, 3, and 11 with PJ C, which was initially characterized as aseptic, suggesting the presence of unspecified bacteria below detectable level in original PJ C. #11 culture in PJ C was judged as non‐assessable (NA) due to the contamination, while colonies of #1 and #3 culture in PJ C were clearly increased.
Figure S2. A legend for the scoring used in the Figure 3b. Bacterial growth was scored according to the colony number as indicated.
Figure S3. Metagenome analysis of the paired duodenal and pancreatic juice from five pancreatic cancer patients. Each colored bar indicates the relative frequency of indicated bacterial species, which are listed on the right panel. D, duodenal juice; P, pancreatic juice.
Table S1. Supplementary table.


