Summary
Objective
This study compared resting blood pressure (BP) using ambulatory BP monitoring (ABPM) responses in two groups of subjects trained in land exercise (LE) and aquatic exercise (AE), and assessed post-exercise hypotension (PEH) using ABPM, after land- and aquatic-based exercises.
Methods
ABPM (24 hours) was used to measure the baseline BP in elderly hypertensive women trained in LE and AE and the PEH induced by exercise. For this, 40 subjects were evaluated at rest and after a land- or aquatic-based exercise session (aerobic: 75% of reserve heart rate combined with resistance exercise).
Results
The daytime BP was lower for AE [systolic BP (SBP) 124 ± 1.0 mmHg, diastolic BP (DBP) 70 ± 1.5 mmHg] than for LE (SBP 134 ± 0.9 mmHg, DBP 76 ± 0.9 mmHg), but there were no differences at night-time. The aquatic exerciseinduced PEH in the second hour was maintained at the 24th hour post-exercise. For land exercise-induced PEH, it was maintained at the 12th hour post-exercise. The SBP and DBP were lower at the 24th hour for AE than for LE.
Conclusion
Elderly hypertensive people trained in AE had lower baseline BP during the daytime. SBP and DBP values were lower for individuals trained in AE, and their PEH was more rapid and longer lasting after AE.
Keywords: aquatic exercise, land exercise, hypertension, elderly
Hypertension (HTN) has been the subject of worldwide study for its clinical aspects or as a health problem. HTN is considered one of the main determinants of cardiovascular morbidity and mortality.1,2 Among non-pharmacological therapies recommended for HTN treatment, exercise training is essential, with reductions of around –3.5 mmHg for systolic and –3 mmHg for diastolic blood pressure (BP) being reported.3
Aerobic exercise for periods of 30 minutes of vigorous or 60 minutes of moderate intensity three to five times a week4 is universally the most recommended measure to lower BP among those with HTN.5-7 Resistance training with nine exercises three times a week for 12 weeks, at 75% intensity on one maximal repetition (1RM), with a volume of six to 10 repetitions, promotes a greater nocturnal reduction (> 10%) in diastolic BP (DBP) among older hypertensive subjects than other forms of training.8
Individuals can benefit from one session of exercise with immediate or short-term effects that persist for up to 24 hours after an acute exercise bout, a response that is termed postexercise hypotension (PEH); this effect is considered an important positive factor in HTN treatment.9-11 Although the modalities of physical exercise (aerobic or resistance exercise) promote different responses in PEH, the magnitudes of PEH that they induce may be distinct. Aerobic exercise seems to promote a higher and longer PEH,12 and the intensity of the exercise appears to have an influence on PEH.13 For resistance training there are conflicting data about its effect on PEH due to variance in factors such as the muscle mass involved, the intensity of exercise, and the interval and volume of sets and rest.13,14
Aquatic physical exercise (AE) offers advantages over land exercise (LE) for the elderly as it involves lower risk of injury than LE owing to water buoyancy, and guards against joint degradation by decreasing weight-bearing loads15-17 and reduced joint load.18 In addition, aquatic-based exercise promotes physiological adjustments resulting from immersion that can affect BP as well as cardiac work, particularly reduction in sympathetic activity and redistribution of blood volume from the lower limbs and abdomen to the upper body.19,20 Therefore, excretion of liquids and electrolytes is increased, together with suppression of levels of the fluid-regulating hormones renin, angiotensin II, aldosterone and arginine vasopressin to control plasma volume,21,22 and peripheral vascular resistance is decreased.23-25
Several studies have established the effectiveness of planned interventions using physical exercise in the treatment of HTN with land and aquatic-based exercises,26-33 but comparisons between the two types of exercise regarding elderly hypertensives trained in different modalities are scarce. In addition, the effects on PEH of land- and aquatic-based exercise during the following 24 hours need further investigation.
In light of the benefits of AE, this study compared resting BP using ambulatory BP monitoring (ABPM), the clinical goldstandard methodology for assessing BP status, in two groups of trained subjects with equivalent cardiorespiratory capacity performing either LE or AE. In addition, using ABPM, we assessed PEH after AE and LE among older women with HTN.
Methods
This was a controlled clinical trial developed at the Exercise Physiology Laboratory (LABFE) of the school of Physical Education of Ouro Preto, Minas Gerais, Brazil. The study protocol was approved by the Research Ethics Committee of the Federal University of Ouro Preto under protocol: 38383314.3.0000.5150.
The study population consisted of 40 elderly hypertensive women, 20 trained in land-based exercise and 20 in aquatic-based exercise. To be included, the subjects had to meet the following criteria: aged over 60 years, hypertensive, female, in regular treatment for BP control, and enrolled in recurrent physical exercise for at least six months before evaluation for a minimum of twice a week. Subjects with symptomatic cardiorespiratory disease or cardiac alterations, the metabolic syndrome, renal or hepatic disease, cognitive impairment, and any other medical contra-indications of physical exercise were excluded.
