Abstract
Acetaminophen (APAP) is a widely used analgesic, but also a main cause of acute liver injury in the United States and many western countries. APAP hepatotoxicity is associated with a sterile inflammatory response as shown by the infiltration of neutrophils and monocytes. While the contribution of the immune cells to promote liver repair have been demonstrated, the direct interactions between macrophages or neutrophils with hepatocytes to help facilitate hepatocyte proliferation and tissue repair remain unclear. The purpose of this study was to investigate the relationship between resident macrophages (Kupffer cells) and hepatocytes with a focus on the chemokine receptor CXCR2. C57BL/6J mice were subjected to an APAP overdose (300mg/kg) and the role of CXCR2 on hepatocytes was investigated using a selective antagonist, SB225002. Additionally, clodronate liposomes were used to deplete Kupffer cells to assess changes in CXCR2 expression. Our data showed that CXCR2 was mainly expressed on hepatocytes and it was induced specifically in hepatocytes around the necrotic area 24 hours after APAP treatment. Targeting this receptor using an inhibitor caused a delayed liver recovery. Depletion of Kupffer cells significantly prevented CXCR2 induction on hepatocytes. In vitro and in vivo experiments also demonstrated that Kupffer cells regulate CXCR2 expression and pro-regenerative gene expression in surviving hepatocytes through production of IL-10. Thus, Kupffer cells support the transition of hepatocytes around the area of necrosis to a proliferative state through CXCR2 expression.
Keywords: Acetaminophen hepatotoxicity, chemokine receptors, regeneration, CXCL14, Kupffer cells, IL-10
INTRODUCTION
Acetaminophen (APAP) is a widely used analgesic and antipyretic that is safe at therapeutic doses. However, APAP overdoses can cause severe hepatotoxicity and is a leading cause of acute liver failure (ALF), accounting for approximately 50% of all ALF cases in the United States and for around 30,000 patients being admitted to hospitals every year for treatment of APAP hepatotoxicity (Bernal and Wendon, 2013; Blieden et al., 2014). N-acetylcysteine (NAC) is the only available treatment for APAP overdose patients (Rumack and Bateman, 2012). NAC is most effective when administered within 8 hours of APAP overdose, with early intervention leading to better prognosis (Smilkstein et al., 1988). However, the effectiveness of NAC is questionable for late-presenting patients. Thus, liver transplantation is often required in patients with acute liver failure secondary to APAP hepatotoxicity. While early injury processes after APAP overdose have been extensively characterized (Ramachandran and Jaeschke, 2019), the role of later events, including the innate immune system’s capacity to modulate liver recovery are not well understood. The contribution of the innate immune response to APAP hepatotoxicity has traditionally been contentious (Jaeschke et al., 2012), however, recent evidence has highlighted that innate immune cells are critical for liver repair (Chauhan et al., 2020; Jaeschke and Ramachandran, 2020). Therefore, a therapeutic strategy that can enhance the ability of the innate immune response to initiate and promote cellular recovery would be highly advantageous, particularly for late-presenting APAP overdose patients when NAC treatment is ineffective.
The injury phase of APAP hepatotoxicity is initiated with the metabolism of APAP to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which can be readily detoxified by glutathione (GSH) at therapeutic doses (Mitchell et al., 1973b; Nelson, 1990). However, an APAP overdose depletes hepatocellular GSH and leads to binding of NAPQI to cellular proteins (Mitchell et al., 1973a,b; Jollow et al., 1973, Cohen et al., 1997). Because liver injury correlated with the presence of mitochondrial protein adducts (Tirmenstein and Nelson, 1989; Qiu et al., 2001; Xie et al., 2015) and mitochondrial dysfunction (McGill et al., 2012; Nguyen et al., 2021a,b), it is concluded that mitochondrial adducts are most critical for the toxicity. Protein adducts on mitochondria initiate an oxidant stress in the cytosol (Nguyen et al., 2021b), which triggers a mitogen activated protein kinase cascade ultimately leading to c-jun N-terminal kinase (JNK) activation, translocation of P-JNK to mitochondria and an amplification of the oxidant stress in the mitochondrial matrix (Hanawa et al., 2008; Saito et al., 2010; Nguyen et al., 2021b). This oxidant stress induces the opening of mitochondrial permeability transition pores (Kon et al., 2004; Masubuchi et al., 2005), loss of the membrane potential, release of intermembrane proteins such as endonuclease G and their translocation to the nucleus where they cause DNA fragmentation (Bajt et al., 2006). Together, these signaling events trigger necrosis in hepatocytes (Jaeschke et al., 2019b).
The hepatocyte death due to necrosis releases damage-associated molecular patterns (DAMPs), which activate resident macrophages (Kupffer cells) to produce chemokines MIP-2, MCP-1, IL-8 and KC. IL-8 levels are elevated in APAP overdose patients (Bonkovsky et al., 2018; James et al., 2005a) and MIP-2 and KC levels are increased in mice after an APAP overdose (Lawson et al., 2000; James et al., 2005b). The main function of these chemokines is thought to facilitate infiltration of neutrophils and monocytes into the site of injury.
CXC chemokines are classified into two groups by the presence of a glutamine-leucine-arginine (ELR) motif. CXCR2 is a chemokine receptor for ELR+ chemokines IL-8, MIP-2 and KC, which are expressed by hepatocytes, neutrophils and macrophages in both humans and mice (Eash et al., 2010; Hogaboam et al., 1999a; Van Sweringen et al., 2011). MIP-2 and KC bind to chemokine receptor CXCR2 and play an important role in neutrophil recruitment (Eash et al., 2010; Shuster et al., 1995). Thus, hepatic ischemia-reperfusion injury, which is critically dependent on neutrophil toxicity (Jaeschke, 2003), was attenuated in CXCR2-deficient mice (Kobuki et al., 2008). However, the deficiency of CXCR2 or the use of a CXCR2 antagonist increased hepatocyte proliferation and accelerated liver repair in this model (Kobuki et al., 2008). In contrast, binding of CXC chemokines to CXCR2 on endothelial cells resulted in proliferation and chemotaxis that promoted angiogenesis (Stricter et al., 2005). The role of CXCR2 in APAP hepatotoxicity is also controversial. A CXCR2 antagonist reduced neutrophil recruitment and protected (Marques et al., 2012), which contradicts many studies on the role of neutrophils in this model (Lawson et al., 2000; Cover et al., 2006, Williams et al., 2010). In contrast, treatment with the CXCR2 agonist MIP-2 showed protection against APAP-induced liver injury and accelerated regeneration (Hogaboam et al., 1999a,b). Thus, there is still a gap of knowledge regarding cell signaling that connects the sterile inflammatory response to recovery in APAP hepatotoxicity, especially the crosstalk between the immune cells and hepatocytes and the expression of specific receptors on hepatocytes in this process. Thus, the objective of the current investigation was to evaluate the expression of the CXCR2 on hepatocytes and asses its functional significance in the pathophysiology of APAP hepatotoxicity and repair.
