Abstract
Background
Travellers’ diarrhoea (TD) is the most common travel-related illness with an estimated 10 million people afflicted annually. Outcome measures to assess the efficacy of primary and secondary TD interventions were historically based on diarrhoea frequency with ≥1 associated gastrointestinal symptom. Furthermore, efficacy determination is often made on the presence or absence of TD, rather than on TD illness severity. Current severity classifications are based on subjective consideration of impact of illness on activity. We sought to develop a standardized scoring system to characterize TD severity to potentially apply as a secondary outcome in future field studies.
Methods
Data on multiple signs and symptoms were obtained from a previously published multisite TD treatment trial conducted by the US Department of Defense (TrEAT TD). Correlation, regression and multiple correspondence analyses were performed to assess impact on activity and a TD severity score was established.
Results
Numerous signs and symptoms were associated with impaired function, with malaise and nausea most strongly associated [odds ratio (OR) 5.9–44.3, P < 0.0001 and OR 2.8–37.1, P < 0.0001, respectively). Based on co-varying symptomatology, a TD severity score accounting for diarrhoea frequency in addition to several signs and symptoms was a better predictor of negative impact on function than any single sign/symptom (X2 = 127.16, P < 0.001). Additionally, there was a significant difference (P < 0.0001) in the mean TD severity score between those with acute watery diarrhoea (3.9 ± 1.9) and those with dysentery or acute febrile illness (6.2 ± 2.0).
Conclusions
The newly developed disease severity score better predicted a negative impact on activity due to TD than did any single sign or symptom. Incorporating multiple parameters into the TD severity score better captures illness severity and moves the field towards current recommendations for TD management by considering symptoms with high functional impact. Further validation of this score is needed in non-military travellers and other settings.
Keywords: Functional impact, illness, score, nausea, vomiting, dysentery
Introduction
Travellers’ diarrhoea (TD) is the most common travel-related illness, with an estimated 10 million people afflicted annually and a reported attack rate of 30–70% depending on destination and season.1–3 TD also significantly affects deployed military personnel, with an estimated attack rate of 30 cases per 100-person months.4–6
Travel medicine is an evolving field that is increasingly important in this era of globalization. The global travel vaccines market was forecasted to reach a value of US$2.94 billion in 2020, with the number of global tourists increasing from 1.0 billion in 2012 to 1.4 billion in 2018, a significant proportion of those travellers journeying from developed countries to areas with endemic diseases.7 With travel projections anticipated to increase, the demand for vaccines will also increase. Moreover, many regulatory authorities strongly recommend or mandate travellers to be vaccinated prior to travelling to disease-prone regions,8 often making vaccines a requirement for travelling internationally. As such, vaccines against TD remain a goal of pivotal importance.
TD vaccines have traditionally focused on the prevention outcome of moderate-to-severe diarrhoea caused by the target pathogen.9–11 However, the definitions of moderate-to-severe diarrhoea have varied across studies. While most, if not all, have based severity on the number of unformed stools in a 24-h period, some have incorporated additional gastrointestinal symptoms.12,13 Table 1 summarizes the clinical endpoints used for field trials testing TD vaccine candidates. These endpoints are critical in interpreting vaccine efficacy as estimates may vary significantly depending on the endpoint utilized. Furthermore, for the traveller, the effect of the illness on function accounting for stool output and other outcomes may be a more informative endpoint than just frequency of loose stools given that functional impairment may impede travel and/or business plans.14,15
Table 1.
Clinical endpoints in TD-vaccine field studies
| Publication | Vaccine candidate | Study population (n) | Primary endpoint definition | Vaccine efficacy (VE) |
|---|---|---|---|---|
| Scerpella et al., 199529 | Killed whole-cell Vibrio cholerae O1 with a recombinant B-subunit of cholera toxin (WC/rBS) | Student travellers to Mexico (n = 502) | ≥4 loose stools in 24 h (or 3 in 8 h) plus an additional symptom | VE against ETEC = 50% (95% CI, 14–71%) beginning 7 days after the second dose. However, no efficacy was demonstrated within 7 days of the second vaccination when 74% of ETEC cases occurred |
| Wiedermann et al., 200030 | Inactivated whole-cell ETEC and cholera vaccines plus recombinant B-subunit of cholera toxin (rCTB) | Austrian travellers to tropical or subtropical destinations (44 different countries in Africa, Asia, Latin-America) (n = 250) | ≥3 liquid stools and ETEC-only pathogen detected in stool | ETEC vaccine VE = 79% (P = 0.119) Cholera vaccine VE = 82% (P = 0.0496) |
| Leyten et al., 200531 | Live-attenuated oral cholera vaccine strain CVD 103-HgR | Travellers to Indonesia, India, Thailand and West Africa (n = 134) | ≥3 loose stools in 24 h, or 2 loose stools plus additional symptoms | Study terminated early as the primary endpoint ≥50% VE not achieved at point of interim analysis |
