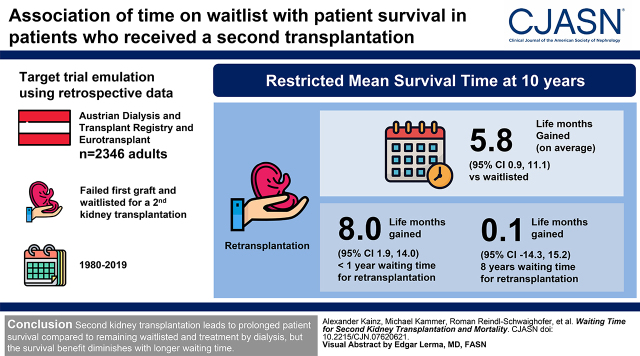
Visual Abstract
Keywords: second kidney transplantation, patient survival, target trial emulation, restricted mean survival time, kidney transplantation, waiting lists
Abstract
Background and objectives
The median kidney transplant half-life is 10–15 years. Because of the scarcity of donor organs and immunologic sensitization of candidates for retransplantation, there is a need for quantitative information on if and when a second transplantation is no longer associated with a lower risk of mortality compared with waitlisted patients treated by dialysis. Therefore, we investigated the association of time on waiting list with patient survival in patients who received a second transplantation versus remaining on the waiting list.
Design, setting, participants, & measurements
In this retrospective study using target trial emulation, we analyzed data of 2346 patients from the Austrian Dialysis and Transplant Registry and Eurotransplant with a failed first graft, aged over 18 years, and waitlisted for a second kidney transplantation in Austria during the years 1980–2019. The differences in restricted mean survival time and hazard ratios for all-cause mortality comparing the treatment strategies “retransplant” versus “remain waitlisted with maintenance dialysis” are reported for different waiting times after first graft loss.
Results
Second kidney transplantation showed a longer restricted mean survival time at 10 years of follow-up compared with remaining on the waiting list (5.8 life months gained; 95% confidence interval, 0.9 to 11.1). This survival difference was diminished in patients with longer waiting time after loss of the first allograft; restricted mean survival time differences at 10 years were 8.0 (95% confidence interval, 1.9 to 14.0) and 0.1 life months gained (95% confidence interval, −14.3 to 15.2) for patients with waiting time for retransplantation of <1 and 8 years, respectively.
Conclusions
Second kidney transplant is associated with patient survival compared with remaining waitlisted and treatment by dialysis, but the survival difference diminishes with longer waiting time.
Introduction
Kidney transplantation is the preferred therapy after kidney failure, as it prolongs survival and improves quality of life (1–3). The median graft survival rates, however, have remained unchanged over the last decades, with a median graft survival of 10–15 years for deceased donor kidneys (4). About 60% of patients with a failing first kidney allograft are eligible to be waitlisted for a second kidney transplantation, and patients awaiting retransplantation represent 28% of all waitlisted patients in Austria (5–7). Observational studies have reported that a second kidney transplantation also offers better survival and quality of life compared with remaining on dialysis, but these findings are generally at a high risk of selection or immortal time bias (8–16). A clinical trial establishing comparative effectiveness of transplantation is, however, infeasible for ethical and logistic reasons.
Decisions regarding a second transplantation need to accommodate different perspectives. At the moment, waiting time for kidney transplant differs between countries and depends on patient-related comorbidities and histocompatibility factors, such as the degree of HLA sensitization, HLA genotype, and ABO blood group. Furthermore, the time spent on dialysis before a transplant is associated with reduced graft survival rates in both first and second kidney transplant recipients (15,17,18). Donor kidneys are a limited good, and roughly 5% of waitlisted patients die each year due to accumulation of comorbidities while treated by dialysis (19,20). Thus, the question arises if it is fair and justifiable to waitlist patients for a subsequent second transplantation if other patients have not received their first graft (21). Recently, the survival difference in terms of life months gained by a first kidney transplantation compared with remaining on dialysis has been estimated to be about 7 months at 10 years of follow-up, but data for a second transplant remain elusive (22).
In this study, we made use of the national dialysis and transplant registry in Austria and data from Eurotransplant. We used the state-of-the-art causal inference methodology of target trial emulation, which comprised the development of a protocol for a hypothetical randomized trial to answer the research questions of interest, and subsequently using the registry data to mimic the target trial, adhering to the identification principles of causal inference to mitigate biases arising in analyses of observational data (23). Using this approach, we evaluated the survival difference of a second kidney transplantation compared with remaining waitlisted on dialysis and how this survival difference changes depending on waiting time (i.e., the time elapsed between graft loss and retransplantation).
