Abstract
Purpose:
To investigate the contribution of radiotherapy to acute and late toxicity in pediatric chest wall sarcoma patients and evaluate dosimetric correlates of higher incidence toxicities such as scoliosis, and pneumonitis.
Methods/Materials:
The data from 23 consecutively treated pediatric patients with chest wall sarcomas of various histologies (desmoid, Ewing, rhabdomyosarcoma, nonrhabdomyosarcoma-soft tissue sarcomas (NRSTS) were reviewed to evaluate the relationship between end-organ radiation dose, clinical factors, and the risk of subsequent late effects (scoliosis, pneumonitis). Cobb angles were used to quantify the extent of scoliosis. Doses to the spine, and lung were calculated from the radiation treatment plan.
Results:
The range of scoliosis identified on follow-up imaging ranged from −47.6 to 64 degrees (median 2.95°). No relationship was identified between either radiation dose to the ipsilateral or contralateral vertebral body or tumor size and the degree or direction of scoliosis. The extent of surgical resection and number and location of resected ribs affected the extent of scoliosis. The dominant predictor of extent of scoliosis at long-term follow-up was the extent of scoliosis following surgical resection. Radiation pneumonitis was uncommon and was not correlated with mean dose or volume of lung receiving 24 Gy. However, one of three surviving patients who received whole pleural surface radiotherapy developed significant restrictive lung disease.
Conclusions:
Acute and late radiotherapy associated toxicities in pediatric chest wall sarcoma patients are modest. The degree of scoliosis following resection is a function of the extent of resection and of the number and location of ribs resected, and the degree of scoliosis at the last follow-up visit is a function of the extent of scoliosis following surgery. Differential radiotherapy dose across the vertebral body does not increase the degree of scoliosis. Severe restrictive pulmonary disease is a late complication of survivors after whole pleural surface radiotherapy.
Keywords: Whole Pleural Surface, IMRT, Sarcoma, Pediatrics, Late Toxicity
Summary
We evaluated 23 pediatric patients with chest wall sarcoma on a prospective trial for acute and late toxicities related to multimodality care, with particular emphasis on restrictive pulmonary disease and scoliosis. Disease control was comparable to that in prior series, with distant failure being the most common progression event. Restrictive pulmonary disease was uncommon but seemed to be compounded by multimodality therapy. We observed a substantial effect of the extent of surgical resection and the number and location of ribs resected on the risk of postoperative scoliosis. The greatest predictor of continued scoliosis was the extent of scoliosis prior to radiotherapy.
Introduction
Pediatric chest wall sarcomas present unique challenges to both primary and adjuvant modalities. The extent and application of surgery and radiotherapy needed to obtain local control must be balanced with the potential for increased acute and late toxicities. Surgical challenges are largely related to balancing the therapeutic impact of sufficient excision with the soft-tissue deficits resulting in late cosmetic or functional deformities.1, 2 Acute complications of consequence include an increased risk of postoperative events such as wound infection and prosthesis revisions.1 Late complications of consequence include an increased risk of scoliosis, restrictive pulmonary disease, and cosmetic aberrations due to scar, tissue and tissue deficits.3
Patient selection remains an issue for neoadjuvant and adjuvant radiotherapy, given the potential for acute and long-term functional and growth deficits.2, 4 Acute complications include an increased risk for skin and wound breakdown, pneumonitis, and soft-tissue induration.5, 6 The late complications of radiotherapy are largely related to its impact on growth and on joint and tissue fibrosis, but may also include second malignancy.4, 7–10 Furthermore, uneven distribution of radiation dose across the bony structures, such as the vertebra, is thought to place patients at increased risk for scoliosis.11 These late complications can substantially affect the development and well-being of pediatric patients.
We systematically evaluated acute and late toxicities in pediatric patients with chest wall sarcoma treated on a prospective clinical trial and noted potential contributors to acute and late toxicity. In subsequent analyses, we evaluated dosimetric correlates of scoliosis and pneumonitis.
Methods
Patient Population
Twenty-three patients (12 female; median age, 12.5 y; range 3.6–20.6 y) with chest wall sarcoma primary tumors composed of various histology’s including desmoid, Ewing Sarcoma, rhabdomyosarcoma, and other NR-STS were identified on a prospective study of radiation effects in the musculoskeletal system between January 10th, 2003 and June 11thk, 2013. The study was approved by the institutional review board. Sixteen patients (69.6%) had localized disease at diagnosis (see Table 1 for tumor characteristics).
