Supplemental Digital Content is available in the text.
Keywords: extracorporeal membrane oxygenation; heart-assisted devices; length of stay; mortality; shock, cardiogenic; transplant
Abstract
Background:
There has been increasing use of extracorporeal membrane oxygenation (ECMO) as bridge to heart transplant (orthotopic heart transplant [OHT]) or left ventricular assist device (LVAD) over the last decade. We aimed to provide insights on the population, outcomes, and predictors for the selection of each therapy.
Methods:
Using the Extracorporeal Life Support Organization Registry between 2010 and 2019, we compared in-hospital mortality and length of stay, predictors of OHT versus LVAD, and predictors of in-hospital mortality for patients with cardiogenic shock that were bridged with ECMO to OHT or LVAD. One hundred sixty-seven patients underwent LVAD versus 234 patients who underwent OHT.
Results:
The overall use of ECMO has increased from 1.7% in 2010 to 22.2% in 2019. Mortality was similar between groups (LVAD: 28.7% versus OHT: 29.1%) while length of stay was longer for OHT (LVAD: 49.6 versus OHT: 59.5 days, P=0.05). Factors associated with OHT included prior transplant (odds ratio [OR]=31.26 [CI, 3.84–780.5]), use of a temporary pacemaker (OR=6.5 [CI, 1.39–50.15]), and increased use of inotropes on ECMO (OR=3.77 [CI, 1.39–11.07]), whereas LVAD use was associated with weight (OR=0.98 [CI, 0.97–0.99]), cardiogenic shock presentation (OR=0.40 [CI, 0.21–0.78]), previous LVAD (OR=0.01 [CI, 0.0001–0.22]), respiratory failure (OR=0.28 [CI, 0.11–0.70]), and milrinone infusion (OR=0.32 [CI, 0.15–0.67]). Older age (OR=1.07 [CI, 1.02–1.12]), cannulation bleeding (OR=26.1 [CI, 4.32–221.3]), and surgical bleeding (OR=6.7 [CI, 1.26–39.9]) in patients receiving LVAD and respiratory failure (OR=5 [CI, 1.17–23.1]) and continuous renal replacement therapy (OR=3.82 [CI, 1.28–11.9]) in patients receiving OHT were associated with increased mortality.
Conclusions:
ECMO use as a bridge to advanced therapies has increased over time, with more patients undergoing LVAD than OHT. Mortality was equal between the 2 groups while length of stay was longer for OHT.
What Is New?
There is increased use of extracorporeal membrane oxygenation as bridged to heart replacement therapies in recent years.
Mortality appears equal between the 2 groups around 30% and different predictors are associated for each subgroup.
Length of stay was shorter for left ventricular assist device recipients despite being sicker before left ventricular assist device implantation.
What are the Clinical Implications?
Heart replacement therapies from extracorporeal membrane oxygenation support are feasible and have recently increased. Outcomes are comparable between the 2 strategies.
Careful selection of these patients is needed as overall mortality still remains high despite advances in cardiogenic shock management.
Prospective studies are needed; however, it is unlikely to occur due to the severity of the patient’s disease.
Despite the historical use of extracorporeal membrane oxygenation (ECMO) for refractory respiratory failure, its use for cardiac indications, including multifactorial cardiogenic shock (CS), cardiac arrest, and postcardiotomy shock, has become increasingly common in the last decade.1 Recent data from the Extracorporeal Life Support Organization (ELSO) demonstrate that the use of ECMO for cardiac indications has rapidly increased by 1180% over the past 15 years.1 In addition, the number of ECMO centers has seen a parallel increase from 135 in 2007 to 463 centers in 2019 representing a 242% increase.2 Yet, it remains a complex, expensive therapy and is associated with high rates of detrimental complications.3 In addition, it requires significant technical expertise and personnel infrastructure for its successful deployment and maintenance. This is important since ECMO does not provide etiologic treatment for the underlying condition; rather, it provides a bridge to patient recovery or to a more definite solution. This notion of a bridge therapy is highlighted in a recent scientific Expert Panel.1
Previous studies have shown an increased risk of in-hospital and long-term mortality in patients that are bridged to orthotopic heart transplant (OHT) with ECMO (28.8% at 1 year) or percutaneous temporary left ventricular assist devices (LVADs; 20.1% at 1 year).4,5 Given existing concerns related to resource utilization and timing in an ECMO to transplant strategy, the alternative ECMO to LVAD strategy has arisen as a plausible option. There is a scarcity of descriptive data for this critically ill cohort of patients despite the rise in ECMO as an option for profound CS. Thus, the aim of this study is to describe the demographics, comorbidities, initial presentation (CS, acute coronary syndrome [ACS], cardiac arrest, etc), hemodynamics, ECMO-related complications, and outcomes for patients with CS treated with ECMO that ultimately go onto OHT versus LVAD.
Methods
Data Source
ELSO is an organization committed to the advancement and optimal use of extracorporeal life support therapies across international member centers. ELSO maintains a patient registry with clinical and outcome data for the purposes of quality improvement and research, available to member centers and contains data for >125 000 patients worldwide.6,7 A map of the participating centers for ELSO can be found at https://www.elso.org/Membership/CenterMap.aspx. Institutional review board approval was waived due to the nature of the study. The data are collected by a standardized collection form that includes demographic and clinical information, hemodynamics, diagnoses by means of International Classification of Diseases, Ninth and Tenth Revisions (ICD-9 and ICD-10), procedures coded by Current Procedures Terminology codes, ECMO indications, ECMO-related information, pre-ECMO medical and mechanical support, ECMO-related complications, duration of hospital stay, and in-hospital mortality. The authors declare that all supporting data are available within the article.
Patient Population
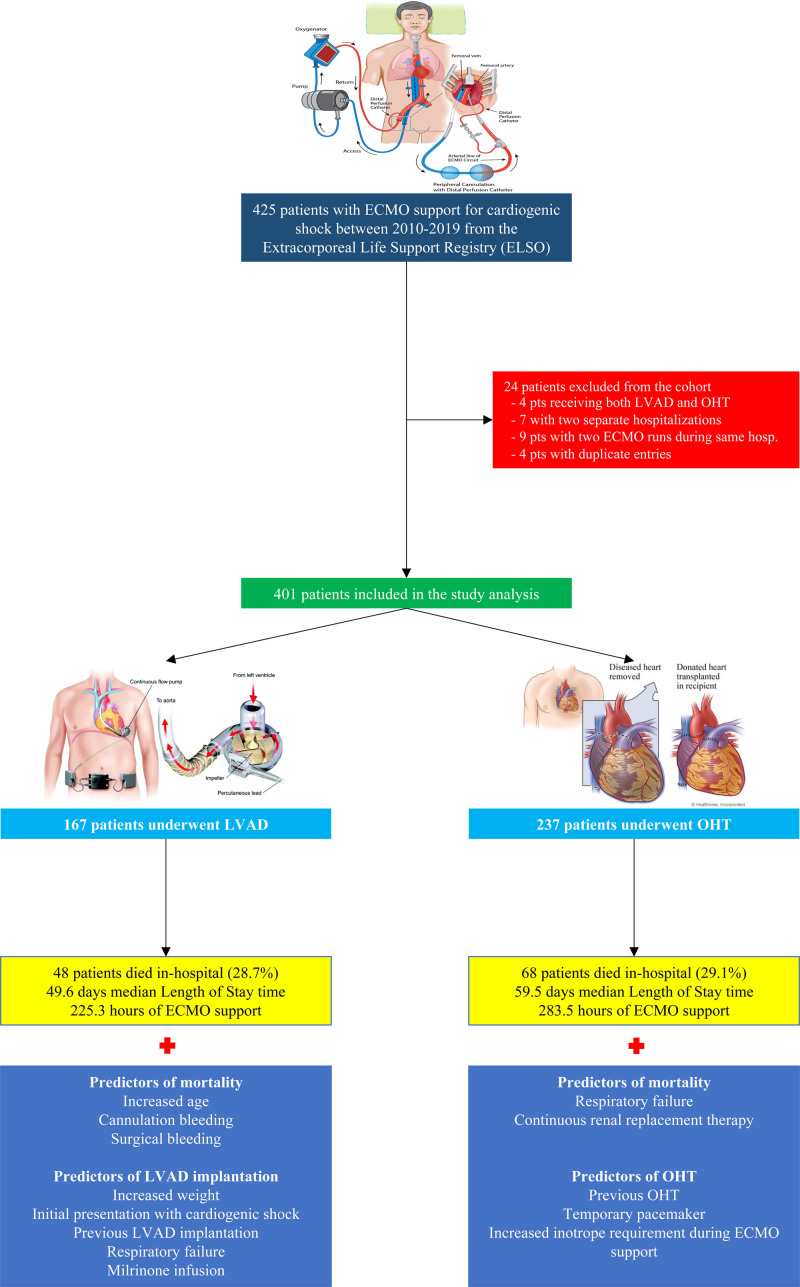
For this study, we queried the ELSO Registry between 2010 and 2019 to identify adult patients (≥18 years of age) with CS treated with ECMO as a bridge to OHT or durable LVAD (Figure 1). Four patients that received both OHT and LVAD during the same admission were excluded from the analysis. For patients with 2 ECMO runs in 2 separate hospitalizations (7 unique IDs in the data set) during the study period, we included only the hospitalization leading to advances therapies. Nine patients had 2 runs of ECMO during the same hospitalization (either before, during, or after LVAD/OHT) for which we included only one run was included. Finally, 4 for patients with duplicate entries only one entry was used.
