Abstract
Purpose
Previous studies support the potential efficacy of venovenous extracorporeal membrane oxygenation (vvECMO) for improving survival in severe acute respiratory distress syndrome (ARDS) cases. Prone positioning (PP) has been shown to improve the outcomes of moderate-to-severe ARDS patients. Few studies and no randomized controlled trials have evaluated the effect of PP performed in ECMO patients.
Methods
We performed a systematic review and meta-analysis examining the effect of prone positioning for ARDS patients receiving vvECMO on survival. All authors were contacted to obtain complementary information not mentioned in the original articles. The main objective was to compare 28-day survival in vvECMO patients with PP to vvECMO patients without PP (controls).
Results
Thirteen studies with a combined population of 1836 patients satisfied the inclusion criteria. PP was associated with a significant improvement in 28-day survival (503 survivors among 681 patients in the PP group [74%; 95% CI 71–77] vs. 450 survivors among 770 patients in the control group [58%, 95% CI 55–62]; RR 1.31 [95% CI 1.21–1.41]; I2 22% [95% CI 0–62%]; P < 0.0001). Survival was also improved in terms of other endpoints (60-day survival, 90-day survival, ICU survival, and hospital survival). In contrast, the duration of mechanical ventilation was increased in vvECMO patients with PP (mean difference 11.4 days [95% CI 9.2–13.5]; 0.64 [95% CI 0.50–0.78]; I2 8%; P < 0.0001).
Conclusion
According to this meta-analysis, survival was improved when prone positioning was used in ARDS patients receiving vvECMO. The impact of this combination on survival should be investigated in prospective randomized controlled trials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-021-06604-x.
Keywords: Mortality, ARDS, Meta-analysis, ECMO, Prone positioning
Take-home message
| Thirteen studies with a combined population of 1836 patients were included in a meta-analysis. Use of prone positioning in acute respiratory distress syndrome (ARDS) patients receiving venovenous extracorporeal membrane oxygenation (ECMO) was associated with a significant improvement in 28-day survival (74 vs 58%, P < 0.001). Survival was also improved regarding other endpoints (60- and 90-day, ICU and hospital). Duration of mechanical ventilation was increased in venovenous ECMO patients who were proned. |
Introduction
Acute respiratory distress syndrome (ARDS) is among the primary causes of intensive care unit (ICU) admission for adult patients. The current coronavirus disease 2019 (COVID-19) pandemic has further highlighted the importance of understanding the best approach to support respiratory function for patients with severe respiratory failure. Venovenous extracorporeal membrane oxygenation (ECMO) is used in patients with severe ARDS to facilitate gas exchange in the setting of profound hypoxemia and to reduce the intensity of mechanical ventilation. Two randomized controlled trials [1, 2] and a recent meta-analysis [3] support the potential efficacy of venovenous ECMO (vvECMO) for severe ARDS in improving survival. Since the results of the PROSEVA study [4], prone positioning (PP) has been considered a strategy to improve the outcomes of ARDS patients with a partial pressure of arterial oxygen to the fractional concentration of oxygen in inspired air (PaO2/FiO2) of less than 150 mmHg. Due to organizational issues and a lack of experience and appropriate medical literature, PP is generally not applied to ECMO patients. However, a rationale for the use of this adjunctive therapy in these patients has been proposed (derecruitment of the dependent part of the lungs related to a positive fluid balance, immobilization, a decreased tidal volume, and limited PEEP levels). A few years ago, some reports indicated that PP in patients receiving ECMO was safe and improved gas exchange [5–7]). More recently, PP has been suggested to be associated with an improved survival rate when applied to ARDS patients receiving ECMO [8–10]. In the absence of randomized controlled trials, we sought to perform a systematic review and meta-analysis examining the effect of prone positioning on survival in ARDS patients receiving ECMO.
Methods
Protocol and registration
The protocol of this study was preregistered on PROSPERO (CRD42021262598). This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (Table S1).
Search strategy and selection criteria
The MEDLINE via PubMed (including In-Process and Epub ahead of print) and Embase databases and the Cochrane Central Register of Controlled Trials database were systematically searched without language restrictions or period limitations. Trial registries including ClinicalTrials.gov were also considered to identify completed and ongoing trials. The electronic search for relevant theoretical references was carried out in April 2021 (more recent publications were considered until September 2021). We searched for cohort studies and randomized controlled trials including adult ARDS patients receiving vvECMO, some of whom were positioned prone during vvECMO (only studies allowing comparisons of patients under ECMO submitted to PP and ECMO patients not turned prone during ECMO). Studies including patients receiving vvECMO without PP during the ECMO period, studies including patients receiving vvECMO with PP prior to the ECMO period but not during ECMO, and studies including patients submitted only to PP and not receiving ECMO were not considered. We searched for studies referring to adults and the following subject terms: [“ECMO (extracorporeal membrane oxygenation)” OR “extracorporeal oxygenation”] AND “prone position” In Abstract AND/OR Title AND/OR keywords.
