Abstract
Background:
Disease progression after frontline therapy for DLBCL is a clinically significant event. Patients who experience early progression or have refractory disease have especially poor outcomes. Simple, clinically applicable prognostic tools are needed for selecting patients for consideration for novel therapies and prognostication in the R/R setting.
Patients and methods
Model building was performed in patients from the SEAL consortium with disease progression after frontline immunochemotherapy. The primary endpoint was overall survival (OS) measured from date of progression. Validation was performed in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER) and Danish LYFO cohorts. Model performance was assessed using time-dependent concordance indices (c-statistic) and calibration with metrics evaluated at two years from progression.
Results
1,234 of 5,112 patients treated with frontline immunochemotherapy in the SEAL consortium developed progressive disease. Time to progression on immunochemotherapy and age at progression were strongly associated with post-progression OS (both p<0.0001). A prognostic model was developed incorporating spline fit for both variables. The model had good concordance in the discovery (0.67) and validation sets (LYFO c=0.64, MER c=0.68) with generally good calibration.
Conclusions
Time to progression on frontline therapy is strongly associated with post-progression OS in DLBCL. We developed and validated a simple to apply clinical prognostic tool in the R/R setting. The useful prediction of expected outcomes in R/R DLBCL and can inform treatment decisions such as considerations for CART therapy as well as trial designs. The model is available in smartphone-based point of care applications.
Keywords: DLBCL, relapsed/refractory, prognostic model
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive lymphoma subtype in the western world with approximately 25,000 new cases diagnosed annually in the United States [1]. Standard frontline therapy of DLBCL consists of CD20 antibody therapy (e.g. rituximab) plus an anthracycline based multi-agent chemotherapy (R-CHOP) [2] [3] [4]. Nearly all DLBCL patients will have disease responsive to R-CHOP or similar immunochemotherapy (IC), and the approximately 80% patients in remission after IC move into a surveillance period without any further treatment. However 30-35% of patients with DLBCL will develop progressive disease within 5 years of starting frontline IC [5] [6]. These patients have poor subsequent outcomes, with only 20-30% achieving durable remission to subsequent therapy [7]. Outcomes in relapsed or refractory (R/R) DLBCL are heterogeneous and patients with early relapsing or refractory disease after R-CHOP have particularly poor outcomes and represent an unmet clinical need [8] [9]. As a result, novel therapies for R/R DLBCL are a particularly active area of drug development. Many clinical trials in the R/R setting are single arm [10] [11] with widely varying eligibility criteria which may include age, transplant eligibility, and/or lines of prior therapy. The heterogeneity of outcomes in the R/R setting [12] and differences in inclusion/exclusion criteria makes it difficult to compare outcomes across these trials. Thus, risk assessment in the setting of R/R DLBCL has both clinical and research utility. The aims of this study were to evaluate clinical predictors of outcome at the time of first relapse or progression of DLBCL after frontline IC and then build and validate a clinically applicable risk model in this setting.
Patients and Methods
SEAL Cohort
Model building was performed on individual patient-level data from R/R DLBCL cases from the Surrogate Endpoint for Aggressive Lymphoma (SEAL) study. Details on the SEAL study [13] and the cohort [14] have been previously published. All patients from the SEAL cohort utilized in the analysis were enrolled on one of 13 randomized clinical trials [2] [3] [4] for newly diagnosed DLBCL and had disease which progressed or relapsed after frontline rituximab plus anthracycline based IC. Time to progression (TTP) on immunochemotherapy was defined as the date from study entry to the first relapse or progression. This study was approved by the Institutional Review Board at the Mayo Clinic; research was conducted in accordance with the Declaration of Helsinki.
LYFO Cohort
The model was validated in a retrospective cohort from the Danish National Lymphoma Register (LYFO), which includes information on nearly all (~95%) lymphoma patients in Denmark [15]. All haematology centers in Denmark prospectively report data to the register, including clinicopathologic information, treatment, disease response, relapse, and vital status. Complete follow-up on vital status is ensured by merging with the Danish Civil Registration System [16]. Patients included in this study were diagnosed in the period 2006-2017 and registered with progression or relapse following frontline IC for DLBCL. Patients with only low-grade disease at relapse were excluded. The use of data from LYFO was approved by the Danish Data Protection Agency (record no: 2008-58-0028).
