Visual Abstract
Keywords: COVID-19, SARS-CoV-2, kidney transplantation, variants of concern, vaccination
Abstract
Background and objectives
Antibody response after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination is impaired in kidney transplant recipients. Emerging variants, such as B.1.617.2 (δ), are of particular concern because of their higher transmissibility and partial immune escape. Little is known about protection against these variants in immunocompromised patients.
Design, setting, participants, & measurements
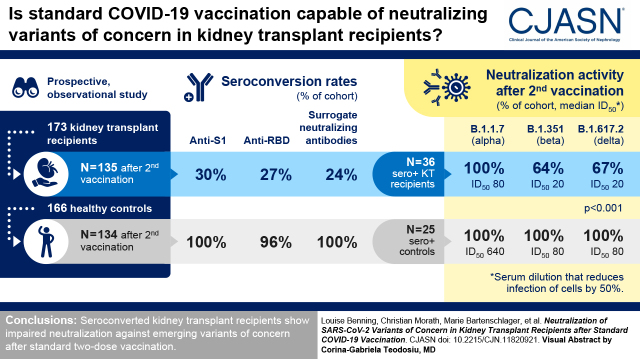
In this prospective two-center study, antispike 1 IgG and surrogate neutralizing antibodies were measured in 173 kidney transplant recipients and 166 healthy controls with different vaccination schedules. In addition, different SARS-CoV-2 epitope antibodies from 135 vaccinated kidney transplant recipients were compared with antibodies in 25 matched healthy controls after second vaccination. In 36 kidney transplant recipients with seroconversion, neutralization against B.1.1.7 (α), B.1.351 (β), and B.1.617.2 (δ) was determined on VeroE6 cells and compared with neutralization in 25 healthy controls.
Results
Kidney transplant recipients had significantly lower seroconversion rates compared with healthy controls. After the second vaccination, antispike 1, antireceptor-binding domain, and surrogate neutralizing antibodies were detectable in 30%, 27%, and 24% of kidney transplant recipients, respectively. This compares with 100%, 96%, and 100% in healthy controls, respectively (P<0.001). Neutralization against B.1.1.7 was detectable in all kidney transplant recipients with seroconversion, with a median serum dilution that reduces infection of cells by 50% of 80 (interquartile range, 80–320). In contrast, only 23 of 36 (64%) and 24 of 36 (67%) kidney transplant recipients showed neutralization against B.1.351 and B.1.617.2, respectively, with median serum dilutions that reduce infection of cells by 50% of 20 (interquartile range, 0–40) and 20 (interquartile range, 0–40), respectively. Neutralization against different variants was significantly higher in healthy controls (P<0.001), with all patients showing neutralization against all tested variants.
Conclusions
Seroconverted kidney transplant recipients show impaired neutralization against emerging variants of concern after standard two-dose vaccination.
Clinical Trial registry name and registration number:
Observational study to assess the SARS-CoV-2 specific immune response in kidney transplant recipients (COVID-19 related immune response), DRKS00024668
Introduction
Patients on hemodialysis and kidney transplant recipients are at high risk for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and more severe coronavirus disease 2019 courses (1,2). Vaccination is strongly recommended in these patients, but the protective immune response is significantly lower, especially if kidney transplant recipients are compared with healthy individuals (3–11). First data have shown a third vaccine dose in kidney transplant recipients to elicit a seroresponse among a substantial number of previous nonresponders (40%–50%) and to augment serotiters above those observed after a second vaccine dose in previous responders (12,13).
Determination of the immune response elicited by vaccination seems reasonable, as there is growing evidence of a correlation between neutralizing antibody levels measured by commercially available assays and protection against (re-)infection (14,15). However, most studies were performed when the SARS-CoV-2 wild-type and B.1.1.7 (α) strains were the predominant variants, and this situation may not translate to the current situation with the emerging variants of concern B.1.351 (β) and B.1.617.2 (δ). In particular, B.1.617.2, which has significantly higher transmissibility and may escape vaccine-induced immunity, is rapidly displacing other strains worldwide (16–18).
Wall et al. (19) reported a four- to six-fold reduction in vaccine-induced neutralization against the B.1.351 and B.1.617.2 variants in healthy individuals. Because neutralizing antibody levels correlate with infection risk, individuals with lower vaccine-induced immune response may not be adequately protected against virus strains with partial immune escape. Recent studies suggest that variants of concern are increasingly responsible for breakthrough infections in two-dose vaccinated healthy individuals, particularly when antibody levels decrease over time (20,21). Immunocompromised cohorts may become particularly susceptible to these variants of concern even after prior SARS-CoV-2 infection or vaccination (22–25).
To date, it is not known whether kidney transplant recipients with seroconversion after two-dose vaccination are protected against emerging variants of concern. Data on the neutralizing effect against new SARS-CoV-2 strains that are rapidly spreading worldwide are urgently needed.
Materials and Methods
Study Design
In this prospective two-center observational cohort study, we included 173 kidney transplant recipients and 166 healthy controls with different SARS-CoV-2 vaccination schemes between December 2020 and June 2021 at the Department of Nephrology (n=161) and the Department of Pediatrics (n=12) of the University Hospital of Heidelberg.