We included randomized controlled trials and observational studies in which patients undergoing invasive mechanical ventilation plus prone positioning during vvECMO were compared with patients submitted to invasive mechanical ventilation plus vvECMO without prone positioning following cannulation. We excluded studies focusing on venoarterial ECMO and those in which the use of extracorporeal CO2 removal was assessed. All authors were contacted to obtain complementary information not mentioned in the original articles regarding mortality at different time points, the duration of mechanical ventilation, and ventilator-free days (VFD) to day 28.
Data extraction
Article selection was first performed by two independent reviewers based on titles and abstracts. They then independently reviewed the full texts of studies that appeared potentially relevant to determine their eligibility for inclusion. Data extraction was also performed by the two independent reviewers with the use of a data collection form. Disagreements were resolved by a third reviewer who had the deciding vote. General and specific characteristics were obtained, including the year of publication, country, study design, the number of patients, age, disease severity, the duration of mechanical ventilation, ventilator-free days, and mortality. If needed, the researchers were contacted to obtain additional results (survival at different timepoints, the duration of mechanical ventilation, VFD to day 28).
Quality assessment
A quality assessment was performed by two independent reviewers at both the individual study level and outcome level. The Newcastle–Ottawa Scale (NOS) was used to assess the risk of bias in each included study [11]. The NOS explores the following domains: selection of the cohort, comparability of exposed and non-exposed participants and the methods for the assessment of outcomes. Studies are then rated as good-, fair-, and poor-quality research. The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) tool was used to assess the overall quality of evidence for each outcome [12]. This tool specifies four levels of certainty (high, moderate, low, and very low) depending on the design of the included studies, the risk of bias, inconsistencies in the results, the indirectness of the evidence, imprecise results, publication bias, large effects, dose–response relationships, and all plausible confounding factors and biases. Disagreements regarding the Newcastle–Ottawa Scale and GRADE assessments were resolved by discussion.
Data analysis
The primary outcome was 28-day survival. Other outcomes included hospital survival, 60-day survival, 90-day survival, ICU survival, ventilator-free days to day 28, the duration of mechanical ventilation, and in-ICU adverse events related to PP. Only exact information was used for each evaluation. As an example, no extrapolation from 90-day survival was implemented to include a study in the hospital survival assessment. Information not provided in the original articles was obtained from the authors (Fig. S1).
Outcomes were pooled using a random-effects model with the inverse variance method [13] to account for statistical heterogeneity among studies, and were summarized as risk ratio (RR) and mean differences (MDs) with their respective 95% confidence intervals (95% CIs). Between-study heterogeneity was measured using I2 statistics (values < 25% indicate low heterogeneity; 25–75%, moderate heterogeneity; and > 75%, considerable heterogeneity), the τ2 with the restricted maximum-likelihood estimator, and P values using the Cochran Q statistic [14, 15].
Sensitivity analyses were performed by serially excluding each study to determine the implications of individual studies for the pooled estimates [16].
We considered several a priori subgroup analyses for survival regarding ARDS aetiology (COVID-19 ARDS vs. non-COVID-19 ARDS) and study design (prospective vs. retrospective; monocentre vs. multicentre). An additional analysis was conducted including studies using a matching procedure to compare proned and non-proned patients [17, 18]. Subgroup analyses were performed using a mixed-effects model [19].
Potential publication bias was assessed by visual inspection of funnel plots, and plot asymmetry was considered suggestive of a reporting bias [20]. Plot asymmetry was tested using Egger’s test based on a weighted linear regression of the treatment effect on its standard error [21].
All analyses were performed using R statistical software version 4.1.1 [22] with the ‘meta’ package [23]. All significance tests were two-tailed, with P < 0.05 considered statistically significant.
Role of the funding source
This study had no funding source. The corresponding author had full access to all study data and had the final responsibility for the decision to submit this article for publication.
Results
Study characteristics
The electronic search recovered 377 citations, 34 of which were selected for full-text assessment (Fig. 1). Thirteen studies with a combined population of 1836 patients satisfied the inclusion criteria, including one randomized controlled trial [1] and 12 observational studies [8–10, 24–32], four of which were observational studies [8–10, 26] with matched controls. All these studies have been published since 2018. ECMO was initiated at a median of 1–7 days following mechanical ventilation initiation, while PP was generally initiated after a few days on ECMO (Table 1). The predominant cause of ARDS was pneumonia, and 7 of the 13 studies involved only COVID patients. In contrast, none of the four studies with matching included COVID patients. The PaO2/FiO2 ratio evaluated prior to PP showed a broad distribution (Table 1). The criteria for using PP were specified in three [9, 10, 25] of the 13 studies. In the four observational studies with matching procedures [8–10, 26], ECMO patients without PP were similar at baseline to those with PP with respect to age, the severity of illness scores, and several other clinical variables (Table 2). At least four of the same five variables (age, sex, SOFA score, and mechanical ventilation duration before ECMO and PP use before ECMO) were always used for matching (Table S2). Duration of prone positioning was generally longer than 12 h and a mean/median of 2 to 3 sessions of proning have been performed (Table S3).