Materials and Methods
Data Sources and Study Population
Our study used observational data from the Austrian Dialysis and Transplant Registry, including patient demographics, transplant data, and mortality (24). The data were supplemented by recipient and donor data from Eurotransplant, comprising the initial dates when an individual was added to or removed from the waiting list. Our analysis included all patients older than 18 years of age who were waitlisted for a second transplant between January 1, 1980 and August 31, 2019. First graft loss was defined as permanent return to dialysis. Patients were followed until death, loss to follow-up, or end of the observation period on August 31, 2019. The study was approved by the ethical committee of the Medical University of Vienna (ethics commission no. 1875/2018) and performed in accordance with the Declaration of Helsinki. The clinical and research activities reported are consistent with the principles of the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Definition of Estimands and Treatment Strategies
The endpoint of interest in our study was overall mortality. Assuming the availability of a suitable organ, we quantified the average survival difference between retransplantation and dialysis for individuals waiting for a second transplant by the difference in restricted mean survival time (RMST) between the treatment strategies “retransplant” versus “remain on the waiting list treated by dialysis and never retransplant in the future.” The RMST can be interpreted as the average survival time when following a population for a specified length of time, and the RMST difference quantifies gain or loss in survival time due to treatment strategy (25,26). We further quantified the survival difference conditional on different waiting times between first graft loss and potential retransplantation. In addition, we report accompanying hazard ratios (HRs) for mortality comparing retransplantation with being waitlisted and treated by dialysis.
Statistical Analyses
Characteristics of the study population at the time of first graft loss were summarized as means (±SD) or medians (first quartile to third quartile, interquartile range [IQR]) for continuous covariates and absolute and relative frequencies for categorical characteristics. Patient survival and functional graft survival (i.e., death-censored graft survival) after retransplantation were computed using the Kaplan–Meier estimator.
A key issue in assessing the effect of transplantation on survival is the lack of a natural control group in observational data. We addressed this by emulating a pragmatic clinical target trial through a series of auxiliary trials in a sequential Cox approach (27,28). Whenever a patient in our study received a second transplantation at a time point T (time of transplant allocation) after the first graft loss, an auxiliary trial was emulated using the observational data. In the trial starting at time T, the treatment group consisted of all individuals who received a transplant at time T after their first graft loss, and the control group consisted of individuals who had not yet received a transplant and who were on the waiting list at time T after their first graft loss. Inclusion and exclusion criteria were re-evaluated for each auxiliary trial, and the time to the observed outcome for eligible patients in that particular trial was measured starting from time T. Patients from the control group who were transplanted during follow-up of a specific auxiliary trial were censored at the time of their transplantation, but the transplantation also initiated another trial in the series of auxiliary trials. Data from all auxiliary trials were stacked and analyzed in a single Cox proportional hazards model using the group assignment as the main exposure. Details on the study design are in Supplemental Material, section 2.1, and Supplemental Figure 1. To assess the survival difference of retransplantation compared with dialysis conditional on the waiting time elapsed since first graft loss, we fitted a second model, adding the starting time of the auxiliary trial (time between first graft loss and transplant allocation, T) and its interaction with the main exposure as covariates. Flexible representations of time using restricted cubic splines were explored, but no relevant departures from linearity were observed.
Because the data were not randomized, we addressed confounding by stabilized inverse probability of treatment weights (IPTW), estimated by pooled logistic regression models following the approach by Hernán et al. (29). As covariates, the model included the variables recipient age and sex, year and duration of first kidney transplant, and duration of dialysis before first transplant, as well as time between first graft loss and initial joining date of the waiting list for the second transplant. Furthermore, individuals who entered an emulated trial in the control group but received a transplantation during the follow-up of that trial were incompatible with the definition of our comparison strategy “remain waitlisted and never retransplant.” We addressed this “nonadherence” by considering individuals in the control group as being censored at the time of their transplantation. This nonrandom censoring pattern was mitigated by yearly stabilized inverse probability of censoring weights (IPCW) for the control groups, estimated using separate Cox models per auxiliary trial (28). The IPCW models used the same covariates as the IPTW model. Further details are provided in Supplemental Material, sections 2.2 and 2.3.
The IPTW and yearly IPCW were multiplied, winsorized at their 0.5% and 99.5% percentiles, and used in the main Cox models for the study outcome as weights. We used 1000 bootstrap resamples of the individuals in which all analysis steps (weight estimation and weighted outcome model) were repeated to provide 95% confidence intervals (95% CIs) for all quantities of interest via the percentile method. Follow-up times (i.e., for the main Cox models and IPCW models in all auxiliary trials) were administratively censored at 15 years to mitigate the influence of individuals with extremely long survival times. We restricted the analysis to a maximum waiting time of 8 years after first graft loss due to inadequate sample sizes in auxiliary trials beyond such waiting times. Because the amount of missing data was low, we conducted a complete case analysis but have considered variables with higher amounts of missingness in the sensitivity analyses. Data preparation was done using SAS software for Windows 9.4 (Cary, NC), and all analyses were conducted using the R statistical software version 4.0.2. Further details on the analysis, including a protocol for the target trial and assumptions for the target trial emulation, are found in Supplemental Material, sections 1 and 2.
