Summary
Coronavirus disease 2019 (COVID-19) is a recently recognised pandemic spreading rapidly from Wuhan, Hubei, to other provinces in China and to many countries around the world. The number of COVID-19-related deaths is steadily increasing. Acute ST-segment elevation myocardial infarction (STEMI) is a disease with high morbidity and mortality rates, and primary percutaneous coronary intervention is usually recommended for the treatment. A patient with diabetes mellitus and hypertension for five years was admitted to the emergency unit with symptoms of fever, cough and dyspnoea. These symptoms were consistent with viral pneumonia and a COVID PCR test was performed, which tested positive three days later. The patient had chest pain on the eighth day of hospitalisation. On electrocardiography, simultaneous acute inferior and anterior STEMI were identified. High levels of stress and increased metabolic demand in these patients may lead to concomitant thrombosis of different coronary arteries, presenting with two different STEMIs.
Keywords: COVID-19, acute anterior wall myocardial infarction, acute inferior wall myocardial infarction
Coronavirus disease 2019 (COVID-19) is a recently recognised pandemic spreading rapidly from Wuhan, Hubei, to other provinces in China and to several countries throughout the world.1 The number of COVID-19-related deaths is steadily increasing.1 Acute ST-segment elevation myocardial infarction (STEMI) is a disease with high morbidity and mortality rates, and primary percutaneous coronary intervention (PPCI) is usually recommended for the treatment.2 Co-existence of cardiovascular disease (CVD) is common in patients with COVID-19 and is related to an increased mortality rate. In this case report, we present a patient who was diagnosed with simultaneous acute anteroseptal and inferior STEMI while he was receiving treatment for COVID-19.
Case report
Our patient (a 55-year-old male, body mass index 26 kg/m2) with diabetes mellitus (DM) and hypertension (HT) for five years was admitted to the emergency unit on 25 March 2020 with symptoms of fever, cough and dyspnoea. He had a history of stable angina pectoris and 2.5 years earlier had had a stent implantation in his left anterior descending (LAD) artery. He had no chest pain on admission.
He was pre-diagnosed with COVID-19 after a thorough physical examination and work-up, including a thoracic computed tomography (CT) scan. There was ground-glass opacity on the CT scan. Azithromycin and oseltamivir treatment were started for COVID-19. His COVID PCR test was positive on 28 March and he was diagnosed with COVID-19. Hydroxychloroquine was added to the treatment regime.
On the morning of 2 April, he experienced chest pain with an increase in dyspnoea. As the level of oxygen saturation decreased, he was intubated. On electrocardiography (ECG), simultaneous acute inferior and anterior STEMI were identified (Fig. 1). It was decided to proceed with the PPCI.
Fig. 1.
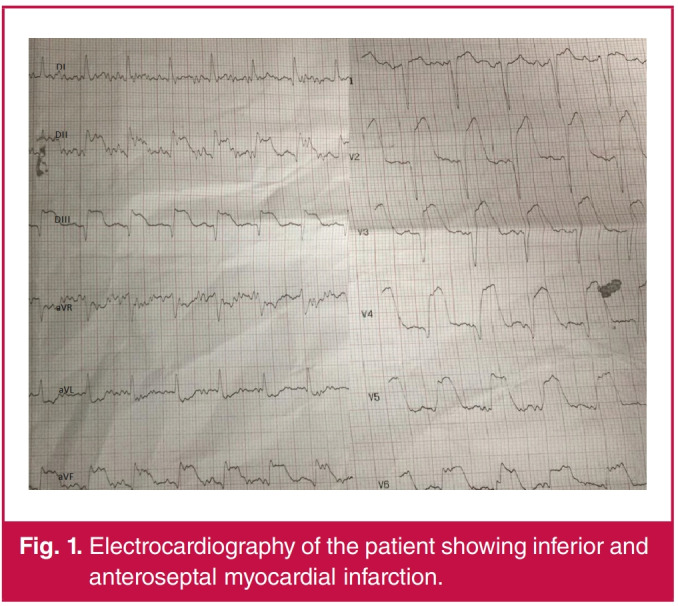
Electrocardiography of the patient showing inferior and anteroseptal myocardial infarction.
All necessary precautions were taken for viral protection. All laboratory staff wore N95 masks and protective goggles with single-use coveralls with an opening for the face. Additionally, the performing physician and the technician wore sterile singleuse laboratory coats.
