Abstract
This study was designed to evaluate the timing of administration of the bovine appeasing substance (BAS) on performance and physiological responses of Bos indicus feedlot cattle. Nellore bulls (n = 100) were ranked by initial body weight (BW; 341 ± 18.5 kg) and assigned to receive BAS (n = 50) or placebo (CON; n = 50) on day −2 of the experiment. Treatments (5 mL) were applied topically to the nuchal skin area of each bull. Bulls were loaded into commercial livestock trailers immediately after treatment administration, transported for 880 km, and unloaded on day −1 at a commercial feedyard. On day 0, bulls within each treatment were again assigned to receive, in a 2 × 2 factorial arrangement, BAS or CON as described previously (25 bulls/treatment combination). Upon treatment administration on day 0, bulls were housed in 12 feedlot pens (3 pens/treatment) for a 108-d feeding period, which was divided into an adaptation (days 0–19), growing (days 20–60), and finishing (days 61–108) phases. Dry matter intake (DMI) was measured daily from days 0 to 108, whereas blood samples and hair from the tail switch were collected on days −2, 0, 19, 60, and 108. Administration of BAS prior to loading (day −2) improved ADG, FE, and DMI during adaptation and across the 108-d feeding period (P ≤ 0.08), resulting in greater (P = 0.03) hot carcass weight and dressing percentage upon slaughter on day 109. A treatment × day interaction was detected for serum glucose concentrations (P = 0.05), which was greater (P = 0.03) on day 60 of the feeding period in bulls receiving CON prior to loading. Administration of BAS at feedlot entry (day 0) improved DMI, ADG, and FE during adaptation (P ≤ 0.05), but it did not impact (P ≥ 0.18) performance and carcass traits during the 108-d feeding period. Bulls administered BAS prior to loading and at feedlot entry had less (P ≤ 0.05) mean serum cortisol concentrations across the 108-d feeding period (loading × feedlot entry interaction; P = 0.10) and greater (P ≤ 0.05) serum insulin concentration on day 60 (loading × feedlot entry × day interaction; P = 0.05). In summary, BAS administration prior to loading increased the overall feedlot performance of Nellore bulls. These outcomes were noted in bulls that received or not a second BAS administration at feedlot entry, suggesting that the benefits of BAS are exploited when this substance is administered before transport to the feedlot.
Keywords: appeasing substance, beef cattle, feedlot entry, performance, stress, transport
Introduction
Transport and feedlot entry are considered as two of the main stressful events that impact welfare and productivity of feedlot cattle (Duff and Galyean, 2007). These events are known to elicit adrenocortical and acute-phase protein responses (APR) that impact cattle immunity, health, and performance (Carroll and Forsberg, 2007). Therefore, alternatives to mitigate stress resultant from mandatory management are warranted to promote health and performance of feedlot cattle (Cooke, 2017). One potential alternative is the administration of the bovine appeasing substance (BAS), a mixture of fatty acids that replicate the composition of the original pheromone produced by cows (Pageat, 2001; Cooke et al., 2020), to cattle exposed to routine stressful management.
Our research group recently demonstrated that BAS administration at weaning alleviated the resultant APR and improved growth of beef calves (Cappellozza et al., 2020; Cooke et al., 2020; Schubach et al., 2020). Administration of BAS at feedlot entry improved average daily gain (ADG) and feed efficiency in high-risk receiving cattle (Colombo et al., 2020). Nonetheless, BAS may be of greater benefit to feedlot cattle if administered prior to transport to mitigate the stress and inflammatory responses resultant from this management (Cooke, 2017), particularly in Bos indicus noncastrated cattle that are more susceptible to these challenges (Cooke, 2014). Hence, we hypothesized that timing of BAS administration impacts performance and alleviates APR in feedlot B. indicus cattle. Therefore, our objective was to evaluate the effects of BAS administration prior to transport and upon feedlot entry on performance and physiological responses of B. indicus cattle.
Materials and Methods
This experiment was conducted at a commercial feedlot operation (Fazenda Flórida), located in Guaiçara, SP, Brazil (21°37′33″ S, 49°47′52″ W, and elevation of 437 m) from May to October 2020. All animals were cared for in accordance with acceptable practices and experimental protocols reviewed and approved by the Nutricorp—Institutional Animal Care and Use Committee (#007/2020) and by the practices outlined in the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Training (FASS, 2010).
Animals and treatments
On day −2 of the study, 100 Bos indicus Nellore bulls were individually weighed (initial body weight [BW] 341 ± 18.5 kg) at the commercial cow-calf ranch they were being reared (Agro Rondinha, Camapuã, MS, Brazil). Bulls were ranked by initial BW and assigned to receive BAS (IRSEA Group, Quartier Salignan, France; n = 50) or placebo (diethylene glycol monoethyl ether; CON; n = 50) in a manner that treatments groups had equivalent initial BW. Bulls were segregated by treatment (2 groups) and immediately processed again for treatment administration, with CON steers processed first to avoid cross-contamination during treatment application (Schubach et al., 2020). Treatments (5 mL) were applied topically to the nuchal skin area of each individual bull, according to Cooke et al. (2020) for dose and route of administration. The placebo used herein is also known as transcutol (Sigma-Aldrich, St. Louis, MO) and used as excipient for the BAS active ingredients. The BAS active ingredient is based on a proprietary mixture of fatty acids including palmitic, oleic, and linoleic acids, added at 1% of the excipient (Pageat, 2001), and estimated to remain in treated animals for a 15-d period according to the manufacturer (Schubach et al., 2020). Following CON administration, bulls were loaded into a commercial livestock trailer and the same procedure was performed for BAS-administered bulls, in a manner that both trailers left the cow-calf farm at the same time (1400 h), transported for 880 km through the same route until arriving at a commercial feedlot (Guaiçara, SP, Brazil) in the evening of day −1. Upon feedlot arrival, bulls within each treatment were unloaded and maintained in two separate nonadjacent paddocks (30 × 20 m), without access to feed and water, as well as with no visual and direct contact until being processed in the morning of day 0.
On day 0, all bulls were individually weighed for BW and assigned to receive, within each treatment in a 2 × 2 factorial design, BAS or CON resulting in four treatment combinations: 1) CON-CON: CON prior to and at feedlot entry (n = 25), 2) CON-BAS: CON prior to transport and BAS at feedlot entry (n = 25), 3) BAS-CON: BAS prior to transport and CON at feedlot entry (n = 25), and 4) BAS-BAS: BAS prior to transport and at feedlot entry (n = 25). Treatments were applied as described for day −2. On day 0, bulls were also ear-tagged, vaccinated against respiratory (5 mL/head; Bayovac Respiratória RD; Bayer SA, São Paulo, SP, Brazil) and clostridial (5 mL/head; Excell-10; Venco Saúde Animal, Londrina, PR, Brazil) pathogens, and administered an anthelmintic (1 mL/50 kg BW; Cydectin, Zoetis) for internal and external parasites.
