Summary
Aim
The aim of this study was to evaluate the temporal relationship between mitochondrial oxidative phosphorylation and mitophagy in rat hearts subjected to ischaemia/reperfusion. Measurements were made at specific points during the experimental protocol (snapshot approach) and by assessments of mitophagic flux, using chloroquine pre-treatment.
Methods
Isolated working rat hearts were subjected to 25 or 30 minutes of global ischaemia/10 minutes of reperfusion. Half of each group received chloroquine (10 mg/kg, intraperitoneally) one hour before experimentation. Mitochondria were isolated after stabilisation, ischaemia and reperfusion, and oxidative phosphorylation was measured polarographically. Mitochondrial mitophagy markers were detected by Western blot analysis.
Results
Mitochondrial oxygen uptake (state 3) and oxidative phosphorylation rate were reduced by ischaemia and increased by reperfusion. Chloroquine pre-treatment increased both parameters. Using a snapshot approach, exposure to ischaemia ± reperfusion had little effect on mitochondrial PINK1, Parkin and p62/SQSTM1 expression. Ischaemia reduced Rab9 expression, and reperfusion upregulated the phospho DRP1, phospho/total DRP1 ratio and Rab9 levels. Chloroquine significantly reduced PINK1, p62/SQSTM1, Rab9 and particularly Parkin expression during reperfusion, without an effect on mitochondrial total and phospho DRP1 levels.
Conclusion:
Ischaemia/reperfusion-induced changes in mitochondrial oxidative phosphorylation function occurred concomitantly with changes in mitophagic flux. Pre-treatment with chloroquine profoundly affected mitochondrial function as well as the pattern of mitophagy during ischaemia/reperfusion.
Keywords: myocardial ischaemia/reperfusion, mitophagy, mitochondrial oxidative phosphorylation, PINK1, Parkin, p62/ SQSTMI, DRP1, Rab9
The mitochondrion has emerged as an important role player in cardiomyocyte survival/death via the permeability transition pore and its capacity to generate free radicals. The deleterious effects of myocardial ischaemia/reperfusion on mitochondrial oxidative phosphorylation function are also well established and attributed to oxidative stress.1-5 Insufficient oxygen delivery to the ischaemic heart leaves the mitochondria in a highly reduced state, causing increased leakage of electrons from the electron transport chain. Re-introduction of oxygen during reperfusion greatly increases this electron leakage.5
Mitophagy refers to the selective removal of dysfunctional mitochondria by autophagosomes. This process is triggered by mitochondrial depolarisation and the generation of reactive oxygen species.6,7 The PINK1/Parkin pathway is the bestcharacterised pathway for mitophagic quality control,8-10 with the autophagic adaptor p62/SQSTM1 being essential for the clearance of damaged mitochondria: p62/SQSTM1 acts as an autophagosomal cargo receptor for ubiquitinated proteins, which are degraded in the autolysosome.8
Direct and indirect evidence however support the existence of alternative mechanisms of mitophagy.9,11 For example, a recent study described an alternative pathway for mitophagy mediated by a protein complex consisting of unc-51-like kinase 1 (Ulk1), Rab9, receptor-interacting serine/threonine protein kinase 1 (Rip1) and dynamin-related protein 1 (DRP1) and it was suggested that this process could be the predominant form of mitophagy in cardiomyocytes during stress.11 Their data also suggest that Ulk1/Rab9-dependent mitophagy targets depolarised mitochondria using a mechanism distinct from that of PINK1/Parkin-dependent mitophagy.
Interpretation of experimental autophagic/mitophagic changes in the setting of ischaemia/reperfusion may present difficulties.12 In a recent article, Gottlieb and co-workers reviewed current methods to measure these processes, indicating that static levels (‘snapshot measurements’) of parameters of autophagy during an experimental protocol and Western blot analysis alone are not sufficient for the evaluation of these events without the direct assessment of flux. Failure to measure flux has resulted in controversial results and much confusion about the significance of autophagy.12
Autophagy is a dynamic process beginning with the induction of autophagosome formation and ending with its degradation in lysosomes.12-14 Evaluation of flux can be done experimentally through lysosomal blockade, or indirectly, inferred from changes in the expression of p62/SQSTM1. Chloroquine has routinely been used for evaluation of autophagic flux: the drug disrupts autophagy by inhibiting the acidification of lysosomes that fuse with the autophagosomes and thereby rescues p62/SQSTM1 breakdown,15,16 indicating flux rather than steady-state levels. Therefore it was advised that Western blots should be paired with the same measurement in the presence of lysosomal blockade with chloroquine or bafilomycin A.12 However, little is known about the effects of chloroquine per se on the mitochondrial mitophagy process in myocardial ischaemia/reperfusion.
As far as we are aware, the temporal relationship between changes in mitochondrial oxidative phosphorylation function and mitophagy during exposure of the heart to ischaemia/ reperfusion injury is still largely unexplored. The aim of this study was to gain more insight into the mitochondrial oxidative phosphorylation processes as well as mitophagy in hearts of rats at the end of an ischaemic episode and after a period of reperfusion. To allow assessment of autophagic flux, an additional series of experiments was performed in rats pre-treated with chloroquine before subjecting the hearts to ischaemia/ reperfusion ex vivo.
Interpretation of the results obtained could be complicated by the fact that chloroquine per se is known to have cardiotoxic effects at both therapeutic and high doses, especially when administered rapidly, including cardiovascular effects such as vasodilation, hypotension, suppressed mechanical function and cardiac arrhythmias.17,18 These negative effects on myocardial function could affect the response of the heart to ischaemia/ reperfusion and therefore the autophagy/mitophagy process.
To evaluate the use of chloroquine as indicator of mitophagic flux in myocardial ischaemia/reperfusion, it was necessary to establish its effects on myocardial function before induction of ischaemia/reperfusion. In this study, rats were therefore treated with a low dose of chloroquine before experimentation and its effects were assessed on myocardial as well as mitochondrial function and mitophagy in a well-characterised ex vivo model of ischaemia/reperfusion. Such an approach would allow evaluation of chloroquine effects on myocardial as well as mitochondrial function and mitophagy after exposure of the heart to ischaemia/reperfusion.