METHODS
Animals.
Eight to ten-week-old male C57BL/6J mice with an average body weight of 20-25g were purchased from Jackson Laboratories (Jackson Lab, Harbor, Maine). All animals were kept in an environmentally controlled room with a 12h light/dark cycle and free access to food and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals.
Experimental design.
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless stated otherwise. Mice were intraperitoneally (i.p.) injected with 300mg/kg APAP (dissolved in warm saline) or saline vehicle after overnight fasting. Animals were refed in all experiments that lasted > 6 hours. The animals were euthanized 24, 48 or 72 hours after APAP treatment. The CXCR2 antagonist, SB225002 (4mg/kg), or 50% DMSO as solvent control were i.p. injected 24 hours after APAP. For experiments with clodronate liposomes, mice were injected with 100μL clodronate liposomes or control liposomes (i.p.) (FormuMax Scientific, Sunnyvale, California). At 72 hours after administration of liposomes, animals were treated with 300mg/kg APAP after overnight fast. The animals were euthanized 24 or 48 hours after APAP treatment. Blood was withdrawn from the vena cava into a heparinized syringe and centrifuged at 18,000g for 3 min to obtain plasma. The liver was removed and rinsed in saline; liver sections were fixed in 10% phosphate buffered formalin or embedded in OCT medium for cryo-sectioning. The remaining parts of the livers were snap frozen in liquid nitrogen.
Biochemical measurements.
Plasma alanine aminotransferase (ALT) activities were measured using an ALT test kit (Point Scientific, Inc, Canton, MI) per the manufacturer’s instruction.
Histology and Immunohistochemistry.
Formalin-fixed mouse tissue samples were embedded in paraffin and 5μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) for evaluation of the areas of necrosis. Additional liver sections were hydrated and went through antigen retrieval by boiling in sodium citrate buffer (pH 6.0). Liver sections were blocked in 3% BSA and serum then incubated with the primary antibodies overnight. Primary antibodies included anti-mouse PCNA (Santa Cruz Biotechnology, cat #sc-56), anti-rabbit p-AKT (Cell Signaling Technology, Danvers, Massachusetts, cat #9271), anti-rabbit MIP-2 (Fisher Scientific, cat #701126), anti-rabbit F4/80 (Cell Signaling Technology, cat #70076S) or rabbit IgG (Cell Signaling Technology, cat# 2729S). Liver sections were washed and incubated in 3% hydrogen peroxide to block endogenous peroxidases. Biotinylated secondary antibody and DAB substrate were used to visualize the signals. Immunofluorescence staining was performed using 5μm cryosections prepared from OCT-embedded tissue. Sections were blocked with 3% BSA and stained with anti-rabbit CXCR2 (Abcam, cat #14935), anti-rat F4/80 (eBioscience, cat #14-4801) and anti-rat Ly6G (Bio Legend, San Diego, CA, cat #127602). Fluorescence conjugated secondary antibodies Alexa Fluor 488 (Invitrogen, cat #A11034) goat anti-rabbit, Alexa Fluor 594 goat anti-rat (Abcam, cat #150160) were used.
Immunocytochemistry.
Primary mouse hepatocytes, isolated as described (Bajt et al., 2004), were plated in a 24-well glass bottom plate in DMEM supplemented with 10% FBS and 1% Glutamax; Kupffer cells were added to some wells after 2 hours with the ratio of 3:1; cell media was then changed to conditioned media. Conditioned media was obtained by culturing primary mouse hepatocytes with 5mM APAP for 15 hours; this was done a day ahead. Twenty hours later, the cells were washed with PBS and fixed in 4% phosphate buffer formalin for 10 min, then washed and blocked with 1% BSA for 30 min. Primary antibodies CXCR2 and F4/80 were incubated overnight. Fluorescent secondary antibody was used for detection.
Western blotting.
Snap frozen tissue was homogenized in a CHAPs containing protein buffer and total protein was measured using the BCA assay (Pierce Scientific, Waltham, Massachusetts). Gel electrophoresis was carried out on protein lysates from individual samples, which were then transferred to a nitrocellulose blot. Densitometry was performed to quantitatively assess differences using ImageJ software. In brief, densitometry was performed serially on blots and normalized to the loading control, β-actin. Antibodies to total p-AKT (cat #9271 ), AKT (cat #4691) and β-actin (cat #4970) were purchased from Cell Signaling Technologies. Horseradish peroxidase-coupled anti-mouse or anti-rabbit IgG was used as the secondary antibody. Proteins were visualized by enhanced chemiluminescence (Amersham Biosciences, Inc, Piscataway, New Jersey).
Isolation of hepatic parenchymal cells.
Hepatocytes were isolated from mice with a 2-step collagenase perfusion technique as described previously (Bajt et al., 2004). Cell viability was more than 80% based on trypan blue exclusion, and cell purity was more than 95%. Cells were plated in William E medium (Life Technologies, Grand Island, NY) containing 100 U/ml penicillin/streptomycin, and 10% fetal bovine serum. After 3 hours, the cells were attached to the bottom of the wells or glass dish, and cells were washed with PBS and changed to 5mM APAP-containing medium.
Isolation of non-parenchymal cells.
NPC were isolated using a protocol adapted from a method described by Watanabe et al. (1992). Under isoflurane anesthesia mice were exsanguinated from the caudal vena cava into heparinized tubes and the blood was placed on ice. The liver was immediately excised, placed in ice-cold PBS and minced with scissors. The tissue was then pressed through a 200-gauge stainless steel mesh into a 50 mL conical tube. The cell suspension was centrifuged at 50 ×g for 2 min to remove hepatocytes and large debris. The supernatant containing non-parenchymal cells was then centrifuged at 350 ×g for 5 min and cells were passed through a 40 μm nylon screen and washed twice in a 15 mL conical bottom tube. Viable, nucleated cells were counted by trypan blue exclusion and brought to a uniform cell density.
Cell staining.
