Abstract
Background:
Tafamidis is approved in many countries for the treatment of transthyretin amyloid cardiomyopathy. This study reports data on the long-term efficacy of tafamidis from an ongoing long-term extension (LTE) to the pivotal ATTR-ACT (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial).
Methods:
Patients with transthyretin amyloid cardiomyopathy who completed ATTR-ACT could enroll in an LTE, continuing with the same tafamidis dose or, if previously treated with placebo, randomized (2:1) to tafamidis meglumine 80 or 20 mg. All patients in the LTE transitioned to tafamidis free acid 61 mg (bioequivalent to tafamidis meglumine 80 mg) following a protocol amendment. In this interim analysis, all-cause mortality was assessed in patients treated with tafamidis meglumine 80 mg in ATTR-ACT continuing in the LTE, compared with those receiving placebo in ATTR-ACT transitioning to tafamidis in the LTE.
Results:
Median follow-up was 58.5 months in the continuous tafamidis group (n=176) and 57.1 months in the placebo to tafamidis group (n=177). There were 79 (44.9%) deaths with continuous tafamidis and 111 (62.7%) with placebo to tafamidis (hazard ratio, 0.59 [95% CI, 0.44–0.79]; P<0.001). Mortality was also reduced in the continuous tafamidis (versus placebo to tafamidis) subgroups of: variant transthyretin amyloidosis (0.57 [0.33–0.99]; P=0.05) and wild-type transthyretin amyloidosis (0.61 [0.43–0.87]; P=0.006); and baseline New York Heart Association class I and II (0.56 [0.38–0.82]; P=0.003) and class III (0.65 [0.41–1.01]; P=0.06).
Conclusions:
In the LTE, patients initially treated with tafamidis in ATTR-ACT had substantially better survival than those first treated with placebo, highlighting the importance of early diagnosis and treatment in transthyretin amyloid cardiomyopathy.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01994889 and NCT02791230.
Keywords: amyloid, cardiomyopathies, heart failure, mutation, phenotype
What Is New?
In the ATTR-ACT (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial), tafamidis was shown to effectively reduce mortality and functional decline in patients with transthyretin amyloid cardiomyopathy over the 30 months of the trial.
This analysis of data from ATTR-ACT and the long-term extension study demonstrates the long-term reduction in mortality (median follow-up of ≈58 months) with the approved dose of tafamidis in patients with transthyretin amyloid cardiomyopathy.
What are the Clinical Implications?
This analysis highlights the importance of early diagnosis and treatment of patients with transthyretin amyloid cardiomyopathy, with patients who initiated tafamidis after ATTR-ACT having poorer outcomes than those on continuous tafamidis treatment.
However, patients who transitioned to treatment with tafamidis still appeared to show a reduction in mortality when compared with an extrapolation of survival with placebo.
These data suggest that tafamidis may provide a survival benefit even in patients with more advanced disease and that all patients with transthyretin amyloid cardiomyopathy could benefit from treatment with tafamidis.
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a fatal disease caused by the deposition of transthyretin amyloid fibrils in the heart.1,2 ATTR-CM can be hereditary (ATTRv), due to mutations in the transthyretin gene (TTR), or be an acquired disorder of older adults (wild-type; ATTRwt).2 Over 130 mutations in TTR have been identified. Many are associated with neuropathy (transthyretin amyloid polyneuropathy)3 whereas others present with a predominant cardiac phenotype.4,5 The most common cardiac variant is the Val122Ile, with a prevalence of 3% to 4% in individuals of Black African ancestry.5,6 Median survival from diagnosis in untreated patients with ATTR-CM is approximately 2.5 years for patients with Val122Ile ATTRv7–9 and 3.6 years for patients with ATTRwt.8,10
ATTRv and ATTRwt result from the dissociation of the transthyretin protein from its native tetramer into monomers that misfold and aggregate as amyloid fibrils.2 Tafamidis is a selective transthyretin stabilizer that prevents tetramer dissociation and amyloidogenesis.11,12 Tafamidis was shown to reduce mortality and functional decline in patients with ATTR-CM in the multicenter, double-blind, placebo-controlled, randomized ATTR-ACT (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial).13 Patients who completed ATTR-ACT were eligible for enrollment in a long-term, open-label extension study (long-term extension [LTE]) in which participants continued (or started) tafamidis for up to 60 months.
Patients enrolled in ATTR-ACT received placebo or tafamidis dosed at 80 or 20 mg once daily. In ATTR-ACT combined with the LTE, there was a significantly greater survival benefit with tafamidis 80 mg compared with 20 mg, and tafamidis 80 mg is the approved dose.14,15 The aim of this analysis was to examine the long-term efficacy of tafamidis 80 mg or equivalent in ATTR-ACT and the LTE.
