Abstract
Reports of immune-related adverse events caused by programmed cell death-ligand 1 (PD-L1) inhibitor have been emerging. Herein, we report a subacute cutaneous lupus erythematosus (SCLE)-like eruption presented after the treatment of durvalumab in a patient with extensive-stage small cell lung carcinoma. A 74-year-old Thai man was referred to our department after experiencing multiple dusky red to brownish papules and patches with scale and erosions on photo-distributed areas after receiving 3 infusion cycles of durvalumab. Histological finding revealed epidermal atrophy with interface changes and superficial perivascular infiltration of lymphocytes. Serum antinuclear antibodies (ANA) was 1:320 and anti-Ro/Sjogren’s-syndrome-related antigen A (anti-Ro/SSA) antibodies were positive (2+). Based on the history and clinicopathological correlation, the diagnosis of SCLE-like eruption due to durvalumab was made. To the best of our knowledge, this is the first case of durvalumab-induced SCLE.
Keywords: programmed cell death-ligand 1 inhibitor, durvalumab, subacute cutaneous lupus erythematosus, immune-related adverse events
Introduction
Immune checkpoint inhibitors (ICIs), which target cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), programmed cell death 1(PD-1) and programmed cell death-ligand 1 (PD-L1), have been established as a dominant paradigm in management of advanced malignancies. Blocking specific molecular targets inhibits cancer cell growth, progression, and spread, often resulting in long-term survival. However, by increasing the activity of the immune system, these blockades can lead to inflammatory effects, which are often termed immune-related adverse events.1 These events have a significant impact on patient’s quality of life and may impair ICIs treatment efficacy due to ICIs dose-limiting effects. Immune-related cutaneous adverse events (irCAEs) are the most common and earliest to occur in ICIs patients; thus, understanding their clinicopathologic features and effective management strategies is critical to a successful oncodermatologic practice.2 Recently, cutaneous connective tissue disease-like eruptions have been observed in patients receiving ICIs, with subacute cutaneous lupus erythematosus (SCLE) being the most common manifestation.3
The newly developed anti-PD-L1 agents also have emerged in drug-related cutaneous adverse events. Regarding the National Comprehensive Cancer Network (NCCN) guidelines, durvalumab is a PD-L1 blocker which has been approved by United State FDA in 2020 as a first-line treatment for adult patients with extensive-stage small cell lung carcinoma (ES-SCLC) in combination with platinum-etoposide as a result of the Phase III CASPIAN trial.2
Case Report
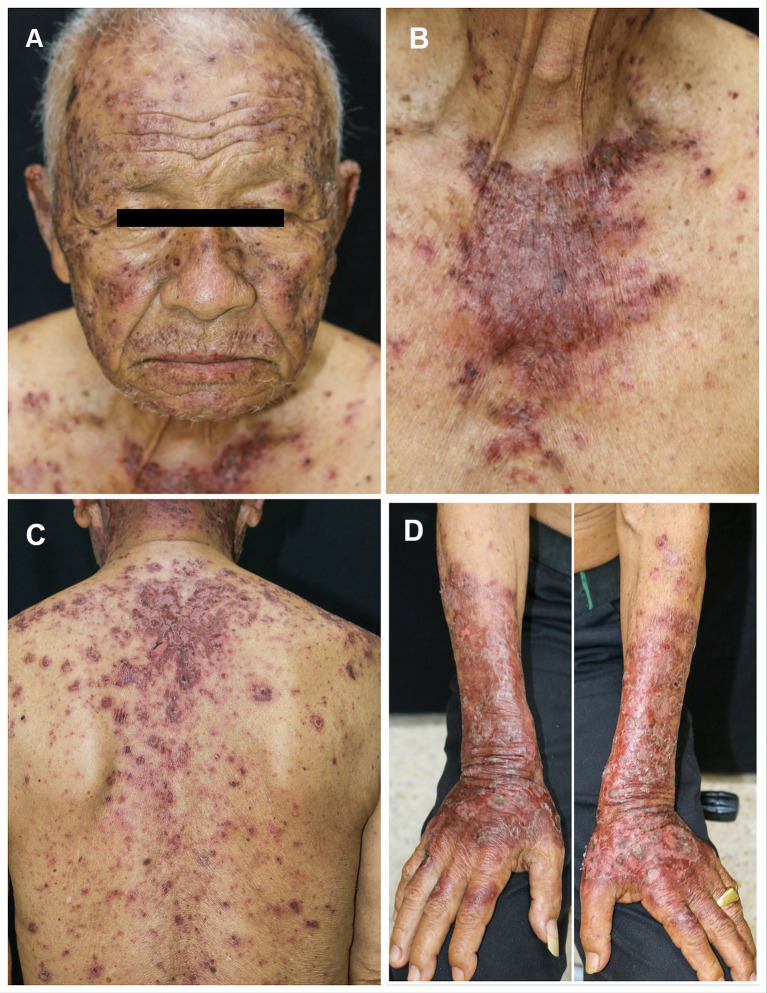