Serum for analysis was obtained from kidney transplant recipients and healthy controls after medians of 21 (interquartile range [IQR], 20–23) and 63 (IQR, 20–83) days after the first vaccination, respectively, and after medians of 21 (IQR, 19–25) and 20 (IQR, 19–21) days after the second vaccination, respectively. Antibodies to the nucleocapsid protein were measured after the first and second vaccinations, and individuals with positivity were excluded because of suspected prior SARS-CoV-2 infection. Antispike 1 (anti-S1) IgG antibodies and SARS-CoV-2–specific neutralizing antibodies measured by a surrogate neutralization test were assessed in 73 and 135 kidney transplant recipients and 115 and 134 healthy controls after the first or second immunization, respectively. Fifty-seven kidney transplant recipients and 45 healthy controls received their first vaccination with the mRNA vaccine BNT162b2 by BioNTech (BNT), and 16 kidney transplant recipients and 70 healthy controls received the chimpanzee adenovirus-vectored vaccine ChAdOx1 nCoV-19 by AstraZeneca (AZ) (Table 1). For second vaccination, 109 kidney transplant recipients received homologous mRNA/mRNA (105 BNT and 4 mRNA-1273 by Moderna), 17 kidney transplant recipients received homologous AZ/AZ, and nine kidney transplant recipients received heterologous AZ/mRNA vaccination (all BNT) (Table 1). Of 134 healthy controls, 82 received homologous mRNA/mRNA (all BNT), 17 received homologous AZ/AZ, and 35 received heterologous AZ/mRNA vaccination (all BNT). The distribution of different vaccination schemes of kidney transplant recipients and healthy controls is shown in Figure 1.
Table 1.
Characteristics of kidney transplant recipients from the University Hospital of Heidelberg assessed for coronavirus disease 2019 vaccine response
| Characteristics | Kidney Transplant Recipients after First Vaccination, n=73 | Kidney Transplant Recipients after Second Vaccination, n=135 | |||
|---|---|---|---|---|---|
| mRNA,a n=57 | AstraZeneca, n=16 | mRNA/mRNA,b n=109 | AstraZeneca/AstraZeneca, n=17 | AstraZeneca/mRNA,c n=9 | |
| Sample collection, d after vaccination (IQR) | 21 (20–23) | 21 (16–23) | 21 (20–26) | 22 (15–26) | 19 (16–22) |
| Vaccine interval, d, (IQR) | — | — | 35 (25–42) | 62 (62–62) | 62 (62–71) |
| Age, yr, (IQR) | 55 (43–62) | 55 (45–63) | |||
| Women, N (%) | 29 (40) | 50 (37) | |||
| Transplant-related data d | |||||
| Time since transplantation, yr, (IQR) | 5 (2–13) | 7 (3–14) | |||
| First transplant, N (%) | 62 (89) | 111 (85) | |||
| Deceased donor, N (%) | 42 (60) | 72 (55) | |||
| Current immunosuppressive medication,e N (%) | |||||
| CNI | 64 (90) | 125 (93) | |||
| MPA | 58 (82) | 110 (82) | |||
| Azathioprine | 2 (3) | 4 (3) | |||
| mTOR inhibitor | 8 (11) | 11 (8) | |||
| Belatacept | 2 (3) | 2 (2) | |||
| Steroids | 68 (96) | 130 (97) | |||
| Primary kidney disease, N (%) | |||||
| Diabetes or vascular | 10 (14) | 11 (8) | |||
| Polycystic kidney disease | 14 (19) | 24 (18) | |||
| GN | 23 (32) | 47 (35) | |||
| Other | 26 (36) | 53 (39) | |||
| Comorbidities, N (%) | |||||
| Hypertension | 59 (81) | 104 (77) | |||
| Chronic artery disease | 12 (16) | 25 (19) | |||
| Diabetes | 14 (19) | 20 (15) | |||
| Chronic lung disease | 11 (15) | 21 (16) | |||
| Chronic liver disease | 4 (6) | 9 (7) | |||
| Malignancy | 20 (27) | 32 (24) | |||
IQR, interquartile range; —, not applicable; CNI, calcineurin inhibitor; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin.
Fifty-seven received BNT162b2 by BioNTech.
One hundred and five received BNT162b2 by BioNTech, and four received mRNA-1273 by Moderna.
Nine received BNT162b2 by BioNTech.
No transplant-related data were available for three patients after the first vaccine dose and for four patients after the second vaccine dose.
No data were available on the immunosuppressive regimen for two patients after the first vaccine dose and for one patient after the second vaccine dose.
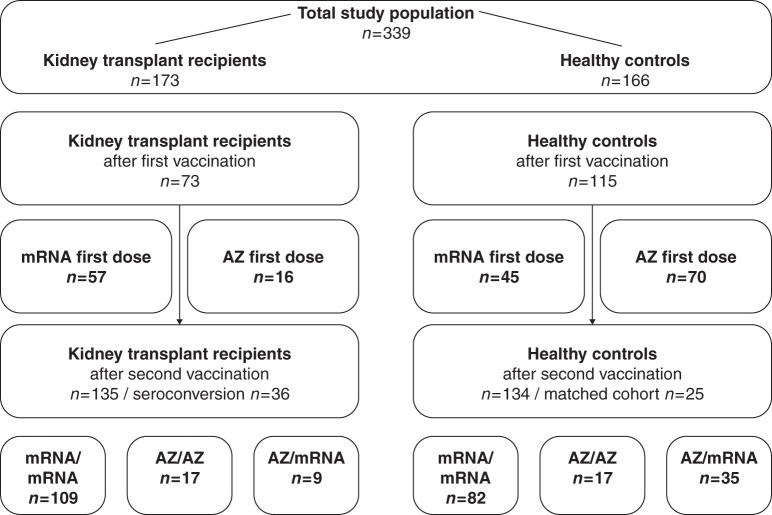
Figure 1.