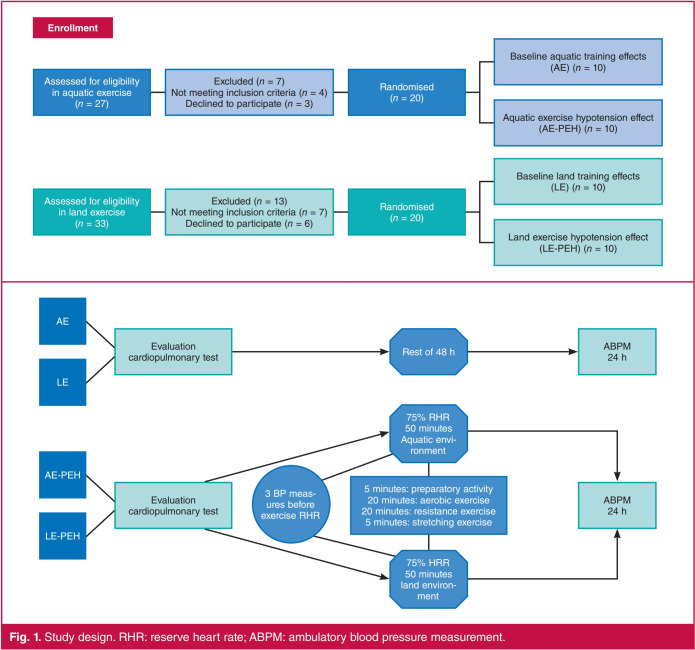
The participants were divided randomly into four groups: individuals enrolled in regular AE (n = 20), of whom the arterial baseline pressures of 10 were evaluated for 24 hours after 48 hours of rest after their last exercise training; and in the other 10 subjects the PEH induced by an AE session was assessed. The other 20 individuals enrolled in regular LE were randomised and evaluated in the same way (Fig. 1).
Fig. 1.
Study design. RHR: reserve heart rate; ABPM: ambulatory blood pressure measurement.
Body mass was measured with a digital scale having a capacity of 150 kg and an accuracy of 100 g (EKSR SUPER 9805). Height measurement was performed with a compact stadiometer fixed to the wall, and with a range of 0 to 2.0 m and an accuracy of 1 mm (Coats Corrente® BA1010).
The heart rate (HR) was measured in both groups at rest and after a cardiopulmonary test. For this a cardiac monitor (PolarR model FT1) was used to collect resting HR. The HR recording was made in the seated position for two minutes after a rest of 10 minutes; the lowest HR reached in this period was used. The maximal HR was measured immediately after completion of the cardiopulmonary test.
For evaluation of the maximum aerobic capacity (VO2 max), the progressive treadmill test was applied following the Balke–Ware protocol.34 The maximal VO2 was evaluated using an opencircuit spirometry VO2000R ventilometer and an InbramedR treadmill.35,36
BP was measured at rest and after cardiopulmonary tests in both groups. For the former, the BP was assessed three times after 10 minutes of rest in a seated position at intervals of one minute, and the result was taken as the mean value, while for the latter, the BP was assessed immediately after completion of the treadmill test with a stethoscope (MissouriR) and a manual aneroid sphygmomanometer (MissouriR) with a precision of 2 mmHg.
The ABPM was started 48 hours after the last training session to evaluate the baseline BP in the AE and LE groups. To evaluate PEH, the ABPM was started immediately after the exercise session. Three devices of the Meditech KFTR brand, model ABPM-04, were used. The BP cuff was worn on the non-dominant arm. Subjects were instructed to maintain their customary daily activities, not to exercise, and to relax and unbend the arm during the recording interval for daytime ABPM. ABPM data were accepted with more than 75% of the measurements effectively taken. Individual BP measurements were revised for missing and erroneous values.
For comparison purposes, data were distributed across the waking period, which consisted of the mean BP of the measurement made every 15 minutes during the periods of the day when the individual was awake (07:00 to 23:00), and the sleep period, during which the BP was measured every 30 minutes and the mean value taken when the individual was asleep (23:00 to 07:00). The result for each hour was then the average of the values recorded during that hour.
For the AE-PEH, each individual remained at rest in a seated position for 15 minutes, then BP was measured three times with a sphygmomanometer and stethoscope. Subsequently the test exercise session was started, which consisted of collective water aerobics with a duration of 50 minutes, comprising five minutes of preparatory activity, 20 minutes of aerobic exercises at 75% of reserve HR (RHR), 20 minutes of strength exercises, and five minutes of stretching. The HR was monitored by a heart rate monitor (POLARR RS800) during the entire session. After the experimental session, ABPM was used to record BP during the following 24-hour period.
