Abstract
Purpose
The diagnosis of tubercular uveitis (TBU) is difficult. The lack of a diagnostic gold standard has contributed to challenges in determining the true prevalence and clinical predictors of TBU. We aimed to determine the proportion of TBU cases in adults with uveitis and to examine clinical features associated with TBU.
Methods
A prospective cohort study of adult uveitis cases after exclusion of other specific etiologies. The diagnosis of TBU was based on a composite reference of: any clinical signs of uveitis; exclusion of other causes of uveitis; and positive QuantiFERON-Gold test, tuberculin skin test, and/or ocular TB polymerase chain reaction.
Results
Of 79 cases analyzed, 49 (62%) had TBU. Female sex (P = 0.001) and chronic uveitis (P = 0.006) cases were more common in the TBU group than the non-TBU group whereas diffuse choroiditis (P = 0.010) and HIV-positive (P = 0.001) cases were less common. Choroidal granulomas (P = 0.176) and serpiginous-like choroiditis (P = 0.292) were more common in TBU group, albeit not significantly. On univariate analysis, female sex (odds ratio, 5.1; P = 0.002), negative HIV status (odds ratio, 0.2; P = 0.001), and chronic uveitis (odds ratio, 4.1; P = 0.008) were associated with TBU. A negative HIV test was associated with TBU on multivariate analysis (P = 0.049).
Conclusions
A high proportion of cases had TBU. Our study did not significantly confirm some of the clinical features associated with TBU reported in other studies.
Translational Relevance
Our study highlights the difficulties in determining the proportion and clinical predictors of TBU, especially in the absence of a gold standard diagnostic test.
Keywords: tuberculosis; tubercular uveitis, prevalence; clinical features; HIV
Introduction
Tuberculosis has varyingly been attributed to causing 0.2% to 32% of uveitis.1,2 Tubercular uveitis (TBU) can present with clinical features involving different ocular structures,3 including broad-based posterior synechiae, retinal vasculitis, optic neuropathy, and choroidopathies such as serpiginous-like choroiditis and choroidal granulomas.4–8 It can result in ocular morbidity, such as visual impairment, chronic hypotony, and blindness.9 Adverse visual sequalae after TBU are associated with a delay in diagnosis, chronic disease, and posterior uveitis with choroiditis.9 Complications of visual impairment could be due to optic neuropathy, macular oedema, glaucoma, vitreous hemorrhage, cataract, and macular scarring.9
The diagnostic difficulties of TBU are due to the inherent problems with the diagnostic tests and obtaining the optimal ocular sample for testing. There is a low positive yield in microbiological or molecular tests of ocular fluids and a low sensitivity and specificity of adjunct immunological tests such as the tuberculin skin test (TST) or TB-specific IFN-γ release assays, such as the QuantiFERON-TB Gold (QFT-G).10–13 Consequently, there is no standardized diagnostic criteria for the diagnosis of TBU.
Although Gupta et al3,4 proposed the diagnosis of TBU being based on varying combinations of suggestive clinical features of TBU, laboratory investigations, exclusion of other causes of uveitis, and response to antitubercular treatment, a number of studies have based the diagnosis on QFT-G and/or TST and/or polymerase chain reaction (PCR) test in the presence of uveitis.14–17 Studies have reported a higher prevalence (44% to 48%) of TB being associated with uveitis if the diagnosis is based on a positive QFT-G.17,18
We undertook a prospective cohort study to determine the proportion of TBU cases in adults with uveitis and to examine the associations of ocular clinical features with TBU.
Materials and Methods
We conducted a prospective cohort study of uveitis cases referred to the Uveitis Clinic at St John Eye Hospital between June 2014 and November 2018. St John Eye Hospital is the Ophthalmology Department of Chris Hani Baragwanath Academic Hospital based in Johannesburg, South Africa, and has the highest prevalence of HIV infection in the world.19 It is a public tertiary care hospital with limited resources serving a low-income population. The study was approved by the Human Research Ethics Committee of the University of the Witwatersrand (M130942) and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants in the study.
Participants were eligible for inclusion in the study if they had any clinical signs of uveitis and were more than 18 years of age. If the participant had bilateral uveitis, only one eye (the eye with worse visual acuity and inflammatory activity) was included for PCR testing and statistical analyses. Individuals were excluded if they (i) had a previous or concurrent TB infection, (ii) had traumatic uveitis or postsurgical uveitis, (iii) were clinically diagnosed uveitis such as acute retinal necrosis, progressive outer retinal necrosis, cytomegalovirus (CMV) retinitis, Behcet's disease, Vogt–Koyanagi–Harada disease, Fuchs heterochromic iridocyclitis, sympathetic ophthalmia, birdshot chorioretinopathy, multiple evanescent white dot syndrome, punctate inner choroidopathy, or acute posterior multifocal placoid pigment epitheliopathy, and (iv) had uveitis caused by toxoplasmosis, syphilis, systemic lupus erythematosus, or sarcoid on blood workup and chest radiography.