Study population to determine humoral immune responses to different severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) two-dose vaccination regimens in kidney transplant recipients and healthy controls. In total, 339 participants were included in this study. Humoral response after vaccination was assessed in 173 kidney transplant recipients and 166 healthy controls. Analysis was done for 73 kidney transplant recipients and 115 healthy controls after first vaccination. Kidney transplant recipients and healthy controls received either an mRNA vaccine (n=57 and n=45, respectively) or an adenoviral vector–based vaccine (AZ; n=16 and n=70, respectively) as the first vaccine dose. Humoral response after the two-dose vaccination was assessed in 135 kidney transplant recipients and 134 healthy controls. Kidney transplant recipients received homologous mRNA/mRNA (n=109), homologous AZ/AZ (n=17), or heterologous AZ/mRNA two-dose vaccination (n=9). Healthy controls received homologous mRNA/mRNA (n=82), homologous AZ/AZ (n=17), or heterologous AZ/mRNA two-dose vaccination (n=35). For analysis of antibodies against various SARS-CoV-2 target epitopes and for analysis of neutralization against different variants of concern, kidney transplant recipients were compared with 25 healthy controls matched for type of vaccine, age, and sex.
In addition, a multiplex assay was performed in all 135 kidney transplant recipients and in 25 healthy controls matched for type of vaccine, sex, and age after the second immunization to detect antibodies against different SARS-CoV-2 target epitopes.
Neutralization against the different variants of concern B.1.1.7 (α), B.1.351 (β), and B.1.617.2 (δ) was assessed further by titration experiments on VeroE6 cells in seroconverted kidney transplant recipients and compared with results in 25 matched healthy controls. Baseline characteristics of seroconverted kidney transplant recipients are given in Supplemental Table 1.
Seropositivity was defined as a result above the respective threshold in at least two of the three commercially available assays: anti-S1 IgG (measured by chemiluminescent immunoassay; cutoff index greater than or equal to one), surrogate neutralizing antibodies (measured by the surrogate virus neutralization test; cutoff ≥30%), and antireceptor-binding domain (anti-RBD) antibodies (measured by the bead-based Luminex assay; cutoff mean fluorescence intensity [MFI] ≥3800). Of the various target epitopes measured by the bead-based Luminex assay, antibodies to the highly immunogenic RBD were selected for determination of seropositivity because they account for up to 90% of the neutralizing SARS-CoV-2–specific antibodies (26).
The study was approved by the ethics committee of the University of Heidelberg and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants or their legal representatives.
Antisevere Acute Respiratory Syndrome Coronavirus 2 Immunoglobulin G for Detection of Antispike 1 and Antinucleocapsid Antibodies
We measured the IgG response against the S1 protein by the SARS-CoV-2 total assay (Siemens, Eschborn, Germany) and the IgG response against the nucleocapsid protein by the Elecsys anti–SARS-CoV-2 assay (Roche, Mannheim, Germany) according to the manufacturers’ instructions. For anti-S1 IgG antibodies, a semiquantitative index of greater than or equal to one was classified as positive. This cutoff for detection gives a specificity of 100% with a sensitivity of 89% for detection of anti-S1 antibodies.
Neutralizing Capacity of Severe Acute Respiratory Syndrome Coronavirus 2 Antibodies Determined by a Surrogate Virus Neutralization Assay
The binding inhibition potency of serum samples was detected by a plate-based SARS-CoV-2 surrogate virus neutralizing assay (Medac, Wedel, Germany) as described previously by us (7,11,22,27). In brief, the surrogate virus neutralizing assay mimics the virus-host interaction by direct protein-protein interaction using purified RBD protein from the viral spike protein and the host cell receptor angiotensin-converting enzyme 2 (28). OD at 450 nm was measured in each well, and the percentage of inhibition was calculated as follows:
A cutoff of ≥30 inhibition of RBD:angiotensin-converting enzyme 2 binding was applied for neutralizing activity according to the manufacturer’s instructions. The test achieves 100% specificity and 95%–100% sensitivity.
Bead-Based Multiplex Analysis of Antibodies against Different Severe Acute Respiratory Syndrome Coronavirus 2 Target Epitopes
We used a bead-based assay for the Luminex platform (LabScreen Covid Plus; One Lambda Inc., West Hill, CA) to determine the IgG antibodies against different SARS-CoV-2 target epitopes. This assay measures antibodies against the SARS-CoV-2 nucleocapsid protein and four distinct fragments of the SARS-CoV-2 spike protein, namely the full spike protein, the S1 protein, the RBD of the spike protein, and the S2 protein (29). The assay was performed according to the manufacturer’s instructions, and MFI was analyzed on a Luminex 200 device (Luminex Corporation, Noord-Brabant, The Netherlands). Individual cutoff values are shown in Supplemental Table 2.
Cells and Viruses
Cultivation of VeroE6 cells and production of SARS-CoV-2 stocks have been reported recently by us and others (22) (Mallm et al., unpublished data). In brief, SARS-CoV-2 variants were isolated from nasopharyngeal and oropharyngeal swabs of RT-PCR–confirmed SARS-CoV-2–positive patients. Virus was amplified in VeroE6 cells, and virus titers of stocks produced in this cell line were determined by plaque assay. Stocks were stored at –80°C until use. In this study, we used UK14P2–B.1.1.7, ZA15P2–B.1.351, and IN2P2–B.1.617.2. We determined neutralization titers in titration experiments on VeroE6 cells as described previously (Mallm et al., unpublished data).
Neutralization Tests against the Variants of Concern B.1.1.7 (α), B.1.351 (β), and B.1.617.2 (δ)
Neutralization titers were determined in titration experiments on VeroE6 cells as described previously by us (30). Briefly, two-fold serial dilutions of vaccine sera were incubated with different SARS-CoV-2 variants. After 1 hour at 37°C, the mixture was added to VeroE6 cells, and cells were fixed in the plates with 5% formaldehyde 24 hours later. Virus replication was determined by immunostaining for the viral nucleoprotein using an in-cell ELISA. Values were normalized to those obtained with cells infected in the absence of patient serum (100% infection) and noninfected cells (0% infection), the latter determining the assay background. Values are demonstrated as a serum dilution that reduces infection of cells by 50% (ID50). A neutralization titer of 1:10 represents the cutoff for detection of this plaque neutralization assay.