Viable hepatocytes were collected using a method adapted from Ben-Moshe et al. (2019). In details, isolated hepatocytes were resuspended in 25mL EBSS then mixed with 22.5ml Percoll (Sigma) and 2.5mL 10xPBS to remove dead cells. The mixture was centrifuged at 600rpm for 10 minutes, supernatant was discarded, and the cells were resuspended in cold FACS buffer (2mM EDTA, 10% FBS in 1x PBS). 100μl of 106 cells were aliquot into FACS tubes and .25μg FcX blocking solution (Biolegend) was added. Cells were incubated on ice for 30 minutes. Next, 2ml of FACS buffer was added to each tube, and centrifuged at 1,000rpm for 3 minutes to wash the cells. Cells were resuspended in 100μl FACS buffer and stained with APC-CD31(BioLegend, cat: 102509), PE/Cy5-CD45 (BioLegend, cat: 103109), APC/Cy7-CXCR2 (BioLegend, cat: 149313) in a dilution of 1:100.
Flow cytometric analysis.
Fc receptor blocking antibody (BioLegend, San Diego, CA) diluted in 1% BSA in PBS was added to 100μL non-parenchymal cell suspension for 30 min on ice. To 100μL FcR-blocked cell suspension, saturating concentration of PerCP-CD45 (BioLegend), PE-CD11b (BioLegend), and FITC-F4/80 (BioLegend) diluted in 1% BSA in PBS were added. Samples were incubated in the dark for 30 min. Pellets were washed three times after incubation with 1% BSA in PBS and measured by flow cytometry (FACSCalibur, BD, Franklin Lakes, NJ). Aqua Zombie (BioLegend) was added to assess cell viability. Mouse monocytes were gated on CD45+CD11b+F4/80+ cells. Data were analyzed using FlowJo-V10.
Flow cytometry and cell sorting.
Cells were sorted by BD FACSAria lllu. DAPI was added to collect viable cells. Hepatocytes were gated by plotting FSC-A and FSC-W plot, viable cells gated by DAPI negative cells compared to unstained cells, hepatocytes were selected by removing CD31 (marker for endothelial cells) and CD45 (marker for NPCs). We then selected APC/Cy7 intensity for CXCR2. The CXCR2hi hepatocytes were collected into 5ml 10% FBS in DMEM media.
RNA sequencing.
(GSE183161) Twenty thousand sorted hepatocytes were collected for library preparation. RNA was extracted using TRIZOL reagent. RNA quantity and integrity were determined using the Agilent TapeStation 4200 using the RNA ScreenTape Assay kit (Agilent Technologies 5067-5576). Libraries were constructed utilizing the Universal plus mRNA-seq with NuQuant library preparation kit (Tecan Genomics 0520-A01) according to the manufacturer’s protocol. Paired-end sequencing was performed using the Illumina NovaSeq 6000 Sequencing System at the KUMC genomics core. Reads were aligned to the mm10 genome with HISAT2, indexed with Samtools, and a count matrix generated using Subread. Differentially expressed genes were determined using the DESeq2 package in R version 4.0.2. Genes with a Log2FC greater than 4 or less than −4 were used as inputs into Metascape (Zhou et al., 2019) for pathway analysis.
Total RNA isolation and real-time PCR analysis.
Total RNA was extracted from liver tissues using TRIZOL reagent (Invitrogen) according to manufacturer’s instructions. RNA (1μg) was reverse-transcribed into cDNA using a High-capacity cDNA Reverse Transcription kit (Applied Biosystems). mRNA expression levels were measured using rt-PCR with power SYBR green master mix (Applied Biosystems). The mRNA level of β-actin was used as an internal control, and each sample was done in duplicate. The relative differences in expression between groups were expressed using cycle time (Ct) values generated by the CFX384 instrument (Bio-Rad). Calculations are made by assuming 1 cycle is equivalent to a 2-fold difference in copy number which is the 2^(−ddCt) formula.
Statistics
All results were expressed as mean ± SE. Comparisons between multiple groups were performed with one-way ANOVA or, where appropriate, by two-way ANOVA, followed by a post hoc Bonferroni test. If the data were not normally distributed, we used the Kruskal–Wallis Test (nonparametric ANOVA) followed by Dunn's Multiple Comparisons Test. P < 0.05 was considered significant.
RESULTS
Induction of CXCR2 in the recovery phase after APAP overdose.
To investigate CXCR2 expression after APAP-induced liver injury, mice were administered an overdose of APAP (300mg/kg) and liver tissues were collected after 24-72 hours. Significant induction of CXCR2 expression was evident in cells around the necrotic area at 24 hours post APAP, which was sustained at 48 hours and decreased slightly by 72 hours (Figure 1 A). Negative and isotype controls were included to assess the reliability of the antibodies used (Suppl. Figure 1B). Examination of liver regeneration along this time course revealed a significant upregulation of proliferating nuclear antigen (PCNA), a marker of liver regeneration, in a similar population of cells around the necrotic area (Figure 1B). This suggests that APAP overdose induces an upregulation of CXCR2 on surviving hepatocytes around the centrilobular area by 24 hours, and these cells also have increased PCNA expression. However, CXCR2 is also known to be expressed on other cell types such as endothelial cells as well as immune cells such as neutrophils and macrophages which are present in the liver at this time after APAP (Addison et al., 2000; Stillie et al., 2009). To confirm the identity of cells showing expression of CXCR2, we separated hepatocytes from the nonparenchymal cell (NPC) fraction and probed for CXCR2 expression by Western blotting. To prevent equal protein loading from masking detection of any CXCR2 expression in the mixed cell types from the NPC fraction, we loaded approximately 3 times more protein in the NPC lanes to compensate. Despite this, it is evident from Figure 1C that CXCR2 expression is restricted to the hepatocyte fraction, indicating that this upregulation was independent of infiltrating immune cells. This is further reiterated in low (Figure 1D) and high magnification images (Suppl. Figure 1A) co-stained for CXCR2 as well as neutrophils (Ly6G) or macrophages (F4/80) at 24 and 48 hours post APAP. There was no significant colocalization of the probes (Figure 1D, Suppl. Figure 1A) indicating that increase in CXCR2 expression was not within these immune cells. Additional experiments evaluating colocalization of endothelial cells using the CD31 marker and CXCR2 also showed no overlap (Suppl. Figure 1C). Taken together, these data support the conclusion that the elevation in CXCR2 expression seen between 24 and 48 hours after an APAP overdose was on the surviving hepatocytes around the area of necrosis, the same population showing elevated PCNA expression indicative of cell proliferation.
Figure 1: CXCR2 expression after an overdose of APAP.
Mice were treated with 300 mg/kg APAP and the tissues were collected after 24, 48 and 72 hours. Immunohistochemical staining for CXCR2 (A) and PCNA (B) in liver sections of untreated controls and APAP-treated animals. (C) Detection of CXCR2 and β-actin by western blotting from the non-parenchymal cell (NPC) fraction and the hepatocyte fraction isolated from control and APAP-treated mice. (D) Colocalization of CXCR2 with F4/80 (macrophages) or Ly6G (neutrophils) in liver sections obtained 24 and 48 hours after APAP administration. (*=central vein).