Methods
Male Wistar rats weighing 230 ± 10 g were used for this study. They had free access to food and water and were kept on a 12-hour day/night cycle in the Central Research Facility of the Faculty of Health Sciences of the University of Stellenbosch. This study was approved by the Committee for Ethical Animal Research of the Faculty of Health Sciences, University of Stellenbosch. The study conformed to the revised South African National Standard for the Care and Use of Animals for Scientific Purposes (South African Bureau of Standards, SANS 10386, 2008).
The experimental protocol followed is summarised in Fig. 1. Rats were divided into two groups, an untreated control and a chloroquine-treated group. One hour before initiation of experimentation, the rats were weighed and the latter group was treated with chloroquine (10 mg/kg, intraperitoneally). The control untreated rats received an equal volume of distilled water, intraperitoneally. Chloroquine was freshly prepared every day (10 mg/ml distilled H2O). Rats were anaesthetised by intraperitoneal injection of sodium pentobarbitone (160 mg/kg). After removal, the hearts were perfused as described below for subsequent preparation of mitochondria.
Fig. 1.

Experimental protocol. Mitochondria were prepared after 40 minutes of stabilisation; after 25 minutes of global ischaemia; after 10 minutes of reperfusion following 25 minutes of global ischaemia; after 30 minutes of global ischaemia; after 10 minutes of reperfusion following 30 minutes of global ischaemia.
The hearts were perfused with modified Krebs-Henseleit bicarbonate buffer (KHB) containing (in mM): NaCl 119, NaHCO3 24.9, KCl 4.7, KH2PO4 1.2, MgSO4.7H2O 0.59, Na2SO4 0.59, CaCl2.H2O 1.25 and glucose 10. KHB was oxygenated and kept at pH 7.4 by gassing with 95% O2/5% CO2 at 37°C. After removal, the hearts were arrested in ice-cold saline, mounted onto the aortic cannula and the left atrium was cannulated via the pulmonary vein.
Hearts were then stabilised for 40 minutes [10 minutes retrograde, followed by 20 minutes working mode (preload 15 cm H2O, afterload 100 cm H2O) and 10 minutes retrograde perfusion]. Perfused hearts were allowed to beat spontaneously and peak systolic pressure was recorded using a Statham pressure transducer (Transpac IV, Abbotts, Sligo, Ireland), which was inserted in the aortic cannula. Pressure signals were recorded in 10-second pulses and analysed using software developed by the University of Stellenbosch Electronic Department.
After stabilisation, hearts were subjected to 25 or 30 minutes of global ischaemia, followed by 10 minutes of reperfusion. Myocardial temperature was thermostatically controlled by inserting a temperature probe into the pulmonary artery. The temperature was monitored at regular intervals and kept at 36.5°C during ischaemia. Measurements of function were heart rate (beats per min), aortic output (AO) (ml/min), cardiac output (CO: coronary flow + aortic output) (ml/min), aortic pressure (PAO) and work total (mW). Work total was calculated as described by Kannengieser et al:19
Work total = 0.00222(PAO – 11.25)(CO) For isolation of subsarcolemmal mitochondria, at the end of the stabilisation, ischaemic or reperfusion periods as described above, the hearts were plunged into ice-cold KE medium (0.18 M KCl/0.01 M EDTA, pH adjusted to 7.4 with Tris base) and homogenised with a Polytron PT 10 homogenizer (4 × 4 seconds, 4°C, setting 2). The mitochondria were isolated by differential centrifugation, as described by Sordahl et al.20 The mitochondrial pellet was divided into two: one half was dispersed in KE medium for immediate measurement of mitochondrial function, while the other half was dissolved in lysis buffer (see below) and stored at –80°C for subsequent Western blot analysis. Protein determination for mitochondrial functional studies was done with the technique of Lowry and co-workers.21
Immediately after preparation, subsarcolemmal mitochondrial oxidative phosphorylation (oxphos) was measured polarographically at 27°C using an oxygraph (Hansatech Instruments, Bannan UK) and Clark electrode. The mitochondrial incubation medium contained (in mM): sucrose 0.25, Tris-HCl 10, pH 7.4, K2HPO4 8.5 and glutamate 5/malate 2 or palmitoyl-L-carnitine 0.45/malate 2 as substrates (pH 7.4). ADP (250–350 nmoles) was added to initiate state 3 respiration.
Parameters investigated were the ADP/O ratio, state 3 respiration (mitochondrial respiration in the presence of ADP) and state 4 respiration (mitochondrial respiration in the absence of ADP). Mitochondrial respiratory rates (states 3 and 4) were expressed as nAtoms oxygen uptake/mg protein/min. The respiratory control index (RCI) was calculated according to the following formula: state 3/state 4 respiration.
The amount of ADP added to the incubation system was obtained spectrophotometrically (molar extinction coefficient of ADP: 15.4 at 259 nm). The oxidative phosphorylation rates (nmoles ATP produced/mg protein/min) were calculated as follows: QO2 (state 3) × ADP/O ratio.
To evaluate the ability of the isolated mitochondria to withstand oxidative stress, mitochondrial preparations were also exposed to 20 minutes of anoxia in the oxygraph chamber, followed by six minutes of re-oxygenation, as described by Essop and co-workers.22 Recovery of respiratory function (state 3) after these interventions was calculated as a percentage of the respiratory rate (state 3) before exposure of the mitochondria to anoxia. Mitochondrial preparations from five to six hearts were studied in each group.