After treatment administration and initial processing on day 0, bulls were housed in 12 feedlot pens (8 or 9 bulls/pen; 3 pens/treatment) for a 108-d feeding period, in a manner that all pens had an equivalent BW at the beginning of the finishing period. Pens from different treatments were not placed side-by-side to avoid the cross-contamination (Schubach et al., 2020), whereas pens were unpaved (18 × 5 m and 1.0 m of linear feedbunk/bull). Bulls received the same diets throughout the 108-d feeding period, which was divided into adaptation (days 0–18), growing (days 19–59), and finishing (days 60–108) phases. Diets within each phase were offered in amounts to ensure ad libitum intake and to result in 5% orts and are described in Table 1.
Table 1.
Composition and nutritional profile of the diets used in the experiment1
| Item | Adaptation | Growing | Finishing |
|---|---|---|---|
| Composition, % DM | |||
| Grass silage | 22.0 | 4.8 | 4.7 |
| Ground corn | 38.3 | 46.6 | 53.0 |
| Whole cottonseed | 17.0 | 20.0 | 20.0 |
| Soybean meal | – | 10.0 | – |
| Peanut meal | 11.0 | – | – |
| Cottonseed meal | – | 5.0 | 5.5 |
| Dried distillers grains | – | – | 9.0 |
| Sugarcane bagasse | 7.2 | 3.4 | 3.0 |
| Soybean molasses | – | 5.9 | – |
| Urea | 1.1 | 0.9 | 1.4 |
| Mineral-vitamin mix2 | 3.2 | 3.0 | 3.0 |
| Water | 0.2 | 0.4 | 0.4 |
| Nutritional profile, % DM3 | |||
| DM | 47.9 | 55.2 | 68.3 |
| CP | 17.0 | 17.8 | 15.9 |
| EE | 4.8 | 4.3 | 6.3 |
| NDF | 45.4 | 37.0 | 27.0 |
| ADF | 27.8 | 21.8 | 14.9 |
| Ash | 7.3 | 7.7 | 7.0 |
| TDN4 | 66.0 | 70.0 | 75.0 |
| Starch | 25.1 | 29.4 | 37.7 |
| NEm, Mcal/kg5 | 1.47 | 1.63 | 1.83 |
| NEg, Mcal/kg5 | 0.90 | 1.02 | 1.20 |
1Experimental period lasted 108 d. Adaptation diet was offered from days 0 to 18, growing diet was offered from days 19 to 59, and finishing diet from days 60 to 108.
2Nutrient composition included 27.7% Ca, 1.7% P, 2.8% Na, 1.7% Mg, 2.3% S, 14 mg Cr, 1,240 mg Mn, 1,800 mg Zn, 455 mg Cu, 20 mg Co, 18 mg Se, 38 mg I, 170 mg F, 77,000 IU Vitamin A, 22,600 IU Vitamin E, 22.0 g tannin, 800 mg virginiamycin, and 500 mg monensin sodium.
3DM, dry matter; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; TDN, total digestible nutrients; NEm, net energy for maintenance; NEg, NE for gain.
4Calculated based on equations described by Weiss et al. (1992).
5Calculated based on equations described by NASEM (2016).
Sampling
Samples of diets offered during the adaptation, growing, and finishing phases were collected at the beginning of each period (days 0, 19, and 60, respectively) and analyzed for nutrient concentration by a commercial laboratory (ESALQLab; Piracicaba, SP, Brazil) as described in Table 1.
Shrunk BW was obtained on days 0 and 108 after 12 h of feed and water withdrawal, whereas unshrunk BW was recorded on days 19 and 60 prior to the morning feeding. Intermediate unshrunk BW, instead of shrunk BW, was obtained to prevent extreme dry matter intake (DMI) fluctuations following management and realimentation (Owens et al., 1998; Krehbiel, 2014), and prevent the APR resultant from feed and water withdrawal (Marques et al., 2012; Marques et al., 2019). Overall average daily gain (ADG) was calculated using final shrunk (day 108) and initial shrunk (day 0) BW, whereas intermediate ADG values were calculated from days 0 to 19, 19 to 60, and 60 to 108. Shrunk BW collected on days 0 and 108 were added an 8% shrink (Cooke et al., 2013) to represent initial and final BW, respectively. Following processing and sample collection on day 108, bulls were road-transported to a commercial packing plant located approximately 5 km away from the feedlot facility (JBS; Lins, SP, Brazil) and slaughtered in the following morning (day 109). Hot carcass weight (HCW) was determined according to equations described by Del Bianco Benedeti et al. (2021), using final BW obtained on day 108 of the study. Based on the HCW results, dressing percent (DP) was calculated by dividing HCW by final BW on day 108 of the study.
From days 0 to 108, feed intake (DM basis) was evaluated from each pen by collecting and weighing offered and non-consumed feed daily. Samples of offered and non-consumed feed were dried following the microwave technique for daily DM calculation. Feed intake of each pen was divided by the number of animals within each pen and expressed as kg per animal/day. Feed efficiency (FE) was calculated using total BW gain during the feedlot period (from days 0 to 108) and total feed intake of each pen during the study. Blood was sampled and hair from the tail switch collected from all bulls on days −2 (prior to treatment administration and loading), 0 (prior to treatment administration at the feedlot), 19, 60, and 108 of the experiment. Blood samples were collected into commercial blood collection tubes (Vacutainer, 10 mL; Becton Dickinson, Franklin Lakes, NJ) containing no additive for serum collection. Hair samples were collected concurrently with blood samples following the methodology described by Schubach et al. (2017).
Laboratorial analyses
Feed samples were analyzed in duplicates by near infrared spectroscopy (NIRS) using a FOSS NIRSystems-500 Transport with ISIScan (v.4.12.0) using Dairy One NIRS calibrations through the FOSS Manager (v.8.10.0). Global NIRS Calibrations (Dairy One Forage Laboratory, Ithaca, NY) were originally developed according to methodologies described in AOAC (2006) and Weiss et al. (1992), and then translated to local calibrations using WinISI (v.4.6.11), as reported by Schenk et al. (2008). All diets were analyzed in duplicates for concentrations of dry matter (DM), crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), ether extract (EE), ash, and total digestible nutrients (TDN), whereas NEm and NEg were calculated according to equations described and reported by NASEM (2016).