Sensitivity Analyses
Sensitivity analyses assessing the robustness of the results and exploring the changes in the magnitude of survival differences in specific subgroups comprise assessments of the proportionality assumptions for the main analysis and the IPCW, aspects of confounding (immunization as expressed by panel reactive antibody, deceased and living donor organs, and changes in transplantation practice over time), and considerations for missing data (missing waiting list dates for second transplantation). Further motivation and details on the sensitivity analyses are found in Supplemental Material, section 4.
Results
Cohort
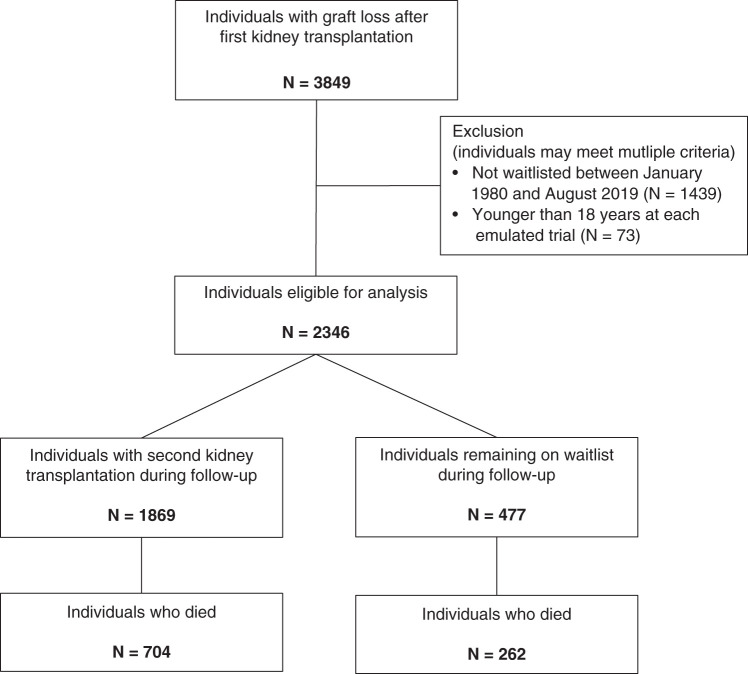
We included 2346 patients who were waitlisted for a second kidney transplantation after their first graft failure (Figure 1). Demographics of patients are provided in Table 1. In total, 1869 (80%) of the waitlisted patients were retransplanted during the study period, and 966 (41%) patients died, among which 262 died while still on the waiting list and 704 died after retransplantation. The median observed time between first graft loss and waitlisting was 0.6 (IQR, 0.2–1.4) years, the median time between first graft loss and retransplantation was 2.5 (IQR, 1.2–4.2) years, and the median time between first graft loss and death was 7.8 (IQR, 3.8–13.7) years. Overall, the median observed follow-up time was 10.7 (IQR, 5.3–18.8) years. The different transplantation dates within the first 8 years after first graft loss resulted in 1478 auxiliary trials, with a median number of 775 patients and 99 deaths per trial. The numbers of individuals and events per trial are shown in Supplemental Figure 2, whereas the observed covariate distributions in each trial are depicted in Supplemental Figure 3. Thirty patients did not enter the analysis due to waiting times longer than 8 years.
Figure 1.
Flow chart of patient inclusion and exclusion from the Austrian Dialysis and Transplant Registry and outcome data.
Table 1.
Cohort characteristics (number of individuals in the analysis: 2346)
| Variable | Statistic | Missing |
|---|---|---|
| Cohort characteristics at first graft loss | ||
| Age, yr | 44±14 | 0 (0%) |
| Women | 917 (39%) | 7 (<1%) |
| Duration of dialysis before first transplantation, yr | 1.5 (0.7–3.1) | 13 (<1%) |
| Year of first transplantation | 1993 (1987–2001) | 0 (0%) |
| Duration of first transplant, yr | 5 (1–10) | 0 (0%) |
| Panel reactive antibody, % | 8 (0–40) | 407 (17%) |
| Follow-up information | ||
| No. of individuals with second transplantation | 1869 (80%) | 0 (0%) |
| Live donor organ second transplantation | 115 (5%) | 40 (2%) |
| Preemptive second transplantationa | 79 (4%) | 0 (0%) |
| Time from first graft loss to waitlisting for second transplantation, yr | 0.6 (0.2–1.4) | 0 (0%) |
| Time from first graft loss to second transplantation, yr | 2.5 (1.2–4.2) | 0 (0%) |
| No. of deaths during follow-up | 966 (41%) | 0 (0%) |
| Time from first graft loss to death, yr | 7.8 (3.8–13.7) | 0 (0%) |
| Follow-up time, yr | 10.7 (5.3–18.8) | 0 (0%) |
Data are reported as mean±SD, median (interquartile range) or absolute frequency (relative frequency in percentage). Missing data are reported by absolute frequency (relative frequency in percentage).