On coronary angiography, the stent in the mid LAD was found to be patent, however it was occluded totally after the stent, and the right coronary artery (RCA) had subtotal occlusion. Initially, a Partner sirolimus-eluting stent of 2.75 × 15 mm was placed in the mid LAD (Fig. 2). After stent implantation, some blood flow was observed beyond the occlusion, however, a thrombotic total occlusion occurred in the distal LAD. Repeated dilatations were performed on this site, however no blood flow was observed. Two Boston Scientific Rebel bare-metal stents of 4.0 × 24 mm and 4.0 × 16 mm were directly implanted (Fig. 2) into the RCA.
In our hospital we have two catheterisation units. The laboratory used for the intervention was sterilised with disinfectant solutions and ultraviolet light and closed for 48 hours. The patient was taken into the intensive care unit. After the intervention, we observed resolution of ST-segment elevations on his ECG (Fig. 3). On a transthoracic echocardiogram (TTE), the ejection fraction was observed at 45% with apical akinesia. In his control TTE performed on 6 April, we observed similar findings after the intervention. The patient was discharged as healthy on 21 April.
Fig. 2.
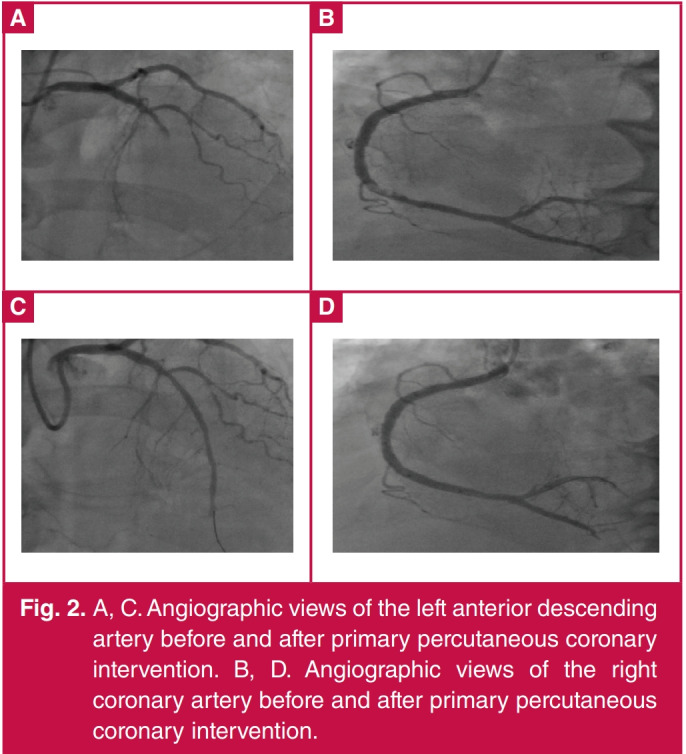
A, C. Angiographic views of the left anterior descending artery before and after primary percutaneous coronary intervention. B, D. Angiographic views of the right coronary artery before and after primary percutaneous coronary intervention.
Fig. 3.
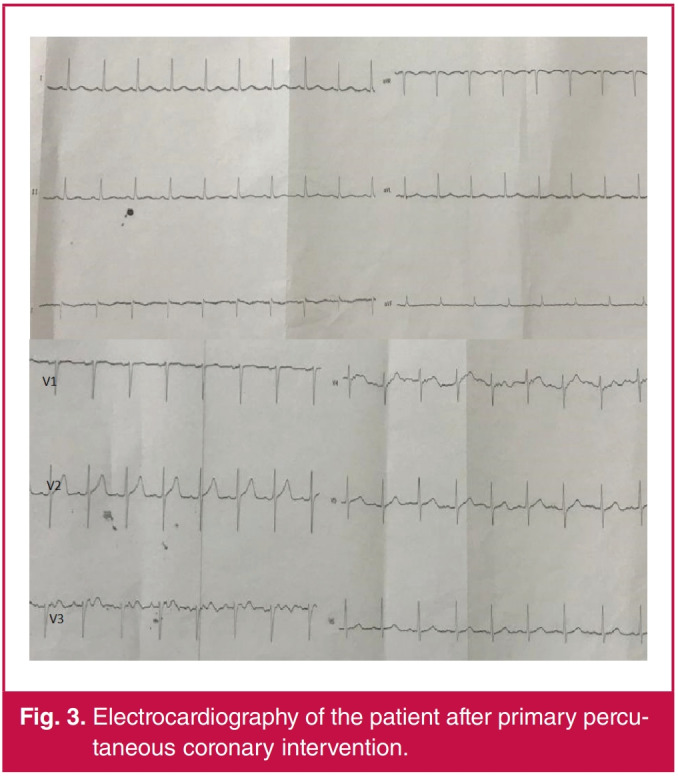
Electrocardiography of the patient after primary percutaneous coronary intervention.
