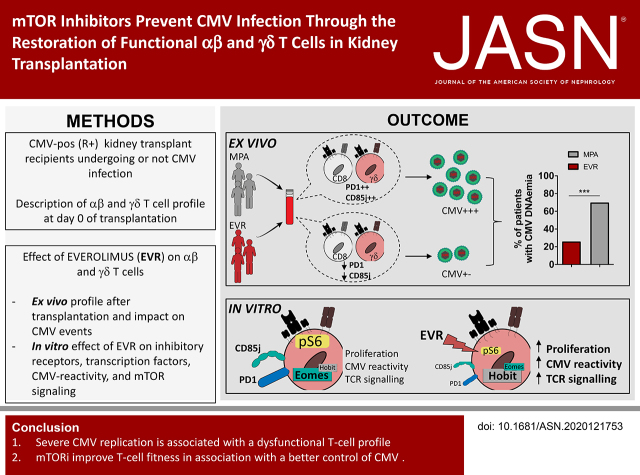
Significance Statement
It has been reported that mTOR inhibitors (mTORis) are associated with a reduction in the incidence of cytomegalovirus (CMV) infection in organ transplant patients who are CMV seropositive (R+), but a mechanistic explanation has been lacking to date. This work showed that a dysfunctional T-cell phenotype (CD85j+ PD-1+) was associated with a higher risk of uncontrolled CMV infection after transplantation in patients who were R+, and that mTORis reduced CMV incidence and severity by reinvigorating αβ and γδ T-cell function. Dysfunctional T-cell phenotype could represent a new biomarker to predict post-transplantation infection in patients who are R+ and to stratify patients who should benefit from treatment with mTORis.
Keywords: CMV, mTOR inhibitors, CMV-specific immunity, kidney transplantation
Visual Abstract
Abstract
Background
The reported association of mTOR-inhibitor (mTORi) treatment with a lower incidence of cytomegalovirus (CMV) infection in kidney transplant recipients (KTR) who are CMV seropositive (R+) remains unexplained.
Methods
The incidence of CMV infection and T-cell profile was compared between KTRs treated with mTORis and mycophenolic acid (MPA), and in vitro mTORi effects on T-cell phenotype and functions were analyzed.
Results
In KTRs who were R+ and treated with MPA, both αβ and γδ T cells displayed a more dysfunctional phenotype (PD-1+, CD85j+) at day 0 of transplantation in the 16 KTRs with severe CMV infection, as compared with the 17 KTRs without or with spontaneously resolving CMV infection. In patients treated with mTORis (n=27), the proportion of PD-1+ and CD85j+ αβ and γδ T cells decreased, when compared with patients treated with MPA (n=44), as did the frequency and severity of CMV infections. mTORi treatment also led to higher proportions of late-differentiated and cytotoxic γδ T cells and IFNγ-producing and cytotoxic αβ T cells. In vitro, mTORis increased proliferation, viability, and CMV-induced IFNγ production of T cells and decreased PD-1 and CD85j expression in T cells, which shifted the T cells to a more efficient EOMESlow Hobithigh profile. In γδ T cells, the mTORi effect was related to increased TCR signaling.
Conclusion
Severe CMV replication is associated with a dysfunctional T-cell profile and mTORis improve T-cell fitness along with better control of CMV. A dysfunctional T-cell phenotype could serve as a new biomarker to predict post-transplantation infection and to stratify patients who should benefit from mTORi treatment.
Clinical Trial registry name and registration number:
Proportion of CMV Seropositive Kidney Transplant Recipients Who Will Develop a CMV Infection When Treated With an Immunosuppressive Regimen Including Everolimus and Reduced Dose of Cyclosporine Versus an Immunosuppressive Regimen With Mycophenolic Acid and Standard Dose of Cyclosporine A (EVERCMV), NCT02328963
Cytomegalovirus (CMV) is, by far, the most common opportunistic infection in recipients of solid allografts and induces direct and indirect morbidity.1 Recipients of solid allografts who are CMV positive (R+) have an intermediate risk of CMV reactivation or superinfection, but represent the vast majority (50%–90%) of transplant recipients around the world.2 A preformed, cell-mediated immunity3,4 contributes to this reduced risk, but sometimes fails to control the virus, leading to clinical infections. A dysfunctional status of CMV-specific T cells could be hypothesized to explain this emergence of CMV disease.
CD8+ αβ T-cell response during CMV infection was described as inflationary, i.e., leading to the lifelong accumulation of highly differentiated/functional T cells5,6 that correlate to a chronic, but well-controlled, low-level viral load.7 These T cells, expressing the transcription factor Hobit, have been distinguished as having “long-lived effector-type” phenotype, and have strong capacities for both IFNγ and granzyme B production and for self-renewal.8–10
Conversely, a dysfunctional effector T-cell profile has been deeply characterized in chronic lymphocytic choriomeningitis virus (LCMV) infection with clone C13 in mice (and also in humans with chronic hepatitis C, hepatitis B, and HIV) in which high virus load persists and is involved in the progressive hyporesponsiveness of antigen-specific effector T cells (for review see Appay et al.5). Dysfunctional state is characterized by an increased expression of inhibitory receptors, such as PD-1, a transcriptional program balance with high level of EOMES/low level of Tbet, and by a decreased ability to produce cytokines and to proliferate.11 Conversely, “Tbethigh/EOMESlow/PD-1low” effector T cells are more functional.11,12
Our hypothesis is that multiple CMV reactivations or higher viral loads could occur in graft recipients that could lead to the emergence of such dysfunctional CMV-specific T cells.9,13–18 Interestingly, among immunosuppressive treatments, mTOR inhibitors (mTORis) have recently been associated with a decreased number of CMV events after solid organ transplantation,19–21 but this association remains unexplained. Direct antiviral action of mTORis was reported, but this has only been observed in vitro and not in all types of CMV-infected cells.22 Alternatively, mTORi effect on CMV events could be due to an effect on CMV-specific immunity. Whereas mTORis have a strong antiproliferative effect on naive cells,23 they also increase the maintenance of CD8 memory T cells after LCMV infection in mice.24 However, data on mTORis effect on human T cells remains elusive, particularly on highly differentiated CMV-specific T cells.
Because mTORis’ positive association with reduced CMV events has been observed in patients who are R+20 but not in patients who are D+R−,25 we hypothesized that mTORis could improve the functioning of preformed, CMV-specific effector T cells.
T-cell response against CMV involves both CD8+ αβ and γδ T cells.26 Only Vδ2neg γδ T cells are involved in the control of CMV, as demonstrated in many different studies (for review, see Couzi et al.27), and their response is very similar to that of CMV-specific CD8+ αβ T cells. These cells share similar kinetics, and acquire the same late effector memory CD45RA+ (TEMRA) phenotype and antiviral functions (cytotoxicity, IFNγ production).28,29 However, Vδ2neg γδ T cells do not recognize viral peptides presented by HLA molecules, but rather they recognize CMV-induced “stress self-antigens” at the surface of infected cells. Very few of these antigens have been described.30
In this study, our first aim was to assess if specific attributes of CMV-specific αβ and γδ T cells in kidney transplant recipients (KTRs) before transplantation could be associated with the risk of developing CMV reactivation after transplantation. We then investigated, in vitro and in vivo, the effect of mTORis on the function and phenotype of CMV-specific effector αβ and γδ T cells to understand why mTORis are associated with a better control of CMV infection in patients.
Methods
Sample and Patients
This ancillary study of a French, multicenter, phase 4 clinical trial comparing the incidence of CMV DNAemia in R+ patients (included at day 0 of transplantation) who received everolimus (EVR) with low doses of ciclosporin or mycophenolic acid (MPA) with standard doses of ciclosporin (see Supplemental Appendix 1 for details) was performed among patients included at Bordeaux University Hospital (83 of 186 patients). Six patients discontinued the study before the first month and were, therefore, excluded (77 remaining participants; for EVR, n=33; for MPA, n=44). Among the patients treated with EVR, six were switched to MPA between month 1 and month 2 and were, therefore, excluded from the study. Consequently, analyses of our ancillary study involved 71 patients: 44 patients treated with MPA and 27 treated with EVR (flow chart in Supplemental Figure 1). Among the 44 patients treated with MPA, deep immunophenotyping of T cells was performed on frozen PBMCs at day 0 of transplantation to compare the basal T-cell profile of patients with “severe CMV” to patients with “well-controlled CMV” in absence of mTORis. Severe CMV infection was defined as CMV DNAemia requiring an antiviral treatment, which included CMV disease and CMV DNAemia for which an antiviral drug was introduced by a physician. Well-controlled CMV was defined either by no post-transplant CMV DNAemia, or by CMV DNAemia with spontaneous resolution without any antiviral treatment. The flow chart of those 44 patients treated with MPA is shown in Supplemental Figure 2. The protocol was approved by an independent ethics committee (CPP number 2013/57). All subjects signed a written informed consent before enrollment. CMV serostatus for each KTR had been determined the day of the graft and was positive for all patients. Immunophenotyping of Vδ2neg γδ T cells was performed at week 1, week 2, and week 24 post-transplantation. PBMCs were isolated and frozen at day 0, month 1, month 3, month 6, and month 12 post-transplantation. The course of CMV infection was pre-emptively monitored by longitudinal whole-blood quantitative nucleic acid testing (QNAT) for CMV viral load every week from day 0 to month 3, and then at months 4, 5, 6, 9, and 12, as previously described.31 Results were given in international units per milliliter, calibrated by the World Health Organization International Standard.32 The lower limit of quantification was 1000 IU/ml and the lower limit of detection was 250 IU/ml; consequently, when the result was “weak positive,” we classified the result as 999 IU/ml. CMV infection was defined as a positive QNAT.33 Antiviral treatment with ganciclovir or valganciclovir was administered at the time of the first positive CMV QNAT result of >2000 IU/ml.