Defined as dialysis vintage of <1 week before second transplantation.
Survival Difference of Retransplantation Compared with Dialysis
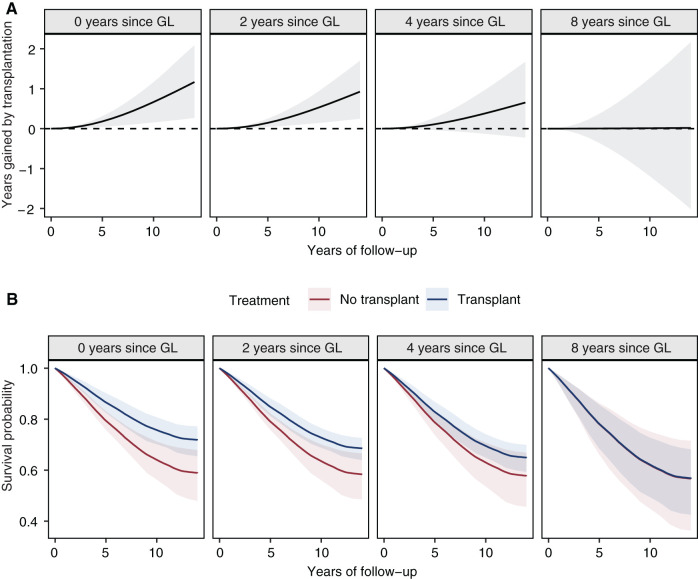
In our analysis of the emulated target trial, receiving a second transplantation led to improved overall survival compared with remaining waitlisted on dialysis, with an HR for mortality of 0.73 (95% CI, 0.53 to 0.95). Over a follow-up of 5 and 10 years, this translates to longer expected RMSTs in the transplantation group by 1.6 (95% CI, 0.3 to 2.9) and 5.8 (95% CI, 0.9 to 11.1) months, respectively. However, differences in average survival time between these two groups diminished with longer waiting time since first graft loss (Figure 2). The HRs comparing the rate of death between the retransplantation and control groups conditional on a patient being transplantable at a given time after first graft loss were 0.62 (95% CI, 0.43 to 0.89), 0.70 (95% CI, 0.53 to 0.91), 0.79 (95% CI, 0.55 to 1.09), and 0.99 (95% CI, 0.50 to 1.96) for 0, 2, 4, and 8 years after first graft loss, respectively. Figure 3 shows the respective difference in RMST and the adjusted survival curves for both treatment strategies at different times elapsed since first graft loss. Within the first year after first graft loss, the survival difference was the highest, and over follow-up times of 5 and 10 years, average survival after retransplantation was longer compared with remaining on dialysis by 2.2 (95% CI, 0.5 to 3.7) and 8.0 (95% CI, 1.9 to 14.0) months, respectively. After a waiting time of 8 years after graft loss, the survival differences between retransplantation and dialysis over 5 and 10 years of follow-up were diminished, with 0.0 (95% CI, −4.2 to 4.3) and 0.1 (95% CI, −14.3 to 15.2) months, respectively.
Figure 2.
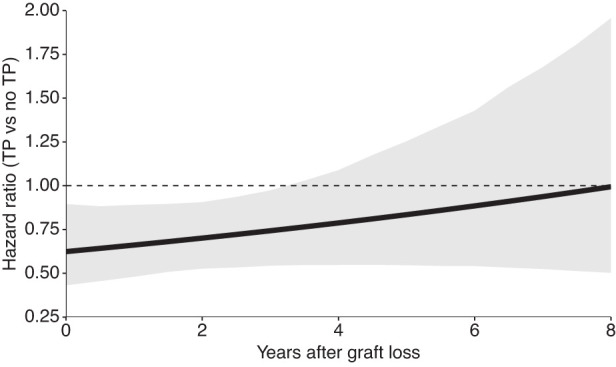
Hazard ratio comparing the effect of the treatment strategies “retransplant” versus “never retransplant—remain on the waiting list with continued dialysis” on mortality, conditional on the waiting time in years elapsed since first graft loss. The hazard ratio was estimated using Cox proportional hazards models incorporating stabilized inverse probability of treatment and stabilized inverse probability of censoring weights. The solid line shows the estimate using a single model fit on the full target trial dataset, and gray bands indicate 95% confidence intervals obtained by 1000 bootstrap resamples. The dashed line presents a hazard ratio of unity (no difference) for reference. TP, retransplant; no TP, never retransplant.