Fig. 1.
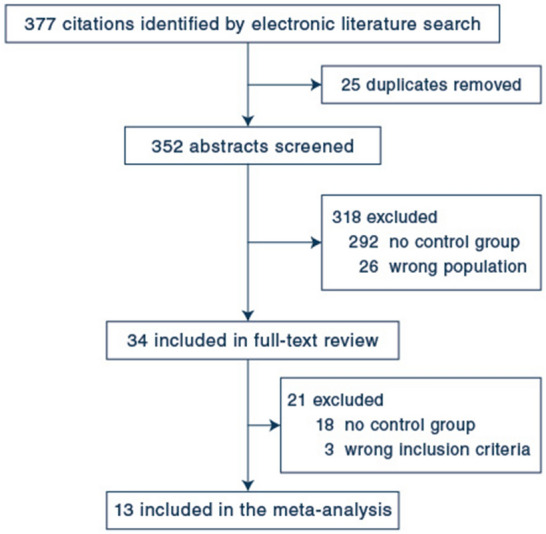
Flow diagram of study selection
Table 1.
Baseline characteristics of included studies
| Combes et al. [1], 2018 | Guervilly et al. [9], 2019 | COVID-ICU [29], 2020 | Garcia et al. [25], 2020 | Jozwiak et al. [28], 2020 | Le Breton et al. [30], 2020 | Rilinger et al. [26], 2020 | |
|---|---|---|---|---|---|---|---|
| Study type | RCTb | Observational | Observational | Observational | Observational | Observational | Observational |
| Country | France, USA, Australia, Canada | France | France, Belgium, Switzerland | France | France | France | Germany |
| N participating ICUs | 42 | 1 | 138 | 1 | 1 | 1 | 1 |
| N | |||||||
| Overall | 124 | 168 | 269 | 25 | 11 | 13 | 158 |
| PP during ECMO | 17 | 91 | 184 | 14 | 6 | 7 | 38 |
| Pneumonia as main cause of ARDS, N (%) | 80 (65) | NA | 269 (100) | 25 (100) | 11 (100) | 13 (100) | 116 (73) |
| Only COVID patients | No | No | Yes | Yes | Yes | Yes | No |
| Mean (SD) or median (IQR) duration of invasive mechanical ventilation before ECMO initiation | 1.4 (0.6–3.7) | 5 (6) | NA | 7 (4–10) | 6 (3–11) | 6 (3.5–6.5) | 1.3 (0.3–5) |
| Mean age (SD), years | |||||||
| ECMO only | NA | 53 (13) | NA | 57 (48–66) | NA | 52 (9) | 52 (39–64) |
| ECMO + PP | 49 (15) | 59 (48–63) | 47 (6) | 56 (44–64) | |||
| Male gender, N (%) | |||||||
| ECMO only | NA | 52 (67) | NA | 10 (91) | NA | 5 (83) | 78 (65) |
| ECMO + PP | 66 (72) | 12 (86) | 5 (71) | 28 (74) | |||
| Mean (SD) SOFA score at cannulation | |||||||
| ECMO only | NA | 11 (4) | NA | NA | NA | 11 (2) | 14 (11–17) |
| ECMO + PP | 10 (4) | 9 (2) | 13 (11–15) | ||||
| Indication for PP | Not specified | specified | Not specified | specified | Not specified | Not specified | Not specified |
| Mean (SD) or median (IQR) days of ECMO before PP | NA | 5 (4) | NA | 1.5 (1–3) | NA | NA | 1.7 (0.5–5) |
| Duration of PP | NA | 12 to 16 h | NA | 16 (15–17) h | NA | NA | 20 (17–21) h |
| Mechanical ventilation protocol | specified | specified | Not specified | specified | Not specified | Not specified | specified |
| Patients who received PP prior ECMO, N (%) | |||||||
| ECMO only | NA | 39 (50) | NA | 11 (100) | 5 (100) | NA | 19 (16) |
| ECMO + PP | 69 (76) | 14 (100) | 6 (100) | 7 (18) | |||
| Mean (SD) or median (IQR) PaO2/FiO2 under ECMO before PP | NA | 135 (57) | NA | 84 (73–108) | NA | NA | 77 (63–107) |
| Corticosteroids on ECMO, N (%) | 80 (65) | NA | NA | 4 (16) | NA | NA | NA |
| Primary outcome | 60-day mortality | 90-day survival | 90-day mortality | 28-day mortality | ICU mortality | ICU mortality | Hospital survival |