For Western blot technique, an aliquot of the mitochondrial pellet was homogenised in 200 μl of lysis buffer containing (in mM): Tris-HCl 20, p-nitrophenyl phosphate 20, EGTA 1, EDTA 1, NaCl 150, tetra-sodium-pyrophosphate 2.5, β-glycerophosphate 1, sodium orthovanadate 1, phenylmethyl sulphonyl fluoride (PMSF) 1, aprotinin 10 μg/ml and leupeptin 10 μg/ml, Triton-X100 1%, pH 7.4 using a Bullet BlenderR (Next Advance Inc, USA) at 4°C for five minutes with a scoop of 0.15 mm zirconium oxide beads equivalent to the pellet size. Samples were then microfuged at 15 000 rpm for 10 minutes to obtain the supernatant, the protein content of which was determined using the Bradford technique.23
The lysates were adjusted accordingly by dilution with lysis buffer to an equal protein concentration, followed by Laemmli sample buffer and boiled for five minutes. Depending on the protein of interest, 30 to 60 μg were loaded and separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) using the standard Bio-Rad Criterion system. The running buffer contained (in mM): Tris 25, glycine 192 and sodium dodecyl sulphate (SDS) 1%.
After separation, the proteins were transferred to a PVDF membrane (ImmobilonTM P, Millipore) using wet electrotransfer. The transfer buffer contained (in mM): Tris-HCl 25, glycine 192, and methanol (20% v/v). Non-specific binding sites on the membranes were blocked with 5% fat-free milk in TBST (Trisbuffered saline + 0.1% Tween 20) for one to two hours at room temperature. After washing with TBST (five by five minutes), membranes were incubated overnight at 4°C with the primary antibodies.
The primary antibodies were diluted in TBST or in 5% fat-free milk in TBST solution. After overnight incubation, membranes were washed with TBST (five by five minutes) and then incubated for one hour at room temperature, with a diluted horseradish peroxidase-labelled secondary antibody (Cell Signaling Technology®). The secondary antibody was either diluted in TBST or in 5% fat-free milk/TBST solution. The following primary antibodies were used: TOM 70 (Santa Cruz, Dallas Tex, USA), PINK1, p62/SQSTM1, Rab9, total (t) and phospho (p) DRP1 (Cell Signaling, Danvers, MA, USA), Parkin (Abcam, Cambridge UK). After thorough washing with TBST, membranes were covered with ECL (enhanced chemiluminescence) detection reagents (Bio-Rad Clarity) and quantified using a ChemiDoc-XRS imager (Bio-Rad).
Twenty-six-well CriterionTM 4–20% pre-cast gradient gels and stain-free technology were used throughout the study. With stain-free technology, the transferred proteins on the PVDF membrane can be visualised in the ChemiDoc to confirm equal loading. Furthermore, the intensity of bands detected by the ECL reaction are normalised to the total proteins that were transferred in each lane, negating the use of a loading control. Four samples/group were included on the same gel plus a sample prepared from a heart of an unperfused age-matched control animal to act as standard for normalisation of all data.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5 software (GraphPad Software, Inc). Comparisons between the groups were performed using one-way ANOVA. If two groups were compared, the unpaired Student’s t-test was used. A p-value ≤ 0.05 was deemed as statistically significant.
Results
Myocardial function
Baseline function of perfused working hearts from untreated and chloroquine-treated rats was monitored over a period of 40 minutes without any interventions. No differences in heart rate, coronary flow and peak systolic pressure were observed between the two groups. However, chloroquine pre-treatment caused a slight but statistically significant reduction in aortic output, cardiac output and work total performed (Fig 2). Since hearts were freeze-clamped after 10 minutes of reperfusion, which was too short for measurement of working heart function, functional recovery during reperfusion could not be evaluated.
Mitochondrial function
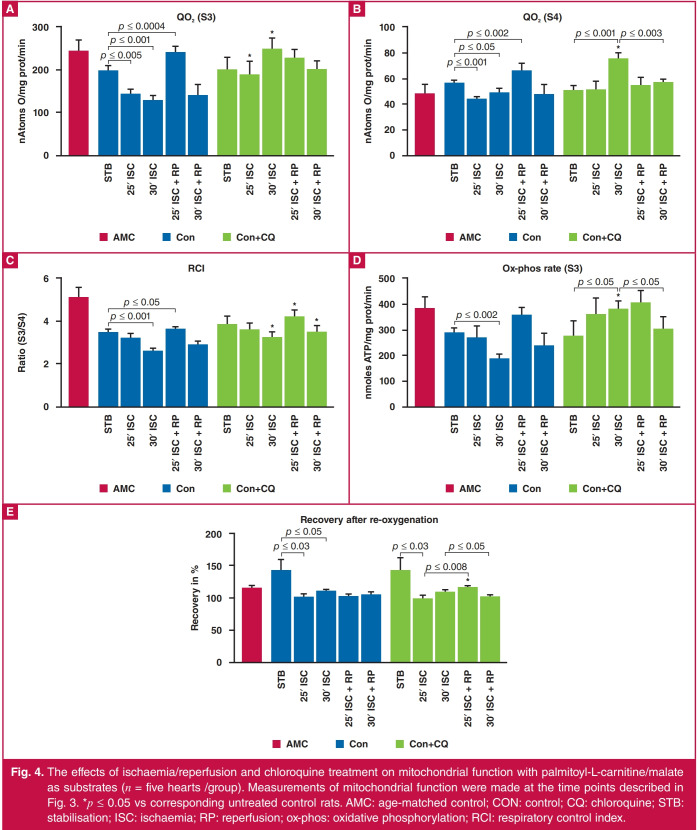
Figs 3 and 4 show the effect of ischaemia/reperfusion on mitochondrial function with glutamate/malate and palmitoyl carnitine/malate, respectively, as substrates. The effects of ischaemia and reperfusion follow a very similar pattern on mitochondrial oxygen uptake state 3 and the ox-phos rate (ADP/O ratio × QO2 state 3). With both substrates, exposure of the isolated heart to 25 or 30 minutes of global ischaemia resulted in a reduction in mitochondrial oxygen uptake as well as ox-phos rate (state 3) compared to stabilisation; with glutamate/ malate as substrates, the change became significant after 30 minutes only, whereas with palmitoyl-L carnitine/malate the reduction in QO2 (state 3) and ox-phos rate was significant after both 25 and 30 minutes of ischaemia.
There was a tendency for these two parameters to increase with reperfusion: with glutamate/malate, the increases observed after both 25 and 30 minutes ischaemia/reperfusion were not significant. With palmitoyl-L carnitine/malate as substrate, the increase in QO2 (state 3) (but not in ox-phos rate) after 25 minutes of ischaemia/reperfusion was significant.