Table 1.
Tumor and Treatment Characteristics
| Characteristic | N | % / M (range) | |
|---|---|---|---|
| Disease Extent | Localized | 16 | 69.6 |
| Metastatic | 7 | 30.4 | |
| Tumor Histology Diagnosis | Desmoid Tumor | 2 | 8.7 |
| Ewing Sarcoma | 9 | 39 | |
| Rhabdomyosarcoma | 3 | 13 | |
| Other NR-STS | 9 | 39 | |
| Tumor Laterality | Right | 15 | 65.2 |
| Left | 8 | 34.8 | |
| Total Radiotherapy Dose | Gy | 23 | 54 (41.4–81.6) |
| Radiotherapy Extent | Focal EBRT | 18 | 78.2 |
| WPS | 3 | 13.0 | |
| Focal EBRT + Brachytherapy Boost | 2 | 8.6 | |
| Radiotherapy Modality * | 3D CRT | 17 | 74 |
| IMRT | 13 | 56.5 | |
| HDR Brachytherapy | 2 | 8.7 | |
| Extent of Resection | Biopsy | 4 | 17.4 |
| Intra-lesional Excision | 2 | 8.6 | |
| Marginal Excision | 8 | 34.8 | |
| Wide Local Excision | 8 | 34.8 | |
| EPP | 1 | 4.3 | |
| Number of Ribs Removed | 0 | 10 | 43.5 |
| 1–2 | 7 | 30.4 | |
| 3+ | 11 | 47.8 | |
| Resected Rib Location | Anterior | 2 | 11 |
| Posterior | 7 | 39 | |
| Anterior & Posterior | 4 | 22 | |
| Superior | 8 | 44 | |
| Inferior | 3 | 17 | |
| Superior & Inferior | 2 | 11 | |
| NA | 10 | 43 | |
| Chemotherapy | None | 3 | 13 |
| MTX+Vinblastine | 2 | 8.6 | |
| VDC/IE | 9 | 39.1 | |
| Ifos/Dox | 4 | 17.4 | |
| VAC | 2 | 8.6 | |
| Cixutumumab, VDC/IE/VAC | 1 | 4.3 |
N - Number, M = Median, Gy = Gray; EBRT = External Beam Radiotherapy; WPS = Whole Pleural Surface; HDR = High Dose Rate, 3D-CRT = 3D conformal radiotherapy, IMRT = Intensity Modulated Radiotherapy, EPP = Extra-pleural pneumonectomy, MTX = Methotrexate, VDC = Vincristine, Doxorubicin, Cyclophosphamide, IE = Ifosfamide, Etoposide, VAC = Vincristine, Actinomycin, Cyclophosphamide, Ifos = Ifosfamide, VI = Vincristine, Irinotecan, NA = Not Applicable
= Multiple patients had components of their radiotherapy plan delivered with mixed radiotherapy modalities and as a result, percentages reported in these cells are the percent of all patients and as a result the total for these cells will be greater than 100%.
Treatment Characteristics
Surgical procedures were categorized as biopsy, intra-lesional, marginal, or wide local excision. Biopsy was defined as having <50% of the total tumor volume resected. An extra-pleural pneumonectomy was performed in one patient. Marginal and wide local excision predominated, at 34.8% each, respectively. Biopsy was performed in 17.4% of cases. The number of ribs removed at the time of resection was documented. No ribs were removed in 43.5%of cases, 1–2 ribs in 30.4% of cases, and ≥3 in 26% of cases. The location of ribs resected was evaluated according to their location along the anterior-posterior direction as demarcated by the mid-axillary line, and the superior-inferior location of resected ribs was defined according to their position above or below the T6 vertebral body.
Chemotherapy was used in 87% of cases. Desmoids were treated with methotrexate and vinblastine. Ewing sarcoma family of tumors were treated with vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide (VDC/IE). Nonrhabdomyosarcoma soft tissue sarcomas were treated with ifosfamide and doxorubicin. Rhabdomyosarcoma was treated with vincristine, actinomycin, and cyclophosphamide, except in one case of high-risk disease in which the patient enrolled on a Children’s Oncology Group study 12additionally received VDC/IE, irinotecan, and cixutumumab, a monoclonal anti-IGF-1R antibody.
