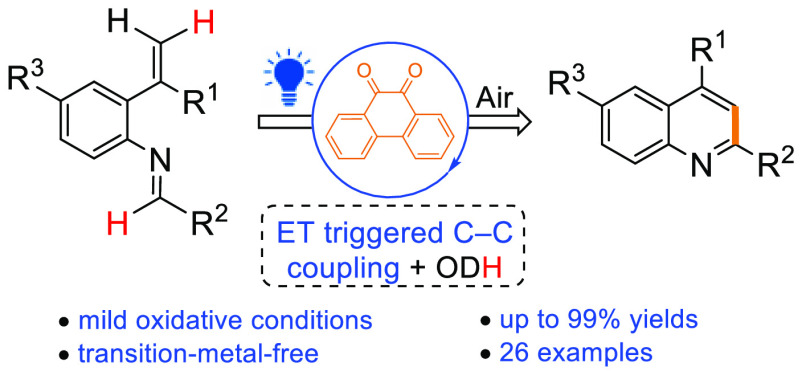
Abstract
Visible-light-excited 9,10-phenanthrenequinone (PQ*) was used as a photocatalyst for the synthesis of polysubstituted quinolines via the electrocyclization of 2-vinylarylimines. Up to quantitative yields of 2,4-disubstituted quinolines were received after 1 h of excitation with blue LEDs at room temperature when MgCO3 was used as an additive in DCM. On the basis of experimental and DFT studies, we propose that PQ* induces one-electron oxidation of the imine substrate that triggers the electrocyclization mechanism.
Quinolines are an important class of N-heterocyclic aromatic compounds as they are ubiquitous structures in natural products and pharmaceuticals.1a The selective functionalization of different positions of the quinoline ring is often hard to achieve and thus substituted quinolines are usually synthesized from precursors carrying functional groups placed at the desired positions.1 One classic example of such a reaction is electrocyclization, which is applicable for a wide range of starting materials to give access to polysubstituted quinolines (Scheme 1a). However, the main drawback of this protocol is the need for harsh reaction conditions, for instance, high temperatures or pressures,2 strong Lewis acid catalysts,3 or short wavelength UV lights.4 Yields typically vary from poor to good with long reaction times up to several days, while dihydroquinolines are often received as side products.
Scheme 1. Electrocyclization and Radical Cyclization Synthetic Protocols to Access Quinoline Derivatives.
Recent developments in methods to generate organic radicals have led to the rise of novel synthetic methodologies to perform organic transformations under mild reaction conditions.5 In particular, visible-light photoredox catalysis has proven to be a useful tool to achieve this goal.6 In the development of quinoline derivative synthesis, in situ created 2-imidoylbiphenyl radicals have provided access to phenanthridines via homolytic aromatic substitution (Scheme 1b). These radicals have been obtained from the reaction of isocyanides with alkyl or aryl radicals, which can be produced by various synthetic or catalytic protocols using, for example, aryl amines and tBuONO,7 boronic acids and Mn(acac)3,8 or the Togni reagent and Bu4NI.9 On the other hand, the photocatalyzed redox approach provides reductive access to the imidoyl radicals from 2,2,2-trifluoro-N-phenylacetimidoyl chlorides.10
In this study, our objective was to combine the simplicity of classic electrocyclization methods for quinoline synthesis with the efficiency and mildness of photoredox radical methods. For a range of pericyclic reactions, activation energies for radical cationic pathways remarkably lower than those for the corresponding neutral reactions have been reported.11
We envisioned that a strong oxidant could transform the imine to an iminium radical cation or a neutral radical that would spontaneously cyclize to dihydroquinoline and subsequently aromatize to quinoline. This scenario was further supported by our previous DFT computational results, which predicted low energy barriers for the radical-cation-mediated cyclization route of 2-vinylarylimines to dihydroquinolines.12
Quinones at their ground states have been frequently used as mediators in various oxidative organic transformations.13 This is in contrast with the relatively low number of studies reported where quinones were studied as photocatalysts, even though their excited states are known to exhibit high oxidation potentials.14 An exception to this is anthraquinone as a photo-oxidant. Besides being reported as a competent electron and hydrogen atom acceptor, it also often acts as a catalyst to produce reactive oxygen species.15 For the current study, the latter feature would be unsuitable, as our interest was to discover catalysts able to oxidize the imine substrates via either single electron transfer (SET) or hydrogen atom transfer (HAT) and be regenerated by atmospheric oxygen afterward.6b,14 In this frame, visible-light-excited 9,10-phenanthrenequinone (PQ) raised our attention as a potential photocatalyst.