MER Cohort
Validation was also performed in patients prospectively enrolled within 9 months of initial diagnosis into the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Cohort (MER). All patients provided informed consent on enrollment in the cohort. Details on the MER have been previously published [17]. Patients included in this study had disease progression, relapse, or initiation of second line therapy after frontline IC for DLBCL. Patients with low-grade only disease at relapse[18] were excluded.
Statistical methods
The primary outcome for R/R DLBCL was overall survival (OS), defined as the date of first progression/relapse to date of death due to any cause. Age and TTP were the only clinical variables available at the time of progression; all other clinical variables were from the time of initial diagnosis only. Cox models were used to evaluate associations between clinical variables and OS from progression; restricted cubic splines were used to model the functional form of both variables. Survival outcomes were also evaluated graphically using Kaplan-Meier curves. Clinical application of the model was a primary goal in model building, recognizing that complete data from the time of initial diagnosis may not always be available at the clinical assessment of progression. Thus, the primary model strategy focused on variables available at progression. Model performance was assessed using the concordance index (c-statistic) [19] and calibration with metrics evaluated at 24 months from progression (OS24). Analyses were performed using SAS/Rv3.6.1.
Results
SEAL Patient Characteristics
5,112 patients were treated with frontline IC in the SEAL cohort. At a median follow-up of 34 months from initiation of frontline IC (range 0 – 191), 1,234 of the 5,112 (24%) patients had disease progression, which comprised the discovery dataset, table 1. The median age at progression was 68 years (IQR 57-74) with 55% being male and 50% having an IPI of 3-5 at diagnosis. At a median follow-up from initial progression of 11 months (range 0-167), 795 patients (64%) had died. The 24 month OS from progression was 35% (95% CI: 32-38).
Table 1.
Patient Characteristics of R/R DLBCL Evaluated in Model
| Characteristics | SEAL All | SEAL* (R/R- IPI Model Subset) |
LYFO Cohort (Validation) |
MER Cohort (Validation) |
|---|---|---|---|---|
| (N=1234) | (N=1036) | (N=592) | (N=305) | |
| Age at Diagnosis (Years) | ||||
| Mean (SD) | 62.6 (12.8) | 64.3 (9.2) | 65.5 (9.1) | 62.1 (9.7) |
| Median | 66 | 66 | 66 | 62 |
| IQR | 56.0, 71.8 | 59.0, 71.1 | 60.0, 72.0 | 56.0, 70.0 |
| Range | 19.0, 87.5 | 39.0, 79.2 | 41.0, 80.0 | 39.0, 79.0 |
| Age at Progression (Years) | ||||
| Mean (SD) | 64.1 (13.2) | 65.5 (9.4) | 66.9 (9.2) | 63.2 (9.8) |
| Median | 67.5 | 67.5 | 68 | 63.2 |
| IQR | 57.4, 73.5 | 59.7, 72.8 | 61.0, 74.0 | 57.4, 71.4 |
| Range | 19.2, 92.0 | 40.1, 79.9 | 41.0, 85.0 | 40.1, 79.8 |
| Sex, n (%) | ||||
| Male | 675 (54.7%) | 572 (55.2%) | 360 (60.8%) | 116 (38.0%) |
| Female | 559 (45.3%) | 464 (44.8%) | 232 (39.2%) | 189 (62.0%) |
| Time to Progression (Months) | ||||
| Mean (SD) | 18.4 (19.5) | 15.3 (12.7) | 16.9 (19.8) | 13.1 (11.9) |
| Median | 11.3 | 10.9 | 9.7 | 8.1 |