For the LE-PEH, each individual remained at rest in a seated position for 15 minutes, then the BP was measured three times with a sphygmomanometer and stethoscope. Subsequently the test exercise session was started, which consisted of aerobic collective gymnastics with a duration of 50 minutes, including five minutes of preparatory activity, 20 minutes of aerobic exercises at 75% of RHR, 20 minutes of resistance exercises, and five minutes of stretching. HR was monitored by a heart rate monitor (POLARR RS800). After the experimental session, ABPM was used to record the BP during the following 24-hour period.
Statistical analysis
The Shapiro–Wilk test was used to evaluate the normality of the numerical data. Data are presented as mean ± standard deviation. An unpaired t-test with Welch’s correction was used to compare the cardiopulmonary response between AE and LE, as well as the magnitude of PEH at the second, 12th and 24th hours after the session.
Two-way ANOVA was used to compare PEH for the sessions by time (second, 12th and 24th hour), as well as determine interaction effects (session and time), followed by Bonferroni’s post hoc test. A 5% significance level was set. All statistical analyses utilised Graph Pad Prism 7.0
Results
Table 1 shows the characteristics of the experimental groups. There were no differences between the groups by age, body mass index, peak VO2, or resting systolic BP (SBP) and DBP.
Table 1. General profile of the two hypertensive groups.
| Age (years) | LE-PEH (n = 10) | AE-PEH (n = 10) | LE (n = 10) | AE (n = 10) |
| Age (years) | 67 ± 3 | 64 ± 3 | 65 ± 3 | 70 ± 2 |
| BMI (kg/m²) | 26.4 ± 3.4 | 25.7 ± 2.8 | 27.3 ± 3.3 | 26.5 ± 5.2 |
| Peak Vo2 (ml/kg/min) | 23.4 ± 3.4 | 22.6 ± 2.1 | 25.7 ± 4.7 | 24.8 ± 3.4 |
| Resting SBP (mmHg) | 140 ± 4.4 | 153 ± 5.0 | 130 ± 8.3 | 128 ± 9.8 |
| Resting DBP (mmHg) | 85 ± 4.3 | 90 ± 5.6 | 82 ± 4.2 | 81 ± 8.1 |
| Antihypertensive drugs (n) | 2 (1–2) | 1 (1–2) | 2 (2–3) | 1 (1–2) |
| Diuretic (%) | 60 | 50 | 60 | 50 |
| Diuretic (%) | 60 | 50 | 60 | 50 |
| ACE inhibitor (%) | 20 | 0 | 20 | 0 |
| ARB (%) | 80 | 80 | 60 | 80 |
AE-PEH: aquatic exercise PEH; LE-PEH: land exercise PEH; AE: aquatic exercise; LE: land exercise; PEH: post-exercise hypotension; VO2; volume of oxygen; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ACE inhibitor: angiotensin converting enzyme inhibitor; ARB: angiotensin II receptor blocker. Data expressed as mean ± SD.
Table 2 shows the HR and BP responses to maximal effort recorded after the cardiopulmonary test. The values of HR and DBP were significantly higher in the LE than the AE group, but there were no differences observed in SBP.
Table 2. Cardiovascular response to maximal effort in the cardiopulmonary test in hypertensive subjects trained in aquatic and land exercise.
| AE (n = 10) | LE (n = 10) | |
| HR (bpm) | 134 (124.9–143.9) | 147 (136.5–157.3)* |
| SBP (mmHg) | 160 (150.9–191.7) | 162.5 (151.3–177.9) |
| DBP (mmHg) | 80.0 (72.4–89.6) | 90.0 (84.1–90.5)* |
AE: aquatic exercise; LE: land exercise; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure. *p < 0.05 when compared to AE. Mann–Whitney test, data expressed in median and 95% confidence interval.
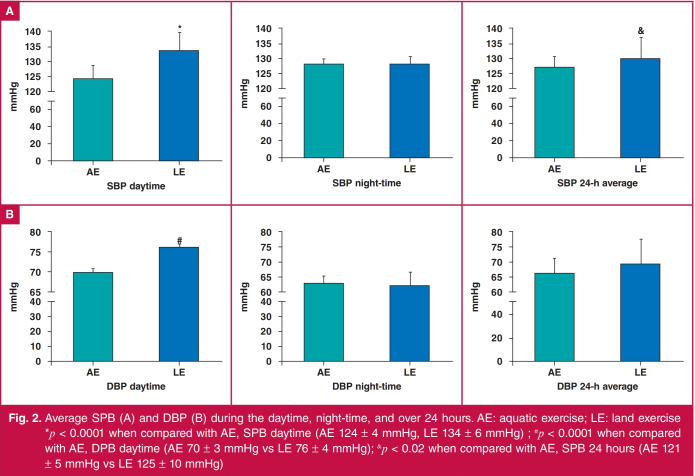
Fig. 2 shows the values of SBP (A) and DBP (B) during the daytime, night-time and total 24 hours (daytime plus night-time). The AE group showed lower values for daytime SBP (124 ± 4 mmHg) and DBP (70 ± 3 mmHg) than the LE group (SBP: 134 ± 6 mmHg, DBP: 76 ± 4 mmHg), as well as for the 24-hour average for SBP (AE: 121 ± 5 mmHg vs LE: 125 ± 10 mmHg). There was no difference between the groups during the night-time or for the 24-hour average of DBP.
