Abstract
We have isolated piroplasms from a patient who developed the first case of human babesiosis in Japan by using NOD/shi-scid mice whose circulating erythrocytes (RBCs) had been replaced with human RBCs (hu-RBC-SCID mice). Following inoculation of the patient's blood specimen into hu-RBC-SCID mice, parasites proliferated within the human RBCs in the mice, resulting in a high level of parasitemia. Parasite DNA was prepared from blood samples of the patient and the mice, and the nuclear small-subunit rRNA gene (rDNA) was amplified and sequenced. Both DNA samples gave rise to identical sequences which showed the highest degree of homology (99.2%) with the Babesia microti rDNA. Because the patient had received a blood transfusion before the onset of babesiosis, we investigated the eight donors who were involved. Their archived blood samples were analyzed for specific antibody and parasite DNA; only a single donor was found to be positive by both tests, and the parasite rDNA sequence from the donor coincided with that derived from the patient. The donor's serum exhibited a high antibody titer against the isolate from the patient, whereas it exhibited only a weak cross-reaction against B. microti strains isolated in the United States. We conclude that the first Japanese babesiosis case occurred due to a blood transfusion and that the etiological agent is an indigenous Japanese parasite which may be a geographical variant of B. microti. Our results also demonstrated the usefulness of hu-RBC-SCID mice for isolation of parasites from humans and for maintenance of the parasite infectivity for human RBCs.
Human babesiosis is a tick-transmitted zoonosis caused by intraerythrocytic protozoan parasites of the genus Babesia (30). Although the first human case was described in a country that was formerly part of Yugoslavia, the disease occurs rarely in Europe (5) and appears more frequently in the United States, particularly in the northeastern and the upper midwestern regions. Clinical manifestations range from asymptomatic to severe and occasionally fatal infections. Two species of Babesia, B. microti and B. divergens, are mainly involved in human infections in the United States and Europe, respectively, although newly emerging species, referred to as WA1 (24), CA1 (23), and MO1 (7), have also been recognized. Meanwhile, only a very few human cases have been reported from outside of the United States and Europe (5, 28, 30). Human babesiosis has not been reported from Japan, despite the presence of B. microti-like parasites among small wild rodents in the country (29).
In contrast to most members of the genus Babesia, which are ubiquitous among wild and domestic mammals and which seem to be highly host specific, the species capable of infecting humans exhibit a wider host range, as determined by experimental infections (4, 29). Nonetheless, some host preference is evident inasmuch as Syrian hamsters (Mesocricetus auratus) and Mongolian gerbils (Meriones unguiculatus) serve as optimal experimental hosts for B. microti (4) and B. divergens (14), respectively. The laboratory mouse (Mus musculus) appears to be less susceptible, although mouse-adapted strains may be selected (15, 25). Other methods of propagating the agents of human babesiosis seem less satisfactory. In vitro cultivation methods are available for B. divergens (37) and WA1 (31), but B. microti has not been continuously maintained by such a method.
We have previously shown that replacement of the circulating red blood cells (RBCs) in SCID mice (C.B-17 scid) with RBCs of other animals species endows the mice with susceptibility to highly host-specific erythroparasitic protozoa of cattle (34, 36), dogs (2), and humans (35). In recent years, this model has been refined by introducing the scid mutation into mouse strains other than the C.B-17 strain. SCID mice with the genetic background of nonobese diabetic (NOD) mice (11) were found to be of significantly improved acceptability for hematopoietic xeno-transplantation (6, 19).
Herein, we demonstrate the first successful use of NOD/shi-scid mice whose circulating RBCs were replaced with human RBCs (hu-RBC-SCID mice) for the isolation of parasites from a Japanese patient who developed symptomatic babesiosis. The isolated parasite was closely related to the reference B. microti strains isolated in the United States, but it differed from those strains antigenically and genetically.
MATERIALS AND METHODS
Case history and blood samples.
The details of the history of the case patient have been reported elsewhere (16). In brief, a 40-year-old male resident of Kobe, Hyogo Prefecture, Japan, was initially hospitalized due to bleeding from a gastric ulcer, for which he received treatments including approximately 2 liters of blood. Nearly a month after cure of the gastric ulcer, he had a fever, malaise, and dark-colored urine and was rehospitalized in the same hospital on 6 February 1999. Since the patient suffered from progressively exacerbating hemolytic anemia, he was transferred on 7 May to the Kobe University Hospital for closer examination, where Babesia-like intraerythrocytic parasites were found in his Giemsa-stained blood smear. Approximately 5 ml of a heparinized blood sample obtained on 24 May (roughly 50% parasitemia) was delivered to Rakuno-gakuen University for identification and isolation of the parasite. The patient was treated with clindamycin and quinine (39), followed by treatment with atovaquone (38), which successfully reduced the parasitemia. Archived blood samples of the donors were provided from Hyogo Red Cross Blood Center of the Japanese Red Cross Society.