| Schmidt et al. [31], 2020 | Chaplin et al. [24], 2021 | Giani et al. [8], 2021 | Lebreton et al. [32], 2021 | Petit et al. [10], 2021 | Yang et al. [27], 2021 | |
|---|---|---|---|---|---|---|
| Study type | Observational | Observational | Observational | Observational | Observational | Observational |
| Country | France | New Zealand | Italy | France | France | China |
| N participating ICUs | 5 | 1 | 6 | 17 | 1 | 21 |
| N | ||||||
| Overall | 83 | 72 | 240 | 302 | 298 | 73 |
| PP during ECMO | 67 | 13 | 107 | 193 | 64 | 51 |
| Pneumonia as main cause of ARDS, N (%) | 83 (100) | 62 (86) | 221 (92) | 302 (100) | 222 (74) | 73 (100) |
| Only COVID patients | Yes | No | No | Yes | No | Yes |
| Mean (SD) or median (IQR) duration of invasive mechanical ventilation before ECMO initiation | 4 (3–6) | NA | 2 (1–6) | 5 (3–7) | 4 (1–10) |
4 (1-7) [n = 59] 1.5 (0–6) [n = 14] |
| Mean (SD) or median (IQR) age, years | ||||||
| ECMO only | NA | 46 (34–56) | 49 (13) | NA | 51 (39–60) | NA |
| ECMO + PP | 45 (36–48) | 48 (13) | NA | 53 (45–61) | ||
| Male gender, N (%) | ||||||
| ECMO only | NA | 39 (66) | 83 (62) | NA | 160 (68) | NA |
| ECMO + PP | 7 (54) | 73 (68) | NA | 43 (67) | ||
| Mean (SD) or median (IQR) SOFA score at cannulation | ||||||
| ECMO only | NA | NA | 10 (4) | NA | 14 (10–17) | NA |
| ECMO + PP | 9 (3) | NA | 13 (9–16) | |||
| Indication for PP | Not specified | Not specified | Not specified | Not specified | Specified | Not specified |
| Mean (SD) or median (IQR) days of ECMO before PP | NA | 8 (5–17.5) | 4 (2–7) | NA | 3 (2–6) | NA |
| Duration of PP | NA | 11 (7–16) | 15 (12–18) ha | NA | 16 h | NA |
| Mechanical ventilation protocol | Specified | Not specified | Specified | Specified | Specified | Not specified |
| Patients who received PP prior ECMO, N (%) | ||||||
| ECMO only | NA | NA | 38 (35) | NA | 141 (60) | NA |
| ECMO + PP | 34 (32) | NA | 55 (86) | |||
| Mean (SD) or median (IQR) PaO2/FiO2 under ECMO before PP | NA | 145 (127–158) | 135 (61) | NA | NA | NA |
| Corticosteroids on ECMO, N (%) | 17 (20) | NA | NA | 84 (28) | NA | 45 (62) |
| Primary outcome | 60-day survival | 6-month survival | Hospital mortality | 90-day survival | 90-day mortality | Hospital mortality |
aMedian (IQR)
bRCT, randomized controlled trial comparing ECMO and controls with ECMO as a late rescue strategy
Table 2.
Main characteristics of the four studies using a propensity-matched analysis
| Author | Guervilly et al. [5] | Rilinger et al. [26] | Giani et al. [8] | Petit et al. [10] | ||||
|---|---|---|---|---|---|---|---|---|
| Year of publication | 2019 | 2020 | 2021 | 2021 | ||||