Figure 1.
Patients from the Extracorporeal Life Support Organization (ELSO) registry undergoing orthotopic heart transplant (OHT) vs left ventricular assist device (LVAD) implantation: study flow, outcomes, and predictors. ECMO indicates extracorporeal membrane oxygenation.
Outcomes and Statistical Analysis
Descriptive statistics were provided for all variables: mean and SD for continuous variables, frequency, and percentage for categorical variables. We compared demographics, comorbid conditions, hemodynamics, pre-ECMO support, ECMO-related information, ECMO complications, in-hospital mortality, and length of stay (LOS) between the 2 groups using Student t tests (continuous variables) and Pearson χ2 tests (categorical variables). Hemodynamic parameters were available for a fraction of patients.
We performed multivariable logistic regression to find predictors of undergoing OHT (versus LVAD) with predictors identified with P<0.05 from univariate logistic regression. We also performed logistic regression analysis to identify predictors of survival within each isolated group (LVAD and OHT). To address missing values, we used imputation with 5 nearest neighbors and excluded predictor variables with >20% missing from the analyses. Sensitivity analysis was conducted to examine the effect of imputation using unimputed data to ensure the direction and significance of the findings were unchanged. As a secondary analysis, we limited to patients with no missing data for the variables cardiac index, mean blood pressure, mean pulmonary artery pressure, and pH and performed a penalized regression (Least Absolute Shrinkage and Selection Operator) to select features that had a significant relationship with OHT versus LVAD.8 We then performed logistic regression analysis using the selected features to interpret the predictors of getting OHT versus LVAD within this subgroup. All statistical tests are 2 sided and at a significance level of 0.05. All analyses were conducted with R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
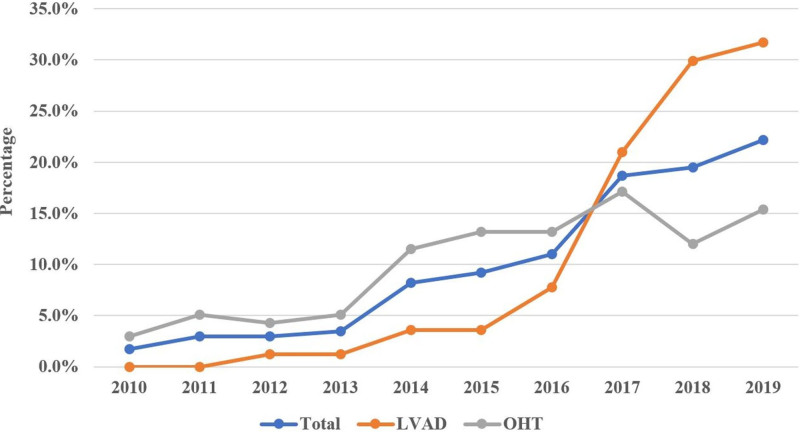
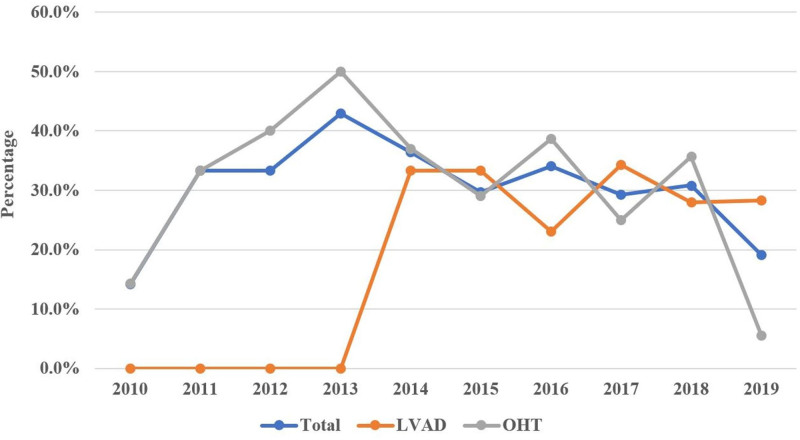
The initial registry query yielded 425 patients with a diagnosis of CS bridged with ECMO to either OHT or LVAD between the years 2010 and 2019. After exclusions, our final data set included 401 patients. Among these, 167 patients underwent LVAD implantation, and 234 underwent OHT during their hospitalization (Figure 1). Among the total number of ECMOs in our study, most cases occurred between the years 2016 to 2019. Of the 401 patients, only 1.7% had received ECMO support in 2010 with that percentage increasing to 22.2% in 2019 (P<0.01; Figure 2). Similar trends have been observed for each strategy individually with increased utilization as bridging strategy towards OHT (from 3% to 15.4%) and LVAD (from 0% to 31.7%) groups over the last decade. While the percentage of OHTs occurring from 2010 until 2016 was higher than LVADs, there was an abrupt increase in the percentage of LVADs implants between 2017 and 2019 surpassing the numbers of OHTs performed.
Figure 2.
Yearly distribution (%) of extracorporeal membrane oxygenation (ECMO) use (1) in all ECMO patients and (2) by strategy group (left ventricular assist device [LVAD] vs orthotopic heart transplant [OHT]). The diagram demonstrates that the majority of ECMO cases in the cohort occurred in recent years.
