Abstract
Objective:
To evaluate the impact of Healthy Heart Africa (HHA), a comprehensive hypertension intervention programme, on hypertension awareness, knowledge, screening and diagnosis among rural communities in Kenya.
Methods:
Individuals from rural households near intervention and matched control healthcare facilities were randomly surveyed at baseline and the end point (after 12 months). A difference-in-differences analysis estimated the impact of HHA.
Results:
This analysis included 838 individuals (intervention, n = 432; control, n = 406) at baseline and 698 (n = 364 and n = 334, respectively) at the end point. At baseline, both groups had high hypertension awareness (> 80%) but poor knowledge. After 12 months, healthcare providers were the primary information source for the intervention group only (p < 0.05). At the end point, respondents’ knowledge of hypertension risk factors, consequences and management trended higher among the intervention versus the control group. Hypertension screening/diagnosis and patient recall of provider recommendations remained unchanged in both groups.
Conclusion:
HHA improved hypertension knowledge but screening and diagnosis remained unchanged after 12 months.
Keywords: awareness, Healthy Heart Africa (HHA), hypertension, Kenya, rural
Hypertension is the leading cause of cardiovascular disease (CVD) worldwide, affecting approximately 31% of the adult population (1.4 billion people) in 2010.1 The growing prevalence of hypertension is a challenge for developing countries, which already face a high burden of infectious diseases, such as human immunodeficiency virus (HIV), malaria and tuberculosis, and have limited resources to dedicate towards hypertension care and control.1
Kenya is one such country facing a growing burden of hypertension, with studies reporting age-standardised prevalence ranging from 18.4 to 22.8%.2,3 According to the recent Kenya STEPwise survey, the overall national prevalence of raised blood pressure (BP) was 23.8%.4 Prevalence increased with age, ranging from 13.2% among Kenyans aged 18 to 29 years to 53.2% for those aged 60 to 69 years.4
Despite the high prevalence of hypertension, overall awareness and control remain low.2,5,6 According to the Kenya STEPwise survey, 56% of Kenyans have never been screened for hypertension, and, of those previously diagnosed with hypertension, only 22.3% were currently taking medication prescribed by a healthcare worker.4 Since hypertension manifests asymptomatically, individuals may not necessarily seek routine BP screening, resulting in late detection and increased risk of stroke, hypertensive heart disease or kidney failure and coronary artery disease.2 Improving the public’s awareness and general knowledge of hypertension may result in positive lifestyle changes and allow for timely detection of hypertension and early prevention of adverse outcomes.
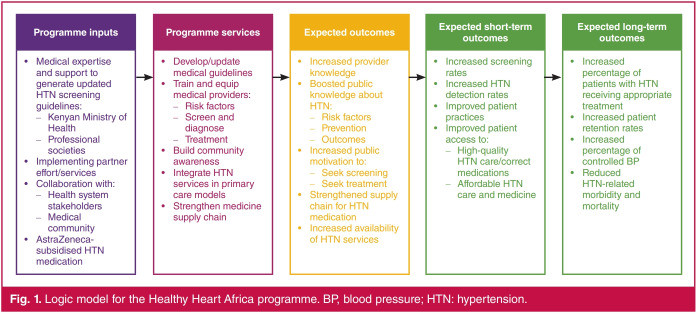
Healthy Heart Africa (HHA), an AstraZeneca-sponsored programme, was designed to provide a model for controlling the growing burden of hypertension in Africa. The HHA programme utilised a collaborative multi-level approach aimed to improve hypertension control through education, BP screening and diagnosis, with longer-term goals of improving retention in care and attainment of treatment goals (Fig. 1). HHA was first initiated in March 2015 across 21 counties in Kenya, including the capital city, Nairobi, and the surrounding areas and parts of western Kenya, and has since expanded across sub-Saharan Africa (Fig. 2).7 Here, we report results from a 12-month prospective, controlled evaluation of the impact of HHA intervention on hypertension awareness and knowledge and the frequency of BP screening and hypertension diagnosis among individuals residing in rural Kenya.
Fig. 1.
Logic model for the Healthy Heart Africa programme. BP, blood pressure; HTN: hypertension.
Fig. 2.