Figure 3.
Treatment comparison in terms of survival time outcomes. (A) The restricted mean survival time (RMST) difference estimated from the weighted Cox proportional hazards model also shown in Figure 2. Each subpanel gives the RMST of the transplantation group minus the RMST for the control group with up to 15 years of follow-up (x axis), conditional on the waiting time in years elapsed since first graft loss (GL). (B) The corresponding estimated adjusted survival curves for both groups in an analogous manner. The difference in RMST in (A) is given by the difference in the area under both survival curves up until a specified time of follow-up and is an estimate of the average difference in life years when the two populations are followed for a certain amount of time. In both (A) and (B), the point estimates were obtained using a single model fit on the full target trial dataset; the shaded areas indicate 95% confidence intervals obtained by 1000 bootstrap resamples.
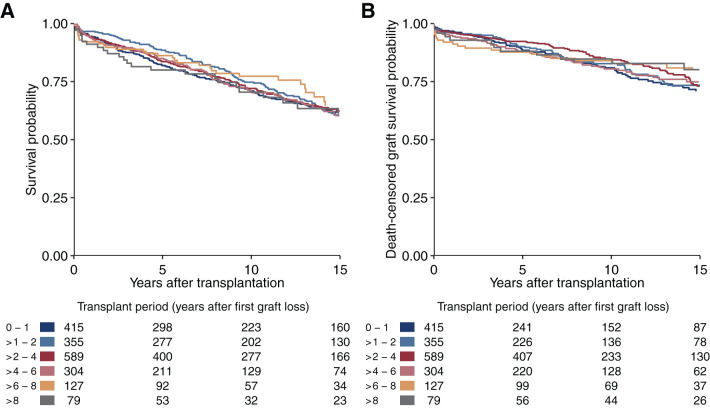
Patient survival from second transplantation onward was similar across different waiting times since first graft loss, as reflected in Figure 3B, blue lines. This observation is also supported by the crude Kaplan–Meier plots stratified by the time period after first graft loss of the second transplantation on the basis of the 1896 individuals who had a transplantation during follow-up (Figure 4A). Figure 4B further shows that graft survival remained stable across the different waiting time periods. The reduced survival difference between retransplantation and dialysis in patients with longer waiting time after first graft loss was mainly driven by an improved relative survival in patients who remained on dialysis, which may be explained by biologically selected long-term survivors (red lines in Figure 3B).
Figure 4.
Patient survival and death-censored graft survival. Kaplan–Meier plots for (A) patient survival and (B) death-censored graft survival after retransplantation, stratified by waiting time after first graft loss. Data are only shown for up to 15 years of follow-up after retransplantation. This figure is on the basis of the 1869 individuals who had a second transplantation during follow-up.
The final winsorized weights combining stabilized IPTW and yearly stabilized IPCW had a range of 0.29–5.23 (median, 1.05; IQR, 0.86–1.32) (Supplemental Figure 4).
Sensitivity Analyses
The general results remain compatible in all sensitivity analyses conducted. The short-term higher risk of death due to transplantation did not affect the survival difference between retransplantation and dialysis beyond 5 years of follow-up (RMST difference at 5 years of 1.6 months; 95% CI, 0.1 to 3.1 and at 10 years of 8.2 months; 95% CI, 3.4 to 12.6). Excluding all data prior to 1994 (the year of the introduction of calcineurin inhibitor–based maintenance immunosuppression as the standard regimen in the Eurotransplant region) to assess the effect of changes in transplantation practice showed a higher survival difference between retransplantation and dialysis compared with the main analysis (HR for retransplantation, 0.47; 95% CI, 0.35 to 0.64; RMST difference at 5 years of 3.8 months; 95% CI, 2.3 to 4.9 and at 10 years of 14.5 months; 95% CI, 8.7 to 19.7), which again diminished over longer waiting times (not shown). An analysis of deceased donor organ recipients only revealed an HR of 0.81 (95% CI, 0.60 to 1.08) for retransplantation, suggesting a higher survival difference for live donor organ recipients. All numerical results are reported in Supplemental Material, section 4.1, and Supplemental Table 1.
Discussion
Our study corroborated an overall survival difference between second kidney transplantation and remaining waitlisted with continued dialysis treatment in patients with a failing first allograft eligible for retransplantation. This survival difference, however, diminished with longer waiting time for retransplantation, with no statistically significant survival difference in individuals with a waiting time of >3 years after first graft loss.