| IQR | 6.3, 22.6 | 6.3, 20.0 | 5.8, 18.1 | 5.1, 18.3 |
| Range | 0.1, 167.0 | 0.1, 59.4 | 1.3, 130.7 | 0.5, 56.6 |
| Ann Arbor Stage (Diagnosis) | ||||
| I & II | 284 (23.0%) | 215 (20.8%) | 123 (20.8%) | 63 (20.7%) |
| III & IV | 949 (77.0%) | 820 (79.2%) | 467 (79.2%) | 242 (79.3%) |
| Missing | 1 | 1 | 2 | |
| ECOG Performance Status (Diagnosis) | ||||
| 0-1 | 998 (80.9%) | 834 (80.5%) | 450 (76.7%) | 236 (77.4%) |
| 2-4 | 236 (19.1%) | 202 (19.5%) | 137 (23.3%) | 69 (22.6%) |
| Extranodal Sites (Diagnosis) | ||||
| <= 1 | 660 (62.3%) | 538 (60.7%) | 378 (63.9%) | 236 (77.4%) |
| > 1 | 400 (37.7%) | 348 (39.3%) | 214 (36.1%) | 69 (22.6%) |
| Missing | 174 | 150 | 0 | |
| LDH Status (Diagnosis) | ||||
| Not Elevated | 384 (31.6%) | 312 (30.5%) | 152 (26.5%) | 72 (23.6%) |
| Elevated | 833 (68.4%) | 712 (69.5%) | 421 (73.5%) | 233 (76.4%) |
| Missing | 17 | 12 | 19 | |
| IPI Score (Diagnosis) | ||||
| 0-1 | 245 (19.9%) | 177 (17.1%) | 79 (13.9%) | 54 (17.7%) |
| 2 | 378 (30.6%) | 318 (30.7%) | 131 (23.1%) | 100 (32.8%) |
| 3 | 393 (31.8%) | 338 (32.6%) | 173 (30.5%) | 102 (33.4%) |
| 4-5 | 218 (17.7%) | 203 (19.6%) | 184 (32.5%) | 49 (16.1%) |
| Induction Treatment (Diagnosis) | ||||
| R-CHOP21 | 599 (48.5%) | 491 (47.4%) | 185 (31.3%) | 223 (73.1%) |
| Other† | 635 (51.5%) | 545 (52.6%) | 407 (68.8%) | 82 (26.9%) |
Not R-CHOP21
SEAL R/R IPI final modelling dataset restricted to age 40-80 and TTP between 0 and 60 month
Abbreviations: IC, Immunochemotherapy; TTP, Time to Progression; R/R, Relapse or Refractory; LDH, Lactate Dehydrogenase; IPI, International Prognostic Index; SEAL, Surrogate Endpoint for Aggressive Lymphoma; LYFO, Danish National Lymphoma Register; MER, Iowa/Mayo SPORE Molecular Epidemiology Resource.
Model development and discovery results
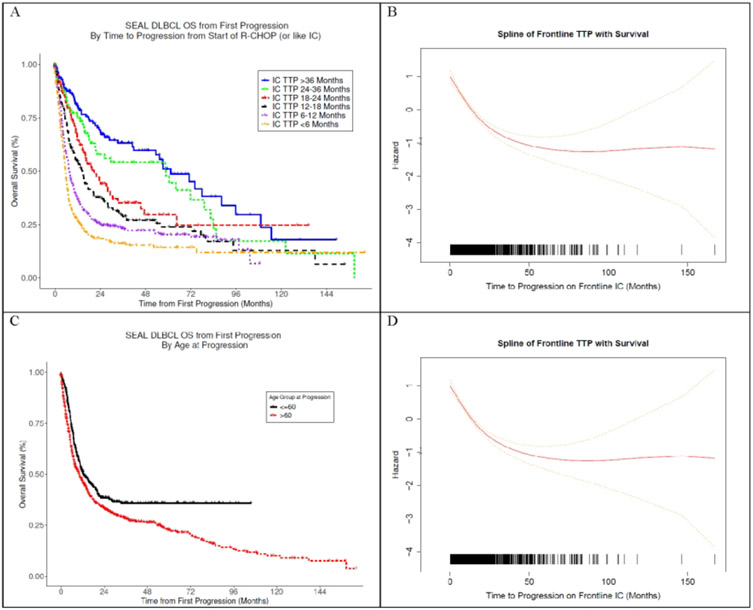
Cox models were used to evaluate the univariate association between clinical variables at the time of progression with post-progression survival (ppOS). TTP was strongly associated with ppOS (per-month linear HR=0.97, 95% CI: 0.96 – 0.98). OS24 ranged from 18% in patients with TTP < 6 months compared to 69% in patients with relapse than 3 years after starting IC treatment, Figure 1A. Examination of the functional form (Figure 1B) shows a continuous association between TTP and ppOS that is monotonic over the range of TTP, with inflection points at around 2 and 4 years after initiation of treatment. Age at the time of progression was also strongly associated with ppOS (per-year HR = 1.01, 95% CI: 1.01-1.02). Outcomes were modestly different using a typical age-60 split (Figure 1C); however, the functional form shows a monotonic linear association above age 50 (Figure 1D). The proposed R/R-IPI model was then built in a Cox model incorporating on the functional forms (splines) of TTP and age at progression. Model parameters were determined using a subset of patients (N=1234) between ages 40-80 years and with TTP between 0-60 months due to sparse data outside these intervals and concerns about model extrapolation. A nomogram representation of the R/R-IPI model is shown in Figure 2. Uncorrected model concordance was 0.67 and the calibration curve is shown in Figure 3A.