SCID mice.
NOD/shi-scid mice (12), which had been developed in the Central Institute of Experimental Animals, Kawasaki, Japan, were maintained in the laboratory animal facility in Rakuno-gakuen University. C.B-17 scid mice were purchased from Japan CLEA (Tokyo, Japan). All mice were housed in a vinyl-film isolator at a temperature of between 22 and 25°C and were provided with a γ-ray-irradiated pellet diet and autoclaved tap water. When needed, the mice were splenectomized and were used for experiments after the surgical wounds had healed completely. Animal experimentation was carried out according to the Laboratory Animal Control Guidelines of Rakuno-gakuen University.
RBC clearance test.
Human type O RBCs were labeled with the fluorescent cell linker dye PKH-26 (Zyanaxis Cell Science, Malvern, Pa.) by a previously described method (10). A dose of 2.5 × 108 labeled RBCs was intravenously injected into NOD/shi-scid and C.B-17 scid mice. Blood samples of approximately 10 μl were collected from the tail veins of the mice at various times after the injection. The numbers of fluorescent cells were enumerated under a fluorescence microscope (BH-2; Olympus, Tokyo, Japan).
Parasite isolation with hu-RBC-SCID mice.
The peripheral RBCs in NOD/shi-scid mice were replaced with human type O RBCs obtained from a healthy volunteer by the method described in our previous reports (20, 35). Transfusions of 0.5 ml of a packed cell volume of human RBCs (approximately 6 × 109) were given to the mice at 2- to 4-day intervals. In order to assist with rapid RBC replacement, 100 μl of anti-mouse RBC rat monoclonal antibody, clone 2E11 (20), and 100 μl of antierythropoietin rabbit serum (20) were also administered to the mice at 2- to 4-day intervals. When more than 90% of the RBCs in the SCID mice were replaced with human RBCs, the mice were infected with 109 RBCs from the patient. Blood samples were collected from the tail veins of the mice daily. The percentage of human RBCs in the total RBCs was measured by flow cytometry (Cyto ACE-150; JASCO Co., Tokyo, Japan) with RBC samples which had been stained with a biotin-labeled anti-human RBC mouse immunoglobulin G Fab fragment (35) and phycoerythrin-labeled streptavidin (Life Technologies, Rockville, Md.). The level of parasitemia was determined by microscopy with Giemsa-stained thin-smear blood films.
Amplification and sequencing of rDNA.
DNA samples were prepared from blood samples with a whole-blood DNA extraction kit (GenTLE; TaKaRa Biochemical, Otsu, Japan). The sequence encoding eukaryotic small-subunit rRNA was amplified from the DNA samples by PCR with the primer set described by Medlin et al. (17). The PCR mixtures contained 400 μM each deoxynucleoside triphosphates, 0.25 μM each primer, 10 to 100 ng of template DNA, and 2.5 U of La Taq DNA polymerase (TaKaRa Biochemical) in 50 μl of the PCR buffer supplied together with the enzyme. Thermal cycling was carried out in a GeneAmp PCR system 9600 thermal cycler (Perkin-Elmer, Norwalk, Conn.), with 30 cycles of denaturation at 94°C for 40 s, annealing at 55°C for 60 s, and extension at 72°C for 90 s. The specific PCR products (1.7 kb) were purified by agarose gel electrophoresis, followed by cloning into the EcoRV site of pZErO-1 (Invitrogen, Groningen, The Netherlands) according to the manufacturer's instruction. The nucleotide sequences were determined by the dideoxy chain-termination method (27) with double-stranded plasmid DNA as the template. Sequencing reactions were carried out with an AutoRead DNA sequencing kit (Pharmacia, Uppsala, Sweden) with fluorescein isothiocyanate-labeled primers. Samples were analyzed with an ALF DNA Sequencer II sequencer (Pharmacia), and sequence data were processed with the associated software (ALF manager, version 3.02). The sequences of both strands of the rRNA gene (rDNA) were determined and were submitted to DDBJ. Detection of the babesial rDNA in the donor's blood samples was carried out by nested PCR with the primer sets described by Persing et al. (22) (primers Bab 1 and Bab 4, followed by primers Bab 2 and Bab 3).