In 2000, Fukuzumi and co-workers reported the pioneering catalytic study on visible-light-activated PQ as a photo-oxidant, where benzylic alcohols were oxidized to the corresponding aldehydes utilizing molecular oxygen as the terminal oxidant.16 In this case, it was concluded that the oxidation step proceeds via a SET mechanism. Since this study, PQ has been reported as a photocatalyst only in a few other studies. Kumar’s team developed a PQ-catalyzed cascade trifluoromethylation and oxidation of 1,6-enynes in which excited-state PQ (PQ*) performs the SET activation of Langlois’ reagent (CF3SO2Na).17 Vila and co-workers observed that PQ* could work as a SET photoredox catalyst in the oxidation of benzoxazinones to iminium cations to couple with indoles in a Friedel–Crafts reaction.18 Wang and co-workers applied PQ* for HAT catalysis to convert aldehydes to acyl radicals, which then reacted with thiosulfonates to access thioesters.19 Recently, Xia and co-workers used a similar procedure to oxidize aldehydes to acyl radicals, which were further utilized in a Pd catalytic cycle.20
We commenced the study of the PQ*-catalyzed cyclization of imine 1a using a 20 mol % PQ loading and blue LED irradiation in acetonitrile, receiving a promising yield of quinoline 2a (43%). Solvent screening exposed DCM as being superior affording fast reactivity, while other solvents (Table 1 and Table S1) resulted in poorer yields even after prolonged reaction times. Trace amounts of water seemed to disturb the reaction significantly, causing imine 1a to hydrolyze to the corresponding aniline and aldehyde. First, we tried to suppress the imine hydrolysis using drying additives. The tested molecular sieves and sulfates, being slightly acidic or neutral in nature, caused only a slight improvement in the yield of 2a and the hydrolysis of 1a still took place (Table S3). Our next attempt was to inhibit the imine hydrolysis with carbonate additives as slightly basic drying agents. The yields improved significantly with all the carbonates tested, and only a trace amount of benzaldehyde was detected. Notably, the highest yield was achieved with MgCO3n-hydrate, indicating that its role was to act as a base rather than as a drying agent. Regardless, all the additives were only sparingly soluble in DCM. We measured the UV–vis spectra of PQ, 1a, and their mixture both with and without MgCO3 (Figure S4) and observed that the additive caused no shifts in the absorbance. This indicates that the carbonate does not have a strong interaction with either the substrate or the catalyst. Hence, we presume that its key effect is to stabilize imines as a non-nucleophilic base.21 The compatibility of various carbonates with quinone photocatalysts has been shown in previous studies.14a,19 Additionally, other tested photocatalysts (anthraquinone, eosin Y, and Acr+–Mes ClO4–) did not provide substantial reactivity (Table S5).
Table 1. Effect of Deviation from the Standard Reaction Conditionsa.
| entry | deviation from the standard conditions | yield (%)b |
|---|---|---|
| 1 | none | 97 |
| 2 | 1 h reaction time | 97 (84)c |
| 3 | no light | 0 |
| 4 | no photocatalyst | 0 |
| 5 | no MgCO3 | 45 |
| 6 | Ar atmosphere | 15 |
| 7 | O2 atmosphere | 84 |
| 8 | MeCN as solvent | 44 |
| 9 | toluene as solvent | 51 |
| 10 | EtOAc as solvent | 48 |
| 11 | 0.12 M concentration | 90 |
| 12 | 0.08 M concentration | 80 |
| 13 | MgSO4 as additived,e | 54 |
| 14 | K2CO3 as additived,e | 83 |
| 15 | 10 mg/mL MgCO3d,e | 94 |
| 16 | 40 mg/mL MgCO3d,e | 96 |
| 17 | 10 mol % PQd | 90 |
| 18 | 20 mol % PQd | 97 |
| 19 | 1 mmol scale, 21 h reaction time | 78c |
Full reaction optimization in Tables S1–S7.
NMR yield using 1,3,5-trimethoxybenzene as an internal standard.
Isolated yield after SiO2 chromatography.