Radiotherapy Plan Parameters
All patients in our series received chest radiation to varying degrees. Dose volume data for the vertebral bodies and lung were calculated across all patients. The following dosimetric parameters for the vertebral body were evaluated: Ipsilateral vertebral body (Dmean), contralateral vertebral body (Dmean), and the ratio of ipsilateral to contralateral vertebral body dose. Additional parameters analyzed included tumor distance from spine, extent of surgical resection, and tumor volume. The following dosimetric parameters were evaluated for the lung: V24 and Dmean (Supplementary Table 1). MIM v.3.0 was used to contour and extract all dose and volume data (Figure 2).
Figure 2.
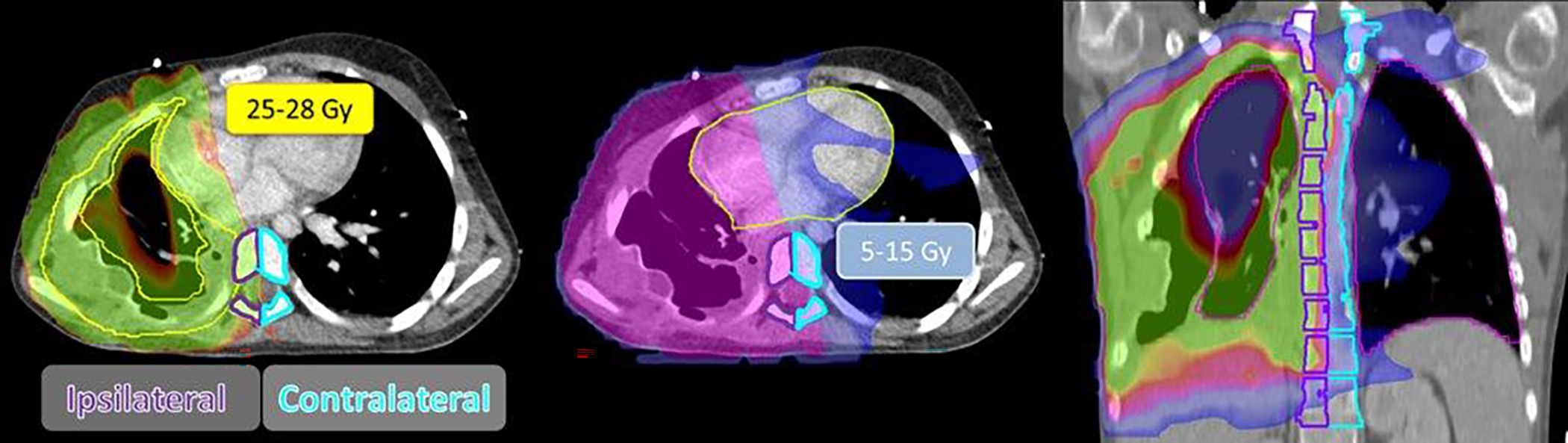
Representative Whole Pleural Surface Intensity-modulated Radiotherapy Plan from a Patient with Ewing Sarcoma.
Toxicity Assessment
Radiotherapy associated toxicities were evaluated and graded prospectively in the context of a clinical trial. Toxicities were categorized per CTCAE sections and graded according to CTCAE v3.0. Higher incidence toxicities of interest including bony hypoplasia and pneumonitis were evaluated for dosimetric correlates. The spine and lungs were systematically evaluated for acute and late toxicities. The number and rate of patients with radiation pneumonitis were tabulated over the follow-up interval (median, 9.25 y). Restrictive lung disease was assessed by spirometry and graded according to Common Terminology Criteria for Adverse Events (CTCAE) v3.0. The degree of spine curvature was assessed at baseline and at the patient’s most recent surveillance imaging (Table 2). Cobb angles were calculated by using either scout CT digitally reconstructed radiographs or anterior-posterior chest X-rays from the data collected at the last follow-up visit (Figure 1, Table 2). Angles away from the side of resection were indicated with a negative sign while those toward the side of resection were considered positive.
Table 2.