| Prone positioning | Yes | No | Yes | No | Yes | No | Yes | No |
| No. patients | 50 | 50 | 38 | 38 | 66 | 66 | 59 | 59 |
| Only COVID patients | No | No | No | No | ||||
| Age | 50 ± 14 | 50 ± 14 | 51.5 (38.5–64) | 55.5 (44–62.5) | 47 ± 12 | 47 ± 14 | 53 (46–61) | 51 (45–59) |
| Male gender, no. (%) | 31 (62) | 34 (68) | 28 (74) | 32 (84) | 43 (65) | 44 (67) | 40 (68) | 42 (71) |
| SOFA score | 10 ± 4 | 11 ± 4 | 13 (11–15) | 12.0 (8.8–16) | 10 ± 3 | 10 ± 3 | 12 (8–16) | 13 (9–17) |
| Pneumonia as main cause of ARDS, no. (%) | 32 (64) | 42 (84) | 33 (87) | 32 (84) | 60 (91) | 60 (91) | 47 (80) | 45 (76) |
| Prone positioning prior ECMO, no | 34 (68) | 29 (58) | 7 (18) | 9 (24) | 16 (31)a | 26 (39) | 50 (85) | 48 (81) |
| Duration invasive mechanical ventilation—ECMO initiation | 4 ± 5 | 4 ± 5 | 2.2 (0.2–7.6) | 1.7 (0.1–6.5) | 2 (1–4) | 2 (0–4) | 4 (1–9) | 4 (2–10) |
aInformation available for 51 patients
Association of PP with survival, ventilator-free days, and mechanical ventilation duration
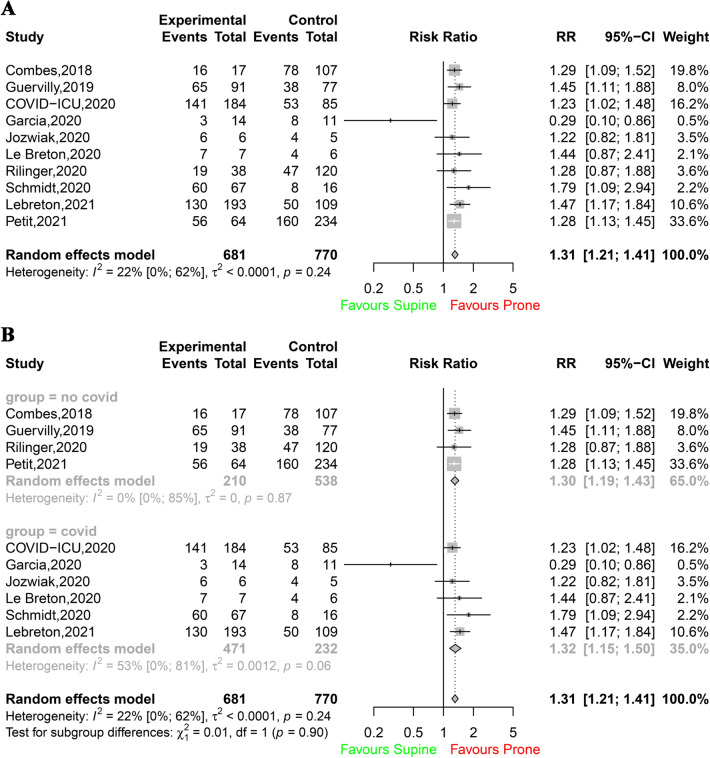
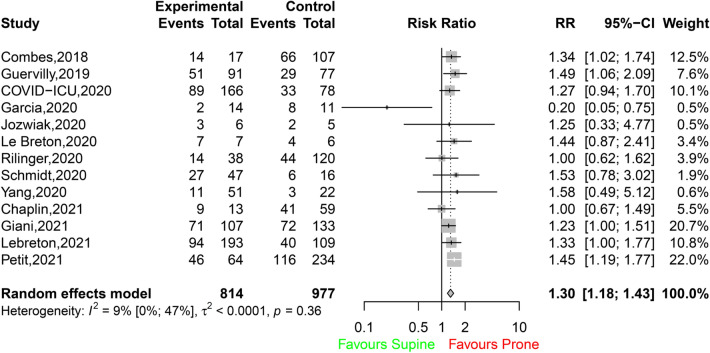
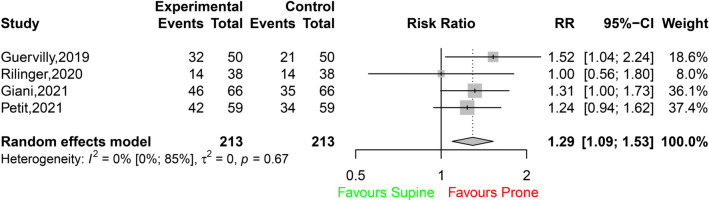
PP was associated with a significant improvement in 28-day survival (503 survivors among 681 patients in the PP group [74%; 95% CI 71–77] vs. 450 survivors among 770 patients in the control group [58%, 95% CI 55–62]; RR 1.31 [95% CI 1.21–1.41]; I2 22% [95% CI 0–62%]; P < 0.0001; Fig. 2A). This difference was observed in both COVID-19 and non-COVID-19 ECMO patients (Fig. 2B), and no significant between-group difference was detected. Additional analyses according to the designs of the studies (single centre or multicentre; prospective or retrospective; Figs. S2, S3) showed that the differences in survival between the prone group and the control group persisted (test for subgroup differences, not significant). Hospital survival was also improved when PP was applied to ECMO patients (438 survivors among 814 patients in the PP group [54%, 95% CI 50–57] vs. 464 survivors among 977 patients in the control group [47%, 95% CI 44–51]; RR 1.30 [95% CI 1.18–1.43]; I2 9% [95% CI 0–47%]; P < 0.0001; Fig. 3). The results were similar when survival was assessed at other timepoints (i.e., 60-, 90-day and ICU survival) (Table S4; Figs. S4, S5, S6). When data from the four observational studies with matching were considered, hospital survival was significantly higher in ECMO patients with PP (134 survivors among 213 patients [63%, 95% CI 56–69]) than in ECMO patients without PP among 213 patients (104 survivors [49%, 95% CI 42–56]; RR 1.29 [95% CI 1.09–1.53]; I2 0% [95% CI 0–85%]; P < 0.0026; Fig. 4).
