Abstract
Objective
Few studies have explored the shared effects of Parkinson’s disease (PD) within patient/caregiver dyads. To fill this gap, we compared stress-health outcomes of patients with those of caregiving-partners, examined individual stress-health associations, and explored stress-health associations within dyads.
Method
A total of 18 PD patient/caregiving-partner dyads (N = 36) reported on disease-specific distress, anxiety, quality of life (QOL), and provided saliva samples for cortisol assessment. This cross-sectional, secondary analysis of a prospective pilot study used Actor-Partner Interdependence Models to test aims.
Results
Patients reported greater anxiety, poorer QOL, and demonstrated flatter cortisol slopes and higher mean bedtime cortisol compared to caregiving-partners. Both patients and caregiving-partners with greater anxiety had elevated bedtime cortisol and poorer QOL. Greater disease-specific distress in an individual was associated with higher diurnal mean cortisol in their partner.
Conclusions
Findings highlight the potential for psychosocial interventions at the dyadic level to reduce shared burden and promote coping among PD patient/caregiving-partner dyads.
Keywords: Parkinson’s disease, Caregiver issues, Anxiety, Quality of life
Introduction
Parkinson’s disease (PD) is characterized by bradykinesia (slowness) and either tremor at rest and/or rigidity (Postuma et al., 2018). Non-motor features of PD, such as cognitive decline, psychological distress (e.g., anxiety, depression), and anticipation of functional decline, can contribute to increased impairment, decreased quality of life (QOL), and disease progression (Chaudhuri, Healy, & Schapira, 2006). For example, PD patients with depressed mood have shown poorer prognosis, cognitive functioning, QOL, and greater decreases in motor function and engagement in activities of daily living (Schrag, 2006). Despite some evidence to suggest non-motor symptoms may be as frequent and debilitating as motor symptoms, non-motor symptoms often remain unrecognized and undertreated (Chaudhuri et al., 2006). Moreover, PD patients demonstrate poorer physiological health outcomes compared to healthy controls, including higher mean cortisol levels and flatter diurnal cortisol slopes (Hartmann, Veldhuis, Deuschle, Standhardt, & Heuser, 1997). Although psychological distress has been associated with physiological biomarkers (e.g., cortisol) in non-PD samples, such as cancer (Lutgendorf et al., 2008), this association is poorly understood in PD.
Caregiving-partners are also susceptible to anticipation of the patient’s functional decline. Common caregiving challenges, including caregiver burden and shifts in relationship roles and dynamics, present additional stress (Schrag, Hovris, Morley, Quinn, & Jahanshahi, 2006). Some caregivers of individuals with chronic illness (e.g., advanced breast cancer) experience comparable levels of distress to their patient-counterparts (Grunfeld et al., 2004) and PD caregivers report higher levels of anxiety and depression than the general population (Martinez-Martin et al., 2008). Yet, minimal research has examined stress-health outcomes among PD caregivers.
Given preliminary evidence that both patients and caregiving-partners experience individual changes in response to PD, it is plausible to hypothesize that shifts in stress and health may have “crossover” dyadic effects, influencing stress-health outcomes of one’s partner. Dyadic influence and shared variance are not accounted for when separately assessing patient-versus-caregiver outcomes—a large limitation within the literature. No known study has explored both individual and dyadic effects of psychological and physiological stress-health factors within PD patient/caregiving-partner dyads.
Using our lab’s published model of stress-health relationships (Salmon, Sephton, & Dreeben, 2011), the present study addressed this gap with three aims. Aim 1 explored differences in four stress-health factors (distress, psychological outcome, cortisol, QOL) between PD patients and their caregiving-partners. Aim 2 examined stress-health relationships within the individual; we hypothesized downward associations consistent with our lab’s model (Fig. 1). Aim 3 explored “crossover” associations within PD patient/caregiving-partner dyads by examining the extent to which PD patients and caregiving-partners influence one another’s stress-health outcomes. Given the nascent literature base, Aims 1 and 3 were exploratory.
Fig. 1.