Figure 1:
(A) Kaplan Meier Curve of Overall Survival from First Progression by Time to Progression (Months) on Frontline Immunochemotherapy (IC) in SEAL Cohort. (B) Functional Form of the Association Between Time to Progression on IC (Continuous Months) and Overall Survival in SEAL Cohort (C) Kaplan Meier Curve of Overall Survival from First Progression by Age at Progression in SEAL Cohort. (D) Functional Form of the Association Between Age at Progression (Continuous Years) and Overall Survival in SEAL Cohort
Figure 2:
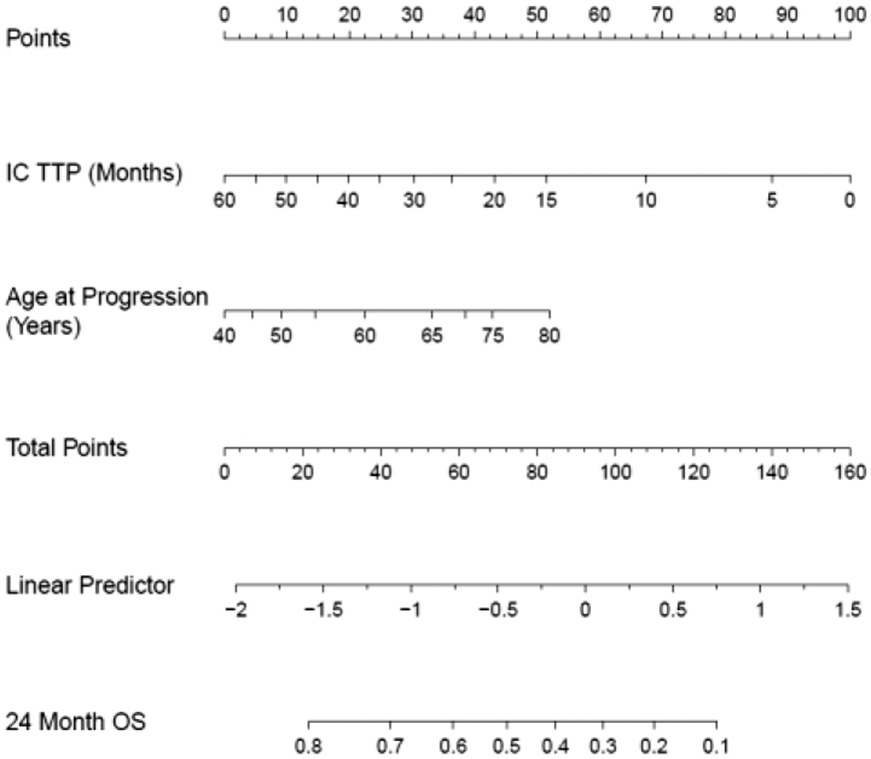
Nomogram for the R/R IPI Model
Figure 3:
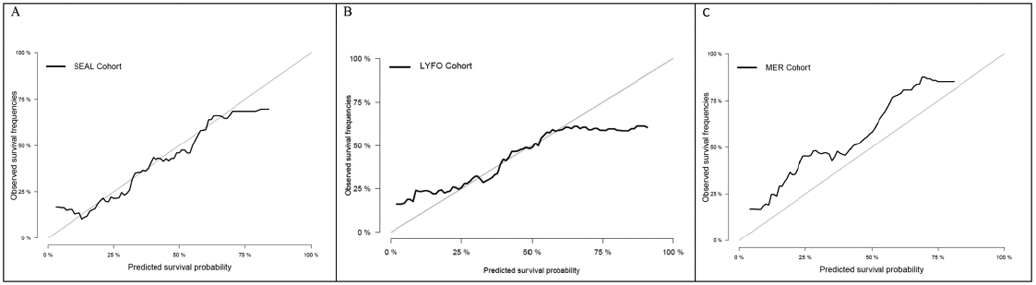
Calibration Curve (A) SEAL Cohort (Discovery) (B) LYFO Cohort (Validation) (C) MER Cohort (Validation)
Model Dissemination
An electronic calculator of the model to facilitate quick and accurate model implementation is currently available at https://qxmd.com/calculate/calculator_682/prognosis-calculator-for-relapsed-refractory-dlbcl as well as in the QxMD Calculate smartphone apps for point-of-care clinical use.
