Abstract
Normal development of germ cells—precursors of egg and sperm cells—is integral for the survival of a species. Prenatal exposure to toxic environmental agents can have profound effects on reproductive health decades after birth,1 yet our understanding of germ cell development has long been hampered by technical challenges.2 In an in vitro study recently published in Environmental Health Perspectives, researchers show for the first time how toxicant exposures at environmentally relevant concentrations can influence the earliest stages of germ cell specification.3 The results add to mounting evidence that the timing of exposure is critical when assessing chemical toxicities.
Multicellular life is made possible by interactions between two cell types: somatic cells that carry out the essential tasks required for life, and germ cells that transfer genetic information to future generations.2 In mammals, germ cell development starts in the epiblast (also known as the primitive ectoderm), a layer of cells that gives rise to the embryo and also contributes to extraembryonic tissues, such as the future amniotic sac. The newly formed primordial germ cells (PGCs) move into extraembryonic tissues, where, guided by cues from neighboring somatic cells, they proliferate and are folded into the embryo.4 Finally, they migrate to their final destination: the developing gonads.5,6
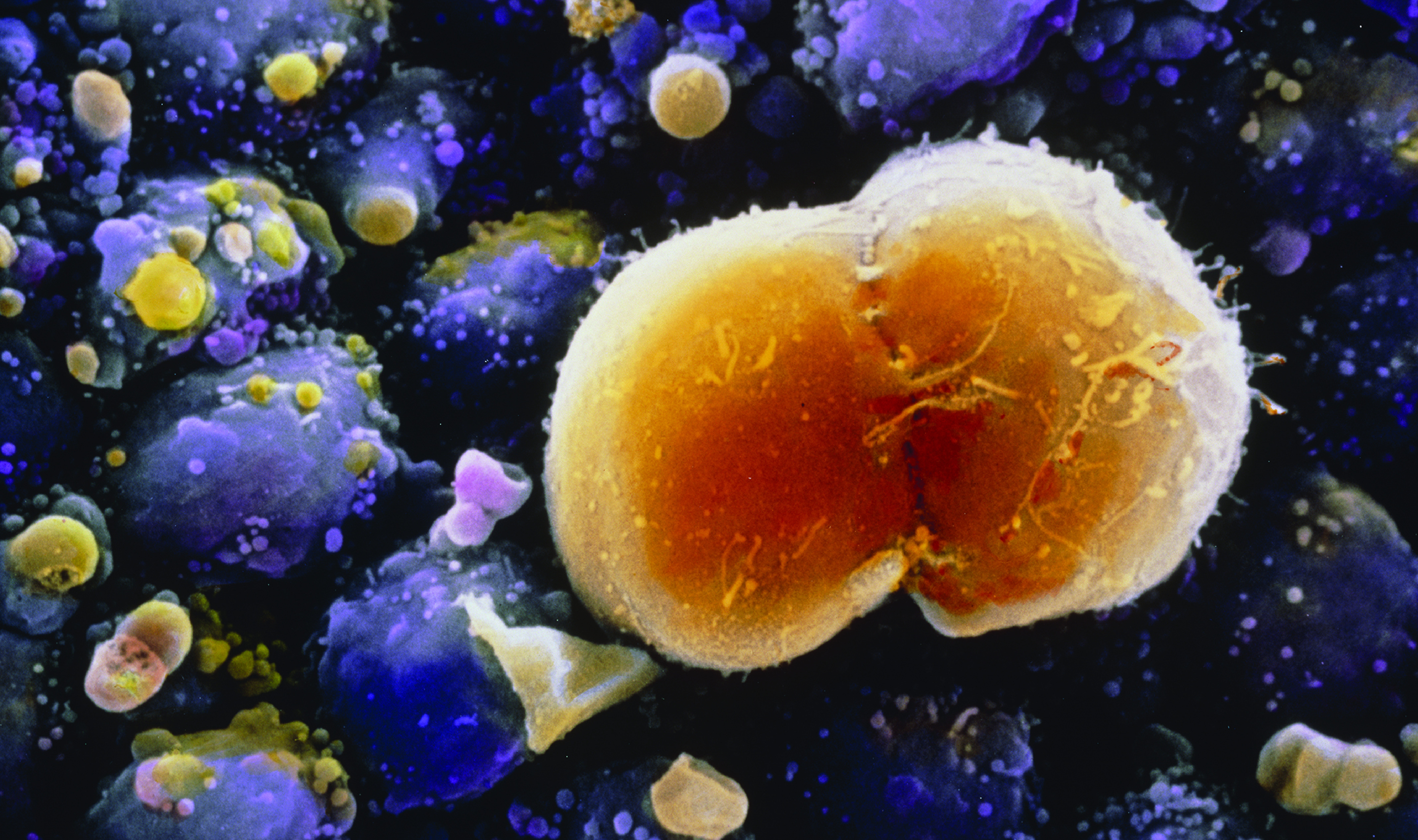
Scanning electron micrograph of a primordial germ cell undergoing division in the ovary of a 7-week-old embryo. Eventually these cells will become oocytes (eggs). Magnification: ×1920 at 6 × 7 cm size. Image: © Professors Pietro M. Motta and Sayoko Makabe/Science Source.
During proliferation and relocation, PGCs undergo a complex reprogramming process known as epigenetic erasure, which involves removal of biochemical modifications to DNA and some of its associated proteins.7,8 Errors that are introduced during this sensitive window can bridge generations and lead to both early-life9,10 and adult-onset outcomes such as infertility,11 cancer,12 and other diseases.