Statistical Analyses
Data are given as median (IQR) or number (N; percent). Different groups were compared using the Mann–Whitney U test in case of continuous variables and the Fisher exact test in case of categorial variables. For the analysis of three or more groups, the Kruskal–Wallis test with the Dunn post-test was applied for continuous variables, and the chi-squared test was applied for categorial variables. To describe the relationship between two different tests analyzing humoral immunity, the Spearman rho was calculated as a nonparametric measure of rank correlation. Statistical significance was assumed at P=0.05. The statistical analysis was performed using GraphPad Prism version 9.0.0 (GraphPad Software, San Diego CA).
Results
Baseline Characteristics
From December 29, 2020 to June 21, 2021, we prospectively enrolled 173 kidney transplant recipients and 166 healthy controls with SARS-CoV-2 vaccination (Figure 1). Anti-S1 IgG and surrogate neutralizing antibodies were measured in 73 and 135 kidney transplant recipients and in 115 and 134 healthy controls after first and second vaccinations, respectively (Figure 1, Table 1). In addition, antibodies against various SARS-CoV-2 target epitopes were measured after second vaccination by multiplex analysis in 135 kidney transplant recipients and compared with results in 25 healthy controls matched for type of vaccine, age, and sex (Figure 1).
Neutralizing activity in VeroE6 cells against different variants of concern was measured in 36 kidney transplant recipients with seroconversion, and activity was compared with that of 25 matched healthy controls.
With median ages of 55 (IQR, 43–62) years at first vaccination and 55 (IQR, 45–63) years at second vaccination, kidney transplant recipients were older than the full healthy control cohort with median ages of 39 (IQR, 30–55) and 43 (IQR, 30–55) years, respectively. At first and second vaccinations, the full healthy control cohort was more likely to be women compared with kidney transplant recipients (Table 1). The use of immunosuppressive drugs in kidney transplant recipients is shown in Table 1.
Lower Humoral Responses after the First and Second Vaccinations in Kidney Transplant Recipients Compared with Healthy Controls
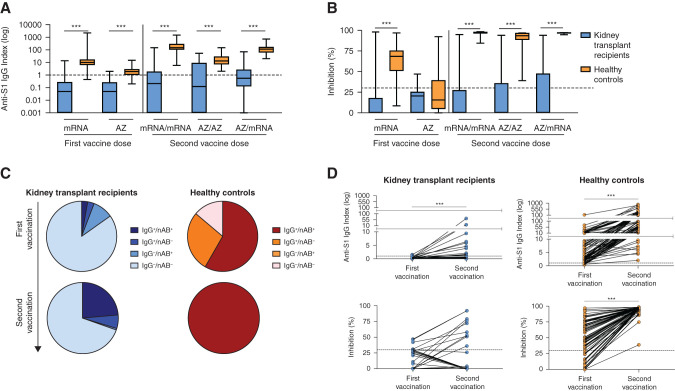
After first vaccination with an mRNA or AZ vaccine, anti-S1 IgG antibodies were significantly lower in kidney transplant recipients with median indices of 0.1 (IQR, 0–0.3) and 0.1 (IQR, 0–0.3) compared with 9 (IQR, 6–17; P<0.001) and 2 (IQR, 1–3; P<0.001), respectively, in the full healthy control cohort (Figure 2A). First vaccination with an mRNA vaccine led to a median surrogate neutralizing antibody activity that was significantly lower in kidney transplant recipients with 0% (IQR, 0%–18%) compared with the full healthy control cohort with 69% (IQR, 51%–75%; P<0.001). First vaccination with AZ led to neutralizing antibody activity that did not differ between groups with a median of 21% (IQR, 0%–26%) in kidney transplant recipients compared with 16% (IQR, 5%–39%) in healthy controls (Figure 2B).
Figure 2.
Antispike 1 (anti-S1) IgG antibodies and neutralizing antibodies in kidney transplant recipients and healthy controls with different SARS-CoV-2 two-dose vaccination regimens. (A) SARS-CoV-2 anti-S1 IgG antibodies were determined by a chemiluminescent immunoassay and are represented logarithmically as an anti-S1 IgG index in kidney transplant recipients and healthy controls with different vaccination regimens. The black dashed line represents the cutoff for detection. A semiquantitative index of greater than or equal to one was classified as positive. (B) The neutralizing capacity of antibodies (nAB) against SARS-CoV-2 was determined by a surrogate virus neutralization test in kidney transplant recipients and healthy controls with different two-dose vaccination regimens. A cutoff of ≥30% binding inhibition was applied according to the manufacturer’s instruction to define positivity. The black dashed line represents the cutoff for detection. (C) Pie charts show the distribution of positivity for anti-S1 IgG and neutralizing antibodies determined by the surrogate virus neutralization test for all kidney transplant recipients and healthy controls after first and second vaccinations. Some individuals had antibodies with neutralizing surrogate activity but no detectable anti-S1 IgG antibodies. This could be due to the presence of antibodies of different isotypes and also due to the independent validation of the two tests, resulting in different cutoffs for detection. (D) Courses of anti-S1 IgG antibodies and nAB measured by the surrogate virus neutralization test are shown for 36 kidney transplant recipients and 83 healthy controls with available sera after both first and second vaccinations. The black dashed lines represent the cutoff for detection for anti-S1 IgG and nAB, respectively. ***P<0.001.