A 74-year-old male with no known underlying disease developed chronic productive cough for 9 months. He had history of heavy smoking (50 pack-years) for several decades. The initial chest radiography revealed a lung mass. His computerized tomography (CT) demonstrated a large lobulated lung mass at the right lower lobe with surrounding reticulonodular opacities. A transbronchial lung biopsy was performed and revealed SCLC on histopathology. He also had leptomeningeal and ipsilateral pleural metastasis. As a result, the diagnosis of ES-SCLC was made. The oncologist began treatment with standard-of-care chemotherapies including carboplatin and etoposide. One month later, he received the second cycle of standard chemotherapies plus durvalumab (Imfinzi®). The therapeutic agents were administered intravenously once a month with no dose adjustments. Before the fourth cycle of treatment (after receiving 3 infusion cycles of durvalumab), he noticed asymptomatic erythematous skin lesions on the extensor surface of his forearms and back. He frequently engaged in outdoor activities with insufficient photoprotection. Three weeks after the fourth cycle, the skin eruptions worsened, becoming darker and more widespread on his face, neck, trunk, and extensor surface of upper extremities. Dermatologic consultation was made. Physical examination showed multiple dusky red to brownish papules and patches covered by scales and crusts with some erosions predominately on sun-exposed areas (face, upper chest, back, dorsum of both forearms) and spared the mucosa (Figure 1A–D). Nikolsky’s sign was positive. A punch biopsy obtained from the lesion on the back revealed a superficial perivascular infiltration and epidermal atrophy with marked interface change (Figure 2A). Remarkably, thin epidermis was necrosed and separated with few dysmaturation of atypical basal keratinocytes (Figure 2B). ANA was detected by using indirect immunofluorescence antibody (IFA) technique showing a titer of 1:320 with a fine speckled pattern. Anti-Ro/SSA was positive (2+) while anti-La/SSB and anti-histone were negative. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were elevated. Durvalumab-induced SCLE was diagnosed in our patient based on history, clinicopathological and serological findings. There was no evidence of systemic lupus erythematosus.
Figure 1.
Multiple dusky red to brownish papules and patches covered by scales and crusts with some erosions predominately on face (A), upper chest (B), back (C), and dorsum of both forearms (D).
Figure 2.
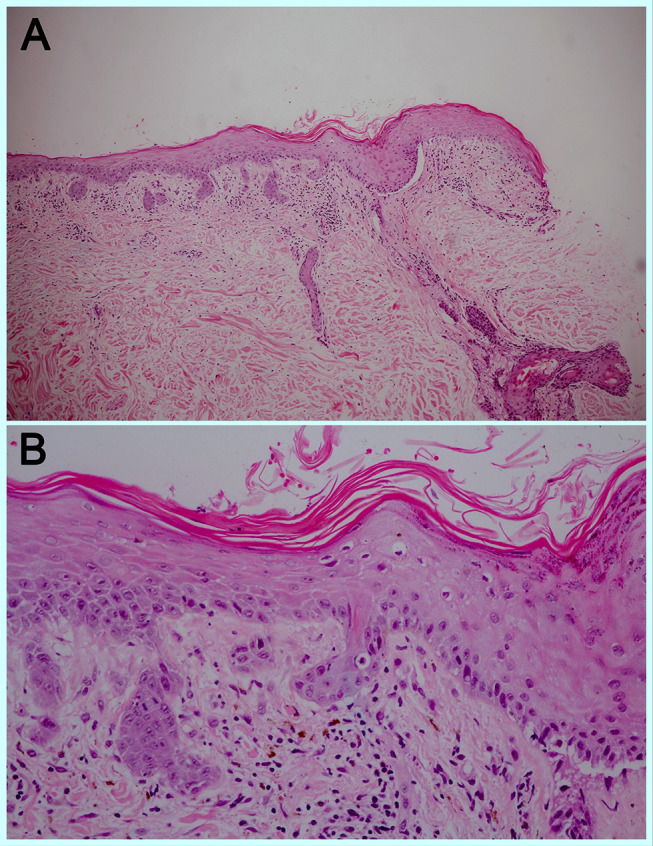
Superficial perivascular infiltration of lymphocytes and melanophages with marked interface change, H&E 100X (A) Epidermal atrophy and few atypical basal keratinocytes, H&E 400X (B).
