Abstract
Glycoprotein G (gG-2) of herpes simplex virus type 2 (HSV-2) is cleaved to a secreted amino-terminal portion and to a cell-associated, heavily O-glycosylated carboxy-terminal portion that constitutes the mature gG-2 (mgG-2). The mgG-2 protein is commonly used as a type-specific antigen in the serodiagnosis of HSV-2 infection. As the amino acid sequence variability of mgG-2 in clinical isolates may affect the performance of such assays, the gG-2 gene was sequenced from 15 clinical HSV-2 isolates. Few mutations were identified, and these were mostly localized outside the epitope regions described earlier. Five isolates were identical to different laboratory strains, indicating that the gG-2 gene is highly conserved over time. In the search for HSV-2 isolates harboring mutations within the immunodominant region of mgG-2, a pool of 2,400 clinical HSV-2 isolates was tested for reactivity with two anti-mgG-2 monoclonal antibodies (MAbs). Ten MAb escape HSV-2 mutants, which all harbored structurally restricted single- or dual-point mutations within the respective epitopes explaining the loss of binding, were identified. Sera from corresponding patients were reactive to mgG-2, as well as to a peptide representing the immunodominant region, suggesting that the point mutations detected did not diminish seroreactivity to mgG-2. The conservation of the gG-2 gene reported here further supports the use of mgG-2 as a type-specific antigen in the diagnosis of HSV-2 infections.
The diagnosis of herpes simplex virus type 1 (HSV-1) and HSV-2 may be performed by detection of type-specific viral antigens or of viral DNA or by demonstration of type-specific HSV antibodies. As it is accepted that the majority of HSV-2 infections are transmitted asymptomatically (32, 38), detection of HSV-2-specific antibodies is important in establishing a diagnosis of infection. Several of the membrane proteins of HSV-2 are highly immunogenic, inducing a strong antibody response in the human host (3, 4). However, most of these antigens induce a cross-reactive antibody response and are not suitable as type-specific antigens.
Glycoprotein G-2 (gG-2) is cleaved during processing (6, 33) into an amino-terminal portion which is secreted and to a cell-associated and highly O-glycosylated carboxy-terminal portion (6, 7, 21, 28, 30, 33). The latter protein, here designated mature gG-2 (mgG-2), is unique among the HSV proteins, as an exclusively type-specific antibody response has been described. Therefore, mgG-2 has been widely used as a prototype antigen for type-discriminating serology (4, 13, 14, 16, 34).
In earlier studies we have localized three regions in mgG-2 containing overlapping, linear, type-specific epitopes for anti-mgG-2 monoclonal antibodies (MAbs) and for purified human anti-mgG-2 antibodies from patients with HSV-2 infection (17). One of these regions, delimited by the amino acids (aa) 552 and 574, was shown to be immunodominant for the human antibody response. Similar peptide sequences encompassing aa 561 to 578 (22) or aa 551 to 570 (10) have been shown to be useful as target peptides in the serotesting of HSV-2-infected patients.
Although approximately half of mgG-2 is unique, showing no sequence similarities to the corresponding protein in HSV-1 (gG-1), the residues in the immunodominant region display, at least partly, a high similarity to those in the gG-1 protein. In addition, this stretch of the gG-1 amino acids was recently shown to be immunogenic, eliciting a type-specific antibody response in most HSV-1-infected patients (37). Thus, sequence variability of this segment of the gG-2 gene in clinical HSV-2 isolates may have consequences for seroreactivity in mgG-2-based assays but has hitherto not been investigated. Furthermore, alterations within the gG-2 gene might contribute to the reported variable and sometimes low sensitivity (range, 77 to 99%) found when using gG-2 antigens (4, 5, 12, 14, 16, 20, 29, 31) or mgG-2-specific peptides (10, 22) in different seroassay formats.
In this study we used two approaches to investigate the sequence variability of the gG-2 gene coding for mgG-2 in clinical HSV-2 isolates. First, we sequenced the gG-2 gene of 15 clinical HSV-2 isolates, including five isolates from patients for which the epitopes have previously been localized for the respective, purified anti-mgG-2 antibody samples (17). Second, we searched for clinical HSV-2 isolates with mutations within the immunodominant region. For this purpose, we used a type-specific anti-mgG-2 MAb in the serotyping of 2,400 clinical HSV-2 isolates. Recently we reported that 13 HSV-2 isolates were unreactive with the MAb used (18). Five of these isolates were shown to harbor frameshift mutations in the gG-2 gene, with complete inactivation of the expression of the protein products in four isolates (19). Two of these patients lacked anti-mgG-2 antibodies, indicating that a few patients can be HSV-2 infected with no detectable antibodies against mgG-2. The remaining eight MAb escape isolates are characterized here, together with two additional clinical HSV-2 isolates which were unreactive with another anti-mgG-2 MAb when 1,000 of the isolates were retested.