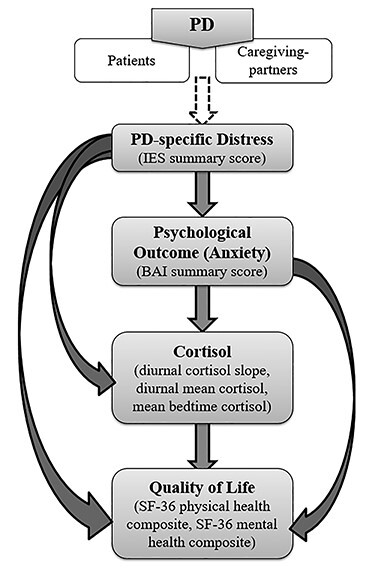
Model of associations tested in the current study; adapted from our research lab’s published model of stress-health relationships (Salmon et al., 2011). Aim 1 tested differences between Parkinson’s disease (PD) patients and their caregiving-partners on the four model factors shown in rectangles. Aim 2 tested individual associations indicated by each of the arrows. Aim 3 tested “crossover” dyadic associations of the same stress-health relationships in Aim 2, within dyads.
Methods
Participants and Procedures
Participants were recruited from the Movement Disorder Clinic of the University-affiliated Rehabilitation Institute to participate in a randomized, prospective pilot study evaluating health-related QOL outcomes among PD patient/caregiver dyads in a mindfulness-based stress reduction intervention compared to usual care. Procedures were approved by the University’s Human Subjects Division. The current study used data collected at baseline of the parent study.
Medical records of patients were screened for eligibility. Upon recruitment, physician collaborators reassessed PD staging criteria, and trained graduate students conducted a Mini-Mental State Examination (MMSE; Folstein, Robins, & Helzer, 1983) to assess cognitive functioning. Patient inclusion criteria included idiopathic PD based on UK PD Brain Bank criteria (Gibb & Lees, 1988), with symptom onset ≥3 years prior; adequate cognitive functioning (MMSE score ≥25); Hoehn and Yahr Stage II to III; ≥40 years old; and proficiency in written and spoken English. Eligible caregiving-partners, identified as “primary caregiver” for the patient, were required to provide ≥4 hr/day of care, demonstrate adequate cognitive functioning (MMSE score ≥28), and proficiency in written and spoken English. Exclusion criteria for patients and caregiving-partners included a serious medical or psychological condition that would impair participation, use of systemic hydrocortisone-based steroids, or history of deep brain stimulation.
Patient/caregiving-partner dyads jointly attended two baseline assessment sessions in the research laboratory. Each participant completed written informed consent, underwent brief mental status evaluation to confirm adequate cognitive functioning, and was given materials for home-based data collection, including a questionnaire packet and salivary cortisol kit. Dyads returned to the laboratory within 1 week to return completed materials.
Measures
Demographics
Participants provided demographic information and a list of medications to clarify potential effects on cortisol.
PD-specific distress
The Impact of Event Scale (IES; Horowitz, Wilner, & Alvarez, 1979) is a 15-item self-report instrument assessing the perceived impact of events considered potentially stressful. Questions were keyed to consider the impact of the PD diagnosis and treatment. A summary score was calculated, with higher scores representing greater PD-specific distress.
Psychological outcome
The Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988a) and Beck Depression Inventory (BDI; Beck, Steer, & Carbin, 1988b) were used to assess levels of anxiety and depression, respectively. The BAI and BDI are well-validated, 21-item self-report instruments measuring severity of anxiety symptoms (BAI) or depressive symptoms (BDI) over the past week. Higher scores suggest greater severity of symptoms. Due to significant correlation between these two measures of psychological outcome (r = .759), only the BAI summary score was used in analyses.
Given somatic symptoms of anxiety on the BAI that may overlap with physical/motor symptoms of PD (e.g., wobbliness in legs, hands trembling) and confound BAI summary scores, significant BAI findings were probed in post hoc analyses examining three subscales: somatic (11 items), subjective (6 items), panic (4 items; Beck et al., 1988a).
Cortisol
Nine salivary cortisol samples were collected at home over 3 days at waking, 30 min after waking, and bedtime. Samples were centrifuged, aliquoted, and frozen at −80 °C until assay. Cortisol levels were assessed using a luminescence immunoassay (IBL International, Hamburg, Germany). Assay sensitivity was 0.003 μg/dl. Inter-assay CV was 8.98% using the low control and 5.95% using the high control. Intra-assay CV was 10.2% for the low control and 7.2% for the high control. Assays yielded a total of 608 cortisol values. Cortisol values were log-transformed, from which three parameters were calculated: diurnal cortisol slope (excluding 30-min post-wake values, regressing six values on collection times), diurnal mean cortisol (including all nine values), and mean bedtime cortisol.