Map of Kenya showing distribution of intervention facilities and matched control facilities.
Methods
HHA collaborated with the Kenyan Ministry of Health and five healthcare service-implementation partners [Academic Model Providing Access to Healthcare (AMPATH); Amref Health Africa, formerly the African Medical and Research Foundation (AMREF); Christian Health Association of Kenya (CHAK); Jhpiego; and Population Services Kenya (PSK)] to improve hypertension education/awareness, screening and primary healthcare services (Table 1). To increase hypertension awareness and knowledge among the public and improve referral to healthcare facilities for hypertension care, the implementing partners conducted education and screening outreach campaigns during market days and at local community events [such as ‘barazas’ (community meetings), ‘chamas’ (an informal co-operative society), roadshows and facility outreach events], church gatherings, public transportation stations and home visits.
Table 1. Healthy Heart Africa implementing partners.
| AMPATH | Amref Health Africa | CHAK | Jhpiego | PSK | |
| Approach | Extension of existing hypertension programme into Ministry of Health sites in rural West Kenya | Community-based screening clustered around Ministry of Health sites in the Kibera slum area | Initial focus on church/religious leaders and expanding outreach efforts across church, community, facility and workplace | Informal integration of the programme into HIV network in Ministry of Health sites with a significant focus on facility-based screening | with significant focus on outreach events and ad hoc screening at various gatherings of people (e.g. bus stops, parties, funerals and sporting events) |
| Implementation sites | Public health dispensaries and primary-care facilities | Public health facilities and Ministry of Health sites in the Kibera slum area | Faith-based facilities and community sites (e.g. markets, group meetings, bus, stops and workplaces) | Public health facilities | Private clinics, pharmacies, and non-traditional sites (e.g. taxi stands, gyms, market places, primary schools, social halls, roadside, youth bases and women’s group) |
AMPATH: Academic Model Providing Access to Healthcare; CHAK: Christian Health Association of Kenya; HIV: human immunodeficiency virus; PSK: Population Services Kenya.
Healthcare facilities participating in the HHA programme received educational materials (examples provided in supplementary information) to use at these outreach events. In addition, participating healthcare facilities received access to key hypertension medications, basic resources (equipment and educational and training materials), and a hypertension diagnosis and treatment protocol, which described hypertension risk factors and management methods but not the consequences of hypertension.7 Of note, during the study period, some healthcare providers who were trained at an intervention facility may have been transferred to either a control or non-participating healthcare facility as part of routine transfer or due to the devolution of the Kenyan government that occurred during the study period.8
A 12-month prospective, controlled study evaluated the effect of HHA intervention on facility services for hypertension care, and the knowledge of hypertension among healthcare providers and the general study population. Two separate surveys, the Facility Survey7 and the Household Survey, were conducted before (baseline) and 12 months after (end point) the implementation of HHA. Initially the Facility Sample included 150 healthcare facilities (75 randomly selected intervention facilities and 75 matched control facilities, paired based on the implementation partner and location) and the Household Sample reflected the catchment areas near 50 facilities (25 intervention facilities chosen at random from the initial 75, and their 25 matched controls). Due to attrition, the final analysis included 132 facilities in the Facility Survey, and the catchment area of 42 facilities in the Household Survey. Sample sizes declined because facilities closed or refused to participate in the end-point survey, or were dropped because their (or their pair’s) treatment status was switched after the initial samples were chosen, and they could not be matched to another comparable facility in the other treatment group.
The impact of HHA on facility services and healthcare providers’ knowledge of hypertension was evaluated by the Facility Survey and has been reported on by Ogola et al.7 Here, we report the results from the Household Survey, which evaluated the effect of HHA intervention on the knowledge of and attitudes toward hypertension and the frequency of BP screening and hypertension diagnosis among the study population.
The Household Survey (supplementary information) was developed by the investigators to evaluate individuals’ awareness and knowledge of hypertension and their attitudes and health-seeking behaviour towards hypertension. The survey was administered to individuals residing near a subset of 25 intervention facilities (randomly selected from the original group of 75 facilities assigned to the intervention) and their matching control facilities at baseline and the end point. The selected facilities were located in rural and urban areas.