Abbreviation: H&E, hematoxylin and eosin.
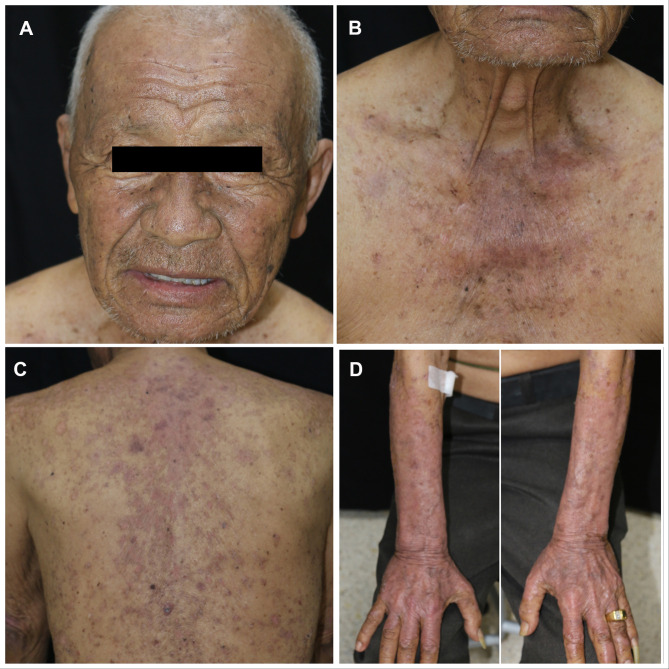
Because the skin lesions progressed to grade 3 (covering more than 30% of body surface area [BSA] with moderate pruritus) according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0,3 all chemotherapies and durvalumab were discontinued. The patient received 1 mg/kg/day of oral prednisolone for the first 2 weeks, followed by hydroxychloroquine (HCQ) 200 mg daily and topical 1.25% hydrocortisone ointment twice daily. Due to significant clinical improvement, topical and systemic corticosteroid was discontinued in the next 2 weeks. Overall, the skin lesions resolved with post-inflammatory hyperpigmentation within one month of drug withdrawal (Figure 3A–D). Furthermore, the patient was advised to undergo strict photo-protection. The standard chemotherapies were resumed without durvalumab and the same hydroxychloroquine dose was maintained for 5 months. During the fifth and sixth cycles of chemotherapies, no new lesions appeared. A CT chest follow-up after 6 months of chemotherapies revealed a reduction in the size of right lung mass with a slightly increased small right pleural effusion and nodular pleural thickening. Unfortunately, the patient failed to follow-up with the oncologist after receiving the sixth cycle of chemotherapies. He passed away 3 months later due to disease progression with no cutaneous eruption recurrence.
Figure 3.
One month after Durvalumab was discontinued, the lesions resolved with post inflammatory hyperpigmentation on face (A), upper chest (B), back (C), and dorsum of both forearms (D).
Discussion
Checkpoint inhibitors are monoclonal antibodies that block CTLA-4, PD-1, or PD-L1 leading to anti-tumor immune response. They can cause a wide range of side effects, most of which are immune-related adverse events that manifest in the gastrointestinal tract, liver, and skin, though any organ can be affected. The diverse groups of cutaneous reactions have been implicated in checkpoint inhibitions. Maculopapular rash, pruritus, psoriasiform and lichenoid eruptions are the most prevalent subtypes.3 Immune-related adverse events occur in 70% of patients treated with PD-1 or PD-1 inhibitor, typically in cutaneous system. Bullous pemphigoid-like, vitiligo-like, and psoriasiform eruptions are frequently reported.3
A large retrospective cohort study found that only 0.025% of patients developed skin lesions resembling de novo cutaneous connective tissue disease. Median time to onset of skin lesions was 8 months (0.5–26). SCLE is the most common, accounting for 72.7% of all cases, followed by SLE with photosensitivity rash, eosinophilic fasciitis, and dermatomyositis (all 9.1%).4 To date, atezolizumab is the only PD-L1 inhibitor associated with SCLE. Table 1 demonstrates a literature review on immune checkpoint inhibitors-associated CLE.
Table 1.