| Sack et al., 200732 | Inactivated whole-cell ETEC vaccine plus recombinant B-subunit of cholera toxin (rCTB) | Travellers to Mexico and Guatemala (n = 672) | Primary vaccine preventable outcome (VPO): ≥3 loose stools in 24 h plus ≥1 gastrointestinal symptom caused by homologous ETEC vaccine strain | VE = 24% (n.s.) |
| Bourgeois et al., 200733 | Inactivated whole-cell ETEC vaccine plus recombinant B-subunit of cholera toxin (rCTB) | Travellers to Mexico and Guatemala (n = 1406) | VPO-ETEC TD: ≥5 unformed or liquid stools in 24 h plus ≥1 gastrointestinal symptom and homologous ETEC vaccine strain isolated within 24 h of episode | VE = −59 (95% CI, −384, 48) |
| Frech et al, 200847 | Heat-labile toxin LT-patch | Travellers to Mexico and Guatemala (n = 170) | Mild TD: 3 loose stools in 24 h Moderate TD: 4–5 loose stools in 24 h and ETEC LT, LT/ST or ST positive Severe TD: ≥6 loose stools in 24 h and ETEC LT, LT/ST or ST positive |
VE against moderate-to-severe TD = 75% (P = 0.007) VE against severe TD = 84% (P = 0.0332) |
| Steffen et al., 201334 | Heat-labile toxin LT-patch | Travellers to India (n = 723) | Mild TD: 3 loose stools in 24 h Moderate TD: 4–5 loose stools in 24 h and ETEC LT, LT/ST or ST positive Severe TD: ≥6 loose stools in 24 h and ETEC LT, LT/ST or ST positive |
VE near zero (P = 1.000) |
| Behrens et al., 201435 | Heat-labile toxin LT-patch | Travellers to Mexico and Guatemala (n = 1644) | VE against moderate-to-severe TD = 34.6% (95% CI, −2.2, 58.9) |
Numerous scoring systems have been developed and validated to address this issue in pediatric studies of diarrhoeal disease.16–24 Furthermore, Porter and colleagues have sought to standardize clinical endpoints and establish disease scoring systems for use in controlled human infection models for enterotoxigenic Escherichia coli (ETEC)25 and Shigella.26 There is no comparable, standardized disease severity score for TD, limiting the interpretation of results within and across studies. A standardized disease severity scoring system optimized for TD is needed to ensure consistency and inform efficacy estimates of interventions targeting prevention and treatment. Therefore, we examined the clinical attributes of TD and, based on the distribution and overlap of those parameters, propose a disease severity score for future validation and utilization.
Methods
Data were obtained from a previously published multisite TD treatment trial (TrEAT TD).27 Eligibility included active-duty US or UK military personnel or beneficiaries, aged ≥18 years of age, deployed to one of five countries (Kenya, Djibouti, Afghanistan, Honduras or Thailand) who presented with TD (≥3 loose stools in 24 h or ≥2 loose stools in 24 h with associated symptoms) of ≤ 96-h duration who were ambulatory at the time of enrollment. Only subjects enrolled in the TrEAT TD study who had complete TD symptom data available were included in this analysis.
In addition to demographic information, site location, disease classification (presenting with acute-watery diarrhoea or febrile or dysentery illness), impact of illness on activity level (normal, decreased ≤50%, decreased >50%, completely unable to function) as well as detailed clinical information on the signs and symptoms of disease were obtained. Symptom severity was based on the maximum observed severity of the TD episode from disease onset to enrollment into TrEAT TD. The following subjective symptoms were documented as not present, mild (present, but not serious or intense); moderate (caused some amount of distress but was manageable); severe (extreme, caused a great deal of discomfort or distress): nausea, tenesmus, malaise/fatigue, faecal incontinence, abdominal cramps and excessive gas/flatulence. Fever severity was based on maximum measured temperature and diarrhoea output based on the maximum number of loose stools in a 24-h period from disease onset to enrollment. The number of loose/liquid stools in the 8 h prior to enrollment and the total number of loose/liquid stools since symptom onset were also analysed. Episodes of vomiting were documented and classified as follows: none (0 episodes in 24 h), mild (1 episode in 24 h), moderate (2 episodes in 24 h), severe (≥3 episodes in 24 h).
Spearman correlations of sign and symptom severity were estimated. Univariable linear regression was utilized to describe the strength of the association between stool output and other TD-attributable signs and symptoms. Separate cumulative odds ordinal logistic regression models with proportional odds were developed to estimate the association between individual clinical signs and TD symptoms as well as metrics of stool output on activity impact. A Classification and Regression Tree (CART) analysis was conducted to determine optimal cut points of the maximum number of loose stools in 24 h for this analysis and inclusion in the scoring system.28 A Multiple Correspondence Analysis (MCA) was performed to graphically illustrate clustering of TD symptom severity. Parameters were assigned a value from 0 to 3 in the final TD score based on grouping within the MCA. The pattern of these outcome groupings were compared with the overlap in symptom severity from the regression analyses, as well as how strongly each symptom correlated with the other, to further inform how each should be weighted within the final scoring algorithm. Based on this iterative process to maximize our ability to differentiate symptoms and predictability on impact on activity to more appropriately characterize the TD illness profile, a TD severity score was developed similar to what has been utilized for ETEC- and Shigella-specific severity scores.25,26 Logistic regression models were utilized to assess the severity score’s ability to predict impact of illness on an individual’s activity.