Interestingly, with glutamate as substrate, exposure of the hearts to 25 or 30 minutes of ischaemia with or without reperfusion had no significant effects on state 4 respiration. On the other hand with palmitoyl-L carnitine as substrate, QO2 state (state 4) was significantly reduced by ischaemia, and increased by reperfusion after 25 minutes of ischaemia.
With glutamate/malate as substrates, a reduction in the RCI values after 25 and 30 minutes of ischaemia and an increase after reperfusion, respectively, were observed. Similar tendencies were observed when palmitoyl-L carnitine/malate were used as substrates.
Pre-treatment with chloroquine had the most marked effects on mitochondrial function of hearts exposed to 30 minutes of ischaemia. With both substrates, pre-treatment with chloroquine caused a significant increase in QO2, states 3 and 4. Similarly, chloroquine treatment prior to exposure to 25 or 30 minutes of ischaemia increased the ox-phos rate. With glutamate/malate as well as palmitoyl carnitine/malate as substrates, the changes were significant after 30 minutes of ischaemia, while the chloroquineinduced increases seen after reperfusion were not significant. In accordance with its effects on QO2 (state 3), the RCI values obtained after 30 minutes of ischaemia were significantly increased when incubated with both substrate combinations.
The ability of mitochondria isolated after stabilisation, ischaemia or reperfusion to withstand oxidative stress was further evaluated by exposing the mitochondria in the oxygraph chamber to anoxia followed by re-oxygenation (Figs 3 and 4). Interestingly, with both substrates, mitochondria isolated after the stabilisation phase showed an increase in the percentage state 3 recovery, compared with the values obtained before exposure to anoxia, while mitochondria isolated after exposure to ischaemia with or without reperfusion showed an almost 100% recovery in QO2 state 3, with no differences between the groups. Similar tendencies were observed in the chloroquine-treated hearts, the only difference being that chloroquine treatment caused an increase in state 3 recovery in mitochondria from hearts reperfused after 25 minutes of ischaemia. This was seen with both substrates.
Mitophagy
Fig. 5A depicts the expression of TOM70, p62, PINK1 and Parkin in mitochondria isolated from rat hearts (with and without chloroquine pre-treatment) at different times during the perfusion protocol. To normalise the data, the same mitochondrial sample isolated from an unperfused heart was included in all Western blots. Interestingly, the stabilisation period of 40 minutes significantly reduced and increased the expression of TOM70 and PINK1, respectively, while not affecting p62 and Parkin. A similar stabilisation-induced reduction in total and phosphorylated DRP1 was observed, in contrast with an increase in Rab9 levels.
Using the snapshot approach, the perfusion protocol had very little effect on the expression of PINK1, Parkin and p62/ SQSTM1; the only significant change being a reduction in the expression of TOM70 at reperfusion, compared to stabilisation. The alternative pathway showed more significant changes: exposure to ischaemia reduced the expression of Rab9, while reperfusion upregulated its levels as well as the pDRP1 and p/t DRP1 ratio (Fig. 5B).
Effects of chloroquine pre-treatment on the mitophagic process in rat hearts exposed to ischaemia and reperfusion are shown in Figs 5A and B. Although chloroquine had no effect on the levels of PINK1, Parkin and p62/SQSTM1, as well as TOM70 after 40 minutes of stabilisation, compared with untreated controls, it markedly increased PINK1 levels after ischaemia, while reducing the expression of PINK1, p62/ SQSTM1 and particularly Parkin during reperfusion, with no effect on TOM70.
Chloroquine had no effect on the levels of mitochondrial total and pDRP1 but caused a reduction in the p/tDRP1 ratio after stabilisation, while not having an effect after ischaemia/ reperfusion, when compared to its untreated counterparts. A marked inhibitory effect during reperfusion was also seen in the expression of Rab9.
Discussion
The aims of this study were: (1) to assess the temporal relationship between ischaemia/reperfusion-induced changes in mitochondrial function and mitophagy (steady state and flux), (2) to evaluate mitophagy by comparing snapshot measurements at specific times during the perfusion protocol with mitophagic flux, obtained by pre-treatment of the experimental animals with chloroquine, as suggested by Gottlieb et al.,12 and (3) to evaluate the appropriateness of chloroquine use in this regard.
Of paramount importance in studies aimed at evaluation of autophagic flux is the presence of the drug at all times throughout the protocol. Chloroquine has been administered one to four hours before experimentation,24-27 a rather long period, which could lead to loss of drug effects. Another approach could be to add chloroquine directly to the perfusate of the isolated rat heart.28 In view of the results obtained by Ma, Zhang and co-workers,24,25 we decided to use a time period of one hour between administration of chloroquine and onset of ischaemia. The marked effects observed during reperfusion after ischaemia (Fig. 4) led us to believe that chloroquine still exerted its effects in the isolated heart after a total perfusion period of 75 to 80 minutes.
Chloroquine (9-aminoquinoline) is an old drug, known for its anti-malarial, anti-rheumatic and immunomodulatory effects. Although cardiac side effects of chloroquine have rarely been reported, it could be severe and irreversible (for review see reference 18). In addition, chloroquine has been shown to protect against ischaemia/reperfusion damage in the heart29,30 and liver31 via inhibition of phospholipase A, preventing phospholipid breakdown. It also is a known inhibitor of autophagy: it disrupts autophagy by inhibiting the acidification of lysosomes that fuse with autophagosomes,15,16 which forms the basis of its use for the study of autophagic flux. These multiple effects of chloroquine could indeed affect the response of the heart to ischaemia/reperfusion injury, mitochondrial function and thus the mitophagic process, apart from its direct effects on the autophagosomal and lysosomal interaction.
Interestingly, in the present study, hearts from rats pre-treated with chloroquine exhibited a slight but significant reduction in aortic and cardiac output (as measured 60 minutes after injection). However the inhibitory effects of chloroquine on myocardial function observed in the present study were rather small but significant (Fig. 2) and unlikely to affect the outcome of the results.
Fig. 2.