Phylogenetic analysis.
The rDNA sequences (accession numbers are given in parentheses) used to construct a phylogenetic tree were from Babesia bigemina (X59604), Babesia divergens (U16370), Babesia canis (L19079), Babesia caballi (Z15104), Babesia equi (Z15105), Babesia rodhaini (AB 049999), B. microti (U09833), Babesia gibsoni (L13729), Theileria parva (AF013418), Theileria annulata (M64243), Theileria taurotragi (L19082), Theileria sergenti (AB000271), Toxoplasma gondii (U03070), WA1 (L13730), and PB-1 (AF081465). The sequences were aligned by use of the Multiple Alignment program in the CLUSTAL V software package (8). Fine adjustments of the aligned sequences were carried out manually. Phylogenetic relationships were analyzed with the aligned sequences by the neighbor-joining method (26) with the Phylogenetic Trees program in CLUSTAL V. Support for tree nodes was calculated by 1,000 bootstrap replicates by using the Bootstrap Tree algorithm.
Indirect fluorescent-antibody test (IFAT).
An approximately 50% suspension of infected RBCs which had 30 to 50% parasitemia was prepared in phosphate-buffered saline (PBS; pH 7.2) containing 50% fetal bovine serum. Roughly 0.3-μl aliquots were spread into each well of a 24-well HT Coating Slide (MS 342 BL; Bokusui Brown, Tokyo, Japan) and were then dried. The slides were fixed in acetone for 5 min and were then immediately transferred into PBS to lyse the RBCs. Following removal of solution by briefly blotting with a filter paper, the slides were placed in a moisturized chamber, and 15 μl of serial twofold dilutions of serum specimens, starting from 1:25, was added to each well. After 1 h of incubation at room temperature, the slides were washed in PBS, and 15 μl of fluorescein isothiocyanate-labeled protein A (EY Laboratories, Inc., San Mateo, Calif.) diluted 1:200 in 5% fetal bovine serum–PBS was added to each well. The slides were incubated at room temperature for 1 h and washed in PBS. Component A of the Slowfade antifade kit (Molecular Probes, Eugene, Oreg.) was mounted onto each well, and cover glasses were placed on the slides. Fluorescent parasites in RBCs were observed with a fluorescence microscope at a magnification of ×200.
Reference strains of B. microti.
The Gray strain (4) was a gift from J. Dickerson, Division of Parasitic Diseases, Centers for Disease Control and Prevention. Strain Gray-Mo, a mouse-adapted substrain of the Gray strain, has been described previously (15). The GI and AJ strains were provided by H. Saeki, Nippon Veterinary and Animal Science University. Syrian hamsters were used for propagation of the Gray strain, and the antibodies in their convalescent-phase sera were used as the specific antibodies. The Gray-Mo, GI and AJ strains were propagated in C.B-17 scid mice, and antisera against these strains were prepared with BALB/c mice.
Nucleotide sequence accession number.
The sequence of the rDNA has been submitted to DDBJ and has been given accession no. AB032434.
RESULTS
Preparation of hu-RBC-SCID mice.
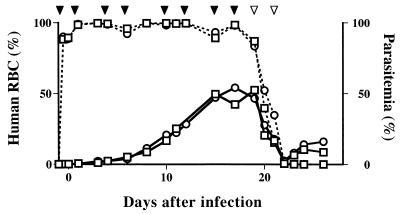
The life span of human RBCs is significantly longer when they are transfused into NOD/shi-scid than when they are transfused into C.B-17 scid mice (Fig. 1). Owing to the superior xeno-transplantation acceptability of RBCs of NOD/shi-scid mice, we were able to generate hu-RBC-SCID mice by repeated transfusion of human RBCs in combination with antibodies directed against mouse RBCs and erythropoietin. Nearly complete substitution could be maintained as long as these treatments continued to be given (Fig. 2).
FIG. 1.
Clearance (mean ± standard deviation) of human RBCs from C.B-17 scid (closed circles; n = 14) and NOD/shi-scid (open circles; n = 17) mice. PKH-26-labeled human RBCs were intravenously injected into mice, and the numbers of fluorescent RBCs in their peripheral blood were measured at various time points.
FIG. 2.