Validation (LYFO)
3,421 patients between ages 40 and 80 years of age with DLBCL were treated with frontline IC between 2006 and 2017 in the LYFO cohort. At a median follow-up of 77 months from diagnosis(range 0-155), 592 of the 3,421(17%) patients had disease progression and comprised the validation cohort. The median age at progression was 68 years (IQR 61-74) with 61% being male and 63% having an IPI of 3-5 at diagnosis. At a median follow-up from initial progression of 79 months (range 0-151), 450 patients (76%) had died and 2-year OS was 35% (95% CI: 31-39), Supplemental Figure S1B. The predicted 2-year survival in the cohort (calibration in the large) was 32%. Concordance of the R/R-IPI model was 0.64, and the calibration curve is shown in Figure 3B.
Validation (MER)
1304 patients with DLBCL were treated with frontline IC between 2002 and 2015 in the MER cohort. At a median follow-up of 85 months from initiation of frontline IC (range 0-204), 305 of the 1304 (23%) patients initiated 2nd line therapy for DLBCL and comprised the validation cohort. The median age at progression was 63 years (IQR 57-71) with 62% being male and 50% having an IPI of 3-5 at diagnosis. At a median follow-up from initial progression of 77 months (range 4-177), 213 patients (70%) had died and 2-year OS was 45% (95% CI: 0.40-0.51), Supplemental Figure S1C. The predicted 2-year survival in the cohort (calibration in the large) was 32%. Concordance of the R/R-IPI model was 0.68, and the calibration curve is shown in Figure 3C.
Sensitivity Analysis
We explored the addition of diagnostic clinical variables to the model. Inclusion of sex and non-age IPI variables from diagnosis resulted in a modest increase in prognostic performance in the SEAL Cohort at increased complexity. Based on these results we did not include additional diagnostic variables as part of the R/R-IPI model. We evaluated the R/R-IPI model in patients between the ages of 40-65 to assess performance in patients who would likely be candidates for stem cell transplant. Results in this age group were consistent with the overall results (Supplemental Figure S3).
Discussion
In this study we present the R/R-IPI, an international prognostic risk calculator for relapsed/refractory DLBCL. The model was developed and subsequently validated in large independent cohorts of international patients and is simple to apply in the clinic. In addition to its clinical utility, the R/R-IPI has potential benefit to the research community. R/R DLBCL is a highly heterogeneous group of patients, and the clinical trials in this space are predominantly single-arm with widely varying eligibility criteria. The R/R-IPI can provide insight on the clinical makeup and expected outcomes of the patients on these studies to assist cross-trial comparability evaluation. Thereby, the R/R IPI can provide benchmarks for decision making in early clinical development and for assessment of real-world effectiveness of approved agents.