The intervention population was defined as individuals residing in the catchment areas of the facilities participating in the HHA programme. The control population was defined as individuals residing in the catchment areas of matched facilities that did not receive HHA intervention. Data for BP screening and hypertension diagnosis were also abstracted from the service delivery registers (used at the point of service) of the selected facilities.
The Household Survey was pilot-tested before fielding by Ipsos Synovate Kenya (Nairobi, Kenya), a contracted survey consulting firm. Trained staff conducted face-to-face interviews with individuals residing in the catchment areas surrounding the participating intervention and control facilities. Each catchment area was divided into four non-overlapping enumeration areas, of which two enumeration areas [one near (1–4 km) and one far (5–7 km) from the facility] were randomly surveyed. For each enumeration area, a random starting point was selected and the right-hand rule (household to the right of the data collector) was used to select every household after 200 metres. The baseline and end-point surveys were conducted at the same enumeration sites; however, different households were randomly surveyed at each time point, resulting in two separate sample populations.
Adults (aged ≥ 18 years) were surveyed if they lived in the same compound, had the same household head and same cooking arrangements, and had lived in the household during the last six months. The surveyors visited each household two additional times to establish contact with adult household members who were not present at the time of the first interview. Households were assigned to the treatment group associated with their local facility (intervention or control); however, the survey did not capture where the survey respondents sought and/ or received care (at the facility to which they were assigned or outside the study area or non-traditional medicine).
This study was reviewed and approved by the Kenyatta National Hospital and the University of Nairobi Ethics and Research Committee (KNH/UON-ERC). Written informed consent was obtained from all survey respondents before the start of the study.
Statistical analysis
Since the survey did not capture where individuals sought care, this analysis was limited to the rural population to minimise the possibility of cross-contamination (study participants residing near one facility but receiving treatment from another facility). Because of the sparse distribution of healthcare facilities in rural areas, the likelihood of individuals seeking care from a healthcare facility other than their locally assigned facility was believed to be lower. The analysis was restricted to individuals residing near seven intervention facilities located in rural areas and the seven matched control facilities. Due to difficulties in matching intervention and control facilities supported by the same implementation partner, select rural intervention facilities were matched to control facilities located in more urban areas.
At baseline and end point, statistical differences between the intervention and control groups with regard to demographics and lifestyle characteristics were evaluated using a t-test for bimodal variables and a chi-squared test for outcomes with more than two values. The impact of HHA intervention, defined as the treatment effect (TE) on hypertension awareness and knowledge, BP screening and patient recall of provider recommendation was assessed using a difference-in-differences (D-in-D) regression analysis, which minimises bias due to other factors that change over the same time frame.
Results
A total of 838 individuals were surveyed at baseline (intervention, n = 432; control, n = 406) and 698 at the end point (intervention, n = 364; control, n = 334). Demographics (age, geographic location and education) were well balanced between the intervention and control groups sampled at baseline and the end point (Table 2). Nevertheless, the two treatment groups at both baseline and end point varied with regard to wealth and lifestyle characteristics. At both baseline and end point, individuals in the intervention group were wealthier and tended to consume one or more servings of fruit per day (p < 0.05 for all). In addition, at the end point, a significantly greater proportion of individuals in the intervention group consumed alcohol and one or more servings of vegetables per day (p < 0.05 for both).
Table 2. Characteristics of survey respondents residing in rural areas.