QOL
Short-Form Health Survey-36 (SF-36; Ware & Sherbourne, 1992) is a 36-item scale assessing health status and QOL. Eight health concepts are assessed from which two composite scores were calculated: overall physical health and overall mental health. Higher scores represent more optimal physical and mental health thought to contribute to greater QOL.
Statistical Analyses
Multilevel modeling with restricted maximum likelihood was used to estimate a series of Actor-Partner Interdependence Models (APIM; Kenny, Kashy, & Cook, 2006; see Supplementary Fig. S1) using IBM SPSS v22.0. Each arrow in Fig. 1 was examined using APIM for a total of 17 models. Although the current sample size limits power for multilevel modeling, other statistical methods (e.g., t-tests, linear regression) do not adequately estimate the nonindependence, or inherent influence among dyad members—a key source of variance in dyadic contexts frequently ignored in the literature. Nonetheless, we emphasize caution with interpretation, as results are preliminary and intended to generate future hypotheses.
One caregiving-partner did not provide saliva for cortisol assessment and was excluded from cortisol analyses, otherwise data were complete. The assumption of nonindependence was tested using Pearson product–moment correlation (Kenny et al., 2006) and was met for each predictor variable. The variable denoting each dyad was entered as an upper level variable, with individual participant scores entered as lower level variables. Predictor variables were grand-mean centered (Enders & Tofighi, 2007) to aid in the interpretation of results. Dyad members were treated as distinguishable based on “role” (1 = PD patient, −1 = caregiving-partner; Kenny et al., 2006) and the residual structure was treated as heterogeneous compound symmetry.
Each APIM gives an estimate of: mean difference between patients and caregiving-partners on an outcome measure (AIM 1); an individual’s predictor associated with their own outcome (“actor effects”; AIM 2), including patient/caregiver status moderating this relationship (actor*role effects; AIM 2); an individual’s predictor associated with their partner’s outcome (“partner effects”; AIM 3), including patient/caregiver status moderating this relationship (partner*role effects; AIM 3).
Results
A total of 19 dyads consented to participate and were enrolled. One dyad withdrew due to time conflicts with the study’s activity schedule. The final sample included 18 dyads, each consisting of one PD patient and their caregiving-partner (N = 36). Demographic information is provided in Table 1. A total of 17 dyads were married/partnered heterosexual couples. One dyad identified as same-gender friends. Patients were early in disease progression and did not report significant motor functioning impairment on the Hoehn and Yahr (1967) scale (M = 1.88, SD = .31) and United Parkinson’s Disease Rating Scale (M = 16.7, SD = 11.6).
Table 1.
Sample characteristics (N = 36)
| Variables | PD Patient (n = 18) | Caregiving-partner (n = 18†) |
|---|---|---|
| Age, Mean (SD) | 63.67 (7.03) | 62.22 (10.44) |
| Gender | ||
| Female | 44.4% | 50.0% |
| Male | 55.6% | 50.0% |
| Marital status | ||
| Never married | 5.6% | 5.6% |
| Currently married | 88.9% | 88.9% |
| Divorced | 0% | 5.6% |
| Widowed | 5.6% | 0% |
| Race | ||
| White | 100% | 100% |
| Education level | ||
| High school | 22.2% | 33.3% |
| Associate/technical degree | 27.8% | 5.6% |
| Bachelor’s degree | 33.3% | 16.7% |
| Master’s degree | 5.6% | 5.6% |
| Doctoral/professional degree | 11.1% | 5.6% |
| Household income | ||
| < $20,000 | 0% | 5.6% |
| $20,000–39,999 | 11.1% | 11.1% |
| $40,000–59,999 | 22.2% | 16.7% |
| $60,000–79,999 | 11.1% | 16.7% |
| $80,000–99,999 | 11.1% | 5.6% |
| ≥$100,000 | 27.8% | 11.1% |
| Employment status | ||
| Employed full-time | 33.3% | 16.7% |
| Employed part-time | 5.6% | 16.7% |
| Homemaker or caregiver | 5.6% | 0% |
| Retired | 50.0% | 33.3% |
| Permanently disabled | 5.6% | 0% |
| Living situation | ||
| Live alone | 11.1% | 11.1% |
| Live with significant other | 83.3% | 50% |
| Live with children/other relatives | 5.6% | 0% |
| Other | 0% | 5.6% |
Note. †Six caregiving-partners did not provide information on education, income, employment status, or living situation due to inadvertent changes in the demographic questionnaire part-way through the study.
