The prevalence of diagnosed CKD is high among individuals older than 65 years. Since GFR declines with normal aging, this phenomenon is due in part to the use of a single, absolute threshold of an eGFR <60 ml/min per 1.73 m2 persisting for at least 3 months to define CKD regardless of age or the concomitant presence of other signs of kidney injury, including abnormal albuminuria. Arguably, the eGFR criteria for CKD diagnosis should be age adapted, in order to account for the normal physiologic age-related decline in eGFR and avoid overdiagnosis of CKD in the elderly (1). Many elderly subjects with a stable eGFR between 45 and 59 ml/min per 1.73 m2 and no accompanying abnormal albuminuria are erroneously labeled as having CKD (1). However, “bona-fide” CKD, of varying etiologies, can also coexist with the physiologic changes in the kidney brought about by normal aging. Here, we attempt to describe the aging-related changes in the kidneys occurring in otherwise healthy individuals as compared with those with CKD.
In response to stress and/or damage, cells can either undergo apoptosis or enter a state of senescence, evidenced by changes in morphology and transcriptional profile, a secretory phenotype, and resistance to apoptosis. Since the ability to regenerate new cells and tissues tends to decrease with aging, restoring a balance between cellular dysfunction and repair progressively diminishes during normal aging. Superimposition of various harmful factors such as oxidative stress (oxygen radicals and profibrogenic mediators) and mitochondrial injury upon the intrinsic age-related changes, as occurs in disease, can further promote cellular and organ deterioration (2). Other disease-specific injurious processes, such as inflammation and ischemia, can further augment these changes. Thus, physiologic aging and disease-related injury commonly coexist, and their inter-relationships are complex and not easily studied. Therefore, a detailed exposition of the kidney alterations in physiologic aging in apparently healthy persons is invaluable in dissecting the contributions of aging per se to observed alterations attending CKD.
Several studies have investigated various aspects of micro- and macrostructural changes and functional alterations of the human kidney in normal, physiologic aging, even among the healthiest populations. Healthy living kidney donors lose up to a half of the functional nephrons they were born with (nephron endowment) by the time they are in their seventies. This attrition of nephrons is accompanied by an increase in global glomerulosclerosis, but not segmental glomerulosclerosis, and the increase in interstitial fibrosis/tubular atrophy (IF/TA) is minimal compared with CKD (3). This nephron loss ultimately leads to a reduction in cortex volume with aging. Whole kidney GFR closely follows this decline in nephron number, maintaining the single nephron GFR unchanged at least before age 70 years (4). Hypertrophy of remaining functional glomeruli is not observed in healthy aging. Correspondingly, albuminuria is also not a feature of healthy aging, unlike CKD. Studies in patients who undergo nephrectomy for renal cancer provide evidence for a decline in whole kidney GFR with aging beyond 70 years even with minimal amounts of IF/TA (5).
While IF/TA appears to be less prominent when due to nephron loss from aging rather than from CKD, the pattern of IF/TA with aging is also informative. Analysis of large wedges of unaffected kidney tissue from patients undergoing radical nephrectomy for tumors reveals that older patients have a more scattered pattern of IF/TA at the same %IF/TA as younger patients (6). This suggests: (1) IF/TA foci atrophy into smaller IF/TA foci with contraction of the cortex, which increases their density; and (2) this atrophy helps explain the minimal extent of %IF/TA in older individuals despite significant loss of nephrons. These findings are also consistent with age-related global glomerulosclerosis where atrophy and disappearance of sclerosed glomeruli leads to underdetection of nephron loss (3). Increased IF/TA density independent of %IF/TA is also prognostic for progressive CKD (6). Taken together, at the same %IFTA, a more long-standing process with more nephron loss has a worse prognosis than a more “acute” process with larger foci of IF/TA (6).
During the past decade, there has been an increased interest in podocyte dysfunction in kidney aging. Podocytes are fully differentiated, long-lived, post-mitotic cells, with a minimal regeneration capacity. Hodgin and colleagues found decreasing podocyte density with older age, from >300 per 106 µm3 in young kidneys to <100 per 106 µm3 in old kidneys (7). Moreover, older age podocytes are stressed with a higher detachment rate. Importantly, the kidneys studied were not restricted to healthy individuals, and the impact of comorbidities with aging may have influenced this finding. If glomerular hyperfiltration is superimposed upon normal aging effects, then podocyte detachment rates may accelerate, leading to more significant reductions in GFR and increases in albuminuria.