| Baseline | End point | |||||
| Intervention (n = 432) | Control (n = 406) | p-value | Intervention (n = 364) | Control (n = 334) | p-value | |
| Geographic region, % | ||||||
| Central or eastern | 63.6 | 63.6 | 75.0 | 72.4 | ||
| Nairobi | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Nyanza | 10.1 | 0.0 | 0.345 | 9.8 | 0.0 | 0.175 |
| Rift Valley | 0.0 | 10.3 | 0.0 | 11.4 | ||
| Western | 26.4 | 26.1 | 15.3 | 16.2 | ||
| Residence location, % | ||||||
| Rural | 93.6 | 99.4 | 69.3 | 99.5 | 0.000** | |
| Urbana 6.4 | 0.6 | 0.014* | 30.7 | 0.5 | 0.000** | |
| Age, years | % | |||||
| 18–24 | 18.7 | 20.0 | 22.4 | 14.3 | ||
| 25–29 | 12.7 | 8.9 | 14.2 | 16.0 | ||
| 30–34 | 10.9 | 12.3 | 14.0 | 10.8 | ||
| 35–39 | 11.2 | 13.3 | 0.647 | 9.3 | 7.6 | 0.128 |
| 40–44 | 11.2 | 8.7 | 11.6 | 9.7 | ||
| 45–49 | 6.6 | 6.9 | 7.0 | 8.8 | ||
| ≥ 50 | 28.8 | 30.0 | 21.6 | 32.8 | ||
| Gender, % | ||||||
| Male | 48.7 | 46.0 | 0.269 | 50.6 | 55.7 | 0.448 |
| Female | 51.3 | 54.0 | 0.269 | 49.4 | 44.3 | 0.448 |
| Education, % | ||||||
| Nursery/kindergarten | 1.8 | 2.0 | 3.5 | 2.7 | ||
| Primary | 47.4 | 48.7 | 37.1 | 48.2 | ||
| Post-primary, vocational | 4.1 | 3.5 | 0.834 | 4.3 | 5.3 | 0.310 |
| Secondary, A-level | 36.4 | 32.2 | 0.834 | 39.3 | 30.1 | |
| College (mid-level) | 6.0 | 6.5 | 11.8 | 6.4 | ||
| University | 1.7 | 1.7 | 1.4 | 1.2 | ||
| No school attended | 2.5 | 4.8 | 2.5 | 6.0 | ||
| Wealth quintile, shillings/month, % | ||||||
| ≤ 653 | 34.3 | 33.5 | 25.6 | 37.9 | ||
| 654–2 158 | 17.2 | 13.6 | 22.4 | 20.6 | ||
| 2 159–2 633 | 25.8 | 51.6 | 0.000** | 15.4 | 38.8 | 0.000** |
| 2 634–3 631 | 21.2 | 0.5 | 0.000** | 32.4 | 1.0 | |
| ≥ 3 632 | 1.5 | 0.8 | 4.2 | 1.7 | ||
| Lifestyle characteristics, % | ||||||
| Non-smoker | 87.8 | 91.5 | 0.392 | 92.0 | 86.6 | 0.104 |
| Does not drink alcohol | 78.0 | 82.8 | 0.177 | 80.2 | 87.4 | 0.023* |
| Consumes ≥ 1 fruit serving/day | 52.0 | 26.3 | 0.001** | 60.8 | 36.0 | 0.010* |
| Consumes ≥ 1 vegetable serving/day | 68.1 | 56.5 | 0.446 | 84.9 | 54.1 | 0.016* |
Table 3. Pearson’s correlation analysis for some parameters (r)
Hypertension awareness (defined as having heard of hypertension) among the intervention group was high at baseline (91.0%) and increased to 94.9% by the end point (Table 3). In contrast, hypertension awareness was much lower at baseline in the control group (79.1%) but had increased to 96.7% by the end point. Of note, the D-in-D method’s underlying assumption of parallel trends could not hold for this outcome, as an increase of 17 percentage points (pp) from an initial level of 91.0% was not feasible in the intervention group.
Table 3. Pearson’s correlation analysis for some parameters (r).
| Control | ES | SLE | SHE | |||
| Parameter | T amplitude (mV) | R amplitude (mV) | QT interval (ms) | dP/dtmax (mmHg/s) | P amplitude (mV) | |
| Nrf2 | –0.944** | 0.041 | 0.333 | –0.362 | –0.182 | –0.817* |
| Nuclear Nrf2 | 0.157 | 0.921** | 0.699 | 0.836* | 0.875* | 0.324 |
| Keap1 | 0.445 | 0.453 | 0.934* | 0.153 | –0.724 | –0.250 |
The data show Pearson’s correlation coefficients (r), n = 6 animals per group. *p < 0.05 and **p < 0.01.
ES: acute exhaustive swimming group; SLE: low-dose salidroside plus exhaustive swimming group; SHE: high-dose salidroside plus exhaustive swimming group; dP/dtmax: peak rate of the increase in pressure.