Demographics and Comorbidities at Baseline
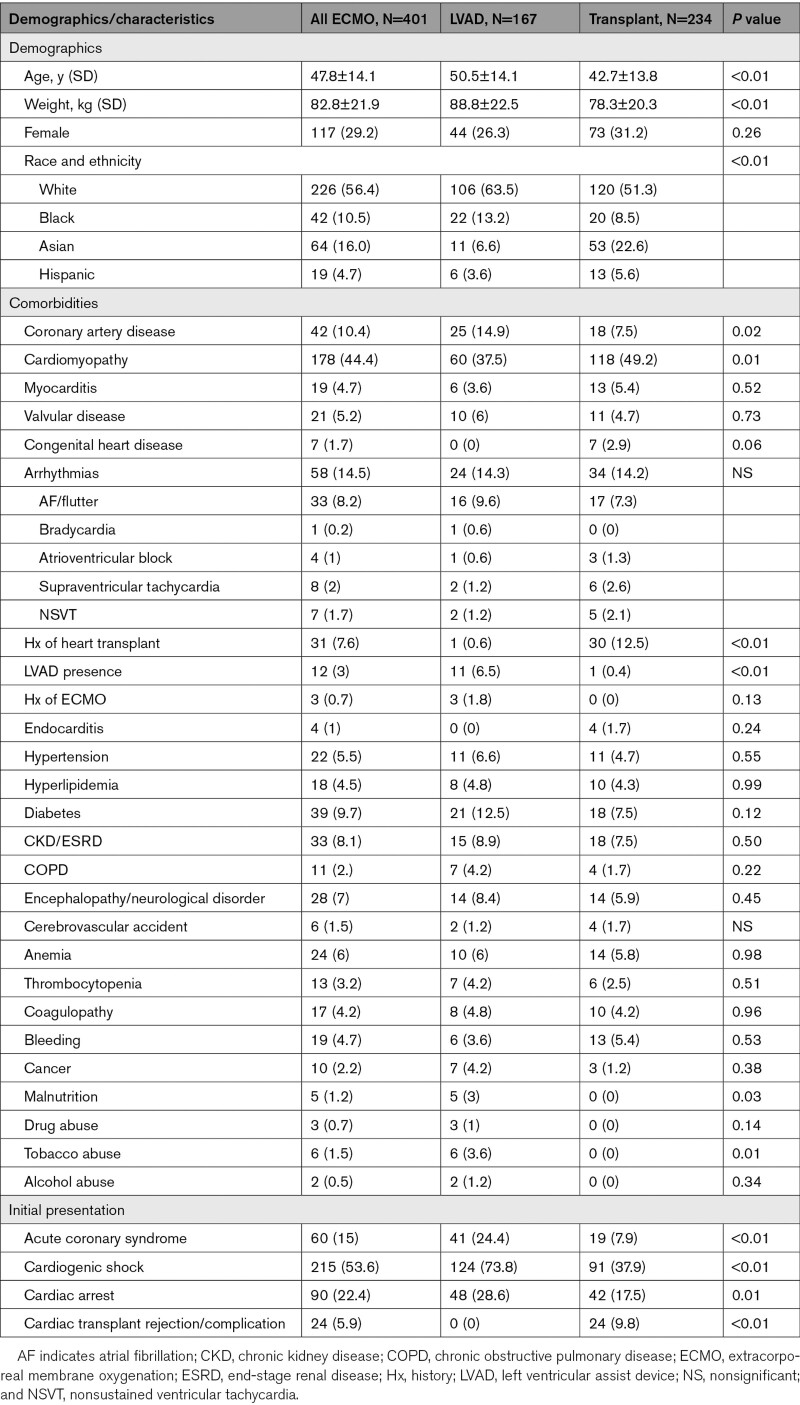
The mean cohort age was 47.8±14.1 years, mean weight was 82.8±21.9 kg, 29.2% were women, and 56.4% were Whites. Overall, 10.4% had prior history of CAD, 44.4% history of previous cardiomyopathy diagnosis, 4.7% with myocarditis, 3% with LVAD presence, and 7.6% history of prior heart transplant. The full set of demographic differences are presented in Table 1. Compared with OHT patients, those who underwent LVAD implantation were more likely to be older (50.5 versus 42.7 years; P<0.01) and have increased weight (88.8 versus 78.3 kg; P<0.01). In addition, they were more likely to have a history of previous LVAD implantation (6.5% versus 0.4%; P<0.01), coronary artery disease (14.9% versus 7.5%; P=0.02), tobacco abuse (3.6% versus 0%; P=0.01), and malnutrition (3% versus 0%; P=0.03). In contrast, patients that received OHT were more likely to have a history of previous cardiomyopathy diagnosis (49.2% versus 37.5%, P=0.01) and history of cardiac transplantation (12.5% versus 0.6%; P<0.01).
Table 1.
Baseline Demographic and Clinical Characteristics
Initial Presentation and Hemodynamics
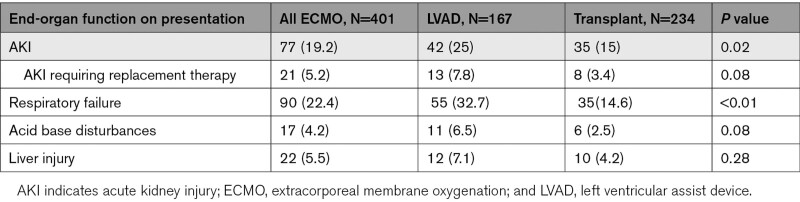
Overall, 53.6% of the cohort presented with CS, 22.4% with cardiac arrest, 15% with ACS, 19.2% with acute kidney injury, and 5.9% with cardiac transplant complications (Tables 1 and 2). Patients that underwent LVAD implantation were more likely to present with ACS (24.4% versus 7.9%, P<0.01), acute kidney injury (25% versus 15%; P=0.02), cardiac arrest (28.6% versus 17.5%; P=0.01), cardiogenic (73.8% versus 37.9%; P<0.01) or septic shock (4.8% versus 0.4%; P=0.01), and respiratory failure (32.7% versus 14.6%; P<0.01), whereas patients that received OHT were more likely to present with cardiac transplant rejection/complications (9.8% versus 0%; P<0.01).
Table 2.
End-Organ Function on Presentation
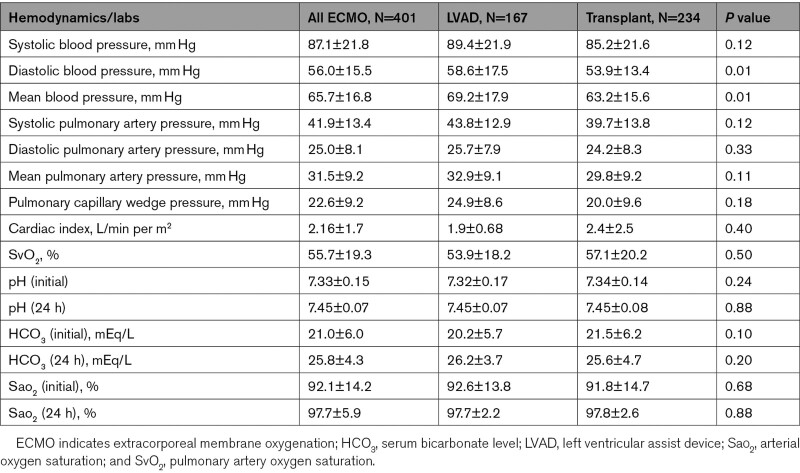
With regards to hemodynamic parameters on presentation, the mean systolic pulmonary arterial pressure was 41.9±13.4 mm Hg, the mean pulmonary artery pressure was 31.5±9.2 mm Hg, the pulmonary artery wedge pressure was 22.6±9.2 mm Hg, and cardiac index was 2.16±1.7 L/min per m2. Hemodynamic parameters did not differ significantly between the 2 groups with the exception of lower diastolic blood pressure (53.9 versus 58.6 mm Hg; P<0.05) and mean arterial pressure (63.2 versus 69.2 mm Hg; P<0.01) which were lower in the transplant group. The hemodynamic profile differences on presentation (before ECMO) are shown in Table 3.
Table 3.