Direct assessment of the comparative effectiveness of transplantation versus remaining on dialysis through a randomized controlled trial is infeasible due to ethical, biologic, and logistic reasons. However, many of the published studies addressing this comparison used conventional observational study designs with a high risk to overestimate potentially better survival with transplantation due to several methodologic issues (8–16). Key among these are immortal time bias due to the wrong attribution of events to the two groups, often caused by ignoring the time-dependent nature of the treatment group status, and selection bias, as outcomes for patients with transplants are compared with individuals on dialysis not eligible for transplantation. We, therefore, applied the state-of-the-art causal inference methodology of target trial emulation to overcome these limitations (23).
Most studies on the survival difference with transplantation have been performed primarily in patients receiving a first kidney allograft, and they provided evidence for improved long-term survival compared with remaining on dialysis. A recent paper using propensity score–matched groups to emulate a target trial in first kidney transplant recipients between 2005 and 2016 from the national French transplant registry reported an overall life expectancy gain of 6.8 months at a follow-up of 10 years in transplanted versus never transplanted patients. However, the authors did not assess changing hazards depending on waiting time (22). Their estimated effect for first kidney transplantation is comparable with results from a study using target trial emulation of our own research group (C. Wallisch, personal communication) and matches the overall survival difference for retransplantation versus remaining waitlisted on dialysis that we found in our cohort.
However, the case for second transplantation differs from first kidney transplantation at several important points. Patients with a failing first graft have usually experienced longer time periods on dialysis treatment but with tapered immunosuppression, leading to an accumulation of comorbidities. In addition, immunologic barriers may arise as a consequence of immunization from the previous transplantation that subsequently reduce the number of compatible donors and increase waiting time for retransplantation. It has previously been reported that longer time on dialysis prior to transplantation is associated with both reduced graft and patient survival following kidney transplantation, but these studies differ in methodology and study population from this work (15,17,18).
In our cohort, survival after retransplantation was fairly similar irrespective of waiting time after first graft loss. The observed reduction in survival difference was mainly a consequence of longer survival in patients remaining on dialysis, conditional on that they survived and were transplant eligible after the respective waiting time after first graft loss. This observation may be explained by the higher mortality in patients remaining on dialysis at earlier time points.
Furthermore, our results on the basis of exclusion of data prior to 1994 indicated a higher survival difference between retransplantation and dialysis in recent decades, as transplantation practice improved over time.
Some limitations of our study need to be taken into account when interpreting the results. As for any observational study, unmeasured confounding may be present, although we used several covariates from the registry data representing the general health status of an individual. Patients in our study were excluded from auxiliary trials after they had been delisted for second transplantation. Because eligibility for waitlisting is on the basis of an overall assessment of comorbidities and fitness, we thereby addressed potential confounding. Our study patients were representative of a central European, predominantly White population, and our data reflect the organ allocation algorithm used within Eurotransplant as well as the relatively higher incidence of deceased donor frequency in Austria compared with other countries. The findings may, therefore, be not directly generalizable to other countries. We did not incorporate temporary, short-term transplant ineligibility due to the lack of data, but we did remove individuals from the analysis after they were delisted. Our study assumes that in each auxiliary trial, an ideal graft would have been available for all individuals.
Key strengths of our study are the state-of-the-art target trial emulation addressing several limitations of prior published works. These include the use of weighting to address confounding, proper definitions and modeling of the compared treatment strategies, and avoiding immortal time bias as well as the almost complete follow-up of transplanted patients in the Austrian registry. Furthermore, we not only provided a marginal estimate of the survival difference of second transplantation compared with waitlisted patients still on dialysis but also accounted for the time between first graft loss and second transplantation to assess the effect of waiting time on the effectiveness of transplantation.
We conclude that early retransplantation for eligible individuals provides an effective treatment strategy to improve overall survival in patients with a failing first allograft. This survival difference may diminish in patients eligible for transplantation with a waiting time for retransplantation longer than 3 years. Nevertheless, patient-reported outcomes show a better quality of life following transplantation compared with remaining on dialysis, which therefore provides a rationale for retransplantation that goes beyond patient survival (30). Our findings could provide further perspectives on allocation priorities as donor organs remain a limited good.