Cumulative Acute and Late Toxicity
| Toxicity Grade | I | II | III | IV |
|---|---|---|---|---|
| Acute Toxicities | ||||
| Dermatitis | 4 | 13 | 6 | 0 |
| Myositis | 5 | 1 | 0 | 0 |
| Musculoskeletal | 2 | 0 | 0 | 0 |
| Weight loss | 2 | 0 | 0 | 0 |
| Pain due to RT | 4 | 4 | 0 | 0 |
| Late Toxicities | ||||
| Bone | 4 | 3 | 3 | 1 |
| Joint | 5 | 4 | 1 | 1 |
| Liver | 0 | 0 | 1 | 0 |
| Lung | 7 | 4 | 0 | 0 |
| Skin | 13 | 2 | 0 | 0 |
| Spinal cord | 1 | 0 | 0 | 0 |
| Subcutaneous tissue | 10 | 2 | 0 | 0 |
| RT Other | 3 | 1 | 1 | 0 |
RT = Radiotherapy
Figure 1.
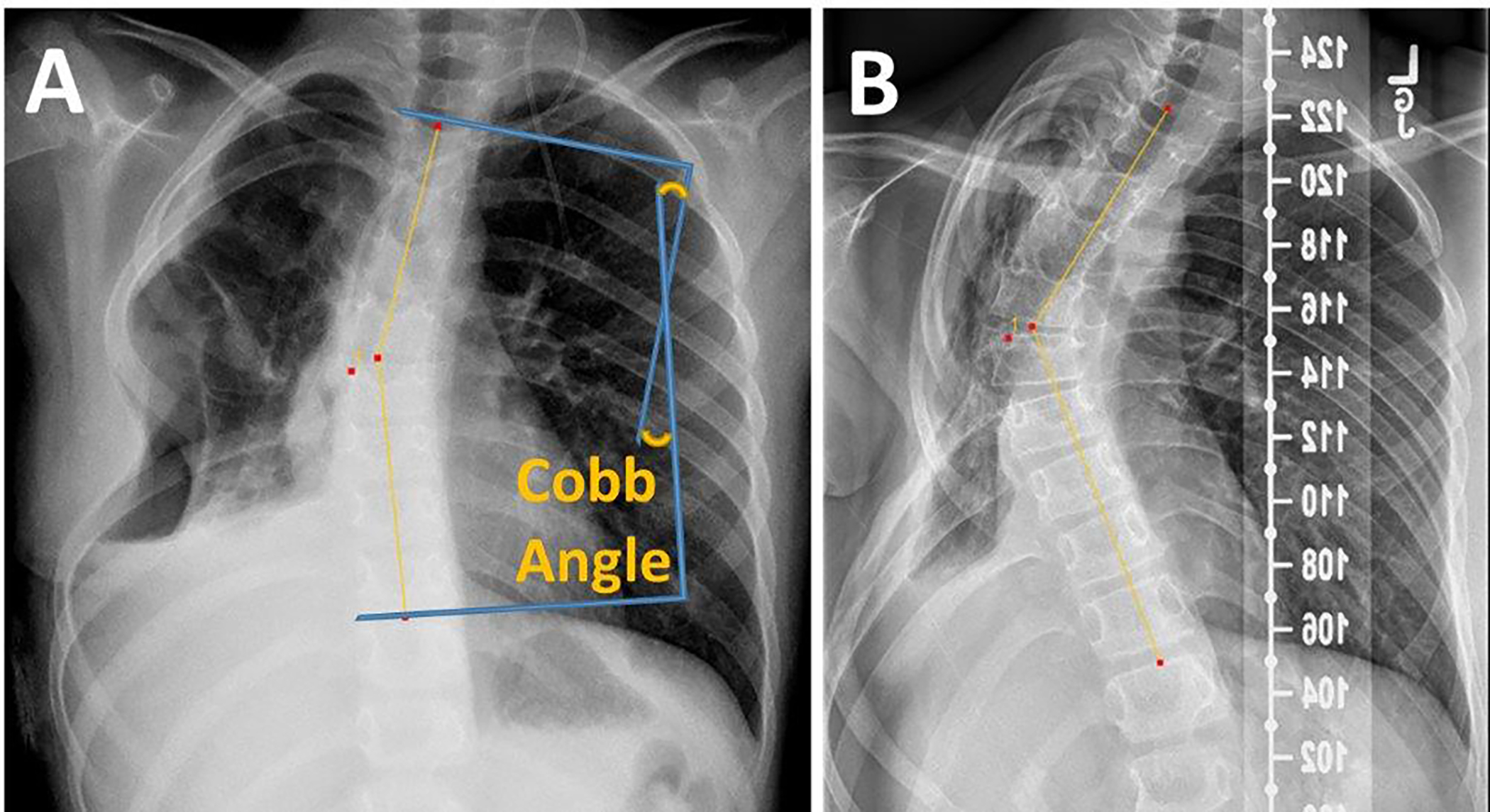
Pre- and Post-Radiotherapy Cobb Angle Measurements
Analyses
Continuous variables were summarized by using the median, range, and interquartile range; categorical data were summarized by using counts and frequencies. Time to event data were summarized by using the Kaplan Meier estimator, and differences across strata were compared by using the log-rank test. Differences in the degree of scoliosis at baseline and change over time were tested across groups via ANOVA. Relationships between vertebral body dose, number of resected ribs, and the degree of scoliosis were evaluated by using linear regression methods. SAS v 9.2 was used for all analyses (Cary, NC).