All individuals presenting to St John Eye Hospital underwent a standard screening protocol for uveitis that included slit-lamp examination and fundoscopy, and investigations including full blood count and differential, erythrocyte sedimentation rate, HIV, CD4+ lymphocyte count if HIV-positive, rapid plasma reagin and Treponema pallidum hemagglutination assay, serum angiotensin converting enzyme levels, Toxoplasma antibodies, antinuclear antibodies, antineutrophil cytoplasmic antibodies, and a chest radiograph to exclude other causes of uveitis. Furthermore, we did tuberculin skin testing using the Mantoux method (0.1 mL containing 2TU RT 23 [Statens Serum Institute, Copenhagen, Denmark] injected intradermally) and QuantiFERON-TB Gold (QFT-G [Cellestis Limited, Carnegie, Victoria, Australia]). Also, ocular fluids (aqueous or vitreous samples) were referred to the National TB Reference Laboratory and tested by TB PCR (Xpert MTB/RIF [Cepheid, Sunnyvale, CA], in-house MPB 64 PCR and in-house IS6110 PCR) and a viral panel PCR (varicella-zoster virus [VZV], herpes simplex virus [1 and 2], CMV, Epstein–Barr virus, and human herpes virus 6). Optical coherence tomography, fluorescein angiography, and lumbar puncture were performed depending on the clinical examination and blood results.
A diagnosis of TBU was made using a composite reference that included (i) any clinical signs of uveitis, (ii) other causes of uveitis were excluded, and (iii) QFT-G, and/or TST, and/or TB PCR of aqueous or vitreous samples were positive. Participants with a positive QFT-G and/or TST were diagnosed with presumed TBU and with positive PCR for TB with confirmed TBU. A TST of 10 mm or more at 48 hours after intradermal injection was considered positive in HIV-negative cases, and a TST of more than 5 mm was considered positive in HIV-positive cases. A QFT-G of 0.35 IU/mL or more was considered positive as per the manufacturer's recommendations. The QFT-G was performed before the TST. Indeterminate QFT-G results were correlated with the TST and the TB-PCR test; if the TST and/or TB-PCR test was positive, the participant was diagnosed with TBU and if these tests were negative, the participant was diagnosed with non-TBU.
All diagnosed TBU cases were treated with antitubercular treatment for 9 months: Rifafour e-275 (rifampicin [R] 150 mg, isoniazid [H] 75 mg, pyrazinamide [Z] 400 mg, and ethambutol hydrochloride [E] 275 mg) for the first 2 months, and RIFINAH-150 (rifampicin 150 mg and isoniazid 100 mg) or RIFINAH-300 (rifampicin 300 mg and isoniazid 150 mg) for 7 months; the dose was weight dependent. If necessary, and depending on the severity of inflammation, TBU cases were additionally treated with topical and/or oral corticosteroids. TBU cases were followed for a further 6 months after completion of antitubercular treatment, totaling 15 months of follow-up. All non-TBU cases were treated with topical steroids and/or systemic corticosteroid and/or immunosuppressive medication and, also followed-up for 15 months. All cases (TBU and non-TBU) were assessed for intraocular inflammation every 6 to 12 weeks for 15 months. Remission was defined as no inflammatory activity and being on 10 mg or less oral prednisone20 for 6 months duration after the completion of 9 months treatment.
Demographic and clinical characteristics were documented in the TBU- and non-TBU groups. Characteristics that were documented and compared included age, gender, HIV status, laterality (unilateral vs. bilateral), TB contact, Bacillus Calmette-Guerin vaccination at birth, clinical course of uveitis, anatomical classification, type of choroiditis, clinical signs suggestive of TBU,4–8 and remission of uveitis. Uveitis was anatomically classified as anterior, intermediate, posterior or panuveitis according to the Standardization of Uveitis Nomenclature criteria.20 The clinical course of the uveitis (acute, recurrent, or chronic) and grading of intraocular inflammation was according to the Standardization of Uveitis Nomenclature criteria.20 The clinical signs suggestive of TBU, from previous studies,4–8 that were documented and evaluated were broad-based posterior synechiae, vasculitis, optic neuropathy, choroidal granulomas, and serpiginous-like choroiditis. A choroidal granuloma (large) was defined as a solitary mass of more than 4 mm; multifocal choroiditis as multiple discrete lesions (each ≤4 mm) or multiple discrete areas of inflammation; and serpiginous-like choroiditis as choroidal lesions that start around the disc and spreading centrifugally that are initially noncontiguous and eventually becoming confluent.3 Diffuse choroiditis was defined as nondiscrete diffuse inflammation of the choroid.