Methods
Data Sharing
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Trial Design
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Data were pooled from ATTR-ACT and an interim analysis of the LTE as of March 20, 2020. ATTR-ACT (NCT01994889) is a phase III, multicenter, international, 3-arm, parallel design, placebo-controlled, double-blind, randomized study.16 Briefly, patients aged ≥18 and ≤90 years with ATTR-CM defined by the presence of either mutated TTR (ATTRv), or wild-type amyloid (ATTRwt) deposits and a medical history of heart failure with at least 1 prior hospitalization due to heart failure, or clinical signs and symptoms associated with heart failure, end-diastolic intraventricular septal wall thickness >12 mm demonstrated by echocardiography, and NT-proBNP (N-terminal pro-B-type natriuretic peptide) concentration ≥600 pg/mL were eligible to enroll. Patients were randomized to tafamidis 80 or 20 mg once daily or matching placebo in a 2:1:2 ratio for 30 months’ treatment. At randomization, patients were stratified by genotype (ATTRv and ATTRwt) and New York Heart Association (NYHA) baseline disease severity classification (NYHA class I and NYHA classes II and III combined).
Patients who completed 30 months’ treatment in ATTR-ACT could enroll in the ongoing LTE (NCT02791230) for up to 60 months. Patients treated with tafamidis continued on the same dose of tafamidis in the LTE (80 or 20 mg). Patients from the placebo group were randomized to receive either tafamidis 80 or 20 mg (in a 2:1 ratio; stratified by genotype [ATTRv and ATTRwt]). In both trials, a dose reduction could be requested if patients experienced adverse events. An actual dose reduction was only possible for patients randomized to 80 mg. In ATTR-ACT, patients receiving 80 mg could have their dose reduced to 40 mg. In the LTE, patients receiving 80 mg could have their dose reduced to 20 mg.
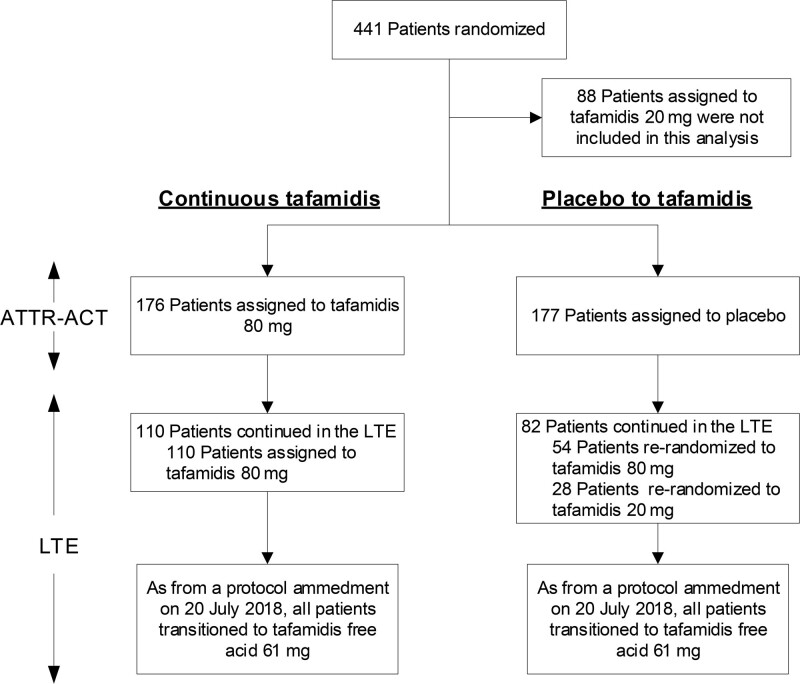
As of July 20, 2018, the LTE protocol was amended to transition all patients in the LTE to tafamidis free acid 61 mg (a new, single-capsule formulation bioequivalent to tafamidis meglumine 80 mg17). The transition to tafamidis free acid 61 mg followed the protocol amendment date, not a specified duration of treatment, with patients treated with tafamidis 80 or 20 mg (in ATTR-ACT and the LTE up to the protocol amendment) for a median of 39 months (Figure 1).
Figure 1.
Patients in ATTR-ACT (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial) and the long-term extension (LTE) in this analysis. Showing the treatment groups (continuous tafamidis and placebo to tafamidis) in ATTR-ACT and the LTE included in this analysis. After 30 months of treatment in ATTR-ACT, patients treated with tafamidis 80 mg could continue in the LTE on the same dose of tafamidis, and patients treated with placebo were randomized (2:1) to tafamidis 80 or 20 mg in the LTE. A total of 110 patients treated with tafamidis 80 mg continued in the LTE. A total of 82 placebo-treated patients continued in the LTE. Following a protocol amendment on July 20, 2018, all patients transitioned to tafamidis free acid 61 mg (bioequivalent to tafamidis 80 mg). ATTR-ACT also included a treatment arm for tafamidis 20 mg, which was not included in this analysis.
Analysis Populations
This analysis compares patients who were first treated with tafamidis 80 mg in ATTR-ACT continuing with tafamidis 80 mg then tafamidis free acid 61 mg in the LTE (continuous tafamidis) to patients first treated with placebo in ATTR-ACT who then received tafamidis in the LTE (placebo to tafamidis). The tafamidis 20 mg arm in ATTR-ACT was not included in this analysis. For both groups, time zero for survival analyses was the time of enrollment in ATTR-ACT. In addition, data were compared with a model-based extrapolation of survival in placebo-treated patients in ATTR-ACT beyond 30 months (extrapolated placebo).