Examining the impact of early environmental exposures on germ cell development has been hampered by its intrinsic complexity: PGCs exist in very limited numbers during embryogenesis and move from one site to another, whereas toxic exposures can span multiple developmental stages from conception through childhood.2 Past studies have therefore been limited to evaluating exposure effects long after PGCs have moved to the developing ovary or testis and differentiated into oocytes or spermatocytes.2 Patricia Hunt, Edward Meyer Distinguished Professor at Washington State University, who was not involved in the new study, notes that the work provides insight into a period of germ cell development that has remained virtually inaccessible until now.
To capture these key developmental events, the investigators used an established mouse embryonic stem cell model of in vitro differentiation into epiblast-like cells (EpiLCs), followed by PGC-like cells.13,14 “We’re standing on the shoulders of [germ cell biology] giants,” says Patrick Allard, senior author of the study. “They have shown that this technology is highly representative of the mammalian in vivo context, so it can now be applied to the question of environmental health.”
The researchers studied the effects of exposure to bisphenol A (BPA), a pervasive environmental contaminant and known endocrine disruptor used primarily in the production of plastics.15 Exposure of EpiLCs to environmentally relevant concentrations of BPA led to greater proliferation, compared with unexposed EpiLCs. However, this effect was also associated with increased DNA damage in BPA-treated cells. Although PGC differentiation was not affected, gene expression analyses revealed BPA-specific alterations to the cells’ gene expression landscape—changes that the authors suggested may affect gamete function later in life. “While one might expect [BPA exposure] to have global effects on the different types of cell lineages, the more subtle perturbations observed are more relevant to the biology of humans or infertility overall,” says Renee Reijo Pera, director of the McLaughlin Research Institute for Biomedical Sciences, who was not involved in the study.
These effects were not observed in a cell line that is genetically male, suggesting that responsiveness to BPA at this stage of germ cell development may be sex specific. “Even though developmental biologists have long thought of PGCs as [an] asexual stage, the differences in gene expression profiles observed highlights the need to pay attention to X-linked gene dosage effects on environmental toxicant sensitivity,” says Steen Ooi, first author of the new paper.
Hunt believes this system can also open the door for more in-depth mechanistic studies. Ooi thinks it would be particularly useful to dissect the epigenetic phenomena underlying how the “memory” of exposure is transmitted to later stages in development. Allard adds that a long-term goal would be to replicate some of these findings in human cells, saying, “We hope to one day develop in vitro systems to recapitulate the entire reproductive cycle in a dish, characterizing every single stage of germ cell development and reproduction.”
Biography
Florencia Pascual, PhD, is a science writer based in Durham, North Carolina.
References
- 1.Hanson M, Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD. 2011. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog Biophys Mol Biol 106(1):272–280, PMID: , 10.1016/j.pbiomolbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira DW, Allard P. 2015. Models of germ cell development and their application for toxicity studies. Environ Mol Mutagen 56(8):637–649, PMID: , 10.1002/em.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ooi SKT, Jiang H, Kang Y, Allard P. 2021. Examining the developmental trajectory of an in vitro model of mouse primordial germ cells following exposure to environmentally relevant bisphenol A levels. Environ Health Perspect 129(9):97013, PMID: , 10.1289/EHP8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesch BJ, Page DC. 2012. Genetics of germ cell development. Nat Rev Genet 13(11):781–794, PMID: , 10.1038/nrg3294. [DOI] [PubMed] [Google Scholar]
- 5.Gardner RL, Rossant J. 1979. Investigation of the fate of 4–5 day post-coitum mouse inner cell mass cells by blastocyst injection. J Embryol Exp Morphol 52:141–152, PMID: , 10.1242/dev.52.1.141. [DOI] [PubMed] [Google Scholar]
- 6.Ginsburg M, Snow MH, McLaren A. 1990. Primordial germ cells in the mouse embryo during gastrulation. Development 110(2):521–528, PMID: , 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- 7.Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, et al. 2007. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development 134(14):2627–2638, PMID: , 10.1242/dev.005611. [DOI] [PubMed] [Google Scholar]
- 8.Guibert S, Forné T, Weber M. 2012. Global profiling of DNA methylation erasure in mouse primordial germ cells. Genome Res 22(4):633–641, PMID: , 10.1101/gr.130997.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassold T, Hunt PA, Sherman S. 1993. Trisomy in humans: incidence, origin and etiology. Curr Opin Genet Dev 3(3):398–403, PMID: , 10.1016/0959-437X(93)90111-2. [DOI] [PubMed] [Google Scholar]
- 10.Hassold T, Hunt P. 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2(4):280–291, PMID: , 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 11.Matzuk MM, Lamb DJ. 2008. The biology of infertility: research advances and clinical challenges. Nat Med 14(11):1197–1213, PMID: , 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oosterhuis JW, Looijenga LHJ. 2019. Human germ cell tumours from a developmental perspective. Nat Rev Cancer 19(9):522–537, PMID: , 10.1038/s41568-019-0178-9. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. 2011. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 146(4):519–532, PMID: , 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Shirane K, Kurimoto K, Yabuta Y, Yamaji M, Satoh J, Ito S, et al. 2016. Global landscape and regulatory principles of DNA methylation reprogramming for germ cell specification by mouse pluripotent stem cells. Dev Cell 39(1):87–103, PMID: , 10.1016/j.devcel.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 15.NIEHS (National institute of Environmental Health Sciences). 2020. Bisphenol A (BPA). https://www.niehs.nih.gov/health/topics/agents/sya-bpa/index.cfm [accessed 10 October 2021].