Fig. 2.
Average SPB (A) and DBP (B) during the daytime, night-time, and over 24 hours. AE: aquatic exercise; LE: land exercise *p < 0.0001 when compared with AE, SPB daytime (AE 124 ± 4 mmHg, LE 134 ± 6 mmHg) ; #p < 0.0001 when compared with AE, DPB daytime (AE 70 ± 3 mmHg vs LE 76 ± 4 mmHg); &p < 0.02 when compared with AE, SPB 24 hours (AE 121 ± 5 mmHg vs LE 125 ± 10 mmHg)
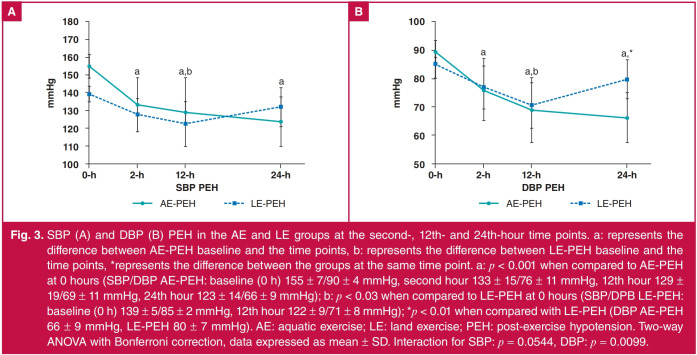
Fig. 3 shows the PEH for SBP (A) and DBP (B) in the AE-PEH and LE-PEH groups at the second, 12th and 24th hours after the exercise session, those times being chosen because the individuals were then awake. The AE-PEH data show that PEH was maintained from the second to the 24th hour, while for the LE group, maintenance of PEH was observed only until the 12th hour. There was no difference in PEH at the second and 12th hour between the groups, but for DBP, at the 24th hour, AE-PEH values were lower (66 ± 9 mmHg) than those of LE-PEH (80 ± 7 mmHg).
Fig. 3.
SBP (A) and DBP (B) PEH in the AE and LE groups at the second-, 12th- and 24th-hour time points. a: represents the difference between AE-PEH baseline and the time points, b: represents the difference between LE-PEH baseline and the time points, *represents the difference between the groups at the same time point. a: p < 0.001 when compared to AE-PEH at 0 hours (SBP/DBP AE-PEH: baseline (0 h) 155 ± 7/90 ± 4 mmHg, second hour 133 ± 15/76 ± 11 mmHg, 12th hour 129 ± 19/69 ± 11 mmHg, 24th hour 123 ± 14/66 ± 9 mmHg); b: p < 0.03 when compared to LE-PEH at 0 hours (SBP/DPB LE-PEH: baseline (0 h) 139 ± 5/85 ± 2 mmHg, 12th hour 122 ± 9/71 ± 8 mmHg); *p < 0.01 when compared with LE-PEH (DBP AE-PEH 66 ± 9 mmHg, LE-PEH 80 ± 7 mmHg). AE: aquatic exercise; LE: land exercise; PEH: post-exercise hypotension. Two-way ANOVA with Bonferroni correction, data expressed as mean ± SD. Interaction for SBP: p = 0.0544, DBP: p = 0.0099.
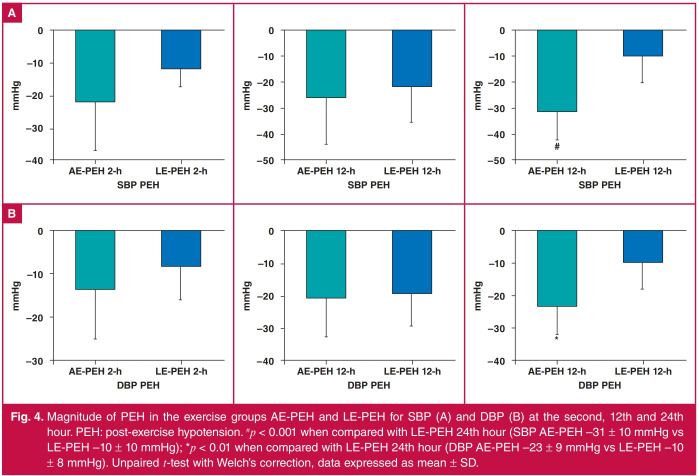
Fig. 4 shows SBP (A) and DBP (B) for the groups AE-PEH and LE-PEH. There was no difference in PEH at the second and 12th hours between the groups, but the 24th hour PEH was higher for the AE group (SBP: −31 ± 11 mmHg, DBP: −23 ± 8 mmHg) than for the LE group (SBP: −10 ± 10 mmHg, DBP: −10 ± 8 mmHg).