Preparation of hu-RBC-SCID mice and proliferation of Babesia parasites in the mice. Two splenectomized NOD/shi-scid mice (○, □) were repeatedly transfused with 6 × 109 human RBCs (closed arrow heads), together with administration of anti-mouse RBCs and antierythropoietin antibodies. The mice were transfused on days 19 and 21 with an equal amount of mouse RBCs (open arrowheads). The mice were infected with the patient's RBCs on day 0, and the peripheral blood samples were examined daily for the rate of replacement with human RBCs (dotted lines) and for levels of parasitemia (solid lines).
Isolation of parasites from the patient.
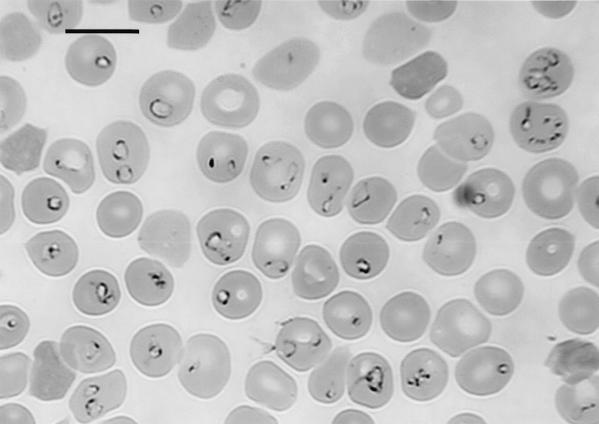
After inoculation of the hu-RBC-SCID mice with the patient's blood sample, increasing numbers of parasites were seen within the human RBCs in the mice (Fig. 2 and 3). A frozen parasite stock, designated the Kobe strain, was prepared by further propagation of the parasites in the other hu-RBC-SCID mice. Although the infected hu-RBC-SCID mice had peak parasitemia that exceeded 50%, they did not show any substantial clinical symptoms. This was in contrast to the patient, who showed severe symptoms, including fever, malaise, joint pain, dark-colored urine, jaundice, anemia, and splenomegaly. When splenectomized NOD/shi-scid mice without human RBC replacement were inoculated with the patient's blood sample, a very few parasitized RBCs were seen in the peripheral blood in the mice for the following few days, but they eventually disappeared. However, a low level of parasitemia was seen in the mice whose results are presented in Fig. 2 even after switching from transfusion with human RBCs to transfusion with mouse RBCs, which resulted in replacement of the RBCs in the mice back into mouse RBCs. Thus, although the parasites in the patient's blood specimen appeared to be poorly infectious for mice, there must be a very small population of the parasites that are capable of replicating in mouse RBCs. In fact, a mouse-adapted substrain (designated strain Kobe-Mo), which is capable of rapidly replicating in mice, could be established after three successive passages in new SCID mice with intact spleens. In hu-RBC-SCID mice, however, this mouse-adapted strain did not replicate as rapidly as the original Kobe strain did, indicating that the adaptation to mice altered the preference of the parasite's infectivity for human RBCs.
FIG. 3.
Photomicrograph of intraerythrocytic piroplasms in a hu-RBC-SCID mouse whose results are presented in Fig. 2. The Giemsa-stained thin-smear blood film was prepared 19 days after inoculation of the patient's blood specimen, when human RBCs made up 87% of the peripheral RBCs of the mouse. Bar, 10 μm.
Analysis of rDNA sequence.
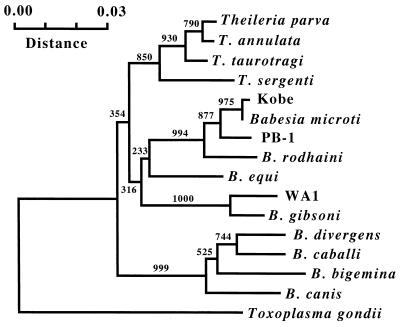
Parasite DNAs were prepared from blood samples of the patient and the mice, and the rDNAs were amplified and sequenced. Identical sequences were obtained from both types of DNA samples. A computer search of the sequences in GenBank showed the highest degree of sequence homology (99.2%) with the rDNA of the Ruebush-Peabody strain of B. microti (accession no. U09833), although differences were seen at 15 positions in the 1,767-bp sequence. We also amplified and sequenced the rDNAs of the Gray, GI, and AJ strains (reference B. microti strains from the United States), showing that these three strains have the same rDNA sequences as that reported for the Ruebush-Peabody strain. A close relationship between the Japanese isolate and the B. microti strains from the United States was further ascertained in a phylogenetic tree constructed with the rDNA sequences of related protozoan species in the phylum Apicomplexa (Fig. 4).