PBMC Cultures for T-Cell Expansions
For γδ T cells, PBMCs were cultured in complete medium (RPMI medium supplemented with 2 mM glutamine and 10% human serum) with 1000 IU/ml recombinant human IL-2 (rIL-2; number 200-02; all from PeproTech) for 28 days with 0, 0.5, or 10 nM of the mTORi EVR (HY-102018; MedChemExpress EU). In some experiments, 10 ng/ml rIL-15 (number 200-15) was added.
For CMV-specific αβ T-cell cultures, PBMCs were cultured in complete medium with 10 IU/ml rIL-2 for 16 days with 0 or 0.5 nM of EVR. PepTivator CMV pp65 (0.6 nmol/ml; 130-093-438; Miltenyi) was added at day 0 and day 9.
Additional experiments were performed, as previously described, for γδ T cells and CMV- specific αβ T-cell cultures with the addition of 0, 25, 75, or 200 ng/ml ciclosporin (Novartis Pharma, Basel, Switzerland) with or without either 0.5 nM EVR or 1000 ng/ml mycophenolate sodium (CellCept, batch B8003; Roche).
Proliferation and Viability Assay for γδ and CMV-Specific αβ T Cells
Proliferation within TEMRA cells was first assessed by 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) assay. PBMCs were labeled with CFSE (number V12883; eBioscience) and cultured with or without 1000 U/ml rIL-2 and 10 ng/ml rIL-15. On day 7 of culture, 2 × 105 cells were incubated with mAbs anti-pan TCRδ (PE), TCRVδ2-PC-7, anti-CD3 (BV510), anti-CD45RA (BV786), and CD27 (BV650); washed; and processed on the BD LSRFortessa. Proliferation was also assessed by counting on a Neubauer cell counting chamber; phenotyping with mAbs anti-CD3 (V450), CD27 (APC), CD45RA (FITC), panδ (PE), and Vδ2 (PC7); and processing using a BD Canto II (BD Biosciences).
For CMV-specific αβ T cells, PBMCs were incubated in 96-well plates with 150 µL of the previously described media. At day 9, 0.6 nmol/ml PepTivator CMV pp65 was added with a protein transport inhibitor (number 554724; BD biosciences) overnight at 37°C, and cells were then stained with anti-CD3 (V450), CD27 (BV650), CD45RA (FITC), pan-αβ (APC), and CD69-PE mAbs. Fixation permeabilization was performed for staining with the anti-IFNγ (BV786) antibody. The assessment of CMV-specific CD8+ T cells was performed using flow cytometry with a gating of double positive CD69+IFNγ+ αβ CD8+ cells, as previously described.34
Absolute numbers are the percentages of CMV-specific αβ and Vδ2neg T cells among PBMCs multiplied by the total PBMC count.
Cell viability was measured by incubating 2 × 105 cultured PBMCs with the viability marker FVS575.
Expression of Coreceptors and Transcription Factors for γδ and CMV-Specific αβ T Cells
For Vδ2neg γδ T cells, 5 × 105 PBMCs at day 21 of culture were stained with either the viability marker FVS575, and then with antibodies anti-CD3 (BV510), PD-1 (BV650), TIM3 (BV711), DNAM-1 (BV786), panδ (PE), Vδ2 (PC7), and KLRG1 (FITC); or the viability marker FVS780, and then with antibodies anti-CD3 (BV510), Vδ2 (Pacific Blue), and either panδ (APC) with Blimp1 (PE), Tbet (PC5.5), or EOMES (Pe-Cy7) alone, or panδ (PE) with Hobit (Alex Fluor 647) (BD Biosciences). PBMCs were permeabilized with the FOXP3 transcription factor staining buffer (Fisher Scientific). For CMV-specific αβ T cells, 5 × 105 PBMCs were stained, as described above, in 96-wells plates were stained with FVS575, and then either with antibodies anti–PD-1 (BV650), KLRG1 (FITC), pan-αβ (APC), CD3 (V450), CD69 (PE), and intracellular staining with anti-IFNγ (BV786), or anti–pan-αβ (PE), CD3 (V450), CD69 (Alexa Fluor 700) antibodies, and then anti-EOMES (PC7), Hobit (Alexa Fluor 647), and IFNγ (BV786) antibodies (processed on the BD LSRFortessa).
S6 and Akt Phosphorylation
On day 21 of culture, 2 × 105 cells from the Vδ2neg γδ cultures were washed and incubated in 400 µl of RPMI (2 mM glutamine, 8% FCS alone or with 0.5 nM EVR) and activated with an anti-Vδ1 mAb (10 µg/ml) for the indicated period of time. As previously described for staining of phosphorylated proteins,35 cells were stained in the phosphoflow buffer with mAbs anti-CD3 (V450), Vδ2 (FITC), and panδ (APC), and with either anti-pS6 (PeCy7), S6 (PE), pAkt T308 (PE), pAkt S473 (FITC), or Akt (PE) (processed on the Canto II).
pSLP-76, p-ERK, and p38 Expression T cells
On day 21 of culture with rIL-2, Vδ2neg γδ T cells were purified with magnetic negative sorting using the pan T-cell isolation kit; αβ−biotin and anti-biotin (Miltenyi Biotec), Vδ2 FITC (Beckman), and anti-FITC (Miltenyi Biotec). Purity was controlled by staining sorted cells with anti-CD3 (V450), panδ (PE), and Vδ2 (PC7), resulting in a 85%–96% purity. Cells were activated with 10 µg/ml of purified UCHT1 (Beckman). Intracellular staining was performed as previously described (processed on Canto II).35
Ex Vivo Phenotyping of γδ T Cells and CMV-Specific CD8+ T Cells
For the complete details regarding antibodies, see Supplemental Table 1.
Flow cytometry phenotyping of patients’ Vδ2neg γδ T cells was performed on whole blood, as previously described,36 using the mAbs anti–CD45-APC, anti–panδ-PE, anti–Vδ2-FITC, anti-CD27 Pe-Cy7, and anti–CD45RA-ECD, and using the Lysing Solution IOTest 3 10× Concentrate (Beckman). The samples were processed on a NAVIOS flow cytometer (Beckman Coulter).
One million frozen PBMCs were used for each multicolor flow cytometry analysis, with the same viability marker FVS575; the same mAbs against CD3 (PerCP), Vδ2 (PC-7), panδ (PE or APC), and CD8 (either BV510 or PE–Texas Red); and IFNγ (BV786) and CD69 (AF-700) for the staining of CMV-specific CD8+ T cells after overnight pp65 stimulation with a protein transport inhibitor. Staining then included either CD45RA (FITC) and CD27 (BV786); CD85j (FITC), CD161 (BV650), CD16 (BV786), and KLRG1 (PE-Vio 615); granulysin (FITC), perforin (APC), and granzyme (BV421); or PD-1 (BV650), TIM-3 (BV711), LAG3 (BV421), and DNAM-1 (BV786). CMV-specific CD8+ T cells were gated as double positive cells for IFNγ and CD69, as previously described.34
PBMCs stained for intracellular markers were permeabilized, fixed using Fixation/Permeabilization Solution (BD Biosciences), and processed on the BD LSRFortessa cytometer (BD Biosciences).
IFNγ Production in Cocultures with CMV-Infected Cells
After 21 days of culture with rIL-2 and rIL-15, Vδ2neg γδ T cells were negatively purified as described above. A total of 50 × 104 cells were incubated per 96-well plate with RPMI, 8% FCS, 2 mM glutamine, and 50 ng/ml rIL-18 (B003-5; MBL International, Woburn MA; see Guerville et al.37) with or without EVR alone, or with either noninfected (NI) or CMV-infected fibroblasts, for 24 hours at 37°C. For CMV-specific αβ T cells, PBMCs were cultured as described above but without pp65 stimulation and, after 7 days, cells were incubated alone or with 0.6 nmol/ml PepTivator CMV pp65 or 25 ng/ml PMA with 1 µg/ml ionomycine at 37°C for 24 hours. An extra well was added to perform an IFNγ and CD69 staining after overnight pp65 stimulation with a protein transport inhibitor.