This research was approved by the Uniformed Services University Institutional Review Board and the Ministry of Defence Research Ethics Committee in compliance with all applicable Federal regulations governing the protection of human subjects.
Results
Data were utilized from 363 subjects (Supplementary Table 1). The mean age was 29.3 years, with a majority being male (93.4%) and white (83.2%). Most subjects presented with acute watery diarrhoea (87.3%) with only 46 (12.7%) presenting with acute dysentery or febrile illness. Most subjects (n = 284, 77.4%) reported some negative impact on activity due to illness, with 45.2% (n = 166) reporting a decrease in activity ≤50%, 26.2% (n = 96) reporting a decrease in activity of >50% and 6% (n = 22) reporting illness that precluded their ability to function.
The frequency of common signs and symptoms and maximum 24-h loose stool output are shown in Supplementary Table 2 and Supplementary Figure 1, respectively. The most common subjective symptoms were abdominal cramps (75.4%) and malaise (64.3%), followed by nausea reported in approximately half the subjects (52.5%), gas (38.9%), tenesmus (29.1%) and faecal incontinence (14.3%). In contrast, the more objective signs of vomiting and fever were less frequently observed (20.4% and 15.8%, respectively). Stooling was not normaly distributed, with the highest proportion of subjects (n = 214; 58.9%) producing between ≥3 and ≤6 loose/liquid stools/24 h with a median of 6 loose stools (interquartile range: 4, 8).
Statistically significant correlations were observed between various signs and symptoms of TD-attributable illness. Among those signs and symptoms that were significantly correlated, the strength of correlation varied (Supplementary Table 3). The strongest correlation observed was between nausea and vomiting (ρ = 0.49; P < 0.001), although only 20.4% of participants reported vomiting. Malaise was positively correlated with all signs and symptoms, with the strongest correlation with nausea (ρ = 0.43; P < 0.001), vomiting (ρ = 0.34; P < 0.001), fever (ρ = 0.30; P < 0.001) and abdominal cramps (ρ = 0.31; P < 0.001). Similarly, abdominal cramps were positively correlated with all analysed signs and symptoms, with smaller correlations observed between nausea (ρ = 0.25; P < 0.001) loose stools (ρ = 0.21; P < 0.001) and tenesmus (ρ = 0.21; P < 0.001). Gas was only significantly correlated with malaise (ρ = 0.13; P = 0.01) and faecal incontinence was only significantly correlated with loose stools (ρ = 0.20; P = 0.01), malaise (ρ = 0.13; P = 0.01), nausea (ρ = 0.11; P = 0.03) and abdominal cramps (ρ = 0.14; P = 0.007). Tenesmus showed small, statistically significant correlations with all signs and symptoms except fever and faecal incontinence.
Numerous signs and symptoms were associated with impaired function; however, severe malaise and nausea were most strongly associated [odds ratio (OR) 44.3, P < 0.0001 and OR 37.1, P < 0.0001, respectively] (Supplementary Table 4). MCA showed co-variability in multiple signs and symptoms with severity being the most common factor associated with similar dimensions in a 2D space (Figure 1). Mild fever, vomiting, faecal incontinence, nausea and malaise clustered tightly with moderate abdominal cramps and faecal incontinence; whereas moderate nausea and vomiting clustered with more severe abdominal cramps and ≥8 loose stools/24 h. As expected, most severe signs and symptoms (with the exception of abdominal cramps and ≥8 loose stools/24 h) tended to cluster together, with moderate fever and tenesmus also included in this grouping, the latter two parameters receiving a maximum of ‘3’ in the final disease severity score. Categories of maximum 24-h stool output were included with the two lowest categories of output (0–1 loose stools/24 h and 2–4 loose stools/24 h) clustering with absence of concurrent signs and symptoms. The highest category of loose stool output (≥8 loose stools/24 h) was most proximal to mild and moderate symptoms.
Figure 1.