FIG. 4.
Phylogenetic tree constructed with rDNA sequences of various apicomplexan parasites. A portion corresponding to bases 26 to 531 in the rDNA sequence of Kobe strain (accession no. AB032434) was included in the neighbor-joining analysis. The numbers show the occurrences of branching in 1,000 bootstrap replicates.
Investigation on infection route.
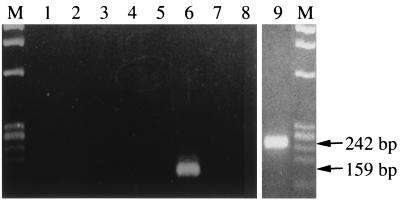
Because the patient had received a blood transfusion nearly a month before the development of symptoms of babesiosis, we suspected that this case might be associated with blood transfusion. Tracing of the blood source revealed eight donors. Their blood samples, cryopreserved at the time of donation, were tested by IFAT and rDNA-based PCR for detection of specific antibody and parasite DNA, respectively. Only a single donor was found to be positive by both the tests (indirect fluorescent-antibody titer of 1:25,600 and a specific band by nested PCR as shown in Fig. 5), whereas all seven other donors were negative (indirect fluorescent-antibody titer of <1:25 and no amplification by nested PCR). The 159-bp rDNA sequence amplified from the positive sample (lane 6) was identical to that of the rDNA from the patient. The sera from the donor and the patient at the convalescent phase exhibited high antibody titers when the Kobe strain was used as the antigen in the IFAT, but they showed only weak cross-reactions against the reference B. microti strains, strains Gray-Mo, GI, and AJ, which were isolated in the United States (Table 1).
FIG. 5.
Results of PCR amplification for the babesial nuclear small-subunit rRNA gene with template DNAs prepared from archived blood samples of the eight donors (lanes 1 to 8, respectively) and from the patient's blood (lane 9). The amplification product after the first-round PCR with primers Bab 1 and Bab 4 and those after the nested PCR with primers Bab 2 and Bab 3 were analyzed for the patient's and the donor's DNA samples, respectively. φ×174/Hae III DNA was used as a DNA size maker (lanes M).
TABLE 1.
Results of IFATs with various B. microti strains
| Serum | Reciprocal titer against:
|
|||
|---|---|---|---|---|
| Kobe | Gray-Mo | GI | AJ | |
| Patienta | 12,800 | 100 | 50 | 50 |
| Donorb | 25,600 | 100 | 100 | 100 |
| Anti-Gray-Mo | 100 | 25,600 | 12,800 | 12,800 |
| Anti-GI | 100 | 12,800 | 12,800 | 12,800 |
| Anti-AJ | 100 | 12,800 | 12,800 | 25,600 |
Serum sample obtained 5 months after the start of quinine and clindamycin treatments.
Serum sample obtained at the time of blood donation.
DISCUSSION
In the present study, we were able to isolate a B. microti-like parasite from the first patient with a clinical case of babesiosis detected in Japan. Whether the parasite should be referred to as a geographical variant of B. microti or a new species (or subspecies) remains unresolved. Analysis of the rDNA sequences indicates that the Japanese isolate is closely related, but not identical, to the reference B. microti strains isolated in the United States. The Japanese and the U.S. parasites had significant differences in their antigenicities. Similar findings have also been reported for a B. microti-like parasite isolated in Taiwan (28). Such local antigenic and genetic variations may have resulted from adaptation of B. microti to the local reservoir and vectors or simply may have resulted from the genetic drift of allopatric populations of this pan-Holarctic species (S. R. Telford, personal communication). Regardless of the clear differences in the antigenicities and rDNA sequences, other criteria, such as morphology, pathogenicity, and ecological niche, clearly place the Asian parasites in the species B. microti. At present, therefore, we should be conservative in drawing conclusions about the specific identity of the organism, pending further descriptions of the interpopulation variability of this parasite.