1 h reaction time, O2 atmosphere.
20 mol % PQ.
With the optimized reaction conditions in hand, we moved on to study the cyclization scope by varying the substitution on the R1–R3 positions, probing electronic effects (R1–R3) and the aromatic or aliphatic substituent effect (R1 and R2). Both 4-methylquinolines 2a–2h and 4-phenylquinolines 2t–2z were obtained in excellent yields in 1–3 h (Scheme 2). All quinolines bearing 4- and 3-substituted aryl groups as R2 substituents were achieved in very good yields, but when the imine substrate had a 2-aryl substituent on the same position the yields of quinolines 2i and 2j were notably lower even though the reaction time was increased to 15 h. A similar substrate 1ad with a 2-nitroaryl substituent was not able to undergo cyclization and instead resulted in a complicated mixture of unknown side products.
Scheme 2. Reaction Scope Study.
1,3,5-Trimethoxybenzene was used as an internal standard
After SiO2 chromatography.
The synthesis of 4-methylquinolines 2k–2o with 2-heteroaryl substituents gave varying results. The reaction with imines 1k and 1l was stopped after 2 h, as the appearance of polymer-like side products was detected during the 1H NMR follow-up. Besides, the reactions with N-heteroaryl substituents did not proceed cleanly, as only 11–39% yields of quinolines 2m–2o were isolated after 15 h. The received lower yields were attributed to the slower conversion of the imines to quinolines as the imine hydrolysis started to compete with the desired reaction. Similarly, hydrolysis was also a problem with imines 1p and 1q with aliphatic substituents on the R2 position, resulting in 31–36% isolated yields of the corresponding quinolines. The instability of these imines was probably due to the lack of the stabilizing conjugation over the imine bond.
Our attempt to further expand the reaction scope to 2,3-diarylquinolines was unsuccessful, as styryl-derivatized imines 1aa–1ac were completely unreactive even when the reaction time was extended to 15 h. In similar fashion, substrate 1r lacking an R1 substituent cyclized rather sluggishly into 2-phenylquinoline 2r, giving only a poor yield of 7% after 15 h. Other unsuitable substrates are listed in the SI. To explain this behavior, we started to investigate the reaction mechanism.
At first, we carried out the reaction with imine 1a under the standard conditions in the presence of varying amount of TEMPO as a radical scavenger. With 2 equiv of TEMPO, the isolated yield of 2a decreased (from 84% to 35%), and a TEMPO-trapped product 3 was identified and isolated with a 16% yield (Scheme 3). Additionally, a trace amount of amide 4 was obtained. Increasing the loading of TEMPO to 5 equiv lowered the quinoline yield to 13%. Moreover, only 4% of adduct 3 could be detected, while the amount of amide 4 increased to 10%, indicating that its formation was associated with the TEMPO additive. The decreased yield suggested a radical character for the reaction, whereas the formation of the TEMPO adduct indicated the presence of a stabilized radical on the 4-position of the quinoline. The presence of long-living radical intermediates was further evaluated by employing cyclopropyl radical clocks with substrates 1q and 1s, which did not deliver any ring opening products.
Scheme 3. Reaction with TEMPO Radical Scavenger.
NMR yields with 5 equiv of TEMPO.
Next, we studied the kinetic isotopic effect with imine 1a and its deuterated derivative 1ah (Figure S3). The isotopic effect did not change the reaction rates, suggesting that the imine proton or hydrogen transfer was not connected to the rate limiting step in the reaction pathway.
To gain theoretical insight into the possible mechanistic routes, we performed computational studies of the initial steps of both HAT and electron transfer–proton transfer (ET-PT) routes of selected imine substrates at the DFT level.22 Interestingly, the HAT mechanism involving imidoyl radicals resulted in very low cyclization barriers (1.5–8.2 kcal/mol) and highly exergonic intermediate energies (−29.6 to −39.3 kcal/mol) for a diverse set of substrates 1a, 1b, 1f, 1r, and 1aa (Figure 1), which was a mismatch with the experimental observation of the reactivities of 1r and 1aa. Instead, the computed radical cation cyclization barriers were 10.5, 11.4, and 12.2 kcal/mol for the smoothly reactive substrates 1f, 1a, and 1b, respectively. Besides, the higher cyclization barriers and endergonic energies of the cyclized intermediates of 1r and 1aa explain well their poor reactivities considering that these steps are reversible.