IFNγ was then measured in supernatants using the Human IFNγ ELISA development kit (number 3420-1H-6; Mabtech).
Preparation of CMV-Infected Fibroblasts
Human foreskin fibroblasts (kindly provided by Dr H. Rezvani, Institut National de la Santé et de la Recherche Médicale, U1035, Bordeaux, France), grown in DMEM containing 8% FCS and 2 mM glutamine, were infected with the TB42/E strain of human CMV at an MOI of 0.1. After virus adsorption overnight at 37°C, cells were washed and covered with fresh growth medium. Cocultures were performed when cytopathic effects were ≥90%, 4 days after infection. NI cells grown in parallel were mock infected using medium alone.
Preparation of Free CMV
To produce free CMV (TB42/E strain), human foreskin fibroblasts were infected at an MOI of 0.1 and incubated at 37°C in culture medium DMEM, 8% bovine serum, and glutamine for 10 days or until cytopathic effects were ≥90%. The supernatant was stored at −80°C. The preparation had a titer of 2.5 × 106 PFU/ml, and the titration was performed as previously described.1 All virus stocks and cells tested negative for the presence of Mycoplasma.
Viability of Vδ2neg γδ T Cells during Coculture with and without Blocking Anti-CD3 Antibody Analyzed with 4′,6-Diamidino-2-Phenylindole
Vδ2neg γδ T cells, after 21 days of culture with 0 and 0.5 nM EVR, were negatively sorted with magnetic beads and cultured in medium alone (either with 0 or 0.5 nM EVR), NI fibroblasts, or CMV-infected fibroblasts with or without blocking anti-CD3 mAb (10 µg/ml) for 24 hours, and cells were stained with 1 µM 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), and, after 15 minutes of incubation at room temperature, cells were analyzed by flow cytometry using the Canto II.
Ex Vivo QuantiFERON-CMV
QuantiFERON-CMV V (number 0350-0201; Qiagen) was performed, as previously described,38 from frozen plasma and read using the QUANTA-Lyser 2 Inova Diagnostics at day 14 (32 patients treated with mTORi and 43 treated with MPA) and month 6 post-transplantation (24 patients treated with mTORi and 39 treated with MPA).Results were analyzed using the CMV version 3.03 software.
Statistical Analyses
The Mann–Whitney U, chi-squared, Fisher, or unpaired t tests were used, as appropriate. Alternatively, the paired t test was used for paired data. Paired tests were used when two sets of data from the same patients were compared, whereas unpaired tests were used to compare data from two different group of patients. P<0.05 was considered statistically significant. Analyses were performed using conventional statistical methods with GraphPad Prism. Figures were obtained with FlowJo software (V.10) and GraphPad Prism.
Results
Increased Percentages of T Cells Expressing Inhibitory Receptors at Baseline in MPA-Treated Patients with Severe CMV Infections
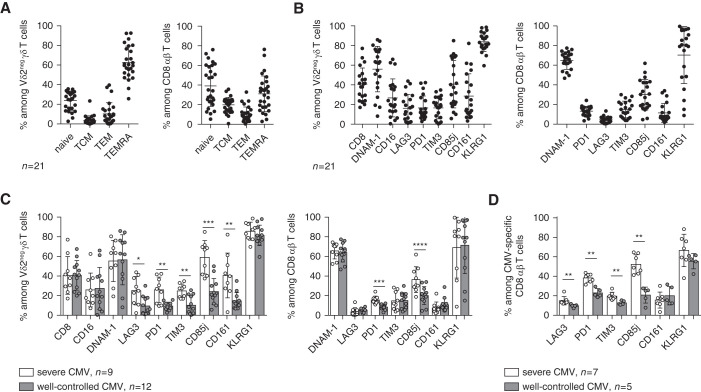
Of the 44 patients treated with MPA, 32 experienced CMV infection: 14 patients with a peak viral load <999 IU/ml which spontaneously cleared and, thus, were classified into the “well-controlled CMV” group, and 18 patients who experienced CMV DNAemia and required treatment were classified into the “CMV-severe” group, six of these patients had CMV disease. Among the 44 patients from the ancillary study who were treated with MPA, 33 had available frozen PBMCs at day 0 of transplantation, and we consequently characterized T-cell phenotypes at day 0 of transplantation (baseline phenotype) in a first subgroup of 21 patients treated with MPA. Nine had “severe CMV,” either CMV disease (n=3) or CMV DNAemia for which an antiviral drug was administered by a physician (n=6; mean [SD] peak viral load of 6916 [3762] IU/ml); and 12 had well-controlled CMV, either no CMV DNAemia (n=6) or CMV DNAemia which spontaneously cleared without any antiviral treatment (n=6, all with a peak viral load <999 IU/ml). TEMRA cells were highly represented in γδ T cells and CD8+ T cells (Figure 1A), as previously observed.29 Both T-cell compartments presented high percentages of cells expressing activation receptors, such as DNAM-1 (CD226; also CD8αα and CD16 for γδ T cells), and KLRG1, a receptor expressed on highly differentiated CD8+ T cells with high cytotoxic but low proliferative capacities in CMV infection.39 A significant proportion of T cells also expressed inhibitory receptors, such as CD85j, PD-1, and TIM3 (Figure 1B). These phenotypes were then compared between patients with post-transplant severe CMV infections (n=9) and patients with well-controlled CMV (n=12). No difference in group differentiation status (Supplemental Figure 3A) or KLRG1, DNAM-1, CD8αα, and CD16 expression (Figure 1C) were observed. However, patients who presented with severe CMV infection had a higher percentage of T cells expressing inhibitory receptors, such as PD-1 and CD85j, when compared with patients with well-controlled CMV (Figure 1C). We validated and extended these results by concomitantly analyzing γδ T cells, total CD8+ T cells (Supplemental Figure 3B), and CMV-specific CD8+ T cells in an internal validation subgroup of 12 additional patients (from the group of 44 patients treated with MPA from the ancillary study): seven patients had “severe CMV infection,” of which three patients had CMV disease and four had CMV DNAemia for which an antiviral drug has been introduced by a physician (mean [SD] peak viral load of 7930 [3976] IU/ml); and five had well-controlled CMV (one patient without CMV DNAemia, four with CMV DNAemia which spontaneously cleared, peak viral load <999 IU/ml). We confirmed that patients with severe CMV infection also displayed significantly higher percentages of PD-1+ and CD85j+ cells in CMV-specific CD8+ T cells (Figure 1D), and we validated those markers in total CD8+ and γδ T cells (Supplemental Figure 3B). At baseline, T cells from patients who were R+ could thus display a pre-existing dysfunctional profile, characterized by expression of inhibitory receptors, correlating to severe CMV infection after transplantation. Finally, PD-1 and CD85j appeared to be the more convincing markers differentially expressed by both γδ T cells and CMV-specific CD8+ T cells. From a clinical perspective, using only two markers would be easier to implement as a routine assay than a multicolor staining.
Figure 1.
Vδ2neg γδ T cells and CD8+ T cells express inhibitory receptors at baseline in patients with severe CMV infections. Vδ2neg γδ T cells and total CD8+ T cells were analyzed for (A) their expression of CD27 and CD45RA and (B) their costimulatory and coinhibitory receptors, in (A and B) all patients who were R+ and treated with MPA (n=21) and (C) by separating patients with well-controlled CMV (n=12) versus patients severe CMV (n=9). (D) Finally, phenotypes were extended to CMV-specific CD8+ T cells in an internal validation cohort of patients with well-controlled CMV (n=5) versus patients with severe CMV (n=7). Each symbol represents an individual donor; large horizontal lines indicate the mean and small horizontal lines indicate the SD. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as determined by the Mann–Whitney U test.
The Proportion of Functional T Cells Is Enhanced by mTORi Treatment and Correlates to a Subsequent Lower Incidence of CMV Infection
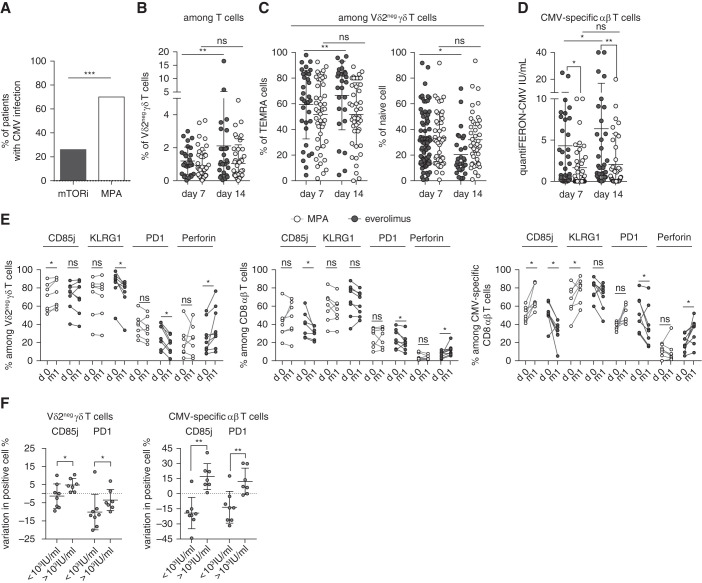
To address the issue of the effect of mTORis on dysfunctional T cells, 44 KTRs treated with MPA were compared with 27 KTRs treated with an mTORi (EVR). As shown in Table 1, no major differences were observed regarding age, sex, CMV serostatus of donor, rank of transplantation, living donor status, expanded criteria donor, and acute rejection. We confirmed that mTORi treatment protects from CMV infection because 26% (seven of 27) of patients treated with mTORis displayed CMV infection, in contrast with 70% (32 of 44) of patients treated with MPA (P<0.001; Figure 2A).