Hemodynamics, Cardiac Indices, and Related Labs
Pre-ECMO Support, ECMO Specifications, and Complications
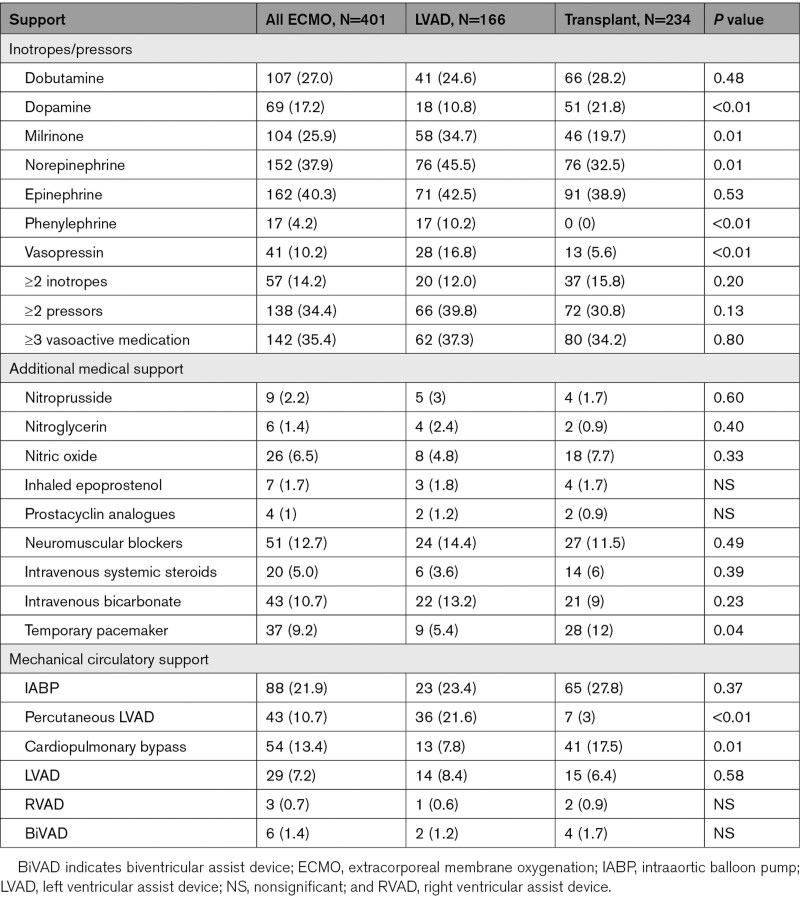
Pre-ECMO medical and mechanical support is illustrated in Table 4. Patients who underwent LVAD implantation were more likely to require to be supported with milrinone (34.7% versus 19.7%; P=0.01), norepinephrine (45.5% versus 32.5%; P=0.01), vasopressin (16.8% versus 5.6%; P<0.01), and percutaneous LVAD (21.6% versus 3%; P<0.01). Conversely, patients that received OHT were more likely to be supported with dopamine infusion (21.8% versus 10.8%; P<0.01) and receive a temporary pacemaker (12% versus 5.4%; P=0.04) compared with their counterparts. There were no differences between when we compared the groups ≥2 pressors infusions (P=0.13), ≥2 inotropes (P=0.20), and ≥3 vasoactive medications (P=0.80).
Table 4.
Pre-ECMO Support
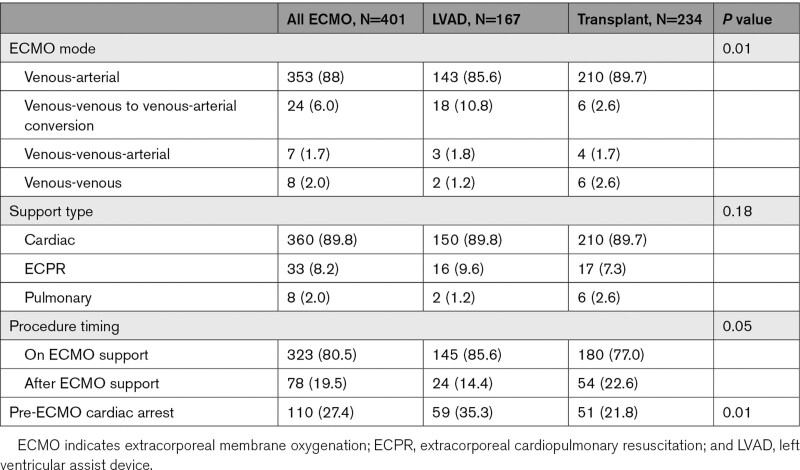
Veno-arterial ECMO was the most common configuration used (88%) in the cohort, whereas conversion from veno-venous to veno-arterial configuration was the second most frequent (6%). Importantly, 27.4% of patients had cardiac arrest before deployment (whether on presentation or in-hospital), more commonly occurring in the LVAD group as compared to OHT (35.3% versus 21.8%, P<0.01). Advanced therapies (OHT and LVAD) during ECMO support (80.5%) were more frequent than those after ECMO wean (19.5%), as shown in Table 5. Finally, there were 9 patients that required ECMO support after OHT. Of those, 2 patients were decannulated before OHT. There were no patients that were supported with ECMO post-LVAD implantation.
Table 5.
ECMO Specifications/Information
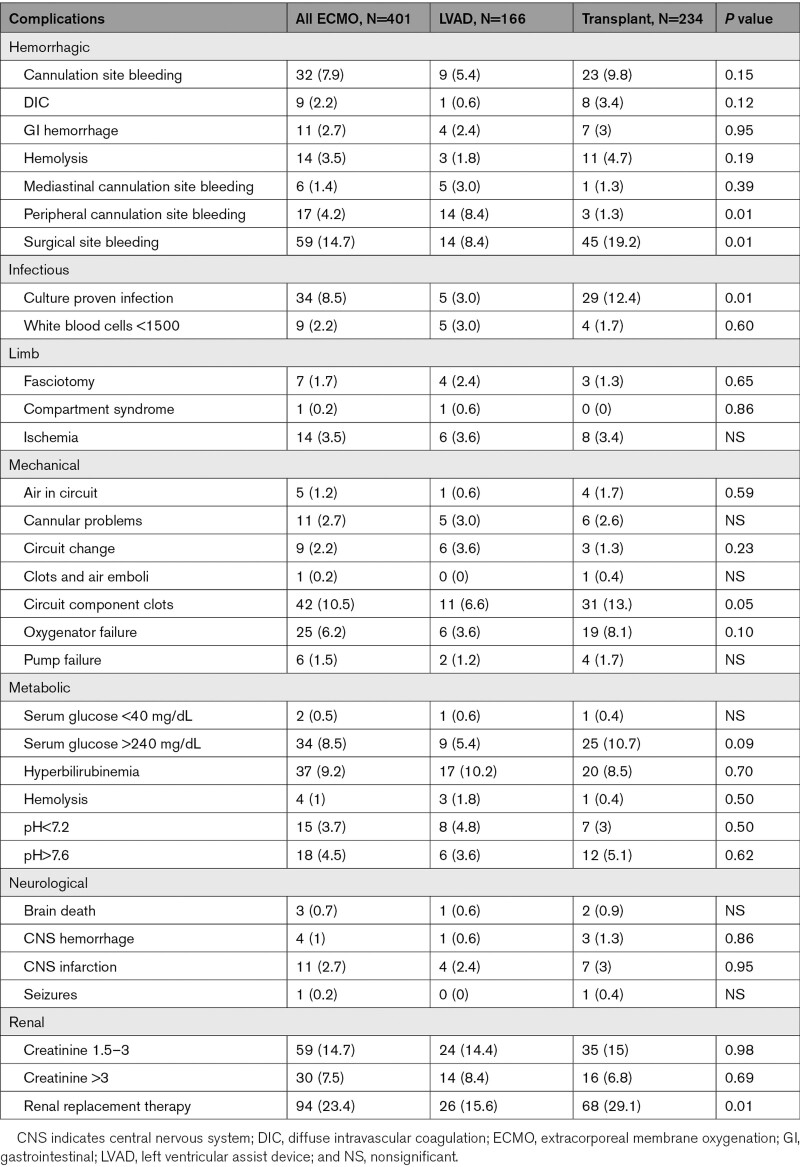
Renal replacement therapy (23.4%), surgical site bleeding (14.7%), worsening kidney function (14.7%), cardiac arrhythmias (14%), and circuit clots (10.5%) were the most common ECMO complications (Table 6). Patients who received OHT were more likely to require additional inotropic support after ECMO deployment (36.3% versus 9.6%; P<0.01), have more surgical site bleeding complications (19.2% versus 8.4%; P=0.004), culture proven infection (12.4% versus 3%; P=0.01), circuit clots (13.2% versus 6.6%; P=0.05), and continuous renal replacement therapy requirement (29.1% versus 15.6%; P=0.002), whereas patients that received LVAD were more likely to have more cannulation site bleeding (8.4% versus 1.3%; P=0.01).
Table 6.