Both studies were approved by the independent review boards or ethics committee at each participating site and were conducted in accordance with the provisions of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
Statistical Analyses
The primary efficacy outcome in the LTE was all-cause mortality, with heart transplant and implantation of a cardiac mechanical assist device treated as death. Differential all-cause mortality in the 2 groups was assessed by Cox proportional hazards model with treatment, genotype (ATTRwt and ATTRv), and NYHA baseline classification (NYHA classes I and II combined and NYHA class III) in the model. Mortality was also assessed by Cox proportional hazards model by genotype (ATTRv and ATTRwt) and by NYHA baseline classification (NYHA class I or II and NYHA class III).
The extrapolated placebo group was constructed from a gamma model based on patient-level data from placebo-treated patients in ATTR-ACT. Other models that provided good statistical fit were evaluated to extrapolate survival beyond 30 months as described previously.18 Briefly, the analysis was conducted based on technical support guidelines from the National Institute for Health and Care Excellence, with multiple models applied to systematically fit different candidate curves to the patient-level data from ATTR-ACT. The candidate curves were evaluated following the model evaluation procedure recommended in the guideline,19 with the gamma distribution selected here.18
Results
Patient Demographics
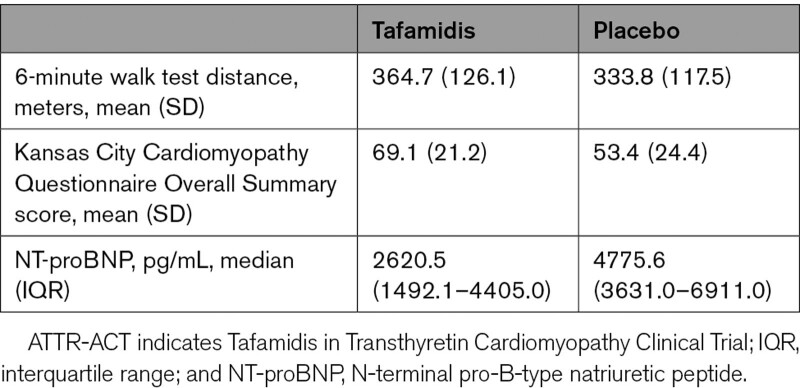
The ATTR-ACT cohort included 176 patients treated with tafamidis 80 mg and 177 treated with placebo. Baseline demographic and clinical characteristics of these groups have been published previously.15 Briefly, tafamidis 80 mg patients were 89.8% male and 77.3% White (compared with 88.7% male and 82.5% White in the placebo group). Tafamidis 80 mg patients (versus placebo) tended to be older (median 76.0 versus 74.0 years). Overall, the majority of patients were ATTRwt (75.9%), with 24.1% ATTRv, of which Val122Ile was the most common mutation.13,15 At month 30 in ATTR-ACT, before enrolling in the LTE, patients treated with placebo had more severe disease than those treated with tafamidis 80 mg (Table 1).
Table 1.
Patient Characteristics at Month 30 in ATTR-ACT