Statistical Analysis
Continuous variables were summarized as means (standard deviation) if normally distributed or medians (interquartile range) if they were skewed. The comparison of means between the TBU and non-TBU groups was performed using the two-sample t-test. The comparison of medians between the two groups was performed using the Wilcoxon rank-sum test. A χ2 or Fisher's exact test was used to compare categorical variables. Univariate logistic regression was used to evaluate the diagnosis of TBU as the outcome with the predictor variables of age, gender, HIV, laterality, TB contacts, Bacillus Calmette-Guerin vaccination at birth, chronicity of uveitis, and clinical signs such as broad-based synechiae, vasculitis, optic neuropathy, serpiginous-like choroiditis, and choroidal granulomas. The selection of variables in the multivariate logistic regression analysis was done by a stepwise regression method using backward elimination, with significance levels of 0.2 or less for inclusion. The estimate of odds ratio (OR) and its relative 95% confidence interval (CI) were calculated. The models were assessed using receiver operating characteristics curves. All analyses were performed using STATA version 16.1 (StataCorp LLC, College Station, TX). For all tests, a P value of less than 0.05 was considered statistically significant.
Results
Forty-nine (62%) of the enrolled 79 cases were diagnosed with TBU, including 41 with presumed TBU and 8 with confirmed TBU (Table 1). Of the 30 non-TBU cases, three were positive for VZV on ocular fluid PCR testing. The three cases with VZV infection had no ocular signs suggestive of acute retinal necrosis, progressive outer retinal necrosis, or CMV at study entry. Additionally, a further four of the 30 non-TBU cases were positive for Epstein–Barr virus on ocular fluid PCR testing. The overall and TBU cases mean age were 40.1 ± 12.2 and 41.8 ± 13.4 years, respectively (Table 1). Twenty-five of the 79 patients (32%) were males, albeit there being a lower percentage (18% [9/49]) among the TBU cases. Thirty cases (38%) were living with HIV, including 24% in those with TBU (Table 1). The median CD4+ cell count of the HIV-positive cases in the TBU group was 233 (interquartile range, 155–473) and the non-TBU group 137 (interquartile range, 105–278). Forty-three (54%), 39 (50%), and 8 (10%) cases had a positive TST, QFT-G, and TB PCR, respectively (Table 2); one case with a positive QFT-G was classified as non-TBU because of a positive VZV PCR test. Forty-nine (62%) cases had at least one positive TB test.
Table 1.
Baseline Characteristics of TBU and Nontubercular Uveitis (Non-TBU) Cases
| Total | TBU | Non-TBU | P Value | |
|---|---|---|---|---|
| No. (%) | 79 (100%) | 49 (62%) | 30 (38%) | |
| Presumed: 41 (84%) | ||||
| Confirmed: 8 (16%) | ||||
| Age (years) | 40.1 ± 12.2 | 41.8 ± 13.4 | 37.5 ± 9.7 | 0.135a |
| Male sex | 25 (32) | 9 (18) | 16 (53) | 0.001 b |
| HIV positive (n = 78) | 30 (38) | 12 (24) | 18 (62) | 0.001 b |
| CD4+ cell count/L in HIV-positive cases (n = 28) | 171 (121–294) | 233 (155–473) | 137 (105–278) | 0.078c |
| Laterality | 0.670b | |||
| Unilateral | 19 (24) | 11 (22) | 8 (27) | |
| Bilateral | 60 (76) | 38 (78) | 22 (73) | |
| Analyzed eye | ||||
| Right | 37 (47) | 17 (35) | 20 (67) | |
| Left | 42 (53) | 32 (65) | 10 (33) | |
| Anterior chamber/vitreous tap | ||||
| Anterior chamber | 43 (54) | 28 (57) | 15 (50) | |
| Vitreous | 36 (46) | 21 (43) | 15 (50) | |
| TB contact | 14 (18) | 7 (14) | 7 (23) | 0.307b |
| BCG vaccination at birth (n = 78) | 66 (85) | 40 (83) | 26 (87) | 0.691b |
Two-sample t test.
χ2 test.
Wilcoxon rank-sum test. Values are number (%), mean ± standard deviation, or median (interquartile range). Boldface entries indicate statistical significance. BCG, Bacille Calmette-Guerin
Table 2.