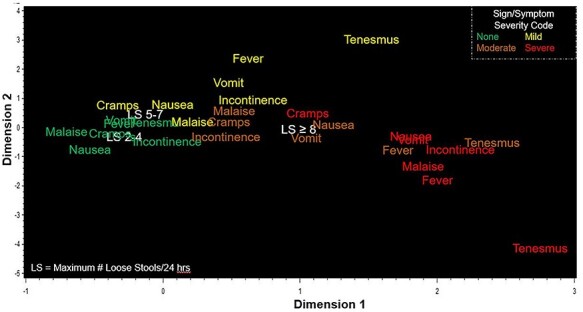
MCA of signs and symptoms of TD (TrEAT TD Dataset)
Based on the grouping of clinical outcomes in the MCA and results of the correlation, univariate logistic regression analyses, a three-component disease score was developed utilizing objective signs, subjective symptoms and stool frequency yielding a score ranging from 0 (no disease) to 9 (most severe disease) (Table 2). To mitigate the unequal distribution of stool output as a measure of TD disease severity, we tried to establish new stool frequency/volume cut-points based on existing data, as was done in the development of scoring systems for ETEC and Shigella.25,26 Stool categories in the scoring system were primarily based on the resulting CART analysis, whereas other signs and symptoms were assigned a value from 0 to 3 in the scoring system based on their clustering pattern in the MCA. For example, mild tenesmus grouped with moderate-to-severe symptoms, resulting in its elevated scoring of ‘2’ in the final disease severity score, representing a subjective symptom weighted according to its placement in the MCA.
Table 2.
TD disease complex score
| Parameter | Outcome | Score |
|---|---|---|
| Objective signs | Severe: ≥3 episodes vomiting OR | 3 |
| Moderate-to-severe fever | 3 | |
| Two episodes vomiting | 2 | |
| One episode vomiting OR Mild fever | 1 | |
| No objective symptoms | 0 | |
| Subjective symptoms | Severe: tenesmus, malaise, nausea, faecal incontinence OR | 3 |
| Moderate tenesmus | 3 | |
| Severe abdominal cramps OR | 2 | |
| Moderate nausea OR | 2 | |
| Mild tenesmus | 2 | |
| Moderate: abdominal cramps, faecal incontinence, malaise OR | 1 | |
| Mild: abdominal cramps, nausea, malaise, faecal incontinence | 1 | |
| No subjective symptoms | 0 | |
| Loose stool output (max. 24 h freq) | ≥8 loose stools/24 h | 3 |
| 5–7 loose stools/24 h | 2 | |
| 2–4 loose stools/24 h | 1 | |
| 0–1 loose stools/24 h | 0 |
There were significant differences in TD score across all classifications of functional impact (Figure 2). As TD score increased, so did the odds of reporting a negative impact on activity (Table 3), with a slight exception between those with a TD score of 6 vs 7. There was significant incremental increase in effect at each level of score with the primary outcome of impact from TD Score = 1 to TD Score = 9. Even subjects with a disease score of 2 had 6.5 times higher odds of reporting a negative impact on activity [OR = 6.5, 95% confidence interval (CI) 1.8–23.9] compared with those with a score of 1. No subjects had a TD Score of 0, as enrollment required diarrhoeal illness of some severity.
Figure 2.
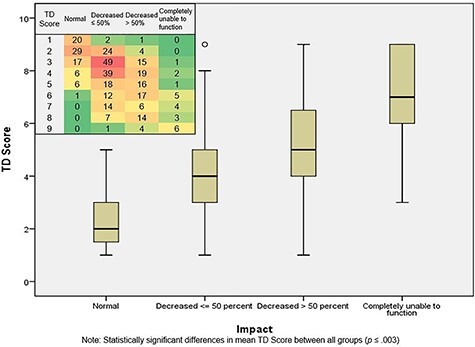
TD Disease Score and impact on activity (TrEAT TD dataset). Note statistically significant differences in mean TD Score between all groups (P ≤ 0.003). Numbers in heat diagram represent number of participants within each category
Table 3.
Ordinal logistic regression analysis of the relationship between TD disease score and impact on activity (TrEAT TD dataset; N = 363)
| Variable | β | Standard error | Adjusted OR | 95% CI for OR | P-value |
|---|---|---|---|---|---|
| Impact on activity (ref: completely unable to function) | |||||
| Normal | 1.87 | 0.61 | 6.41 | 1.94–21.17 | 0.002 |
| Decreased ≤ 50% | 4.63 | 0.64 | 102.67 | 29.20–360-98 | <0.0001 |
| Decreased ≥ 50% | 7.12 | 0.69 | 1241.16 | 322.77–4772-69 | <0.0001 |
| TD Score (ref.: TD Score 1) | |||||
| TD Score 2 | 1.88 | 0.66 | 6.53 | 1.78–23.86 | 0.005 |
| TD Score 3 | 3.20 | 0.65 | 24.53 | 6.77–88.56 | <0.0001 |
| TD Score 4 | 3.93 | 0.67 | 50.84 | 13.64–189.55 | <0.0001 |
| TD Score 5 | 4.09 | 0.70 | 59.95 | 15.27–235.38 | <0.0001 |
| TD Score 6 | 5.12 | 0.72 | 167.54 | 40.97–685.19 | <0.0001 |
| TD Score 7 | 4.84 | 0.74 | 126.00 | 29.66–535.23 | <0.0001 |
| TD Score 8 | 5.42 | 0.76 | 225.17 | 51.04–993.39 | <0.0001 |
| TD Score 9 | 7.26 | 0.90 | 1422.84 | 244.69–8273.63 | <0.0001 |
There was a significant difference (P < 0.0001) in the mean TD Score between those with acute watery diarrhoea (3.9 ± 1.9) and those with dysentery or acute febrile illness (6.2 ± 2.0) (Supplementary Table 5; Supplementary Figure 2). Additionally, disease severity varied significantly across study sites (Supplementary Table 5; Supplementary Figure 2), with Thailand yielding the highest mean score [M = 6.4, standard deviation (SD) = 1.4], compared with Honduras (M = 4.5, SD = 2.2, P = 0.02), Kenya (M = 3.6, SD = 2.1, P = 0.001) and Djibouti (M = 4.0, SD = 1.7, P = 0.05). There was also a statistically significant increase in mean TD Score for Afghanistan (M = 5.3, SD = 2.0) compared with Kenya and Djibouti (P < 0.001). There were no statistically significant differences in mean TD Scores between Kenya, Honduras and Djibouti.