Blood samples were placed immediately on ice after collection, centrifuged (2,500 × g for 30 min; 4 °C) for serum harvest, and stored at −20 °C on the same day of collection. All samples were analyzed for serum concentrations of haptoglobin (Cooke and Arthington, 2013b), glucose (Carysta High Volume Chemistry Analyzer; Zoetis), cortisol (radioimmunoassay kit #07221106, MP Biomedicals, Santa Ana, CA; Colombo et al., 2019), and insulin (PI-12K; Millipore Sigma, Burlington, MA). The intra- and inter-assay CV were 5.6% and 6.1% for cortisol, 3.8% and 7.2% for haptoglobin, and 8.6% and 8.5% for insulin. All glucose samples were analyzed in a single assay with an intra-assay CV of < 5%. Hair samples were analyzed for cortisol concentrations as described by Schubach et al. (2017).
Statistical analysis
All data were analyzed using pen as the experimental unit, using the MIXED procedure of SAS (version 9.4; SAS Inst. Inc., Cary, NC), and Satterthwaite approximation to determine the denominator degrees of freedom for tests of fixed effects. All data were analyzed as a 2 × 2 factorial design. Model statements contained the effects of treatment at loading, treatment at feedlot entry, day for repeated measures (DMI during adaptation and metabolic data), and all resultant interactions. Values from blood and hair samples collected on day −2 were used as a covariate in each respective analysis. Random statement for DMI and FE included pen (treatment at loading × treatment at feedlot entry), whereas BW, ADG, carcass traits, and physiological data included bull (pen) and pen (treatment at loading × treatment at feedlot entry) as random variables. The specified term for repeated statements was day, with pen(treatment at loading × treatment at feedlot entry) as subject for DMI, and bulls (pen) as subject for metabolic data. The covariance structure used was compound symmetry, which provided the smallest Akaike Information Criterion and hence the best fit for all variables analyzed herein. All results are reported as least square means or adjusted to the covariate values obtained prior to treatment application for blood and hair variables. Significance was set at P ≤ 0.05 and tendencies were determined if P > 0.05 and ≤ 0.10. Repeated measures are reported according to main treatment effect if no higher-order interactions were detected.
Results
Treatment administration at loading and/or feedlot entry
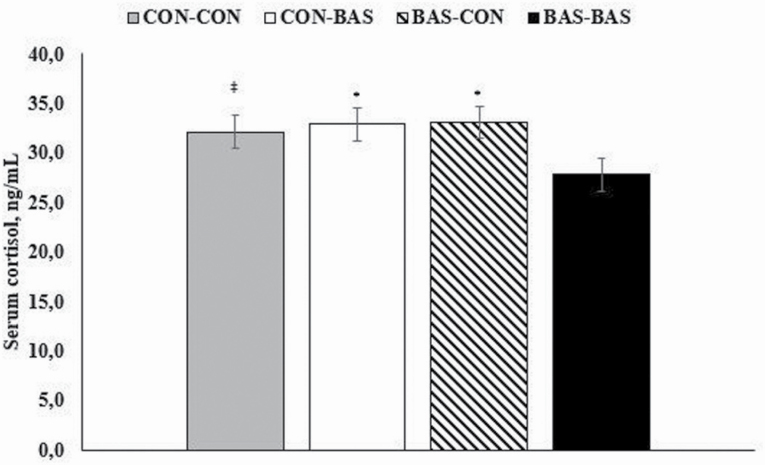
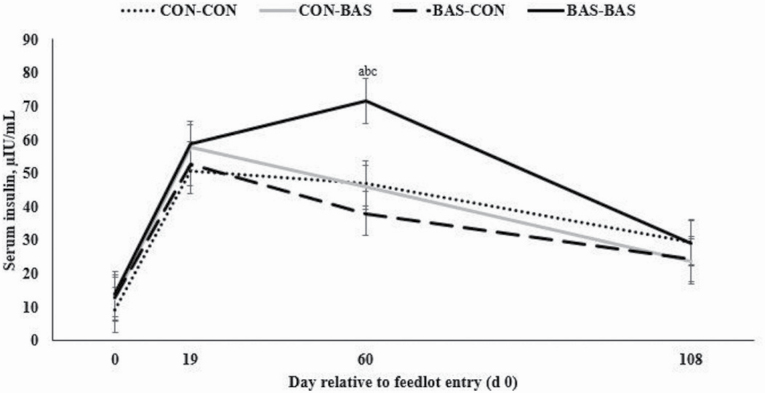
A treatment at loading × treatment at feedlot entry interaction tended to be detected for serum cortisol concentrations (P = 0.10; Figure 1), which was less (P ≤ 0.05) during the experiment in bulls administered BAS at both loading and feedlot entry compared with all other treatment combinations (Figure 1). A treatment at loading × treatment at feedlot entry × day interaction was observed for serum insulin concentration (P = 0.05), which was greater (P ≤ 0.05) in bulls administered BAS at both loading and feedlot entry on d 60 compared with all other treatment combinations (Figure 2).
Figure 1.
Serum cortisol concentration of Bos indicus bulls receiving (BAS) or not (CON) prior to transport to the feedlot (at loading) and/or feedlot entry. A loading × feedlot entry interaction tended to be observed herein (P = 0.10). * denotes differences at P ≤ 0.05 level and ‡ denotes differences at 0.05 < P ≤ 0.10.
Figure 2.
Serum insulin concentration of Bos indicus bulls receiving (BAS) or not (CON) prior to transport to the feedlot (at loading) and/or feedlot entry. A loading × feedlot entry × day interaction was observed herein (P = 0.05). Different letters denote differences at P ≤ 0.05 level: a = CON-CON vs. BAS-BAS (P = 0.02); b = CON-BAS vs. BAS-BAS (P = 0.01); c = BAS-CON vs. BAS-BAS (P < 0.01).
No interactions between the main factors (treatment at loading × treatment at feedlot entry) were noted for feedlot performance data (P ≥ 0.29); hence, only main factors effects are presented and discussed herein for these responses (Table 2).
Table 2.