Figure 1.

Computed initial steps in HAT and ET-PT pathways.
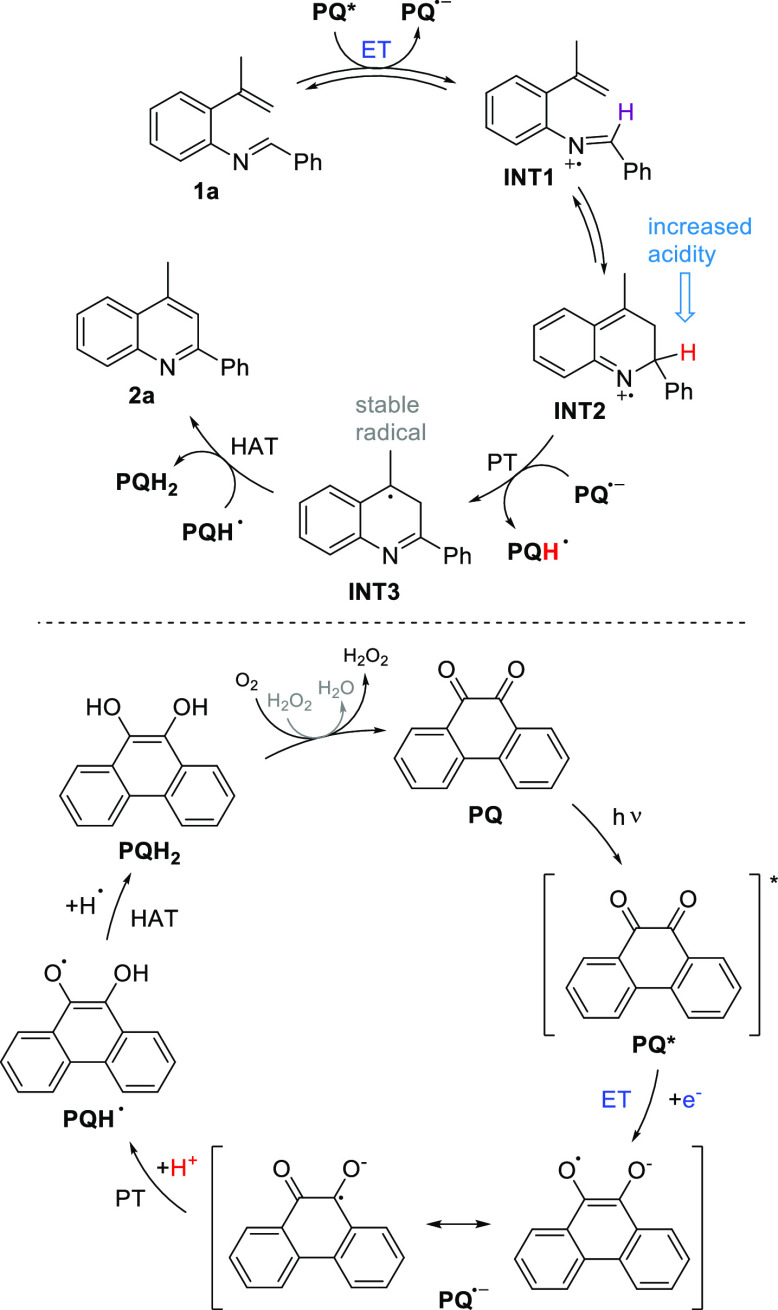
Computed pKas of the iminium radical cation INT1 and the cyclized intermediate INT2 (Scheme 4) resulted in values of 45–51 and 17–20 in DCM, respectively (Table S10). This indicates the dramatically increased acidity for the proton laying on the 2-position of INT2, favoring the C–C coupling step before the deprotonation event in the mechanistic route.23
Scheme 4. Proposed Reaction Mechanism.
The computed oxidation potentials (E1/2ox) for the studied imine substrates are in the range of 1.1–1.5 V (versus SCE), being lower than the reported excited state reduction potential E1/2* of PQ (3PQ*/PQ̇– = 1.6 V vs SCE).16,17 This suggests that PQ* is a strong enough oxidant for the imines to operate with the SET mechanism (SI).