Clinical Characteristics of TBU and Nontubercular Uveitis (Non-TBU)
| Total | TBU | Non-TBU | P Value | |
|---|---|---|---|---|
| No. (%) | 79 (100) | 49 (62) | 30 (38) | |
| Presumed: 41 (84) | ||||
| Confirmed: 8 (16) | ||||
| Anatomical classification | ||||
| Anterior | 1 (1) | 1 (2) | 0 (0) | |
| Intermediate | 2 (3) | 1 (2) | 1 (3) | |
| Posterior | 1 (1) | 0 (0) | 1 (3) | |
| Panuveitis | 75 (95) | 47 (96) | 28 (94) | 0.632a |
| Course of uveitis (n = 77) | ||||
| Acute | 24 (32) | 10 (21) | 14 (48) | |
| Chronic | 47 (61) | 35 (73) | 12 (42) | 0.006 b |
| Recurrent | 6 (7) | 3 (6) | 3 (10) | |
| Fundus pathology | ||||
| Choroiditis type | ||||
| Multifocal (multiple lesions, each <4 mm) | 52 (66) | 34 (69) | 18 (60) | 0.393b |
| Serpiginous-like | 4 (5) | 4 (8) | 0 (0) | 0.292a |
| Diffuse | 11 (14) | 3 (6) | 8 (27) | 0.010 b |
| Granulomas (≥4 mm) | 7 (9) | 6 (13) | 1 (3) | 0.176b |
| Vasculitis only | 2 (2) | 0 (0) | 2 (7) | |
| No fundus lesions | 3c (4) | 2 (4) | 1 (3) | |
| Clinical signs5–8,d suggestive of intraocular tuberculosis | ||||
| Broad-based posterior synechiae | 18 (23) | 11 (22) | 7 (23) | 0.928b |
| Vasculitis | 39 (50) | 22 (46) | 17 (57) | 0.352b |
| Optic neuropathy | 4 (5) | 1 (2) | 3 (10) | 0.151a |
| Choroidal granulomas | 7 (12) | 6 (13) | 1 (3) | 0.176b |
| Serpiginous-like choroiditis | 4 (5) | 4 (8) | 0 (0) | 0.292a |
| Remission for 6 months duration after completing ATT (TBU, n = 35) or completing anti-inflammatory medication (non-TBU, n = 17) | 0.073b | |||
| Yes | 18 (35) | 15 (43) | 3 (18) | |
| No | 34 (65) | 20 (57) | 14 (82) | |
| Positive TST | 43 (54) | 43 (88) | 0 (0) | |
| Quantiferon-TB Gold (n = 78) | ||||
| Positive | 39 (50) | 38 (79) | 1e (3) | |
| Mtb PCR (n = 78) | ||||
| Negative | 67 (86) | 38 (79) | 29 (97) | |
| Indeterminatef | 3 (4) | 2 (4) | 1 (3) | |
| Positive | 8 (10) | 8 (17) | 0 (0) | |
| Viral PCR (n = 78) | ||||
| Varicella zoster virus | 3 (4) | 0 (0) | 3 (10) | |
| Epstein-Barr virus | 5 (6) | 1 (2) | 4 (13) | |
| Negative | 70 (90) | 47 (98) | 23 (77) |
Fishers exact test.
χ2 test.
Three cases had no fundus lesions; one had anterior uveitis and two intermediate uveitis.
References of studies suggestive of clinical signs of intraocular tuberculosis.
One case with a positive QuantiFERON test was classified as non-TBU because of a positive PCR for VZV.
Cycle threshold >35 cycles. Values are number (%). Boldface entries indicate statistical significance. ATT, antituberculosis treatment; Mtb, Mycobacterium tuberculosis detected by IS6110 and MPB64 [none detected by Gene Xpert]); PCR, polymerase chain reaction.
Chronicity of uveitis (Table 2) was more common in TBU (73%) than non-TBU cases (42%) (P = 0.006). Ninety-six percent and 94% of TBU and non-TBU cases had panuveitis (P = 0.632), respectively (Table 2). The presence of diffuse choroiditis was lower in the TBU (6%) than non-TBU (27%) group (P = 0.010) (Table 2). The prevalence of broad-based posterior synechiae (P = 0.928), vasculitis (P = 0.352), and optic neuropathy (P = 0.151) was not significantly different between the two groups. The prevalence of choroidal granulomas (13% vs. 3%; P = 0.176) and serpiginous-like choroiditis (8% vs. 0%; P = 0.292) was higher in the TBU group than the non-TBU group, although the difference was not significant. The proportion of cases in remission for 6 months duration after completing 9 months treatment (Table 2) was higher in TBU (43%) than non-TBU groups (18%), although it was not a significant difference (P = 0.073).
On univariate regression analysis (Table 3), the odds of TBU were higher in female cases (OR, 5.08; 95% CI, 1.83–14.06; P = 0.002) and cases with chronic uveitis (OR, 4.08; 95% CI, 1.44–11.59; P = 0.008), but lower in cases living with HIV (OR, 0.19; 95% CI, 0.07–0,54; P = 0.001). The multivariate logistic regression model was most predictive for the outcome when the following variables were included in the analysis: gender, HIV, chronic uveitis, and diffuse choroiditis (χ2 goodness-of-fit test P = 0.373; area under the receiver operating characteristics curve, 0.803) (Table 4 and Fig.). A negative HIV test significantly predicted a diagnosis of TBU on multivariate analysis (OR, 0.313; 95% CI, 0.099–0.994; P = 0.049).