Feedlot performance of Bos indicus bulls receiving (BAS) or not (CON) prior to transport to the feedlot and/or at feedlot entry1,2
| Loading | Feedlot entry | SEM | P-value3 | |||||
|---|---|---|---|---|---|---|---|---|
| Item | CON | BAS | CON | BAS | L | F | L × F | |
| Body weight,4 kg | ||||||||
| Day −2 | 341.2 | 341.1 | 341.1 | 341.2 | 2.66 | 0.97 | 0.98 | 0.99 |
| Day 0 | 302.8 | 300.0 | 302.3 | 300.5 | 2.54 | 0.44 | 0.62 | 0.97 |
| Day 19 | 336.1 | 342.0 | 337.3 | 340.8 | 2.89 | 0.15 | 0.40 | 0.61 |
| Day 60 | 395.9 | 405.4 | 399.3 | 402.1 | 3.61 | 0.06 | 0.59 | 0.75 |
| Day 108 | 457.1 | 471.3 | 461.8 | 466.6 | 4.59 | 0.03 | 0.46 | 0.98 |
| Average daily gain, kg/d | ||||||||
| Days 0–19 | 1.789 | 2.210 | 1.880 | 2.119 | 0.0706 | < 0.0001 | 0.02 | 0.44 |
| Days 19–60 | 1.447 | 1.548 | 1.516 | 1.479 | 0.0457 | 0.16 | 0.59 | 0.67 |
| Days 60–108 | 1.274 | 1.372 | 1.301 | 1.345 | 0.0411 | 0.10 | 0.45 | 0.53 |
| Overall | 1.430 | 1.586 | 1.483 | 1.533 | 0.0301 | < 0.001 | 0.24 | 0.80 |
| Dry matter intake, kg/d | ||||||||
| Days 0–19 | 6.52 | 6.90 | 6.53 | 6.88 | 0.102 | 0.03 | 0.04 | 0.88 |
| Days 19–60 | 9.50 | 9.76 | 9.40 | 9.86 | 0.157 | 0.27 | 0.07 | 0.30 |
| Days 60–108 | 9.48 | 9.93 | 9.53 | 9.88 | 0.152 | 0.07 | 0.14 | 0.30 |
| Overall | 8.97 | 9.33 | 8.95 | 9.35 | 0.127 | 0.08 | 0.06 | 0.29 |
| Feed efficiency,5 g/kg | ||||||||
| Days 0–19 | 274 | 321 | 287 | 307 | 6.0 | < 0.001 | 0.05 | 0.14 |
| Days 19–60 | 154 | 159 | 163 | 150 | 6.1 | 0.58 | 0.18 | 1.00 |
| Days 60–108 | 135 | 139 | 137 | 136 | 3.0 | 0.39 | 0.79 | 0.97 |
| Overall | 160 | 170 | 167 | 164 | 3.2 | 0.05 | 0.57 | 0.67 |
| Carcass traits6 | ||||||||
| Hot carcass weight, kg | 260.1 | 268.9 | 263.1 | 266.1 | 3.12 | 0.03 | 0.46 | 0.93 |
| Dressing percent, % | 56.88 | 57.03 | 56.93 | 56.99 | 0.052 | 0.03 | 0.40 | 0.93 |
1Experimental period lasted 108 d and animals were slaughtered on the morning of day 109.
2Day −2, prior to transport to the feedlot; Day 0, feedlot entry; Day 19, end of the adaptation period; Day 60, end of the growing period; Day 108, end of the finishing period.
3L, Effects of treatment at loading (day −2); F, Effects of treatment at feedlot entry (day 0); L × F, loading × feedlot entry interaction.
4Bull initial and final BW were calculated based on shrunk BW and added an 8% shrink (Cooke et al., 2013c).
5Feed efficiency was calculated using total BW gain (in grams), and total feed intake (kg of dry matter) of each pen during each and overall experimental period.
6Calculated using the equations proposed by Del Bianco Benedeti et al. (2021).
Treatment administration at loading.
Initial BW (day −2) and on day 0 were similar (P ≥ 0.44) between treatments (Table 2). Administration of BAS increased ADG during the adaptation period (P < 0.01), tended to increase ADG during the finishing period (P = 0.10), resulting in greater (P < 0.01) overall ADG during the experiment (Table 2). As a result, BW on day 60 tended to be greater (P = 0.06), whereas final BW was greater (P < 0.01) in bulls administered BAS compared with CON (Table 2). Accordingly, BAS administration increased (P = 0.03) HCW and DP compared with CON (Table 2).
Daily DMI was greater (P = 0.03) for BAS vs. CON bulls during the adaptation period, and tended to be greater for BAS bulls throughout the experiment (P = 0.08; Table 2). Bulls administered BAS also had improved (P ≤ 0.05) FE during the adaptation and throughout the experiment compared with CON bulls (Table 2).
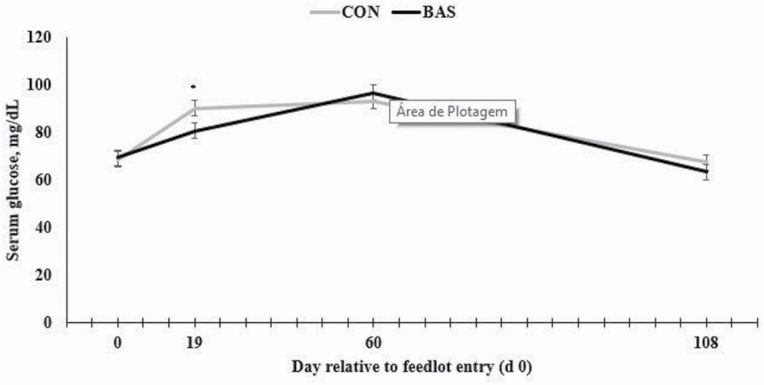
A treatment × day interaction was detected for serum glucose concentrations (P = 0.05), which was greater (P = 0.03) in CON bulls compared with BAS cohorts on d 19 of the experiment (Figure 3). No treatment effects were observed (P ≥ 0.24) for concentrations of cortisol in tail switch hair, or serum haptoglobin, cortisol, and insulin (Table 3).
Figure 3.
Serum glucose concentration of Bos indicus bulls receiving (BAS) or not (CON) prior to transport to the feedlot (at loading). A treatment × day interaction was observed herein (P = 0.05). * denotes differences at P ≤ 0.05 level.
Table 3.