CXCR2 expression is required for hepatocytes recovery and regeneration after APAP.
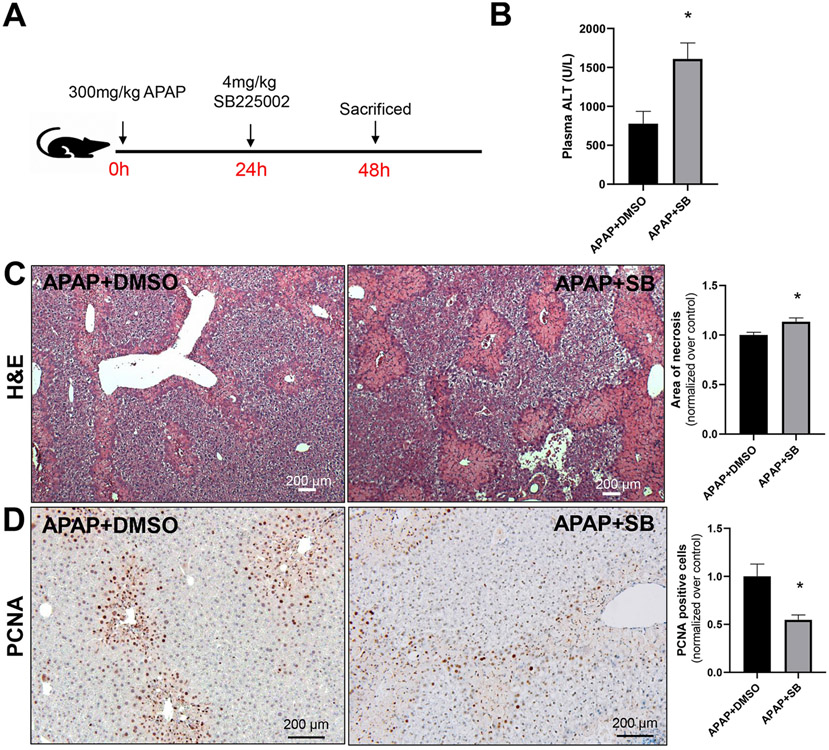
To further explore the role of CXCR2, we utilized the potent and selective CXCR2 antagonist SB225002 (White et al., 1998). Since our data indicated that CXCR2 was induced strongly on hepatocytes with no noticeable expression on immune cells at 24 hours after APAP treatment, systemic administration of the antagonist would selectively influence hepatocyte CXCR2 expression. Mice were treated with 4 mg/kg of the CXCR2 antagonist SB225002 24 hours after APAP overdose at which time there would be no interference with APAP metabolism or injury and CXCR2 expression was upregulated (Figure 2A). Examination of liver injury by measuring plasma ALT activities at 48 hours post APAP showed significant recovery in control mice with moderately elevated ALT levels (Figure 2B). Interestingly, mice treated with SB225002 showed a delay in liver recovery with significantly higher ALT levels at 48 hours (Figure 2B). This was accompanied by a delay in shrinking of necrotic areas evident in H&E-stained liver sections (Figure 2C). These data suggest a delay in liver recovery in mice that were treated with the CXCR2 antagonist. In addition, expression of PCNA, a marker for liver regeneration, was also significantly decreased in mice with inhibition of CXCR2 (Figure 2D).
Figure 2: Importance of CXCR2 for liver recovery after APAP overdose.
(A) Animals were treated with APAP (300 mg/kg) and 24 hours later with the selective CXCR2 antagonist, SB225002 (4 mg/kg) or vehicle (DMSO). Plasma and liver tissues were collected 48 hours after APAP injection. (B) Plasma ALT activities; (C) representative H&E-stained liver sections and quantitation of the areas of necrosis; (D) immunohistochemical detection of PCNA-positive hepatocytes with quantifications. Bars represent means ± SE of n=5 animals per group. *P<0.05 (compared to APAP/DMSO).
Kupffer cells mediate CXCR2 expression on hepatocytes.
Immune cell infiltration after APAP hepatotoxicity is an important prerequisite for liver recovery and regeneration, and Kupffer cells have been shown to play an important role in this process (Ju et al., 2002; You et al., 2013). Closer examination of the liver sections co-stained with CXCR2 and either Ly6G or F4/80 showed that F4/80 positive Kupffer cells were in close proximity to hepatocytes, unlike neutrophils which were located within the necrotic area (Figure 1D, Suppl. Figure 1A). This raised the possibility that Kupffer cells influence CXCR2 induction through production of chemokines or cytokines. To target Kupffer cells and test this hypothesis, we depleted Kupffer cells using liposomal clodronate three days prior to administration of an APAP overdose. As predicted, macrophage depleted animals had significantly higher ALT levels after 24 hours, and liver recovery was delayed at 48 hours after APAP overdose (Figure 3A). Immunohistochemistry of F4/80 (Figure 3B) indicated that depletion of Kupffer cells also delayed immune cell infiltration such that very few bone marrow-derived macrophages were evident at 24 hours post APAP unlike mice treated with control liposomes. This effect seems to be due to the lack of resident Kupffer cells and not circulating monocytes, since evaluation of circulating monocytes 3 days after clodronate treatment and prior to APAP administration showed no change, unlike hepatic Kupffer cells, which were evidently depleted (Suppl. Figure 2). It should be noted, however, that a role for other immune cells, such as dendritic cells in this response cannot be entirely ruled out. Notably, however, macrophage (F4/80+) staining recovered by 48 hours in both groups. Furthermore, there was a significant reduction of CXCR2, especially in the cells around the necrotic area, by 24 hours after the APAP overdose, the time point when Kupffer cells were depleted (Figure 3C,D). Interestingly, CXCR2 expression recovered by 48 hours, by which time macrophage recruitment was also evident (Figure 3C,D). This suggests that Kupffer cells were required for elevated CXCR2 expression in this hepatocyte population. Since macrophages are a known source of IL-1β, TGF-β, TNF-α, MIP-2, KC and IL-10 especially after liver injury (Roth et al., 2020), we then examined putative mediators which could be involved. Our data indicated that no significant change was evident in the level of TGF-β though other cytokines like KC, IL-1β and IL-10 showed a significant decrease in mice with Kupffer cell depletion in whole liver samples (Figure 3E), indicating that any of these mediators could be playing a role in the Kupffer cell-hepatocyte crosstalk. Interestingly, we also observed a significant elevation of the CXCR2 ligand, MIP-2 in livers of animals with Kupffer cell depletion (Suppl. Figure 3A) which was localized to hepatocytes (Suppl. Figure 3B). Because a MIP-2 gradient is involved in neutrophil recruitment by 6 hours after APAP, lack of MIP-2 in Kupffer cell-depleted animals delayed neutrophil recruitment to the site of necrosis by 24 hours after APAP (Suppl. Figure 3C). Taken together, these data indicate that upregulation of CXCR2 on surviving hepatocytes around the necrotic area after APAP hepatotoxicity was dependent on secretion of cytokines by Kupffer cells. Identification of the mediators involved was the focus of the next series of in vitro and in vivo experiments.