Discussion
Since research on TD and related vaccines, prophylaxis or treatments began, primary efficacy endpoints have varied.27,29–34 Some studies have attempted to characterize TD symptom severity in the context of interference with daily activities,32,33 whereas others have used stool-based endpoints.34,35 Uniformly consistent in these studies is the focus on diarrhoea frequency as a key metric in primary endpoints. However, loose stool frequency alone may not be an inadequate predictor of disease severity, and other symptoms may more strongly predict traveller disability.11 This was the first attempt to integrate stool frequency and associated symptoms into a comprehensive TD severity score based on functional impact outcome assessment. In our score derivation, while an increase in diarrhoea frequency was significantly associated with a greater likelihood to report a negative impact on activity, it remained a poor predictor of impact on activity compared with other symptoms. For example, the odds of reporting a negative impact on activity while having ≥8 loose stools/24 h (OR = 8.51) was not as great as when afflicted with severe incontinence (OR 10.8), nausea (OR 116.7) or malaise (OR 44.29). Additionally, the likelihood of reporting a negative impact on activity with a TD Score of ‘2’ or ‘3’ was only slightly lower (OR 6.53) and approximately three times higher (OR 24.53), respectively, compared with the sole outcome of maximum stool output (Supplementary Figure 3). This indicates that stool frequency alone may not be a useful predictive measure of serious disease and indicates that symptoms beyond diarrhoea frequency contribute as much, if not more, to a severe illness profile impacting a traveller’s function.
Reported symptomology within the TrEAT TD dataset reflected trends similar to previous TD studies,12,32,33,36 especially regarding abdominal cramps, nausea, vomiting and fever. Malaise, tenesmus, gas and faecal incontinence have not been as consistently reported across TD studies, yet ~64%, 29%, 39% and 14% of TrEAT TD participants reported those symptoms, respectively. Tenesmus is a common symptom of infectious gastroenteritis often associated with pathogens that cause dysentery and inflammatory enteritis.38–40 Given the pathogen distribution in TrEAT TD, with ETEC and EAEC infections isolated as a sole pathogen in 24.6% and 38.6% of subjects, respectively, the high frequency of reported tenesmus is surprising. Additional efforts are needed to more completely validate data collection instruments used to obtain self-collected symptomology. Tenesmus is more commonly associated with pathogens that cause dysentery and inflammatory enteritis. Self-reporting of tenesmus may be subject to bias is a symptom that is easily misunderstood by study participants and therefore often inaccurately reported. It could be there was not enough rigor on assuring participants truly understood how to report this accurately, thus potentially contributing disproportionately to the disease severity score and should be further confirmed in prospective studies.
Each sign and symptom (except gas and tenesmus) was significantly associated with the maximum 24-h stool output as measured by frequency. The lack of significant association between gas and stool frequency was consistent with its negligible effect on activity and, given its lack of correlation with all other signs and symptoms except malaise, it was subsequently excluded as a parameter in the TD disease complex score. In contrast, while tenesmus was prevalent in TrEAT TD and significantly associated with a negative impact on activity, it was not significantly associated with stool output. Because of the prevalence of tenesmus in the TrEAT TD, its significant association with subject activity and its significant clustering with more severe symptoms in the MCA, tenesmus was an important clinical parameter to include in the TD complex score. Despite not being significantly associated with stool output but shown in correlation, various regression and multiple correspondence analyses to be a meaningful clinical parameter, tenesmus is an excellent example of how other symptoms, independent of stool frequency, might play an important role in TD severity. Application of this score in future studies would need to provide guidance to those collecting these same symptom parameters, and application retrospectively to datasets where there may have been symptom misclassification would need to be done cautiously. Based on these results, using stool frequency as the sole parameter to define TD illness is likely suboptimal. While this score proposes new stool frequency cut-points for the TD score, it was on a single dataset and would benefit from further study to see if these cut-points are consistent. It is important to emphasize that a relatively high proportion of subjects reported for care within 24 h of illness onset. Therefore, diarrhoea frequency may be lower than what would be observed if subjects waited longer to report illness.