Anti-S1 IgG antibodies were significantly lower in kidney transplant recipients after homologous mRNA/mRNA and AZ/AZ or heterologous AZ/mRNA standard two-dose vaccination with medians of 0.2 (IQR, 0–2), 0.1 (IQR, 0–9), and 0.5 (IQR, 0.1–3), respectively, compared with the full healthy control cohort with medians of 146 (IQR, 103–291), 13 (IQR, 7–29), and 116 (IQR, 62–170), respectively (for all P<0.001) (Figure 2A). This finding is reflected in a significantly lower surrogate neutralizing antibody capacity in kidney transplant recipients after homologous mRNA/mRNA and AZ/AZ or heterologous AZ/mRNA standard two-dose vaccination with medians of 0% (IQR, 0%–29%), 0% (IQR, 0%–36%), and 0% (IQR, 0%–48%), respectively, compared with the full healthy control cohort with medians of 97% (IQR, 96%–98%), 94% (IQR, 89%–97%), and 97% (IQR, 97%–97%), respectively (for all P<0.001) (Figure 2B). Distribution of anti-S1 IgG and/or surrogate neutralizing antibody positivity from first to second vaccination is shown in Figure 2C. Paired analyses of anti-S1 IgG and surrogate neutralizing antibodies from first to second vaccination are shown in Figure 2D.
Antibodies against Different Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein Epitopes Are Lower after Second Vaccination in Kidney Transplant Recipients Compared with Healthy Controls
After second vaccination, 135 kidney transplant recipients compared with 25 healthy controls matched for type of vaccine, sex, and age had significantly lower reactivity against different measured spike protein epitopes. Median MFI values of kidney transplant recipients were 914 (IQR, 0–14,089) for the full spike, 0 (IQR, 0–4220) for the S1, 227 (IQR, 0–6859) for the RBD, and 0 (IQR, 0–788) for the S2 protein compared with 23,166 (IQR, 22,425–23,543), 16,682 (IQR, 14,407–18,242), 19,650 (18,120–20,304), and 8908 (IQR, 6006–13,513) in matched healthy controls, respectively (for all P<0.001) (Supplemental Figure 1A). The individual distribution of immune reactivity against different spike protein epitopes and the proportional contribution of each antibody target to the total MFI value in kidney transplant recipients and healthy controls are shown in Supplemental Figure 1, B and C, respectively.
Neutralizing Activity (VeroE6 Cells) against Variants of Concern in Kidney Transplant Recipients with Seroconversion after Two-Dose Vaccination
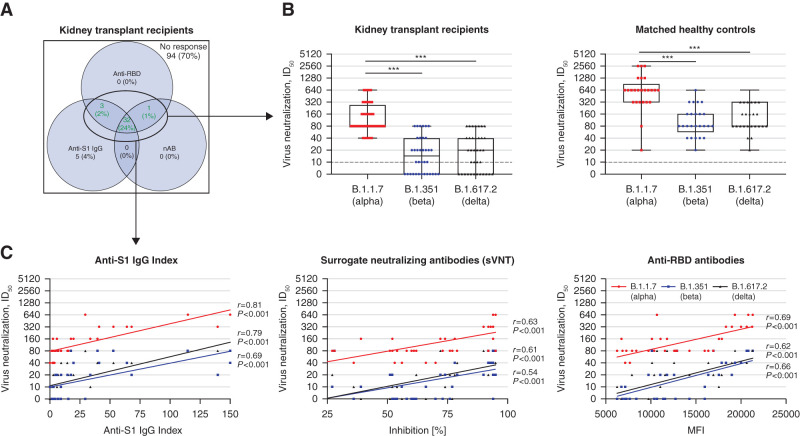
After the second vaccine dose, neutralization activity against different variants of concern was analyzed by a neutralization assay on VeroE6 cells in 36 seropositive kidney transplant recipients and compared with results in 25 matched healthy controls (Figure 3A).
Figure 3.
Neutralization of the variants of concern B.1.1.7 (α), B.1.351 (β), and B.1.617.2 (δ) in VeroE6 cells in all 36 seroconverted kidney transplant recipients and 25 healthy controls after two-dose vaccination against SARS-CoV-2. (A) The positivity of anti-S1 IgG index (chemiluminescent immunoassay), surrogate neutralizing antibodies (surrogate virus neutralization assay; nAB), and antireceptor-binding domain (anti-RBD) antibodies (multiplex bead-based assay) is shown in a color-coded Venn diagram for all 135 kidney transplant recipients after two-dose vaccination. Seroconversion was defined if at least two of the following antibodies were detectable: anti-S1 IgG, nAB, or anti-RBD IgG antibodies. Neutralization activity against variants of concern in VeroE6 cells was performed in 36 seroconverted kidney transplant recipients, represented in the Venn diagram by the green numbers. (B) Neutralizing activity against the variants of concern B.1.1.7 (α), B.1.351 (β), and B.1.617.2 (δ) was determined in a SARS-CoV-2 infection assay using VeroE6 target cells and serial two-fold dilutions of sera from all 36 kidney transplant recipients with seroconversion and 25 matched healthy controls after two-dose SARS-CoV-2 vaccination. The black dashed lines represent the cutoff for detection. (C) The correlation between anti-S1 IgG index (left panel), neutralizing SARS-CoV-2 antibodies measured by a surrogate virus neutralization test (center panel), and anti-RBD antibodies (right panel) with the neutralizing activity of the different variants of concern B.1.1.7 (α), B.1.351 (β), and B.1.617.2 (δ) in kidney transplant recipients was evaluated by Spearman correlation analysis. ID50, serum dilution that reduces infection of cells by 50%. ***P<0.001. sVNT, surrogate neutralizing antibodies.