Based on the experimental reactivity, TEMPO-trapped intermediates, and the computational studies, we propose a reaction mechanism for the electrocyclization (Scheme 4). After the visible-light excitation of PQ to its excited triplet state (PQ*),16 it induces SET from 1a, generating a radical on the nitrogen atom. The radical cation intermediate INT1 next cyclizes to the dihydroquinoline cation radical INT2 carrying an acidic proton, which is removed by the radical anion PQ•– resulting in two neutral radical species, PQH• and INT3. The formation of INT3 is supported by the TEMPO-trapped product 3 (Scheme 3). In the final stage, HAT yields phenanthrene-9,10-diol PQH2 and quinoline 2a. The reduced PQH2 is then readily oxidized back to PQ by molecular oxygen. The key role of oxygen was proven by conducting the standard cyclization reaction under an argon atmosphere (Table 1, entry 6), where only a stoichiometric amount of 2a was obtained.
In summary, we have developed a mild and efficient method to selectively synthesize polysubstituted quinolines using visible-light-excited PQ as a photocatalyst. The performed preliminary mechanistic studies suggest that excited-state PQ oxidizes imines to iminium cation radicals that trigger the cyclization step, leading to the subsequent deprotonation and dehydrogenation steps and the final formation of quinolines. Time-resolved photophysical studies are underway in our laboratory to acquire more detailed information on the reaction mechanism.
Acknowledgments
The Finnish National Centre for Scientific Computing (CSC) is recognized for computational resources. Gudrun Silvennoinen (Department of Chemistry, University of Helsinki) is acknowledged for performing high-resolution mass spectrometry.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c03934.
Experimental procedures, full reaction optimization, 1H NMR reaction monitoring, mechanistic studies, computational procedures and details, and 1H and 13C NMR spectra for all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Selected quinoline reviews:; a Matada B. S.; Pattanashettar R.; Yernale N. G. A Comprehensive Review on the Biological Interest of Quinoline and Its Derivatives. Bioorg. Med. Chem. 2021, 32, 115973. 10.1016/j.bmc.2020.115973. [DOI] [PubMed] [Google Scholar]; b Prajapati S. M.; Patel K. D.; Vekariya R. H.; Panchal S. N.; Patel H. D. Recent Advances in the Synthesis of Quinolines: A Review. RSC Adv. 2014, 4, 24463–24476. 10.1039/C4RA01814A. [DOI] [Google Scholar]; c Xuan D. D. Recent Progress in the Synthesis of Quinolines. Curr. Org. Synth. 2019, 16, 671–708. 10.2174/1570179416666190719112423. [DOI] [PubMed] [Google Scholar]
- Qiang L. G.; Baine N. H. A Convenient Synthesis of Substituted Quinolines by Thermal Electrocyclic Rearrangement of o-Vinyl Anils under Nonacidic Conditions. J. Org. Chem. 1988, 53, 4218–4222. 10.1021/jo00253a011. [DOI] [Google Scholar]
- a Zhang X.; Xu X.; Yu L.; Zhao Q. Bronsted Acid-Mediated Reactions of Aldehydes with 2-Vinylaniline and Biphenyl-2-Amine. Tetrahedron Lett. 2014, 55, 2280–2282. 10.1016/j.tetlet.2014.02.090. [DOI] [Google Scholar]; b Youn S. W.; Bihn J. H. Trifluoroacetic Acid-Mediated Facile Construction of 6-Substituted Phenanthridines. Tetrahedron Lett. 2009, 50, 4598–4601. 10.1016/j.tetlet.2009.05.071. [DOI] [Google Scholar]; c Majumdar K. C.; Ponra S.; Nandi R. K. BF3·OEt2-Mediated 1,3-Hydride Shift Followed by 6π Electrocyclization: An Efficient Route for the Synthesis of Pyridopyrimidine, Pyranoquinoline, and Phenanthroline Derivatives. Eur. J. Org. Chem. 2011, 2011, 6909–6915. 10.1002/ejoc.201101065. [DOI] [Google Scholar]