Table 3.
Univariate Logistic Regression of Variables Predicting TBU
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Age | 1.031 | 0.990–1.074 | 0.138 |
| Gender (female) | 5.079 | 1.834–14.064 | 0.002 |
| HIV positive | 0.198 | 0.073–0.535 | 0.001 |
| Laterality (bilateral) | 1.256 | 0.439–3.594 | 0.671 |
| TB contacts | 0.548 | 0.171–1.755 | 0.311 |
| BCG received at birth | 0.769 | 0.210–2.816 | 0.692 |
| Course of uveitis | |||
| Acute (reference group) | 1.0 | ||
| Chronic | 4.083 | 1.438–11.590 | 0.008 |
| Recurrent | 1.400 | 0.233–8.421 | 0.713 |
| Choroiditis type | |||
| Multifocal | 1.511 | 0.584–3.908 | 0.394 |
| Serpiginous | 1 | ||
| Diffuse | 0.564 | 0.351–0.906 | 0.018 |
| Choroidal granulomas | 4.047 | 0.463–35.396 | 0.206 |
| Clinical signs5–8,a suggestive of intraocular tuberculosis | |||
| Broad-based posterior synechiae | 0.951 | 0.323–2.800 | 0.928 |
| Vasculitis | 0.647 | 0.258–1.621 | 0.353 |
| Optic neuropathy | 0.188 | 0.019–1.892 | 0.156 |
| Choroidal granulomas | 4.047 | 0.463–35.396 | 0.206 |
| Serpiginous-like choroiditis | 1 |
Boldface entries indicate statistical significance.
References of studies suggestive of clinical signs of intraocular tuberculosis.
Table 4.
Multivariate Logistic Regression Model for Variables Predicting TBUa
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| HIV | 0.313 | 0.099–0.994 | 0.049 |
| Gender (female) | 2.831 | 0.859–9.325 | 0.087 |
| Chronic uveitis | 1.483 | 0.844–2.608 | 0.171 |
| Diffuse choroiditis | 0.630 | 0.372–1.067 | 0.086 |
n = 76, χ2 goodness-of-fit test P = 0.373, area under the receiver operating characteristics curve = 0.803).
Figure.
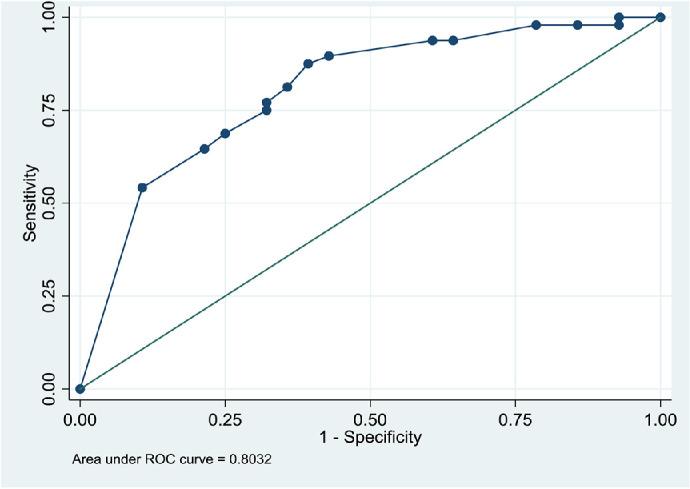
Receiver operating characteristics (ROC) curve of the multivariate regression model predicting TBU.
Discussion
The proportion of TBU cases in our study was 62%. The reported prevalence of TBU in several studies from high TB-burden countries range from 22% to 48%1,17,21–24 and from non-high TB-burden countries range from 7% to 44%.18,25 Although we report a higher proportion of TBU cases, direct comparison of our data with these studies is not possible because the prevalence of TBU reported in these studies is in a uveitis population that included cases with other etiologies. Our study excluded uveitis cases that had a specific etiology, such as acute retinal necrosis, progressive outer retinal necrosis, CMV retinitis, Behcet's disease, Vogt–Koyanagi–Harada disease, Fuchs heterochromic iridocyclitis, sympathetic ophthalmia, birdshot chorioretinopathy, multiple evanescent white dot syndrome, acute posterior multifocal placoid pigment epitheliopathy, punctate inner choroidopathy toxoplasmosis, syphilis, systemic lupus erythematosus, and sarcoid. The other reason for the high proportion of TBU cases in our study is that the diagnosis of TBU was based on a composite reference, including any of the following three positive tests—QFT-G, TST, and/or PCR. If a single test alone was used to make the diagnosis, the prevalence would have been lower. For example, the QFT-G positivity rate in our study was 50%. In high TB-burden countries the QFT-G positivity rate in all uveitis cases is reported at between 36% and 48%.17,26 Gineys et al.,18 from a low TB-burden country, reported a QFT-G positivity of 44% and proposed the treatment of TBU based on a positive QFT-G and TST. As mentioned elsewhere in this article, the greater proportion of patients with TBU may be due to an overestimation of the prevalence of TBU. Alternatively, it may reflect an accurately high proportion of TBU as evidenced by the higher proportion of TBU cases in remission for the duration of 6 months after completing antitubercular treatment compared to non-TBU cases (43% vs. 18%). The difference was, however, not significant and a larger sample size may have shown significance between the two groups.