Aim 1
Anxiety. PD patients reported significantly higher anxiety (M = 11.83, SD = 10.12) than caregiving-partners (M = 3.44, SD = 3.71), t(21) = 3.302, p = .003. Post hoc analysis revealed patients reported significantly higher somatic symptoms (M = 7.22, SD = 5.00) and subjective symptoms of anxiety (M = 2.72, SD = 3.36) than caregiving-partners (M = 1.94, SD = 2.18, t(23) = 4.104, p < .001; M = 0.94, SD = 1.21, t(21) = 2.113, p = .047, respectively).
Cortisol. Patients demonstrated significantly flatter diurnal cortisol slopes (M = −0.08, SD = .04) and significantly higher mean bedtime cortisol (M = −2.55, SD = .62) compared to caregiving-partners (M = −0.14, SD = .07, t(33) = 2.819, p = .008; M = −3.17, SD = .72, t(33) = 2.746, p = .010, respectively).
QOL. Patients reported significantly lower QOL due to poorer overall physical health (M = 41.21, SD = 10.06) compared to caregiving-partners (M = 49.96, SD = 10.01), t(34) = −2.615, p = .013.
No other significant differences emerged.
Aim 2
Anxiety and cortisol
Results revealed a significant actor effect of anxiety on mean bedtime cortisol: holding one’s partner’s anxiety constant, individuals with greater anxiety had higher bedtime cortisol (t(31) = 2.36, p < .05). Post hoc analysis revealed a significant actor effect of somatic anxiety on bedtime cortisol: holding the partner’s somatic symptoms constant, individuals with greater somatic symptoms of anxiety had higher bedtime cortisol (t(28) = 2.57, p < .05).
Anxiety and QOL
Significant actor effects of anxiety on QOL emerged: holding the partner’s anxiety constant, individuals with greater anxiety had poorer QOL due to both overall physical health (t(32) = −4.01, p < .001) and overall mental health (t(23) = −5.16, p < .001).
Post hoc analysis revealed a significant actor effect of somatic anxiety on QOL: holding the partner’s somatic symptoms constant, individuals with greater somatic symptoms of anxiety had poorer QOL due to both physical health (t(30) = −3.95, p < .001) and mental health (t(22) = −4.21, p < .001).
A significant actor by role interaction emerged for subjective anxiety on QOL due to physical health. Simple slopes were computed separately for PD patients and caregiving-partners to explore this interaction. The actor effect of subjective anxiety on physical health was significant for patients (t(15) = −3.98, p = .001), but not caregiving-partners (t(15) = 1.29, p > .05); holding caregiving-partners’ subjective symptoms constant, PD patients with greater subjective symptoms of anxiety reported poorer physical health. Results also revealed a significant actor effect of subjective anxiety on QOL due to mental health: holding the partner’s subjective symptoms constant, individuals with greater subjective symptoms of anxiety reported poorer overall mental health (t(28) = −5.36, p < .001).
Lastly, a significant actor effect emerged for panic anxiety on QOL due to mental health: holding the partner’s panic symptoms constant, individuals with greater panic symptoms of anxiety, had poorer overall mental health (t(23) = −3.66, p < .01).
No other significant actor effects emerged.
Aim 3
PD-specific distress and cortisol
Results revealed a significant partner effect of distress on diurnal mean cortisol: holding an individual’s distress constant, greater partner distress was associated with higher diurnal mean cortisol for the individual (t(28) = −2.75, p < .05).
No other significant partner effects emerged.