Table 1.
Clinical parameters of patients
| Characteristics | EVR (n=27) | MPA (n=44) |
|---|---|---|
| Recipients | ||
| Age, yr mean (SD) | 64.2 (15.5) | 62.6 (13.5) |
| Male, n (%) | 15 (64) | 32 (73) |
| Donors, n (%) | ||
| CMV-seropositive donors | 14 (51.8) | 23 (52.6) |
| Living donor | 5 (14.8) | 3 (6.8) |
| Rank of transplantation, first | 1 (3.7) | 2 (4.5) |
| Expanded criteria deceased donor | 16 (59.2) | 23 (52.2) |
| Ciclosporin whole blood trough concentrations, day 7, mean (SD) | 172.1 (71.4) | 163.3 (51.2) |
| Ciclosporin whole blood trough concentrations, day 14, mean (SD) | 210.7 (65.2) | 223.8 (55.6) |
| Ciclosporin whole blood trough concentrations, month 6, mean (SD) | 88.7 (34.3) | 117.2 (30.9) |
| EVR whole blood trough concentration, day 7, mean (SD) | 4.2 (1.8) | |
| EVR whole blood trough concentration, day 14, mean (SD) | 6.3 (2.3) | |
| EVR whole blood trough concentration, month 6, mean (SD) | 4.9 (1.3) | |
| Biopsy proven acute rejection (12 mo of follow-up), n (%) | 8 (29.6) | 11 (25) |
Figure 2.
High percentage of functional T cells in patients treated with mTORis correlated with a lower incidence of CMV infection. (A) Incidence of CMV DNAemia in 27 patients treated with mTORis and in 44 patients treated with MPA at month 12 post-transplantation. (B and C) Whole blood staining of Vδ2neg γδ T cells and their expression of CD27 and CD45RA. Frequencies of Vδ2neg γδ T cells among (B) T cells, and (C) of TEMRA (CD27neg CD45RA+) and naive (CD27hi CD45RA+) cells among Vδ2neg γδ T cells at day 7 and 14 post-transplantation (n=27 EVR, n=44 MPA). Each symbol represents an individual donor; large horizontal lines indicate the mean and small horizontal lines indicate the SD. (D) CMV-specific αβ T cells were analyzed with QuantiFERON-CMV (IU/mL) at day 7 (n=27 EVR, n=41 MPA) and 14 post-transplantation (n=27 EVR, n=43 MPA). Each symbol represents an individual donor; large horizontal lines indicate the mean and small horizontal lines indicate the SD. (E) Proportions among Vδ2neg γδ T cells (left); among total CD8+ T cells (middle); and CMV-specific CD8+ T cells (right) of CD85j+, perforin+, KLRG1+, and PD-1+ cells, compared between day 0 (d0) and month 1 (m1) post-transplantation in patients treated with mTORis (n=8) and MPA (n=7). Each symbol represents an individual donor. (F) Difference of CD85j and PD-1+ cell percentages between month 1 and day 0 (value m1 minus value day 0) was calculated and compared in patient groups with a post-transplantation CMV viral load >1000 or <1000 IU/ml (including 0 IU/ml). *P<0.05, **P<0.01, ***P<0.001, as determined for all unpaired data by the Mann–Whitney U test and for paired data by Wilcoxon test.
Between day 7 and day 14, the total and TEMRA γδ T-cell percentages increased significantly in patients treated with mTORis, whereas this change was not observed in patients treated with MPA (before any CMV replication; Figure 2, B and C). For CMV-specific αβ T-cell immunity assessed by QuantiFERON-CMV (Figure 2D), a significant difference between patients treated with mTORis versus MPA already exists at day 7, but QuantiFERON-CMV significantly increased between day 7 and day 14 in patients treated with mTORis, but not in those treated with MPA.
We then compared the effect of MPA (n=7 patients) and mTORis (n=8 patients) on the evolution of the T-cell phenotype before any CMV replication. mTORi treatment was associated with a decreased percentage of PD-1+ and CD85j+ T cells (in γδ T-cells, CD85j was unchanged), together with an increase of perforin+ T cells, whereas MPA was associated with increased or stable PD-1+ and CD85j+ cell percentages (Figure 2E). Importantly, there was a direct association between the decrease in the percentage of CD85j- or PD-1–expressing T cells during the first month of transplantation and an absence of or a low level (<1000 IU/ml) of CMV DNAemia (Figure 2F).
mTORi treatment increased the percentage of T cells expressing a functional profile, which is associated with a low incidence of CMV infection post-transplantation.
mTORi Treatment Is Associated with Spontaneously Cleared CMV Replication that Correlates to an Enhanced Specific T-Cell Response
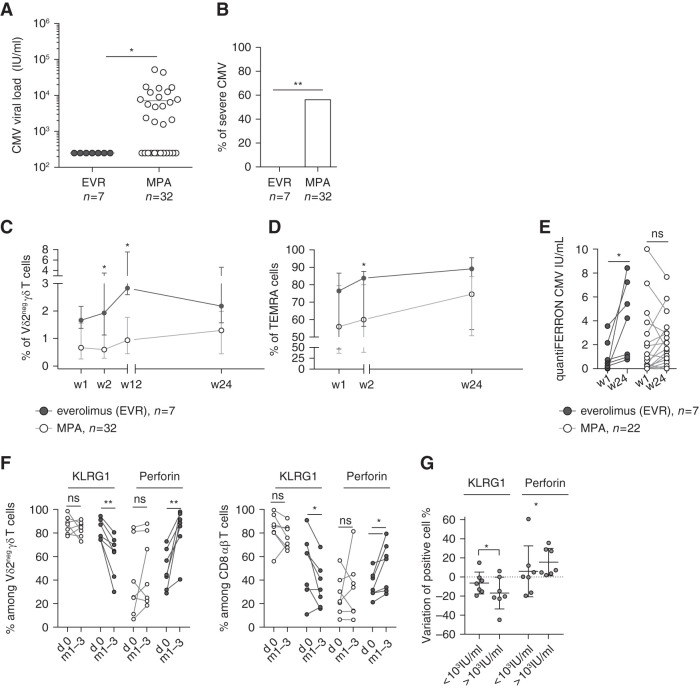
We then focused on patients who developed post-transplantation CMV infection. The mean (SD) of CMV infection occurrence was 51 (37) days post-transplantation.
Viral load was much lower (never exceeding 999 IU/ml) in patients treated with mTORis (n=7) than those treated with MPA (n=32; mean 7482 IU/ml) (Figure 3A). Moreover, patients treated with mTORis did not require any antiviral treatment because all of the patients spontaneously cleared the virus. In contrast, 56.25% (18 of 32) of patients treated with MPA required antiviral treatment (P=0.009; Figure 3B). Six patients treated with MPA experienced CMV disease, whereas none of the patients treated with EVR did (data not shown).
Figure 3.
Better response of T cells in patients treated with mTORis than in those treated with MPA correlated with a lower severity of CMV infection. (A) Maximal CMV viral load (IU/ml) in patients treatd with mTORis (n=7) and MPA (n=32). Each symbol represents the CMV viral load value for an individual patient; large horizontal lines indicate the mean. (B) Proportion of patients treated with MPA (n=38) and mTORis (n=7) who experienced CMV DNAemia. *P<0.05, as determined by the Fisher exact test. (C and D) Whole blood staining of Vδ2neg γδ T cells and their expression of CD27 and CD45RA. Proportion of (C) Vδ2neg γδ T cells among T cells and of (D) TEMRA (CD27neg CD45RA+) among Vδ2neg γδ T cells at week 1, 2, 12, and 24 post-transplantation in patients treated with mTORis (n=7) and MPA (n=32). Each symbol represents the median (interquartile range) value for each group of patients. (E) QuantiFERON-CMV (IU/ml) at week 1 and week 24 in patients treated with mTORis (n=7) and MPA (n=22) who experienced CMV DNAemia post-transplantation. Each symbol represents an individual donor. (F) Proportions among Vδ2neg γδ T cells (left) and among total CD8+ T cells (right) of perforin+ and KLRG1+ cells, compared between day 0 and during CMV DNAemia (either month 1 or month 3) in patients treated with mTORis (n=7) and MPA (n=7). Each symbol represents an individual donor. (G) Difference of KLRG1 and perforin+ Vδ2neg γδ T-cell percentages between month 1 and day 0 were calculated and compared in patient groups with a post-transplantation CMV viral load ≥1000 IU/ml. *P<0.05, **P<0.01, as determined for paired data by the Wilcoxon test and for unpaired data by Mann–Whitney U test.