The proportion of TrEAT TD subjects who experienced acute dysentery or febrile illness compared with acute watery diarrhoea remains consistent with data reported in other studies, with ~10% of TD cases being dysenteric across most travel destinations.41 There was a statistically significant difference in the mean TD score between the two illness profiles, due to inclusion of fever, and more severe tenesmus and abdominal pain reported in the acute dysentery or febrile illness group. While more subjects in the acute watery diarrhoea group experienced higher maximum 24-h stool frequency, the presence of more severe objective signs and subjective symptoms were not observed and lowered the overall mean TD score in this group. The higher mean TD score of the acute dysentery or febrile illness group is reflective of a recent expert panel of recommended standard TD definitions, in which severe TD includes all dysenteric cases.1,27
TD score differences across TrEAT TD sites were in large part driven by the TD syndrome distribution, with Thailand yielding the highest mean score due to enrollment being restricted to those presenting with acute dysentery or febrile diarrhea (ADF). Nevertheless, mean TD scores across TrEAT TD sites aligns with the geographic differences in distribution of pathogens and manifestation of clinical illness. For example, Salmonella and Campylobacter are the most commonly isolated TD pathogens in Southeast Asia,42,43 both of which are associated more with dysenteric cases or illness with fever.44 The rate of multipathogen recovery is also highest in Southeast Asia (~16%).42 ETEC is the most commonly isolated pathogen in the Middle East, followed by ETEC and EAEC in Africa,42 which correlate to the slightly milder disease profile observed in participants enrolled in Afghanistan and Kenya as those etiologies are more associated with acute watery diarrhoea. Norovirus features heavily into the etiology of TD in Latin and South America, and with a clinical manifestation of increased vomiting, perhaps that is what contributed to a mean TD Score of 4.52 in Honduras, the third highest among the six study sites. The regional variability in TD score and etiology were aligned and potentially provides another metric to help guide more targeted treatment or eventual vaccination efforts.
This analysis was conducted using data from a predominantly active-duty military population potentially limiting its external validity. As Mary Roach stated in her 2016 New York Times magazine article describing the TrEAT TD study, ‘For every person who shows up at the morning sick call, four tough it out’.45 As a result, it should be considered that it may differ from the routine travel population.
TrEAT TD utilized diary cards to collect symptoms from subjects while ill and prior to enrollment, thus limiting the risk of recall bias. However, it is possible that response bias persisted in reporting of symptoms. For example, a participant who experienced a severe symptom might have been more likely to report other signs and symptoms as he/she was more focused on what might have been making him/her feel unwell. In contrast, a participant who experienced mild symptoms with little impact on activity might have been less focused on feeling unwell and recorded fewer symptoms. While reporting bias may be a potential limitation, previous studies with military participants report a moderate rate of care-seeking behaviour for their TD.37,42 Furthermore, a recent review revealed an increase in care-seeking behaviour for the treatment of TD among both military and long-term travel populations.42
Conclusion
This TD disease severity score aims to advance beyond historical measures focused solely on loose stool frequency. In addition to more accurately predicting functional impact of TD, this holistic approach begins to address recommendations that disease severity should be based on an individual’s assessment that his/her illness is tolerable, distressing or incapacitating.14 Our assessment of the role of multiple TD-attributable signs and symptoms on functional impairment assists in understanding TD as a complex syndrome and advances the field beyond solely stool output-based endpoints.14 Furthermore, an ordinal score increases statistical power to differentiate treatment effects in interventional trials.26,46 This research reinforces recently published workshop-derived conclusions regarding the need for disease scoring algorithms for vaccine efficacy trials.11 Future research is needed to further develop and validate this score in other traveller populations and settings, and application and refinement of the score could be considered for evaluation of other TD preventive and treatment measures.
Supplementary Material
Disclaimer
The views expressed are those of the authors and do not necessarily reflect the official views or policies of the Uniformed Services University of the Health Sciences, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the US Department of Defense or the Departments of the Army, Navy, or Air Force or the UK Ministry of Defence. Authors are military service members or federal/contracted employee of the US government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that ‘copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C. 101 defines a US Government work as work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Author contributions
C.K.P., M.S.R., R.G., J.F., P.C. and D.R.T. were involved in primary data collection. N.M., C.K.P. and M.S.R. planned the analysis. N.M. performed all statistical analysis. N.M. and C.K.P. wrote the manuscript. All other authors contributed to the interpretation of results and critical review and revision of the manuscript and have approved the final version.
Funding
This work (IDCRP-065) was conducted by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USU) through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been supported with federal funds from the Bureau of Medicine and Surgery, Uniformed Services University of the Health Sciences (USU grant agreement HU0001-11-1-0022, USU project G187V2) and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (interagency agreement Y1-AI-5072).
Conflict of interest: None declared.