There was a significantly greater proportion of females and HIV-negative cases in the TBU group. These findings were similar to the study by Smit et al.,24 which was also conducted in South Africa. Although several studies27–29 have shown that females were strongly associated with extrapulmonary TB, some studies16,24,30 on TBU showed a female preponderance, whereas others14,31 (including the Collaborative Ocular Tuberculosis Study [COTS]-1) did not. This gender effect in extrapulmonary TB is thought to be related to access to health care, socioeconomic, and hormonal factors.28,29 Although on univariate analysis female gender was significantly associated with TBU, this significance was lost in the multivariate analysis. There was a significantly higher proportion of HIV-negative cases in the TBU group. Because the diagnosis of TBU in most cases was based on a positive QFT-G or TST, a lower proportion of TBU cases being HIV positive may be due to higher false negative TB tests secondary to immunosuppression. This finding is supported by the lower median CD4+ cell count in the non-TBU group. Because the sensitivity of these immunological tests is decreased in immunosuppressed individuals,12,32 the diagnosis of TBU could have been underestimated in the HIV-positive cases. The T-spot.TB (Oxford Immunotech, Oxford, UK) test is more sensitive in diagnosing TB in HIV-positive individuals with lower CD4+ cell counts33 and might have yielded more TBU cases in our study; however, it is not available at our hospital. A study from Cape Town in South Africa also found a higher proportion of HIV negative cases diagnosed with possible intraocular TB24; the QFT-G test was used to support the diagnosis. In our study, a negative HIV test was significantly associated with TBU on both univariate and multivariate analyses.
The most common anatomic diagnosis in the TBU and non-TBU groups was panuveitis. The high prevalence of panuveitis reflects the referral pattern in our hospital; most anterior and intermediate uveitis cases are treated in the outpatient area and rarely referred to our uveitis clinic. TBU predominantly manifested as panuveitis in several studies.16,18,34 In the COTS study, the most common anatomic presentation was posterior uveitis followed by panuveitis.31 There was a significantly greater proportion of cases with chronic uveitis in the TBU group compared with the non-TBU group. If there was selection bias in that only chronic cases who did not respond to immunosuppressive treatment were referred, then this difference would have been reflected in both the TBU and non-TBU groups. Chronic uveitis was associated significantly with TBU on univariate analysis, but not on multivariate analysis.
Choroidal granulomas and serpiginous-like choroiditis are commonly associated with TBU.5,8,35 In our study, there was a greater proportion of cases with choroidal granulomas and serpiginous-like choroiditis in the TBU group than in the non-TBU group; however, the difference was not significant and this lack may possibly be due to the small sample of cases with these signs. Diffuse choroiditis was significantly associated with non-TBU on univariate analysis, but not on multivariate analysis.
Broad-based posterior synechiae, retinal vasculitis and optic neuropathy are signs reportedly associated with TBU.4,5 In our study, there was no significant difference of these signs between the TBU group and the non-TBU group. The reason for this may be due to the small number of cases with these clinical signs.
The limitations of our study include the limited number of cases in the subgroups, selection bias, and, as with all studies on TBU, the lack of a gold standard for the diagnosis of TBU. The limited number of cases in the clinical subgroups meant that, although differences in clinical signs were found between the TBU and non-TBU groups, statistical significance may not have been attained because of this. Selection bias may have contributed to the high proportion of TBU and the high proportion of panuveitis. The exclusion of uveitis cases with other etiologies probably resulted in a higher proportion of TBU, and the possible frequent referral of cases with panuveitis may have resulted in a higher proportion of these cases. The lack of a gold standard for the diagnosis of TBU and the reliance mainly on QFT-G and/or TST for its diagnosis may have contributed to the high proportion of TBU cases. Also, the lack of a gold standard makes the determination of the predictors difficult. However, it is because of this diagnostic difficulty we were looking for clinical predictors for the diagnosis of TBU. The strengths of the study include it being a prospective cohort study with a comprehensive ophthalmic and investigative evaluation, including QFT-G, TST, and TB PCR.