During the course of CMV infection (all occurring between month 1 and month 3 post- transplantation), γδ T-cell expansion (Figure 3C) and the increase in the proportion of TEMRA cells (Figure 3D) were higher in patients treated with mTORis than in those treated with MPA. Moreover, the CMV-specific αβ T-cell response was also improved because IFNγ, measured by QuantiFERON-CMV, increased significantly during the course of CMV infection only in patients treated with mTORis (P=0.02; Figure 3E).
Immunophenotyping was performed on day 0 and on the closest time point of CMV replication (month 1 or month 3, depending on when CMV replication occurred for each patient) to analyze the effect of both the immunosuppressive drug and CMV replication.
The proportion of γδ T cells and total CD8+ T cells expressing perforin increased more significantly in patients treated with mTORis, as compared with those treated with MPA (Figure 3F). KLRG1 was expressed on significantly less T cells in patients treated with mTORis (Figure 3F). Finally, the decrease of KLRG1+ cells and the increase of perforin+ cells among γδ T cells was directly associated with low viral loads (<999 IU/ml), independently of the immunosuppressive treatment (Figure 3G).
Thus, the better control of CMV infection in patients treated with mTORis, as compared with those treated with MPA, was associated with better expansion and functional reinforcement of T cells.
Long-Term In Vitro Treatment of T Cells with Therapeutic Doses of mTORis Increase Their Proliferation and Viability
Proliferation and viability were assessed in vitro in R+ KTR PBMCs comprising predominantly TEMRA cells among both γδ T cells (>95%) and CMV-specific αβ T cells (up to 85%) (Supplemental Figure 4) at day 0.
TEMRA γδ T cells can proliferate despite their late-differentiated phenotype, as shown through CFSE experiments at day 7 of culture (Supplemental Figure 4B), and through the strong increase in cell percentages (Supplemental Figure 4C) and numbers (Supplemental Figure 4D).
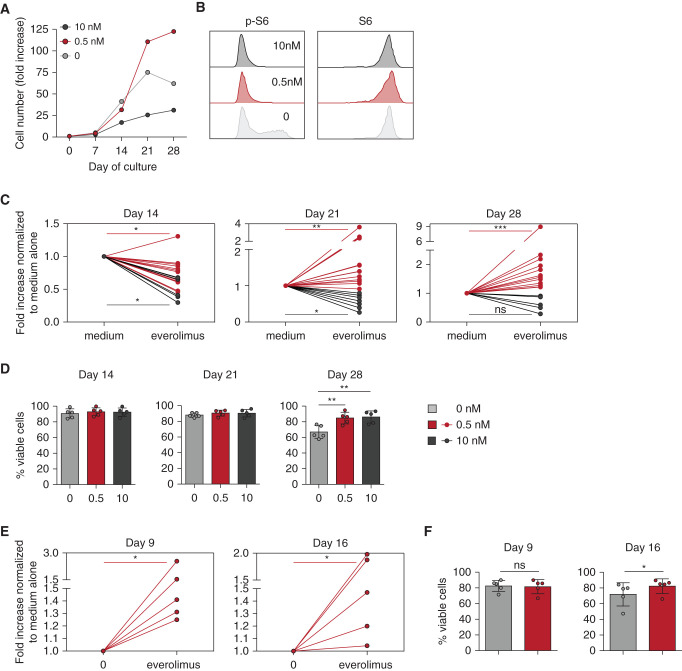
mTORi was then added either at a dose mimicking an antiproliferative effect (10 nM), or at the dose used in immunosuppressive treatment of solid-organ transplantation (0.5 nM) for γδ T cells and only at the lowest dose for αβ T cells.
As expected and shown in Figure 4A, the high dose of mTORi inhibited γδ T-cell proliferation. In contrast, culture performed with low-dose mTORi (0.5 nM) resulted in better proliferation than in the absence of the mTORi (Figure 4, A and C). Yet, the dose of 0.5 nM was as potent as that of 10 nM to inhibit S6 phosphorylation in TEMRA γδ T cells, while not affecting the expression of the total S6 protein level (Figure 4B). Interestingly, low and high doses of mTORis were associated with an increased viability of cells during the late period of culture (day 28; Figure 4D). As with γδ T cells, low-dose, mTORi-treated, CMV-specific αβ T cells showed better proliferation (Figure 4E) and viability (Figure 4F). Hence, a low-dose mTORi improves the proliferation and viability of TEMRA T cells, consistent with our previous observations in patients treated with mTORis (Figure 2).
Figure 4.
Long-term in vitro mTORi treatment improves proliferation and viability of Vδ2neg γδ T cells and CMV-specific αβ T cells. PBMCs from KTRs who were R+ were incubated with IL-2, with or without IL-15, for Vδ2neg γδ T cells, and IL-2 alone for CMV-specific αβ T cells, and with the indicated doses of EVR. Proliferation and viability of Vδ2neg γδ T cells at day 14, 21, and 28 of culture and of CMV-specific αβ T cells at day 9 and 16 of culture were performed. (A) Representative donor for proliferation of Vδ2neg γδ T cells. (B) Representative flow cytometry staining of S6 and phospho-S6 (p-S6) among Vδ2neg γδ T cells at day 14 of culture. (C) Proliferation of Vδ2neg γδ T cells, represented as fold increases normalized to culture with medium alone, at day 14 (0.5 nM EVR, n=13; 10 nM EVR, n=6), 21 (0.5 nM EVR, n=15; 10 nM EVR, n=8), and 28 (0.5 nM EVR, n=12; 10 nM EVR, n=6). (D) Vδ2neg γδ T-cell viability tested by flow cytometry live-dead staining (n=5). (E) Proliferation of CMV-specific αβ T cells, represented as fold increases normalized to culture with medium alone (0.5 nM EVR, n=5). (F) Viability of CMV-specific αβ T cells tested by flow cytometry live-dead staining (n=5). For (C), (D), (E), and (F), each symbol represents an individual donor. *P<0.05, **P<0.01, ***P<0.001, as determined by Wilcoxon test.
Low Dose of mTORi Promotes the Functional Profile of T Cells
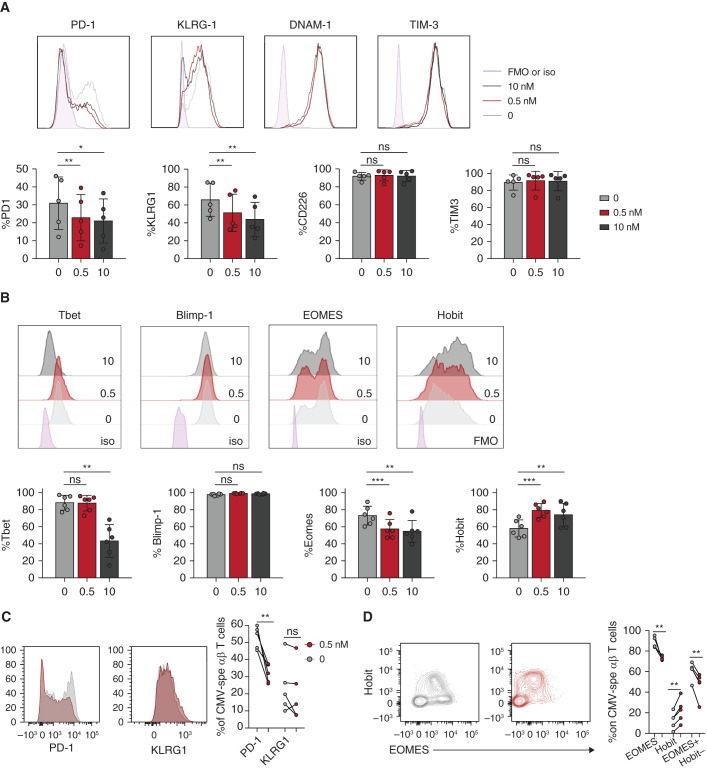
When cultured in the presence of a low or high dose of mTORis, a decrease in the proportion of PD-1+ and KLRG1+ γδ T cells was observed, whereas DNAM-1 and TIM-3 receptors were expressed at the same levels (Figure 5A). These results suggested that mTORis were able to modify in vitro the dysfunctional profile of γδ T cells observed earlier in vivo at baseline (Figure 1), recapitulating the effect of mTORis observed in patients after transplantation.
Figure 5.
Low-dose mTORi improves the functional profile of Vδ2neg γδ T cells and CMV-specific αβ T cells. Vδ2neg γδ T cells (after 21 days) and CMV-specific αβ T cells (after 16 days) were analyzed after in vitro culture, with or without EVR, of PBMCs from KTRs who were R+. Frequencies of (A) PD-1, KLRG1, DNAM-1, and TIM-3; and (B) Tbet, Blimp-1, EOMES, and Hobit among Vδ2neg γδ T cells for one representative donor (top) and for five donors (bottom). Frequencies of (C) PD-1 and KLRG1 and of (D) EOMES and Hobit among CMV-specific αβ T cells for one representative donor (left) and five donors (right). Each symbol represents an individual donor. *P<0.05, **P<0.01, as determined by the Wilcoxon test. FMO, fluorescence minus one; iso, control isotype.