Overall, the gG-2 gene was well conserved, especially within the epitope regions. The MAb escape isolates displayed structurally restricted point mutations within the MAb epitopes, explaining the observed lack of binding. Sera from patients harboring these strains all showed retained reactivity to mgG-2, suggesting that these mutations did not affect the antibody response to mgG-2.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney cells (GMK-AH1) were cultured in Eagle's minimal essential medium supplemented with 2% calf serum and antibiotics. A local laboratory HSV-2 strain with the designation B4327UR, first isolated in 1970 (15); the HSV-2 strain 333, first described in 1971 (9); and an HSV-2 HG52 mutant (JH2609) devoid of mgG-2 expression (11) (kindly provided by M. Brown, Glasgow University, Glasgow, United Kingdom) were used. The laboratory strain 333 has been passaged 5 times at our laboratory since 1993, while strain B4327UR was passaged 10 times before sequencing.
The clinical HSV-2 strains which were characterized in this study were all isolated from nonimmunocompromised patients presenting with clinical genital lesions or lesions from the buttock or foot. The isolates were passaged once in GMK-AH1 cells for preparation of viral stocks.
MAbs.
Three type-specific anti-mgG-2 MAbs were used as reagents in this study. The corresponding epitopes were mapped to the following linear stretches of amino acids: 552PPPPEHR558 for MAb O1.B9.E5, 557HRGGPEE563 for MAb O1.C5.B2, and 579ATGLAFRTP587 for MAb O3.G11.H7 (17). Note that the first two epitopes are overlapping and present within the immunodominant region of mgG-2 described earlier (17). The localization of the epitopes was confirmed for the same MAbs in another study (10).
Clinical MAb escape HSV-2 isolates.
Recently, consecutive clinical HSV isolates received at the Department of Clinical Virology in Göteborg were typed by using a type-1-specific MAb (gC-1), a type-common MAb (gE), and the type-2-specific anti-mgG-2 MAb O1.C5.B2 in an enzyme immunoassay (EIA) (18). In all, 2,400 HSV-1 and 2,400 HSV-2 isolates were examined. Thirteen clinical HSV isolates were reported to be reactive with the type-common MAb but unreactive with both of the type-specific MAbs. All these isolates were confirmed to be HSV-2 positive by PCR (18). Five of the 13 isolates were shown to harbor frameshift mutations within the gG-2 gene (19), while the remaining eight isolates were clearly reactive with the anti-mgG-2 MAbs O1.B9.E5 and O3.G11.H7 and were further characterized in this study. In addition, the last 1,000 of the 2,400 clinical HSV-2 isolates were retested in an EIA using the MAb O1.B9.E5.
Sequencing of the gG-2 gene in HSV-2 isolates by PCR.
In an earlier study we mapped linear epitopes for purified anti-mgG-2 antibodies from five HSV-2-infected patients (17). As the anti-mgG-2 antibodies presented variable endpoint titers in an enzyme-linked immunosorbent assay (ELISA) and were mapped to different peptides within the described epitope regions, we investigated whether these differences could be explained by sequence variability of the gG-2 gene in the respective HSV-2 isolate. These five isolates, designated VI-769, VI-2031, VI-2086, VI-964, and VI-1217 (designations refer to patients 1 to 5 in the study described earlier [17]), were therefore included for sequencing. In addition, 10 randomly selected clinical HSV-2 isolates and the strains 333 and B4327UR were sequenced. The gene segment coding for mgG-2 was sequenced for these 17 HSV-2 isolates. The set of primers used has been described elsewhere (19).
Sequencing of the gG-2 gene segment covering the epitopes for MAbs O1.B9.E5, O1.C5.B2, and O3.G11.H7 was performed for the detected MAb escape HSV-2 isolates by using the sense primer 5′-CGCCAACGTTTCGGTCGCC (nucleotides [nt] 1530 to 1548) and the antisense primer 5′-GGGGTGGTTTGTTGGGGTTC (nt 1780 to 1761). The gG-2 gene (US4), within the HindIII l fragment of the HSV-2 reference strain HG52 (23), is numbered as nt 1 to 2097. Viral HSV-2 DNA for PCR amplification was prepared by using a QIAmp Blood Kit (Qiagen) after one passage of the viral stock in GMK-AH1 cells. The amplified products were extracted by using the Qiaex II Gel Extraction Kit, and PCR cycle sequencing was performed using fluorescent-labeled stop nucleotides. After precipitation with ethanol, the samples were analyzed using an ABI Prism 310 automated capillary sequence reader (Applied Biosystems). The sequences were compared with the HSV-2 laboratory strain B4327UR.
Antibody reactivity of patient sera to mgG-2.
Serum samples were available from seven patients harboring MAb escape HSV-2 isolates. Serum was also included from the two patients with isolates carrying a single amino acid substitution at the end of the immunodominant region of mgG-2 (VI-3089 and VI-4530) (Fig. 1) identified in the sequencing of the randomly selected HSV-2 isolates. Detection of antibodies to Helix pomatia lectin-purified mgG-2 and to a recombinant gG-1 antigen was performed by indirect ELISA as described earlier (19, 37).