Results
Patterns of Failure and Disease Outcomes
Treatment outcomes are summarized in Supplementary Table 1 and Supplementary Figure 2. Four patients experienced local failure in the treated field, with a 5-year local control estimate of 81.4%. Two patients’ disease recurred in adjacent lymph node regions that were out-of-field relative to their original radiation plans. One of the regional failures was in the contralateral internal mammary nodal chain after whole pleural surface radiotherapy to the contralateral thoracic cavity. Two additional patients’ treatments failed within the radiotherapy field: one in the high-dose region, and the other in the initial, pre-cone-down, low-dose region. The patient whose treatment failed in the initial/low-dose region experienced failure along the pleural surface outside of the high dose cone down to gross residual disease. Six patients experienced distant failure, with a 5-year estimate of distant control of 75.3%. Thirteen patients were alive at last follow-up for a 5-year overall survival rate of 60.6%. Following treatment failure, only 12% of patients were alive at 5 years.
Toxicity
Acute toxicities were primarily limited to radiation dermatitis, with 6 patients (26.1%) experiencing grade 3 radiation dermatitis (Table 2). Pain secondary to radiation therapy was grade 1 in 4 patients (17.4%) and grade 2 in 4 (17.4%) patients. Radiation-related myositis was documented as grade 1 in 5 patients (21.7%) and grade 2 in 1 patient (4.3%).
Late toxicities were primarily limited to bone/joint, skin, and subcutaneous tissues (Table 2). Overall, grade 3 toxicity was noted in 6 patients; grade 4 toxicity was noted in 2 patients. Grade 3 toxicities included skin, bone/joint, and liver. Three late grade 3 bone/joint toxicities included severe kyphosis requiring a surgical correction. One patient had concomitant radicular pain consistent with nerve root injury. In one patient who was treated with whole pleural surface radiotherapy for alveolar rhabdomyosarcoma with pleural contamination, (grade 4, RT other – Table 2) disabling hepatic insufficiency developed secondary to sinusoidal obstruction syndrome 4 months after therapy, requiring a 3-week hospitalization. This patient was treated concurrently with vincristine and irinotecan during radiation. The mean liver dose was 13 Gy. Grade 4 toxicities included rib fracture with persistent nonunion which resulted in pseudoarthroses around the fracture of the right posterolateral fifth rib and the right posterolateral tenth rib.
Pulmonary toxicity was infrequent for patients receiving limited target volumes. Grade 2 pneumonitis was observed in four patients. Of the three patients who were treated with whole pleural surface radiotherapy, 2 succumbed to disease while one was alive after 15 months and experienced substantial right thoracic hypoplasia resulting in a severe restrictive pulmonary deficit, with a forced vital capacity of 48% predicted (1.74 L), total lung capacity of 47% predicted (2.29 L), and residual volume of 47% predicted (0.47 L) (Supplementary Figure 2).