In conclusion, because of the inherent problems in the diagnostic tests for TBU, including in persons living with HIV, the true prevalence of TBU is difficult to determine. However, based on the higher remissions achieved in the TBU cases, we found these tests, especially QFT-G and TST, to be useful in the diagnosis of TBU. TBU was associated with a chronic course and HIV-negative cases. In terms of the other clinical predictors of TBU, we could not significantly confirm the clinical features associated with TBU reported in other studies because of the limited number of cases in the clinical subgroups. Thus, there is a need for a large multicenter prospective cohort study to determine the clinical predictors of TBU.
Acknowledgments
The authors thank support from the National TB Reference Laboratory staff.
Disclosure: H.D. Alli, None; N. Ally, None; I. Mayet, None; L. Joseph, None; S.V. Omar, None; S.A. Madhi, None
References
- 1. Win MZA, Win T, Myint S, Shwe T, Sandar H.. Epidemiology of uveitis in a tertiary eye center in myanmar. Ocul Immunol Inflamm. 2017; 25(Suppl 1): S69–S74. [DOI] [PubMed] [Google Scholar]
- 2. Kotake S, Furudate N, Sasamoto Y, Yoshikawa K, Goda C, Matsuda H.. Characteristics of endogenous uveitis in Hokkaido, Japan. Graefes Arch Clin Exp Ophthalmol. 1996; 234(10): 599–603. [DOI] [PubMed] [Google Scholar]
- 3. Gupta V, Gupta A, Rao NA.. Intraocular tuberculosis-an update. Surv Ophthalmol. 2007; 52(6): 561–587. [DOI] [PubMed] [Google Scholar]
- 4. Gupta A, Sharma A, Bansal R, Sharma K.. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015; 23(1): 7–13. [DOI] [PubMed] [Google Scholar]
- 5. Gupta A, Bansal R, Gupta V, Sharma A, Bambery P.. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. 2010; 149(4): 562–570. [DOI] [PubMed] [Google Scholar]
- 6. Ang M, Hedayatfar A, Zhang R, Chee S-P.. Clinical signs of uveitis associated with latent tuberculosis. Clin Exp Ophthalmol. 2012; 40(7): 689–696. [DOI] [PubMed] [Google Scholar]
- 7. Babu RB, Sudharshan S, Kumarasamy N, Therese L, Biswas J.. Ocular tuberculosis in acquired immunodeficiency syndrome. Am J Ophthalmol. 2006; 142(3): 413–418. [DOI] [PubMed] [Google Scholar]
- 8. Agrawal R, Testi I, Mahajan S, et al.. The Collaborative Ocular Tuberculosis Study (COTS) Consensus (CON) group meeting proceedings. Ocul Immunol Inflamm. 2020: 1–11, doi: 10.1080/09273948.2020.1716025. [DOI] [PubMed] [Google Scholar]
- 9. Gunasekeran DV, Gupta B, Cardoso J, Pavesio CE, Agrawal R.. Visual morbidity and ocular complications in presumed intraocular tuberculosis: an analysis of 354 cases from a non-endemic population. Ocul Immunol Inflamm. 2018; 26(6): 865–869. [DOI] [PubMed] [Google Scholar]
- 10. Arora SK, Gupta V, Gupta A, Bambery P, Kapoor GS, Sehgal S.. Diagnostic efficacy of polymerase chain reaction in granulomatous uveitis. Tuber Lung Dis. 1999; 79(4): 229–233. [DOI] [PubMed] [Google Scholar]
- 11. Sudheer B, Lalitha P, Kumar AL, Rathinam S.. Polymerase chain reaction and its correlation with clinical features and treatment response in tubercular uveitis. Ocul Immunol Inflamm. 2018; 26(6): 845–852. [DOI] [PubMed] [Google Scholar]
- 12. Huebner RE, Schein MF, Bass JB.. The tuberculin skin test. Clin Infect Dis. 1993; 17(6): 968–975. [DOI] [PubMed] [Google Scholar]
- 13. Ang M, Htoon HM, Chee S-P.. Diagnosis of tuberculous uveitis: clinical application of an interferon-gamma release assay. Ophthalmology. 2009; 116(7): 1391–1396. [DOI] [PubMed] [Google Scholar]
- 14. Manousaridis K, Ong E, Stenton C, Gupta R, Browning AC, Pandit R.. Clinical presentation, treatment, and outcomes in presumed intraocular tuberculosis: experience from Newcastle upon Tyne, UK. Eye (Lond). 2013; 27(4): 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grajewski RS, Caramoy A, Frank KF, et al.. Spectrum of uveitis in a German tertiary center: review of 474 consecutive patients. Ocul Immunol Inflamm. 2015; 23(4): 346–352. [DOI] [PubMed] [Google Scholar]
- 16. Sanghvi C, Bell C, Woodhead M, Hardy C, Jones N.. Presumed tuberculous uveitis: diagnosis, management, and outcome. Eye (Lond). 2011; 25(4): 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. La Distia Nora R, Sitompul R, Bakker M, et al.. Tuberculosis and other causes of uveitis in Indonesia. Eye (Lond). 2018; 32(3): 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gineys R, Bodaghi B, Carcelain G, et al.. QuantiFERON-TB gold cut-off value: implications for the management of tuberculosis-related ocular inflammation. Am J Ophthalmol. 2011; 152(3): 433–440. [DOI] [PubMed] [Google Scholar]
- 19. UNAIDS Programme Coordinating Board sees South Africa's AIDS response first-hand. Accessed 11 December 2020. Available from: https://www.unaids.org/en/resources/presscentre/featurestories/2018/november/pcb-field-visit-south-africa.