Review of Reports on Checkpoint Inhibitors-Associated CLE
| Study | Age (yrs)/Sex | Tumor Type | History of Autoimmune Disease | ICIs | Time to Rash Onset | Cutaneous Manifestations | Autoimmune Serologies | Histologic/DIF Results | Treatment | ICI Interruption | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Our case | 74/M | SCLC | None | Durvalumab | 3 infusion cycles (2 mo) | Multiple dusky red to brownish papules and patches with scale and erosions on the face, neck, trunk, and extensor surface of upper extremities | ANA: 1:320, fine speckled pattern Anti-Ro (SSA): positive Anti-La (SSB): negative Anti-dsDNA: negative Anti-Smith: negative |
H&E: superficial perivascular infiltration, epidermal atrophy with marked interface change Thin epidermis was necrosed and separated with dysmaturation of atypical basal keratinocytes DIF: NA |
HCQ 200 mg/day, prednisolone 1 MKD, low-potency topical corticosteroid | Permanently discontinued | Improved within 1 mo |
| Liu et al 201825 | 58/F | NSCLC | AIHA | Nivolumab | 5 mo | Monomorphous, violaceous papules and polycyclic, annular papulosquamous plaques on the back and chest | ANA: NA Anti-Ro (SSA): positive Anti-La (SSB): NA Anti-dsDNA: NA Anti-cardiolipin: positive |
H&E: epidermal atrophy, interface dermatitis with lymphocytic and histiocytic infiltrate, moderate basal vacuolar damage, and colloid bodies DIF: negative |
HCQ 400 mg/day, prednisolone 37.5 mg/day, superpotent topical corticosteroid | Discontinued and restarted 5 months later | Improved |
| Blakeway et al 201920 | Case 1 79/F | Melanoma | None | Pembrolizumab | 3 infusion cycles | Annular scaly rash on the face, arms, torso, and legs | ANA: negative Anti-Ro (SSA): NA Anti-La (SSB): NA Anti-dsDNA: NA |
H&E: vacuolar interface pattern, colloid bodies in the spinous and basal layers, and moderately dense perivascular infiltrate of lymphocytes in upper dermis DIF: granular deposition of C3 in the BMZ |
Superpotent topical corticosteroid | Discontinued and restarted with no recurrence | Improved within 3 weeks |
| Case 2 75/M | Melanoma | None | Pembrolizumab | 9 infusion cycles | Widespread, symmetrical “lupus-like” dermatosis on the torso, arms and legs | ANA: negative Anti-Ro (SSA): NA Anti-La (SSB): NA Anti-dsDNA: NA |
H&E: vacuolar interface pattern, colloid bodies in the spinous and basal layers, mildly dense perivascular infiltrate of lymphocytes in upper dermis, and increased dermal mucin DIF: granular deposition of IgG, IgA and IgM in the BMZ |
Superpotent topical corticosteroid | Discontinued and restarted with no recurrence | Improved within 3 weeks | |
| Zitouni et al 201924 | Case 1 72/F | Melanoma | Autoimmune hepatitis | Nivolumab | 13 infusion cycles plus 2 mo after ICI discontinuation | Pruritic, nummular erythematous plaques on the back and arms | ANA: 1:640 Anti-Ro (SSA): positive Anti-La (SSB): positive Anti-dsDNA: negative |
H&E: lymphoid inflammatory infiltrates predominantly in perivascular areas, and focal lesions of the dermis and epidermis DIF: no C3 or IgG depositions |
HCQ 400 mg/day | Permanently discontinued | Improved within 4 mo |
| Case 2 43/M | NSCLC | None | Nivolumab | 2 infusion cycles (1 mo) | Annular erythematous eruption on the dorsal aspect of the hands, arms, and chest | ANA: 1:320 Anti-Ro (SSA): >600 IU/mL Anti-La (SSB): NA Anti-dsDNA: NA |
H&E: discrete lymphoid perivascular inflammatory infiltrates DIF: scarce C3 deposits along the BMZ |
HCQ 400 mg/day, prednisolone 1 MKD, potent topical corticosteroid | Permanently discontinued | Severe flare-up after 2 weeks of the treatment | |
| Kosche et al 201926 | 75/F | Serous ovarian cancer | None | Ipilimumab and Nivolumab | 2 infusion cycles | Pruritic, erythematous, red-brown, scaly plaques with an arcuate appearance on the back, abdomen, arms, and legs | ANA: 1:160, speckled pattern Anti-Ro (SSA): >8.0 Anti-La (SSB): negative Anti-dsDNA: negative Anti-Smith: negative |
H&E: interface lymphocytic infiltrate and focal basal vacuolar change DIF: NA |
HCQ 400 mg/day, quinacrine 100 mg/day, prednisone 40 mg/day, mid-strength topical corticosteroid | Discontinued and switched to pembrolizumab | Improved within 1 week and later flared up |
| Ogawa-Momohara et al 202022 | 80/M | Melanoma | None | Pembrolizumab | 5 infusion cycles | Multiple annular erythema on the trunk | ANA: NA Anti-Ro (SSA): positive Anti-La (SSB): NA Anti-dsDNA: negative |
H&E: strong liquefaction degeneration and dense superficial dermal and perivascular lymphocytic infiltration DIF: no C3 or IgG depositions |
Prednisolone 1 MKD, topical corticosteroid | Discontinued at the 9th cycle | Improved within 3 mo |