Family and friends were the primary source of information on hypertension for both the intervention and control groups at baseline and the control group at the end point. However, by the end point, a healthcare provider or facility became the primary source of information for individuals in the intervention group (TE, 19.4 pp; p < 0.05; Table 3).
In general, the intervention group experienced an increase in knowledge of individual risk factors for hypertension. Significant improvement was observed in individuals’ knowledge of tobacco use as a risk factor for hypertension with intervention (TE, 4.0 pp; p < 0.05; Table 3). Within 12 months, individuals’ knowledge of three or more hypertension risk factors also showed a trend toward improvement in the intervention group [TE, 3.8 pp; p = not significant (NS)].
A positive improvement in individuals’ knowledge of hypertension management was seen in the intervention group. Identification of alcohol reduction as a method for managing hypertension significantly increased four-fold in the intervention group (TE, 8.4 pp; p < 0.01). In addition, positive trends were seen in the proportion of individuals who identified salt reduction as a method for hypertension management (TE, 1.0 pp; p = NS) in the intervention group. Individuals’ knowledge of three or more or five or more methods for managing hypertension also improved three-fold (TE, 3.7 pp; p = NS) and 17-fold (TE, 1.7 pp; p = NS), respectively, in the intervention group.
Similarly, individuals’ knowledge of hypertension-related consequences showed a trend toward improvement among the intervention group (Table 3). Knowledge of possible complications during delivery significantly increased among respondents with intervention (TE, 3.3 pp; p < 0.01). Similarly, a positive trend was seen in individuals’ knowledge of stroke (increased from 19.6% at baseline to 22.4% at the end point), heart failure (from 6.2 to 9.0%, respectively), aneurysm (from 3.8 to 4.1%, respectively) and death (from 43.7 to 52.3%, respectively) with intervention; however, these changes were not significant relative to the control. Individuals’ knowledge of three or more consequences of hypertension increased two-fold in the intervention group.
Despite these changes in knowledge surrounding hypertension, the proportion of individuals screened for BP and diagnosed with hypertension slightly decreased from baseline to the end point in the intervention group (Table 4).
Table 4. Impact of Healthy Heart Africa on hypertension diagnosis and provider’s recommendation.
| Baseline | End point | Treatment effect (SE), percentage point | |||
| Intervention (n = 432) | Control (n = 406) | Intervention (n = 364) | Control (n = 334) | ||
| Individuals who reported being screened for BP, % | 74.3 | 62.6 | 72.9 | 77.6 | –19.9 (7.8)* |
| Last time BP screening was performed, % | |||||
| ≤ 6 months | 38.7 | 24.4 | 37.8 | 39.4 | –15.2 (4.6)** |
| 7–12 months | 10.2 | 8.8 | 12. | 1 16.3 | 7.2 (4.8) |
| ≥ 12 months | 24.8 | 27.8 | 22.2 | 21.9 | 0.7 (4.1) |
| BP screening location, % | |||||
| Public hospital | 29.3 | 29.0 | 27.1 | 19.9 | 8.3 (7.8) |
| Public health centre or dispensary | 40.9 | 24.1 | 35.9 | 44.6 | –16.2 (9.0) |
| Private hospital | 5.6 | 8.9 | 13.3 | 18.2 | –2.7 (3.4) |
| Private health centre or dispensary | 11.3 | 6.1 | 15.5 | 13.7 | –4.6 (6.4) |
| At screening event | 1.2 | 0.8 | 2.5 | 8.1 | –7.4 (2.9) |
| Other | 7.7 | 10.4 | 15.0 | 21.1 | –2.0 (1.6) |
| Individuals who reported being diagnosed with hypertension, % | 14.9 | 8.8 | 12.9 | 10.3 | –0.03 (3.3) |
| Timing of hypertension diagnosis, % | |||||
| ≤ 6 months | 3.8 | 2.9 | 2.7 | 2.9 | –1.2 (3.0) |
| 7–12 months | 2.1 | 1.0 | 3.8 | 1.3 | 2.5 |
| > 12 months | 8.8 | 4.9 | 5.5 | 5.1 | –1.3 (2.7) |
| Individuals’ recall of healthcare providers’ recommendation, % | |||||
| ≥ 1 healthcare providers’ recommendation | 13.4 | 8.8 | 9.8 | 9.3 | –2.3 (3.5) |
| ≥ 3 healthcare providers’ recommendations | 0.1 | 2.1 | 0.5 | 1.4 | 1.2 (0.9) |
| Medication | 13.1 | 7.1 | 7.2 | 8.2 | –5.0 (2.5) |
| Reduction in salt | 2.2 | 3.8 | 4.0 | 4.0 | 2.0 (1.6) |
| Lose weight | 0.1 | 2.0 | 0.6 | 1.3 | 1.9 (1.7) |
| Reduce alcohol consumption | 0.2 | 0.7 | 0.6 | 0.0 | 1.3 (0.5)* |
| Exercise | 1.4 | 2.3 | 1.3 | 1.3 | 1.3 (1.5) |
| Reduce stress | 3.5 | 2.1 | 3.8 | 3.6 | 0.4 (1.3) |
| Home remedies | 0.3 | 0.9 | 0.1 | 0.7 | 0.3 (0.7) |
| Unable to recall | 0.9 | 0.1 | 1.6 | 0.6 | 0.8 (1.5) |
*p < 0.05; **p < 0.01 vs control. BP: blood pressure; SE: standard error.