Fig. 4.
Magnitude of PEH in the exercise groups AE-PEH and LE-PEH for SBP (A) and DBP (B) at the second, 12th and 24th hour. PEH: post-exercise hypotension. #p < 0.001 when compared with LE-PEH 24th hour (SBP AE-PEH –31 ± 10 mmHg vs LE-PEH –10 ± 10 mmHg); *p < 0.01 when compared with LE-PEH 24th hour (DBP AE-PEH –23 ± 9 mmHg vs LE-PEH –10 ± 8 mmHg). Unpaired t-test with Welch’s correction, data expressed as mean ± SD.
Discussion
The main finding of this study was that elderly hypertensive subjects trained in AE had different baseline BP responses from land-trained subjects. During the daytime, SBP and DBP values were lower for aquatic-trained hypertensive subjects. In addition, the PEH induced by AE was more rapid and lasted longer than that induced by LE, based on data recorded 24 hours after the exercise session. Another interesting result was the cardiovascular response after a cardiopulmonary test: maximal HR and DBP were higher for land-trained than aquatic-trained subjects.
Both training environments have been shown to be efficacious in reducing BP, but aquatic training caused a more impressive reduction (−10.58 mmHg) than that due to land aerobic training (−3.5 mmHg) or resistance training (−1.8 mmHg).3 The baseline data show that AE induced lower BP values, an effect appearing during the awake period, which could be due to higher sympathetic tonus activity during the awake period than at night, as data show that in the daytime there is a prevalence of sympathetic tonus.37
AE modulates the sympathetic drive differently from that observed for LE. In AE, one should consider the effect of hydrostatic pressure, which induces an increase in blood concentration in the thorax38 and reflexively decreases the heart rate. Increased venous return during immersion stimulates cardiopulmonary receptors, which decrease sympathetic activity and total peripheral resistance.39 Bradycardia also occurs during immersion.40 In addition, data reported in the literature show that aquatic-based exercise induces a different response associated with renal sympathetic nerve activity,23 as well as higher suppression of the vasopressin and renin–angiotensin systems, from that of physical activities on land.41,42
The maximal response to the cardiopulmonary test shows that both groups had the same VO2 max, but, interestingly, hypertensives trained in AE had lower HR and DBP during maximal effort. The chronic effect of AE ameliorates arterial peripheral resistance, and the decrease in levels of epinephrine, norepinephrine and endothelin-1 associated with an increase in nitric oxide levels can improve the BP response during exercise, including DBP.43 We found that elderly hypertensive subjects had a better profile of cardiovascular responses during AE. The DBP decreases during aquatic cycle ergometer exercise were greater than in the case of the same exercise intensity on land.44
Our data show that PEH for SBP and DBP lasted for 24 hours after AE, which was longer than for LE. Similarly, Ngomane30 showed that heated AE was more effective in producing PEH for 11–18 hours after a bout of exercise than LE. The higher PEH after AE was observed in reduced SBP and DBP during the daytime, but there was no difference found in any other haemodynamic variable assessed: arterial stiffness, endothelial reactivity or heart rate variability. Our findings likewise corroborate the results of Bocalini,45 who verified that water ergometric exercise was effective in promoting a higher magnitude of PEH in older hypertensive women with more apparent outcomes in untreated women, than LE.
Concerning the mechanisms associated with PEH, several have been presented in the literature as playing a major role in these effects on BP: reduction in sympathetic activity,46 attenuation of cardiac adrenergic receptor sensitivity, decreased catecholamine synthesis with changes in renin and angiotensin release as a result,47 lesser peripheral vascular resistance48 and stroke volume,49 and synthesis of vasopressin21 and endothelins.50 The mechanism whereby AE creates lasting PEH however needs better elucidation.
Our study used a session of combined aerobic and resistance exercises for AE, and PEH was longer and started earlier (two hours after the exercise session) than for LE. This result is in agreement with Ferrari,51 who used concurrent training, aerobic plus resistance training, to show a reduction in BP in the first hour after training in hypertensive subjects participating in LE, but such an effect may not last as long as that of aerobic exercise alone. Similarly, Cunha32 found that moderate-intensity AE elicited PEH for SBP and DBP for over 21 hours. Pinto52 assessed the effect of concurrent training in water on normotensive subjects to show a similar effect on PEH from resistance and aerobic exercise.