All seropositive kidney transplant recipients showed neutralizing activity against the B.1.1.7 (α) variant with a median ID50 of 80 (IQR, 80–320) (Figure 3B), whereas only 23 of 36 (64%) and 24 of 36 (67%) seropositive kidney transplant recipients showed neutralization activity against the variants of concern B.1.351 (β) and B.1.617.2 (δ), respectively, with median ID50 values of 20 (IQR, 0–40) and 20 (IQR, 0–40), respectively (Figure 3B). In contrast, all matched healthy controls showed neutralizing activity against all tested variants (Figure 3B). With a median ID50 of 640 (IQR, 320–1280) for B.1.1.7, 80 (IQR, 60–160) for B.1.351, and 80 (IQR, 80–320) for B.1.617.2, matched healthy controls had significantly higher neutralization activity against the different variants of concern as compared with kidney transplant recipients (for all P<0.001) (Figure 3B). In both kidney transplant recipients and matched healthy controls, the neutralization activity against the variants of concern B.1.351 and B.1.617.2 was significantly lower compared with activity against B.1.1.7 (for all P<0.001) (Figure 3B).
The different assays for detection of anti-S1, anti-RBD, and surrogate neutralizing antibodies correlated well with the neutralizing activity (VeroE6 cells) against the variants of concern B.1.1.7, B.1.351, and B.1.617.2 (Figure 3C). Anti-S1 IgG antibodies correlated most strongly with the ID50 in the neutralization assay on VeroE6 cells of the different variants of concern.
Discussion
Emerging variants of concern, such as B.1.617.2 (δ), are rapidly displacing other strains due to their significantly higher transmissibility and ability to escape vaccine-induced immunity. Kidney transplant recipients with lower neutralizing antibody levels may become particularly susceptible to these variants (22).
This is the first study to provide an in-depth characterization of humoral responses to different variants of concern in kidney transplant recipients. Seroconversion rates were reduced after second vaccination in kidney transplant recipients, and neutralizing antibody levels were significantly lower compared with levels in healthy controls. We show that all kidney transplant recipients with detectable seroconversion by commercially available assays exhibited neutralizing activity against the B.1.1.7 (α) variant. However, 36% and 33% of seroconverted kidney transplant recipients did not show neutralizing activity against the B.1.351 (β) and B.1.617.2 (δ) variants, respectively. Anti-S1, anti-RBD, and surrogate neutralizing antibodies correlated well with the neutralizing activity against the different variants of concern, with anti-S1 antibodies showing the best correlation.
A limitation of studies examining humoral and cellular immune responses after coronavirus disease 2019 vaccination is a largely unknown correlate of protection against symptomatic infection or severe disease progression. Two recently published studies by Feng et al. (31) and Khoury et al. (32) used data from vaccine studies and convalescent cohorts to analyze the correlation between neutralization levels found in vitro and the observed protection from SARS-CoV-2 infection. An estimated 50% protection against symptomatic SARS-CoV-2 infection was achieved at ID50 levels in the range from 1:10 to 1:80 (31,32). However, these correlates of protection may vary significantly between different SARS-CoV-2 strains, and further research and validation are needed.
The lack of cellular immunity data against several variants of concern is another limitation of our study. Cellular immunity appears to be impaired in kidney transplant recipients, as both the frequency and function of spike-specific T-helper cells are reduced after two-dose mRNA vaccination (5,33). Sattler et al. (5) hypothesized that reduced IL-2 production by spike-specific T-helper cells combined with the direct effects of immunosuppressive therapy could explain the absence of vaccine-specific CD8+ T cells in these individuals.
Humoral responses after different homologous or heterologous vaccination regimens are shown separately in our study. However, the number of patients in different subgroups was too small to make a formal comparison or draw conclusions.
In summary, this study shows that a large proportion of kidney transplant recipients may not be adequately protected against the emerging variants B.1.351 (β) and B.1.617.2 (δ) with the standard vaccination regimens currently used in the healthy general population. Even if seroconversion is detectable by various commercially available assays, neutralization of these variants may not be sufficient to protect against infection. Additional vaccinations appear to be required in kidney transplant recipients to maintain high levels of neutralizing antibodies, especially when B.1.617.2 (δ) or other variants with partial escape from neutralizing antibodies become more prevalent.
Disclosures
R. Bartenschlager reports receiving research funding from Johnson & Johnson. C. Nusshag is funded by the Physician Scientist Programme of Heidelberg Faculty of Medicine. M. Schaier reports employment with and ownership interest in TolerogenixX GmbH. B. Tönshoff received research grants, travel grants, and lecture fees from and participated in advisory boards for Astellas, Bristol-Myers Squibb, Novartis, and Roche. All remaining authors have nothing to disclose.
Funding
Funding for this study was from Dietmar Hopp Stiftung grant 1DH2111111. R. Bartenschlager is supported by the program for surveillance and control of SARS-CoV-2 mutations of the State of Baden-Württemberg, the German Federal Research Network Applied Surveillance and Testing within the Network University Medicine, the DKFZ@fightCOVID initiative, and the Helmholtz Association Initiative and Networking Fund Project Virological and Immunological Determinants of COVID-19 Pathogenesis—Lessons to Get Prepared for Future Pandemics grant KA1-Co-02 “COVIPA.” L. Benning is funded by the Rahel Goitein-Strauss Program of the Heidelberg Faculty of Medicine. C. Speer is funded by the Physician Scientist Program of the Heidelberg Faculty of Medicine.
Supplementary Material
Acknowledgments
We thank Iris Arnold and Sabine Bönisch at the Department of Nephrology, Verena Backendorf at the Department of Immunology, and Heeyoung Kim at the Department of Infectious Diseases, Molecular Virology (all at Heidelberg University Hospital) for their technical support.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11820921/-/DCSupplemental.