- a Butković K.; Vuk D.; Marinić Ž.; Penić J.; Šindler-Kulyk M. Synthesis and Photochemistry of 3-(o-Stilbeneyl)-4-H/Me/Ph-Sydnones; Intramolecular Cyclization to 1,2-Benzodiazepines and/or Quinolines. Tetrahedron 2010, 66, 9356–9362. 10.1016/j.tet.2010.10.013. [DOI] [Google Scholar]; b Elferink V. H. M. M.; Bos H. J. T. T. Novel Photochemical and Thermal Electrocyclization of Fused Quinolines. J. Chem. Soc. Chem. Commun. 1985, 882–883. 10.1039/c39850000882. [DOI] [Google Scholar]
- a Curran D. P. The Design and Application of Free Radical Chain Reactions in Organic Synthesis. Part 1. Synthesis 1988, 1988, 417–439. 10.1055/s-1988-27600. [DOI] [Google Scholar]; b Curran D. P. The Design and Application of Free Radical Chain Reactions in Organic Synthesis. Part 2. Synthesis 1988, 1988, 489–513. 10.1055/s-1988-27620. [DOI] [Google Scholar]; c Studer A.; Curran D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem., Int. Ed. 2016, 55, 58–102. 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- Selected reviews:; a Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]; c Crespi S.; Fagnoni M. Generation of Alkyl Radicals: From the Tyranny of Tin to the Photon Democracy. Chem. Rev. 2020, 120, 9790–9833. 10.1021/acs.chemrev.0c00278. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Visible Light Photocatalysis in Organic Chemistry; Stephenson C., Yoon T., MacMillan D. W. C., Eds.; Wiley-VHC, 2018. 10.1002/9783527674145. [DOI] [Google Scholar]
- Xia Z.; Huang J.; He Y.; Zhao J.; Lei J.; Zhu Q. Arylative Cyclization of 2-Isocyanobiphenyls with Anilines: One-Pot Synthesis of 6-Arylphenanthridines via Competitive Reaction Pathways. Org. Lett. 2014, 16, 2546–2549. 10.1021/ol500923t. [DOI] [PubMed] [Google Scholar]
- Tobisu M.; Koh K.; Furukawa T.; Chatani N. Modular Synthesis of Phenanthridine Derivatives by Oxidative Cyclization of 2-Isocyanobiphenyls with Organoboron Reagents. Angew. Chem., Int. Ed. 2012, 51, 11363–11366. 10.1002/anie.201206115. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Mück-Lichtenfeld C.; Daniliuc C. G.; Studer A. 6-Trifluoromethyl-Phenanthridines through Radical Trifluoromethylation of Isonitriles. Angew. Chem., Int. Ed. 2013, 52, 10792–10795. 10.1002/anie.201306082. [DOI] [PubMed] [Google Scholar]
- Dong X.; Xu Y.; Liu J. J.; Hu Y.; Xiao T.; Zhou L. Visible-Light-Induced Radical Cyclization of Trifluoroacetimidoyl Chlorides with Alkynes: Catalytic Synthesis of 2-Trifluoromethyl Quinolines. Chem. Eur. J. 2013, 19, 16928–16933. 10.1002/chem.201303149. [DOI] [PubMed] [Google Scholar]
- a Staykov A.; Areephong J.; Browne W. R.; Feringa B. L.; Yoshizawa K. Electrochemical and Photochemical Cyclization and Cycloreversion of Diarylethenes and Diarylethene-Capped Sexithiophene Wires. ACS Nano 2011, 5, 1165–1178. 10.1021/nn102806z. [DOI] [PubMed] [Google Scholar]; b Mahmood T.; Kosar N.; Ayub K. DFT Study of Acceleration of Electrocyclization in Photochromes under Radical Cationic Conditions: Comparison with Recent Experimental Data. Tetrahedron 2017, 73, 3521–3528. 10.1016/j.tet.2017.05.031. [DOI] [Google Scholar]; c Donoghue P. J.; Wiest O. Structure and Reactivity of Radical Ions: New Twists on Old Concepts. Chem. Eur. J. 2006, 12, 7018–7026. 10.1002/chem.200600554. [DOI] [PubMed] [Google Scholar]