Scoliosis
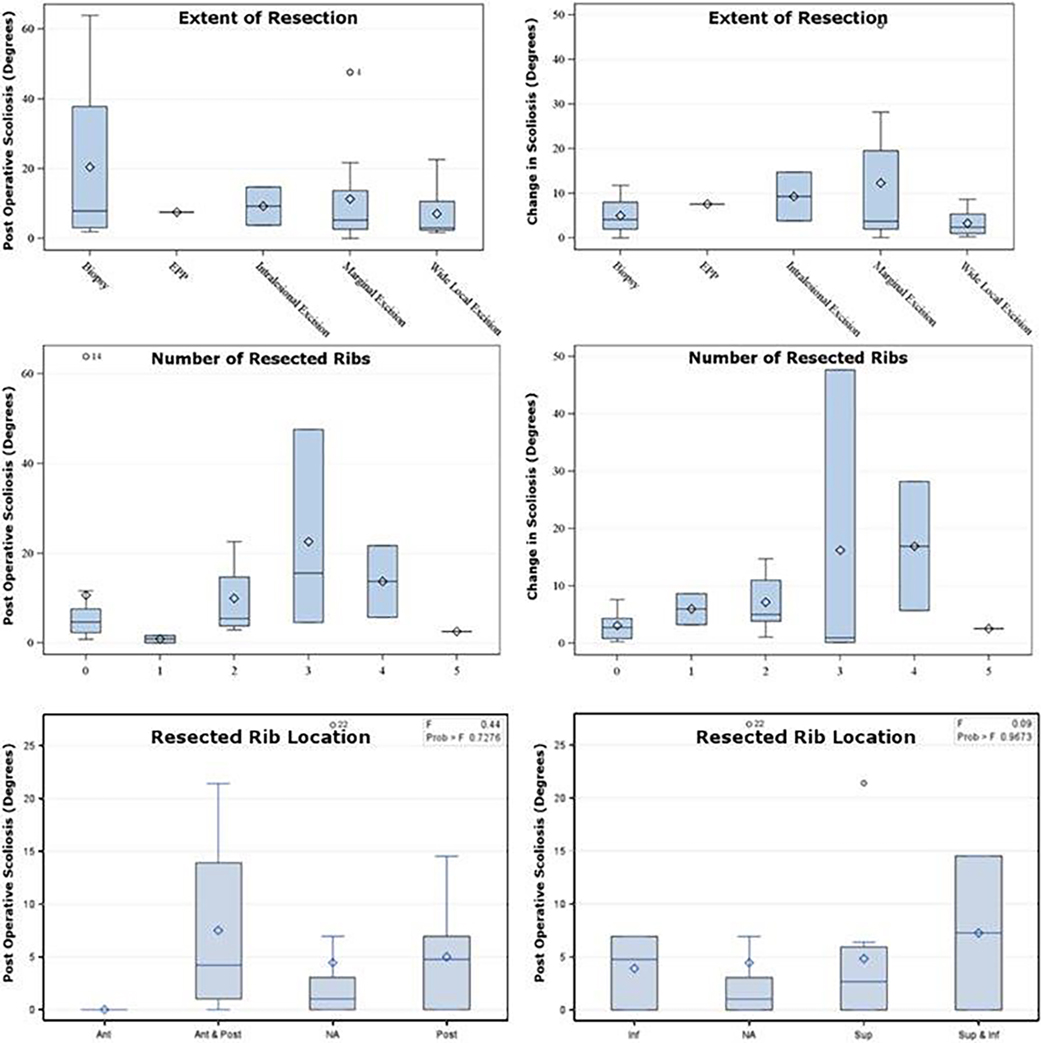
The Cobb angle was measured following surgery on the first post-operative CT or chest x-ray, and at last follow-up (Supplementary Table 3). The median, range and interquartile range of Cobb angles following surgery and at last follow-up was 0°, (−21.5–27), and [0–4.8], and 2.95°, (−47.6–63.9), and [−3.8–5.7], respectively. The median, range and interquartile range of absolute change in scoliosis measurements was 4°, (0–47.6), and [1.12–8.7]. Patients with more extensive resections at diagnosis did not have substantially different rates of scoliosis at diagnosis; however, patients who received a larger extent of resection experienced a trend toward increased scoliosis away from the site of resection over time (p=0.12) (Figure 3a). There was a trend toward an increasing degree of scoliosis among patients with a larger number of ribs removed (Figure 3b). We also observed a trend toward increasing degree of scoliosis immediately postoperatively in patients who had the more posterior portion of the rib or the entire rib (anterior and posterior) resected (Figure 3). No relationship was observed between the change in scoliosis with age, and either the ipsilateral or contralateral dose, absolute differential dose, or the ratio of dose to the ipsilateral and contralateral vertebral body dose (Figure 4). The biggest predictor of increased degree of scoliosis at last follow-up visit was the extent of scoliosis immediately post resection (R-square, 0.69) (Figure 5).
Figure 3.
Degree of Scoliosis (degrees) at Diagnosis and Absolute Change in Scoliosis (degrees) by Number of Ribs Removed and Extent of Resection.