Fig. 2.
A Survival at day 28. B Survival at day 28 according to the ARDS aetiology
Fig. 3.
Hospital survival
Fig. 4.
Hospital survival for the four studies using matching
In contrast, the duration of mechanical ventilation was increased when PP was applied to ECMO patients (mean difference 11.38 days [95% CI 9.24–13.53]; I2 8% [95% CI 0–65%]; P < 0.0001; Fig. S7), whereas the number of ventilator-free days to day 28 slightly decreased (mean difference − 1.29 day [95% CI − 2.39 to − 0.19]; I2 67% [95% CI 30–84%]; P = 0.02; Fig. S8).
The GRADE assessment of the certainty of the evidence supporting the association of PP with better outcomes was low or very low (Table S5), mainly due to the observational study design described in the quality assessment analysis (Table S6).
The associated funnel plots were globally symmetrical for the different outcomes, although the limited number of studies does not allow the exclusion of publication bias (Fig. S9). The P values of Egger’s regression intercept were all > 0.05, suggesting that the asymmetry can be considered statistically nonsignificant. Sensitivity analyses based on a serially exclusion process for each study did not change the effect on survival endpoints, confirming the robustness of our findings (Table S7; Fig. S10).
Association between PP and adverse events
Four studies [9, 10, 24, 30] included data for ECMO-related complications comparing the patients according to the use of PP. In six other studies [1, 26–28, 31, 32], these ECMO-related complications were presented without comparisons according to PP use. Specific complications due to the positioning procedure were assessed in only two studies [8, 24]. In the Chaplin et al. study [24], complications were evaluated in only 40 of the 72 included patients. No major complications (tracheal tube displacement, chest tube displacement, and intravenous catheter displacement) occurred, and six (21%) reversible pressure injuries were observed. In the Giani et al. study [8], no major complications due to PP were reported. A few minor complications assessed only in the prone group were also described in this latter study [11]. Given the inconsistent reporting of these outcomes across the studies, the adverse events were not pooled.
Discussion
This systematic review and meta-analysis of 13 studies (total N = 1836) showed that PP of ARDS patients receiving venovenous ECMO was associated with a significant improvement in 28-day survival compared with supine positioning of ECMO patients. All other timepoints, including hospital survival, showed a beneficial effect of PP on patients receiving ECMO.
Lung inflation is significantly more homogeneous in the prone position than in the supine position, inducing a more homogeneous distribution of the distending forces causing lung stress [33]. In contrast, the distribution of pulmonary blood flow is only minimally altered by gravity when turning patients prone, and the remaining blood flow predominantly perfuses (nondependent) dorsal regions, resulting in a better ventilation-to-perfusion relationship [33]. However, the improved outcome associated with PP (and documented in the PROSEVA trial) [4] is probably not related only to increased oxygenation [34] but rather to decreased levels of lung stress and strain and corresponding ventilator-induced lung injuries (VILIs). During ECMO, the supine position, the positive fluid balance, and the use of sedatives and myorelaxants may cause an increase in collapsed lung units in the dependent parts of the lungs. However, the rationale for using PP in ECMO patients must be counterbalanced by the risk of complications, especially in unexperienced centres.
The design, execution, and completion of randomized controlled trials regarding ECMO are difficult and require a long time [1, 2]. Difficulties include the need to very rapidly randomize patients with a high risk of imminent death, the inclusion of many centres in different countries, the establishment of a management protocol regarding other measures (such as mechanical ventilation settings, the use of neuromuscular blocking agents, and weaning strategies) and an ethical aspect (lack of equipoise). Indeed, PP has demonstrated a clear beneficial effect regarding outcomes [4], but many intensivists in Europe or elsewhere are reluctant to implement this technique for severe ARDS patients even if receiving ECMO. As a result, a large study evaluating the effect of PP on survival among ECMO patients with severe ARDS in the near future is unlikely. Thus, our meta-analysis provides clinicians with the most comprehensive synthesis of available evidence for the efficacy of PP in adult patients with severe ARDS placed under venovenous ECMO. Two studies aiming to evaluate the time to successful ECMO weaning are in progress (ClinicalTrials.gov Identifiers: NCT04607551 and NCT04139733) and may reinforce our conclusions.
A recent systematic review related to PP in ECMO patients has been published [35]. The cumulative survival (pooling survival proportions from the longest postdischarge timepoint reported) in patients with PP was 57% [35]. The cumulative survival among patients who were submitted to PP compared to those without PP was nonsignificant [35]. However, this latter meta-analysis [35] included only 7 studies, whereas 13 were included in the present study. Another major difference from the present study is that the primary outcome of interest was pooled cumulative survival based on studies reporting varying survival interval data, as well as survival to ICU discharge, hospital discharge, and 30-, 60- and 90-day survival [35]. In the present study, the timepoints considered were based on the exact rates obtained from the authors when not reported in the original studies.