The hu-RBC-SCID mice were found to serve as a useful experimental tool for isolation of Babesia parasites from humans. Among the various erythroparasitic protozoan species which infect humans, only two, Plasmodium falciparum (33) and B. divergens (37), can be isolated with in vitro cultivation systems with human RBCs. Since such a system has not yet been available for B. microti, inoculation of a patient's sample into susceptible laboratory animals is currently the sole method for the isolation and propagation of parasites. Syrian hamsters have been used as the host of choice owing to their excellent susceptibility. However, several reports have documented unsuccessful parasite isolation with hamsters even in cases in which Babesia parasites were observed in the patient's blood specimens by microscopy (3, 7, 23). We have also attempted to use hamsters for isolation of parasites from the first Japanese patient found to have babesiosis. Although the parasites were capable of infecting hamsters, the levels of parasitemia varied greatly and did not increase to a level as high as that observed in hu-RBC-SCID mice (M. Tsuji, unpublished data). Our SCID mouse system appears to have some additional advantages over hamsters. For instance, propagation of parasites in immunodeficient mice may be an ideal method for minimization of the possible antigenic variation that has been detected in some Babesia species, including B. bovis (1), B. rodhaini (32), and B. microti (9). The hu-RBC-SCID mouse system may be a useful method which can directly test whether Babesia parasites obtained from wild animals have the ability to infect human RBCs. Furthermore, propagation of human-derived parasites in human RBCs may prevent selection of those variants that are more infectious for rodents. The Gray strain of B. microti, which was isolated from the American index case patient in 1969 (4), has subsequently been maintained by serial passage in hamsters. Although putatively highly infectious to humans, our preliminary study demonstrated that this strain propagated very poorly in hu-RBC-SCID mice (M. Tsuji, unpublished data), suggesting that it may no longer represent the prototypic agent of human babesiosis. An analogous finding was obtained when we compared the replication of the human-derived Kobe strain and its mouse-adapted substrain, Kobe-Mo, in hu-RBC-SCID mice.
We were able to demonstrate that the patient was infected by a blood transfusion from an asymptomatic carrier. Of the eight blood donors involved in the transfusion, only a single individual was found to be positive for the specific antibody and the parasite DNA. The donor was in good health at about the time of blood donation and did not recall any tick bites, contact with wild animals, or blood transfusion. Thus, how the donor obtained the parasite infection is not clear at this time. However, since neither the patient nor the donor had a history of travel abroad, it is reasonable to assume that the infection occurred in Japan. Even though the case of human babesiosis described here was the first case of babesiosis detected in Japan, it may simply be that human babesiosis has been undetected in the country for many years. More than 15 years ago, Shiota et al. (29) reported the presence of a Babesia sp. (probably B. microti) in Apodemus speciosus (family Muridae), which seemed to be endemic to Japan. However, rDNA sequence information for that parasite is lacking, and no conclusion may be made about its relationship to the Kobe strain.
Human babesiosis in the United States has been studied extensively since 1969, and the roles of Peromyscus leucopus and Ixodes dammini as the reservoir and the tick vector, respectively, have well been established (30). The northeastern United States is known as the area of endemicity, and special attention has been paid not only to human babesiosis but also to Lyme borreliosis and human granulocytic ehlichiosis in that region because of cotransmission by the same tick vector (13, 18). By analogy, it may be that Ixodes persulcatus, the main vector of Lyme borreliosis in Japan (21), serves as the vector of human babesiosis as well and that human granulocytic ehrlichiosis will soon be discovered as a public health burden in Japan. In the United States, physicians' awareness of tick-transmitted diseases contributes to a rapid diagnosis and appropriate case management. In this first Japanese case of babesiosis, however, the unavailability of information about the disease resulted in a significant delay in the time to diagnosis, and the patient was left untreated for nearly 4 months. Japanese investigators therefore urgently need to study the reservoir, vector ticks, regions of endemicity, and prevalence of babesiosis and associated tick-borne infections. In addition, a practical assay method for the rapid, sensitive, and specific detection of the Japanese B. microti-like parasites may be required in order to establish appropriate measures for prevention of transfusion-associated infections.
ACKNOWLEDGMENTS
We thank S. R. Telford III, Harvard School of Public Health, for critical review of and helpful discussions on the manuscript. We also thank S. K. Rai, Kobe University School of Medicine, and M. Otake and Y. Saito, Rakuno-gakuen University, for excellent technical assistance.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (grants 11660316 and 12450139) and by Gakujutsu-Frontier Cooperative Research in Rakuno-gakuen University.