Metabolic data of Bos indicus bulls receiving (BAS) or not (CON) prior to transport to the feedlot (at loading) and/or at feedlot entry1
| Loading | Feedlot entry | SEM | P-value2 | |||||
|---|---|---|---|---|---|---|---|---|
| Item | CON | BAS | CON | BAS | L | F | L × F | |
| Haptoglobin, µg/mL | 2.49 | 2.34 | 2.42 | 2.40 | 0.182 | 0.57 | 0.95 | 0.94 |
| Cortisol, ng/mL | 32.5 | 30.4 | 32.6 | 30.3 | 1.18 | 0.25 | 0.21 | 0.10 |
| Glucose, mg/dL | 79.9 | 77.4 | 77.1 | 80.1 | 2.45 | 0.51 | 0.41 | 0.19 |
| Insulin, µIU/mL | 33.0 | 39.1 | 34.5 | 37.7 | 3.45 | 0.24 | 0.53 | 0.31 |
| Hair cortisol, pg/mg of hair | 2.44 | 2.38 | 2.34 | 2.48 | 0.066 | 0.54 | 0.17 | 0.49 |
1Blood samples were collected on days −2, 0, 19, 60, and 108 of the experimental period. Samples collected on day 0 were used as covariates in each respective analysis.
2L, Effects of treatment at loading (day −2); F, Effects of treatment at feedlot entry (day 0); L × F, loading × feedlot entry interaction.
Treatment administration at feedlot entry
Bulls administered BAS at feedlot entry had greater (P ≤ 0.05) ADG, DMI, and FE during the adaptation period compared with CON bulls (Table 2). Administration of BAS, however, did not impact bull BW during the experiment (P ≥ 0.40; Table 2). Bulls administered BAS also tended (P ≤ 0.07) to have greater DMI during the growing phase and throughout the experiment compared with CON (Table 2).
No other treatment differences were noted (P ≥ 0.14) for bull performance during the experiment, including overall ADG, final BW, and carcass traits (Table 2). No treatment effects were also observed (P ≥ 0.24) for concentrations of cortisol in tail switch hair or serum haptoglobin, cortisol, glucose, and insulin (Table 3).
Discussion
The primary goal of the present experiment was to evaluate whether the timing of BAS administration would lead to differences on physiologic responses and feedlot performance of B. indicus bulls. To the best of our knowledge, this is the first experiment evaluating overall performance and metabolic responses of beef animals receiving BAS at loading and at feedlot entry. Previous studies from our research group (Colombo et al., 2020; Cooke et al., 2020) reported positive effects of BAS at the beginning of the feedlot period, but none has evaluated the animals until slaughter. More specifically, Cooke et al. (2020) reported greater ADG in B. indicus beef feedlot animals for the first 15-d post-BAS administration, whereas ADG and FE was improved over a 45-d receiving period in Angus-influenced steers (Colombo et al., 2020).
Mammals have developed a mechanism to detect and conduct pheromone molecules, such as BAS, that are suspended in the atmosphere up to the target organs. This mechanism, known as “Flehmen Reflex,” causes the animal to elevate and extend its head, to retract the superior lip, and to expose the ear-jaw articulation, which in turn, allows the inhalation of a specific substance (Crowell-Davis and Houpt, 1985). In ruminants, 2 locals were thought to be involved in pheromone perception: main olfactory epithelium (MOE) and vomeronasal organ (VNO; Kekan et al., 2017), which recognizes pheromones that carry specific intraspecies chemosensory signals (Halpern and Martinez-Marcos, 2003). Moreover, the VNO express neural receptors that are activated by specific binders or pheromones (V1r; Grus et al., 2005), likely stimulating a neuroendocrine cascade independent of an animal cognitive recognition (Patra et al., 2012). The VNO neurons have the ability to encode stimulus strength and when it reaches its threshold, an entire neural subpopulation is activated to reach an action potential threshold and conduct a strong electrochemical signal to the brain (Kekan et al., 2017). This specific signal could stimulate the hypothalamus to exhibit an appropriate neuroendocrine response unique to the specific subpopulation of neurons stimulated in the VNO, such as alleviating an APR by reducing the release of the stress-related cascade initiated by corticotropin releasing hormone in ruminants (Cooke and Bohnert, 2011). Indeed, Hervet et al. (2021) recently demonstrated that BAS-administered bulls had a reduced blood mRNA expression of pro-inflammatory-related genes, such as interleukin (IL)-12 and IL-6, and greater blood mRNA of IL-8 following weaning and feedlot entry.
Effects of treatment administration at loading
Administration of BAS prior to transport (at loading) improved ADG, DMI, and FE of feedlot B. indicus bulls, resulting in greater HCW and DP upon slaughter. These outcomes demonstrate the potential of BAS in improving performance and carcass traits of feedlot cattle exposed to stressors resultant from transport and feedlot entry, which are known to impact their health and productivity (Loerch and Fluharty, 1999; Marques et al., 2012; Cooke et al., 2013a; Cooke et al., 2013b; Cooke, 2017; Marques et al., 2019). The observed improvement in performance might be primarily explained by the fact that BAS-administered animals had greater FE throughout the experimental period, a trait that is severely impacted in stressed animals (Guarnieri Filho et al., 2014; Cooke, 2017). In fact, a stressor, when perceived by the animal, might lead to the inflammatory cascade that acts as nutrient sink, removing the nutrients from an anabolic- to a catabolic-state (Johnson, 1997). During the adaptation phase, DMI and ADG were also greater for BAS- vs. CON-treated, but DMI only tended to be greater during the entire experimental period, highlighting the window of action of BAS (15-d period; Cappellozza et al., 2020; Cooke et al., 2020). Nonetheless, the exact mechanism(s) by which BAS positively impacts FE for an extended period (108 d) is unknown and definitely warrants further investigation.
Administration of BAS at loading and at feedlot entry significantly reduced mean serum cortisol concentrations when compared with all other groups, but this effect was not observed in hair cortisol samples. Conversely to our results, Schubach et al. (2020) demonstrated that BAS administration in B. indicus × B. taurus steers at weaning reduced hair cortisol concentrations in samples obtained 14-d post-weaning, but no differences were observed for plasma cortisol. Cortisol, in turn, causes tissue mobilization that will supply energy to metabolism to fight the ongoing inflammation and, consequently, restore homeostasis (Carroll and Forsberg, 2007). Upon tissue mobilization, NEFA are released and might be recognized by the immune system as a disruption in homeostasis (Abbas and Lichtman, 2007), triggering an APR in ruminants (Cooke and Bohnert, 2011; Cooke et al., 2012).