Discussion
Dyadic data analysis tested whether the impact of a diagnosis of PD on stress and health is shared among PD patient/caregiving-partner dyads. In the current sample, PD patients demonstrated greater anxiety, flatter cortisol slopes, higher mean bedtime cortisol, and poorer QOL compared to caregiving-partners. Patients were early in the disease trajectory, highly functioning, and required minimal assistance, suggesting “hidden” non-motor symptoms could be affecting stress-health factors among PD patients. It is possible a PD diagnosis may not become as impactful for caregiving-partners until both motor and non-motor symptoms worsen and the toll of caregiving increases (Schrag et al., 2006).
Several significant stress-health associations were observed within the individual. For both patients and caregiving-partners, somatic symptoms of anxiety were significantly associated with elevated bedtime cortisol, whereas subjective and panic symptoms were not. It is possible that both greater somatic anxiety (i.e., feeling hot, shaky, lightheaded) and higher bedtime cortisol—a biomarker associated with poorer health and a prognostic factor in other disease populations (Sephton, Sapolsky, Kraemer, & Spiegel, 2000)—are driven by elevated sympathetic arousal. Higher levels of overall anxiety were also associated with poorer QOL due to both overall physical and mental health. Interestingly, subjective symptoms of anxiety were only significantly associated with poorer physical health among PD patients. Subjective items, such as “scared” and “fear of losing control,” may capture the PD patients’ experience given the neurodegenerative nature of the disease and anticipated functional decline, thereby reducing perceived QOL due to physical health. Findings highlight the importance of routine screening of anxiety symptoms in PD patients and accompanying caregiving-partners at medical appointments, and consideration for use of behavioral interventions in a dyadic format.
For both patients and caregivers, overall secretion of cortisol was elevated if their partner endorsed higher levels of PD-related distress. Interestingly, the only dyadic effect observed was disease-specific, and the outcome—elevated cortisol—is particularly relevant given the potential exacerbating effects of elevated and/or dysregulated hypothalamic–pituitary-axis activity on the progression of neurodegeneration in PD (Soares, Pereira, Altmann, de Almeida, & Rieder, 2019).
Limitations
Sample size and statistical power were limited by the pilot nature of the parent study; however, to address a major gap in the literature (accounting for inherent, shared variance in dyadic samples), the authors prioritized use of the APIM over more simplistic, inadequate methods. Our sample of White individuals with relatively high levels of education and socioeconomic status may indicate sample bias, thereby limiting generalizability. Our inclusion criteria contributed to a sample of high-functioning patients with good balance control and no dementia, and caregiving-partners who provided limited care. Thus, we cannot generalize our findings to patients with greater disability, including medication increases on cortisol, and to caregiving-partners under increased caregiving stress/burden. Nonetheless, considering the stress-health associations observed in this study, there is value in understanding the non-motor symptoms associated with a PD diagnosis in its early stages among patients and their caregiving-partners.
Future Directions
Future research with larger, more diverse samples of PD patient/caregiving-partner dyads is needed to replicate these findings and enhance power for dyadic data analyses. Longitudinal investigations are also needed to understand how the individual and “crossover” associations of stress-health factors among PD patient/caregiving-partner dyads may change as the disease, and the need for caregiving, progresses.
Conclusion
Using a model of stress-health associations, the present study explored the extent to which a diagnosis of PD is a shared experience among patients and their caregiving-partners early in the disease trajectory. This investigation highlights the importance of using dyadic data analyses to account for shared variance and reveal potential “crossover” effects within dyads. Improving our understanding of how chronic conditions affect dyadic relationships may help modify existing evidence-based interventions to target both members of the patient/caregiving-partner dyad to address common factors, such as anxiety. Future investigations of PD patient/caregiving-partner dyads across the spectrum of disease progression will further our understanding of non-motor features prior to onset of motor symptoms and the compounded nature of the two.
Supplementary Material
Acknowledgements
We would like to express our gratitude to Samuel Dreeben, PhD and our research assistants for their assistance with data collection and preprocessing and study management. We thank the study participants for their time and efforts in data collection and for their contribution to the progress of biobehavioral research on Parkinson’s disease and caregiving.
Funding
This work was supported by an Intramural Research Incentive Grant: Multidisciplinary Research Grant from the University of Louisville Office of the Executive Vice President for Research and Innovation [to Principal Investigator P.S. and Co-Investigators S.E.S. and I.L].