Baseline function of working rat hearts during stabilisation: effect of chloroquine pre-treatment (n = 10 hearts/group). CON: control; CQ: chloroquine pre-treatment (10 mg/kg); HR: heart rate (beats/min); W total: work total (mW).
Unfortunately functional recovery during reperfusion could not be assessed in our working heart model, since hearts were freeze-clamped after 10 minutes of reperfusion only, when working heart measurements could not yet be done. However, other studies from our laboratory32 showed that pre-treatment (one hour) with chloroquine had no effect on the ischaemiainduced infarction after 35 minutes of regional ischaemia/120 minutes of reperfusion. It also was without effect on functional recovery during reperfusion after 20 minutes of global ischaemia, suggesting that the changes in mitochondrial function and mitophagy observed in the present study were not caused by the effects of chloroquine on function.
Mitochondrial function after ischaemia/reperfusion
Subsarcolemmal mitochondria were used for the purpose of this study. As expected, exposure of the hearts to ischaemia/ reperfusion had marked effects on the parameters of oxidative phosphorylation, regardless of the substrate used. Chloroquine pre-treatment increased mitochondrial QO2 states 3 and 4, the ox-phos rate and RCI of mitochondria isolated after 30 minutes of global ischaemia in particular, while having relatively little effect on mitochondrial behaviour during reperfusion (Figs 3, 4).
Fig. 3.
Effects of ischaemia/reperfusion and chloroquine pre-treatment on mitochondrial function with glutamate/malate as substrates (n = 5 hearts/group). Measurements of mitochondrial function were made after 40 minutes of stabilisation; after 25 minutes of global ischaemia; after 10 minutes of reperfusion following 25 minutes of global ischaemia; after 30 minutes of global ischaemia; after 10 minutes of reperfusion following 30 minutes of global ischaemia. Mitochondria were also prepared from hearts of age-matched control rats for comparison purposes. A. QO2 (state 3) (nAtoms oxygen/mg protein/min); B. QO2 (state 4) (nAtoms oxygen/mg protein/min); C. RCI (state 3/state 4); D. ox-phos rate (nmoles ATP/mg prot/min); E. percentage recovery after re-oxygenation. *p ≤ 0.05 vs corresponding untreated control rats. AMC: age-matched control; CON: control; CQ: chloroquine; STB: stabilisation; ISC: ischaemia; RP: reperfusion; ox-phos: oxidative phosphorylation; RCI: respiratory control index.
Fig. 4.
The effects of ischaemia/reperfusion and chloroquine treatment on mitochondrial function with palmitoyl-L-carnitine/malate as substrates (n = five hearts /group). Measurements of mitochondrial function were made at the time points described in Fig. 3. *p ≤ 0.05 vs corresponding untreated control rats. AMC: age-matched control; CON: control; CQ: chloroquine; STB: stabilisation; ISC: ischaemia; RP: reperfusion; ox-phos: oxidative phosphorylation; RCI: respiratory control index.
The effects of ischaemia/reperfusion on mitochondrial function of the isolated rat heart model are well established: exposure to a relatively short period of ischaemia is characterised by metabolic, ultrastructural and functional changes.33
Inactivation of mitochondrial respiratory complexes during ischaemia is known to be time dependent, progressive and heterogeneous: a reduction in mitochondrial state 3 is known to occur in ischaemic hearts from rats and rabbits (see for example, references 34–36). As was also observed in the present study, reperfusion after an ischaemic incident is associated with improvement in subsarcolemmal mitochondrial ox-phos rate.33,35 Interestingly, mitochondrial oxygen uptake (state 4) after reperfusion appeared to be higher with palmitoyl-L-carnitine/ malate as substrates, and may indicate a degree of uncoupling in the presence of fatty acids in the incubation medium.
Chloroquine pre-treatment resulted in an upregulation in state 3 respiration after exposure of the hearts to 25–30 minutes of global ischaemia (Figs 3, 4). This may be due to inhibition of phospholipase A, but this remains to be determied. In contrast, in vivo treatment with anti-malarials (chloroquine, primaquine and quinine) adversely affected oxidative energy metabolism in rat liver mitochondria, namely a marked depression in states 3 and 4 respiration rates, while these drugs also had uncoupling effects on sites II and III phosphorylation.37
High-dose chloroquine has been shown to be metabolically cardiotoxic by inducing lysosomal and mitochondrial dysfunction in a rat model of pressure overload hypertrophy.38 It has also been reported that the negative inotropic and chronotropic effects of chloroquine on isolated perfused rabbit hearts were due to a reduction in mitochondrial calcium binding and accumulation.39 These negative effects of chloroquine on mitochondrial function are in contrast with those observed in the present study and may be due to differences in dosage and experimental conditions.
Mitophagy in ischaemia/reperfusion
Mitophagy, responsible for the degradation and recycling of damaged mitochondria, is a dynamic cellular process from the formation of autophagosome, autophagosome–lysosome fusion to final degradation of mitochondria and has been shown to be essential for cardioprotection under both physiological and pathophysiological conditions (for review see reference 40). It is also generally accepted that the PINK1/Parkin mitophagy pathway is a major mitochondrial quality-control mechanism and critical for maintenance of function at baseline in adult hearts.41
As far as we are aware, the temporal relationship between ischaemia/reperfusion-induced changes in mitochondrial ox-phos function and mitophagy has received little attention. It is well established that mitochondrial depolarisation induced by various stimuli is a common trigger for mitophagy. In the present study, markers of mitophagy were evaluated in mitochondria isolated from perfused hearts after stabilisation, exposure to ischaemia alone as well as after reperfusion following ischaemia. Mitochondrial PINK1, Parkin, p62/SQSTM1 and TOM70 expression were used in the present study as indicators of mitophagy in view of the fact that Parkin-mediated mitophagy required loss of mitochondrial membrane potential.42,43 According to Gottlieb and co-workers,12 p62/SOSTM1 expression can be regarded as a useful marker of autophagy: it is a polyubiquitinbinding protein that is degraded by autophagy and its protein levels are inversely related to autophagic activity.44,45 Therefore accumulation of p62/SQSTM1 would be indicative of impaired autophagosome clearance in the case of a snapshot approach.