ECMO Complications
Mortality, LOS, and Independent Predictors
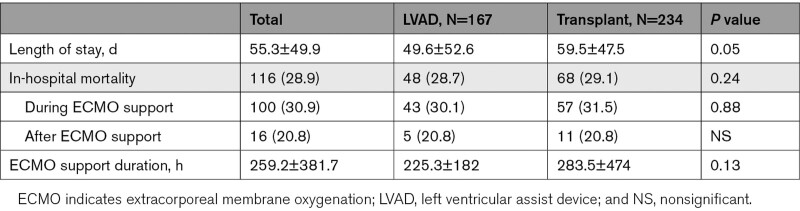
All-cause in-hospital mortality was 28.9% and was not different between the groups ([28.7% versus 29.1%; P=0.24], Table 7). Figure 3 shows the temporal variation of in-hospital mortality throughout the study period. As the number of procedures increased overtime, combined in-hospital mortality ranged between 19% and 36% without significant differences between the 2 groups. However, there was a trend towards reduced mortality in the OHT group in 2019. In-hospital mortality for patients who underwent the procedure while on ECMO versus underwent the procedure after ECMO decannulation was similar (P=0.11). Total LOS was 55.3±49.9 days and was significant longer for patients that underwent OHT compared with those that received an LVAD (59.5 versus 49.6 days; P=0.05), as shown in Table 7. The ECMO therapy duration between the 2 groups did not differ significantly (225.3 versus 283.5 hours, P=0.13).
Table 7.
Outcomes
Figure 3.
All-cause mortality temporal trends (1) in all extracorporeal membrane oxygenation (ECMO) patients and (2) by strategy group (left ventricular assist device [LVAD] vs orthotopic heart transplant [OHT]).
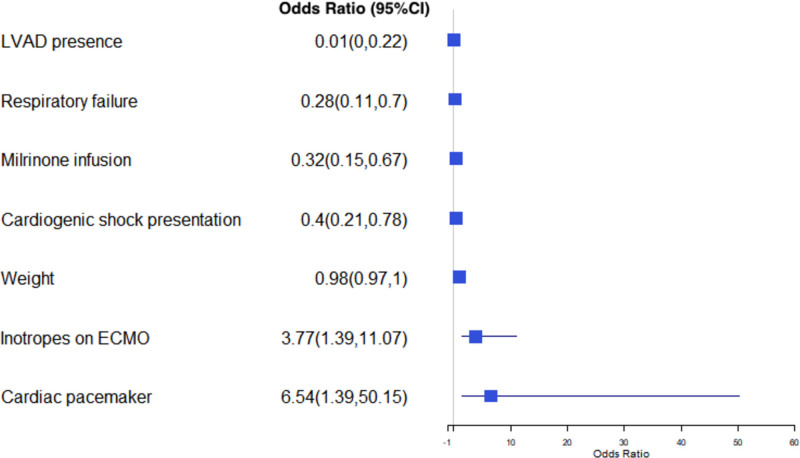
Using the modified imputed logistic regression analysis, we identified predictors of undergoing LVAD versus OHT. Higher weight (OR=0.98 [CI, 0.97–0.99]; P=0.01), CS presentation (OR=0.40 [CI, 0.21–0.78]; P=0.01), history of LVAD implantation (OR=0.01 [CI, 0.0001–0.22]; P=0.05), respiratory failure (OR=0.28 [CI, 0.11–0.70]; P=0.01), and milrinone infusion (OR=0.32 [CI, 0.15–0.67]; P=0.01) were independently associated with LVAD implantation, whereas prior cardiac transplant (OR=31.26 [CI, 3.84–780.5]; P=0.01), use of a temporary pacemaker (OR=6.5 [CI, 1.39–50.15]; P=0.03), and increased requirement of inotropes during ECMO support (OR=3.77 [CI, 1.39–11.07]; P=0.01) were independently associated with undergoing OHT (Figure 4). These predictors were confirmed with logistic regression after Least Absolute Shrinkage and Selection Operator with the addition of pre-ECMO arrest (OR=0.5 [CI, 0.25–0.99]; P=0.05) as an additional predictor for LVAD implantation. In a secondary analysis incorporating hemodynamics in the predictive model, no additional independent predictors were identified. There were no significant differences in additional sensitivity analyses performed excluding patients with extracorporeal resuscitation and venous-venous ECMO indications (Tables S1 through S3).
Figure 4.
Predictors of undergoing left ventricular assist device (LVAD) vs orthotopic heart transplant (OHT). An odds ratio (OR) <1 denotes that an LVAD strategy is more likely, whereas an OR >1 denotes that an OHT strategy is more likely. ECMO indicates extracorporeal membrane oxygenation.
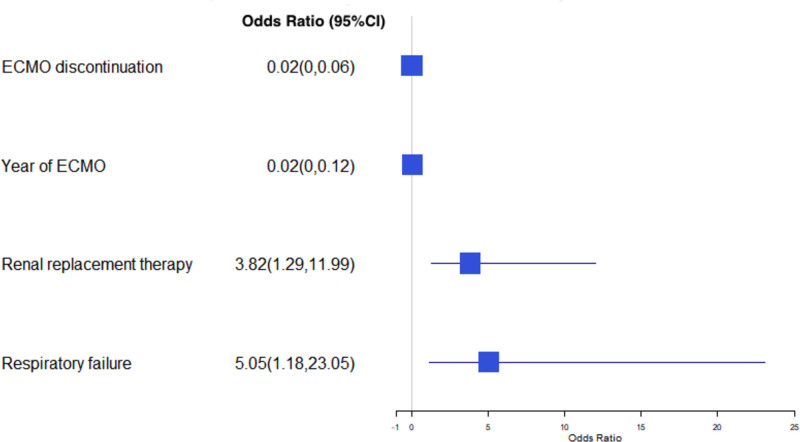
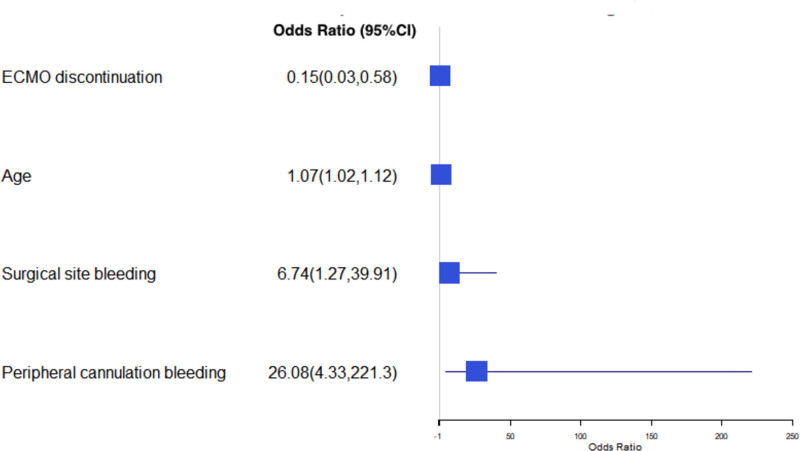
Using a different model assessing survival for each group individually, while respiratory failure (OR=5 [CI, 1.17–23.1]; P=0.03) and continuous renal replacement therapy (OR=3.82 [CI, 1.28–11.9]; P=0.02) were associated with increased mortality, using ECMO to bridge to OHT in the most contemporary years (OR=0.023 [CI, 0.003–0.017]; P<0.01) and discontinuing ECMO before OHT (OR=0.015 [CI, 0.003–0.06]; P<0.01) were strongly related with improved in-hospital survival after heart transplantation (Figure 5). Furthermore, increasing age (OR=1.07 [CI, 1.02–1.12]; P=0.01), cannulation bleeding (OR=26.1 [CI, 4.32–221.3]; P=0.01), and surgical bleeding (OR=6.7 [CI, 1.26–39.9], P=0.03) were associated with increased mortality in patients that underwent LVAD implantation (Figure 6). There were no significant differences in additional sensitivity analyses performed excluding patients with extracorporeal resuscitation and venous-venous ECMO indications (Tables S1 through S3).