Our study has several important limitations. Our primary results mainly result from observational studies responsible for the low or very low certainty of our findings. Due to the design of these studies, the criteria regarding the use of PP were provided in less than 25% of them. Despite having obtained from the authors many additional numbers not mentioned in their original manuscript, few missing data remained. As stated above, obtaining the results of a large interventional trial regarding the survival effect of PP in ECMO patients is very unlikely in the next 5 years. To minimize clinical heterogeneity, we performed a prespecified analysis including only the four studies using a matching process [8–10, 26]. This additional analysis confirmed the beneficial effect of proning on the survival of ECMO patients. Second, although visual inspection of funnel plots did not suggest publication bias, definitive confidence in excluding bias was limited by the small number of studies included in our plot. Finally, we could not analyse the risks of proning ECMO patients, such as cannula-associated colonization or infection, skin lesions, catheter accidental removal or ECMO circuit dysfunctions.
The use of prone positioning in adult patients with severe ARDS for venovenous ECMO was associated with improved survival across our meta-analysis. Awaiting prospective randomized controlled trials, prone positioning may be considered in adults with severe ARDS at expert, high-volume centres.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This manuscript was edited for proper English language, grammar, punctuation, spelling, and overall style by American Journal Experts.
Author contributions
LP and LB conceived and coordinated the study; LP wrote the manuscript. LB and CG critically reviewed the manuscript; MS, DH, MP, AC, GL, JR, MG, CB, TD, MJ, TW, DR, RP, and CG collected the additional required information; LB and AL performed the statistical analyses. All authors revised the manuscript and approved the final version.
Funding
No financial support.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
LP received consultancy fees from Air Liquide MS, Faron and MSD. MS received consultancy fees from Getinge, Xenios FMC and Drager. DH has no conflicts of interest. AC received grants and personal fees from Maquet, Xenios and Baxter. MP has no conflicts of interest. GL reports lecture fees from Livanova and Abiomed. JR has no conflicts of interest. MG has no conflicts of interest. CLB has no conflicts of interest. TD has no conflicts of interest. TW received consulting fees from Abbott Medical, Boehringer Ingelheim, AstraZeneca, Bayer, Abiomed and Novartis. DR has no conflicts of interest. MJ has no conflicts of interest. RP has no conflicts of interest. AL has no conflicts of interest. CG received consultancy fees from Xenios FMC. LB received consultancy fees from Lundbeck and Janssen.
Ethics approval
Not applicable. All studies have been independently reviewed and approved by the local Institutional Review Board.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A, Eolia Trial Group R, Ecmonet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 2.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, collaboration Ct Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 3.Munshi L, Del Sorbo L, Adhikari NKJ, Hodgson CL, Wunsch H, Meade MO, Uleryk E, Mancebo J, Pesenti A, Ranieri VM, Fan E. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14:S280–S288. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 4.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L, Group PS Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 5.Guervilly C, Hraiech S, Gariboldi V, Xeridat F, Dizier S, Toesca R, Forel JM, Adda M, Grisoli D, Collart F, Roch A, Papazian L. Prone positioning during veno-venous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome in adults. Minerva Anestesiol. 2014;80:307–313. [PubMed] [Google Scholar]
- 6.Kimmoun A, Roche S, Bridey C, Vanhuyse F, Fay R, Girerd N, Mandry D, Levy B. Prolonged prone positioning under VV-ECMO is safe and improves oxygenation and respiratory compliance. Ann Intensive Care. 2015;5:35. doi: 10.1186/s13613-015-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kipping V, Weber-Carstens S, Lojewski C, Feldmann P, Rydlewski A, Boemke W, Spies C, Kastrup M, Kaisers UX, Wernecke KD, Deja M. Prone position during ECMO is safe and improves oxygenation. Int J Artif Organs. 2013;36:821–832. doi: 10.5301/ijao.5000254. [DOI] [PubMed] [Google Scholar]
- 8.Giani M, Martucci G, Madotto F, Belliato M, Fanelli V, Garofalo E, Forlini C, Lucchini A, Panarello G, Bottino N, Zanella A, Fossi F, Lissoni A, Peroni N, Brazzi L, Bellani G, Navalesi P, Arcadipane A, Pesenti A, Foti G, Grasselli G. Prone positioning during venovenous extracorporeal membrane oxygenation in acute respiratory distress syndrome. A multicenter cohort study and propensity-matched analysis. Ann Am Thorac Soc. 2021;18:495–501. doi: 10.1513/AnnalsATS.202006-625OC. [DOI] [PubMed] [Google Scholar]