Figure 4.

Relationship between Scoliosis and Vertebral Body Dose. A) Scoliosis at last follow-up visit as a function of the dose to the ipsilateral vertebral body (relative to tumor), B) Scoliosis at last follow-up visit as a function of the dose to the contralateral vertebral body (relative to tumor), C) Scoliosis at last follow-up visit as a function of the ipsilateral:contralateral vertebral body dose, D) Change in scoliosis from diagnosis to last-follow-up visit as a function of the ipsilateral:contralateral vertebral body dose.
Figure 5.

Change in Scoliosis (degrees) from Diagnosis by Initial Degree of Scoliosis Post-resection.
Discussion
We systematically examined the disease control and acute and late toxicities associated with multimodality therapy, with a particular emphasis on pulmonary toxicity and factors predictive of scoliosis in children with chest wall sarcomas. We found excellent local control but increased risk for distant/metastatic failure at later time points, and the greatest predictors of scoliosis were extent of surgical resection and number of resected ribs. Furthermore, patients with scoliosis prior to radiotherapy were at increased risk for progressive scoliosis later in life. Pulmonary restrictive disease was limited but seemed to be compounded by the extent of surgical resection and use of comprehensive pleural surface radiotherapy.
Comparison of local control metrics to other series remains challenging secondary to the diverse disease groups and varied histological subtypes. Other examinations of chest wall disease control include a series that noted a 5-year disease-free survival rate of 82% (95% CI, 62–92%).13 Other data about body wall nonrhabdomyosarcoma soft tissue sarcoma suggests that the risk for distant failure largely dominates the pattern of failure, with 10-year cumulative incidence rates of 15.2–23.5%.14 Similar trends have been noted in chest wall rhabdomyosarcoma and Ewing sarcoma.15, 16
A significant concern with combined modality therapy for chest wall sarcomas includes the risk for restrictive pulmonary disease. Extensive surgical resections seemed to contribute to the anatomic etiology via reduction in thoracic cavity volume and/or resection of associated lung parenchyma. In our series, radiotherapy contributed to both anatomic and intrinsic etiologies for restrictive lung disease. Anatomic restriction seemed to be related to reduced thoracic cavity volume due to soft-tissue and bony hypoplasias. Intrinsic restrictive disease seemed to be caused by pulmonary fibrosis. Anatomic and intrinsic restrictive lung disease seemed to be more pronounced in cases in which more comprehensive radiotherapy volumes were required, such as whole pleural surface radiotherapy. Although evidence of late pulmonary toxicity was observed in several cases, acute toxicity was infrequent, similar to a prior analysis of predictors of pneumonitis.5 In pediatric patients, risk of pneumonitis seemed to be lower than that in the adult population and may have a higher threshold (i.e., V24) and be related to certain systemic therapies, such as bleomycin.
Crucial to any analysis of the role of radiotherapy in scoliosis is the evaluation of dose gradients across vertebral bodies.11 The rates of minor and major pathologic changes are noted to be dose-dependent, with the recommendation that dose thresholds of 20 Gy and dose gradients of 35 Gy should be avoided.11 We were unable to show that, in the context of surgical resection, dose gradients across vertebral bodies cause increased rates of scoliosis. Although we observed no statistically significant difference, this may be due to lack of power enabling observation of only the most significant contributors to scoliosis.
Conclusions
The incidence and grade of radiotherapy associated toxicities were acceptable and comparable to other clinical series. Radiotherapy parameters were not significantly correlated with the degree or direction of scoliosis across any of the vertebral body dosimetric parameters evaluated. Our data suggest that uneven dose distributions have little effect on the development of scoliosis. Higher mean lung doses and higher lung V24 did not increase the risk of pneumonitis. Comprehensive whole pleural surface radiotherapy of the thoracic cavity may cause severe late restrictive pulmonary disease in long-term survivors.
Supplementary Material
Supplementary Table 1. Dosimetric Parameters
Supplementary Table 2. Patient Outcomes
Supplementary Table 3. Scoliosis at Baseline and Follow-up
Supplementary Figure 1. Composite Bilateral Lung Dose-Volume Histogram
Supplementary Figure 2. Patient with a Severe Restrictive Pulmonary Deficit
Supplementary Figure 3. Time to Event Outcomes
Acknowledgment:
Supported in part by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest Notification: The authors have no active or potential conflicts of interest to declare.