Disclosures
M. Naik reports receiving research funding from the Berlin Institute of Health; receiving travel expenditures from Abbot Pharma (Symposium at Zurich 2012), Neovii (The Transplantation Society 2018 Madrid), Pfizer (Deutsche Transplantationsgesellschaft 2011, European Society for Organ Transplantation 2013, American Transplant Congress 2017), and TevaPharm (American Transplant Congress 2011); serving as a scientific advisor or member of the Pfizer German Sirolimus Study Group Advisory Board (2010–2015); and ownership of shares of Alexion Pharmaceuticals, Bayer, Fresenius Medical Care, and Dr. Reddys. M. Naik is a participant in the Berlin Institue of Health–Charité Digital Clinician Scientist Program, which is funded by the Charité Universitätsmedizin Berlin and the Berlin Institute of Health. R. Oberbauer reports receiving research funding from Chiesi, Fresenius, Novartis, Roche, and Sandoz; receiving honoraria from Chiesi, Neovii, Sandoz, and Teva; patents and inventions with Amgen (sold patent, no royalties); serving as a scientific advisor or member of Amgen, Astellas, Chiesi, and Novartis; receiving speaker honoraria from Amgen, Astellas, Chiesi, Fresenius, Novartis, and Sandoz; and other interests/relationships with the Austrian Society of Nephrology, the European Society of Nephrology, and the European Society of Organ Transplantation. O. Viklicky reports receiving honoraria from Astellas, Chiesi, and Fresenius and serving as a scientific advisor or member of Bayer. All remaining authors have nothing to disclose.
Funding
This study was supported by Vienna Science and Technology Fund grant LS16-019. S. Strohmaier received funding from the European Union’s H2020 Marie Skłodowska-Curie Actions grant 795292.
Supplementary Material
Acknowledgments
We are grateful to all persons responsible for the maintenance of the Austrian Dialysis and Transplant Registry. We thank Dr. Georg Heinze, Dr. Christine Wallisch, and Dr. Maria Haller for the discussions on the study design and their complementary work on first transplantations.
Funding sources were not involved in the preparation of the article; in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Waitlist Mortality for Second Kidney Transplants,” on pages 6–7.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07620621/-/DCSupplemental.
Supplemental Material. Emulated trial protocol, extended statistical methods, supplemental figures, and sensitivity analyses.
Supplemental Figure 1. Visualization of the study design.
Supplemental Figure 2. Sample size for each emulated trial in the target trial dataset.
Supplemental Figure 3. Covariate distributions in each auxiliary trial.
Supplemental Figure 4. Histogram of relative frequencies of inverse probability weights used as observations weights in the final Cox analysis models.
Supplemental Table 1. Summary of the results for effect of second transplantation for all sensitivity analyses.
References
- 1.Meier-Kriesche HU, Kaplan B: Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A paired donor kidney analysis. Transplantation 74: 1377–1381, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, Leichtman AB, Kaplan B: Effect of waiting time on renal transplant outcome. Kidney Int 58: 1311–1317, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Wekerle T, Segev D, Lechler R, Oberbauer R: Strategies for long-term preservation of kidney graft function. Lancet 389: 2152–2162, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Heaphy EL, Poggio ED, Flechner SM, Goldfarb DA, Askar M, Fatica R, Srinivas TR, Schold JD: Risk factors for retransplant kidney recipients: Relisting and outcomes from patients’ primary transplant. Am J Transplant 14: 1356–1367, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Schold JD, Augustine JJ, Huml AM, O’Toole J, Sedor JR, Poggio ED: Modest rates and wide variation in timely access to repeat kidney transplantation in the United States. Am J Transplant 20: 769–778, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eurotransplant : Active kidney-only waiting list (at year-end) in 2020, by country, by characteristic, 2021. Available at: https://statistics.eurotransplant.org/index.php?search_type=waiting+list&search_organ=kidney&search_region=Austria&search_period=2020&search_characteristic=active+WL&search_text=&search_collection=. Accessed March 17, 2021
- 8.Florit EA, Bennis S, Rodriguez E, Revuelta I, De Sousa E, Esforzado N, Cofán F, Ricart MJ, Torregrosa JV, Campistol JM, Oppenheimer F, Diekmann F: Pre-emptive retransplantation in patients with chronic kidney graft failure. Transplant Proc 47: 2351–2353, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Girerd S, Girerd N, Aarnink A, Solimando E, Ladrière M, Kennel A, Rossignol P, Kessler M, Frimat L: Temporal trend and time-varying effect of preemptive second kidney transplantation on graft survival: A 30-year single-center cohort study. Transplant Proc 48: 2663–2668, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Girerd S, Girerd N, Duarte K, Giral M, Legendre C, Mourad G, Garrigue V, Morelon E, Buron F, Kamar N, Del Bello A, Ladrière M, Kessler M, Frimat L: Preemptive second kidney transplantation is associated with better graft survival compared with non-preemptive second transplantation: A multicenter French 2000-2014 cohort study. Transpl Int 31: 408–423, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Goldfarb-Rumyantzev