There are important limitations of the model that must be considered when applying in clinical practice. The SEAL cohort provides a large dataset for modeling but is limited by the lack of several key data elements that would potentially aid in model development and interpretation. Details at the time of progression not available in the SEAL frontline clinical trial based cohort. OS data were submitted by each trial to the SEAL consortium and long-term follow-up was limited to a few older trials. Most importantly, details on second line therapy were not available in the SEAL cohort. The model underestimated actual survival in the MER cohort and overestimated survival in high-risk patients in the LYFO cohort. It is difficult to pinpoint the precise cause of this due to unavailable clinical details in the SEAL cohort but selection bias of relapsed/refractory patients is unlikely due to all patients being followed from frontline therapy. However, there is likely a wide variation in management and therapy selection in the relapsed/refractory setting between the cohorts. Outcomes in relapsed/refractory DLBCL are heterogeneous [20] and long-term second remissions are historically obtained almost exclusively through high dose chemotherapy and autologous stem cell transplant. Significant patient selection is involved in the choice of second-line therapy, with younger and healthier patients being considered for stem cell transplant. Age is an imperfect clinical indicator for transplant eligibility, and criteria for pursuing a salvage therapy and transplant approach varies by country and provider. The treatment landscape in relapsed/refractory DLBCL is rapidly evolving with recent approval of chimeric antigen receptor T-cell (CAR-T) and targeted agents. It is likely that any model based on historical data, including our proposed model, will underestimate survival when applied to current patients who may have access to recently approved effective therapies. Future evaluation and recalibration of the model in patients treated in the current treatment era will be needed.
These limitations should not overshadow the strengths of the study. Modeling was performed in over 1000 patients with relapse or progression of DLBCL, which to our knowledge is the largest cohort of patients for outcome modeling in this setting All patients used in modeling were followed from diagnosis which greatly limits potential selection bias compared to a retrospective identification of patients with relapsed/refractory disease. The model was validated in two independent, international cohorts from Europe and the United States also followed from diagnosis. The fact that this study utilized international data from both clinical trials and a real-world setting also strengthens the external validity of the model as it can be applied to patients enrolled in clinical trials as well as an often more fragile patient population treated in the real-world setting. The model provides prognostic concordance [21] [22] that is comparable to the IPI [23], the standard prognostic index at the time of initial diagnosis in DLBCL. Importantly the model is simple to apply in the clinic. Age and time of progression from frontline therapy are readily available clinical details to any clinician globally seeing a patient with progressive DLBCL. While not novel, these variables are established prognostic factors[9] and highly unlikely to be spurious findings in these data. We have made the model freely available in an easy point of care calculator via the QxMD Calculate app and future iterations of the model can be easily incorporated into the existing framework.
Characterizing the outcomes of R/R DLBCL is becoming increasingly important as more treatment options now exist. High dose therapy with autologous stem-cell transplant is still considered the standard of care and curative treatment approach for transplant eligible patients, whereas elderly and frail patients historically were limited to more palliative therapies. The treatment landscape of R/R DLBCL has changed significantly with the introduction of CAR-T, as well as recent approvals of targeted agents in this setting such as tafasitamab and polatuzumab vedotin. CAR-T therapies are complex cellular therapies with limited access and high costs. Decision support tools applicable to daily clinical practice are very useful to better identify patients that may do well with conventional approaches and those who should be considered for the more complex and costly CAR-T therapy immediately.
In summary, we present the first externally validated prognostic model for relapsed/refractory DLBCL. The model was developed and validated in large international cohorts of patients from the clinical trial as well as real world setting making external validity high. Age and frontline time to progression are simple clinical variables yet provide comparable prognostic information to the IPI in the newly diagnosed setting. The model is available via point of care smartphone for easy implementation in the clinic.
Supplementary Material
Highlights.
Age and time to progression on frontline therapy are strong prognostic factors in relapse/refractory DLBCL
The R/R-IPI model has good concordance in relapsed/refractory DLBCL but may underestimate survival when applied in the current treatment era
The R/R-IPI model is available via web and smartphone applications for clinical utilization
Funding:
This work was supported by the National Cancer Institute at the National Institutes of Health [University of Iowa/Mayo Clinic SPORE P50CA097274, Lymphoma Epidemiology of Outcomes 1U01CA195568].
Footnotes
Disclosures:
Maurer: research funding from Nanostring, Celgene, Morphosys, Genentech and advisory board/consulting from Kite, Pfizer, Morphosys; Farooq: honoraria from Kite; Brown: advisory board from BMS, Incyte, Roche, Takeda; Cunningham: research funding from Amgen, Sanofi, Merrimack, AstraZeneca, Celgene, MedImmune, Bayer, 4SC, Clovis, Eli Lilly, Jannsen, Merck; Jorgensen: advisory board from Gilead, Novartis, Roche, BMS; Poeschel: travel grants from Abbvie, Amgen, and Roche; Tilly personal fees and non-financial support from Roche, personal fees from Karyopharm, AstraZeneca, BMS, Servier, Janssen-Cilag; El-Galaly: employment by Roche. All remaining authors have declared no conflicts of interest.