| Marano et al 201927 | Case 1 60/M | SCLC | None | Nivolumab | 2 infusion cycles (2 weeks) | Pruritic, erythematous macules and scaly papules coalescing into annular plaques on photo-distributed areas | ANA: 1:40 Anti-Ro (SSA): positive Anti-La (SSB): NA Anti-dsDNA: NA |
H&E: interface dermatitis Colloidal iron staining: increased dermal mucin DIF: NA |
HCQ 400 mg/day, prednisone 60 mg/day, potent topical corticosteroid | Discontinued and restarted | Improved |
| Case 2 60/F | NSCLC | None | Pembrolizumab | 3 infusion cycles (6 weeks) | Painful and pruritic, edematous, crusted and scaly erythematous papules coalescing into plaques on the face, upper back, chest, arms, forearms, and interphalangeal areas on the dorsal hands | ANA: 1:2560 Anti-Ro (SSA): positive, Anti-La (SSB): positive Anti-dsDNA: NA Anti-histone: positive |
H&E: interface dermatitis with adnexal involvement and increased dermal mucin DIF: IgG, IgA, IgM cytoid bodies, linear fibrin deposits at the BMZ, and granular IgG and C3 staining |
Prednisone 60 mg/day, intravenous infliximab, topical corticosteroid | Permanently discontinued | Improved within 1 mo | |
| Bui et al 20219 | Case 1 54/F | SCLC | ICI-associated psoriasis | Nivolumab | 20 mo | Annular eruption on the trunk and extremities | ANA: 1:5120, speckled pattern Anti-Ro (SSA): >8.0 Anti-La (SSB): >8.0 anti-dsDNA: negative |
H&E: focal interface dermatitis, focal lichenoid dermal lymphocytes infiltrate, and mild dermal mucin deposition DIF: NA |
HCQ 200 mg/day, potent topical corticosteroid | Continued with no interruption | Complete clearance within 6 mo |
| Case 2 54/F | Ovarian cancer | None | PD-1 inhibitor | 4 mo | Annular eruption on the upper extremities and trunk | ANA: negative Anti-Ro (SSA): negative Anti-La (SSB): negative Anti-dsDNA: negative |
H&E: interface dermatitis, epidermal spongiosis, superficial dermal perivascular lymphocytes infiltrate with rare eosinophils, follicular plugging and subtle dermal mucin deposition DIF: granular C3, IgM, and IgG along the BMZ |
Potent topical corticosteroid | Discontinued and restarted 1 month later | Improved within 2 mo | |
| Case 3 57/F | Breast cancer | ICI-associated Sjogren’s syndrome | Atezolizumab | 11.5 mo | Annular eruption on the upper extremities and trunk | ANA: 1:320, speckled pattern Anti-Ro (SSA): >8.0 Anti-La (SSB): negative Anti-dsDNA: negative |
H&E: interface dermatitis, focal lichenoid infiltrate, superficial to mid-dermal perivascular lymphocytic infiltrate, perifollicular plugging and increased dermal mucin deposition DIF: negative |
Superpotent topical corticosteroid | Permanently discontinued 2 mo prior to rash onset for colitis | Improved within 1 mo | |
| Case 4 65/M | SCLC | None | Pembrolizumab | 3 mo | Eruption on the trunk and extremities | ANA: 1:320, speckled pattern Anti-Ro (SSA): >8.0 Anti-La (SSB): negative Anti-dsDNA: NA |
H&E: prominent interface dermatitis, focal vesicle formation, lichenoid infiltrate, prominent dyskeratotic keratinocytes with epidermal necrosis, and superficial to mid-dermal perivascular, periadnexal lymphocytic infiltrate and follicular plugging DIF: negative |
HCQ 400 mg/day, potent topical corticosteroid | Permanently discontinued | Improved within 2 mo | |
| Case 5 60/M | Melanoma | None | Nivolumab | 0.5 mo | Annular eruption on the extremities and trunk | ANA: 1:320, speckled pattern Anti-Ro (SSA): >8.0 Anti-La (SSB): negative Anti-dsDNA: NA |
H&E: prominent interface dermatitis, lichenoid infiltrate, clefting, prominent superficial to deep dermal perivascular, periadnexal lymphocytic infiltrate and increased dermal mucin deposition DIF: negative |
Potent topical corticosteroid | Continued with no interruption | Improved within 2 mo | |
| Takeda et al 202128 | 49/F | Oropharyngeal carcinoma | None | Pembrolizumab | 2 weeks | Persistent erythema, and purple discoloration on the fingers, lower legs, and feet | ANA: 1:1280, speckled pattern Anti-Ro (SSA): positive Anti-La (SSB): NA Anti-dsDNA: negative Anti-Smith: positive Anti-phospholipid: negative ANCA: negative Cryoglobulin: negative C3: Low, C4: normal |
NA | HCQ 200 mg/day, prednisolone 30 mg/day | NA | NA |
Abbreviations: AIHA, autoimmune hemolytic anemia; ANCA, antineutrophil cytoplasmic antibodies; Anti-dsDNA, Anti-double stranded DNA; BMZ, basement membrane zone; C, complement; DIF, direct immunofluorescence; F, female; HCQ, hydroxychloroquine; H&E, hematoxylin and eosin stain; ICIs, immune checkpoint inhibitors; IU/mL, International Unit/mL; M, male; MKD, mg/kg/day; mo, months; NA, not available; NSCLC, non-small cell lung cancer.