We then tested whether mTORis were acting on transcription factors regulating IFNγ, i.e., Tbet, EOMES, Hobit, and Blimp-1. Low and high mTORi doses resulted in an increase of Hobit and a decrease of EOMES, which may favor high IFNγ production.40 Low-dose mTORis maintained, whereas a high dose decreased, Tbet expression (Figure 5B). As with γδ T cells, CMV-specific αβ T cells treated with a low mTORi dose showed a lower expression of EOMES along with an increased expression of Hobit (Figure 5D), whereas PD-1+ cells showed reduced expression (Figure 5C). The low mTORi dose also reduced the percentage of CD85j+ cells in both T cell subsets (Supplemental Figure 5).
Finally, we wondered if this new phenotype induced by mTORi correlated with a better antiviral function. γδ T cells treated with a low-dose mTORi produced increased amounts of IFNγ against CMV-infected cells, which was remarkably not the case when a high mTORi dose was used (Figure 6A), in accordance with mTORi inhibitory action on Tbet expression at high dose (Figure 5B). Regarding CMV-specific αβ T cells, PBMCs activated with CMV peptides after 7 days of mTORi culture consisted of a similar quantity of CMV-specific αβ T cells (IFNγ+CD69+ αβ T cells; Supplemental Figure 6), but showed an increased production of IFNγ (Figure 6B), as compared with PBMCs cultured without mTORis.
Figure 6.
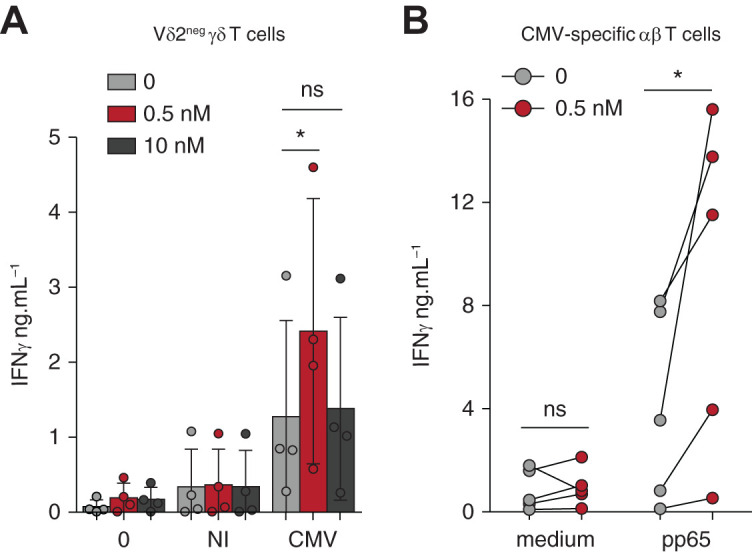
Low-dose mTORi improves the response of Vδ2neg γδ T cells and CMV-specific αβ T cells to CMV. (A) Vδ2neg γδ T cells were purified and cultured in medium alone or with NI or CMV-infected fibroblasts for 24 hours, and ELISA for IFNγ was performed (n=4 donors). (B) PBMCs, from donors who were R+, were cultured for 7 days and then maintained in medium alone or stimulated with 0.6 nmol/L of pp65 PepTivator for 24 hours, and ELISA for IFNγ was performed (n=5). Each symbol represents an individual donor and, in (A), large horizontal lines indicate the mean and small horizontal lines indicate the SD. *P<0.05, **P<0.01, as determined by the Wilcoxon test.
In summary, a low mTORi dose improves functional fitness of T cells with the reinforcement of CMV-induced IFNγ production. mTORis drive an increased percentage of “Hobithigh/EOMESlow/PD-1low” cells, which was previously associated with an efficient antiviral potential.10,12
mTORi Improvement of the T-Cell Dysfunctional Profile Is Maintained when Combined with Ciclosporin
To mimic the patients’ immunosuppressive regimen, we also performed γδ and CMV-specific αβ T cell culture combining either 0, 25, 75, or 200 ng/ml ciclosporin alone or in combination with 0.5 nM EVR or with 1000 ng/ml mycophenolate sodium. Mycophenolate sodium strongly inhibited the proliferation of both γδ and CMV-specific αβ T cells. Ciclosporin inhibited the proliferation of γδ and CMV-specific αβ T cells in a dose-dependent manner (Supplemental Figure 7A). However, for the same concentration of ciclosporin, EVR had a significant effect on increasing proliferation (Supplemental Figure 7A) and on decreasing PD-1 and CD85j expression (Supplemental Figure 7B). Consequently, our in vitro observations about the effects of EVR and ciclosporin were confirmed and led us to extrapolate what we observed ex vivo in patients.
Effect of mTORis on TCR Engagement and Signaling of γδ T Cells
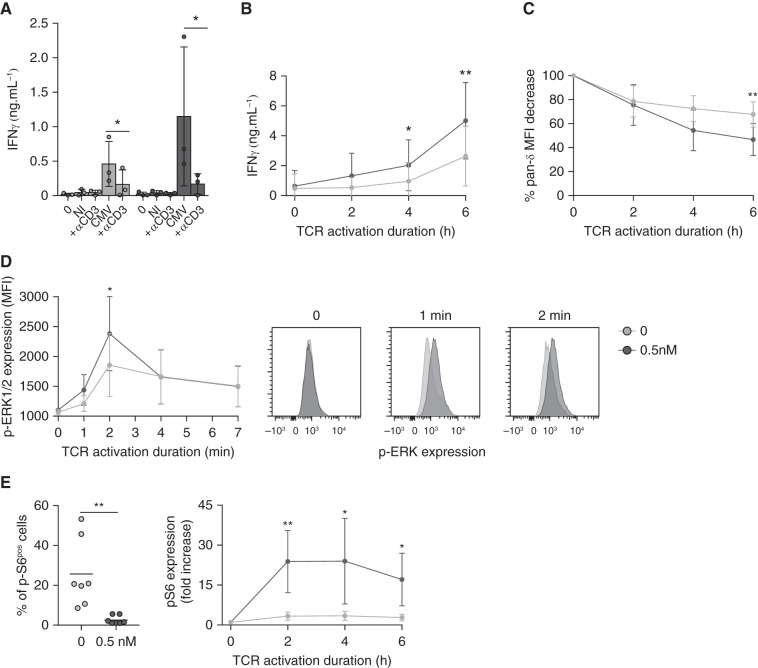
Because it was previously demonstrated that dysfunctional T cells are hyporesponsive to TCR signaling,41,42 and because we could obtain large numbers of γδ T cells (compared with CMV-specific αβ T cells), we investigated the TCR signaling efficiency of γδ T cells with or without mTORi. TCR neutralization significantly inhibited the production of IFNγ by γδ T cells against CMV-infected cells (Figure 7A), and even more when γδ T cells were preincubated with an mTORi, without affecting viability (Supplemental Figure 8). mTORis may amplify the intrinsic ability of γδ T cells to respond to the TCR engagement. In agreement with this hypothesis, in mTORi-treated γδ T cells, T cell production of IFNγ after anti-TCR Vδ1 activation was increased (Figure 7B), along with an increased internalization of the TCR (Figure 7C). TCR signaling–induced phosphorylation of the kinase ERK was enhanced in mTORi-treated γδ T cells (Figure 7D), whereas no difference was observed for MAPK-38 or SLP-76, suggesting the mTORi was acting downstream of this very proximal event of TCR signaling (Supplemental Figure 9).
Figure 7.
TCR engagement and signaling of Vδ2neg γδ T cells are improved by mTORi. (A) Vδ2neg γδ T cells were purified and cultured in medium alone, with NI or CMV-infected fibroblasts, with or without a blocking anti-CD3 mAb (10 µg/ml) for 24 hours, and ELISA for IFNγ was performed (n=4 donors). (B) Vδ2neg γδ T cells among the total PBMCs were specifically stimulated via their TCR by an anti-Vδ1 mAb (10 µg/ml), for 2, 4, and 6 hours, and then ELISA for IFNγ was performed, and (C) cells were stained for γδ TCR downregulation analysis by flow cytometry (n=4 donors). (D) Vδ2neg γδ T cells were purified and stimulated with an anti-CD3 antibody (UCHT1, 10 µg/ml) for 0, 1, 2, 4, and 7 minutes. Erk 1/2 phosphorylation was measured by flow cytometry (one representative donor, right; in four donors, left). (E) Vδ2neg γδ T cells among the total PBMCs were specifically stimulated via their TCR by an anti-Vδ1 mAb (10 µg/ml) for 2, 4, and 6 hours, and S6 phosphorylation was measured by flow cytometry. Basal levels before stimulation are represented for seven donors (left), and activation kinetics normalized to the basal level for each condition (0 and 0.5 nM EVR) (right) for four donors are represented. Each symbol represents an individual donor, large horizontal lines indicate the mean and small horizontal lines indicate the SD in (A) and (E, left). Each symbol represents the median of the results for four donors, and the small horizontal lines represent the ranges in (B), (C), (D), and (E, right). *P<0.05, **P<0.01, as determined by the Wilcoxon test.