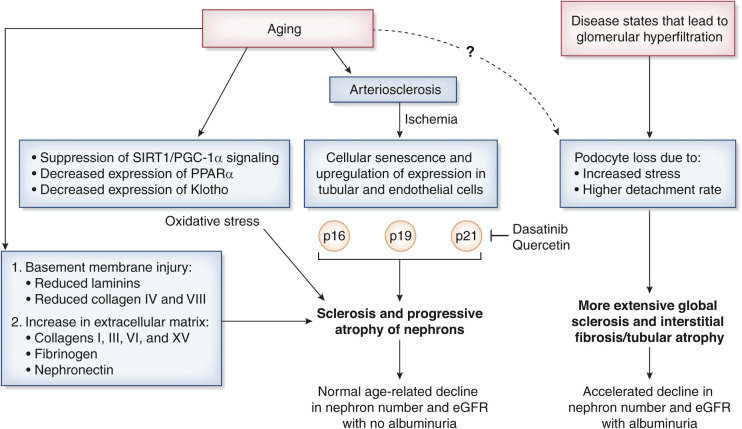
A conceptual diagram of processes that impact aging or that lead to accelerated aging and nephron loss is shown in Figure 1. Ischemia and progressive cellular senescence may be another important aspect of kidney aging. The primary events underlying an ischemia-related hypothesis for kidney aging are that arterio- and arteriolo-sclerosis causes an ischemia-driven collapse of the glomerular architecture and, ultimately, global glomerulosclerosis. As a result of chronic ischemia, a series of molecular changes ensue, such as upregulation of p16, p19, and p21 expression in tubular cells (8). Mesangial expansion and IF/TA in aging mice can be linked with inflammation, apoptosis, and oxidative stress (9). The evidence for oxidative stress as a potential causative factor was supported by a systematically decreased expression of Sirt1, PGC-1α, ERR-1α, PPARα, and Klotho in the oldest mice (24 months of age). It has been suggested that novel therapeutic approaches targeting these signaling molecules could alleviate processes that influence the rate of aging in kidneys. Of importance, ischemia-related cellular senescence markers accumulate in human kidneys supplied by stenotic renal arteries (8). Senolytic agents (dasatinib and quercetin) hold promise in reducing kidney atrophy, damage, and declining function by clearing p21-positive senescent cells (8). Future studies may determine a role for senolytic agents in treating age-related changes in the kidney (10).
Figure 1.
Conceptual diagram of nephron loss from aging as well as the impact of states of glomerular hyperfiltration leading to accelerated nephron loss and albuminuria.
Molecular evidence suggests that kidney aging and some causes of CKD share common biologic processes. Proteomic profiling of extracellular matrix composition in mouse and human kidneys has revealed a proteomic signature common for both kidney aging and disease (11). The main findings were a reduction in components of basement membrane (such as laminins, and collagens type IV and VIII) and increased amounts of extracellular matrix proteins (collagens I, III, VI, and XV, and fibrinogens and nephronectin). Collagen VI increased early in both aging and disease models, possibly as an attempt to strengthen the thinning of basement membranes underlying progression of IF/TA.
Absence of albuminuria in normal age-related eGFR decline suggests that this functional decline is not tightly and causally linked to podocytopenia; however, in disease processes that lead to glomerular hyperfiltration, the presence of albuminuria suggests a direct link to podocyte dysbiosis. Further work is needed to clarify these distinct pathways as mechanisms for progressive decline in nephron number. Many challenges exist, but much potential clinical utility may also accrue as we become more facile in distinguishing age-related from disease-related changes in the kidney. Overlap in the cellular biology of normal aging and at least some forms of progressive CKD is evident. Development of senolytic therapeutic agents holds promise in hopefully slowing down the effects of aging per se and the accelerated forms of aging that can accompany some forms of CKD.