Discussion
Individuals’ natural awareness of hypertension may be limited as the disease manifests asymptomatically and care may not be sought until sudden, severe and irreversible consequences occur. Little is known about the optimal method to rapidly educate the general population of a low- to moderate-income developing country about hypertension. The purpose of this 12-month prospective study was to assess the impact of HHA intervention on the status of awareness and knowledge of hypertension among the rural Kenyan population.
General awareness of hypertension among survey respondents was high (approximately 80 to 90%) at baseline; however, the specific knowledge of risk factors and actions was poor, indicating that information from primary sources, namely friends and family, was not necessarily accurate. At baseline, less than 5% of respondents could correctly identify three or more known risk factors for hypertension. Similarly, respondents appeared to be largely unaware that smoking, alcohol consumption and family history of heart disease were possible risk factors for hypertension or associated CVD.
Within 12 months, HHA was successful in conveying information about hypertension to individuals residing in the intervention areas. This is demonstrated by the significant increase in the percentage of respondents in the intervention areas who reported healthcare providers as their primary source of hypertension information compared with the control group.
In general, knowledge of hypertension showed a trend toward improvement among the HHA intervention group. By the end point, more individuals residing in the intervention areas identified tobacco use as a risk factor for hypertension and reducing alcohol consumption as a method for managing hypertension. The observed improvement is of importance because both alcohol and tobacco consumption have been associated with the high prevalence of hypertension in Kenya.9,10 According to the 2015 Kenya STEPwise survey, approximately 13.3% of survey respondents, aged 18–69 years, reported current use of a tobacco product (manufactured or hand-rolled cigarettes, pipes or shisha).4 In addition, 19.3% of respondents reported current alcohol use, with 12.7% consuming alcohol on a daily basis and 12.7% reporting heavy episodic drinking (six or more drinks on a single occasion).4 Therefore it is believed that improving individuals’ awareness of the association between tobacco and alcohol use and hypertension may lead to the adoption of a healthier lifestyle over time.
Individuals’ knowledge of hypertension-related consequences remained relatively unchanged. This was expected to a certain extent, as the programme intervention did not educate healthcare providers or the general public on the consequences of hypertension.7 In addition, HHA intervention areas did not have a significant change in the number of individuals screened for BP or diagnosed with hypertension, emphasising the need for more innovative outreach activities/approaches to identify individuals at high risk for hypertension, including those who may not necessarily visit healthcare facilities.
To the best of our knowledge, this is the first study to characterise and intervene to improve individuals’ awareness and knowledge of hypertension among the rural population in Kenya. Previous studies have reported a low degree of awareness among Kenyans, but clinically meaningful comparisons were not possible due to a lack of a standardised definition for hypertension awareness.2,5,6,11
However, a recent qualitative study based on a series of focus group discussions with 53 individuals with HIV-1 conducted at the Kenyatta National Referral and Teaching Hospital Comprehensive Care Centre reported a gap between hypertension awareness and knowledge, similar to what was seen in this study.11 Respondents commonly referred to hypertension as ‘pressure’ and, although all of the participants had heard of the term, most were unable to adequately describe it. Stress followed by fatty foods, excessive salt intake, and physical inactivity were the most frequently cited causes of hypertension. All respondents demonstrated some knowledge regarding treatment modalities for hypertension; however, most believed that hypertension could not be prevented.