References
- 1. Centers for Disease Control and Prevention [CDC] . Travelers’ Diarrhea. 2019. https://wwwnc.cdc.gov/travel/yellowbook/2020/preparing-international-travelers/travelers-diarrhea (4 Novmember 2020, date last accessed).
- 2. Shah N, Ramsey DJ, DuPont HL. Global etiology of travelers' diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg 2009; 80:609–14. [PubMed] [Google Scholar]
- 3. Steffen R, Hill DR, DuPont HL. Traveler's diarrhea: a clinical review. JAMA 2015; 313:71–80. [DOI] [PubMed] [Google Scholar]
- 4. Jaep K. A systematic review of Shigella epidemiology in travelers. Oral presentation at the 1st International VASE Conference, Washington, D.C. (2016 June). [Google Scholar]
- 5. Riddle MS, Sanders JW, Putnam SD, Tribble D. Incidence, etiology, and impact of diarrhea among long-term travelers (US Military and similar populations): a systematic review. Am J Trop Med Hyg 2006; 74:891–900. [PubMed] [Google Scholar]
- 6. Porter CK, Olson S, Hall A, Riddle MS. Travelers’ diarrhea: an update on the incidence, etiology, and risk in military deployments and similar travel populations. Mil Med 2017; 182:4–10. [DOI] [PubMed] [Google Scholar]
- 7. IMARC Group . Travel Vaccines Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2021-2026. 2019. https://www.imarcgroup.com/travel-vaccines-market (8 August 2021, date last accessed).
- 8. Centers for Disease Control and Prevention (CDC) . Travel Vaccines. 2018. https://wwwnc.cdc.gov/travel/page/travel-vaccines (3 June 2021, date last accessed).
- 9. Connor BA, Dawood R, Riddle MS, Hamer DH. Cholera in travellers: a systematic review. J Travel Med 2019; 26:taz085. 10.1093/jtm/taz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. López-Gigosos R, Segura-Moreno M, Díez-Díaz R et al. Commercializing diarrhea vaccines for travelers. Hum Vaccin Immunother 2014; 10:1557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Porter CK, Gutierrez R, Kotloff K. Clinical endpoints for efficacy studies. Vaccine 2019; 37:4814–22. [DOI] [PubMed] [Google Scholar]
- 12. Stoney RJ, Han PV, Barnett ED et al. Travelers diarrhea and other gastrointestinal symptoms among Boston-Area international travelers. Am J Trop Med Hyg 2017; 96:1388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiwanitkit V. A report on a cluster of travelers’ diarrhea among Cambodian students visiting Thailand. J Travel Med 2007; 14:245–7. [DOI] [PubMed] [Google Scholar]
- 14. Riddle MS, Connor BA, Beeching NJ et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med 2017; 24:S63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Travelers' Diarrhea Deployment Health Guideline Expert Panel . Management of acute diarrheal illness during deployment: a deployment health guideline and expert panel report. Mil Med 2017; 182:34–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark HF, Offit PA. Vaccines for rotavirus gastroenteritis universally needed for infants. Pediatr Ann 2004; 33:536–43. [DOI] [PubMed] [Google Scholar]
- 17. Friedman JN, Goldman RD, Srivastava R, Parkin PC. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr 2004; 145:201–7. [DOI] [PubMed] [Google Scholar]
- 18. Gorelick MH, Shaw KN, Murphy KO. Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics 1997; 99:6. [DOI] [PubMed] [Google Scholar]
- 19. Jauregui J, Nelson D, Choo E et al. External validation and comparison of three pediatric clinical dehydration scales. PLoS One 2014; 9:e95739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinlin LM, Freedman SB. Evaluation of a clinical dehydration scale in children requiring intravenous rehydration. Pediatrics 2012; 129:e1211–9. [DOI] [PubMed] [Google Scholar]
- 21. Lee GO, Richard SA, Kang G et al. A comparison of diarrheal severity scores in the MAL-ED multisite community-based cohort study. J Pediatr Gastroenterol Nutr 2016; 63:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levine AC, Glavis-Bloom J, Modi P et al. Empirically derived dehydration scoring and decision tree models for children with diarrhea: assessment and internal validation in a prospective cohort study in Dhaka, Bangladesh. GHSP 2015; 3:405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259–67. [DOI] [PubMed] [Google Scholar]
- 24. WHO and UNICEF Joint Statement . Clinical Management of Acute Diarrhoea 2004. http://www.unicef.org/publications/files/ENAcute_Diarrhoea_reprint.pdf (28 October 2020, date last accessed).
- 25. Porter CK, Riddle MS, Alcala AN et al. An evidenced-based scale of disease severity following human challenge with enteroxigenic Escherichia coli. PLoS One 2016; 11:e0149358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porter CK, Lynen A, Riddle MS et al. Clinical endpoints in the controlled human challenge model for Shigella: a call for standardization and the development of a disease severity score. PLoS One 2018; 13:e0194325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riddle MS, Connor P, Fraser J et al. Trial Evaluationg Ambulatory Therapy of Travelers’ Diarrhea (TrEAT TD) Study: a randomizaed controlled trial comparing 3 single-dose antibiotic regimens with loperamide. Clin Infect Dis 2017; 65:2008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis RJ, Street WC. An introduction to Classification and Regression Tree (CART) analysis. Presented at the 2000 Society for Academic Emergency Medicine (SAEM) Annual Meeting; May 22–25; San Francisco, California.