Figure 5.
Predictors of mortality in the orthotopic heart transplant (OHT) group. An odds ratio (OR) >1 denotes increased mortality, whereas an OR <1 denotes decreased mortality. ECMO indicates extracorporeal membrane oxygenation.
Figure 6.
Predictors of mortality in left ventricular assist device (LVAD) group. An odds ratio (OR) >1 denotes increased mortality, whereas an OR <1 denotes decreased mortality. ECMO indicates extracorporeal membrane oxygenation.
Supplemental Analyses
To address the impact of the heart transplant organ allocation policy changes in 2018, we performed additional analyses for OHT patients between 2011 to 2017 and 2019 periods (Tables S4 through S7). Patients that underwent OHT in 2019 had more frequently chronic kidney disease/end-stage renal disease (22.2% versus 5.9%), presented with CS (58.3% versus 35.3%), and underwent OHT on ECMO more commonly (94.4% versus70%) as compared to the 2011 to 2017 patients. However, in-hospital mortality was significantly lower in 2019 patients (5.6% versus 32.9). There were no other differences that were observed.
In addition, given that 20% of patients underwent advanced therapies after decannulation, we sought to identify predictors of decannulation. Overall, patients that were decannulated had ECMO circuit clots. There were no other differences that were observed. In-hospital mortality, LOS, and ECMO duration did not differ significantly. A discontinuation intent (OR=3.6; P=0.01), cardiac arrest presentation (OR=1.94; P=0.05), and intraaortic balloon pump support (OR=1.86; P=0.05) were predictive of decannulation before OHT/LVAD (Tables S8).
Finally, we assessed the impact of ACS presentation in this population (Tables S9 through S12). Non-ACS OHT patients were more likely to be younger, have a history of cardiomyopathy, whereas non-ACS LVAD patients were more likely to be heavier, have a history of CAD, present with CS, and cardiac arrest. There were no differences in in-hospital mortality. Regarding ACS patients, ACS LVAD patient were more likely to be older, have increased weight, present with CS, have cardiac arrest and undergo on-pump LVAD compared with ACS OHT. ACS OHT patients have a greater LOS.
Discussion
As the trend of ECMO use as intermediary to advanced heart failure therapies has been rising over the past several years, there has been an increased demand for evidence supporting these practices. In this present analysis, we sought to elucidate various aspects of patients bridged with ECMO towards either LVAD or OHT. Our findings from this analysis can be summarized as follows: (1) there has been an increase in use of ECMO as bridge to either therapy in the last decade, (2) the cumulative in-hospital mortality remains high at 28.9%, however, the temporal trends do not appear to differ between the 2 groups, (3) 80% of LVAD/OHT occurred during ECMO support with LVAD more than OHT, (4) patients that underwent OHT had significantly longer stay in the hospital compared with LVAD, (5) increased weight, CS presentation, prior LVAD, respiratory failure, and milrinone infusion independently predicted LVAD implantation, whereas prior transplant, use of a temporary pacemaker, and increased requirement of inotropes on ECMO were independently associated with undergoing OHT, and (4) respiratory failure and continuous renal replacement therapy were associated with increased mortality in OHT, whereas age, surgical, and cannulation bleeding predicted mortality in LVAD.
Our cohort demographics are different than cohorts where ECMO has been used for a different indication such as postcardiotomy shock, CS, and cardiac arrest in which patients were older and had more comorbid conditions.9–12 Hernandez-Montfort et al13 using a contemporary cohort of CS patients, showed that patients receiving heart replacement therapies were younger, had lower weight, and had less comorbidities compared with those who recovered or died. Since all patients in our cohort received heart replacement therapies, these age and weight differences likely reflect the unique selection process in deploying ECMO for patients with reversible causes of cardiomyopathy (myocarditis) or those who are thought to be appropriate candidates for OHT/LVAD. In fact, the decision-making process early after deployment is identical as ECMO is not an etiologic treatment but merely a temporizing measure while more durable therapies (recovery, LVAD, OHT) can be considered. Of note, patients who received LVAD were older that those receiving OHT (50.5 versus 42.7 years; P<0.001) with increased number of comorbid conditions at the time of implantation.
The same notion of selection bias is likely applicable for the characteristics of presentation for these patients. Overall, patients in the LVAD group were more critically ill on presentation as evidenced by the observation that they were more likely to present with CS, cardiac arrest, ACS, and acute kidney injury when compared with the OHT group. Although data regarding acute presentation of patients with CS that ended up undergoing OHT or LVAD have been scarce, the preponderance of more severe presentation in patients with LVAD is unlikely due to chance effect. Based on their risk profile and comorbid conditions, this group is more likely to exhibit a poor outcome posttransplantation. In addition, despite the improvement of neurologically favorable results post-extracorporeal resuscitation after cardiac arrest, overall outcomes are still meager. As such, physicians faced with an ethical dilemma of poor posttransplant outcome for a finite resource may opt for stabilization with durable LVADs. Of note, although ACS presentation was higher in LVAD group (24.4% versus 7.9%, P<0.001), the overall ACS presentation was as low as 15% in our population. While this might seem counterintuitive, prior evidence shows that CS complicates about 5% to 10% of acute myocardial infarction.14
The use of ECMO as bridge to advanced therapies has increased in the last decade due to technological improvements, increasing availability and familiarity of medical staff, its ability to provide biventricular support and ease of implantation in the catheterization lab.1 Interestingly, the cases of OHT after ECMO have recently decreased along with a concurrent increase in LVAD as shown in Figure 1. Similar declining ECMO to OHT trends have been observed in the United States.15 These inversing trends have to be interpreted within the context of outcomes for this population. While superior to other ECMO populations, mortality in patients bridged to LVAD/OHT remains unacceptably high. We found a cumulative in-hospital mortality of ≈30% that was similar in both groups. A single-center experience from France had comparable in-hospital mortality of 38.5% and 29% in OHT and LVAD, respectively.16 Contemporary data from the United Network for Organ Sharing and International Society for Heart and Lung Transplantation (ISHLT) databases found a 1-year survival rate of 68% and 71.2%, respectively, after OHT, with ECMO use independently associated with mortality.4,15 However, patients who survive exhibit a superior long-term outcome after OHT showing an annual survival rate of 90%.4 This latter finding may be partially responsible for the declining numbers of OHT.
Pulmonary artery pressure is an important determinant of future therapies (OHT versus LVAD) and long-term outcomes. OHT is avoided in patients with elevated pulmonary pressures given the high incidence of graft right ventricular failure postoperatively with a subsequent increase in mortality.17 In that context, LVAD treatment is preferred as it has been shown to normalize pulmonary pressures re-introducing future eligibility for heart transplant.18 In our cohort, the mean pulmonary artery pressure was elevated at 31.5 mm Hg. Although not statistically significant, mean pulmonary artery pressure was numerically higher in LVAD group (32.9 versus 29.8 mm Hg). Same trends are seen in wedge pressure (24.9 versus 20.0 mm Hg). Although numerically congruent with previous reports definite conclusions on the hemodynamic profiling of the population cannot be made given the low numbers provided by the registry.15,19,20
Patients that underwent heart transplantation had significant longer hospital stay than those undergoing LVAD implantation (59.5 versus 49.6 days; P=005). The LVAD group exhibited a lower LOS despite higher age and more severe presentation. This finding could be attributed to a few plausible explanations. First, patients that underwent transplant may have to wait longer times until a suitable graft becomes available. Second, in our analysis, the OHT group experienced more complications, including worsening hemodynamic instability, surgical bleeding, circuit clots, and renal replacement, that could contribute to prolonged hospitalization. Third, this difference could reflect physician’s practices and comfort leading to a more conservative management of the OHT group. In patients with ECMO, post-LVAD LOS was recently found to be 29 days, whereas for OHT could be up to 69 days.21 Indeed, perioperative ECMO use has been found to be an independent predictor of prolonged LOS in both LVAD and OHT populations.22,23
Increased weight, CS presentation, respiratory failure, prior LVAD implantation, and milrinone infusion independently predicted LVAD implantation. While increased weight is likely the result of the selection process for heart transplant, CS presentation, and respiratory failure would suggest a relatively sicker subgroup of patients. As increased body mass index and mechanical ventilation has been previously identified as independent predictors of poor posttransplant outcomes, our findings appear rational.15 CS in patients with previous LVAD is likely to result from LVAD malfunction or right ventricular failure. While there is no way to know that from our data, previous LVAD strongly predicted recurrent LVAD implantation. In this case, LVAD malfunction and surgical considerations may have impacted our results. On the contrary, prior heart transplant and the use of a temporary pacemaker predicted OHT. While the former appears a logical finding, the latter does not have a straightforward cause. A possible explanation could include cardiac sarcoidosis and giant cell myocarditis as concomitant causes of atrioventricular block and CS.24 In the same context, use of a temporary pacemaker could be an indicator of a global biventricular cardiomyopathy that would favor transplant over LVAD.