A total of 110 patients treated with tafamidis 80 mg continued in the LTE (and continued to receive tafamidis 80 mg). A total of 82 placebo-treated patients continued in the LTE, 54 of whom were randomized to tafamidis 80 mg and 28 to tafamidis 20 mg. As of the data cutoff for this analysis, the median follow-up was 58.5 months in the continuous tafamidis group and 57.1 months in the placebo to tafamidis group.
All-Cause Mortality in ATTR-ACT and the LTE
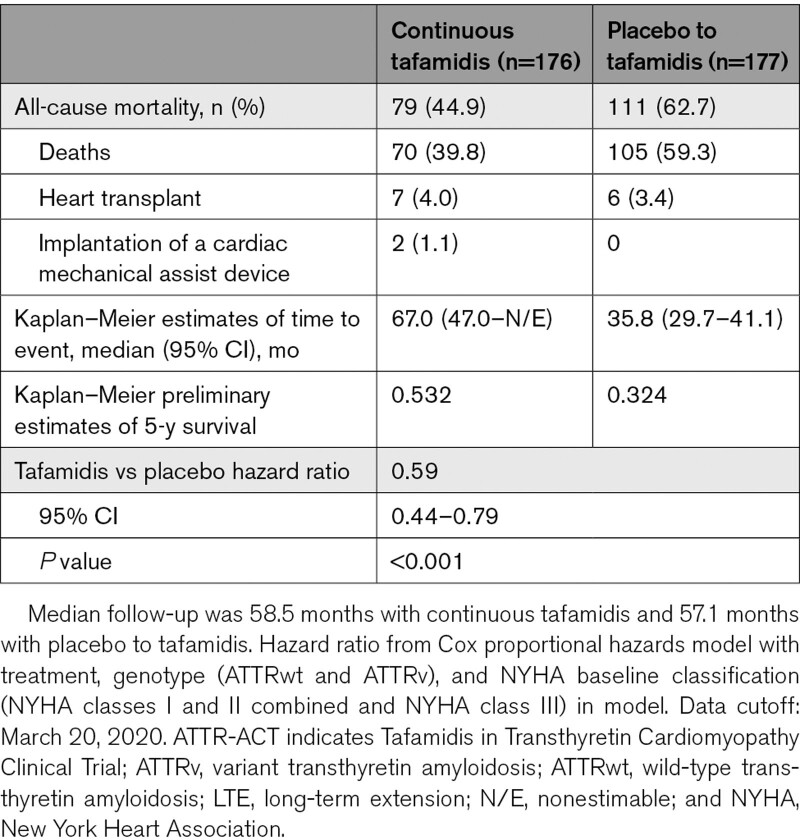
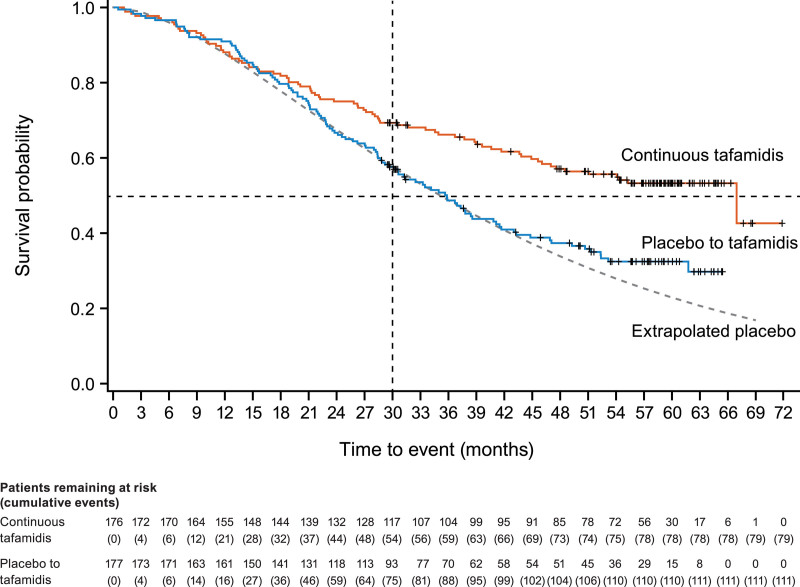
There was a significant 41% reduction in the risk of all-cause mortality in patients with continuous tafamidis treatment compared with those first receiving placebo (hazard ratio, 0.59 [95% CI, 0.44–0.79]; P<0.001; Table 2). Median (95% CI) survival was 35.8 (29.7–41.1) months in the placebo to tafamidis group. While median survival was 67.0 (47.0–N/E) months in the continuous tafamidis group the high degree of censoring before this time point suggests that the estimate is subject to change. The survival curves for the 2 cohorts diverged after ≈17 months (Figure 2). The preliminary 5-year survival rate was 53.2% with continuous tafamidis treatment and 32.4% in the placebo to tafamidis group.
Table 2.
All-Cause Mortality in ATTR-ACT and the LTE
Figure 2.
Kaplan–Meier plot of observed time to all-cause mortality in ATTR-ACT (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial) and the long-term extension (LTE) and compared with model-based extrapolation of time to all-cause mortality with placebo. Time to all-cause mortality (with heart transplant and implantation of a cardiac mechanical assist device treated as death) shown for all patients treated with tafamidis 80 mg in ATTR-ACT continuing with tafamidis 80 mg, then tafamidis free acid 61 mg in the LTE (continuous tafamidis) compared with patients treated with placebo in ATTR-ACT continuing with tafamidis (20, 80, or 61 mg) in the LTE (placebo to tafamidis). The extrapolated placebo curve (dotted line) is a model-based extrapolation of survival in placebo-treated patients in ATTR-ACT beyond 30 months.18 Data cutoff: March 20, 2020.
Based on post hoc analyses using Cox proportional hazards model, there was no significant interaction of treatment with NYHA baseline classification (P=0.73) and genotype (P=0.58).
Time to Death Compared With an Extrapolated Placebo Model
Median overall survival was 35.2 months in the extrapolated placebo group. The survival curve for the placebo to tafamidis group diverged from the extrapolated placebo curve after ≈44 months in favor of patients treated with tafamidis in the LTE (Figure 2).