FIG. 1.
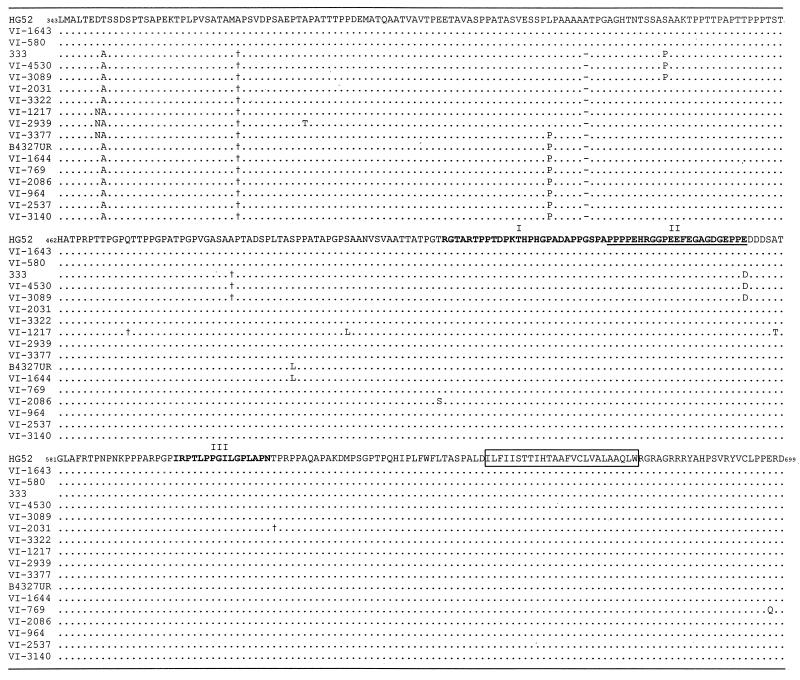
Deduced amino acid sequences of the mgG-2 protein of 15 clinical HSV-2 isolates and for the laboratory strains 333 and B4327UR. The amino acid sequence of mgG-2 of the HSV-2 reference strain HG52 (23) is aligned at the top for comparison, starting from the proposed cleavage site. The identified missense mutations are depicted for each isolate, as well as silent mutations (†) and a deletion of 3 bases coding for the amino acid alanine (−). The three epitope regions (I to III) are marked above the sequence, and the immunodominant region is underlined. The predicted transmembrane region is boxed.
Serum reactivity to a 23-mer peptide representing the immunodominant region of mgG-2 (552PPPPEHRGGPEEFEGAGDGEPPE574) (Fig. 1) was determined. The peptide was synthesized (KJ Ross-Petersen AS, Horsholm, Denmark) by 9-fluorenylmethoxycarbonyl chemistry, purified by high-pressure liquid chromatography (>90% purity), and covalently coupled amino-terminally to bovine serum albumin fraction V (Sigma Chemical Co.) at an approximately 10:1 (peptide/bovine serum albumin) molar ratio by using N-succinimidyl 3-(2-pyridyldithio)-propionate (Pharmacia Biotech) according to conditions given by the manufacturer. Maxisorp microtiter plates (Nalge Nunc Int.) were coated with peptides at a concentration of 0.1 μg/ml in carbonate buffer (pH 9.6) overnight at 4°C. Before sera were added at twofold dilutions and incubated for 1 h at 37°C, the plates were blocked with 2% skim milk in phosphate-buffered saline and washed three times in phosphate-buffered saline containing 0.05% Tween 20. Peroxidase-conjugated goat anti-human immunoglobulin G (Jackson ImmunoResearch Laboratory), added at a 1:3,000 dilution, was used as conjugate, and O-phenylenediamine was used as the substrate. The protocol was optimized in the development of a type-specific peptide-based assay for detection of anti-mgG-2 antibodies in HSV-2-infected patients (unpublished data). End point titers were expressed as the reciprocal of the dilution, giving a mean absorbance value from duplicate wells greater than the cutoff. The cutoff was defined for mgG-2 as the mean absorbance value of HSV-1-specific sera plus 0.2 optical density (OD) units, for the mgG-2 peptide as the mean absorbance values of five HSV-negative sera, and for the gG-1 antigen as the mean absorbance values of three HSV-negative sera plus 3 standard deviations.
Statistical analysis.
The end point titers for the test group of nine serum samples, drawn from patients harboring point mutations within the immunodominant region, were calculated by using the mgG-2 protein and the mgG-2-derived peptide as antigens and were compared to those for a control group representing 30 Western blot-positive HSV-2 sera, collected from patients with verified HSV-2 infection (isolation positive). The Mann-Whitney test (two-tailed) was used for comparison.