There are pitfalls however in determining autophagic activity based on snapshot measurements only during an experimental protocol,12 since static levels give an incomplete indication of autophagy without assessment of flux. The interruption of autophagy by chloroquine-induced inhibition of the acidification of lysosomes that fuse with autophagosomes15,16 will rescue p62/ SQSTM1. Therefore, increased accumulation of this marker in the presence of chloroquine will be indicative of increased autophagic flux.
Evaluation of the temporal relationship between mitochondrial ox-phos and mitophagy was initially done using snapshot measurements made at different times during the ischaemia/ reperfusion protocol. In view of the fact that depolarisation of the mitochondrial membrane leads to activation of mitophagy as well as the gross ultrastructural changes visible after exposure of the perfused heart to 25 minutes of global ischaemia,33 changes in the PINK1/Parkin pathway were expected. However, apart from a reduction in TOM70 expression during ischaemia/reperfusion, no significant changes in mitochondrial PINK1, Parkin and p62/ SQSTM1 expression were observed throughout the perfusion protocol, suggesting that removal of damaged mitochondria by the mitophagic process probably occurs at a later stage.
Based on these assumptions, it was concluded that the changes in mitochondrial oxidative phosphorylation caused by 25 minutes of ischaemia are not yet associated with measureable changes in the PINK1/Parkin mitophagy pathway. In view of the role of TOM70 in the import of PINK1 into mitochondria,46 it is possible that changes in TOM70 precede the mitophagic process.
In contrast with our findings, increases in mitophagy after exposure to ischaemia/reperfusion were reported in many studies using a longer period of ischaemia. For example, increased expression of PINK1 and Parkin was reported in mouse (30 minutes of ischaemia/two hours of reperfusion)47 and rat hearts (30 minutes of regional ischaemia/two hours of reperfusion).48 Short episodes of ischaemia/reperfusion (three minutes of ischaemia/three minutes of reperfusion) have also been shown to translocate Parkin from the cytosol to the mitochondria.49 This suggests that the perfusion protocol may have an important effect on the mitophagy process. It should be kept in mind that these studies were done in the absence of chloroquine.
Pre-treatment with chloroquine had a profound effect on the parameters studied: not only did the drug influence the expression of a number of markers of mitophagy when compared to their corresponding untreated counterparts, but it also affected the pattern of the response to ischaemia and reperfusion. Apart from a significant increase in PINK1 levels after ischaemia, the most significant changes induced by chloroquine occurred during the reperfusion period: the reduction in PINK1, Parkin and p62/SQSTM1 levels observed at this stage suggested a downregulation of mitophagic flux during reperfusion. In contrast, the significant upregulation of PINK1 levels during ischaemia may be indicative of increased mitophagy occurring at this stage. Therefore, based on flux measurements after chloroquine treatment, it appears that the changes in mitochondrial oxidative phosphorylation function induced by exposure of hearts to 25 minutes of global ischaemia coincides with changes in mitophagic flux (in contrast with using snapshot evaluation of events).
The possibility of chloroquine wash-out during reperfusion accounting for the reduction in expression of PINK1, Parkin, p62/SQSTMI and Rab 9 is unlikely, since one would expect values to be similar to those obtained in the absence of treatment. In addition, our results are in agreement with those obtained by Ma and co-workers,13 namely that the increase in p62/SQSTM1 in chloroquine-treated hearts subjected to 30 minute of ischaemia/90 minutes of reperfusion was not affected by chloroquine treatment, indicating impaired mitophagic flux under these conditions.
In addition to the conventional markers of mitophagy, the effects of ischaemia/reperfusion as well as chloroquine pre-treatment were also evaluated by determining their effects on mitochondrial fission, as indicated by expression and phosphorylation of the DRP1. DRP1, the master mediator of fission, is located in the cytosol and when activated, translocates to the mitochondrial outer membrane where it interacts with other proteins such as human fission factor (Fis 1), and mitochondrial fission factor (MFF) (for a review see references 8 and 9). Once at the outer mitochondrial membrane, DRP1 mediates mitochondrial fragmentation and loss of membrane potential, and facilitates release of cytochrome C.50
Our results show that while the expression of total mitochondrial DRP1 was not changed by the ischaemia/ reperfusion protocol, its phosphorylation was increased, especially after reperfusion, causing a significant increase in the pDRP1/tDRP1 ratio at this stage. Chloroquine had no significant effect on mitochondrial fission during ischaemia/ reperfusion, as reflected by the pattern of unchanged DRP1 levels during the ischaemia/reperfusion protocol, apart from a reduction in pDRP1/tDRP1 ratio after stabilisation.
Interestingly, in the presence of chloroquine, the very significant reduction in the expression of the PINK1/Parkin/ p62 pathway occurring upon reperfusion, indicating reduced mitophagic flux, did not coincide with changes in fission. In view of the assumption that fission is a prerequisite for mitophagy, the relationship between these events needs to be evaluated.
The link between the PINK1/Parkin pathway and fission has been studied in COS cells.51 Workers have shown that Parkin promotes fission independently from PINK1 and this effect depends on pathways involved in DRP1 phosphorylation. Loss of mitochondrial electron potential leads to recruitment of DRP1 to mitochondria in the proximity of PINK1 and Parkin, suggesting that mitochondrial division occurs at sites where the PINK1/Parkin-dependent mitochondrial clearance programme is initiated.
Although the translocation of DRP1 from the cytosol to the mitochondria was not investigated in our study, previous studies from our laboratory showed translocation occurring in hearts exposed to ischaemia (unpublished data). Translocation of DRP1 from the cytosol to the mitochondria is known to occur in ex vivo hearts after exposure to 30 minutes of global ischaemia/90 minutes of reperfusion, as well as in primary cultured cardiomyocytes subjected to ischaemia/re-oxygenation.52
The effect of chloroquine on the alternative non-canonical autophagy pathway was also evaluated by determining the expression of an essential regulator of this process, Rab9, a small GTP-binding protein, during exposure of the heart to ischaemia/reperfusion. While Rab9 expression did not change significantly during the ischaemia/reperfusion protocol in untreated hearts, chloroquine almost completely abolished its expression during reperfusion (Fig. 5). The results obtained indicate that chloroquine also very significantly inhibited the alternative autophagy pathway during reperfusion, suggesting that this pathway should be considered when evaluating the effects of this drug on autophagy/mitophagy.