This gap between awareness and knowledge/understanding is not strictly limited to hypertension and extends to individuals’ awareness and understanding of CVDs. A systematic review evaluating awareness and knowledge of CVDs in sub-Saharan Africa found that awareness, when reported, was high; however, knowledge and understanding of CVDs and CVD risk factors were poor.12 Although limited, the data suggest that individuals may benefit from intervention efforts designed to not only raise awareness but also improve general understanding and knowledge of hypertension. The HHA programme’s positive impact on knowledge of hypertension may help to address this critical gap in communication, and, when coupled with the previously reported facility-level improvements in provider education and ability to diagnose and treat hypertension,7 may lead to greater utilisation of hypertension services and, in turn, timely diagnosis and treatment of hypertension.
There are some inherent limitations associated with this analysis. The generalisability of the data is limited, in part, by study design. This study was not designed to collect nationally representative findings and therefore data interpretation is limited to individuals residing near the study sites. In addition, this study did not capture where respondents received care. Potential ramifications of receiving care at distant sites were mitigated by focusing on the rural population, which, due to limited access to healthcare facilities, is more likely to receive care at the local facility. The study design did not the capture frequency and type of study- and non-study-related hypertension awareness/education events conducted within the study area, which may affect programme evaluation.
Furthermore, the short duration of this study may not be sufficient for evaluating the impact of the HHA programme, as significant changes in individuals’ behaviours and attitudes towards hypertension care may require a longer period of time in this setting. The impact of the HHA programme may be underestimated as HHA-trained healthcare providers from intervention facilities may have been moved and replaced with untrained healthcare providers, a by-product of routine transfer and the devolution of the Kenyan government,8 which occurred during the 12-month study period.
Conclusion
Little is known about how to rapidly improve control of hypertension in low- to moderate-income countries. The results from this study may help to develop more realistic expectations on the anticipated rate of improvement in individuals’ awareness and knowledge of hypertension and health-seeking behaviour towards hypertension care. In this study, individuals residing in rural Kenya demonstrated a high degree of hypertension awareness; however, their medical knowledge of hypertension was quite poor.
The HHA programme was able to demonstrate an improvement in individuals’ knowledge of hypertension within 12 months of programme implementation. These initial improvements may eventually lead to longer-term changes in individuals’ attitudes and behaviours, including lifestyle changes and healthcareseeking practices toward hypertension care. Additional studies are needed to determine realistic time frames for improvements in lifestyle, regular BP screening, adherence to hypertension care and reduction in the negative outcomes of hypertension.
Acknowledgments
The authors thank all the individuals involved with programme implementation and data collection, field monitoring and data analysis for this study. We acknowledge the efforts of the HHA partners involved with this study: Academic Model Providing Access to Healthcare (AMPATH); Amref Health Africa, formerly African Medical and Research Foundation (AMREF); Christian Health Association of Kenya (CHAK); Jhpiego; Population Services Kenya (PSK); Abt Associates; Ipsos Synovate Kenya; and Savannah Informatics Ltd.
Elizabeth Macgregor-Skinner, formerly of Abt Associates, contributed to study design and data analysis and interpretation and Doug Johnson, formerly of Abt Associates, contributed to the study design. The authors also thank Alec van Gelder, Javier Jimenez, Clive Pickering, Ian MacTavish, Allan Mackenzie and Kennedy Njau of AstraZeneca, for their support of the Healthy Heart Africa programme and this study, and Mary Beth DeYoung of AstraZeneca for providing critical review of the manuscript. Disha Patel, PhD, of inScience Communications, Springer Healthcare (Philadelphia, PA, USA), provided medical writing support, which was funded by AstraZeneca.