Figure 3: Kupffer cell depletion prevents APAP-induced CXCR2 expression and delays monocyte recruitment.
Mice were treated with clodronate- or empty liposomes 3 days before 300 mg/kg APAP administration. Plasma and liver tissues were collected 24 or 48 hours after APAP injection. (A) Plasma ALT activities; (B) immunohistochemical detection of F4/80-positive macrophages in liver sections; (C) immunohistochemical detection of CXCR2 in liver sections; (D) quantification of hepatic CXCR2 expression; (E) hepatic mRNA expression of selected cytokines and chemokines 24 hours after APAP treatment in mice pretreated with control- or clodronate liposomes. Bars represent means ± SE of n=5 animals per group. *P<0.05 (compared to APAP/control liposomes). (*=central vein).
IL-10 secreted from Kupffer cells is critical for CXCR2 upregulation on surviving hepatocytes to initiate liver recovery.
To further investigate the role of Kupffer cells in mediating CXCR2 expression on hepatocytes, we performed in vitro experiments using Kupffer cells isolated from control mice. To better mimic the in vivo environment, we activated isolated Kupffer cells with conditioned media from primary mouse hepatocytes treated with 5mM APAP for 15 hours. Our data indicate that these Kupffer cells showed a significant increase in IL-10 mRNA expression level compared to cells not exposed to conditioned media (Figure 4A). Co-culture of these activated Kupffer cells with hepatocytes significantly increased hepatocyte CXCR2 expression (Figure 4B). Furthermore, treatment of primary mouse hepatocytes with recombinant IL-10 also increased CXCR2 expression (Figure 4C). Our data indicate that activated Kupffer cells produce IL-10, which is a key factor in the induction of CXCR2 expression on surviving hepatocytes necessary for proliferation and recovery. We hypothesized that if IL-10 was the mediator from Kupffer cells inducing CXCR2 expression on hepatocytes, direct administration of IL-10 should rescue CXCR2 expression in Kupffer cell-depleted animals. Thus, to confirm the role of IL-10 in vivo, we treated Kupffer cell-depleted mice with 2μg IL-10 at 10 hours after APAP overdose and examined CXCR2 expression at 24 hours after APAP. As seen in Figure 4D, IL-10 treatment rescued CXCR2 expression in Kupffer cell-depleted animals confirming the role of this mediator in vivo. The identification of IL-10 as the important cytokine for the induction of CXCR2 selectively in surviving hepatocytes around the area of necrosis now allowed us to move on to the next step; namely what pathways were upregulated in these cells as consequence of CXCR2 overexpression? Since these hepatocytes were a selective population and thus measurements in the whole liver had limited utility, we used a cell sorting approach to explore this.
Figure 4: Kupffer cells secrete IL-10 to induce CXCR2 expression on hepatocytes.
(A) Cytokine mRNA expression in Kupffer cells isolated from mice that have been activated by conditioned media (CM) for 15 hours. The condition medium was obtained after treating hepatocytes with 5mM APAP for 15 hours. Bars represent means ± SE of n=3 separate incubations. *P<0.05 (compared to Kupffer cells without conditioned media) (B) Co-culture of hepatocytes exposed to CM in the presence or absence of Kupffer cells for 24 hours. Cells were stained for CXCR2 (green), F4/80 (red) and nucleus (blue). (C) Expression of CXCR2 on hepatocytes after exposure to 10ng/mL IL-10 for 24 hours (evaluated by western blotting and its densitometric quantitation). (D) Expression of CXCR2 in livers of Kupffer cell-depleted mice, i.e. clodronate treatment 3 days before exposure to 300 mg/kg APAP. One group was treated with 2μg of recombinant IL-10 at 10 hours after APAP. Livers were obtained 24 hours after APAP treatment and liver sections were stained with CXCR2 (red) and nucleus (blue). (*=central vein).
Surviving hepatocytes around the area of necrosis which showed elevation of CXCR2 expression include genes involved in cell proliferation, chemotaxis and ECM remodeling.
Previous reports have indicated that the response of hepatocytes to CXCR2 activation is dependent on ligand concentrations, with low concentrations of MIP-2 promoting proliferation, whereas high concentrations induced cell injury (Wilson et al., 2015). The contradictory effects of MIP-2 in hepatic ischemia-reperfusion injury (Kuboki et al., 2008; Lentsch et al., 1998) and APAP hepatotoxicity (Hogaboam et al., 1999a,b) might be due to the level of ligand as well as different role of CXCR2 expression on immune cells or hepatocytes. Since CXCR2 was expressed on the hepatocyte cell surface and its expression was selectively elevated in cells around the area of necrosis by 24 hours after APAP, we used FACS sorting to collect CXCR2hi hepatocytes 24 hours after APAP overdose (Figure 5A). To enhance hepatocyte purity, we used CD31 as endothelial cells marker and CD45 as non-parenchymal cell marker during FACS sorting to eliminate these cells (Suppl. Figure 4A). Our RNAseq data of the collected CXCR2hi hepatocyte population showed a distinct upregulation of genes involved in cell proliferation, chemotaxis (Figure 5B) and ECM remodeling (Suppl. Figure 4B), which confirmed that selective upregulation of CXCR2 on surviving hepatocytes around the area of necrosis induced their proliferation, tissue reconstruction and localization of immune cells for liver recovery. Interestingly, CXCL14 was among the genes significantly elevated in this cell population (Figure 5C). Since CXCL14 is an important chemokine that regulates cell chemotaxis, angiogenesis, and differentiation (Lu et al., 2016), its production in these CXCR2 overexpressing hepatocytes is likely to be important in the context of recovery after APAP-induced liver injury. CXCR2 is known to activate PI3K for chemotaxis, adhesion, and granule release in neutrophils (Thelen and Didichenko, 1997), and ART is the primary mediator of PI3K-initiated signaling that promotes cell cycle progression and cell survival through activation of a variety of factors (Chang et al., 2003). Examination of AKT phosphorylation in liver tissue sections 24 and 48 hours after APAP treatment indicated that positive cells are in the border of the necrotic area, which suggests an overlap with CXCR2 expression after APAP overdose (Figure 6A). Immunohistochemistry of CXCL14 also demonstrated a localization restricted to the necrotic area (Figure 6A). The level of CXCL14 was decreased in the CXCR2 antagonist treatment group (Figure 6B,C), and AKT phosphorylation revealed a significant decrease at 48h post APAP in mice where CXCR2 was inhibited (Figure 6B,C). The role of hepatocyte AKT in downstream signaling after CXCR2 activation was further confirmed in vitro, where treatment of primary mouse hepatocytes with 10ng/mL of the CXCR2 ligand MIP-2 showed similar increase in AKT phosphorylation (Figure 6D). Together with our in vivo data, this suggests that the downstream signaling of CXCR2 in hepatocytes involves activation of the AKT pathway to produce CXCL14 and other mediators. Though we treated mice with CXCR2 antagonist at 24 hours after APAP to minimize any interference with immune cell recruitment, there was a partial reduction in the number of neutrophils, as indicated by staining for Ly6G in liver sections, with no significant changes in number of macrophages (F4/80) (Figure 7A,B). Collectively, our data indicate that CXCR2 expression on hepatocytes during the recovery phase after APAP is important for mediating liver recovery through Akt signaling and CXCL14 secretion.