Fig. 5.
Effect of ischaemia/reperfusion and chloroquine on the expression of TOM 70, p62/SQSTM1 (p62), PINK and Parkin (A); pDRP-1/total DRP, total DRP, pDRP and Rab9 (B). Western blot analysis was done on mitochondria (n = four hearts/group) isolated from hearts after 40 minutes of stabilisation, after 25 minutes of global ischaemia; and after 10 minutes of reperfusion following 25 minutes of global ischaemia. *p < 0.05 vs corresponding stabilisation. #p < 0.05 vs corresponding ischaemia. AMC: age-matched controls; STB: stabilisation; ISC: ischaemia: RP: reperfusion; VE: negative control.
Interestingly, chloroquine had its most significant effects during reperfusion, as was seen in the expression of p62, PINK1, Parkin and Rab9. The significance of the upregulated state 3 ox-phos rate during reperfusion (Figs 3, 4) in the observed changes in mitophagy needs to be further investigated. Although the purpose of chloroquine treatment was to block the autophagosome–lysosome interaction to allow evaluation of flux, the data suggest that the effects of this treatment on mitochondrial function per se may contribute to the effects on mitophagy as seen specifically in reperfusion.
Conclusion
The results show that data obtained by snapshot measurements of mitophagy differed markedly from flux measurements using chloroquine pre-treatment. Reperfusion in particular is associated with a significant inhibition of mitophagic flux. Whether this indicates a salvage attempt to rescue damaged mitochondria, as seen in the marked improvement in ultrastructural appearance upon reperfusion,33 remains to be determined.
The data obtained underscore the necessity of evaluation of the process of mitophagy in the absence and presence of lysosomal blockade, as suggested by Gottlieb and co-workers.12 However the data also demonstrate that chloroquine per se affected the response of the mitochondria to ischaemia, which, in turn, may have affected the mitophagy process.
Another complicating factor in the interpretation of the results is that the drug may have cardioprotective actions in a rat heart model, attributed to its phospholipase A2 effects,29,30 which may lead to an underestimation of the effects of ischaemia/ reperfusion on mitophagy. This possibility, however, was not reflected by changes in infarct size and functional recovery after global ischaemia.32 Therefore although measurement of flux is a prerequisite for evaluation of mitophagy, interpretation of data should be done with care in view of the multiple other effects of chloroquine on the heart.
References
- 1.Chen Y-R, Zweier JL. Cardiac mitochondria and ROS generation. Circ Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari R. Importance of oxygen free radicals during ischemia and reperfusion in the experimental and clinical setting. Oxygen free radicals and the heart. Am J Cardiovasc Pathol. 1992;4:103–114. [PubMed] [Google Scholar]
- 3.Ferrari R, Ceconi C, Curello S, Cargnoni A, Pasini E, De Giuli F, Albertini A. Role of oxygen free radicals in ischemic and reperfused myocardium. Am J Clin Nutr. 1991;53:215S–222S. doi: 10.1093/ajcn/53.1.215S. [DOI] [PubMed] [Google Scholar]
- 4.Watts JA, Kline JA. Bench to bedside: the role of mitochondrial medicine in the pathogenesis and treatment of cellular injury. Acad Emergency Med. 2003;10:985–997. doi: 10.1111/j.1553-2712.2003.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 5.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Scherz-Shouval R, Shvets E, Fass E. et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Embo J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song M, Chen, Gong G. et al. Super-suppression of mitochondrial reactive oxygen species signalling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anzell AR, Maizy R, Przyklenk K, Sanderson TH. Mitochondria quality control and disease: insights into ischaemia/reperfusion injury. Mol Neurobiol. 2018;55(3):2547–2564. doi: 10.1007/s12035-017-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nah J, Miyamoto S, Sadoshima J. Mitophagy as a protective mechanism against myocardial stress. Compr Physiol. 2017;7:1407–1424. doi: 10.1002/cphy.c170005. [DOI] [PubMed] [Google Scholar]
- 10.Dorn GW 2nd. Central Parkin: the evolving role of Parkin in the heart. Biochem Biophys Acta. 2016;1857:1307–1312. doi: 10.1016/j.bbabio.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito T, Nah J Oka S. et al. An alternative mitophagy mediated by Rab9 protects the heart against ischemia. J Clin Invest. 2019;129(2):802–819. doi: 10.1172/JCI122035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb RA, Andres AM, Sin J, Taylor DP. Untangling autophagy measurements: all fluxed up. Circ Res. 2015;116:504–514. doi: 10.1161/CIRCRESAHA.116.303787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Dian A. Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy. 2012:1394–1396. doi: 10.4161/auto.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y. Restoration of autophagic flux in myocardial tissues is required for cardioprotection of sevoflurane postconditioning in rats. Acta Pharmacol Sunica. 2014;35:758–769. doi: 10.1038/aps.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verschooten L, Barrette K, Van Kelst S. et al. Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS One. 2012;7:e48264. doi: 10.1371/journal.pone.0048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger ME, Huang JS, Yin W, McMasters KM, McNally LR. Inhibition of autophagy with chloroquine is effective in melanoma. J Surg Res. 2013;184:274–281. doi: 10.1016/j.jss.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: From malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonnesmann E, Kandolf R, Lewaiter T. Chloroquine cardiomyopathy – a review of the literature. Immunopharm Immunotoxicol. 2013;35:434–442. doi: 10.3109/08923973.2013.780078. [DOI] [PubMed] [Google Scholar]
- 19.Kannengieser GJ, Opie LH, van der Werff TJ. Impaired cardiac work and oxygen uptake after reperfusion of regionally ischaemic myocardium. J Mol Cell Cardiol. 1979;11:197–207. doi: 10.1016/0022-2828(79)90464-4. [DOI] [PubMed] [Google Scholar]