GenBank accession numbers.
The gG-2 gene sequences have been assigned to GenBank with the following accession numbers: VI-448 (AJ270567), VI-449 (AJ270566), VI-429 (AJ270568), VI-2944 (AJ270565), VI-287 (AJ270569), VI-1529 (AJ270570), VI-216 (AJ270572), VI-3023 (AJ270571), VI-1070 (AJ270573), VI-1915 (AJ270574), VI-1643 (AJ252251), VI-580 (AJ252252), strain 333 (AJ252253), VI-4530 (AJ252254), VI-3089 (AJ252255), VI-2031 (AJ252256), VI-3322 (AJ252257), VI-1217 (AJ252258), VI-2939 (AJ252259), VI-3377 (AJ252260), strain B4327UR (AJ252261), VI-1644 (AJ252262), VI-769 (AJ252263), VI-2086 (AJ252264), VI-964 (AJ252265), VI-2537 (AJ252266), and VI-3140 (AJ252267).
RESULTS
Nucleotide sequence analysis of the gG-2 gene.
DNA sequencing of the 15 clinical HSV-2 isolates and two laboratory HSV-2 strains identified few alterations compared to the HSV-2 reference strain HG52 (23) (Fig. 1). As one of the cleavage sites of the gG-2 precursor protein has been proposed to occur between the amino acids arginine342 and leucine343 (R. L. Courtney, personal communication) the latter residue was aligned at the amino-terminal end of the protein. Eleven different alleles of the gene were identified, based on point mutations that mostly were localized to specific sites and a deletion of 3 nt coding for alanine.
Two clinical isolates each were identical to the laboratory strains HG52 and 333, and one isolate was identical to the laboratory strain B4327UR. The clinical HSV-2 isolates VI-3089 and VI-4530 (identical to strain 333) harbored a single-point mutation (E574→D) localized at the carboxy-terminal end of the immunodominant region. The other isolates showed no mutations in the three epitope regions, including the five isolates (VI-769, VI-2031, VI-2086, VI-964, and VI-1217) for which the antibody responses against mgG-2 in the respective hosts have been characterized in detail (17). Note that asparagine was substituted for aspartate349 in isolates VI-1217, VI-2939, and VI-3377, thus generating a new potential N glycosylation site (349NAS351).
Reactivity of clinical MAb escape HSV-2 isolates.
Eight of 2,400 clinical HSV-2 isolates were unreactive with MAb O1.C5.B2 but reactive with MAbs O1.B9.E5 and O3.G11.H7, and 2 of 1,000 retested clinical HSV-2 isolates were unreactive with MAb O1.B9.E5. Moreover, these two isolates were unreactive with MAb O3.G11.H7 (Table 1).
TABLE 1.
Reactivity of anti-mgG-2 MAbs to 10 clinical antibody escape HSV-2 isolates in a virus-infected GMK-AH1 cell EIA
| Isolate or cell line | EIA reactivity of MAba
|
||
|---|---|---|---|
| O1.B9.E5 | O1.C5.B2 | O3.G11.H7 | |
| B4327URb | +++ | +++ | +++ |
| GMK-AH1 | 0.2 | 0.1 | 0.2 |
| HG52 JH2609c | − | − | − |
| VI-448d | +++ | − | +++ |
| VI-449d | +++ | − | +++ |
| VI-429 | +++ | − | +++ |
| VI-2944 | +++ | − | +++ |
| VI-287 | +++ | − | +++ |
| VI-1529 | +++ | − | +++ |
| VI-216 | +++ | − | ++ |
| VI-3023 | +++ | − | +++ |
| VI-1070 | − | +++ | − |
| VI-1915 | − | +++ | − |
−, OD values that are less than or equal to the reactivity to uninfected cells plus 0.15 OD units; +++, OD values of >2.0; ++, OD values between 1.5 and 2.0.
A local laboratory HSV-2 strain was used as the positive control virus.
An HSV-2 strain HG52 mutant which lacks gG-2 expression was used as the negative control virus.
Two isolates were tested from the same patient.
Sequencing of gG-2 gene for MAb escape HSV-2 isolates.
The 10 identified MAb escape HSV-2 isolates all harbored nucleotide substitutions with a resulting amino acid replacement within the respective MAb epitope (Fig. 2B). The isolates, which were unreactive with MAb O1.B9.E5, exhibited two missense mutations within the epitope. The C1660→T nucleotide mutation resulted in an amino acid substitution of P554→S, and 1666GAA1668 nucleotides were changed to AGA with the amino acid substitution of E556→R. Three additional missense mutations were found for both isolates: first, a C1562→T nucleotide mutation resulting in a T521→M amino acid substitution; second, a G1627→A nucleotide mutation with the amino acid substitution of A543→T (data not shown); and finally, a T1745→C nucleotide mutation with the following amino acid substitution of L582→P. The latter alteration was localized within the epitope described for MAb O3.G11.H7, explaining the observed unreactivity in EIA for this MAb. Isolates unreactive with MAb O1.C5.B2 (Fig. 2B) had either a G1678→A nucleotide mutation resulting in a G560→R amino acid substitution (four isolates) or a G1684→C nucleotide mutation with an amino acid substitution of E562→Q (four isolates). The isolates VI-448 and VI-449 were recovered from the same patient (VI-448 was isolated from the vulva and VI-449 from the foot 2 weeks later) and showed the same mutation. Except for isolate VI-216, which also displayed a D577→H amino acid substitution, no other alterations were found in comparison to strain HG52 (data not shown).