Moreover, whereas S6 phosphorylation was decreased at baseline in mTORi-treated γδ T cells (Figure 7E, left), the induction of S6 phosphorylation upon TCR triggering was much more important in mTORi-treated than in untreated cells, which was not associated with feedback activation of Akt (Supplemental Figure 10, A and B).
Overall, inhibition of the mTOR pathway conditions γδ T cells to respond more efficiently to an antigenic challenge, as occurs during CMV infection.
Discussion
It has been well established that mTORi treatment is associated with a reduction in the incidence of CMV infection in organ transplant patients who are R+,19,20 but a mechanistic explanation for this effect has been lacking to date. In this study, we suggest that a multimodal functional action on both αβ and γδ effector T cells is involved. In contrast with mTORi’s immunosuppressive functions on naive allogenic T cells, our overall results indicate that mTORi treatment of specific effector T cells improves their response to CMV through improving expansion and viability, and in restoring their functional phenotype, as characterized by increased cytotoxic potential, increased IFNγ production, and decreased expression of inhibitory checkpoints.
Our first original observation was to describe, at baseline, a higher proportion of T cells with a dysfunctional phenotype in patients who were R+ and were unable to control CMV infection without antiviral treatment. We characterized this dysfunctional phenotype through the expression of several inhibitory receptors (PD-1, LAG3, TIM3, CD161, CD85j). As usually described, effector memory T cells that are maintained after CMV infection have a specific profile of CD45RA re-expression; low PD-1 expression; and high KLRG1, Tbet, and Hobit expression without loss of function.6 Here, we described that, in in vitro cultured cells from KTRs who were R+, among effector memory T cells, a percentage of dysfunctional cells expressed PD-1, a high level of EOMES, and a low level of Hobit, with the same level of Tbet. This profile of dysfunctional T cells is usually described in a viral context of a persistent, uncontrolled viral load during chronic phases of infection (e.g., LCMV),43,44 whereas the effector T-cell response described during CMV infection was called inflationary5,6 and was correlated to a well-controlled, low viral load. However, patients who are R+ and are awaiting a kidney graft could be in a relative immunosuppressive state due to ESKD,45 to previous immunosuppressive treatment of their renal disease, or to previous kidney transplantation. This context could lead to a chronically higher viral load favoring the emergence of those dysfunctional CMV effector T cells. Interestingly, documenting the presence of these dysfunctional T cells before the introduction of an immunosuppressive drug regimen may be used to predict the risk of CMV reactivation after transplantation in patients who are R+ to avoid useless treatment. Here, PD-1 and CD85j, expressed on both CMV-specific CD8+ and γδ T cells, were the most discriminating markers for predicting severe CMV infection in patients who were R+. In particular, CD85j is a well-known inhibitory receptor that is induced on both αβ46 and γδ T cells47 during CMV infection, and that binds the CMV-encoded UL18 protein with high affinity.48
Second, we observed that mTORis improved the functional profile (decreased inhibitory receptors, increased functionality) of effector T cells, together with a lower incidence and severity of CMV infection after transplantation. In addition, during the course of CMV infection, a decreased proportion of T cells expressing the inhibitory receptor KLRG1 and an increased proportion of perforin-producing T cells were directly associated with better control of CMV, independently of immunosuppressive treatments; these changes were then shown to be mostly induced by mTORis.
In vitro, a low dose of mTORi induced a long-lived cell profile (PD-1 low, Tbet high, and EOMES low), which was associated with increased proliferation, viability, and IFNγ production in both γδ and αβ T cells. These characteristics were previously associated with improved functionality of effector T cells.11,12 Moreover, mTORi also increased the amount of Hobit+ T cells, which are known to be long-lived effector cells in the context of CMV.8–10
Although the effects of mTORis have been well-studied in naive cells and, notably, in allogenic naive cells, it has been poorly evaluated in effector T cells. Our in vitro results suggest that late-differentiated effector cells present a high basal level of p-S6 that limits their capacity for further S6 phosphorylation during activation, and this could be involved in their low IFNγ production after TCR engagement. Conversely, when the basal level was decreased by mTORi pretreatment, phosphorylation of S6 and IFNγ production was much more inducible during activation. Altogether, these results suggest that mTORis are able to dampen basal S6 phosphorylation in highly differentiated T cells, allowing them to respond more efficiently upon activation.
Finally, we also observed that mTORi-treated T cells improved TCR signaling. This is of particular interest because late effector memory T cells are mostly dependent on TCR signaling, rather than cytokine signals, to persist during chronic infection.49,50 Chronic antigen activation could lead to increased basal mTOR levels in T cells, hampering their capacity to respond properly during a new viral challenge. Decreasing the basal level of mTOR pathway activation could sensitize effector T cells to better respond to a new TCR activation.
Altogether, those findings have highlighted the involvement of dysfunctional T cells in CMV infection risk after transplantation. Expression of CD85j and PD-1 could be promising prospective markers to better manage prevention strategies in patients who are R+. We believe these results provide a clinical and mechanistic understanding of the effect of mTORis on the restoration of CMV-specific late effector T cells, leading to better control of CMV after transplantation. These new insights could guide better use of mTORis in patients who are R+ and contribute to improved outcomes for the most frequent opportunistic infection in solid organ transplantation.
Disclosures
L. Couzi reports having consultancy agreements with, and receiving honoraria from, Biotest, Hansa, and Novartis; and receiving research funding from Novartis. J. Déchanet-Merville reports serving as a scientific advisor for, or member of, American Gene Technologies. M. Mamani-Matsuda reports serving as a reviewer for the For Woman in Sciences program of the L’Oreal-Unesco Foundation (fellowships). P. Merville reports having other interests in/relationships with Agence Biomédecine; having consultancy agreements with Astellas and Bristol Myers Squibb; serving as a scientific advisor for, or member of, Bristol Myers Squibb and Novartis; and receiving honoraria from CSL Behring and Sanofi. All remaining authors have nothing to disclose.
Funding
This work was supported by the Agence Nationale de la Recherche international grant ANR-19-CE18-0024 – TEPEE.
Supplementary Material
Acknowledgments
We thank Catherine Rio, the coordinator nurse of Kidney Transplant Unit. We thank Guillaume Rebillon for his English reading and corrections. We thank Audrey Montero, a clinical research associate. We thank Ligue Nationale Contre le Cancer (J. Déchanet-Merville), Fondation pour la Recherche Médicale (H. Kaminski), and Fondation du rein (J. Déchanet-Merville) for their global support.
H. Kaminski was involved in designing research studies, conducting experiments, acquiring data, analyzing data, and writing the manuscript; N. Yared, G. Marseres, M.-J. Nokin, A. Zouine, V. Pitard, S. Loizon, X. Gauthereau, I. Garrigue, and B. Pinson were involved in acquiring and analyzing data, and writing the manuscript; L. Couzi, I. Pellegrin, R. Thiébaut, R. V. Durán, M. Mamani-Matsuda, and M. Capone were involved in analyzing data and writing the manuscript; and P. Merville and J. Déchanet-Merville were involved in supervising research, designing research studies, in analyzing data, and writing the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Disarming the Old Foe. Restoring T-Cell Immune Function with mTor-Inhibitors to Tackle Cytomegalovirus Infection,” on pages 6–8.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020121753/-/DCSupplemental.
Supplemental Appendix 1. Immunosuppressive regimen of patients.
Supplemental Table 1. References of flow cytometry material.
Supplemental Figure 1. Flow chart of the ancillary study.
Supplemental Figure 2. Flow chart of MPA-treated patients of the ancillary study.
Supplemental Figure 3. Vδ2neg γδ T cells and CD8+ αβ T cells phenotype at baseline in CMV seropositive patients.
Supplemental Figure 4. Phenotype of Vδ2neg γδ T cells and CMV-specific αβ T cells during in vitro culture.
Supplemental Figure 5. Low dose of mTORi decrease the number of Vδ2neg γδ T cells and CMV-specific αβ T cell expressing CD85j.
Supplemental Figure 6. mTORi does not affect CMV-specific αβ T cell frequencies during in vitro culture.
Supplemental Figure 7. mTORi improvement of the T cell dysfunctional profile is maintained when combined with ciclosporin.
Supplemental Figure 8. Blocking anti-CD3 mAb had no effect on Vδ2neg γδ T cell viability.
Supplemental Figure 9. mTORi does not affect SLP-76 and MAP kinase 38 signaling after TCR stimulation of Vδ2neg γδ T cells.