Finally, we showed that respiratory failure and continuous renal replacement therapy conveyed a 5-fold and a 4-fold increase, respectively, in in-hospital mortality for OHT patients. Moonsamy et al15 have confirmed our finding in a recent report where pretransplant dialysis and ventilator dependency conferred a 1.8- to 3-fold and 2- to 2.5-fold increase in posttransplant mortality, respectively.15 Regarding LVAD, while increasing age weakly predicted in-hospital mortality (OR=1.07; P=0.003), cannulation bleeding and surgical site bleeding conferred an astounding 26- and 7-fold respective increase in in-hospital mortality. Saeed et al found that age among body mass index, sex, lactate, atrial fibrillation, and prior surgery has been an important predictor of mortality post-LVAD.25–28 Congruent with our report, an ISHLT Mechanically Assisted Circulatory Support registry study found increased rates of bleeding, cerebrovascular accidents, and mortality after mechanical circulatory support in patients undergoing LVAD implantation.29 Others have shown that ECMO duration >7 days has prognostic implications on mortality post-LVAD.30 While this could be a reflection of ECMO-related complications due to prolonged therapy, it may represent a surrogate for peripheral vascular disease complications after LVAD implantation, although causal inferences cannot be made. Vascular disease has been found to be a strong predictor of mortality in this population.31,32
Limitations
The findings of our analysis should be interpreted within the context of its limitations. First, the ELSO database uses ICD-9 and ICD-10 for coding purposes from which most of our data regarding diagnoses and procedures are derived. Thus, the use of administrative data set for this study may make it prone to errors of coding, including miscoding, under coding, or over coding. However, we think that such effect is negligible as much of our data match data from previous studies. Second, our ability to find differences or predictors in hemodynamic parameters might have been impacted by missing patient data in the ELSO database. To address this problem and increase the reliability of our findings, we have created a separate logistic regression model including only the population that had nonmissing hemodynamic data. Importantly, complete hemodynamic profiling that has been shown to predict outcomes before mechanical circulatory support initiation was not available on the basis of the ELSO database.33 Third, the ELSO database does not provide the breadth, or the longitudinal outcomes provided by other databases. However, the goal of our study was to describe the ECMO population undergoing OHT/LVAD along with perioperative factors that affect in-hospital outcomes. We think that our study provides a distinct wealth of information that pertains to acute cardiovascular care physicians.
Conclusions
ECMO use has been increasing in the recent year as bridge to advanced therapies with LVAD case more than OHT. The LVAD group had a more severe presentation and comorbidities, but hemodynamics did not differ between the 2 groups. Mortality remains high overall, and OHT has longer LOS. Further studies are needed to confirm our results.
Article Informaton
Sources of Funding
None.
Disclosures
Dr Tonna is supported by a Career Development Award from the National Institutes of Health/National Heart, Lung, and Blood Institute (K23 HL141596). Dr Tonna received speaker fees and travel compensation from LivaNova and Philips Healthcare, unrelated to this work. The other authors report no conflicts.
Supplemental Material
Tables S1–S12
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACS
- acute coronary syndrome
- CS
- cardiogenic shock
- ECMO
- extracorporeal membrane oxygenation
- ELSO
- Extracorporeal Life Support Organization
- ICD
- International Classification of Diseases
- LOS
- length of stay
- LVAD
- left ventricular assist device
- OHT
- orthotopic heart transplant
- OR
- odds ratio
This manuscript was sent to Frank W. Sellke, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 55.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.121.008777.
Contributor Information
Ioannis Mastoris, Email: ioannis.mastoris@outlook.com.
Joseph E. Tonna, Email: joseph.tonna@hsc.utah.edu.
Jinxiang Hu, Email: jhu2@kumc.edu.
Andrew J. Sauer, Email: asauer@kumc.edu.
Nicholas A. Haglund, Email: nhaglund@kumc.edu.
Peter Rycus, Email: prycus@elso.org.
Yu Wang, Email: y183w954@kumc.edu.
William J. Wallisch, Email: john.wallisch@gmail.com.
Travis O. Abicht, Email: TABICHT@kumc.edu.
Matthew R. Danter, Email: mdanter@kumc.edu.
Ryan J. Tedford, Email: tedfordr@musc.edu.
James C. Fang, Email: james.fang@hsc.utah.edu.
References
- 1.Guglin M, Zucker MJ, Bazan VM, Bozkurt B, El Banayosy A, Estep JD, Gurley J, Nelson K, Malyala R, Panjrath GS, et al. Venoarterial ECMO for adults: JACC scientific expert panel. J Am Coll Cardiol. 2019; 73:698–716. doi: 10.1016/j.jacc.2018.11.038 [DOI] [PubMed] [Google Scholar]
- 2.The Extracorporeal Life Support Organization (ELSO) Accessed November 25, 2020. www.elso.org.