Durvalumab has been approved as a first-line treatment for adult patients with ES-SCLC in combination with standard-of-care chemotherapies, etoposide plus either carboplatin or cisplatin (platinum-etoposide). The regimen demonstrated a statistically significant improvement in overall survival. The most common adverse events of durvalumab (more than 20%) in patients with ES-SCLC are neutropenia, anemia, nausea, and alopecia.2 The increase in frequency of dermatologic toxicities has been noticed suggesting the possible association between the drug and cutaneous adverse events. The cutaneous lesions possibly associated with durvalumab consist of psoriasiform eruptions,5 cutaneous sarcoidosis,6 and dermatomyositis.7 To the best of our knowledge, no case of durvalumab-related CLE has ever been documented in the literature.
According to our case, the dusky red lesions and skin denudation can be explained by severe apoptotic injury of the epidermis, which correlates with epidermal necrosis and separation in histologic features. Interface dermatitis with foci of vacuolar alteration of basal keratinocytes alternating with areas of lichenoid dermatitis, prominent epidermal atrophy, follicular plugging, and mild or absent basement membrane thickening are all classic features of SCLE skin biopsy.9 The median time interval between drug exposure and the appearance of drug-induced SCLE (DI-SCLE) was reported to be 6 weeks (3 days to 11 years). The period of ICIs use before the onset of SCLE appears longer, averaging 4 months (2 weeks to 20 months).8,9 In our case, anti-cancer drugs consisting cytotoxic chemotherapies and durvalumab were the new medications initiated before the skin eruption in preceding 3 months and 2 months, respectively. However, DI-SCLE due to chemotherapeutic agents is uncommon, with few published cases associated with taxanes, tamoxifen, capecitabine, 5-fluorouracil, pemetrexed, and carboplatin.10 There is no report on etoposide-associated SCLE in the literature. Moreover, anti-Ro/SSA can be positive in up to 80% in patients with ICIs-associated SCLE, whereas anti-La/SSB positivity occurs in only 25%.9 The percentage of anti-Ro/SSA and ANA positivity among DI-SCLE associated with other drugs and ICIs-related SCLE were roughly the same, at 80% and 82%, respectively. However, positive anti-La/SSB is more common in classic DI-SCLE, accounting for approximately 48%.8 Anti-histone antibodies are almost always positive in DI-SLE and patients usually lack cutaneous manifestations. Approximately one-third of the classic DI-SCLE triggers (ie, thiazide diuretics, calcium channel blockers, and terbinafine) has also been reported to be associated with anti-histone antibodies;8 however, our patient had a negative result and no systemic involvement. Future studies are required to determine the frequency and pattern of LE-associated autoantibodies in ICI-related SCLE.
We conclude that the clinicopathological features, drug history and its compatible temporal relationship with rash development and positive lupus-associated serology (ie, ANA and anti-Ro/SSA) makes durvalumab associated SCLE-like eruption the most likely diagnosed. In this patient, the skin lesion resolved within one month of discontinuing immunotherapy, without recurrence after resuming the original chemotherapies.