AS, Hurdle JF, Baird BC, Stoddard G, Wang Z, Scandling JD, Barenbaum LL, Cheung AK: The role of pre-emptive re-transplant in graft and recipient outcome. Nephrol Dial Transplant 21: 1355–1364, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Johnston O, Rose CL, Gill JS, Gill JS: Risks and benefits of preemptive second kidney transplantation. Transplantation 95: 705–710, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, Callard SE, Dickinson DM, Schmouder RL, Leichtman AB: Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: Multivariate analyses from the United States Renal Data System. Transplantation 66: 1651–1659, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Rao PS, Schaubel DE, Wei G, Fenton SS: Evaluating the survival benefit of kidney retransplantation. Transplantation 82: 669–674, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Wong G, Chua S, Chadban SJ, Clayton P, Pilmore H, Hughes PD, Ferrari P, Lim WH: Waiting time between failure of first graft and second kidney transplant and graft and patient survival. Transplantation 100: 1767–1775, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Clark S, Kadatz M, Gill J, Gill JS: Access to kidney transplantation after a failed first kidney transplant and associations with patient and allograft survival: An analysis of national data to inform allocation policy. Clin J Am Soc Nephrol 14: 1228–1237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haller MC, Kainz A, Baer H, Oberbauer R: Dialysis vintage and outcomes after kidney transplantation: A retrospective cohort study. Clin J Am Soc Nephrol 12: 122–130, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prezelin-Reydit M, Combe C, Harambat J, Jacquelinet C, Merville P, Couzi L, Leffondré K: Prolonged dialysis duration is associated with graft failure and mortality after kidney transplantation: Results from the French transplant database. Nephrol Dial Transplant 34: 538–545, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Lassalle M, Monnet E, Ayav C, Hogan J, Moranne O, Couchoud C; REIN registry : 2017 Annual report digest of the Renal Epidemiology Information Network (REIN) registry. Transpl Int 32: 892–902, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Miller J, Wey A, Musgrove D, Son Ahn Y, Hart A, Kasiske BL, Hirose R, Israni AK, Snyder JJ: Mortality among solid organ waitlist candidates during COVID-19 in the United States. Am J Transplant 21: 2262–2268, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivlin MM: Why the fair innings argument is not persuasive. BMC Med Ethics 1: E1, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenain R, Boucquemont J, Leffondré K, Couchoud C, Lassalle M, Hazzan M, Foucher Y: Clinical trial emulation by matching time-dependent propensity scores: The example of estimating impact of kidney transplantation. Epidemiology 32: 220–229, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Hernán MA, Robins JM: Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183: 758–764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ÖDTR : ÖDTR - Österreichisches Dialyse- und Transplantationsregister, 2021. Available at: https://oedtr.i-med.ac.at/. Accessed November 19, 2021
- 25.Kim DH, Uno H, Wei LJ: Restricted mean survival time as a measure to interpret clinical trial results. JAMA Cardiol 2: 1179–1180, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royston P, Parmar MK: Restricted mean survival time: An alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 13: 152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaubel DE, Wolfe RA, Port FK: A sequential stratification method for estimating the effect of a time-dependent experimental treatment in observational studies. Biometrics 62: 910–917, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Gran JM, Røysland K, Wolbers M, Didelez V, Sterne JA, Ledergerber B, Furrer H, von Wyl V, Aalen OO: A sequential Cox approach for estimating the causal effect of treatment in the presence of time-dependent confounding applied to data from the Swiss HIV Cohort Study. Stat Med 29: 2757–2768, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Hernán MA, Brumback B, Robins JM: Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11: 561–570, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Ju A, Josephson MA, Butt Z, Jowsey-Gregoire S, Tan J, Taylor Q, Fowler K, Dobbels F, Caskey F, Jha V, Locke J, Knoll G, Ahn C, Hanson CS, Sautenet B, Manera K, Craig JC, Howell M, Rutherford C, Tong A, Harden P, Hawley C, Holdaas H, Israni A, Jesse M, Kane B, Kanellis J, Kiberd B, Kim J, Larsen C, Leichtman A, Lentine K, Malone A, Mannon R, Oberbauer R, Patzer R, Peipert JD, Phan HA, Poggio E, Reed R, Scandling J, Tang I, Watson C, Contrares D, Contreras P, Cross D, Juodvalkis E, Koide D, Koide J, Kozarewicz A, Kozarewicz L, Kozarewicz R, Koritala A, Lisiecki E, Lipuma C, Lyman M, Mueller R, Mueller G, Noble L, Nolan N, Nolan S, Thomas J, Urbancyzk L, Zerante J, Zerante S; SONG-Tx Life Participation Workshop Investigators ; Health professionals (*includes 2 patients from the SONG-Tx Graft Health Expert Working Group); Patients and family members: Establishing a core outcome measure for life participation: A Standardized Outcomes in Nephrology-Kidney Transplantation Consensus Workshop Report. Transplantation 103: 1199–1205, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.