- 3.Keebler ME, Haddad EV, Choi CW, McGrane S, Zalawadiya S, Schlendorf KH, Brinkley DM, Danter MR, Wigger M, Menachem JN, et al. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail. 2018; 6:503–516. doi: 10.1016/j.jchf.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 4.Yin MY, Wever-Pinzon O, Mehra MR, Selzman CH, Toll AE, Cherikh WS, Nativi-Nicolau J, Fang JC, Kfoury AG, Gilbert EM, et al. Post-transplant outcome in patients bridged to transplant with temporary mechanical circulatory support devices. J Heart Lung Transplant. 2019; 38:858–869. doi: 10.1016/j.healun.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Pagani FD, Lynch W, Swaniker F, Dyke DB, Bartlett R, Koelling T, Moscucci M, Deeb GM, Bolling S, Monaghan H, et al. Extracorporeal life support to left ventricular assist device bridge to heart transplant: a strategy to optimize survival and resource utilization. Circulation. 1999; 100(19 suppl):II206–II210. doi: 10.1161/01.cir.100.suppl_2.ii-206 [DOI] [PubMed] [Google Scholar]
- 6.ECMO Registry of the Extracorporeal Life Support Organization (ELSO). February, 2021, Ann Arbor, Michigan [Google Scholar]
- 7.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, Bartlett RH, Tonna JE, Hyslop R, Fanning JJ, et al. ; Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020; 396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tibshirani R. Regression Shrinkage and selection via the LASSO. J Royal Stat Soc Series B (Methodol). 1996; 58:267–288 [Google Scholar]
- 9.Xie A, Phan K, Tsai YC, Yan TD, Forrest P. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: a meta-analysis. J Cardiothorac Vasc Anesth. 2015; 29:637–645. doi: 10.1053/j.jvca.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 10.Chang CH, Chen HC, Caffrey JL, Hsu J, Lin JW, Lai MS, Chen YS. Survival analysis after extracorporeal membrane oxygenation in critically ill adults: A Nationwide Cohort Study. Circulation. 2016; 133:2423–2433. doi: 10.1161/CIRCULATIONAHA.115.019143 [DOI] [PubMed] [Google Scholar]
- 11.Biancari F, Dalén M, Perrotti A, Fiore A, Reichart D, Khodabandeh S, Gulbins H, Zipfel S, Al Shakaki M, Welp H, et al. Venoarterial extracorporeal membrane oxygenation after coronary artery bypass grafting: results of a multicenter study. Int J Cardiol. 2017; 241:109–114. doi: 10.1016/j.ijcard.2017.03.120 [DOI] [PubMed] [Google Scholar]
- 12.Choi DS, Kim T, Ro YS, Ahn KO, Lee EJ, Hwang SS, Song SW, Song KJ, Shin SD. Extracorporeal life support and survival after out-of-hospital cardiac arrest in a nationwide registry: a propensity score-matched analysis. Resuscitation. 2016; 99:26–32. doi: 10.1016/j.resuscitation.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Montfort J, Sinha SS, Thayer KL, Whitehead EH, Pahuja M, Garan AR, Mahr C, Haywood JL, Harwani NM, Schaeffer A, et al. Clinical outcomes associated with acute mechanical circulatory support utilization in heart failure related cardiogenic shock. Circ Heart Fail. 2021; 14:e007924. doi: 10.1161/CIRCHEARTFAILURE.120.007924 [DOI] [PubMed] [Google Scholar]
- 14.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014; 3:e000590. doi: 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moonsamy P, Axtell AL, Ibrahim NE, Funamoto M, Tolis G, Lewis GD, D’Alessandro DA, Villavicencio MA. Survival after heart transplantation in patients bridged with mechanical circulatory support. J Am Coll Cardiol. 2020; 75:2892–2905. doi: 10.1016/j.jacc.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 16.Rousse N, Juthier F, Pinçon C, Hysi I, Banfi C, Robin E, Fayad G, Jegou B, Prat A, Vincentelli A. ECMO as a bridge to decision: recovery, VAD, or heart transplantation? Int J Cardiol. 2015; 187:620–627. doi: 10.1016/j.ijcard.2015.03.283 [DOI] [PubMed] [Google Scholar]
- 17.Vakil K, Duval S, Sharma A, Adabag S, Abidi KS, Taimeh Z, Colvin-Adams M. Impact of pre-transplant pulmonary hypertension on survival after heart transplantation: a UNOS registry analysis. Int J Cardiol. 2014; 176:595–599. doi: 10.1016/j.ijcard.2014.08.072 [DOI] [PubMed] [Google Scholar]
- 18.Selim AM, Wadhwani L, Burdorf A, Raichlin E, Lowes B, Zolty R. Left ventricular assist devices in pulmonary hypertension group 2 with significantly elevated pulmonary vascular resistance: a bridge to cure. Heart Lung Circ. 2019; 28:946–952. doi: 10.1016/j.hlc.2018.04.299 [DOI] [PubMed] [Google Scholar]
- 19.Akin S, Soliman O, de By TMMH, Muslem R, Tijssen JGP, Schoenrath F, Meyns B, Gummert JF, Mohacsi P, Caliskan K; EUROMACS investigators. Causes and predictors of early mortality in patients treated with left ventricular assist device implantation in the European Registry of Mechanical Circulatory Support (EUROMACS). Intensive Care Med. 2020; 46:1349–1360. doi: 10.1007/s00134-020-05939-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess NR, Hickey GW, Sultan I, Kilic A. Extracorporeal membrane oxygenation bridge to heart transplant: trends following the allocation change. J Card Surg. 2020; 36:40–47. doi: 10.1111/jocs.15118 [DOI] [PubMed] [Google Scholar]
- 21.Ouyang D, Gulati G, Ha R, Banerjee D. Incidence of temporary mechanical circulatory support before heart transplantation and impact on post-transplant outcomes. J Heart Lung Transplant. 2018; 37:1060–1066. doi: 10.1016/j.healun.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 22.Crawford TC, Magruder JT, Grimm JC, Suarez-Pierre A, Patel N, Sciortino CM, Zehr KJ, Mandal K, Tedford RJ, Russell SD, et al. A comprehensive risk score to predict prolonged hospital length of stay after heart transplantation. Ann Thorac Surg. 2018; 105:83–90. doi: 10.1016/j.athoracsur.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 23.Loyaga-Rendon RY, Boeve T, Tallaj J, Lee S, Leacche M, Lotun K, Koehl DA, Cantor RS, Kirklin JK, Acharya D. Extracorporeal membrane oxygenation as a bridge to durable mechanical circulatory support. Circulation: Heart Failure. 2020; 13:e006387. doi: 10.1161/CIRCHEARTFAILURE.119.006387 [DOI] [PubMed] [Google Scholar]
- 24.Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol. 2011; 4:303–309. doi: 10.1161/CIRCEP.110.959254 [DOI] [PubMed] [Google Scholar]
- 25.Harvey L, Holley C, Roy SS, Eckman P, Cogswell R, Liao K, John R. Stroke after left ventricular assist device implantation: outcomes in the continuous-flow era. Ann Thorac Surg. 2015; 100:535–541. doi: 10.1016/j.athoracsur.2015.02.094 [DOI] [PubMed] [Google Scholar]
- 26.Sandner SE, Zimpfer D, Zrunek P, Rajek A, Schima H, Dunkler D, Zuckermann AO, Wieselthaler GM. Age and outcome after continuous-flow left ventricular assist device implantation as bridge to transplantation. J Heart Lung Transplant. 2009; 28:367–372. doi: 10.1016/j.healun.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 27.Sabashnikov A, Mohite PN, Zych B, García D, Popov AF, Weymann A, Patil NP, Hards R, Capoccia M, Wahlers T, et al. Outcomes and predictors of early mortality after continuous-flow left ventricular assist device implantation as a bridge to transplantation. ASAIO J. 2014; 60:162–169. doi: 10.1097/MAT.0000000000000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeed D, Potapov E, Loforte A, Morshuis M, Schibilsky D, Zimpfer D, Riebandt J, Pappalardo F, Attisani M, Rinaldi M, et al. ; Durable MCS after ECLS Study Group. Transition from temporary to durable circulatory support systems. J Am Coll Cardiol. 2020; 76:2956–2964. doi: 10.1016/j.jacc.2020.10.036 [DOI] [PubMed] [Google Scholar]
- 29.Ton VK, Xie R, Hernandez-Montfort JA, Meyns B, Nakatani T, Yanase M, Shaw S, Pettit S, Netuka I, Kirklin J, et al. Short- and long-term adverse events in patients on temporary circulatory support before durable ventricular assist device: an IMACS registry analysis. J Heart Lung Transplant. 2020; 39:342–352. doi: 10.1016/j.healun.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 30.Tsyganenko D, Gromann TW, Schoenrath F, Mueller M, Mulzer J, Starck C, Krabatsch T, Stein J, Falk V, Potapov E. Predictors of mid-term outcomes in patients undergoing implantation of a ventricular assist device directly after extracorporeal life support. Eur J Cardiothorac Surg. 2019; 55:773–779. doi: 10.1093/ejcts/ezy351 [DOI] [PubMed] [Google Scholar]
- 31.Ullah W, Sattar Y, Darmoch F, Al-Khadra Y, Mir T, Ajmal R, Moussa-Pacha H, Glazier J, Asfour A, Gardi D, et al. The impact of peripheral arterial disease on patients with mechanical circulatory support. Int J Cardiol Heart Vasc. 2020; 28:100509. doi: 10.1016/j.ijcha.2020.100509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Idris A, Al-Khadra Y, Kabach A, Zaitoun A, Kaki A, Alraies MC. Peripheral arterial disease and left ventricular assist device surgical outcomes: an analysis of the national inpatient sample. J Card Fail. 2018; 24:S108. doi: 10.1016/j.cardfail.2018.07.404 [Google Scholar]
- 33.Garan AR, Kanwar M, Thayer KL, Whitehead E, Zweck E, Hernandez-Montfort J, Mahr C, Haywood JL, Harwani NM, Wencker D, et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. JACC Heart Fail. 2020; 8:903–913. doi: 10.1016/j.jchf.2020.08.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.