FIG. 2.
Aligned deduced amino acid sequence of mgG-2 for the laboratory HSV-2 strain B4327UR, covering the epitopes described for MAbs O1.B9.E5, O1.C5.B2, and O3.G11.H7. The immunodominant region is in bold (A). Detected amino acid substitutions for 10 clinical MAb escape HSV-2 isolates are depicted in bold (B).
Seroreactivity in patients harboring isolates with point mutations within the immunodominant region.
The nine sera from the test group were reactive to mgG-2 and the mgG-2 peptide (Table 2) as were the 30 tested HSV-2-positive (determined by Western blotting) sera in the control group (data not shown). The means of the ranks for the test group (n = 9) and the control group (n = 30) were 16.3 versus 21.1 for the mgG-2 antigen (P = 0.25) and 19.4 versus 20.2 for the mgG-2 peptide (P = 0.86).
TABLE 2.
Seroreactivity in an ELISA for sera of patients harboring HSV-2 isolates with point mutations within the immunodominant region of mgG-2
| Patient serum | End point titer
|
||
|---|---|---|---|
| mgG-2 antigena | mgG-2 peptideb | gG-1 antigenc | |
| 448 and 449d | 800 | 400 | 800 |
| 429 | 400 | 400 | 800 |
| 2944 | 3,200 | 800 | 3,200 |
| 287 | 200 | 400 | 400 |
| 216 | 12,800 | 6,400 | 800 |
| 3023 | 100 | 200 | 6,400 |
| 1915 | 200 | 200 | 800 |
| 4530 | 400 | 800 | 400 |
| 3089 | 800 | 400 | — |
H. pomatia lectin-purified mgG-2.
A 23-mer peptide representing the immunodominant region of mgG-2.
Recombinant gG-1. —, end point titer of less than 50.
Two isolates from the same patient.
DISCUSSION
The purpose of this study was to determine the variability of the gG-2 gene coding for mgG-2 among clinical HSV-2 isolates, with special interest in the antibody binding regions. Sequencing of 15 clinical HSV-2 isolates and 2 laboratory strains identified 11 different genetic alleles, indicating that several HSV-2 strains circulate in the Swedish population. Two clinical isolates were identical to each of the laboratory strains 333 and HG52. These strains were first described in 1971 (9, 36), indicating that the gG-2 gene is highly conserved over time. As strain 333 was isolated from a patient from North America, identical alleles of the gG-2 gene exist in the populations of two continents.
The local laboratory HSV-2 strain B4327UR has been passaged in cell culture 10 times since 1970. Although the previous number of passages of the strains 333 and HG52 is not documented in detail, the present finding of identical clinical isolates suggests that the gG-2 gene is conserved despite multiple cell culture passages. This suggestion is in agreement with a recently published study where clinical HSV-2 isolates were passaged five times without detectable alteration in the DNA sequences of the gB, gC, and gD genes (35). The conservation of investigated HSV-2 genes in the cell culture system probably reflects the accuracy of the viral DNA polymerase. Moreover, the conservation of the gG-2 gene among clinical HSV-2 isolates may be due to the low replication rate and establishment of latency but could also suggest a functional importance of the mgG-2 protein in vivo.
Except for two clinical HSV-2 isolates which harbored identical, single missense mutations at the very end of the carboxy terminus of the immunodominant region, the three epitope regions were conserved among the isolates. No mutations were found within these regions for the five isolates whose epitopes were localized in detail for purified human anti-mgG-2 antibody samples from their respective hosts. Hence, the variable end point titers and the unique pattern of binding to the different peptides covering the epitope regions that was previously presented for these five patient sera (17) could not be explained by the gG-2 gene sequence per se but may depend on the individual immune response.
In the search for clinical HSV-2 isolates carrying mutations within the immunodominant region, we identified 10 clinical MAb escape HSV-2 isolates, all of which harbored single or dual amino acid substitutions at specific sites within the MAb epitopes. The ability of single-point mutations within a MAb epitope to abolish antibody binding is well known and previously described for other HSV glycoproteins (8, 24, 25, 27, 39). The lack of reactivity in such mutants may be explained by a few residues being of key importance for binding because they provide most of the binding energy of the antigen-antibody complex (26). However, the reason why such a structurally limited number of residues is selected for substitution within the epitope as reported here is not well understood but has been described for different MAb escape mutants of HSV (24, 25, 39), as well as for influenza virus mutants (1).