REFERENCES
- 1.Allred D A, Cinque R M, Lane T J, Ahrens K P. Antigenic variation of parasite-derived antigens on the surface of Babesia bovis-infected erythrocytes. Infect Immun. 1994;62:91–98. doi: 10.1128/iai.62.1.91-98.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai S, Tsuji M, Kim S-J, Nakade T, Kanno Y, Ishihara C. Babesia canis infection in canine-red blood cell-substituted SCID mice. Int J Parasitol. 1998;28:1429–1435. doi: 10.1016/s0020-7519(98)00094-0. [DOI] [PubMed] [Google Scholar]
- 3.Dobroszycki J, Herwaldt B L, Boctor F, Miller J R, Linden J, Eberhard M L, Yoon J J, Ali N M, Tanowitz H B, Graham F, Weiss L M, Wittner M. A cluster of transfusion-associated babesiosis cases traced to a single asymptomatic donor. JAMA. 1999;281:927–930. doi: 10.1001/jama.281.10.927. [DOI] [PubMed] [Google Scholar]
- 4.Gleason N N, Healy G R, Western K A, Benson G D, Schultz M G. The Gray strain of Babesia microti from a human case established in laboratory animals. J Parasitol. 1970;56:1256–1257. [PubMed] [Google Scholar]
- 5.Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters T P M. Human babesiosis. Ann Trop Med Parasitol. 1998;92:489–501. doi: 10.1080/00034989859465. [DOI] [PubMed] [Google Scholar]
- 6.Greiner D L, Hesselton R A, Shultz L D. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 7.Herwaldt B L, Persing D H, Precigout E A, Goff W L, Mathiesen D A, Taylor P W, Eberhard M L, Gorenflot A F. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann Intern Med. 1996;124:643–650. doi: 10.7326/0003-4819-124-7-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Higgins D G, Bleasby A J, Fuchs R. Clustal V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 9.Homer M J, Bruinsma E S, Lodes M J, More M H, Telford III S, Krause P J, Reynolds L D, Mohamath R, Benson D R, Houghton R L, Reed S G, Persing D H. A polymorphic multigene family encoding an immunodominant protein from Babesia microti. J Clin Microbiol. 2000;38:362–368. doi: 10.1128/jcm.38.1.362-368.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishihara C, Tsuji M, Hagiwara K, Hioki K, Arikawa J, Azuma I. Transfusion with xenogeneic erythrocytes into SCID mice and their clearance from the circulation. J Vet Med Sci. 1994;56:1149–1154. doi: 10.1292/jvms.56.1149. [DOI] [PubMed] [Google Scholar]
- 11.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 12.Koyanagi Y, Tanaka Y, Tanaka R, Misawa N, Kawano Y, Tanaka T, Miyasaka M, Ito M, Ueyama Y, Yamanoto N. High levels of viremia in hu-PBL-NOD-scid mice with HIV-1 infection. Leukemia. 1997;3:109–112. [PubMed] [Google Scholar]
- 13.Krause P J, Telford III S R, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing D H. Concurrent Lyme disease and babesiosis; evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 14.Lewis D, Williams H. Infection of the Mongolian gerbil with the cattle piroplasma Babesia divergens. Nature. 1979;278:170–171. doi: 10.1038/278170a0. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara J, Koura M, Kamiyama T. Infection of immunodeficient mice with a mouse-adapted substrain of the Gray strain of Babesia microti. J Parasitol. 1993;79:783–786. [PubMed] [Google Scholar]
- 16.Matsui T, Inoue R, Kajimoto K, Tamekane A, Katayama Y, Shimoyama M, Chihara K, Saito-Ito A, Tsuji M. First documentation of human babesiosis in Japan. Rinsho Ketsueki. 2000;41:628–634. . (In Japanese with an English summary.) [PubMed] [Google Scholar]
- 17.Medlin L, Elwood H J, Stickel S, Sogin M L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell P D, Reed K D, Hofkes J M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore J M, Kumar N, Shultz L D, Rajan T V. Maintenance of the human malaria parasite, Plasmodium falciparum, in scid mice and transmission of gametocytes to mosquitoes. J Exp Med. 1995;181:2265–2270. doi: 10.1084/jem.181.6.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura Y, Tsuji M, Arai S, Ishihara C. A method for rapid and complete substitution of the circulating erythrocytes in SCID mice with bovine erythrocytes and use of the substituted mice for bovine hemoprotozoa infections. J Immunol Methods. 1995;188:247–254. doi: 10.1016/0022-1759(95)00222-7. [DOI] [PubMed] [Google Scholar]