Supplemental Figure 1. Antibodies against different SARS-CoV-2 target epitopes in kidney transplant recipients and healthy controls with different SARS-CoV-2 two-dose vaccination regimens.
Supplemental Table 1. Characteristics of 36 kidney transplant recipients with seroconversion after standard two-dose COVID-19 vaccination.
Supplemental Table 2. Cutoff values for defining positivity for antibodies against different SARS-CoV-2 target epitopes by a bead-based multiplex assay.
References
- 1.Hilbrands LB, Duivenvoorden R, Vart P, Franssen CFM, Hemmelder MH, Jager KJ, Kieneker LM, Noordzij M, Pena MJ, Vries H, Arroyo D, Covic A, Crespo M, Goffin E, Islam M, Massy ZA, Montero N, Oliveira JP, Roca Muñoz A, Sanchez JE, Sridharan S, Winzeler R, Gansevoort RT; ERACODA Collaborators : COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol Dial Transplant 35: 1973–1983, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B: Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: 430–436, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Hanoy M, Laurent C, Lebourg L, Etienne I, Lemoine M, Le Roy F, Nezam D, Plantier JC, Boyer O, Guerrot D, Candon S: Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol 32: 2147–2152, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danthu C, Hantz S, Dahlem A, Duval M, Ba B, Guibbert M, El Ouafi Z, Ponsard S, Berrahal I, Achard JM, Bocquentin F, Allot V, Rerolle JP, Alain S, Touré F: Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol 32: 2153–2158, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattler A, Schrezenmeier E, Weber UA, Potekhin A, Bachmann F, Straub-Hohenbleicher H, Budde K, Storz E, Proß V, Bergmann Y, Thole LM, Tizian C, Hölsken O, Diefenbach A, Schrezenmeier H, Jahrsdörfer B, Zemojtel T, Jechow K, Conrad C, Lukassen S, Stauch D, Lachmann N, Choi M, Halleck F, Kotsch K: Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest 131: 150175, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon-Arevalo H, Choi M, Stefanski A-L, Halleck F, Weber U, Szelinski F, Jahrsdörfer B, Schrezenmeier H, Ludwig C, Sattler A, Kotsch K, Potekhin A, Chen Y, Burmester GR, Eckardt KU, Guerra GM, Durek P, Heinrich F, Ferreira-Gomes M, Radbruch A, Budde K, Lino AC, Mashreghi MF, Schrezenmeier E, Dörner T: Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol 6: eabj1031, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Speer C, Göth D, Benning L, Buylaert M, Schaier M, Grenz J, Nusshag C, Kälble F, Kreysing M, Reichel P, Töllner M, Hidmark A, Ponath G, Schnitzler P, Zeier M, Süsal C, Morath C, Klein K: Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin J Am Soc Nephrol 16: 1073–1082, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi SG, Knight RJ, Graviss EA, Moore LW, Nguyen DT, Ghobrial RM, Gaber AO, Huang HJ: Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation 105: e72–e73, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, Martzloff J, Perrin P, Moulin B, Fafi-Kremer S, Caillard S: Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 99: 1498–1500, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco-Zevallos J, Casals-Urquiza J, Cofan F, Moreno A, Rovira J, Banon-Maneus E, Ramirez-Bajo MJ, Ventura-Aguiar P, Pérez-Olmos A, Garcia-Pascual M, Pascal M, Vilella A, Trilla A, Ríos J, Palou E, Juan M, Bayés B, Diekmann F: Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant 21: 2727–2739, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speer C, Morath C, Töllner M, Buylaert M, Göth D, Nusshag C, Kälble F, Schaier M, Grenz J, Kreysing M, Reichel P, Hidmark A, Ponath G, Schnitzler P, Zeier M, Süsal C, Klein K, Benning L: Humoral responses to single-dose BNT162b2 mRNA vaccination in dialysis patients previously infected with SARS-CoV-2. Front Med (Lausanne) 8: 721286, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A: Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 385: 661–662, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, Selzner N, Schiff J, McDonald M, Tomlinson G, Kulasingam V, Kumar D, Humar A: Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 385: 1244–1246, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Levin EG, Rubin C, Indenbaum V, Tal I, Zavitan M, Zuckerman N, Bar-Chaim A, Kreiss Y, Regev-Yochay G: Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 385: 1474–1484, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banham GD, Godlee A, Faustini SE, Cunningham AF, Richter A, Harper L; COVID-HD Birmingham Study Group : Hemodialysis patients make long-lived antibodies against SARS-CoV-2 that may be associated with reduced reinfection. J Am Soc Nephrol 32: 2140–2142, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Liu Y, Xia H, Zou J, Weaver SC, Swanson KA, Cai H, Cutler M, Cooper D, Muik A, Jansen KU, Sahin U, Xie X, Dormitzer PR, Shi PY: BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature 596: 273–275, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Xie X, Liu Y, Liu J, Zhang X, Zou J, Fontes-Garfias CR, Xia H, Swanson KA, Cutler M, Cooper D, Menachery VD, Weaver SC, Dormitzer PR, Shi PY: Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med 27: 620–621, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, Liu J, Errico JM, Xie X, Suryadevara N, Gilchuk P, Zost SJ, Tahan S, Droit L, Turner JS, Kim W, Schmitz AJ, Thapa M, Wang D, Boon ACM, Presti RM, O’Halloran JA, Kim AHJ, Deepak P, Pinto D, Fremont DH, Crowe JE Jr., Corti D, Virgin HW, Ellebedy AH, Shi PY, Diamond