Discussion
COVID-19 is a pandemic spreading over approximately 208 countries/regions. By 6 April 2020, more than 1 287 742 patients had become infected worldwide and it had caused the death of more than 70 000 people.
The prevalence of CVD is higher among those with COVID-19, and myocardial injury occurs in more than 7% of patients due to the infection (22% of them critically ill).3 COVID-19 infection can directly affect the cardiovascular system and the presence of CVD also facilitates COVID-19 infection.4 The Chinese Centre for Disease Control and Prevention reported in the recently published largest case series in mainland China that the overall mortality rate was 2.3% (1 023 deaths in 44 672 confirmed cases); however, the mortality rate increased up to 10.5% in patients with a history of CVD.5
STEMI is still a significant underlying factor for increased morbidity and mortality rates all around the world, although there has been a decrease in incidence and an increase in survival rates recently.6 Thrombotic occlusion occurs in a coronary artery at the site of a ruptured or eroded plaque and it leads to STEMI.6 Characteristic symptoms, and changes in the ECG are the basis for diagnosis, and elevated cardiac enzymes subsequently confirm the diagnosis.6 If it is performed by experienced specialists timeously, mechanical reperfusion via PPCI is superior to fibrinolytic therapy.6
For patients with COVID-19, there are two possible pathophysiological mechanisms: (1) type-I MI caused by anxiety-induced catecholamine discharge, prothrombotic system activation triggered by severe inflammatory activation, and plaque rupture, and (2) type-II MI caused by decreased oxygen delivery due to acute inflammation, respiratory failure and hypoxia.7,8
Moreover, Huang et al. stated that high concentrations of interleukin-1β, interferon-γ (IFN- γ), IFN-γ-inducible protein 10 and monocyte chemoattractant protein-1 could be observed in COVID-19 patients, thereby leading to activated T-helper-1 cell responses.9,10 It was also suggested that invasion by the virus of angiotensin converting enzyme-II, which is abundantly present in myocytes and vascular endothelial cells and is also the binding site of the coronavirus, may be the direct cause of cardiac involvement.9
In a published study, it was reported that a total of 40% of patients with positive test results for COVID-19 had cardiovascular or cerebrovascular disease and 7% had the possibility to develop acute cardiac injury. In case reports, on the other hand, the first causes of admission to hospital due to COVID-19 were heart failure, acute MI, myocarditis and sudden cardiac arrest.10-12 Shi et al. found that 19.7% of patients experienced cardiac injury, and the report showed for the first time that cardiac injury was independently related to an increased risk of mortality in patients with COVID-19 infection.13
It was also reported that in a significant number of patients with COVID-19 infection, levels of high-sensitivity cardiac troponin were increased.14 In a retrospective analysis of 191 patients hospitalised due to COVID-19, it was observed that levels of troponin were increased in more than 50% of patients who died.14 In other words, increasing levels of troponin in patients presenting with COVID-19 infection is an important indicator of mortality.14
Li et al. reported in a meta-analysis of six studies including 1 527 patients that the prevalence of CVD in patients with COVID-19 was 16.4%.15 The prevalence of CVD was higher in patients who required intensive care than those who did not require it.15 It was also reported that at least 8% of the patients were troponin positive.15
In their analysis, Guo et al. showed that while in-hospital mortality rates of intensive care patients who were without CVD and had normal troponin levels was 7.62%, it was 69.44% in patients with known CVD and high troponin levels.1 In a study evaluating 187 patients, troponin elevation, which is an indication of myocardial injury, was observed in 52 (27.8%) patients, and while the mortality rate was 59.6% in patients with high troponin levels, it was 8.9% in those with normal troponin levels.1
For the first time, acute MI was identified at the autopsy of a 53-year-old woman with chronic renal failure in Jinyintan Hospital (data not published; obtained via personal communication with a pathologist from the Chinese Academy of Science).14
In this study, we present a case of MI with anterior and inferior ST elevation on ECG, with the sudden onset of chest pain and increasing dyspnoea while receiving treatment for COVID-19. These patients may be hypoxic due to pneumonia, and both catecholaminergic discharge due to stress and plaque rupture with prothrombotic system activation induced by inflammatory activation related to COVID-19 may develop and result in STEMI. Moreover, in these patients, increased thrombophilia and arterial and venous embolisms are observed.