Since the type-specific anti-mgG-2 MAbs can be used to detect clinical HSV-2 isolates either directly from patient specimens or after cell culture isolation, it is noteworthy that some isolates will be unreactive in such assays, due to point mutations within the epitope of the used MAb. Moreover, two isolates harbored coupled point mutations within two different MAb epitopes (O1.B9.E5 and O3.G11.H7), indicating that a reagent mixture of these two MAbs would not increase the sensitivity. However, by using a mixture of MAbs O1.C5.B2 and O1.B9.E5 or O1.C5.B2 and O3.G11.H7, false-negative results due to point mutations within the MAb epitopes in the typing of HSV-2 isolates were eliminated in this study. Such a mixture of MAbs would therefore identify all gG-2-positive clinical HSV-2 isolates.
A few clinical HSV-2 isolates (5 of 2,400) lacking the expression of mgG-2 on the viral particles and infected cell membranes due to frameshift mutations (19) will be unreactive with any of the used anti-mgG-2 MAbs. For these isolates, the use of type-specific MAbs directed against proteins other than mgG-2 or other techniques such as PCR are needed for a correct HSV-2 typing.
When the immunoglobulin G reactivity of sera from patients harboring gG-2 point mutations was analyzed, the antibody response to mgG-2 and a peptide representing the immunodominant region of mgG-2 showed similar end point titers in this group compared to the titers for control patient sera. These results argue against these mutations having been selected as an escape mechanism from the B-cell immune response during human HSV-2 infection. Similar results were found in a recently published study where point mutations detected in the gD-2 and gB-2 genes and a deletion of bases within the gB-2 gene among five investigated clinical HSV-2 isolates did not confer resistance to neutralization by guinea pig or human antisera raised against recombinant gB-2 or gD-2 proteins (35).
Although epitopes of human anti-mgG-2 antibodies have been localized to a limited region of mgG-2 (10, 17, 22), the fine specificity of those epitopes may differ, or residues other than those described here for the MAbs may have a key importance for binding. As the initial screening of clinical HSV-2 isolates using the two MAbs could detect only substitutions of amino acids that were essential for binding of the antibody, we further investigated whether residues within the epitope other than those described here could abolish the MAb binding. When the MAbs were tested for peptide libraries representing the epitopes, it could be shown that multiple substitutions within the epitope abolished binding to the MAbs (unpublished observation). These findings indicated that such mutations, if appearing in clinical HSV-2 isolates, would most likely be unreactive in the initial screening. Thus, at least the amino-terminal half of the immunodominant region of mgG-2, which was covered by the two MAbs used, is highly conserved, and a restricted repertoire of point mutations is presented among clinical HSV-2 isolates.
The finding that the antibody response to mgG-2 was retained, despite identified point mutations within the immunodominant region, suggests that sequence variability of HSV-2 isolates may be a minor problem for the performance of type-specific mgG-2-based seroassays. Instead, it is likelier that the techniques and cell lines used for the production of the antigen and the methods selected for detection of anti-mgG-2 antibodies, as well as individual host factors determining antibody responses, are more important.
In conclusion, the conservation of the gG-2 gene in clinical HSV-2 isolates documented here validates the use of mgG-2 as the prototype antigen in type-discriminating serology as well as for typing of HSV-2 isolates. Moreover, the conservation of the gG-2 gene of clinical isolates reported here might also be a prerequisite if mgG-2 is to be included as a constituent in the development of an HSV-2-specific vaccine.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Society of Göteborg, Swedish Medical Research Council (MFR, no. 11225), the LUA foundation at Sahlgren's Hospital, the Central Committee for Animal Research (CFN or centrala försöksdjursnämnden), and the Swedish Society for Medical Research.
We thank Ann-Sofi Andersson, Carolina Gustafsson, Antonina Krotochwil, and Anette Roth for skillful technical assistance.