In cattle, glucose concentration has been positively associated with feed intake and BW gain (Vizcarra et al., 1998; Hersom et al., 2004; Cappellozza et al., 2014). Hence, considering the performance data herein, one would expect that BAS-administered animals would have greater glucose concentration vs. CON cohorts. Nonetheless, two factors related to glucose concentrations should be mentioned herein: 1) blood glucose concentrations in cattle are fairly stable due to the role of insulin, which may have prevented proper assessment of treatment effects on glucose flux herein (Marston et al., 1995) and 2) glucose synthesis is stimulated during a stressful situation in order to supply energy to the animal counteract the ongoing inflammatory cascade (Carroll and Forsberg, 2007), indicating that stress and inflammatory response might override the performance on the increases of circulating glucose.
The lack of main treatment effects on haptoglobin and insulin observed herein was unexpected, given the aforementioned performance data, the association between performance and these metabolites during a stress-related response (Qiu et al., 2007; Rodrigues et al., 2015; Cooke, 2017), as well as previous reports from our research group demonstrating that BAS alleviates APR in beef animals (Cooke et al., 2020; Schubach et al., 2020). In fact, Schubach et al. (2020) reported that mean haptoglobin concentration was reduced in BAS-administered beef B. indicus × B. taurus steers over a 42-d period (Schubach et al., 2020), and, therefore, it was expected similar results in the present experiment. Nonetheless, the lack of effects on haptoglobin corroborate with others (Colombo et al., 2020), even when a performance benefit has been reported, as observed herein. Other factors might also impact overall hormone and metabolite responses, such as environment, cattle breed, and sampling schedule, and additional studies are warranted to better understand the effects of BAS on hormone and metabolite response of B. indicus feedlot cattle. In fact, breed differences among studies might play a key role in impacting metabolic and health-related responses in beef animals, as B. indicus animals are more temperamental than B. taurus cohorts (Cooke, 2014), and this is the first experiment evaluating overall health and performance of B. indicus feedlot cattle receiving BAS.
Effects of treatment administration at feedlot entry
When BAS was administered to B. indicus bulls at feedlot entry, immediate benefits on ADG, FE, and DMI were observed up to the end of the adaptation period (day 19), without lasting performance effects throughout the feedlot period, as reported by others in a feedlot setting (Cooke et al., 2020).
It may be speculated that the lack of treatment effects on overall feedlot performance when BAS was administered at feedlot entry only could be related to the fact that a greater majority of stressors had already been placed and occurred, such as transportation itself, environmental alterations, as well as feed and water restriction, that might immediately trigger a stress-related APR and lead to productive losses for a prolonged period of time (Marques et al., 2012; Marques et al., 2019). In agreement with these results, Cooke et al. (2020) demonstrated that performance of B. indicus bulls was only improved for the first 15-d following feedlot entry and BAS administration. Moreover, the animals used in the study by these authors (Cooke et al., 2020) had already arrived and maintained at the feedlot facility for a period of time prior to the beginning of the experiment and BAS administration. However, it still must be determined whether the amount of stress employed during a routine and daily management procedure might lead to different results following BAS utilization in different livestock production systems.
Effects of treatment administration at loading × feedlot entry
Similarly to what has been discussed for the feedlot entry, the lack of adding BAS shots at loading and feedlot entry might be related to the timing of stress occurrence. Therefore, based on our data, it is logical to suggest that in order to maximize performance of beef animals facing several stressors (i.e., transport, feed and water withdrawal, novel environment, management, commingling, and feedlot entry), BAS should be administered prior to the encounter of such stressful events.
Besides the lack of performance, administering BAS in both periods yielded positive effects on serum cortisol and insulin. Insulin concentrations were expected to follow the same pattern to what has been aforementioned reported for glucose, considering that circulating concentration of insulin is tightly regulated by nutrient intake and plasma glucose concentration (Vizcarra et al., 1998; Nussey and Whitehead, 2001; Cappellozza et al., 2014). Others have also reported immediate and transient increases in circulating insulin concentrations upon a pathogen encounter (Steiger et al., 1999; Waldron et al., 2003; Rodrigues et al., 2015), as insulin synthesis and release are enhanced during an inflammatory response (Eizirik et al., 1995; Andersson et al., 2001) to increase energy utilization by the body to restore homeostasis (Waggoner et al., 2009). In the present study, serum insulin was greater in BAS-BAS animals only on day 60 of the study and the reason(s) involved in this response are currently unknown, as performance was not positively impacted when BAS was administered at loading and feedlot entry. Nonetheless, to the best of our knowledge, this is the first research evaluating 1) the effects of a stressful, nonpathogenic, situation on serum insulin concentration and 2) the effects of BAS on insulin response of cattle undergoing a stressful situation, such as transport, feed and water deprivation, and feedlot entry, and, therefore, still warrant further investigation.
Overall conclusions
Administering BAS prior to transport improved DMI, ADG, and FE during the adaptation period (19 d) in an immediate manner, and such improvements were noted until the end of the 108-d feeding period. These outcomes demonstrate the short- and long-term efficacy of this technology in improving nutrient utilization in B. indicus feedlot cattle. Conversely, administration of BAS at feedlot entry improved performance only during the adaptation period, given that major source of stress in this experimental design (transport) had already occurred, which limited the benefits of BAS. Administration of two doses of BAS (at loading and feedlot entry) did not result in additive effects when compared with administration at loading only.
Acknowledgments
Financial support of this research was provided by Nutricorp (Araras, SP, Brazil). We would like to thank all the staff personnel from Faz. Flórida (Guaiçara, SP, Brazil) for their assistance throughout the experiment.