Our case presented with HT and DM, however, there was no history of chest pain or dyspnoea. On the fourth day of hospitalisation, sudden onset of chest pain with ST-segment elevation in the anteroseptal and inferior leads on ECG were observed. The Turkish Society of Cardiology (TSC), in its recently published expert opinion report, recommended thrombolytic therapy as the first option during STEMI.4 They also recommended PPCI in cases of wide anterior wall infarcts.4 We considered PPCI in our patient because of ST elevations in the V1-6 and inferior leads on ECG, in agreement with the recommendations of the TSC. PPCI was performed with coronary angiography for the LAD and right coronary arteries.
In COVID-19 patients, the incidence of CVD together with multiple cardiovascular risk factors is high. It is difficult to evaluate chest pain in these patients as they are isolated in intensive care units and the number of intubated patients is high. Moreover, ECG and TTE are performed less often due to strict isolation of these patients. In retrospective analysis, troponin was determined to be positive in approximately half of the deceased patients. With co-morbid coronary artery disease and positive troponin results, these patients were identified as a group with a significantly higher mortality rate.
Conclusion
Serial ECG monitoring and performing echocardiography as required in these patients in the intensive care unit could help to diagnose STEMI or non-STEMI accurately. A true diagnosis of MI may lead to the administration of appropriate treatment and lowering of mortality rates. On the other hand, a diagnosis of MI during an autopsy suggests that the reported rate of MI diagnosis is lower than the actual rate. High levels of stress and increased metabolism in these patients may lead to thrombosis of several coronary arteries with the concurrent occurrence of two different STEMIs.
Contributor Information
Mustafa Yolcu, Email: yolcudoctor@gmail.com, Department of Cardiology, Faculty of Medicine, Istanbul Yeni Yuzyil University, Gaziosmanpasa Hospital, Gaziosmanpasa, Istanbul, Turkey.
Fusun Gunesdogdu, Department of Family Medicine, Faculty of Medicine, Istanbul Yeni Yuzyil University, Gaziosmanpasa Hospital, Gaziosmanpasa, Istanbul, Turkey.
Metin Bektas, Department of Anesthesiology, Faculty of Medicine, Istanbul Yeni Yuzyil University, Gaziosmanpasa Hospital, Gaziosmanpasa, Istanbul, Turkey.
References
- 1.Guo T, Fan Y, Chen M. et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). J Am Med Assoc Cardiol. doi: 10.1001/jamacardio.2020.1017. Mar 27, 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibanez B, James S, Agewall S. et al. ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3.Clerkin KJ, Fried JA, Raikhelkar R. et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. doi: 10.1161/CIRCULATIONAHA.120.046941. Mar 21, 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Aktoz M, Altay H, Aslanger E. et al. Türk Kardiyoloji Derneği Uzlaşı Raporu: COVID-19 Pandemisi ve Kardiyovasküler Hastalıklar Konusunda Bilinmesi Gerekenler. Turk Kardiyoloji Dernegi Arsivi. 2020;48(Supp1):1–48. doi: 10.5543/tkda.2020.36713. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Mc Googan JM. Characteristics of and important lessons from the corona virus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. Feb 24, 2020. [Epub ahead of print] [Google Scholar]
- 6.Choudhury T, West NE, El-Omar M. ST-elevation myocardial infarction. Clin Med (Lond) 2016;16(3):277–282. doi: 10.7861/clinmedicine.16-3-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Januzzi JL. Troponin and BNP use in COVID-19 Cardiol Mag. Am Coll Cardiol. Available at: https:// www.acc.org/latest-in-cardiology/ articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid-19. Accessed Mar 23, 2020. [Google Scholar]
- 8.Driggin E, Madhavan VM, Bikdeli B. et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Cardiology. Troponin and BNP use in COVID-19. Available at: https://www.acc.org/latest-in-cardiology/articles/ 2020/03/18/15/25/troponin-and-bnp-use-in-covid19. [Google Scholar]
- 10.Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID-19: the protocols from Sichuan Provincial People’s Hospital. Intensive Care Med. doi: 10.1007/s00134-020-05993-9. Mar 11, 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel corona-virus-infected pneumonia in Wuhan, China. J Am Med Assoc. doi: 10.1001/jama.2020.1585. Feb 7, 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi S, Qin M, Shen B. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. J Am Med Assoc Cardiol. doi: 10.1001/jamacardio.2020.0950. March 25, 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, Yang J, Zhao F. et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. doi: 10.1007/s00392-020-01626-9. Mar 11, 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