REFERENCES
- 1.Air G M, Laver W G, Webster R G. Mechanism of antigenic variation in an individual epitope on influenza virus N9 neuraminidase. J Virol. 1990;64:5797–5803. doi: 10.1128/jvi.64.12.5797-5803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley R, Benedetti J, Corey L. Humoral immune response to HSV-1 and HSV-2 viral proteins in patients with primary genital herpes. J Med Virol. 1985;17:153–166. doi: 10.1002/jmv.1890170208. [DOI] [PubMed] [Google Scholar]
- 3.Ashley R L, Militoni J. Use of densitometric analysis for interpreting HSV serologies based on Western blot. J Virol Methods. 1987;18:159–168. doi: 10.1016/0166-0934(87)90121-2. [DOI] [PubMed] [Google Scholar]
- 4.Ashley R L, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley R L, Wu L, Pickering J W, Tu M C, Schnorenberg L. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simplex virus type-specific antibodies. J Clin Microbiol. 1998;36:294–295. doi: 10.1128/jcm.36.1.294-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balachandran N, Hutt-Fletcher L M. Synthesis and processing of glycoprotein gG of herpes simplex virus type 2. J Virol. 1985;54:825–832. doi: 10.1128/jvi.54.3.825-832.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dall'Olio F, Malagolini N, Campadelli-Fiume G, Serafini-Cessi F. Glycosylation pattern of herpes simplex virus type 2 glycoprotein G from precursor species to the mature form. Arch Virol. 1987;97:237–249. doi: 10.1007/BF01314424. [DOI] [PubMed] [Google Scholar]
- 8.Dolter K E, Goins W F, Levine M, Glorioso J C. Genetic analysis of type-specific antigenic determinants of herpes simplex virus glycoprotein C. J Virol. 1992;66:4864–4873. doi: 10.1128/jvi.66.8.4864-4873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duff R, Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971;233:48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- 10.Grabowska A, Jameson C, Laing P, Jeansson S, Sjögren-Jansson E, Taylor J, Cunningham A, Irving W L. Identification of type-specific domains within glycoprotein G of herpes simplex virus type 2 (HSV-2) recognized by the majority of patients infected with HSV-2, but not by those infected with HSV-1. J Gen Virol. 1999;80:1789–1798. doi: 10.1099/0022-1317-80-7-1789. [DOI] [PubMed] [Google Scholar]
- 11.Harland J, Brown M. Generation of a herpes simplex virus type 2 variant devoid of XbaI sites: removal of the 0.91 map coordinate site results in impaired synthesis of glycoprotein G-2. J Gen Virol. 1988;69:113–124. doi: 10.1099/0022-1317-69-1-113. [DOI] [PubMed] [Google Scholar]
- 12.Hashido M, Lee F K, Inouye S, Kawana T. Detection of herpes simplex virus type-specific antibodies by an enzyme-linked immunosorbent assay based on glycoprotein G. J Med Virol. 1997;53:319–323. doi: 10.1002/(sici)1096-9071(199712)53:4<319::aid-jmv2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Ho D W T, Field P R, Irving W L, Packham D R, Cunningham A L. Detection of immunoglobulin M antibodies to glycoprotein G-2 by Western blot (immunoblot) for diagnosis of initial herpes simplex virus type 2 genital infections. J Clin Microbiol. 1993;31:3157–3164. doi: 10.1128/jcm.31.12.3157-3164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho D W T, Field P R, Sjögren-Jansson E, Jeansson S, Cunningham A L. Indirect ELISA for the detection of HSV-2 specific IgG and IgM antibodies with glycoprotein G (gG-2) J Virol Methods. 1992;36:249–264. doi: 10.1016/0166-0934(92)90056-j. [DOI] [PubMed] [Google Scholar]
- 15.Jeansson S, Molin L. On the occurrence of genital herpes simplex virus infection. Clinical and virological findings and relation to gonorrhoea. Acta Dermato-Venereol. 1974;54:479–485. [PubMed] [Google Scholar]
- 16.Lee F K, Coleman M, Pereira L, Bailey P D, Tatsuno M, Nahmias A J. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J Clin Microbiol. 1985;22:641–644. doi: 10.1128/jcm.22.4.641-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liljeqvist J-Å, Trybala E, Svennerholm B, Jeansson S, Sjögren-Jansson E, Bergström T. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J Gen Virol. 1998;79:1215–1224. doi: 10.1099/0022-1317-79-5-1215. [DOI] [PubMed] [Google Scholar]
- 18.Liljeqvist J-Å, Svennerholm B, Bergström T. Typing of clinical herpes simplex virus type 1 and 2 isolates with monoclonal antibodies. J Clin Microbiol. 1999;37:2717–2718. doi: 10.1128/jcm.37.8.2717-2718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liljeqvist J-Å, Svennerholm B, Bergström T. Herpes simplex virus type 2 glycoprotein G-negative clinical isolates are generated by single frameshift mutations. J Virol. 1999;73:9796–9802. doi: 10.1128/jvi.73.12.9796-9802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez C, Arvin A M, Ashley R L. Immunity to herpesvirus infections in humans. In: Roizman B, Whitley R J, Lopez C, editors. The human herpesviruses. New York, N.Y: Raven Press Ltd.; 1993. pp. 397–425. [Google Scholar]