Glossary
Abbreviations
- ADG
average daily gain
- APR
acute-phase response
- BAS
bovine appeasing substance
- BW
body weight
- DMI
dry matter intake
- DP
dressing percent
- FE
feed efficiency
- HCW
hot carcass weight
- TDN
total digestible nutrients
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abbas, A. K., and Lichtman A. H.. . 2007. Cellular and molecular immunology. 6th ed. Saunders Co., Philadelphia, PA. [Google Scholar]
- Andersson, A. K., Flodstrom M., and Sandler S.. . 2001. Cytokine-induced inhibition of insulin release from mouse pancreatic β-cells deficient in inducible nitric oxide synthase. Biochem. Biophys. Res. Commun. 281:396–403. doi: 10.1006/bbrc.2001.4361 [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists . 2006. Official methods of analysis. 18th ed. Association of Official Analytical Chemists, Arlington, VA. [Google Scholar]
- Cappellozza, B. I., Bastos J. P., and Cooke R. F.. . 2020. Administration of an appeasing substance to Bos indicus-influenced beef cattle improves performance after weaning and carcass pH. Livest. Sci. 238:104067. doi: 10.1016/j.livsci.2020.104067 [DOI] [Google Scholar]
- Cappellozza, B. I., Cooke R. F., Reis M. M., Moriel P., Keisler D. H., and Bohnert D. W.. . 2014. Supplementation based on protein or energy ingredients to beef cattle consuming low-quality cool-season forages: II. Performance, reproductive, and metabolic responses of replacement heifers. J. Anim. Sci. 92:2725–2734. doi: 10.2527/jas.2013-7442. [DOI] [PubMed] [Google Scholar]
- Carroll, J. A., and Forsberg N. E.. . 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. North Am. Food Anim. Pract. 23:105–149. doi: 10.1016/j.cvfa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Colombo, E. A., Cooke R. F., Brandão A. P., Wiegand J. B., Schubach K. M., Duff G. C., Gouvêa V. N., and Cappellozza B. I.. . 2020. Administering an appeasing substance to optimize performance and health responses in feedlot receiving cattle. J. Anim. Sci. 98:1–8. doi: 10.1093/jas/skaa339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo, E. A., Cooke R. F., Millican A. A., Schubach K. M., Scatolin G. N., Rett B., and Brandão A. P.. . 2019. Supplementing an immunomodulatory feed ingredient to improve thermoregulation and performance of finishing beef cattle under heat stress conditions. J. Anim. Sci. 97:4085–4092. doi: 10.1093/jas/skz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, R. F. 2014. Temperament and acclimation to human handling influence growth, health, and reproductive responses in Bos taurus and Bos indicus cattle. J. Anim. Sci. 92:5325–5333. doi: 10.2527/jas.2014-8017 [DOI] [PubMed] [Google Scholar]
- Cooke, R. F. 2017. Nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 33:1–11. doi: 10.15232/pas.2016-01573 [DOI] [Google Scholar]
- Cooke, R. F., and Arthington J. D.. . 2013b. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J. Anim. Physiol. Anim. Nutr. (Berl). 97:531–536. doi: 10.1111/j.1439-0396.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- Cooke, R. F., Bohnert D. W., Moriel P., Hess B. W., and Mills R. R.. . 2011. Effects of polyunsaturated fatty acid supplementation on forage digestibility, performance, and physiological responses of feeder cattle. J. Anim. Sci. 89:3677–3689. doi: 10.2527/jas.2010-3515 [DOI] [PubMed] [Google Scholar]
- Cooke, R. F., Cappellozza B. I., Guarnieri Filho T. A., and Bohnert D. W.. . 2013a. Effects of flunixin meglumine administration on acute-phase and performance responses of transported feeder cattle. J. Anim. Sci. 91:5500–5506. doi: 10.2527/jas.2013-6336 [DOI] [PubMed] [Google Scholar]
- Cooke, R. F., Carroll J. A., Dailey J., Cappellozza B. I., and Bohnert D. W.. . 2012. Bovine acute-phase response after different doses of corticotropin-releasing hormone challenge. J. Anim. Sci. 90:2337–2344. doi: 10.2527/jas.2011-4608. [DOI] [PubMed] [Google Scholar]
- Cooke, R. F., Guarnieri Filho T. A., Cappellozza B. I., and Bohnert D. W.. . 2013c. Rest stops during transport: impacts on performance and acute-phase protein responses of feeder cattle. J. Anim. Sci. 91:5448–5454. doi: 10.2527/jas.2013-6357 [DOI] [PubMed] [Google Scholar]
- Cooke, R. F., Millican A., Brandão A. P., Schumaher T. F., de Sousa O. A., Castro T., Farias R. S., and Cappellozza B. I.. . 2020. Short communication: administering an appeasing substance to Bos indicus-influenced beef cattle at weaning and feedlot entry. Animal 14:566–569. doi: 10.1017/S1751731119002490. [DOI] [PubMed] [Google Scholar]
- Crowell-Davis, S., and Houpt K. A.. . 1985. The ontogeny of flehmen in horses. Anim. Behav. 33:739–745. doi: 10.1016/S0003-3472(85)80005-1 [DOI] [Google Scholar]
- Del Bianco Benedeti, P., Valadares Filho S. C., Chizzotti M. L., Marcondes M. I., and de Sales Silva F. A.. . 2021. Development of equations to predict carcass weight, empty body gain, and retained energy of Zebu beef cattle. Animal 15:100028. doi: 10.1016/j.animal.2020.100028. [DOI] [PubMed] [Google Scholar]
- Duff, G. C., and Galyean M. L.. 2007. Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik, D. L., Sandler S., Welsh N., Juntti-Berggren L., and Berggren P. O.. . 1995. Interleukin-1 B-induced stimulation of insulin release in mouse pancreatic islets is related to diacylglycerol pro-duction and protein kinase C activation. Mol. Cell. Endocrinol. 111:159–165. doi: 10.1016/0303-7207(95)03561-K [DOI] [PubMed] [Google Scholar]
- FASS. 2010. Guide for the care and use of agricultural animals in agricultural research and teaching, 3rd ed. Savoy (IL): Federation of Animal Science Societies. [Google Scholar]
- Grus, W. E., Shi P., Zhang Y. P., and Zhang J.. . 2005. Dramatic variation of the vomeronasal pheromone receptor gene repertoire among five orders of placental and marsupial mammals. Proc. Natl. Acad. Sci. U. S. A. 102:5767–5772. doi: 10.1073/pnas.0501589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri Filho, T. A., Cooke R. F., Cappellozza B. I., Reis M. M., Marques R. S., and Bohnert D. W.. . 2014. Effects of meloxicam administration on physiological and performance responses of transported feeder cattle. J. Anim. Sci. 92:4137–4144. doi: 10.2527/jas.2014-7783. [DOI] [PubMed] [Google Scholar]