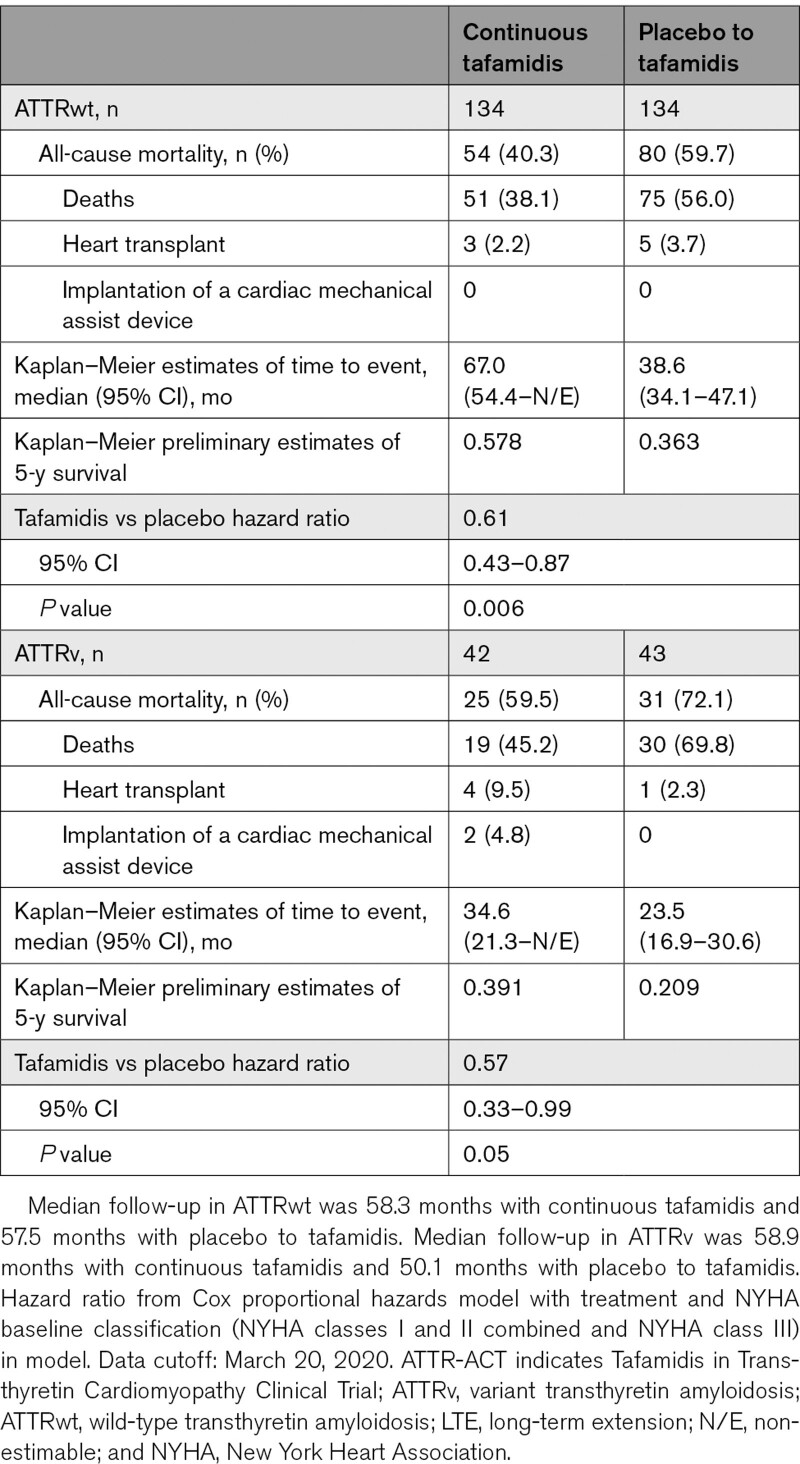
All-Cause Mortality in ATTR-ACT and the LTE by Genotype and NYHA Class
Mortality reductions were generally consistent across the subgroups. In patients with continuous tafamidis treatment, there was a 39% reduction in the risk of all-cause mortality in patients with ATTRwt (hazard ratio, 0.61 [95% CI, 0.43–0.87]; P=0.006), and a 43% reduction in patients with ATTRv (0.57 [0.33–0.99]; P=0.05), compared with the placebo to tafamidis group (Table 3). The preliminary 5-year survival rate in patients with ATTRwt was 57.8% with continuous tafamidis treatment and 36.3% in the placebo to tafamidis group. In patients with ATTRv, the preliminary 5-year survival rate was 39.1% with continuous tafamidis treatment and 20.9% in the placebo to tafamidis group.
Table 3.
All-Cause Mortality in ATTR-ACT and the LTE by Genotype
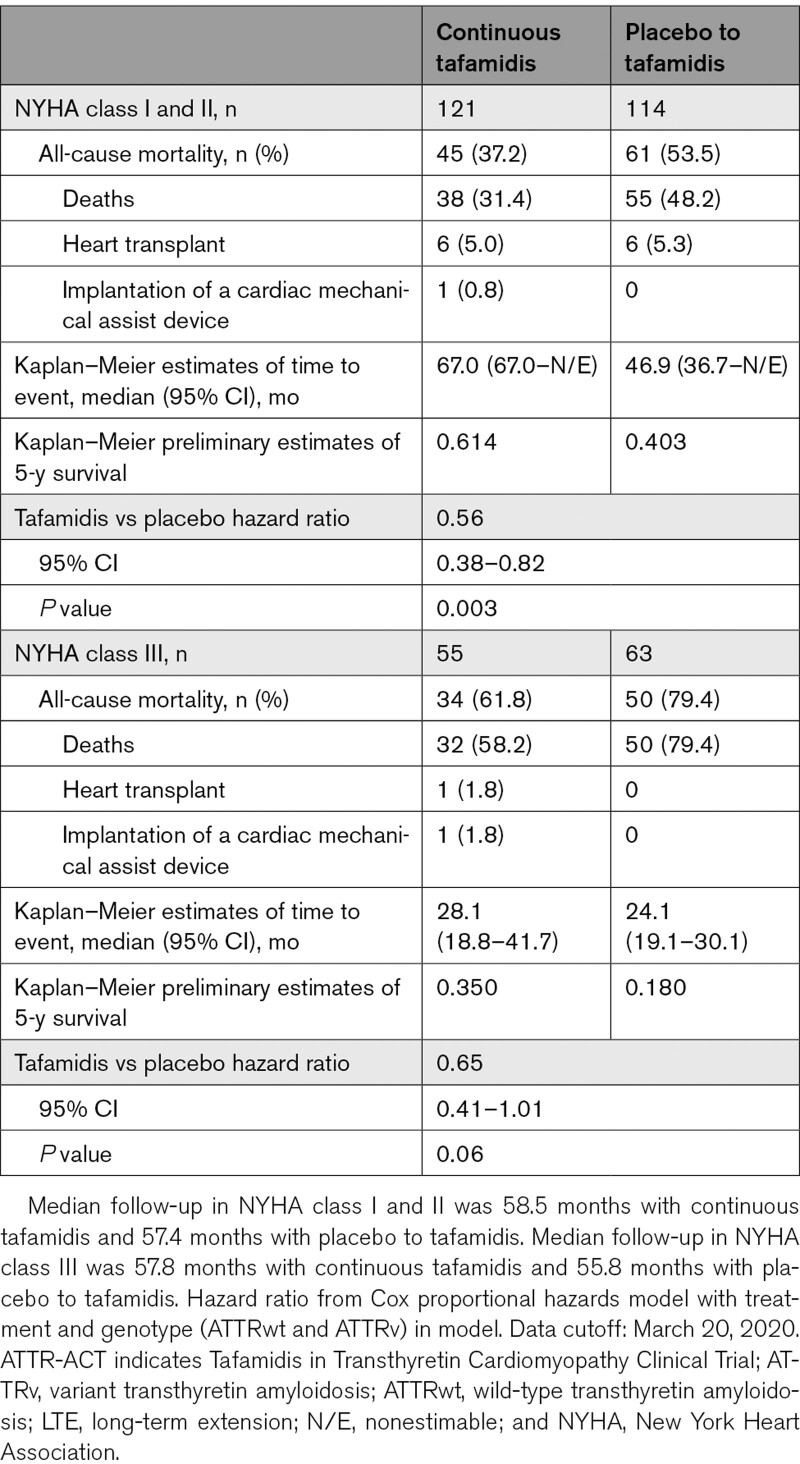
Similarly, there was a 44% reduction in the risk of all-cause mortality in patients with baseline NYHA class I or II (0.56 [0.38–0.82]; P=0.003), and a 35% reduction in patients with baseline NYHA class III (0.65 [0.41–1.01]; P=0.06) in the continuous tafamidis group compared with the placebo to tafamidis group (Table 4). The preliminary 5-year survival rate in patients with baseline NYHA class I or II was 61.4% with continuous tafamidis treatment and 40.3% in the placebo to tafamidis group. In patients with baseline NYHA class III, the preliminary 5-year survival rate was 35.0% with continuous tafamidis treatment and 18.0% in the placebo to tafamidis group.
Table 4.
All-Cause Mortality in ATTR-ACT and the LTE by NYHA Class
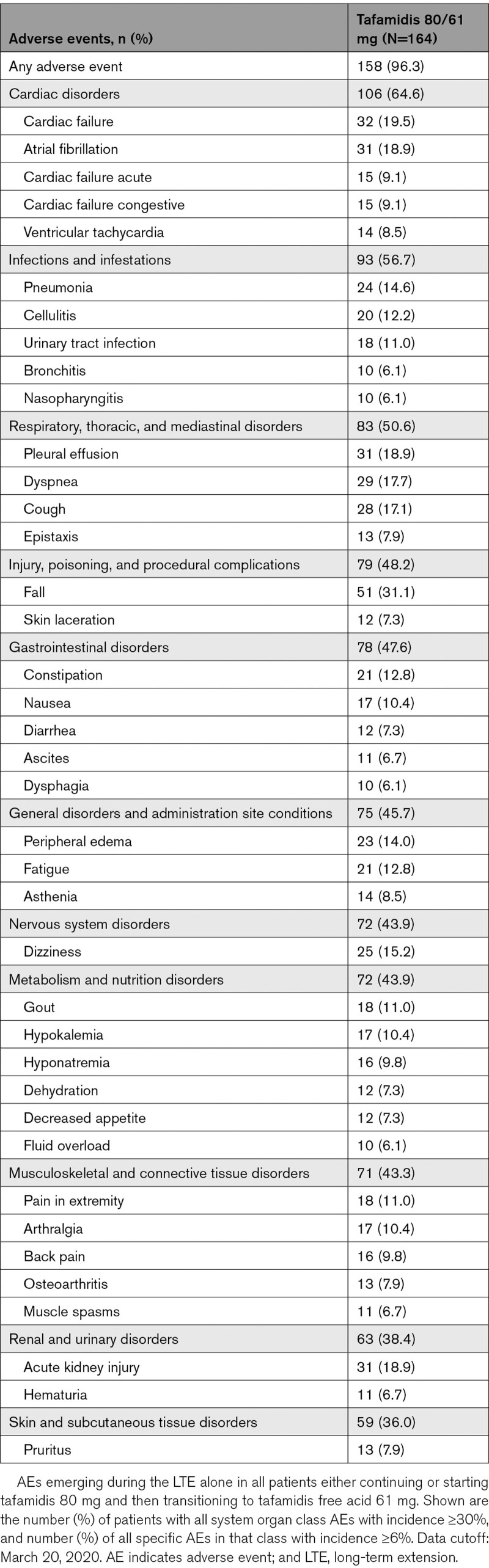
Safety in the LTE
Safety outcomes in ATTR-ACT have been published previously with the safety profiles of tafamidis 80 mg, tafamidis 20 mg, and placebo shown to be similar.13,15 In the LTE alone, there were 164 patients treated with tafamidis 80 mg transitioning to tafamidis free acid 61 mg. Incidence and types of adverse events (Table 5) were similar, or lower, than that with pooled tafamidis (80 and 20 mg) or placebo in ATTR-ACT.13 No new safety concerns emerged in patients treated with tafamidis 80 mg or tafamidis free acid 61 mg in the LTE.