This study/analysis was funded by AstraZeneca. GY and ENO have served as consultants for Abt Associates. GY is currently a visiting professor at the University of Nairobi, Kenya. FOO was an employee of Abt Associates at the time of the survey and is currently serving as the chief of party for the US Agency for International Development Ethiopia Performance Monitoring and Evaluation Service Activity. JLH is an employee of Abt Associates. AM is an employee of AstraZeneca and has stock/stock options in AstraZeneca.
Contributor Information
Gerald Yonga, Email: yongag@gmail.com, Aga Khan University, Nairobi, Kenya.
Ashling Mulvaney, AstraZeneca, London, United Kingdom.
Elijah N Ogola, Clinical Medicine and Therapeutics, University of Nairobi, Nairobi, Kenya.
References
- 1.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K. et al. Global disparities of hypertension prevalence and control. A systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi MD, Ayah R, Njau EK, Wanjiru R, Kayima JK, Njeru EK. et al. Prevalence of hypertension and associated cardiovascular risk factors in an urban slum in Nairobi, Kenya: a population-based survey. BMC Public Health. 2014;14:1177. doi: 10.1186/1471-2458-14-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van de Vijver SJ, Oti SO, Agyemang C, Gomez GB, Kyobutungi C. Prevalence, awareness, treatment and control of hypertension among slum dwellers in Nairobi, Kenya. J Hypertens. 2013;31(5):1018–1024. doi: 10.1097/HJH.0b013e32835e3a56. [DOI] [PubMed] [Google Scholar]
- 4.Kenyan Ministry of Health. Kenya STEPwise survey for non-communicable diseases risk factors 2015 report. Nairobi, Kenya. 2015.http://www. health.go.ke/wp-content/uploads/2016/04/Executive-summary-6-2.pdf. Accessed May 31, 2017 [Google Scholar]
- 5.Republic of Kenya. Kenya national strategy for the prevention and control of non-communicable diseases. 2015.http://ilakenya.org/wp-content/ uploads/2015/08/Kenya-national-strategy-for-NCDs-2015-2020.pdf. Accessed May 31, 2017 [Google Scholar]
- 6.Hendriks ME, Wit FW, Roos MT, Brewster LM, Akande TM, de Beer IH. et al. Hypertension in sub-Saharan Africa: cross-sectional surveys in four rural and urban communities. PLoS One. 2012;7(3):e32638. doi: 10.1371/journal.pone.0032638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogola EN, Okello FO, Herr JL, Macgregor-Skinner E, Mulvaney A, Yonga G. Healthy Heart Africa – Kenya: a 12-month prospective evaluation of program impact on health care providers’ knowledge and treatment of hypertension. Global Heart. 2019;14(1):61–70. doi: 10.1016/j.gheart.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Williamson T, Mulaki A. Devolution of Kenya’s health system. The role of Health Policy Project. Health Policy Project, Washington, DC. 2015.https://www.healthpolicyproject.com/pubs/719_KenyaDevolutionBrief. pdf. Accessed May 31, 2017 [Google Scholar]
- 9.Van de Vijver S, Akinyi H, Oti S, Olajide A, Agyemang C, Aboderin I. et al. Status report on hypertension in Africa –consultative review for the 6th Session of the African Union Conference of Ministers of Health on NCDs. Pan Afr Med J. 2013;16:38. doi: 10.11604/pamj.2013.16.38.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloomfield GS, Mwangi A, Chege P, Simiyu CJ, Aswa DF, Odhiambo D. et al. Multiple cardiovascular risk factors in Kenya: evidence from a health and demographic surveillance system using the WHO STEPwise approach to chronic disease risk factor surveillance. Heart. 2013;99(18):1323–1329. doi: 10.1136/heartjnl-2013-303913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temu TM, Bahiru E, Bukachi F, Bloomfield GS, Muiruri P, Farquhar C. Lay beliefs about hypertension among HIV-infected adults in Kenya. Open Heart. 2017;4(1):e000570. doi: 10.1136/openhrt-2016-000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boateng D, Wekesah F, Browne JL, Agyemang C, Agyei-Baffour P, Aikins Ad-G. et al. Knowledge and awareness of and perception towards cardiovascular disease risk in sub-Saharan Africa: a systematic review. PLoS One. 2017;12(12):e0189264. doi: 10.1371/journal.pone.0189264. [DOI] [PMC free article] [PubMed] [Google Scholar]