Disclosures
A. Denic reports employment with Mayo Clinic. R.J. Glassock reports consultancy agreements with American Journal of Nephrology, Anteris Bio, Aurinia, BioCryst, Bioscience, Calliditas, ChemoCentryx, Equillium, Forsee Pharma, Horizon, Ionis, Karger Publications, NIH, Novartis, Omeros, Otsuka Pharma, RenaSight (Natera), River3Renal, Sentien, Therini Bio, Travere (Retrophin), UpToDate (Wolters-Kluwer), Vertex, Vivace, and Walden; ownership interest in Reata, Inc; receiving honoraria from Aurinia, EcoR1, Karger Publications, and Wolters-Kluwer (UpToDate); serving as a scientific advisor or member of American Journal of Nephrology, BioCryst, Calliditas, JASN, Novartis, Otsuka, RenaSight, Travere, University Kidney Research Organization, and UpToDate; speakers bureau for Aurinia; and other interests/relationships with ASN-Open Forum Communities. A.D. Rule reports employment with Mayo Clinic; serving as an Associate Editor of JASN and a Section Editor of Mayo Clinic Proceedings; serving as a scientific advisor or member of NIDDK – CKD Biomarker Consortium External Expert Panel; and other interests/relationships with UpToDate.
Funding
This study was supported by the U.S. Department of Health and Human Services National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK90358.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Delanaye P, Jager KJ, Bökenkamp A, Christensson A, Dubourg L, Eriksen BO, Gaillard F, Gambaro G, van der Giet M, Glassock RJ, Indridason OS, van Londen M, Mariat C, Melsom T, Moranne O, Nordin G, Palsson R, Pottel H, Rule AD, Schaeffner E, Taal MW, White C, Grubb A, van den Brand JAJG: CKD: A call for an age-adapted definition. J Am Soc Nephrol 30: 1785–1805, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H, Fogo AB: Cell senescence in the aging kidney. J Am Soc Nephrol 21: 1436–1439, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, Kremers WK, Lerman LO, Rule AD: The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol 28: 313–320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, Poggio E, Glassock RJ, Rule AD: Single-nephron glomerular filtration rate in healthy adults. N Engl J Med 376: 2349–2357, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P, Gupta S, Mothi SS, Rennke HG, Leaf DE, Waikar SS, McMahon GM: Histopathologic correlates of kidney function: Insights from nephrectomy specimens. Am J Kidney Dis 77: 336–345, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Ricaurte Archila L, Denic A, Mullan AF, Narasimhan R, Bogojevic M, Thompson RH, Leibovich BC, Sangaralingham SJ, Smith ML, Alexander MP, Rule AD: A higher foci density of interstitial fibrosis and tubular atrophy predicts progressive CKD after a radical nephrectomy for tumor. J Am Soc Nephrol 32: 2623–2633, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC: Glomerular aging and focal global glomerulosclerosis: A podometric perspective. J Am Soc Nephrol 26: 3162–3178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SR, Puranik AS, Jiang K, Chen X, Zhu XY, Taylor I, Khodadadi-Jamayran A, Lerman A, Hickson LJ, Childs BG, Textor SC, Tchkonia T, Niewold TB, Kirkland JL, Lerman LO: Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J Am Soc Nephrol 32: 1987–2004, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim JH, Kim EN, Kim MY, Chung S, Shin SJ, Kim HW, Yang CW, Kim YS, Chang YS, Park CW, Choi BS: Age-associated molecular changes in the kidney in aged mice. Oxid Med Cell Longev 2012: 171383, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzin R, Stasi A, Ranieri E, Netti GS, Cantaluppi V, Gesualdo L, Stallone G, Castellano G: Targeting premature renal aging: from molecular mechanisms of cellular senescence to senolytic trials. Front Pharmacol 12: 630419, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randles M, Lausecker F, Kong Q, Suleiman H, Reid G, Kolatsi-Joannou M, Tian P, Falcone S, Davenport B, Potter P, Van Agtmael T, Norman J, Long D, Humphries M, Miner J, Lennon R: Identification of an altered matrix signature in kidney aging and disease. J Am Soc Nephrol 32: 1713–1732, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]