Conclusion
Our study shows that elderly hypertensive individuals who exercised in water had lower SBP and DBP during the day than those trained in land exercise. In addition, hypotension was induced more quickly (two hours) by the exercise session after water-based exercise and lasted longer (24 hours) than that induced by land-based exercise. These data show that waterbased exercise has a different pressure control than land-based exercise, such that water-based exercise constitutes a potential clinical approach for the treatment of hypertension.
Acknowledgments
This study was supported by the Pro-Rectory of Research and Postgraduate of the Federal University of Ouro Preto (PROPP-UFOP).
Contributor Information
Lenice K Becker, Email: lenice@ufop.edu.br.
Raimundo M Nascimento-Neto, Department of Medicine, Federal University of Ouro Preto, Brazil.
References
- 1.Pescatello LS, MacDonald HV, Ash GI, Lamberti LM, Farquhar WB, Arena R. et al. Assessing the existing professional exercise recommendations for hypertension: a review and recommendations for future research priorities. Mayo Clinic Proceedings, Elsevier, 2015. doi: 10.1016/j.mayocp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S. et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. . J Am Heart Assoc. 2013;2(1):e004473. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M. et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 5.Johnson HM, Olson AG, LaMantia JN, Kind AJ, Pandhi N, Mendonca EA. et al. Documented lifestyle education among young adults with incident hypertension. J Gen Int Med. 2015;30(5):556–564. doi: 10.1007/s11606-014-3059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pescatello LS, MacDonald HV, Lamberti L, Johnson BTJC. Exercise for hypertension: a prescription update integrating existing recommendations with emerging research. Curr Hypertens Rep. 2015;17(11):87. doi: 10.1007/s11906-015-0600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moraes-Silva IC, Mostarda CT, Silva-Filho AC, Irigoyen MC. Hypertension and exercise training: evidence from clinical studies.Exercise for Cardiovascular Disease Prevention and Treatment. Springer. 2017;65 doi: 10.1007/978-981-10-4304-8_5. [DOI] [PubMed] [Google Scholar]
- 8.Bertani RF, Campos GO, Perseguin DM, Bonardi JMT, Ferriolli E, Moriguti JC. et al. Resistance Exercise training is more effective than interval aerobic training in reducing blood pressure during sleep in hypertensive elderly patients. J Strength Cond Res. 2018;32(7):2085–2090. doi: 10.1519/JSC.0000000000002354. [DOI] [PubMed] [Google Scholar]
- 9.Cavalcante PAM, Rica RL, Evangelista AL, Serra AJ, Figueira Jr A, Pontes Jr FL. et al. Effects of exercise intensity on postexercise hypotension after resistance training session in overweight hypertensive patients. Clin Interv Aging. 2015;10:1487. doi: 10.2147/CIA.S79625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Freitas Brito A, do Socorro Brasileiro-Santos M, de Oliveira CVC, da Cruz Santos AJTJoS, Research C. Postexercise hypotension is volume-dependent in hypertensives: autonomic and forearm blood responses. J Strength Cond Res. 2019;33(1):234–241. doi: 10.1519/JSC.0000000000001735. [DOI] [PubMed] [Google Scholar]
- 11.Imazu AA, Goessler KF, Casonatto J, Polito MDJB. The influence of physical training status on postexercise hypotension in patients with hypertension: a cross-sectional study. Blood Press Monit. 2017;22(4):196–201. doi: 10.1097/MBP.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 12.Keese F, Farinatti P, Pescatello L, Monteiro WJT. A comparison of the immediate effects of resistance, aerobic, and concurrent exercise on postexercise hypotension. J Strength Cond Res. 2011;25(5):1429–1436. doi: 10.1519/JSC.0b013e3181d6d968. [DOI] [PubMed] [Google Scholar]
- 13.Keese F, Farinatti P, Pescatello L, Cunha F, Monteiro WJI. Aerobic exercise intensity influences hypotension following concurrent exercise sessions. Int J Sports Med. 2012;33(02):148–153. doi: 10.1055/s-0031-1291321. [DOI] [PubMed] [Google Scholar]
- 14.De Freitas Brito A, de Oliveira CVC, do Socorro Brasileiro-Santos M, da Cruz Santos AJC. Resistance exercise with different volumes: blood pressure response and forearm blood flow in the hypertensive elderly. Clin Interv Aging. 2014;9:2151. doi: 10.2147/CIA.S53441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva LE, Valim V, Pessanha APC, Oliveira LM, Myamoto S, Jones A. et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther. 2008;88(1):12–21. doi: 10.2522/ptj.20060040. [DOI] [PubMed] [Google Scholar]