- 20. Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005; 140(3): 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dogra M, Singh R, Agarwal A, et al.. Epidemiology of uveitis in a tertiary-care referral institute in North India. Ocul Immunol Inflamm. 2017; 25(Sup1): S46–S53. [DOI] [PubMed] [Google Scholar]
- 22. Biswas J, Kharel Sitaula R, Multani P. Changing uveitis patterns in South India - comparison between two decades. Indian J Ophthalmol. 2018; 66(4): 524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schaftenaar E, Meenken C, Baarsma GS, et al.. Uveitis is predominantly of infectious origin in a high HIV and TB prevalence setting in rural South Africa. Br J Ophthalmol. 2016; 100(10): 1312–1316. [DOI] [PubMed] [Google Scholar]
- 24. Smit DP, Esterhuizen TM, Meyer D.. The prevalence of intraocular tuberculosis in HIV-positive and HIV-negative patients in South Africa using a revised classification system. Ocul Immunol Inflamm. 2018; 26(6): 830–837. [DOI] [PubMed] [Google Scholar]
- 25. Yeo TK, Ho SL, Lim WK, Teoh SC.. Causes of visual loss associated with uveitis in a Singapore Tertiary Eye Center. Ocul Immunol Inflamm. 2013; 21(4): 264–269. [DOI] [PubMed] [Google Scholar]
- 26. Pathanapitoon K, Kunavisarut P, Sirirungsi W, Rothova A.. Looking for ocular tuberculosis: prevalence and clinical manifestations of patients with uveitis and positive QuantiFERON-TB Gold test. Ocul Immunol Inflamm. 2018; 26(6): 819–826. [DOI] [PubMed] [Google Scholar]
- 27. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR.. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009; 49(9): 1350–1357. [DOI] [PubMed] [Google Scholar]
- 28. Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN.. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect Dis. 2008; 8: 8, doi: 10.1186/1471-2334-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cailhol J, Decludt B, Didier C.. Sociodemographic factors that contribute to the development of extrapulmonary tuberculosis were identified. J Clin Epidemiol. 2005; 58(10): 1066–1071. [DOI] [PubMed] [Google Scholar]
- 30. Manandhar A. Patterns of uveitis and scleritis in Nepal: a tertiary referral center study. Ocul Immunol Inflamm. 2017; 25: S54–S62. [DOI] [PubMed] [Google Scholar]
- 31. Agrawal R, Gunasekeran DV, Grant R, et al.. Clinical features and outcomes of patients with tubercular uveitis treated with antitubercular therapy in the Collaborative Ocular Tuberculosis Study (COTS)-1. JAMA Ophthalmol. 2017; 135(12): 1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leidl L, Mayanja-Kizza H, Sotgiu G, et al.. Relationship of immunodiagnostic assays for tuberculosis and numbers of circulating CD4+ T-cells in HIV infection. Eur Respir J. 2010; 35(3): 619–626. [DOI] [PubMed] [Google Scholar]
- 33. Bocchino M, Bellofiore B, Matarese A, Galati D, Sanduzzi A.. IFN-gamma release assays in tuberculosis management in selected high-risk populations. Expert Rev Mol Diagn. 2009; 9(2): 165–177. [DOI] [PubMed] [Google Scholar]
- 34. Al-Mezaine HS, Kangave D, Abu El-Asrar, AM. Patterns of uveitis in patients admitted to a University Hospital in Riyadh, Saudi Arabia. Ocul Immunol Inflamm. 2010; 18(6): 424–431. [DOI] [PubMed] [Google Scholar]
- 35. Bansal R, Gupta A, Gupta V, Dogra MR, Sharma A, Bambery P.. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012; 119(11): 2334–2342. [DOI] [PubMed] [Google Scholar]