Figure 5: Transcriptomic analysis of CXCR2hi hepatocytes around the necrotic areas.
(A) Illustration of the necrotic areas with the layers of CXCR2hi hepatocytes. (B) Gene enrichment pathways from CXCR2hi hepatocytes isolated from control animals and mice treated with 300 mg/kg APAP for 24 hours. (C) Chemotaxis gene profiles of CXCR2hi hepatocytes isolated from control animals and mice treated with 300 mg/kg APAP for 24 hours. CXCL14 (arrow) was one of the most induced genes of this category. Adjusted p-value is obtained by comparing with control samples with n=3 for each group.
Figure 6: Downstream signaling of CXCR2 on hepatocytes involves the AKT pathway.
(A) Immunohistochemical detection of p-AKT and CXCL14 in liver tissue from animals treated with 300 mg/kg APAP. (B) Western blots from whole liver tissues with quantifications (C); the data represent means ± SE of n=3 samples per group; *P<0.05 (versus DMSO). Samples were obtained from control animals and mice treated with 300 mg/kg APAP for 48 hours and the selective CXCR2 antagonist SB225002 or its vehicle DMSO at 24 hours after APAP. (D) Western blots from primary mouse hepatocytes treated with 5mM APAP with or without 10ng/ml MIP-2 for 24 hours. (*=central vein).
Figure 7: Effect of the CXCR2 antagonist SB225002 on hepatic neutrophil and macrophage localization.
(A) Immunohistochemical detection of F4/80-positive macrophages and Ly6G-positive neutrophils in livers of mice treated with 300 mg/kg APAP for 48 hours. Groups of mice were treated with the selective CXCR2 antagonist SB225002 or the vehicle DMSO 24 hours after APAP treatment. (B) Quantification of inflammatory cells in liver sections from n=5 animals per group using ImageJ. Bars represent mean ± SE. *P<0.05 (compared to APAP-DMSO). (*=central vein).
DISCUSSION
The objective of the current study was to determine the functional role of the chemokine receptor CXCR2 on hepatocytes, and the contribution of Kupffer cells in modulating CXCR2 expression during the recovery phase of APAP hepatotoxicity. Using different approaches to target CXCR2 receptors including treatment with a selective antagonist and specific CXCR2hi hepatocyte cell sorting, we confirmed the important role of surviving hepatocytes that expressed high level of CXCR2 in liver repair and Kupffer cells releasing IL-10 to induce CXCR2 on hepatocytes.
CXCR2 expression pattern on hepatocytes after APAP overdose.
CXCR2 is a chemokine receptor that is typically expressed on neutrophils and is essential for neutrophil mobilization from the bone marrow to the blood and recruitment from the blood to the site of injury (Eash et al., 2010). CXCR2 is also expressed on endothelial cells and is responsible for angiogenesis (Addison et al., 2000). Its role on hepatocytes has been demonstrated under several pathophysiological conditions when signaling through CXCR2 can directly affect hepatocyte viability (Allen et al., 2011; Dorman et al., 2005; Kuboki et al., 2008; Ren et al., 2003). In APAP-induced liver injury, we found that CXCR2 was induced on hepatocytes around the necrotic area 24 hours after APAP overdose, and the level of induction gradually increased until the shrinkage of the necrotic area was complete. Though neutrophils, macrophages and endothelial cells can also express this receptor, our data indicated that CXCR2 is absent on these liver cells of control and APAP-treated mice after 24 hours. Therefore, we were able to target primarily hepatocytes using the potent and highly selective CXCR2 antagonist SB225002 (White et al., 1998) at 24 hours post APAP, which caused a delay in both ALT reduction and shrinkage of necrotic areas, suggesting that CXCR2 contributes to liver recovery after APAP overdose.
IL-10 modulates CXCR2 induction on hepatocytes.
Kupffer cells initiate the inflammatory response by releasing chemotactic mediators, which are necessary for neutrophil and monocyte recruitment into the liver. Production of inflammatory mediators such as TNF-α also influence chemokine and adhesion molecules expression in the liver (Jaeschke and Hasegawa, 2006; Lentsch et al., 2000). Though the number of Kupffer cells were reduced, and the liver is occupied more by monocyte-derived macrophages 24 hours after APAP (Dambach et al., 2002; Holt et al., 2008), a significant population of Kupffer cells are always present throughout the time course of APAP-induced liver injury. Kupffer cells have been shown to directly influence other cells in the liver including hepatocytes and neutrophils in various pathological states (Calvente et al., 2019; Yang et al., 2019). In this study, we used clodronate liposomes to deplete Kupffer cells (van Rooijen and Hendrikx, 2010). However, it has been shown that clodronate liposome treatment can deplete other phagocytes besides Kupffer cells including monocytes and dendritic cells (Moreno, 2018; Kozicky and Sly, 2019). Our data confirmed the depletion of Kupffer cells 3 days after treatment but did not show a significant effect on circulating monocytes. Nevertheless, an effect on other immune cells such as dendritic cells cannot be completely ruled out. Thus, our conclusions that we have mainly a Kupffer cell-mediated effect has to be considered with this caveat in mind. Based on these clodronate liposome data we were able to show that CXCR2 induction on hepatocytes was dependent on the presence of Kupffer cells. Previous studies using the same approaches have indicated that Kupffer cell depletion reduced IL-10 expression and aggravated APAP-induced liver injury (Ju et al., 2002). The absence of IL-10 in whole body IL-10-deficient mice enhanced pro-inflammatory cytokine formation by macrophages during the injury phase, which upregulated inducible nitric oxide synthase in hepatocytes to further amplify the cell death mechanisms (Bourdi et al., 2002). Since IL-10 receptors are also expressed on hepatocytes, we hypothesized that an effect of IL-10 secretion by Kupffer cells could be to induce CXCR2 on hepatocytes during the repair phase. Our data clearly support this concept as condition medium from APAP-treated hepatocytes triggered IL-10 gene transcription in isolated Kupffer cells and Kupffer cell-hepatocyte co-cultures induced CXCR2. Importantly, this process was confirmed in vivo when Kupffer cell-depleted animals failed to upregulate CXCR2, and exogenous IL-10 treatment was able to again induce CXCR2 on hepatocytes.