References
- 1.Dingemann C, Linderkamp C, Weidemann J, Bataineh ZA, Ure B, Nustede R. Thoracic wall reconstruction for primary malignancies in children: short- and long-term results. Eur J Pediatr Surg. 2012;22:34–39. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg H, van Rijn RR, Merks JH. Management of tumors of the chest wall in childhood: a review. J Pediatr Hematol Oncol. 2008;30:214–221. [DOI] [PubMed] [Google Scholar]
- 3.Soyer T, Karnak I, Ciftci AO, Senocak ME, Tanyel FC, Buyukpamukcu N. The results of surgical treatment of chest wall tumors in childhood. Pediatr Surg Int. 2006;22:135–139. [DOI] [PubMed] [Google Scholar]
- 4.Schuck A, Ahrens S, Konarzewska A, et al. Hemithorax irradiation for Ewing tumors of the chest wall. Int J Radiat Oncol Biol Phys. 2002;54:830–838. [DOI] [PubMed] [Google Scholar]
- 5.Hua C, Hoth KA, Wu S, et al. Incidence and correlates of radiation pneumonitis in pediatric patients with partial lung irradiation. Int J Radiat Oncol Biol Phys. 2010;78:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krasin MJ, Hoth KA, Hua C, Gray JM, Wu S, Xiong X. Incidence and correlates of radiation dermatitis in children and adolescents receiving radiation therapy for the treatment of paediatric sarcomas. Clin Oncol (R Coll Radiol). 2009;21:781–785. [DOI] [PubMed] [Google Scholar]
- 7.Krasin MJ, Wiese KM, Spunt SL, et al. Jaw dysfunction related to pterygoid and masseter muscle dosimetry after radiation therapy in children and young adults with head-and-neck sarcomas. Int J Radiat Oncol Biol Phys. 2012;82:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2004;60:265–274. [DOI] [PubMed] [Google Scholar]
- 9.Kaste SC, Ahn H, Liu T, et al. Bone mineral density deficits in pediatric patients treated for sarcoma. Pediatr Blood Cancer. 2008;50:1032–1038. [DOI] [PubMed] [Google Scholar]
- 10.Hua C, Shukla HI, Merchant TE, Krasin MJ. Estimating differences in volumetric flat bone growth in pediatric patients by radiation treatment method. Int J Radiat Oncol Biol Phys. 2007;67:552–558. [DOI] [PubMed] [Google Scholar]
- 11.Dorr W, Kallfels S, Herrmann T. Late bone and soft tissue sequelae of childhood radiotherapy. Relevance of treatment age and radiation dose in 146 children treated between 1970 and 1997. Strahlenther Onkol. 2013;189:529–534. [DOI] [PubMed] [Google Scholar]
- 12.Malempati S Temozolomide, Cixutumumab, and Combination Chemotherapy in Treating Patients With Metastatic Rhabdomyosarcoma. ClinicalTrials.gov Identifier: NCT01055314. https://clinicaltrials.gov/ct2/show/NCT01055314?term=NCT01055314&rank=12010.
- 13.Girelli L, Luksch R, Podda MG, et al. Surgical approach to primary tumors of the chest wall in children and adolescents: 30 years of mono-institutional experience. Tumori. 2016;102:89–95. [DOI] [PubMed] [Google Scholar]
- 14.Navid F, Billups CA, Krasin MJ, et al. Body wall and visceral nonrhabdomyosarcoma soft tissue sarcomas in children and adolescents. J Pediatr Surg. 2009;44:1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes-Jordan A, Stoner JA, Anderson JR, et al. The impact of surgical excision in chest wall rhabdomyosarcoma: a report from the Children’s Oncology Group. J Pediatr Surg. 2008;43:831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chui CH, Billups CA, Pappo AS, Rao BN, Spunt SL. Predictors of outcome in children and adolescents with rhabdomyosarcoma of the trunk--the St Jude Children’s Research Hospital experience. J Pediatr Surg. 2005;40:1691–1695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Dosimetric Parameters
Supplementary Table 2. Patient Outcomes
Supplementary Table 3. Scoliosis at Baseline and Follow-up
Supplementary Figure 1. Composite Bilateral Lung Dose-Volume Histogram
Supplementary Figure 2. Patient with a Severe Restrictive Pulmonary Deficit
Supplementary Figure 3. Time to Event Outcomes