- 21.Marsden H S, Buckmaster A, Palfreyman J W, Hope R G, Minson A C. Characterization of the 92,000-dalton glycoprotein induced by herpes simplex virus type 2. J Virol. 1984;50:547–554. doi: 10.1128/jvi.50.2.547-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsden H S, MacAulay K, Murray J, Smith I W. Identification of an immunodominant sequential epitope in glycoprotein G of herpes simplex virus type 2 that is useful for serotype-specific diagnosis. J Med Virol. 1998;56:79–84. doi: 10.1002/(sici)1096-9071(199809)56:1<79::aid-jmv13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.McGeoch D J, Moss H W M, McNab D, Frame M C. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J Gen Virol. 1987;68:19–38. doi: 10.1099/0022-1317-68-1-19. [DOI] [PubMed] [Google Scholar]
- 24.Minson A C, Hodgman T C, Digard P, Hancock D C, Bell S E, Buckmaster E A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986;67:1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- 25.Muggeridge M I, Wu T T, Johnson D C, Glorioso J C, Eisenberg R J, Cohen G H. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology. 1990;174:375–387. doi: 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- 26.Novotny J, Bruccoleri R E, Saul F A. On the attribution of binding energy in antigen-antibody complexes McPC 603, D1.3, and HyHEL-5. Biochemistry. 1989;28:4735–4749. doi: 10.1021/bi00437a034. [DOI] [PubMed] [Google Scholar]
- 27.Olofsson S, Bolmstedt A, Biller M, Mårdberg K, Leckner J, Malmström B G, Trybala E, Bergström T. The role of a single N-linked glycosylation site for a functional epitope of herpes simplex virus type 1 envelope glycoprotein gC. Glycobiology. 1999;9:73–81. doi: 10.1093/glycob/9.1.73. [DOI] [PubMed] [Google Scholar]
- 28.Olofsson S, Lundström M, Marsden H, Jeansson S, Vahlne A. Characterization of a herpes simplex virus type 2-specified glycoprotein with affinity for N-acetylgalactosamine-specific lectins and its identification as g92K or gG. J Gen Virol. 1986;67:737–744. doi: 10.1099/0022-1317-67-4-737. [DOI] [PubMed] [Google Scholar]
- 29.Parkes D L, Smith C M, Rose J M, Brandis J, Coates S R. Seroreactive recombinant herpes simplex virus type 2-specific glycoprotein G. J Clin Microbiol. 1991;29:778–781. doi: 10.1128/jcm.29.4.778-781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roizman B, Norrild B, Chan C, Pereira L. Identification and preliminary mapping with monoclonal antibodies of a herpes simplex virus type 2 glycoprotein lacking a known type 1 counterpart. Virology. 1984;133:242–247. doi: 10.1016/0042-6822(84)90447-1. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Martinez D, Schmid D S, Whittington W, Brown D, Reeves W C, Chatterjee S, Whitley R J, Pellett P E. Evaluation of a test based on baculovirus-expressed glycoprotein G for detection of herpes simplex virus type-specific antibodies. J Infect Dis. 1991;164:1196–1199. doi: 10.1093/infdis/164.6.1196. [DOI] [PubMed] [Google Scholar]
- 32.Slomka M J. Seroepidemiology and control of genital herpes: the value of type specific antibodies to herpes simplex virus. Commun Dis Rep CDR Rev. 1996;6:41–45. [PubMed] [Google Scholar]
- 33.Su H K, Fetherston J D, Smith M E, Courtney R J. Orientation of the cleavage site of the herpes simplex virus glycoprotein G-2. J Virol. 1993;67:2954–2959. doi: 10.1128/jvi.67.5.2954-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svennerholm B, Olofsson S, Jeansson S, Vahlne A, Lycke E. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J Clin Microbiol. 1984;19:235–239. doi: 10.1128/jcm.19.2.235-239.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terhune S S, Coleman K T, Sekulovich R, Burke R L, Spear P G. Limited variability of glycoprotein gene sequences and neutralizing targets in herpes simplex virus type 2 isolates and stability on passage in cell culture. J Infect Dis. 1998;178:8–15. doi: 10.1086/515590. [DOI] [PubMed] [Google Scholar]
- 36.Timbury M C. Temperature-sensitive mutants of herpes simplex virus type 2. J Gen Virol. 1971;13:373–376. doi: 10.1099/0022-1317-13-2-373. [DOI] [PubMed] [Google Scholar]
- 37.Tunbäck P, Liljeqvist J-Å, Löwhagen G-B, Bergström T. Glycoprotein G of herpes simplex virus type 1—identification of type-specific epitopes by human antibodies. J Gen Virol. 2000;81:1033–1040. doi: 10.1099/0022-1317-81-4-1033. [DOI] [PubMed] [Google Scholar]
- 38.Wald A, Koutsky L, Ashley R L, Corey L. Genital herpes in a primary care clinic. Demographic and sexual correlates of herpes simplex virus type 2 infections. Sex Transm Dis. 1997;24:149–155. doi: 10.1097/00007435-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Wu C-T B, Levine M, Homa F, Highlander S L, Glorioso J C. Characterization of the antigenic structure of herpes simplex virus type 1 glycoprotein C through DNA sequence analysis of monoclonal antibody-resistant mutants. J Virol. 1990;64:856–863. doi: 10.1128/jvi.64.2.856-863.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]