- 16.Suomi R, Collier DJA. Effects of arthritis exercise programs on functional fitness and perceived activities of daily living measures in older adults with arthritis. Arch Phys Med Rehabil. 2003;84(11):1589–1594. doi: 10.1053/s0003-9993(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 17.Reichert T, Costa RR, Barroso BM, da Rocha VMB, Delevatti RS, Kruel LFM. Aquatic training in upright position as an alternative to improve blood pressure in adults and elderly: a systematic review and meta-analysis. Sports Med. 2018;48(7):1727–1737. doi: 10.1007/s40279-018-0918-0. [DOI] [PubMed] [Google Scholar]
- 18.Kutzner I, Richter A, Gordt K, Dymke J, Damm P, Duda GN. et al. Does aquatic exercise reduce hip and knee joint loading? In vivo load measurements with instrumented implants. PloS One. 2017;12(3):e0171972. doi: 10.1371/journal.pone.0171972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenleaf J, Morse J, Barnes P, Silver J, Keil LJJ. Hypervolemia and plasma vasopressin response during water immersion in men. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(6):1688–1693. doi: 10.1152/jappl.1983.55.6.1688. [DOI] [PubMed] [Google Scholar]
- 20.Harrison M, Keil L, Wade C, Silver J, Geelen G, Greenleaf JJJ. Effect of hydration on plasma volume and endocrine responses to water immersion. J Appl Physiol. 1986;61(4):1410–1417. doi: 10.1152/jappl.1986.61.4.1410. [DOI] [PubMed] [Google Scholar]
- 21.Epstein MJ. Renal effects of head-out water immersion in humans: a 15-year update. Physiol Rev. 1992;72(3):563–621. doi: 10.1152/physrev.1992.72.3.563. [DOI] [PubMed] [Google Scholar]
- 22.Norsk P, Bonde-Petersen F, Warberg JJE. Central venous pressure and plasma arginine vasopressin during water immersion in man. Eut J Appl Physiol Occup Physiol. 1985;54(1):71–78. doi: 10.1007/BF00426302. [DOI] [PubMed] [Google Scholar]
- 23.Pendergast DR, Moon RE, Krasney JJ, Held HE, Zamparo P. Human Physiology in an aquatic environment. Compr Physiol. 2015;5(4):1705–1750. doi: 10.1002/cphy.c140018. [DOI] [PubMed] [Google Scholar]
- 24.Pendergast DR, Lundgren CEJ. The underwater environment: cardiopulmonary, thermal, and energetic demands. J Appl Physiol. 2009;106(1):276–283. doi: 10.1152/japplphysiol.90984.2008. [DOI] [PubMed] [Google Scholar]
- 25.Gabrielsen A, Bie P, Christensen N, Frandsen E, Galatius S, Pump B. et al. Systemic vascular resistance during brief withdrawal of angiotensin converting enzyme inhibition in heart failure. Scand J Clin Lab Invest. 2002;62(4):245–254. doi: 10.1080/003655102760145799. [DOI] [PubMed] [Google Scholar]
- 26.Boutcher Y, Boutcher SJ. Exercise intensity and hypertension: what’s new? J Hum Hypertens. 2017;31(3):157. doi: 10.1038/jhh.2016.62. [DOI] [PubMed] [Google Scholar]
- 27.Larsen MK, Matchkov VVJM. Hypertension and physical exercise: the role of oxidative stress. Medicina. 2016;52(1):19–27. doi: 10.1016/j.medici.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Cornelissen VA, Fagard RHJH. Effects of endurance training on blood pressure, blood pressure–regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46(4):667–675. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 29.Pierce GLJ. Aortic stiffness in aging and hypertension: prevention and treatment with habitual aerobic exercise. Curr Hypertens Rep. 2017;19(11):90. doi: 10.1007/s11906-017-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngomane AY, Fernandes B, Guimaraes GV, Ciolac EG. Hypotensive effect of heated water-based exercise in older individuals with hypertension. Int J Sports Med. 2019;40(4):283–291. doi: 10.1055/a-0828-8017. [DOI] [PubMed] [Google Scholar]
- 31.Guimaraes GV, Fernandes-Silva MM, Drager LF, de Barros Cruz LG, Castro RE, Ciolac EG. et al. Hypotensive effect of heated water-based exercise persists after 12-week cessation of training in patients with resistant hypertension. Can J Cardiol. 2018;34(12):1641–1647. doi: 10.1016/j.cjca.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Cunha RM, Vilaca-Alves J, Noleto MV, Silva JS, Costa AM, Silva CNF. Acute blood pressure response in hypertensive elderly women immediately after water aerobics exercise: A crossover study. et al. Clin Exp Hypertens. 2017;39(1):17–22. doi: 10.1080/10641963.2016.1226891. [DOI] [PubMed] [Google Scholar]