Table 5.
Treatment-Emergent Adverse Events (All Causalities) in the LTE Alone
Discussion
ATTR-ACT, the largest randomized controlled trial in ATTR-CM, demonstrated that tafamidis improved survival and stabilized functional capacity, health status, and quality of life in patients with ATTR-CM.13 The LTE is an open-label trial without an equivalent control group, but this analysis shows that, in comparison to patients who first received placebo in ATTR-ACT, the mortality reduction observed in patients initially treated with tafamidis is maintained. This trend was apparent in patients with ATTRwt and patients with ATTRv, and in patients with less and more severe disease (by NYHA class) at baseline.
While the reduction in mortality was similar in patients with ATTRwt and patients with ATTRv (≈40% in each), there was a greater reduction in patients with NYHA class I or II (44%) than NYHA class III (35%). Together with results from ATTR-ACT,13,20 these data support the use of tafamidis in all patients with ATTR-CM but emphasize the importance of early diagnosis and treatment.
By virtue of their treatment with active drug in ATTR-ACT, patients in the continuous tafamidis group were generally healthier than those who entered the LTE having received placebo. Thus, the subsequently poorer outcomes in the latter are not surprising; however, given the demonstrable benefit of tafamidis therapy, it is reasonable to conjecture that survival in this group would have been even worse without treatment. This hypothesis is supported by the comparison with a gamma model based on placebo-treated patient data in ATTR-ACT, which extrapolated survival beyond 30 months. Survival in the placebo to tafamidis cohort and the model extrapolated placebo curve diverged after ≈44 months in favor of the LTE treatment arm. The timing of this divergence, ≈14 months after the start of the LTE, is similar to that seen in ATTR-ACT where the tafamidis and placebo survival curves diverged ≈17 months after the start of therapy.
There are limitations in extrapolating survival using a model-based approach, where alternate models could result in different estimates of survival. Nevertheless, the estimated median survival for the extrapolated placebo curve (35.2 months) was similar to the observed median survival in patients transitioning from placebo to active drug in the LTE (35.8 months), which supports the accuracy of the model.
In ATTR-ACT, tafamidis had a safety profile comparable to placebo, with the majority of adverse events being of mild or moderate severity and discontinuations due to adverse events less common with tafamidis than with placebo.13 There was also no difference in the safety profile of tafamidis 80 and 20 mg.13,15 No new safety concerns emerged in the LTE.
Conclusions
Patients initially randomized to placebo in ATTR-ACT had poorer survival in the LTE than those randomized to tafamidis from the start of ATTR-ACT, highlighting the importance of early diagnosis and treatment. Nevertheless, survival appeared to improve in patients following transition to treatment with tafamidis, suggesting that initiation of tafamidis in patients with more advanced disease may still provide a survival benefit.
Article Information
Acknowledgments
The authors thank Jeffrey H. Schwartz (Pfizer, now retired) for his contribution to the studies and the statistical analysis plans.
Sources of Funding
This study was sponsored by Pfizer. Medical writing support was provided by Joshua Fink, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Disclosures
Dr Elliott has received consultancy fees from Pfizer and Alnylam and educational grants from Pfizer. Dr Drachman has received consultancy fees from Alnylam and Eidos. Dr Gottlieb has received consultancy fees from Pfizer. Dr Hoffman has received consultancy fees from Celgene/BMS. Dr Lenihan has received consultancy fees from Prothena and Eidos. B. Ebede, B. Gundapaneni, B. Li, and Dr Sultan are full-time employees of Pfizer and hold stock and/or stock options. Dr Shah has received research grants from the National Institutes of Health (R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423), the American Heart Association (No. 16SFRN28780016), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer and consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Axon Therapeutics, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardiora, CVRx, Cytokinetics, Eisai, GlaxoSmithKline, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Regeneron, Sanofi, Shifamed, Tenax, and United Therapeutics. Dr Hummel reports no conflicts.
Nonstandard Abbreviations and Acronyms
- ATTR-ACT
- Tafamidis in Transthyretin Cardiomyopathy Clinical Trial
- ATTR-CM
- transthyretin amyloid cardiomyopathy
- ATTRv
- variant transthyretin amyloidosis
- ATTRwt
- wild-type transthyretin amyloidosis
- LTE
- long-term extension
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- NYHA
- New York Heart Association
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Brian M. Drachman, Email: drachman@pennmedicine.upenn.edu.