Surviving hepatocytes influence immune cells to facilitate liver repair.
Liver repair processes involve hepatocyte proliferation, and reconstruction of extracellular matrix and the vasculature (Bhushan and Apte, 2019; Mehendale, 2005; Michalopoulos and Bhushan, 2021). APAP hepatotoxicity induces cell death by necrosis in the area around the central vein (Gujral et al, 2002). Previous studies have demonstrated that mitochondrial biogenesis is active in hepatocytes around the necrotic area and these hepatocytes are also proliferating to replace dead cells (Du et al., 2017; Jaeschke et al., 2019a). Here we showed novel transcriptomic analysis of the proliferating hepatocytes around the necrotic area that also induced CXCR2. Since these hepatocytes are the closest to the necrotic area, they also contributed to extracellular remodeling by upregulation of genes such as Nid2, Col3a1 and MMP23. Additionally, immune cells like neutrophils and macrophages stay mainly within the necrotic area, and they are also key factors for proper recovery after APAP overdose (Calvente et al., 2019; Dambach et al., 2002; Holt et al., 2008; Young et al., 2013). Thus, chemotaxis of these immune cells to the necrotic area is critical. While MCP-1 is certainly a key factor for monocyte recruitment (Dambach et al., 2002; Holt et al., 2008), our RNAseq data demonstrated that these surviving hepatocytes also control immune cell localization by upregulation of chemotactic cytokines like CXCL14.
In summary, our study demonstrated an important link between Kupffer cell activation and the expression and function of the chemokine receptor CXCR2 on hepatocytes following APAP overdose (Figure 8). CXCR2 expression is regulated by IL-10 released from Kupffer cells. In addition, the CXCR2hi hepatocytes around the area of necrosis induce the expression of pro-regenerative genes and generate other chemotactic factor such as CXCL14 to further promote inflammatory cell recruitment into the area of necrosis. Thus, Kupffer cells support the transition of hepatocytes around the area of necrosis to a proliferative state and at the same time assist in recruiting inflammatory cells to remove necrotic cell debris. These data are particularly intriguing because they show a connection between various innate immune cells and hepatocytes to contribute to liver recovery through CXCR2.
Figure 8: Kupffer cells activate hepatocyte recovery by IL-10 mediated induction of CXCR2 after APAP-induced liver injury.
APAP-induced hepatocyte necrosis causes release of DAMPs, which result in infiltration of monocyte-derived macrophages and activation of resident Kupffer cells to secrete IL-10, which selectively activates CXCR2 expression on surviving hepatocytes around the area of necrosis. These IL-10 activated cells then undergo proliferation accompanied by release of additional cytokines such as CXCL14. This figure includes templates from Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 generic license (https://smart.servier.com).
Supplementary Material
ACKNOWLEDGEMENT
We acknowledge the Flow Cytometry Core Laboratory, which is sponsored, in part, by the NIH/NIGMS COBRE grant P30 GM103326
FUNDING
This work was funded in part by a grant from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01 DK102142 and DK125465, and National Institute of General Medicine (NIGMS) funded Liver Disease COBRE grants P20 GM103549 and P30 GM118247.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher's embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature's terms of use for AM versions of subscription articles: www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM (2000) The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol 165:5269–77. [DOI] [PubMed] [Google Scholar]
- Allen K, Jaeschke H, Copple BL (2011) Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol 178:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H (2006) Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci 94:217–25. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Jaeschke H (2001) Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol 281:G1188–95. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H (2004) Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci 80:343–9. [DOI] [PubMed] [Google Scholar]
- Ben-Moshe S, Shapira Y, Moor AE, Manco R, Veg T, Bahar Halpern K, Itzkovitz S (2019) Spatial sorting enables comprehensive characterization of liver zonation. Nat. Metab 1, 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal W, Wendon J (2013) Acute liver failure. N Engl J Med 369:2525–34. [DOI] [PubMed] [Google Scholar]
- Blieden M, Paramore LC, Shah D, Ben-Joseph R (2014) A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States. Expert Rev Clin Pharmacol 7:341–8. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL, Barnhart HX, Foureau DM, Steuerwald N, Lee WM, Gu J, Fontana RJ, Hayashi PJ, Chalasani N, Navarro VM, Odin J, Stolz A, Watkins PB, Serrano J; US Drug-Induced Liver Injury Network and the Acute Liver Failure Study Group (2018) Cytokine profiles in acute liver injury-Results from the US Drug-Induced Liver Injury Network (DILIN) and the Acute Liver Failure Study Group. PLoS One 13:e0206389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR (2002) Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 35:289–98. [DOI] [PubMed] [Google Scholar]
- Bhushan B, Apte U (2019) Liver Regeneration after Acetaminophen Hepatotoxicity: Mechanisms and Therapeutic Opportunities. Am J Pathol 189:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvente CJ, Tameda M, Johnson CD, Del Pilar H, Lin YC, Adronikou N, De Mollerat Du Jeu X, Llorente C, Boyer J, Feldstein AE (2019) Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. J Clin Invest 129:4091–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA (2003) Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 17:590–603. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Sheriff L, Hussain MT, Webb GJ, Patten DA, Shepherd EL, Shaw R, Weston CJ, Haidar D, Bourke S, Bhandari R, Watson S, Adams DH, Watson SP, Lalor PF (2020) The platelet receptor CLEC-2 blocks neutrophil mediated hepatic recovery in acetaminophen induced acute liver failure. Nat Commun 11:1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA (1997) Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol 143:1–12. [DOI] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H (2006) Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 216:98–107. [DOI] [PubMed] [Google Scholar]
- Dorman RB, Gujral JS, Bajt ML, Farhood A, Jaeschke H (2005) Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am J Physiol Gastrointest Liver Physiol 288:G880–6. [DOI] [PubMed] [Google Scholar]
- Du K, Ramachandran A, McGill MR, Mansouri A, Asselah T, Farhood A, Woolbright BL, Ding WX, Jaeschke H (2017) Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol 108:339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC (2010) CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest 120:2423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H (2002) Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67:322–8. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N (2008) Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 283:13565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Bone-Larson CL, Steinhauser ML, Lukacs NW, Colletti LM, Simpson KJ, Stricter RM, Kunkel SL (1999a) Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J 13:1565–74. [DOI] [PubMed] [Google Scholar]
- Hogaboam CM, Simpson KJ, Chensue SW, Steinhauser ML, Lukacs NW, Gauldie J, Stricter RM, Kunkel SL (1999b) Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther 6:573–84. [DOI] [PubMed] [Google Scholar]