- 9.Guervilly C, Prud'homme E, Pauly V, Bourenne J, Hraiech S, Daviet F, Adda M, Coiffard B, Forel JM, Roch A, Persico N, Papazian L. Prone positioning and extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: time for a randomized trial? Intensive Care Med. 2019;45:1040–1042. doi: 10.1007/s00134-019-05570-9. [DOI] [PubMed] [Google Scholar]
- 10.Petit M, Fetita C, Gaudemer A, Treluyer L, Lebreton G, Franchineau G, Hekimian G, Chommeloux J, Pineton de Chambrun M, Brechot N, Luyt CE, Combes A, Schmidt M. Prone-positioning for severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005145. [DOI] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thomas J (2021) Cochrane handbook for systematic reviews of interventions. https://training.cochrane.org/handbook/current. Accessed 4 Jan 2022
- 13.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Stat Med. 2002;21:1503–1511. doi: 10.1002/sim.1183. [DOI] [PubMed] [Google Scholar]
- 18.Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ. 1994;309:1351–1355. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci. 2013;14:134–143. doi: 10.1007/s11121-013-0377-7. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Team RDC (2021) The R project for statistical computing. https://www.r-project.org/. Accessed 4 Jan 2022
- 23.Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 24.Chaplin H, McGuinness S, Parke R. A single-centre study of safety and efficacy of prone positioning for critically ill patients on veno-venous extracorporeal membrane oxygenation. Aust Crit Care. 2021;34:446–451. doi: 10.1016/j.aucc.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Garcia B, Cousin N, Bourel C, Jourdain M, Poissy J, Duburcq T, C-g LIC. Prone positioning under VV-ECMO in SARS-CoV-2-induced acute respiratory distress syndrome. Crit Care. 2020;24:428. doi: 10.1186/s13054-020-03162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rilinger J, Zotzmann V, Bemtgen X, Schumacher C, Biever PM, Duerschmied D, Kaier K, Stachon P, von Zur MC, Zehender M, Bode C, Staudacher DL, Wengenmayer T. Prone positioning in severe ARDS requiring extracorporeal membrane oxygenation. Crit Care. 2020;24:397. doi: 10.1186/s13054-020-03110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Hu M, Yu Y, Zhang X, Fang M, Lian Y, Peng Y, Wu L, Wu Y, Yi J, Zhang L, Wang B, Xu Z, Liu B, Yang Y, Xiang X, Qu X, Xu W, Li H, Shen Z, Yang C, Cao F, Liu J, Zhang Z, Li L, Liu X, Li R, Zou X, Shu H, Ouyang Y, Xu D, Xu J, Zhang J, Liu H, Qi H, Fan X, Huang C, Yu Z, Yuan S, Zhang D, Shang Y. Extracorporeal membrane oxygenation for SARS-CoV-2 acute respiratory distress syndrome: a retrospective study from Hubei, China. Front Med (Lausanne) 2020;7:611460. doi: 10.3389/fmed.2020.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jozwiak M, Chiche JD, Charpentier J, Ait Hamou Z, Jaubert P, Benghanem S, Dupland P, Gavaud A, Pene F, Cariou A, Mira JP, Nguyen LS. Use of venovenous extracorporeal membrane oxygenation in critically-ill patients with COVID-19. Front Med (Lausanne) 2020;7:614569. doi: 10.3389/fmed.2020.614569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Network C-IGobotR Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Breton C, Besset S, Freita-Ramos S, Amouretti M, Billiet PA, Dao M, Dumont LM, Federici L, Gaborieau B, Longrois D, Postel-Vinay P, Vuillard C, Zucman N, Lebreton G, Combes A, Dreyfuss D, Ricard JD, Roux D. Extracorporeal membrane oxygenation for refractory COVID-19 acute respiratory distress syndrome. J Crit Care. 2020;60:10–12. doi: 10.1016/j.jcrc.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, Baron E, Beurton A, Chommeloux J, Meng P, Nemlaghi S, Bay P, Leprince P, Demoule A, Guidet B, Constantin JM, Fartoukh M, Dres M, Combes A, Groupe de Recherche Clinique en ReSidPeIRaSU, Paris-Sorbonne E-Ci Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, Lansac E, Sage E, Cholley B, Megarbane B, Cronier P, Zarka J, Da Silva D, Besset S, Morichau-Beauchant T, Lacombat I, Mongardon N, Richard C, Duranteau J, Cerf C, Saiydoun G, Sonneville R, Chiche JD, Nataf P, Longrois D, Combes A, Leprince P, Paris E-C-i Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, Munshi L, Papazian L, Pesenti A, Vieillard-Baron A, Mancebo J. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert RK, Keniston A, Baboi L, Ayzac L, Guerin C, Proseva I. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:494–496. doi: 10.1164/rccm.201311-2056LE. [DOI] [PubMed] [Google Scholar]
- 35.Poon WH, Ramanathan K, Ling RR, Yang IX, Tan CS, Schmidt M, Shekar K. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2021;25:292. doi: 10.1186/s13054-021-03723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.