Stephen S. Gottlieb, Email: sgottlie@medicine.umaryland.edu.
James E. Hoffman, Email: j.hoffman4@med.miami.edu.
Scott L. Hummel, Email: scothumm@med.umich.edu.
Daniel J. Lenihan, Email: cardio-oncologydoc@protonmail.com.
Ben Ebede, Email: ben.ebede@pfizer.com.
Balarama Gundapaneni, Email: Balarama.Gundapaneni@pfizer.com.
Benjamin Li, Email: Benjamin.Li@pfizer.com.
Marla B. Sultan, Email: marla.b.sultan@pfizer.com.
Sanjiv J. Shah, Email: sanjiv.shah@northwestern.edu.
References
- 1.Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M, Ciliberti P, Biagini E, Salvi F, Branzi A. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010; 7:398–408. doi: 10.1038/nrcardio.2010.67 [DOI] [PubMed] [Google Scholar]
- 2.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin Amyloid Cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019; 73:2872–2891. doi: 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol. 2011; 10:1086–1097. doi: 10.1016/S1474-4422(11)70246-0 [DOI] [PubMed] [Google Scholar]
- 4.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015; 22:171–174. doi: 10.3109/13506129.2015.1051219 [DOI] [PubMed] [Google Scholar]
- 5.Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, Mosley TH, Butler KR, Boerwinkle E, Solomon SD. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015; 372:21–29. doi: 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah KB, Mankad AK, Castano A, Akinboboye OO, Duncan PB, Fergus IV, Maurer MS. Transthyretin Cardiac Amyloidosis in Black Americans. Circ Heart Fail. 2016; 9:e002558. doi: 10.1161/CIRCHEARTFAILURE.115.002558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez-Lopez E, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018; 39:2799–2806. doi: 10.1093/eurheartj/ehx589 [DOI] [PubMed] [Google Scholar]
- 8.Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY, Falk RH, Cheung KN, Patel AR, Pano A, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J. 2012; 164:222–228.e1. doi: 10.1016/j.ahj.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 9.Givens RC, Russo C, Green P, Maurer MS. Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medical center. Aging Health. 2013; 9:229–235. doi: 10.2217/ahe.13.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild-type Transthyretin Cardiac Amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016; 68:1014–1020. doi: 10.1016/j.jacc.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 11.Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, Packman J, Powers ET, Wiseman RL, Foss TR, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. 2012; 109:9629–9634. doi: 10.1073/pnas.1121005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waddington Cruz M, Benson MD. A review of Tafamidis for the treatment of Transthyretin-related amyloidosis. Neurol Ther. 2015; 4:61–79. doi: 10.1007/s40120-015-0031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, et al. ; ATTR-ACT Study Investigators. Tafamidis treatment for patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018; 379:1007–1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 14.VYNDAQEL and VYNDAMAX Highlights of Prescribing Information. https://www.fda.gov/media/126283/download. Accessed January 21, 2021.
- 15.Damy T, Garcia-Pavia P, Hanna M, Judge DP, Merlini G, Gundapaneni B, Patterson TA, Riley S, Schwartz JH, Sultan MB, et al. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. 2021; 23:277–285. doi: 10.1002/ejhf.2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer MS, Elliott P, Merlini G, Shah SJ, Cruz MW, Flynn A, Gundapaneni B, Hahn C, Riley S, Schwartz J, et al. ; ATTR-ACT Study Investigators. Design and rationale of the phase 3 ATTR-ACT Clinical Trial (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial). Circ Heart Fail. 2017; 10:e003815. doi: 10.1161/CIRCHEARTFAILURE.116.003815 [DOI] [PubMed] [Google Scholar]
- 17.Lockwood PA, Le VH, O’Gorman MT, Patterson TA, Sultan MB, Tankisheva E, Wang Q, Riley S. The bioequivalence of Tafamidis 61-mg free acid capsules and Tafamidis Meglumine 4 × 20-mg capsules in healthy volunteers. Clin Pharmacol Drug Dev. 2020; 9:849–854. doi: 10.1002/cpdd.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Alvir J, Stewart M. Extrapolation of survival benefits in patients with Transthyretin Amyloid Cardiomyopathy receiving Tafamidis: analysis of the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial. Cardiol Ther. 2020; 9:535–540. doi: 10.1007/s40119-020-00179-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latimer N. NICE DSU Technical Support Document 14: Survival analysis for Economic Evaluations Alongside Clinical Trials - Extrapolation With Patient-Level Data. 2013. Accessed June 12, 2019. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf [PubMed]
- 20.Rapezzi C, Elliott P, Damy T, Nativi-Nicolau J, Berk JL, Velazquez EJ, Boman K, Gundapaneni B, Patterson TA, Schwartz JH, et al. Efficacy of Tafamidis in patients with hereditary and wild-type Transthyretin Amyloid Cardiomyopathy: further analyses from ATTR-ACT. JACC Heart Fail. 2021; 9:115–123. doi: 10.1016/j.jchf.2020.09.011 [DOI] [PubMed] [Google Scholar]