- Holt MP, Cheng L, Ju C (2008) Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol 84:1410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H (2003) Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 284:G15–26. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Duan L, Nguyen N, Ramachandran A (2019a) Mitochondrial Damage and Biogenesis in Acetaminophen-induced Liver Injury. Liver Res 3:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Hasegawa T (2006) Role of neutrophils in acute inflammatory liver injury. Liver Int, 26:912–9. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Ramachandran A (2020) Mechanisms and pathophysiological significance of sterile inflammation during acetaminophen hepatotoxicity. Food Chem Toxicol 138:111240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Ramachandran A, Chao X, Ding WX (2019b) Emerging and established modes of cell death during acetaminophen-induced liver injury. Arch Toxicol 93:3491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML (2012) Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int 32:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, Kurten RC, Lamps LW, McCullough S, Hinson JA (2005a) Tumour necrosis factor receptor 1 and hepatocyte regeneration in acetaminophen toxicity: a kinetic study of proliferating cell nuclear antigen and cytokine expression. Basic Clin Pharmacol Toxicol 97:8–14. [DOI] [PubMed] [Google Scholar]
- James LP, Simpson PM, Farrar HC, Kearns GL, Wasserman GS, Blumer JL, Reed MD, Sullivan JE, Hinson JA (2005b) Cytokines and toxicity in acetaminophen overdose. J Clin Pharmacol 45:1165–71. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB (1973) Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 187:195–202. [PubMed] [Google Scholar]
- Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR (2002) Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol 15:1504–13. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ (2004) Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40:1170–9. [DOI] [PubMed] [Google Scholar]
- Kozicky LK, Sly LM (2019) Depletion and Reconstitution of Macrophages in Mice. Methods Mol Biol 1960:101–12. [DOI] [PubMed] [Google Scholar]
- Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Edwards MJ, Lentsch AB (2008) Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology 48:1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JA Farhood A, Hopper RD, Bajt ML, Jaeschke H (2000) The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci 54:509–16. [DOI] [PubMed] [Google Scholar]
- Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ (2000) Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 32:169–73. [DOI] [PubMed] [Google Scholar]
- Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ (1998) Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology 27:1172–7. [DOI] [PubMed] [Google Scholar]
- Lu J, Chatterjee M, Schmid H, Beck S, Gawaz M (2016) CXCL14 as an emerging immune and inflammatory modulator. J Inflamm (Lond) 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, Lopes GA, Russo RC, Avila TV, Melgaço JG, Oliveira AG, Pinto MA, Lima CX, De Paula AM, Cara DC, Leite MF, Teixeira MM, Menezes GB (2012) Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56:1971–82. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T (2005) Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol 42:110–6. [DOI] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H (2012) Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol 264:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehendale HM (2005) Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol 33:41–51. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, Bhushan B (2021) Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol 18:40–55. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB (1973a) Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther 187:185–94. [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB (1973b) Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187:211–7. [PubMed] [Google Scholar]
- Moreno SG. Depleting Macrophages In Vivo with Clodronate-Liposomes. (2018) Methods Mol Biol 1784:259–62. [DOI] [PubMed] [Google Scholar]
- Nelson SD (1990) Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis 10:267–78. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Akakpo JY, Weemhoff JL, Ramachandran A, Ding WX, Jaeschke H (2021a) Impaired protein adduct removal following repeat administration of subtoxic doses of acetaminophen enhances liver injury in fed mice. Arch Toxicol 95:1463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NT, Du K, Akakpo JY, Umbaugh DS, Jaeschke H, Ramachandran A (2021b) Mitochondrial protein adduct and superoxide generation are prerequisites for early activation of c-jun N-terminal kinase within the cytosol after an acetaminophen overdose in mice. Toxicol Lett 338:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL (1998) Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem 273:17940–53. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H (2019) Acetaminophen Hepatotoxicity. Semin Liver Dis 39:221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Carpenter A, Hogaboam C, Colletti L (2003) Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Pathol 163:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth K, Strickland J, Copple BL (2020) Regulation of macrophage activation in the liver after acute injury: Role of the fibrinolytic system. World J Gastroenterol 26:1879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumack BH, Bateman DN (2012) Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol (Phila) 50:91–8. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H (2010) c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 246:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster DE, Kehrli ME Jr, Ackermann MR (1995) Neutrophilia in mice that lack the murine IL-8 receptor homolog. Science 269:1590–1. [DOI] [PubMed] [Google Scholar]
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH (1988) Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 319:1557–62. [DOI] [PubMed] [Google Scholar]
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW (2009) The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol 86:529–43. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP (2005) CXC chemokines in angiogenesis. Cytokine Growth Factor Rev 16:593–609. [DOI] [PubMed] [Google Scholar]
- Thelen M, Didichenko SA (1997) G-protein coupled receptor-mediated activation of PI 3-kinase in neutrophils. AnnN Y Acad Sci 832:368–82. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD (1989) Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem 264:9814–9. [PubMed] [Google Scholar]
- Van Sweringen HL, Sakai N, Tevar AD, Burns JM, Edwards MJ, Lentsch AB (2011) CXC chemokine signaling in the liver: impact on repair and regeneration. Hepatology 54:1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N, Hendrikx E (2010) Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol 605:189–203. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Ohtsuka K, Kimura M, Ikarashi Y, Ohmori K, Kusumi A, Ohteki T, Seki S, Abo T (1992) Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods 146:145–54. [DOI] [PubMed] [Google Scholar]
- White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM (1998) Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem 273:10095–8. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H (2010) Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD 18-deficient mice. Liver Int 30:1280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GC, Kuboki S, Freeman CM, Nojima H, Schuster RM, Edwards MJ, Lentsch AB (2015) CXC chemokines function as a rheostat for hepatocyte proliferation and liver regeneration. PLoS One 10:e0120092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Du K, Dorko K, Kumer SC, Schmitt TM, Ding WX, Jaeschke H (2015) Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol Appl Pharmacol 289:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Tao Y, Wu Y, Zhao X, Ye W, Zhao D, Fu L, Tian C, Yang J, He F, Tang L (2019) Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat Commun 10:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Q, Holt M, Yin H, Li G, Hu CJ, Ju C (2013) Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem Pharmacol 86:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.