The pathomechanism of ICIs-associated SCLE remains unclear, but many potential theories have been proposed. Firstly, the multi-hit hypothesis presumes that the PD-1 or PD-L1 blockade may unmask an immune response to a previously tolerated medication, resulting in SCLE phenotype.11,12 Another mechanism is that SCLE may be stimulated by ultraviolet (UV)-B radiation resulting in translocation of Ro (SSA) antigen and increase of cell membrane antigen expression via epitope spreading.13–15 Our patient frequently engaged in outdoor activities with inadequate photoprotection which may have aggravated the symptoms.16 Lastly, focusing on mechanism of action, anti-PD-1 or PD-L1 may modulate humoral immunity which enhances pre-existing autoantibodies and unmask latent auto-immunity.17–19 Therefore, anti-PD-L1 might be involved with immune-related adverse events, not only by increasing the activity of the immune system against antigens in both cancers and healthy tissue, but also by increasing levels of pre-existing autoantibodies. These mechanisms may explain the positive autoantibodies in our case.
No prospective trials have defined the best treatment approach for immune-related cutaneous adverse events. Systemic corticosteroids may be indicated for widespread or severe disease while moderate- to high-potency topical corticosteroids are first-line treatments for mild to moderate eruptions.3,20–22 In our case, the rash dramatically improved with 1 mg/kg/day of oral prednisolone. The use of antimalarial drugs has typically been recommended as the first-line systemic treatment for CLE, with response rates ranging from 31% to 91%.23 There is no strong evidence on using antimalarials in treatment for ICIs-associated SCLE. However, HCQ administered at 200–400 mg/day has been employed with good outcome and without any adverse events.9,24–28 In a previous study, approximately 40% showed serologic improvement at the 8-month follow-up in drug-induced CLE with positive anti-Ro/SSA.29
In conclusion, ICIs are increasingly used in cancer therapy, the clinical-pathologic correlation is important to diagnose specific dermatologic adverse events. Prospective trials are required to define the best treatment approaches and recommendations for minimizing immunotherapy interruption and avoiding immunosuppressive medications whenever possible.
Funding Statement
Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
Consent Statement
Written informed consent was provided by the patient to have the case details and any accompanying images published. Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, Phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/s0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 3.Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255–1268. doi: 10.1016/j.jaad.2020.03.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui AN, Singer S, Hirner J, et al. De novo cutaneous connective tissue disease temporally associated with immune checkpoint inhibitor therapy: a retrospective analysis. J Am Acad Dermatol. 2021;84(3):864–869. doi: 10.1016/j.jaad.2020.10.054 [DOI] [PubMed] [Google Scholar]
- 5.Lin WH, Lee KY, Lee WR, Shih YH. Durvalumab-induced de novo annular psoriasiform drug eruption successfully treated with a combination of narrowband ultraviolet B phototherapy and topical treatment. J Dermatol. 2020;47(9):1041–1045. doi: 10.1111/1346-8138.15371 [DOI] [PubMed] [Google Scholar]
- 6.Rousseau PM, Raimbourg J, Robert M, Dansette D, Dréno B, Peuvrel L. First case of cutaneous sarcoidosis within tattoos under durvalumab. Int J Dermatol. 2019;58(9):e168–e170. doi: 10.1111/ijd.14484 [DOI] [PubMed] [Google Scholar]
- 7.Coustal C, Du Thanh A, Roubille F, Assenat E, Maria ATJ. Rare cutaneous toxicity of immune checkpoint inhibitors: a case of durvalumab-induced dermatomyositis. Eur J Cancer. 2021;155:25–27. doi: 10.1016/j.ejca.2021.06.031 [DOI] [PubMed] [Google Scholar]
- 8.Lowe GC, Henderson CL, Grau RH, Hansen CB, Sontheimer RD. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164(3):465–472. doi: 10.1111/j.1365-2133.2010.10110.x [DOI] [PubMed] [Google Scholar]
- 9.Bui AN, Hirner J, Singer SB, et al. De novo subacute cutaneous lupus erythematosus-like eruptions in the setting of programmed death-1 or programmed death ligand-1 inhibitor therapy: clinicopathological correlation. Clin Exp Dermatol. 2021;46(2):328–337. doi: 10.1111/ced.14449 [DOI] [PubMed] [Google Scholar]