- Halpern, M., and Martínez-Marcos A.. . 2003. Structure and function of the vomeronasal system: an update. Prog. Neurobiol. 70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Hersom, M. J., Wettemann R. P., Krehbiel C. R., Horn G. W., and Keisler D. H.. . 2004. Effect of live weight gain of steers during winter grazing: III. Blood metabolites and hormones during feedlot finishing. J. Anim. Sci. 82:2059–2068. doi: 10.2527/2004.8272059x. [DOI] [PubMed] [Google Scholar]
- Hervet, C., Bouiller J., Guiadeur M., Michel L., Brun-Lafleur L., Aupiais A., Zhu J., Mounaix B., Meurens F., Rennois F., . et al. 2021. Appeasing pheromones against bovine respiratory complex and modulation of immune transcript expressions. Animals. 11:1545. doi: 10.3390/ani1106154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- Kekan, P. M., Ingole S. D., Sirsat S. D., Bharucha S. V., Kharde S. D., and Nagvekar A. S.. . 2017. The role of pheromones in farm animals – a review. Agric. Rev. 38:83–93. doi: 10.18805/ag.v38i02.7939 [DOI] [Google Scholar]
- Krehbiel, C. R. 2014. Invited review: applied nutrition of ruminants: fermentation and digestive physiology. Prof. Anim. Sci. 30:129–139. doi: 10.15232/S1080-7446(15)30100-5 [DOI] [Google Scholar]
- Loerch, S. C., and Fluharty F. L.. 1999. Physiologic changes and digestive capabilities of newly received feedlot cattle. J. Anim. Sci. 77:1113–1119. [DOI] [PubMed] [Google Scholar]
- Marques, R. S., Cooke R. F., Francisco C. L., and Bohnert D. W.. . 2012. Effects of twenty-four hour transport or twenty-four hour feed and water deprivation on physiologic and performance responses of feeder cattle. J. Anim. Sci. 90:5040–5046. doi: 10.2527/jas.2012-5425. [DOI] [PubMed] [Google Scholar]
- Marques, R. S., Bohnert D. W., de Sousa O. A., Brandão A. P., Schumaher T. F., Schubach K. M., Vilela M. P., Rett B., and Cooke R. F.. . 2019. Impact of 24-h feed, water, or feed and water deprivation on feed intake, metabolic, and inflammatory responses in beef heifers. J. Anim. Sci. 97:398–406. doi: 10.1093/jas/sky397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston, T. T., Lusby K. S., Wettemann R. P., and Purvis H. T.. 1995. Effects of feeding energy or protein supplements before or after calving on performance of spring-calving cows grazing native range. J. Anim. Sci. 73:657–664. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine . 2016. Nutrient requirements of beef cattle model. 8th rev. ed. National Academic Press, Washington, DC. [Google Scholar]
- Nussey, S. S., and Whitehead S. A.. . 2001. Endocrinology: an integrated approach. BIOS Scientific, Oxford, UK. [PubMed] [Google Scholar]
- Owens, F. N., Secrist D. S., Hill W. J., and Gill D. R.. . 1998. Acidosis in cattle: a review. J. Anim. Sci. 76:275–286. doi: 10.2527/1998.761275x. [DOI] [PubMed] [Google Scholar]
- Pageat, P. 2001. Appeasing Pheromones to decrease stress, anxiety, and aggressiveness. US Patent 6,169,113 B1. Jan. 2, 2001. [Google Scholar]
- Patra, M. K., Barman P., and Kumar H.. . 2012. Potential application of pheromones in reproduction of farm animals – a review. Agric. Rev. 33:82–86. [Google Scholar]
- Qiu, X., Arthington J. D., Riley D. G., Chase C. C. Jr., Philips W. A., Coleman S. W., and Olson T. A.. . 2007. Genetic effects on acute phase protein response to the stress of weaning and transportation in beef calves. J. Anim. Sci. 85:2367–2374. doi: 10.2527/jas.2006-843 [DOI] [PubMed] [Google Scholar]
- Rodrigues, M. C., Cooke R. F., Marques R. S., Cappellozza B. I., Arispe S. A., Keisler D. H., and Bohnert D. W.. . 2015. Effects of vaccination against respiratory pathogens on feed intake, metabolic, and inflammatory responses in beef heifers. J. Anim. Sci. 93:4443–4452. doi: 10.2527/jas.2015-9277. [DOI] [PubMed] [Google Scholar]
- Schenk, J. S., Workman J. J. Jr., and Westerhaus M. O.. . 2008. Application of NIR spectroscopy to agricultural products. In: Burns, D. A., and Ciurczak, E. W. (eds). Handbook of near-infrared analysis, 3rd ed. CRC Press, Boca Raton, FL; p. 347–386. [Google Scholar]
- Schubach, K. M., Cooke R. F., Brandão A. P., Lippolis K. D., Silva L. G. T., Marques R. S., and Bohnert D. W.. . 2017. Impacts of stocking density on development and puberty attainment of replacement beef heifers. Animal 11:2260–2267. doi: 10.1017/S1751731117001070. [DOI] [PubMed] [Google Scholar]
- Schubach, K. M., Cooke R. F., Daigle C. L., Brandão A. P., Rett B., Ferreira V. S. M., Scatolin G. N., Colombo E. A., Pohler K. G., and Cappellozza B. I.. . 2020. Administering an appeasing substance to beef calves at weaning to optimize productive and health responses during a 42-d preconditioning program. J. Anim. Sci. doi.org: 10.1093/jas/skaa269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger, M., Senn M., Altreuther G., Werling D., Sutter F., Kreuzer M., and Langhans W.. . 1999. Effect of a prolonged low-dose lipopolysaccharide infusion on feed intake and metabolism in heifers. J. Anim. Sci. 77:2523–2532. doi: 10.2527/1999.7792523x. [DOI] [PubMed] [Google Scholar]
- Vizcarra, J. A., Wettemann R. P., Spitzer J. C., and Morrison D. G.. . 1998. Body condition at parturition and postpartum weight gain influence luteal activity and concentrations of glucose, insulin, and nonesterified fatty acids in plasma of primiparous beef cows. J. Anim. Sci. 76:927–936. doi: 10.2527/1998.764927x. [DOI] [PubMed] [Google Scholar]
- Waggoner, J. W., Löest C. A., Turner J. L., Mathis C. P., and Hallford D. M.. . 2009. Effects of dietary protein and bacterial lipopolysaccharide infusion on nitrogen metabolism and hormonal responses of growing beef steers. J. Anim. Sci. 87:3656–3668. doi: 10.2527/jas.2009-2011. [DOI] [PubMed] [Google Scholar]
- Waldron, M. R., Nishida T., Nonnecke B. J., and Overton T. R.. . 2003. Effect of lipopolysaccharide on indices of peripheral and hepatic metabolism in lactating cows. J. Dairy Sci. 86:3447–3459. doi: 10.3168/jds.S0022-0302(03)73949-6. [DOI] [PubMed] [Google Scholar]
- Weiss, W. P., Conrad H. R., and St. Pierre N. R.. . 1992. A theoretically-based model for predicting total digestible nutrient values of forages and concentrates. Anim. Feed Sci. Technol. 39:95–110. doi: 10.1016/0377-8401(92)90034-4 [DOI] [Google Scholar]