- Mäkelä M. K.; Bulatov E.; Malinen K.; Talvitie J.; Nieger M.; Melchionna M.; Lenarda A.; Hu T.; Wirtanen T.; Helaja J. Carbocatalytic Cascade Synthesis of Polysubstituted Quinolines from Aldehydes and 2-Vinyl Anilines. Adv. Synth. Catal. 2021, 363, 3775–3782. 10.1002/adsc.202100711. [DOI] [Google Scholar]
- a Wendlandt A. E.; Stahl S. S. Quinone-Catalyzed Selective Oxidation of Organic Molecules. Angew. Chem., Int. Ed. 2015, 54, 14638–14658. 10.1002/anie.201505017. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Morales-Rivera C. A.; Floreancig P. E.; Liu P. Predictive Model for Oxidative C-H Bond Functionalization Reactivity with 2,3-Dichloro-5,6-Dicyano-1,4-Benzoquinone. J. Am. Chem. Soc. 2017, 139, 17935–17944. 10.1021/jacs.7b08902. [DOI] [PubMed] [Google Scholar]; c Wirtanen T.; Muuronen M.; Hurmalainen J.; Tuononen H. M.; Nieger M.; Helaja J. Intermolecular Oxidative Dehydrogenative 3,3′-Coupling of Benzo[b]Furans and Benzo[b]Thiophenes Promoted by DDQ/H+: Total Synthesis of Shandougenine B. Org. Chem. Front. 2016, 3, 1738–1745. 10.1039/C6QO00331A. [DOI] [Google Scholar]
- a Itoh A.Chapter 2: Quinones. In Photoorganocatalysis in Organic Synthesis; Fagnoni M., Protti S., Ravelli D., Eds.; Catalytic Science Series, Vol. 18; World Scientific, 2019; pp 39–70. 10.1142/Q0180;. [DOI] [Google Scholar]; b Lerch S.; Unkel L. N.; Wienefeld P.; Brasholz M. Ground- and Excited-State Quinones: Perspectives in Organocatalysis and Visible-Light Photocatalysis. Synlett 2014, 25, 2673–2680. 10.1055/s-0034-1379363. [DOI] [Google Scholar]
- Cervantes-González J.; Vosburg D. A.; Mora-Rodriguez S. E.; Vázquez M. A.; Zepeda L. G.; Villegas Gómez C.; Lagunas-Rivera S. Anthraquinones: Versatile Organic Photocatalysts. ChemCatChem. 2020, 12, 3811–3827. 10.1002/cctc.202000376. [DOI] [Google Scholar]
- Fukuzumi S.; Itoh S.; Komori T.; Suenobu T.; Ishida A.; Fujitsuka M.; Ito O. Photochemical Reactions of Coenzyme PQQ (Pyrroloquinolinequinone) and Analogues with Benzyl Alcohol Derivatives via Photoinduced Electron Transfer. J. Am. Chem. Soc. 2000, 122, 8435–8443. 10.1021/ja001351g. [DOI] [Google Scholar]
- Jana S.; Verma A.; Kadu R.; Kumar S. Visible-Light-Induced Oxidant and Metal-Free Dehydrogenative Cascade Trifluoromethylation and Oxidation of 1,6-Enynes with Water. Chem. Sci. 2017, 8, 6633–6644. 10.1039/C7SC02556D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostoll-Berenguer J.; Blay G.; Pedro J. R.; Vila C. 9,10-Phenanthrenedione as Visible-Light Photoredox Catalyst: A Green Methodology for the Functionalization of 3,4-Dihydro-1,4-Benzoxazin-2-Ones through a Friedel-Crafts Reaction. Catalysts 2018, 8, 653. 10.3390/catal8120653. [DOI] [Google Scholar]
- Zhang Y.; Ji P.; Hu W.; Wei Y.; Huang H.; Wang W. Organocatalytic Transformation of Aldehydes to Thioesters with Visible Light. Chem. Eur. J. 2019, 25, 8225–8228. 10.1002/chem.201900932. [DOI] [PubMed] [Google Scholar]
- Wang H.; Li T.; Hu D.; Tong X.; Zheng L.; Xia C. Acylation of Arenes with Aldehydes through Dual C–H Activations by Merging Photocatalysis and Palladium Catalysis. Org. Lett. 2021, 23, 3772–3776. 10.1021/acs.orglett.1c01184. [DOI] [PubMed] [Google Scholar]
- Cordes E. H.; Jencks W. P. On the Mechanism of Schiff Base Formation and Hydrolysis. J. Am. Chem. Soc. 1962, 84, 832–837. 10.1021/ja00864a031. [DOI] [Google Scholar]
- pw6b95d3/def2-tzvp//pbe0-D3bj/def2-svp (CPCM DCM). For further information, see the SI.
- The corresponding DFT-computed pKaH of PQ semiquinone is 61 (see the SI).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.