This study was presented in part at the 61st American Society of Hematology Annual Meeting and Exposition, Orlando, December 7 - 10, 2019
References
- 1.Teras LR, DeSantis CE, Cerhan JR et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA: A Cancer Journal for Clinicians 2016; 66: 443–459. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Lepage E, Brière J et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. New England Journal of Medicine 2002; 346: 235–242. [DOI] [PubMed] [Google Scholar]
- 3.Habermann TM, Weller EA, Morrison VA et al. Rituximab-CHOP Versus CHOP Alone or With Maintenance Rituximab in Older Patients With Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology 2006; 24: 3121–3127. [DOI] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Trümper L, Österborg A et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. The Lancet Oncology 2006; 7: 379–391. [DOI] [PubMed] [Google Scholar]
- 5.Maurer MJ, Ghesquières H, Jais J-P et al. Event-Free Survival at 24 Months Is a Robust End Point for Disease-Related Outcome in Diffuse Large B-Cell Lymphoma Treated With Immunochemotherapy. Journal of Clinical Oncology 2014; 32: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobsen LH, Bøgsted M, Brown PdN et al. Minimal Loss of Lifetime for Patients With Diffuse Large B-Cell Lymphoma in Remission and Event Free 24 Months After Treatment: A Danish Population-Based Study. Journal of Clinical Oncology 2017; 35: 778–784. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg JW. Relapsed/Refractory Diffuse Large B-Cell Lymphoma. ASH Education Program Book 2011; 2011: 498–505. [DOI] [PubMed] [Google Scholar]
- 8.Crump M, Neelapu SS, Farooq U et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017; 130: 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gisselbrecht C, Glass B, Mounier N et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010; 28: 4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelapu SS, Locke FL, Bartlett NL et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine 2017; 377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster SJ, Bishop MR, Tam CS et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine 2018; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 12.Farooq U, Maurer MJ, Thompson CA et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol 2017; 179: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Q, Schmitz N, Ou F-S et al. Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Diffuse Large B-Cell Lymphoma: An Individual Patient–Level Analysis of Multiple Randomized Trials (SEAL). Journal of Clinical Oncology 2018; 36: 2593–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer MJ, Habermann TM, Shi Q et al. Progression-free survival at 24 months (PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials. Annals of Oncology 2018; 29: 1822–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arboe B, El-Galaly TC, Clausen MR et al. The Danish National Lymphoma Registry: Coverage and Data Quality. PLOS ONE 2016; 11: e0157999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011; 39: 22–25. [DOI] [PubMed] [Google Scholar]
- 17.Cerhan JR, Link BK, Habermann TM et al. Cohort Profile: The Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) Cohort Study. Int J Epidemiol 2017; 46: 1753–1754i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Farooq U, Link BK et al. Late Relapses in Patients With Diffuse Large B-Cell Lymphoma Treated With Immunochemotherapy. J Clin Oncol 2019; Jco1900014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerds TA, Kattan MW, Schumacher M, Yu C. Estimating a time-dependent concordance index for survival prediction models with covariate dependent censoring. Statistics in medicine 2013; 32: 2173–2184. [DOI] [PubMed] [Google Scholar]
- 20.Farooq U, Maurer MJ, Thompson CA et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. British Journal of Haematology 2017; 179: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurer MJ, Jais JP, Ghesquieres H et al. Personalized risk prediction for event-free survival at 24 months in patients with diffuse large B-cell lymphoma. Am J Hematol 2016; 91: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biccler J, Eloranta S, de Nully Brown P et al. Simplicity at the cost of predictive accuracy in diffuse large B-cell lymphoma: a critical assessment of the R-IPI, IPI, and NCCN-IPI. Cancer Medicine 2018; 7: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A Predictive Model for Aggressive Non-Hodgkin's Lymphoma. New England Journal of Medicine 1993; 329: 987–994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.