- 33.Arca EA, Martinelli B, Martin LC, Waisberg CB, Franco RJ. Aquatic exercise is as effective as dry land training to blood pressure reduction in postmenopausal hypertensive women. Physiother Res Int. 2014;19(2):93–98. doi: 10.1002/pri.1565. [DOI] [PubMed] [Google Scholar]
- 34.Araujo CGS. Manual de teste de esforco. Rio de Janeiro: Ao livro tecnicio, 1984 [Google Scholar]
- 35.Neder JA, Dal Corso S, Malaguti C, Reis S, De Fuccio MBd, Schmidt H. et al. The pattern and timing of breathing during incremental exercise: a normative study. Eur Respir J. 2003;21(3):530–538. doi: 10.1183/09031936.03.00045402. [DOI] [PubMed] [Google Scholar]
- 36.Araujo CG.. Fisiologia do exercicio fisico e hipertensao arterial: uma breve introducao. Hipertensao. 2001;4(3):78–83. [Google Scholar]
- 37.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328(5):303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 38.Park KS, Choi JK, Park YS.. Cardiovascular regulation during water immersion. Appl Hum Sci. 1999;18(6):233–241. doi: 10.2114/jpa.18.233. [DOI] [PubMed] [Google Scholar]
- 39.Mourot L, Bouhaddi M, Gandelin E, Cappelle S, Dumoulin G, Wolf J-P. et al. Cardiovascular autonomic control during short-term thermoneutral and cool head-out immersion. Aviat Space Environ Med. 2008;79(1):14–20. doi: 10.3357/asem.2147.2008. [DOI] [PubMed] [Google Scholar]
- 40.Graef FI, Kruel LFM. Frequencia cardiaca e percepcao subjetiva do esforco no meio aquatico: diferencas em relacao ao meio terrestre e aplicacoes na prescricao do exercicio-uma revisao. Revista brasiliera de medicina do esporte. 2006;12(4):221–227. [Google Scholar]
- 41.Reilly T, Dowzer CN, Cable NJ. The physiology of deep-water running. J Sports Sci. 2003;21(12):959–972. doi: 10.1080/02640410310001641368. [DOI] [PubMed] [Google Scholar]
- 42.Gabrielsen A, Videbaek R, Johansen L, Warberg J, Christensen N, Norsk PJ. Immediate baroreflex-neuroendocrine interactions in humans during graded water immersion. J Gravit Physiol. 1996;3(2):22. [PubMed] [Google Scholar]
- 43.De Barros Cruz LG, Bocchi EA, Grassi G, Guimaraes GV. Neurohumoral and endothelial responses to heated water-based exercise in resistant hypertensive patients. Circ J. 2017;81(3):339–345. doi: 10.1253/circj.CJ-16-0870. [DOI] [PubMed] [Google Scholar]
- 44.Gomes SG, Silva LG, Santos TM, Totou NL, Souza PM, Pinto K. et al. Elderly hypertensive subjects have a better profile of cardiovascular and renal responses during water-based exercise. J Exerc Physiol Online. 2016;19(4) [Google Scholar]
- 45.Bocalini DS, Bergamin M, Evangelista AL, Rica RL, Pontes FLJ, Figueira AJ. et al. Post-exercise hypotension and heart rate variability response after water- and land-ergometry exercise in hypertensive patients. PLoS One. 2017;12(6):e0180216. doi: 10.1371/journal.pone.0180216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulics JM, Collins HL, DiCarlo SEJA. Postexercise hypotension is mediated by reductions in sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 1999;276(1):H27–H32. doi: 10.1152/ajpheart.1999.276.1.H27. [DOI] [PubMed] [Google Scholar]
- 47.Pontes Junior FL, Prestes J, Leite RD, Rodriguez DJ. Influence of aerobic exercise on physiopathological mechanisms of systemic hypertension. Brasiliera de Ciencias do Esporte. 2010;32(2–4):229–244. [Google Scholar]
- 48.Halliwill JR.. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev. 2001;29(2):65–70. doi: 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Rondon MUPB, Alves MJN, Braga AMF, Teixeira OTU, Barretto ACP, Krieger EM. et al. Postexercise blood pressure reduction in elderly hypertensive patients. J Am Coll Cardiol. 2002;39(4):676–682. doi: 10.1016/s0735-1097(01)01789-2. [DOI] [PubMed] [Google Scholar]
- 50.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K. et al. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108(5):530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- 51.Ferrari R, Umpierre D, Vogel G, Vieira PJC, Santos LP, de Mello RB. et al. Effects of concurrent and aerobic exercises on postexercise hypotension in elderly hypertensive men. Exp Gerontol. 2017;98:1–7. doi: 10.1016/j.exger.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Pinto SS, Umpierre D, Ferreira HK, Nunes GN, Ferrari R, Alberton CL. Postexercise hypotension during different water-based concurrent training intrasession sequences in young women. J Am Soc Hypertens. 2017;11(10):653–659. doi: 10.1016/j.jash.2017.08.002. [DOI] [PubMed] [Google Scholar]