- 10.González García A, Sifuentes Giraldo WA, Grillo Fernández E, Zea Mendoza A. Subacute cutaneous lupus erythematosus associated with pemetrexed plus Carboplatin chemotherapy. J Clin Rheumatol. 2014;20(8):449–450. doi: 10.1097/rhu.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 11.Lin JH, Dutz JP, Sontheimer RD, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Clin Rev Allergy Immunol. 2007;33(1–2):85–106. doi: 10.1007/s12016-007-0031-x [DOI] [PubMed] [Google Scholar]
- 12.Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152(10):1128–1136. doi: 10.1001/jamadermatol.2016.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2(2):85–95. doi: 10.1038/nri724 [DOI] [PubMed] [Google Scholar]
- 14.Chanprapaph K, Limtong P, Ngamjanyaporn P, Suchonwanit P. Trichoscopic signs in dermatomyositis, systemic lupus erythematosus, and systemic sclerosis: a comparative study of 150 patients. Dermatology. 2021;1–11. doi: 10.1159/000520297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149(9):1071–1075. doi: 10.1001/jamadermatol.2013.4957 [DOI] [PubMed] [Google Scholar]
- 16.Chanprapaph K, Ploydaeng M, Pakornphadungsit K, Mekwilaiphan T, Vachiramon V, Kanokrungsee S. The behavior, attitude, and knowledge towards photoprotection in patients with cutaneous/systemic lupus erythematosus: a comparative study with 526 patients and healthy controls. Photochem Photobiol Sci. 2020;19(9):1201–1210. doi: 10.1039/d0pp00073f [DOI] [PubMed] [Google Scholar]
- 17.Hasan Ali O, Bomze D, Ring SS, et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J Am Acad Dermatol. 2020;82(4):854–861. doi: 10.1016/j.jaad.2019.08.045 [DOI] [PubMed] [Google Scholar]
- 18.Seree-aphinan C, Chanprapaph K, Rattanakaemakorn P, et al. Inactivated COVID-19 Vaccine Induces a Low Humoral Immune Response in a Subset of Dermatological Patients Receiving Immunosuppressants. Front Med. 2021;8:769845. doi: 10.3389/fmed.2021.769845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583–589. doi: 10.1093/annonc/mdw640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blakeway EA, Elshimy N, Muinonen-Martin A, Marples M, Mathew B, Mitra A. Cutaneous lupus associated with pembrolizumab therapy for advanced melanoma: a report of three cases. Melanoma Res. 2019;29(3):338–341. doi: 10.1097/cmr.0000000000000587 [DOI] [PubMed] [Google Scholar]
- 21.Chanprapaph K, Pomsoong C, Kositkuljorn C, Suchonwanit P. Intramuscular corticosteroid therapy in the treatment of alopecia areata: a time-to-event analysis. Drug Des Devel Ther. 2022;16:107–116. doi: 10.2147/DDDT.S342179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa-Momohara M, Muro Y, Goto K, et al. Subacute cutaneous lupus erythematosus with melanocyte elimination induced by pembrolizumab. J Dermatol. 2020;47(6):e217–e219. doi: 10.1111/1346-8138.15316 [DOI] [PubMed] [Google Scholar]
- 23.Chasset F, Bouaziz JD, Costedoat-Chalumeau N, Francès C, Arnaud L. Efficacy and comparison of antimalarials in cutaneous lupus erythematosus subtypes: a systematic review and meta-analysis. Br J Dermatol. 2017;177(1):188–196. doi: 10.1111/bjd.15312 [DOI] [PubMed] [Google Scholar]
- 24.Zitouni NB, Arnault JP, Dadban A, Attencourt C, Lok CC, Chaby G. Subacute cutaneous lupus erythematosus induced by nivolumab: two case reports and a literature review. Melanoma Res. 2019;29(2):212–215. doi: 10.1097/cmr.0000000000000536 [DOI] [PubMed] [Google Scholar]
- 25.Liu RC, Sebaratnam DF, Jackett L, Kao S, Lowe PM. Subacute cutaneous lupus erythematosus induced by nivolumab. Australas J Dermatol. 2018;59(2):e152–e154. doi: 10.1111/ajd.12681 [DOI] [PubMed] [Google Scholar]
- 26.Kosche C, Owen JL, Choi JN. Widespread subacute cutaneous lupus erythematosus in a patient receiving checkpoint inhibitor immunotherapy with ipilimumab and nivolumab. Dermatol Online J. 2019;25(10). doi: 10.5070/D32510045821 [DOI] [PubMed] [Google Scholar]
- 27.Marano AL, Clarke JM, Morse MA, et al. Subacute cutaneous lupus erythematosus and dermatomyositis associated with anti-programmed cell death 1 therapy. Br J Dermatol. 2019;181(3):580–583. doi: 10.1111/bjd.17245 [DOI] [PubMed] [Google Scholar]
- 28.Takeda K, Nakano K, Udagawa S, et al. Chilblain lupus-like cutaneous reaction associated with systemic lupus erythematosus induced by immune checkpoint inhibitor. Rheumatology. 2021. doi: 10.1093/rheumatology/keab670 [DOI] [PubMed] [Google Scholar]
- 29.Srivastava M, Rencic A, Diglio G, et al. Drug-induced, Ro/SSA-positive cutaneous lupus erythematosus. Arch Dermatol. 2003;139(1):45–49. doi: 10.1001/archderm.139.1.45 [DOI] [PubMed] [Google Scholar]