References
- Beck, A. T., Epstein, N., Brown, G., & Steer, R. A. (1988a). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893. [DOI] [PubMed] [Google Scholar]
- Beck, A. T., Steer, R. A., & Carbin, M. G. (1988b). Psychometric properties of the Beck depression inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. [Google Scholar]
- Chaudhuri, K. R., Healy, D. G., & Schapira, A. H. (2006). Non-motor symptoms of Parkinson's disease: Diagnosis and management. The Lancet Neurology, 5(3), 235–245. [DOI] [PubMed] [Google Scholar]
- Enders, C. K., & Tofighi, D. (2007). Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychological Methods, 12(2), 121. [DOI] [PubMed] [Google Scholar]
- Folstein, M. F., Robins, L. N., & Helzer, J. E. (1983). The mini-mental state examination. Archives of General Psychiatry, 40(7), 812. [DOI] [PubMed] [Google Scholar]
- Gibb, W., & Lees, A. (1988). The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry, 51(6), 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld, E., Coyle, D., Whelan, T., Clinch, J., Reyno, L., Earle, C. C. et al. (2004). Family caregiver burden: Results of a longitudinal study of breast cancer patients and their principal caregivers. CMAJ, 170(12), 1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, A., Veldhuis, J. D., Deuschle, M., Standhardt, H., & Heuser, I. (1997). Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: Ultradian secretory pulsatility and diurnal variation. Neurobiology of Aging, 18(3), 285–289. [DOI] [PubMed] [Google Scholar]
- Hoehn, M. M., & Yahr, M. D. (1967). Parkinsonism: Onset, progression and mortality. Neurology Genetics, 17(5), 427. [DOI] [PubMed] [Google Scholar]
- Horowitz, M., Wilner, N., & Alvarez, W. (1979). Impact of event scale: A measure of subjective stress. Psychosomatic Medicine, 41(3), 209–218. [DOI] [PubMed] [Google Scholar]
- Kenny, D., Kashy, D., & Cook, W. (2006). The analysis of dyadic data. New York, NY: Guilford. [Google Scholar]
- Lutgendorf, S. K., Weinrib, A. Z., Penedo, F., Russell, D., DeGeest, K., Costanzo, E. S. et al. (2008). Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. Journal of Clinical Oncology, 26(29), 4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin, P., Arroyo, S., Rojo-Abuin, J. M., Rodriguez-Blazquez, C., Frades, B., & de Pedro Cuesta, J. (2008). Burden, perceived health status, and mood among caregivers of Parkinson's disease patients. Movement Disorders, 23(12), 1673–1680. [DOI] [PubMed] [Google Scholar]
- Postuma, R. B., Poewe, W., Litvan, I., Lewis, S., Lang, A. E., Halliday, G. et al. (2018). Validation of the MDS clinical diagnostic criteria for Parkinson's disease. Movement Disorders, 33(10), 1601–1608. [DOI] [PubMed] [Google Scholar]
- Salmon, P. G., Sephton, S. E., & Dreeben, S. J. (2011). Mindfulness-Based Stress Reduction. In Herbert, J. D., & Forman, E. M. (Eds.), Acceptance and mindfulness in cognitive behavior therapy: Understanding and applying the new therapies (, pp. 132–163). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Schrag, A. (2006). Quality of life and depression in Parkinson's disease. Journal of the Neurological Sciences, 248(1–2), 151–157. [DOI] [PubMed] [Google Scholar]
- Schrag, A., Hovris, A., Morley, D., Quinn, N., & Jahanshahi, M. (2006). Caregiver-burden in Parkinson's disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism & Related Disorders, 12(1), 35–41. [DOI] [PubMed] [Google Scholar]
- Sephton, S. E., Sapolsky, R. M., Kraemer, H. C., & Spiegel, D. (2000). Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute, 92(12), 994–1000. [DOI] [PubMed] [Google Scholar]
- Soares, N. M., Pereira, G. M., Altmann, V., de Almeida, R. M. M., & Rieder, C. R. (2019). Cortisol levels, motor, cognitive and behavioral symptoms in Parkinson’s disease: A systematic review. Journal of Neural Transmission, 126(3), 219–232. [DOI] [PubMed] [Google Scholar]
- Ware, J. E., & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Medical Care, 30, 473–483. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