- 21.Nakao M, Miyamoto K, Uchikawa K, Fujita H. Characterization of Borrelia burgdorferi isolated from Ixodes persulcatus and Ixodes ovatus ticks in Japan. Am J Trop Med Hyg. 1992;47:505–511. doi: 10.4269/ajtmh.1992.47.505. [DOI] [PubMed] [Google Scholar]
- 22.Persing D H, Mathiesen D, Marshall W F, Telford S R, Spielman A, Thomford J W, Conrad P A. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persing D H, Herwaldt B L, Glaser C, Lane R S, Thomford J W, Mathiesen D, Krause P J, Phillip D F, Conrad P A. Infection with a Babesia-like organism in northern California. N Engl J Med. 1995;332:298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 24.Quick R E, Herwaldt B J, Thomford J W, Garnett M E, Eberhard M L, Wilson M, Spach D H, Dickerson J W, Telford III S R, Steingart K R, Pollock R, Persing D H, Kobayshi J M, Juraneck D D, Conrad P A. Babesiosis in Washington state: a new species of Babesia? Ann Intern Med. 1993;119:284–290. doi: 10.7326/0003-4819-119-4-199308150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Ruebush M J, Hanson W L. Susceptibility of five strains of mice to Babesia microti of human origin. J Parasitol. 1979;65:430–433. [PubMed] [Google Scholar]
- 26.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Sanger F, Nicklen S, Cloulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih C-M, Liu L-P, Chung W-C, Ong S J, Wan C-C. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450–454. doi: 10.1128/jcm.35.2.450-454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiota T, Kurimoto H, Haguma N, Yoshida Y. Studies on Babesia first found in murine in Japan: epidemiology, morphology and experimental infection. Zentbl Bakteriol Mikrobiol Hyg Reihe A. 1984;256:347–355. [PubMed] [Google Scholar]
- 30.Telford S R, III, Gorenflot A, Brasseur P, Spielman A. Babesial infections in humans and wildlife. In: Kreier J P, editor. Parasitic protozoa. 2nd ed. Vol. 5. New York, N.Y: Academic Press. Inc.; 1993. pp. 1–47. [Google Scholar]
- 31.Thomford J W, Conrad P A, Telford III S R, Mathiesen D, Bowman B H, Spielman A, Eberhard M L, Herwaldt B L, Quick R E, Persing D H. Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J Infect Dis. 1994;169:1050–1056. doi: 10.1093/infdis/169.5.1050. [DOI] [PubMed] [Google Scholar]
- 32.Thoongsuwan S, Cox M W. Antigenic variation of the haemosporidian parasite, Babesia rodhaini, selected by in vitro treatment with immune globulin. Ann Trop Med Parasitol. 1973;67:373–385. doi: 10.1080/00034983.1973.11686903. [DOI] [PubMed] [Google Scholar]
- 33.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji M, Hagiwara K, Takahashi K, Ishihara C, Azuma I, Siddiqui W A. Theileria sergenti proliferates in SCID mice with bovine erythrocytes transfusion. J Parasitol. 1992;78:750–752. [PubMed] [Google Scholar]
- 35.Tsuji M, Ishihara C, Arai S, Hiratai R, Azuma I. Establishment of a SCID mouse model having circulating human red blood cells and a possible growth of Plasmodium falciparum in the mouse. Vaccine. 1995;13:1389–1392. doi: 10.1016/0264-410x(95)00081-b. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji M, Terada Y, Arai S, Okada H, Ishihara C. Use of the Bo-RBC-SCID mouse model for isolation of a Babesia parasite from grazing calves in Japan. Exp Parasitol. 1995;81:512–518. doi: 10.1006/expr.1995.1144. [DOI] [PubMed] [Google Scholar]
- 37.Vayrynen R, Tuomi J. Continuous in vitro cultivation of Babesia divergens. Acta Vet Scand. 1982;23:471–472. doi: 10.1186/BF03546800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wittner M, Lederman J, Tanowitz H B, Rosenbaum G S, Weiss L M. Atovaquone in the treatment of Babesia microti infections in hamsters. Am J Trop Med Hyg. 1996;55:219–222. doi: 10.4269/ajtmh.1996.55.219. [DOI] [PubMed] [Google Scholar]
- 39.Wittner M, Rowin K S, Tanowitz H B, Hobbs J F, Saltzman S, Wenz B, Hirsch R, Chisholm E, Healy G R. Successful chemotherapy of transfusion babesiosis. Ann Intern Med. 1982;96:601–604. doi: 10.7326/0003-4819-96-5-601. [DOI] [PubMed] [Google Scholar]