- 29. Scerpella EG, Sanchez JL, Mathewson JJ III et al. Safety, immunogenicity, and protective efficacy of the whole-cell/recombinant B subunit (WC/rBS) oral cholera vaccine against travelers' diarrhea. J Travel Med 1995; 2:22–7. [DOI] [PubMed] [Google Scholar]
- 30. Wiedermann G, Kollaritsch H, Kundi M, Svennerholm AM, Bjare U. Double-blind, randomized, placebo-controlled pilot study evaluating efficacy and reactogenciity of an oral ETEC B-subunit-inactivated whole cell vaccine against travelers' diarrhea (preliminary report). J Travel Med 2000; 7:27–9. [DOI] [PubMed] [Google Scholar]
- 31. Leyten EMS, Soonawala D, Schultsz C et al. Analysis of efficacy of CVD 103-HgR live oral cholera vaccine against all-cause travellers' diarrhoea in a randomised, double-blind, placebo-controlled study. Elsevier 2005; 23:5120–6. [DOI] [PubMed] [Google Scholar]
- 32. Sack DA, Shimko J, Torres O et al. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. coli diarrhoea of travellers to Guatemala and Mexico. Vaccine 2007; 25:4392–400. [DOI] [PubMed] [Google Scholar]
- 33. Bourgeois AL, Halpern J, Grahek SL, et al. Vaccination of Travelers to Guatemala (GU) and Mexico (MX) with an Oral Killed Vaccine (OKV) for Enterotoxigenic E. coli (ETEC): Impact of Vaccine “Take” on Risk of ETEC Disease and Infection with Enteric Pathogens. Vaccines for Enteric Diseases (VED) Conference. Lisbon, Portugal. 2007. [Google Scholar]
- 34. Steffen R, Cramer JP, Burchard G et al. Efficacy of a travelers' diarrhea vaccine system in travelers to India. J Travel Med 2013; 20:374–9. [DOI] [PubMed] [Google Scholar]
- 35. Behrens RH, Cramer JP, Jelinek T et al. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers' diarrhoea: a phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect Dis 2014; 14:197–204. [DOI] [PubMed] [Google Scholar]
- 36. Riddle MS, Rockabrand DM, Schlett C et al. A prospective study of acute diarrhea in a cohort of United States military personnel on deployment to the Multinational Force and Observers, Sinai. Am J Trop Med Hyg 2011; 84:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Putnam SD, Sanders JW, Frenck RW et al. Self-reported description of diarrhea among military populations in operations Iraqi freedom and enduring freedom. J Travel Med 2006; 13:92–9. [DOI] [PubMed] [Google Scholar]
- 38. Adachi JA, Jiang ZD, Mathewson JJ et al. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clin Infect Dis 2001; 32:1706–9. [DOI] [PubMed] [Google Scholar]
- 39. Hebbelstrup Jensen B, Olsen KEP, Struve C, Krogfelt KA, Petersen AM. Epidemiology and clinical manifestations of enteroggregative Escherichia coli. Clin Microbiol Rev 2014; 27:614–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGregor AC, Wright SG. Gastrointestinal symptoms in travellers. Clin Med 2015; 15:93–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steffen R. Epidemiology of travellers’ diarrhea. J Travel Med 2017; 24:S2–5. [DOI] [PubMed] [Google Scholar]
- 42. Olson S, Hall A, Riddle MS, Porter CK. Travelers’ diarrhea: update on the incidence, etiology and risk in military and similar populations – 1990-2005 versus 2005–2015, does a decade make a difference? Trop dis travel med vaccines 2019; 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tribble DR. Antibiotic therapy for acute Watery diarrhea and dysentery. Mil Med 2017; 182:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tribble D, Zell E, Bradley W, et al. Campylobacteriosis outcomes assessed by baseline immune status in controlled human Campylobacter jejuni infection in humans. The 9th International Conference on Vaccines for Enteric Diseases (VED), Albufeira, Portugal. 2017. [Google Scholar]
- 45. Roach M. Diarrhea is the Wartime Enemy No One Mentions. New York Magazine . June 9, 2016. https://www.thecut.com/2016/06/diarrhea-is-the-wartime-enemy-no-one-mentions.html (10 September 2020, date last accessed).
- 46. Roozenbeek B, Lingsma HF, Perel P et al. The added value of ordinal analysis in clinical trials: an example in traumatic brain injury. Crit Care 2011; 15:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frech SA, Dupont HL, Bourgeois AL et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet 2008; 37:2019–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