MS: Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med 27: 717–726, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, Daniels R, Hobson P, Hatipoglu E, Ngai Y, Hussain S, Nicod J, Goldstone R, Ambrose K, Hindmarsh S, Beale R, Riddell A, Gamblin S, Howell M, Kassiotis G, Libri V, Williams B, Swanton C, Gandhi S, Bauer DL: Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 397: 2331–2333, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, Caspi I, Levy R, Leshchinsky M, Ken Dror S, Bergerzon G, Gadban H, Gadban F, Eliassian E, Shimron O, Saleh L, Ben-Zvi H, Keren Taraday E, Amichay D, Ben-Dor A, Sagas D, Strauss M, Shemer Avni Y, Huppert A, Kepten E, Balicer RD, Netzer D, Ben- Shachar S, Stern A: Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med 27: 1379–1384, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen AE, Cohen S, Bryson-Cahn C, Liu C, Pergam SA, Lynch J, Schippers A, Strand K, Whimbey E, Mani NS, Zelikoff AJ, Makarewicz VA, Brown ER, Bakhash SAM, Baker NR, Castor J, Livingston RJ, Huang ML, Jerome KR, Greninger AL, Roychoudhury P: Variants of concern are overrepresented among post-vaccination breakthrough infections of SARS-CoV-2 in Washington State [published online ahead of print June 24, 2021]. Clin Infect Dis 10.1093/cid/ciab581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speer C, Benning L, Töllner M, Nusshag C, Kälble F, Reichel P, Schaier M, Bartenschlager M, Schnitzler P, Zeier M, Süsal C, Morath C, Bartenschlager R: Neutralizing antibody response against variants of concern after vaccination of dialysis patients with BNT162b2. Kidney Int 100: 700–702, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsapepas D, Paget K, Mohan S, Cohen DJ, Husain SA: Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am J Kidney Dis 78: 314–317, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang KM, Berlinrut I, Wallach FR: A case of severe COVID-19 despite full vaccination with mRNA-1273 SARS-CoV-2 vaccine (Moderna) in a kidney transplant recipient. Transpl Infect Dis 23: e13710, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta RB, Silveira FP: COVID-19 after two doses of mRNA vaccines in kidney transplant recipients [published online ahead of print July 31, 2021]. Am J Transplant 10.1111/ajt.16778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccoli L, Park Y-J, Tortorici MA, Czudnochowski N, Walls AC, Beltramello M, Silacci-Fregni C, Pinto D, Rosen LE, Bowen JE, Acton OJ, Jaconi S, Guarino B, Minola A, Zatta F, Sprugasci N, Bassi J, Peter A, De Marco A, Nix JC, Mele F, Jovic S, Rodriguez BF, Gupta SV, Jin F, Piumatti G, Lo Presti G, Pellanda AF, Biggiogero M, Tarkowski M, Pizzuto MS, Cameroni E, Havenar-Daughton C, Smithey M, Hong D, Lepori V, Albanese E, Ceschi A, Bernasconi E, Elzi L, Ferrari P, Garzoni C, Riva A, Snell G, Sallusto F, Fink K, Virgin HW, Lanzavecchia A, Corti D, Veesler D: Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 183: 1024–1042.e21, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benning L, Töllner M, Hidmark A, Schaier M, Nusshag C, Kälble F, Reichel P, Buylaert M, Grenz J, Ponath G, Klein K, Zeier M, Süsal C, Schnitzler P, Morath C, Speer C: Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines (Basel) 9: 857, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, Hu Z, Chen VC, Young BE, Sia WR, Tan YJ, Foo R, Yi Y, Lye DC, Anderson DE, Wang LF: A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 38: 1073–1078, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Bray RA, Lee J-H, Brescia P, Kumar D, Nong T, Shih R, Woodle ES, Maltzman JS, Gebel HM: Development and validation of a multiplex, bead-based assay to detect antibodies directed against SARS-CoV-2 proteins. Transplantation 105: 79–89, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Tönshoff B, Müller B, Elling R, Renk H, Meissner P, Hengel H, Garbade SF, Kieser M, Jeltsch K, Grulich-Henn J, Euler J, Stich M, Chobanyan-Jürgens K, Zernickel M, Janda A, Wölfle L, Stamminger T, Iftner T, Ganzenmueller T, Schmitt C, Görne T, Laketa V, Olberg S, Plaszczyca A, Cortese M, Bartenschlager R, Pape C, Remme R, Huzly D, Panning M, Weigang S, Giese S, Ciminski K, Ankerhold J, Kochs G, Schwemmle M, Handgretinger R, Niemeyer CM, Engel C, Kern WV, Hoffmann GF, Franz AR, Henneke P, Debatin KM, Kräusslich HG: Prevalence of SARS-CoV-2 infection in children and their parents in southwest Germany. JAMA Pediatr 175: 586–593, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I, Humphries HE, Jepson B, Kelly EJ, Plested E, Shoemaker K, Thomas KM, Vekemans J, Villafana TL, Lambe T, Pollard AJ, Voysey M; Oxford COVID Vaccine Trial Group : Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 27: 2032–2040, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP: Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27: 1205–1211, 2021 [DOI] [PubMed] [Google Scholar]
- 33.Stumpf J, Siepmann T, Lindner T, Karger C, Schwöbel J, Anders L, Faulhaber-Walter R, Schewe J, Martin H, Schirutschke H, Barnett K, Hüther J, Müller P, Langer T, Pluntke T, Anding-Rost K, Meistring F, Stehr T, Pietzonka A, Escher K, Cerny S, Rothe H, Pistrosch F, Seidel H, Paliege A, Beige J, Bast I, Steglich A, Gembardt F, Kessel F, Kröger H, Arndt P, Sradnick J, Frank K, Klimova A, Mauer R, Grählert X, Anft M, Blazquez-Navarro A, Westhoff TM, Stervbo U, Tonn T, Babel N, Hugo C: Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur 9: 100178, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.