Supplemental Figure 10. No effect of mTORi on Akt phosphorylation after TCR stimulation of Vδ2neg γδ T cells.
References
- 1.Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. ; The Transplantation Society International CMV Consensus Group : The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 102: 900–931, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. : Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol 29: e2034, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Jarque M, Crespo E, Melilli E, Gutiérrez A, Moreso F, Guirado L, et al. : Cellular immunity to predict the risk of cytomegalovirus infection in kidney transplantation: A prospective, interventional, multicenter clinical trial. Clin Infect Dis 71: 2375–2385, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Kaminski H, Jarque M, Halfon M, Taton B, Di Ascia L, Pfirmann P, et al. : Different impact of rATG induction on CMV infection risk in D+R- and R+ KTRs. J Infect Dis 220: 761–771, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Appay V, van Lier RAW, Sallusto F, Roederer M: Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 73: 975–983, 2008 [DOI] [PubMed] [Google Scholar]
- 6.van den Berg SPH, Pardieck IN, Lanfermeijer J, Sauce D, Klenerman P, van Baarle D, et al. : The hallmarks of CMV-specific CD8 T-cell differentiation. Med Microbiol Immunol (Berl) 208: 365–373, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makwana N, Foley B, Fernandez S, Lee S, Irish A, Pircher H, et al. : CMV drives the expansion of highly functional memory T cells expressing NK-cell receptors in renal transplant recipients. Eur J Immunol 47: 1324–1334, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Braun J, Frentsch M, Thiel A: Hobit and human effector T-cell differentiation: The beginning of a long journey. Eur J Immunol 45: 2762–2765, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Hertoghs KM, Moerland PD, van Stijn A, Remmerswaal EB, Yong SL, van de Berg PJ, et al. : Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J Clin Invest 120: 4077–4090, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oja AE, Vieira Braga FA, Remmerswaal EB, Kragten NA, Hertoghs KM, Zuo J, et al. : The transcription factor hobit identifies human cytotoxic CD4+ T cells. Front Immunol 8: 325, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, et al. : The epigenetic landscape of T cell exhaustion. Science 354: 1165–1169, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. : Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338: 1220–1225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Mare-Bredemeijer EL, Shi XL, Mancham S, van Gent R, van der Heide-Mulder M, de Boer R, et al. : Cytomegalovirus-induced expression of CD244 after liver transplantation is associated with CD8+ T cell hyporesponsiveness to alloantigen. J Immunol 195: 1838–1848, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, et al. : Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol 175: 8218–8225, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Huygens A, Lecomte S, Tackoen M, Olislagers V, Delmarcelle Y, Burny W, et al. : Functional exhaustion limits CD4+ and CD8+ T-cell responses to congenital cytomegalovirus infection. J Infect Dis 212: 484–494, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Kallemeijn MJ, Boots AMH, van der Klift MY, Brouwer E, Abdulahad WH, Verhaar JAN, et al. : Ageing and latent CMV infection impact on maturation, differentiation and exhaustion profiles of T-cell receptor gammadelta T-cells. Sci Rep 7: 5509, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato T, Nishida T, Ito Y, Murase M, Murata M, Naoe T: Correlations of programmed death 1 expression and serum IL-6 level with exhaustion of cytomegalovirus-specific T cells after allogeneic hematopoietic stem cell transplantation. Cell Immunol 288: 53–59, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Sester U, Presser D, Dirks J, Gärtner BC, Köhler H, Sester M: PD-1 expression and IL-2 loss of cytomegalovirus- specific T cells correlates with viremia and reversible functional anergy. Am J Transplant 8: 1486–1497, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Pascual J, Berger SP, Witzke O, Tedesco H, Mulgaonkar S, Qazi Y, et al. ; TRANSFORM Investigators : Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J Am Soc Nephrol 29: 1979–1991, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tedesco-Silva H, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes-Freitas T, et al. : Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. Am J Transplant 15: 2655–2664, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Tedesco-Silva H, Pascual J, Viklicky O, Basic-Jukic N, Cassuto E, Kim DY, et al. ; TRANSFORM Investigators : Safety of everolimus with reduced calcineurin inhibitor exposure in de novo kidney transplants: An analysis from the randomized TRANSFORM study. Transplantation 103: 1953–1963, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Poglitsch M, Weichhart T, Hecking M, Werzowa J, Katholnig K, Antlanger M, et al. : CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am J Transplant 12: 1458–1468, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Bak S, Tischer S, Dragon A, Ravens S, Pape L, Koenecke C, et al. : Selective effects of mTOR inhibitor sirolimus on naïve and CMV-specific t cells extending its applicable range beyond immunosuppression. Front Immunol 9: 2953, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. : mTOR regulates memory CD8 T-cell differentiation. Nature 460: 108–112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristelli MP, Esmeraldo RM, Pinto CM, Sandes-Freitas TV, Felipe C, Lobo CF, et al. : The influence of mTOR inhibitors on the incidence of CMV infection in high-risk donor positive-recipient negative (D+/R-) kidney transplant recipients. Transpl Infect Dis 20: e12907, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Kaminski H, Marsères G, Cosentino A, Guerville F, Pitard V, Fournié JJ, et al. : Understanding human γδ T cell biology toward a better management of cytomegalovirus infection. Immunol Rev 298: 264–288, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Couzi L, Pitard V, Moreau JF, Merville P, Déchanet-Merville J: Direct and indirect effects of cytomegalovirus-induced γδ T cells after kidney transplantation. Front Immunol 6: 3, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couzi L, Pitard V, Netzer S, Garrigue I, Lafon ME, Moreau JF, et al. : Common features of gammadelta T cells and CD8(+) alphabeta T cells responding to human cytomegalovirus infection in kidney transplant recipients. J Infect Dis 200: 1415–1424, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, et al. : Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood 112: 1317–1324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S, et al. : Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc Natl Acad Sci U S A 114: 3163–3168, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrigue I, Doussau A, Asselineau J, Bricout H, Couzi L, Rio C, et al. : Prediction of cytomegalovirus (CMV) plasma load from evaluation of CMV whole-blood load in samples from renal transplant recipients. J Clin Microbiol 46: 493–498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preiksaitis JK, Hayden RT, Tong Y, Pang XL, Fryer JF, Heath AB, et al. : Are we there yet? Impact of the first international standard for cytomegalovirus DNA on the harmonization of results reported on plasma samples. Clin Infect Dis 63: 583–589, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum : Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 64: 87–91, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. : Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202: 673–685, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marçais A, Marotel M, Degouve S, Koenig A, Fauteux-Daniel S, Drouillard A, et al. : High mTOR activity is a hallmark of reactive natural killer cells and amplifies early signaling through activating receptors. eLife 6: e26423, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaminski H, Couzi L, Garrigue I, Moreau JF, Déchanet-Merville J, Merville P: Easier control of late-onset cytomegalovirus disease following universal prophylaxis through an early antiviral immune response in donor-positive, recipient-negative kidney transplants. Am J Transplant 16: 2384–2394, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Guerville F, Daburon S, Marlin R, Lartigue L, Loizon S, Pitard V, et al. : TCR-dependent sensitization of human γδ T cells to non-myeloid IL-18 in cytomegalovirus and tumor stress surveillance. OncoImmunology 4: e1003011, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, et al. : Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON- CMV. Transpl Infect Dis 9: 165–170, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. : Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27: 281–295, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieira Braga FA, Hertoghs KM, Kragten NA, Doody GM, Barnes NA, Remmerswaal EB, et al. : Blimp-1 homolog Hobit identifies effector-type lymphocytes in humans. Eur J Immunol 45: 2945–2958, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Sandu I, Cerletti D, Claassen M, Oxenius A: Exhausted CD8+ T cells exhibit low and strongly inhibited TCR signaling during chronic LCMV infection. Nat Commun 11: 4454, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, et al. : Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med 187: 1383–1393, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R: Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77: 4911–4927, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. : Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188: 2205–2213, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, Gasparini A, Ishigami J, Mzayen K, Su G, Barany P, et al. : eGFR and the risk of community-acquired infections. Clin J Am Soc Nephrol 12: 1399–1408, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustafson CE, Qi Q, Hutter-Saunders J, Gupta S, Jadhav R, Newell E, et al. : Immune checkpoint function of CD85j in CD8 T cell differentiation and aging. Front Immunol 8: 692, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rey J, Fauriat C, Kochbati E, Orlanducci F, Charbonnier A, D’Incan E, et al. : Kinetics of cytotoxic lymphocytes reconstitution after induction chemotherapy in elderly AML patients reveals progressive recovery of normal phenotypic and functional features in NK cells. Front Immunol 8: 64, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prod’homme V, Griffin C, Aicheler RJ, Wang EC, McSharry BP, Rickards CR, et al. : The human cytomegalovirus MHC class I homolog UL18 inhibits LIR-1+ but activates LIR-1- NK cells. J Immunol 178: 4473–4481, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R: Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A 101: 16004–16009, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin H, Blackburn SD, Blattman JN, Wherry EJ: Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med 204: 941–949, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.