- 20.Sordahl LA, Besch HR Jr, Allen JC, Crow C, Lindenmayer GE, Schwartz A. Enzymatic aspects of the cardiac muscle cell: mitochondria, sarcoplasmic reticulum and noncovalent cation active transport system. Methods Achiev Exp Pathol. 1971;5:287–346. [PubMed] [Google Scholar]
- 21.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Essop MF, Chan WY, Valle A, Garcia-Palmer FJ, du Toit EF. Impaired contractile function and mitochondrial respiratory capacity in response to oxygen deprivation in a rat model of prediabetes. Acta Physiol (Oxford) 2009;197:289–296. doi: 10.1111/j.1748-1716.2009.02024.x. [DOI] [PubMed] [Google Scholar]
- 23.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Ma S, Wong Y, Chen Y, Cao F. The role of autophagy in myocardial ischemia/reperfusion injury. Biochim Biophys Acta. 2015;1852:271–276. doi: 10.1016/j.bbadis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Yao YT, Jiang NX. et al. Restoration of autophagic flux in myocardial tissues is required for cardioprotection of sevoflurane postconditioning in rats. Acta Pharm Sinica. 2014;35:758–769. doi: 10.1038/aps.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwai-Kanai E, Yuan H, Huang C. A method to measure cardiac autophagic flux. in vivo. Autophagy. 2008;4:322–329. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos JC, Queliconi BB, Bozi LH. et al. Exercise re-establishes autophagic flux and mitochondrial quality control in heart failure. Autophagy. 2017;13:1304–1317. doi: 10.1080/15548627.2017.1325062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giricz Z, Varga ZV, Kanesos G. et al. Autophagosome formation is required for cardioprotection by chloramphenicol. Life Sci. 2017;186:11–16. doi: 10.1016/j.lfs.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hostetler KY, Jellison EJ. Role of phospholipases in myocardial ischaemia: effect of cardioprotective agents on the phospholipases A of heart cytosol and sarcoplasmic reticulum in vitro. Mol Cell Biochem. 1989;88:77–82. doi: 10.1007/BF00223427. [DOI] [PubMed] [Google Scholar]
- 30.Bourke L, McCormick J, Taylor V. et al. Hydroxychloroquine protects against cardiac ischaemia/reperfusion injury in vivo via enhancement of ERK1/2 phosphorylation. PLoS One. 2015;10:e0143771. doi: 10.1371/journal.pone.0143771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang H, Liu A, Dahmen U, Dirsch O. Dual role of chloroquine in liver ischemia reperfusion injury: reduction of liver damage in early phase, but aggravation in late phase. Cell Death Dis. 2013;4:e694. doi: 10.1038/cddis.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smit M. The manipulation of autophagy during early and late reperfusion: effect on myocardial protection. PhD thesis, University of Stellenbosch, 2018 [Google Scholar]
- 33.Edoute Y, van der Merwe E, Sanan D. et al. Normothermic ischemic arrest of the isolated working rat heart. Effects of time and reperfusion on myocardial ultrastructural, mitochondrial oxidative function and mechanical recovery. Circ Res. 1983;53:663–678. doi: 10.1161/01.res.53.5.663. [DOI] [PubMed] [Google Scholar]
- 34.Rouslin W. Mitochondrial complexes I II, III, IV and V in myocardial ischemia and outolysis. Am J Physiol. 1983;244:H743–H748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- 35.Lesnefsky EJ, Chen Q, Moqhaddas S. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 36.Asimakis GK, Conti VR. Myocardial ischemia: correlation with mitochondrial adenine nucleotide and respiratory function. J Mol Cell Cardiol. 1984;16:43–47. doi: 10.1016/s0022-2828(84)80615-x. [DOI] [PubMed] [Google Scholar]
- 37.Katewa SD, Katyare SS. Treatment with antimalarials adversely affects the oxidative energy metabolism in rat liver mitochondria. Drug Chem Toxicol. 2004;27:41–53. doi: 10.1081/dct-120027898. [DOI] [PubMed] [Google Scholar]
- 38.Chaanine AH, Gordon RE, Nonnenmacher M, Kohlbrenner E, Benard L, Haijar RJ. High-dose chloroquine is metabolically cardiotoxic by inducing lysosomes and mitochondria dysfunction in a rat model of pressure overload hypertropgy. Physiol Rep. 2015;3:e12413. doi: 10.14814/phy2.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Essien EE, Ette EI. Effects of chloroquine and didesethylchloroquine on rabbit myocardium and mitochondria. J Pharm Pharmacol. 1986;38:543–546. doi: 10.1111/j.2042-7158.1986.tb04635.x. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi S, Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochem Biophys Acta. 2015;1852:252–261. doi: 10.1016/j.bbadis.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Shires SE, Gustafsson AB. Mitophagy and heart failure. J Mol Med (Berlin) 2015;93:253–262. doi: 10.1007/s00109-015-1254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rikka S, Quinsay MN, Thomas RL, Kubli DA. et al. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ. 2011;18:721–731. doi: 10.1038/cdd.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakai A, Yamaguchi O, Takeda T. et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 44.Pankiv S, Clausen TH, Lamark T. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 45.Komatsu M, Waguri S, Koike M. et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 46.Kato H, Lu Q, Rapaport D. et al. Tom70 is essential for pink 1 import into mitochondria. PloS One. 2013;8:e58435. doi: 10.1371/journal.pone.0058435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H, Zhang Y, Hu S, Shi C. et al. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC-HK2-mPTP-mitophagy axis. J Pineal Res. 2017;63:e12413. doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji W, Wei S, Hao P. et al. Aldehyde dehydrogenase 2 has cardioprotective effects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front Pharmacol. 2016;7:101. doi: 10.3389/fphar.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Andres AU, Ratliff EP. et al. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM 1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for controlling cardiovascular disease. Br J Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buhlman L, Damiano M, Bertolin G. et al. Functional interplay between Parkin and Drp1 in mitochondrial fission and clearance . Biochim Biophys Acta. 2014;1843:2012–2026. doi: 10.1016/j.bbamcr.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 52.Disatnik MH, Ferreira JC, Campos JC, Gomes KS. et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]